Abstract
Transient receptor potential (TRP) A1 and TRPM8 are ion channels that have been localized to afferent nociceptive nerves. These TRP channels may be of particular relevance to respiratory nociceptors in that they can be activated by various inhaled irritants and/or cold air. We addressed the hypothesis that mouse vagal sensory nerves projecting to the airways express TRPA1 and TRPM8 and that they can be activated via these receptors. Single cell RT-PCR analysis revealed that TRPA1 mRNA, but not TRPM8, is uniformly expressed in lung-labelled TRPV1-expressing vagal sensory neurons. Neither TRPA1 nor TRPM8 mRNA was expressed in TRPV1-negative neurons. Capsaicin-sensitive, but not capsaicin-insensitive, lung-specific neurons responded to cinnamaldehyde, a TRPA1 agonist, with increases in intracellular calcium. Menthol, a TRPM8 agonist, was ineffective at increasing cellular calcium in lung-specific vagal sensory neurons. Cinnamaldehyde also induced TRPA1-like inward currents (as measured by means of whole cell patch clamp recordings) in capsaicin-sensitive neurons. In an ex vivo vagal innervated mouse lung preparation, cinnamaldehyde evoked action potential discharge in mouse vagal C-fibres with a peak frequency similar to that observed with capsaicin. Cinnamaldehyde inhalation in vivo mimicked capsaicin in eliciting strong central-reflex changes in breathing pattern. Taken together, our results support the hypothesis that TRPA1, but not TRPM8, is expressed in vagal sensory nerves innervating the airways. TRPA1 activation provides a mechanism by which certain environmental stimuli may elicit action potential discharge in airway afferent C-fibres and the consequent nocifensor reflexes.
The majority of nerve fibres innervating the respiratory tract are bronchopulmonary afferent C-fibres. Peripheral terminals of at least a proportion of the sensory C-fibres are observed in the vicinity of the bronchial epithelium (Lamb & Sparrow, 2002). This localization allows the detection of physical and chemical irritants and the subsequent increase in central, local and peripheral reflex activity. The activation profile of these nerves is consistent with their nociceptive function, i.e. they provide the organ with a sense of its own potential injury. Accordingly, they can be activated by inflammatory mediators, acidic solutions, anosmotic solutions, and excessive, potentially harmful, mechanical distension of the tissue. Unlike the nociceptors in the somatosensory system that transmit pain messages when activated, bronchopulmonary C-fibres evoke sensations and defensive reflexes such as cough, changes in respiration pattern, mucus production, airway smooth muscle contraction, vasodilatation and dyspnoea (Coleridge & Coleridge, 1984; Lee, 2006). Excessive activation of bronchopulmonary C-fibres may be a major contributor to the symptoms of inflammatory airway diseases.
The ion channel, transient receptor potential vanilloid-1 (TRPV1), is a defining feature of bronchopulmonary nociceptors. TRPV1 confers on the nerve the ability to respond not only to pungent ingredients of hot peppers such as capsaicin, but also to increases in hydrogen ion concentrations, autacoids that act through certain G-protein coupled receptors (GPCRs), lipoxygenase products of arachidonic acid, and extreme increases in temperature (Jia & Lee, 2007). TRPV1 is a member of a large family of related ion channels. At present, the TRP receptor family consists of 28 channels which belong to seven subfamilies including canonical (TRPC), melastatin (TRPM), ankyrin (TRPA), vanilloid (TRPV), polycystin (TRPP), no mechanoreceptor potential C (NOMPC; TRPN) and mucolipin (TRPML) channels (Pedersen et al. 2005). Among these channels, TRPA1 and TRPM8 (along with TRPV1) may be of particular relevance to the physiology of bronchopulmonary C-fibres.
TRPA1, which is expressed in DRG, trigeminal and jugular/nodose nociceptors (Nagata et al. 2005), is not activated by capsaicin, but is stimulated by other pungent agents including cinnamaldehyde, allicin and allyl isothiocyanate, the active ingredient in mustard oil, wasabi and horseradish (Jordt et al. 2004; Bandell et al. 2004). Like TRPV1, TRPA1 may also be gated by autacoids that act through certain GRCRs, e.g. bradykinin acting via B2 receptors (Bandell et al. 2004). The mechanisms underlying GPCR-dependent activation of TRPA1, however, appear to be different from those involved in TRPV1 activation. TRPA1 has an N-terminal EF-hand calcium-binding domain making it sensitive to any stimulus that increases intracellular calcium (Doerner et al. 2007; Zurborg et al. 2007). This mechanism provides a pathway through which inflammatory mediators may increase the activation rate of TRPA1 containing nerves. Other potentially endogenous activators of TRPA1 that may be relevant to pulmonary pathophysiology are certain prostanoids products of oxidative stress such as 4-hydroxy-2-nonenal (Trevisani et al. 2007; Taylor-Clark et al. 2008). Certain environmental irritants such as isothiocyanates and acrolein, a highly toxic and reactive α,β-unsaturated aldehyde present in vehicle exhaust, and smoke from tobacco products (Hales et al. 1988; Hales et al. 1992) may directly activate TRPA1 (Bautista et al. 2006), providing a mechanism by which air-pollutants can lead to nasal and bronchial C-fibre activation. Cool temperatures (< 17°C) (Story et al. 2003) activate not only TRPA1 but also TRPM8 (< 26°C) (McKemy et al. 2002), which mediates the cold perception of menthol. This may be of relevance to cold air-induced respiratory reflexes. Based on these observations, TRPM8 and TRPA1, if expressed in bronchopulmonary C-fibres, may be key ion channels through which disparate stimuli activate airway nociceptive nerves. Although theoretically intriguing, at present, the extent to which bronchopulmonary C-fibres can be activated via these TRP channels is unknown. This question is specifically addressed in the present study.
Methods
Animal experiments
All experiments were performed with approval from the Bezirksregierung Hannover or the Johns Hopkins Animal Use and Care Committee.
Retrograde labelling and cell dissociation
Bronchopulmonary afferent neurons of C57/BL6 mice (male, 6–8 weeks) were retrograde-labelled using DiI (DiC18(3); Molecular Probes, Eugene, OR, USA) solution (0.1%, 50 μl; dissolved in 10% DMSO and 90% normal saline). Under anaesthesia (2 mg ketamine and 0.2 mg xylazine i.p. per mouse), mice were orotracheally intubated, and DiI was instilled into the tracheal lumen 5–9 days before an experiment.
After the animals were killed by CO2 asphyxiation, the jugular/nodose ganglia were dissected and cleared of adhering connective tissue. Isolated ganglia were incubated in the enzyme buffer (2 mg ml−1 collagenase type 1A and 2 mg ml−1 dispase II in Ca2+-, Mg2+-free Hanks' balanced salt solution) for 30 min at 37°C. Neurons were dissociated by trituration with three glass Pasteur pipettes of decreasing tip pore size, then washed by centrifugation (three times at 1000 g for 2 min) and suspended in L-15 medium containing 10% fetal bovine serum (FBS). The cell suspension was transferred onto poly d-lysine/laminin-coated coverslips. After the suspended neurons had adhered to the coverslips for 2 h, the neuron-attached coverslips were flooded with the L-15 medium (10% FBS) and used within 8 h.
Single-cell RT-PCR
First strand cDNA was synthesized from single lung-labelled jugular/nodose cells by using the SuperScript(tm) III CellsDirect cDNA Synthesis System (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's recommendations.
Cell picking
Coverslips of retrogradelly labelled, dissociated neurons were constantly perfused by Locke's solution and identified by using fluorescence microscopy. Single cells were harvested into a glass-pipette (tip diameter 50–150 μm) pulled with a micropipette puller (Model P-87, Sutter Instruments Co., Novato, CA, USA) by applying negative pressure. The pipette tip was then broken in a PCR tube containing resuspension buffer (1 μl) and RNAse inhibitor (RNAseOUT, 2 U μl−1), immediately snap frozen and stored on dry ice. From one coverslip, one to four cells were collected. A sample of the bath solution from the vicinity of a labelled neuron was collected from each coverslip for no-template experiments (bath control).
RT-PCR
Samples were defrosted, lysed (10 min at 75°C) and treated with DNAse I. Then, poly(dT) and random hexamer primers (Roche Applied Bioscience) were added. Half of the volume was reverse transcribed by adding SuperscriptIII RT for cDNA synthesis, whereas water was added to the remaining sample, which was used in the following as RNA control.
PCR
Three microlitres of each sample (cDNA, RNA control or bath control) was used for PCR amplification of mouse β-actin, TRPV1, TRPA1 and TRPM8 receptors by the HotStar Taq Poymerase Kit (Qiagen) according to the manufacturer's recommendations in a final volume of 20 μl. After an initial activation step at 95°C for 15 min, cDNAs were amplified with custom-synthesized intron-spanning primers (Invitrogen) (Table 1) by 45 cycles of denaturation at 94°C for 30 s, annealing at 60°C for 30 s and extension at 72°C for 1 min followed by a final extension at 72°C for 10 min. Products were then visualized in ethidum bromide-stained 1.5% agarose gels.
Table 1.
Sequence of primers used for analysis of murine TRPA1 receptor transcripts
| Gene | Primer | Sequence (5′-to 3′) | GenBank | Product length |
|---|---|---|---|---|
| β-Actin | Forward | CTG GTC GTC GAC AAC GGC TCC | NM_007393 | 238 bp |
| Reverse | GCC AGA TCT TCT CCA TG | |||
| TRPV1 | Forward | TCA CCG TCA GCT CTG TTG TC | NM_001001445 | 229 bp |
| Reverse | GGG TCT TTG AAC TCG CTG TC | |||
| TRPM8 | Forward | GGC TGG AGA TGA GAT TGT GAG | AF481480 | 313 bp |
| Reverse | GCT GAA GTG GGT GGA GAA GA | |||
| TRPA1 | Forward | GGA GCA GAC ATC AAC AGC AC | AY231177 | 393 bp |
| Reverse | GCA GGG GCG ACT TCT TAT C |
Drug preparations and applications
trans-Cinnamaldehyde, l-menthol and capsaicin were dissolved in ethanol and diluted with the appropriate buffer to the final concentrations. The final ethanol concentration was 0.1% for calcium measurements and patch clamp experiments, 0.1–0.3% for extracellular recordings, and 20% for lung function measurement by head-out body-plethysmography. Cinnamaldehyde was purchased from TCI America (Portland, OR, USA). Capsaicin was purchased from Sigma-Aldrich (St Louis, MO, USA). Fura-2 AM was purchased from Molecular Probes (Eugene, OR, USA). L-15 and Hanks' balanced salt solution (HBSS) were purchased from Invitrogen Corp. (Gibco).
Ca2+ imaging
The intracellular [Ca2+]free measurements were done with dissociated jugular/nodose neurons irrespective of DiI labelling in six animals. The coverslip was loaded with Fura-2 AM (8 μm) in L-15 medium containing 20% FBS and incubated for 40 min at 37°C. The coverslip was placed in a custom-built chamber (600 μl bath volume) that was superfused with Locke's solution (at 35°C; composed of (mm): 136 NaCl, 5.6 KCl, 1.2 MgCl2, 2.2 CaCl2, 1.2 NaH2PO4, 14.3 NaHCO3 and 10 dextrose; pH 7.3–7.4) for 20 min before the experiment by an infusion pump (8 ml min−1). Changes in intracellular [Ca2+]free were measured by digital microscopy (Universal: Carl Zeiss, Inc., Thornwood, NY, USA) with in-house equipment for ratiometric recording of single cells. A field of cells was monitored by sequential dual excitation, 352 and 380 nm, and the analysis of the image ratios used methods previously described (MacGlashan., 1989). The ratio images were acquired every 6 s. Superfused buffer was stopped 20 s prior to drug applications. In each experiment, the cells on the coverslip were exposed to cinnamaldehyde (100 μm), menthol (100 μm), capsaicin (1 μm) and KCl (75 mm) for 1 min each. Between each stimulus, the cells were washed with fresh buffer for at least 3 min for the cells to recover prior to the addition of the second stimulus. Each set of images for the Ca2+ measurements also included a brightfield image of the field of cells under study. Cells that had an average diameter (long and short axis) of over 15 μm were analysed. Those cells that failed to respond to capsaicin were considered healthy neurons only if they responded to KCl with a rapid rise in Ca2+.
Whole-cell patch clamp recording
The jugular/nodose neurons labelled from the airways were identified by fluorescence microscopy using 560 nm of excitation filter and 480 nm of emission filter. To maintain intracellular signal pathways and a native intracellular Cl−concentration, a gramicidin-perforated whole-cell patch-clamp technique was employed using a Multiclamp 700A amplifier and Axograph 4.9 software (Axon Instruments). A pipette (1.5–3 MΩ) was filled with a solution composed of (mm): 140 KCl, 1 CaCl2, 2MgCl2, 10 Hepes, 11 EGTA and 10 dextrose; titrated to pH 7.3 with KOH; 304 mosmol l−1. Gramicidin was dissolved in DMSO and mixed with the pipette solution to give 1 μg ml−1 just prior to each recording. Cell membrane potential was held at −60 mV. During the experiments, the cells were continuously superfused (6 ml min−1) by gravity with Locke's solution. In order to stimulate the cells, vehicle (0.1 % ethanol), cinnamaldehyde (100 μm), menthol (100 μm) and capsaicin (1 μm) were added to the buffer. All recordings were done at 35°C.
Extracellular recording
The animals were killed by CO2 asphyxiation followed by exsanguination. The innervated isolated trachea/bronchus was prepared as previously described (Riccio et al. 1996). Briefly, the airways and lungs with their intact extrinsic innervation (vagus nerve including jugular/nodose ganglia) were taken and placed in a dissecting dish containing Krebs-bicarbonate buffer solution composed of (mm): 118 NaCl, 5.4 KCl, 1.0 NaH2PO4, 1.2 MgSO4, 1.9 CaCl2, 25.0 NaHCO3 and 11.1 dextrose, and equilibrated with 95% O2 and 5% CO2 (pH 7.2–7.4). Connective tissue was trimmed away leaving the trachea and lungs with their intact nerves. The airways were then pinned to the larger compartment of a custom-built two-compartment recording chamber which was lined with silicone elastomer (Sylgard). The jugular/nodose ganglion of one side was gently pulled into the adjacent compartment of the chamber through a small hole and pinned. Both compartments were separately superfused with the buffer with a flow rate of 1.5 ml min−1 which was warmed by a warming jacket (39–42°C) to keep airway tissues and ganglia at 37°C. A sharp glass electrode was pulled by a Flaming–Brown micropipette puller (P-87; Sutter Instrument Co.) and filled with 3 m NaCl solution. The electrode was gently inserted into the jugular/nodose ganglion so as to be placed near the cell bodies. The recorded action potentials were amplified (Microelectrode AC amplifier 1800; A-M Systems, Everett, WA, USA), filtered (0.3 kHz of low cut-off and 1 kHz of high cut-off), and monitored on an oscilloscope (TDS340; Tektronix, Beaverton, OR, USA) and a chart record (TA240; Gould, Valley View, OH, USA). The scaled output from the amplifier was captured and analysed by a Macintosh computer using NerveOfIt software (Phocis, Baltimore, MD, USA). For measuring conduction velocity, an electrical stimulation (S44; Grass Instruments, Quincy, MA, USA) was applied on the core of the receptive field. The conduction velocity was calculated by dividing the distance along the nerve pathway by the time delay between the shock artifact and the action potential evoked by electrical stimulation. If a C-fibre (<1 m s−1) was found, the recording was started. One millilitre of vehicle, cinnamaldehyde (30, 100 or 300 μm), menthol (300 μm or 1 mm) or capsaicin (1 μm) was intratracheally applied for 10 s.
Assessment of lung function
In order to identify the role of TRPA1 on afferent sensory nerve function, reactivity of sensory airway nerves was measured in response to cinnamaldehyde aerosol by head-out body-plethysmography (HBP) in conscious, restrained and spontaneously breathing Balb/c mice (female, 10 weeks, n = 16) as previously described (Nassenstein et al. 2006). Baseline measurements were followed by aerosol provocation with vehicle (20% EtOH in PBS) and increasing doses of cinnamaldehyde. The average of the length of pause prior to expiration (time of braking, Tb (s); Vijayaraghavan et al. 1994) was calculated from the breaths during cinnamaldehyde aerosol challenge and compared with the corresponding data obtained from vehicle control values. Tb reflects a change in breathing pattern that is directly dependent on sensory stimulation by a central reflex loop (Remmers et al. 1986).
Statistical analysis
There was little (< 1 Hz) or no background activity in the extracellular recordings. In all experiments, a single unit was recorded. The action potential discharge evoked by vehicle and cinnamaldehyde stimulation were quantified off-line and segregated into consecutive 1 s bins. The response was considered to be terminated when the number of spikes in the bins declined to < 2 × baseline. The total number of action potentials recorded following vehicle, cinnamaldehyde, or capsaicin application was counted. The peak frequency evoked by a stimulus was quantified as the maximum number of action potentials that occurred within any 1 s bin. Data obtained by intracellular [Ca2+]free measurement were expressed as the 352/380 ratio. If a cell lacked a robust response to capsaicin (1 μm) or KCl (75 mm), or had an averaged diameter (long and short axis) of less than 15 μm, it was not included in the analysis. A cell was considered as cinnamaldehyde-, capsaicin- or KCl positive if the drug-induced peak increase was greater than 2 × standard deviation above the mean baseline 352/380 ratio. Student's t test for paired or unpaired data, ANOVA, or the ‘closed test procedure’ was used when appropriate. The ‘closed test procedure’ was used in connection with hierarchically ordered hypotheses to identify differences between the response to vehicle and different doses of cinnamaldehyde, which were measured by means of head-out body-plethysmography. The basic assumption of a possible effect of different cinnamaldehyde doses on the breathing pattern was that of a monotone dose–response relationship. The sequence of hypotheses was ordered by applied cinnamaldehyde doses. Beginning with the highest dose, each comparison with the vehicle control group was performed by conducting a paired two-sided t test with Welch's correction. The procedure stopped as the first non-significant result occurred. P < 0.05 was considered statistically significant. All data are expressed as means ± s.e.m.
Results
TRPA1 is expressed in vagal nerve afferents innervating the airways
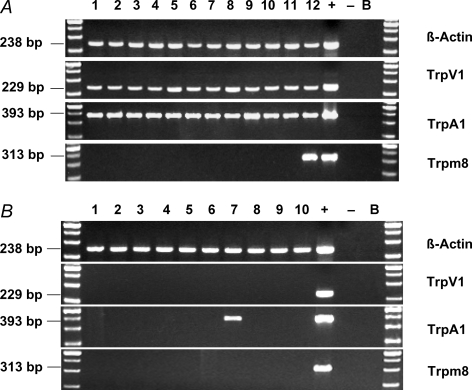
In order to address the hypothesis that TRPV1 expressing nerves innervating mouse lungs also express TRPA1, we used single neuron RT-PCR. The retrograde labelled neurons were individually isolated and, after reverse transcription, PCR was performed using β-actin-, TRPV1-, TRPA1-, and TRPM8-specific primers. TRPA1 mRNA expression could be observed in all investigated TRPV1+ cells (12/12 neurons). In TRPV1− cells, TRPA1 mRNA expression was detected in only 1 out of 10 cells. TRPM8 was completely lacking in TRPV1− neurons, and was observed in only 1 of 12 TRPV1+ neurons. No specific PCR products were seen in RNA controls of each individual cell and bath controls, whereas β-actin, TRPV1, TRPA1 and TRPM8 mRNA could be consistently amplified from the cDNA of a whole vagal ganglion which was used as positive control. All PCR products showed the expected lengths (Fig. 1).
Figure 1. TRPA1, but not TRPM8, mRNA is expressed in vagal airway neurons.
Single cell RT-PCR of TRPV1+ (A) or TRPV1− (B) jugular/nodose cells; 1–12 or 1–10, respectively, represent 12 or 10 individual jugular/nodose ganglion neurons retrograde lung-labelled from the lungs. Samples in which the reverse transcriptase was omitted (‘−’) served as negative control. cDNA obtained from a whole jugular/nodose ganglion served as positive control (‘+’); B = bath control (bath solution was used as a template)
Cinnamaldehyde increases intracellular [Ca2+]free in vagal nerve afferents innervating the airways
In order to investigate TRPA1 and TRPM8 at a functional level we first studied the effect of cinnamaldehyde, a selective TRPA1 agonist, and menthol, a TRPM8 stimulus, on intracellular [Ca2+]free in dissociated vagal sensory neurons in general (not necessarily lung-specific neurons). Neurons were identified based on their morphology and by a KCl-induced intracellular [Ca2+]free increase. Altogether 189 neurons were investigated; 112/189 (59.3%) neurons responded to capsaicin, whereas the remaining 77 (40.7%) were capsaicin insensitive. Half (52/112, 46.4%) of these capsaicin-sensitive neurons also responded to cinnamaldehyde with an increase in intracellular [Ca2+]free. Menthol stimulated calcium increases in 14/112 (12.5%) capsaicin-sensitive neurons. Most cells which were activated by menthol also responded to cinnamaldehyde (10/14, 71.4%) (Table 2).
Table 2.
Cinnamaldehyde and menthol induced increases in intracellular [Ca2+]free in vagal sensory neurons
| Lung labelled neurons | Non-lung labelled neurons | |||
|---|---|---|---|---|
| Capsaicin+ | Capsaicin− | Capsaicin+ | Capsaicin− | |
| Cinnamaldehyde+ | 15/15 (100%) | 0/9 (0%) | 52/112 (46.4%) | 8/77 (10.4%) |
| Menthol+ | 0/15 (0%) | 0/9 (0%) | 14/112 (12.5%) | 3/77 (2.2%) |
Number (%) of neurons that responded positively with an increase in intracellular [Ca2+]free. A positive response was taken as an increase in calcium that was greater than 2 × standard deviation above the mean baseline 352/380 ratio.
The vast majority (69/77; 89.6%, or 74/77, 96.1%, respectively) of capsaicin-insensitive neurons (presumably non-nociceptive neurons) were found to be insensitive to cinnamaldehyde and menthol (Table 2).
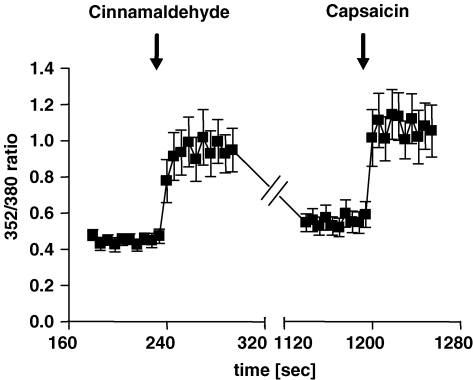
In order to investigate if vagal neurons innervating the airways exhibit a response pattern consistent with the ganglion as a whole, we evaluated the calcium response in neurons which were retrograde-labelled from the lungs. Consistent with the single-cell RT-PCR data, 15/24 (62.5%) lung-specific DiI+ neurons were responsive to capsaicin and all 15 capsaicin-sensitive neurons (100%) showed a distinct increase in intracellular [Ca2+]free after stimulation with cinnamaldehyde. The mean response to cinnamaldehyde (100 μm) was comparable to the capsaicin (1 μm)-induced response and significantly different from the mean baseline intracellular [Ca2+]free of each individual cell (P < 0.01). None of the nine neurons labelled from the lung that were capsaicin insensitive, responded to cinnamaldehyde (0/9) (Table 2 and Fig. 2).
Figure 2. Cinnamaldehyde increases intracellular [Ca2+]free in retrogradely labelled vagal afferents.
Effect of cinnamaldehyde (100 μm) and capsaicin (1 μm) on intracellular [Ca2+]free (expressed as 352/380 ratio) in dissociated DiI-labelled bronchopulmonary jugular/nodose afferents; mean ± s.e.m., n = 15.
Also consistent with the single-cell RT-PCR data showing that TRPM8 expression was lacking in lung-labelled neurons, menthol (100 μm) had no effect on the intracellular calcium concentration in any lung-labelled neuron studied (0 of 24 responded) (Table 2 and Fig. 2).
Cinnamaldehyde depolarizes vagal nerve afferents
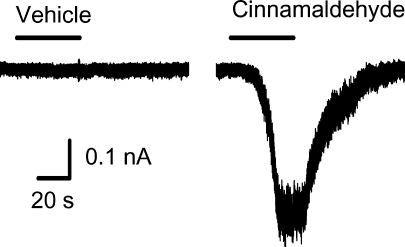
We next evaluated whether the results with calcium were consistent with TRPA1 activation using whole cell patch clamp techniques. In 7 of 7 capsaicin-sensitive lung labelled neurons, cinnamaldehyde (100 μm) caused an immediate inward current that averaged −15.93 ± 6.35 pA pF−1. In contrast, neither menthol (100 μm) (−2.22 ± 0.52 pA pF−1, n = 6) nor vehicle (−0.42 ± 0.11 pA pF−1) had an effect on inward currents. This current density is of a magnitude similar to what was observed with TRPV1 activation using capsaicin (Fig. 3).
Figure 3. Cinnamaldehyde induced inward current response in gramicidin-perforated patch clamp recordings.
All recorded jugular/nodose neurons (n = 7) were labelled from lungs and airways. Depicted is a typical trace of inward current induced by vehicle (0.1% EtOH) and cinnamaldehyde (100 μm) application.
Cinnamaldehyde evokes action potential discharge in bronchopulmonary vagal C-fibres
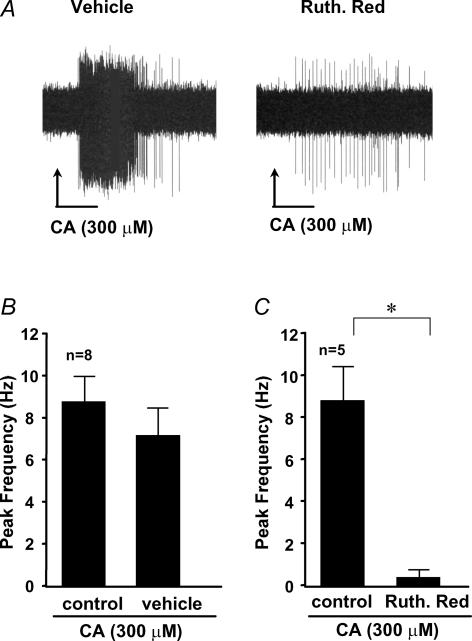
It would be imprudent to infer information about electrical activity at the nerve terminals in the tissue from studies carried out at the distant cell body. We therefore next evaluated whether cinnamaldehyde and menthol can evoke action potential discharge from C-fibres in the vagal-innervated ex vivo lung preparation. We have previously noted that afferent nerves with conduction velocities less than 0.7 m s−1 are routinely sensitive to capsaicin in this preparation. We addressed the hypothesis that cinnamaldehyde would evoke action potential discharge in this subpopulation of nerves. We evaluated 13 C-fibres with conduction velocities less than 0.7 m s−1 (range 0.4–0.6 m s−1). All fibres responded robustly to cinnamaldehyde (300 μm) with a peak action potential discharge frequency of 8.5 ± 1.5 impulses s−1 (Fig. 4). By comparison, capsaicin (0.3 μm) caused action potential discharge with a peak frequency of 10.2 ± 2.3 impulses s−1 (n = 6). In the first three experiments we determined that 10 μmtrans-cinnamaldehyde was ineffective, and 100 μm gave a response that was smaller than that observed with 300 μm. Concentrations greater than 300 μm were not studied. Treating the tissue with cinnamaldehyde a second time 15 min after the initial treatment resulted in a response that was not significantly different from the initial response (Fig. 4B), i.e. the response did not desensitize. Treating the tissue with ruthenium red (10 μm for 15 min) to block TRPA1 (and TRPV1) virtually abolished the response to cinnamaldehyde (Fig. 4C). Menthol (300 μm or 1 mm), also induced an action potential discharge (13 ± 5.9 impulses s−1) in 3 out of 4 experiments (data not shown).
Figure 4. Cinnamaldehyde stimulation of nerve terminals evokes action potential discharges.
A, representative trace of extracellular recordings in response to cinnamaldehyde (CA) during perfusion with vehicle (Krebs-bicarbonate buffer solution) and after 15 min pretreatment with ruthenium red (30 μm). B, repeated stimulation with cinnamaldehyde evokes similar action potential discharges (mean ± s.e.m., n = 8). C, cinnamaldehyde-induced action potential discharge can be blocked by pretreatment with ruthenium red (mean ± s.e.m.; *P < 0.05; n = 5).
Inhalation of cinnamaldehyde evokes reflexes in vivo consistent with C-fibre activation
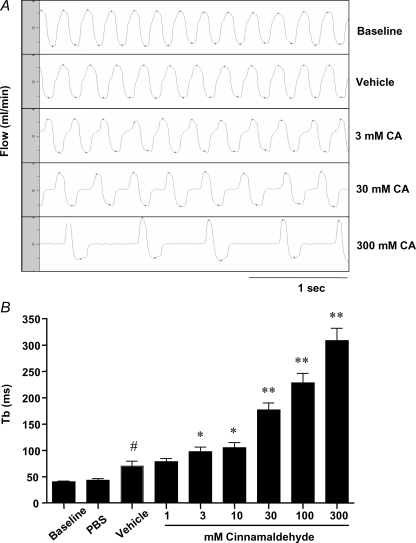
It has previously been noted that stimuli that activate respiratory C-fibres in the mouse, such as capsaicin, consistently evoke a breaking response in respiration. We next evaluated whether cinnamaldehyde is capable of evoking this stereotypical nocifensor reflex. The time of braking (Tb) baseline values were comparable to previously published data (Nassenstein et al. 2006). As described before, the aerosol of the vehicle control caused a slight prolongation of Tb. In contrast, cinnamaldehyde evoked a dose-dependent marked prolongation of Tb, significantly greater than the vehicle control, and of a similar magnitude to that observed previously with capsaicin (Nassenstein et al. 2006) (Fig. 5).
Figure 5. Cinnamaldehyde aerosol provocations elicit central reflexes.
The time of braking (Tb) was measured in response to vehicle (20% EtOH in PBS) and increasing doses of cinnamaldehyde. A, breathing pattern of a mice while breathing room air (baseline), during inhalation of vehicle (20% EtOH in PBS), and during cinnamaldehyde aerosol provocation, respectively. Scale bar = 1 s. B, quantification of Tb during inhalation of increasing concentrations of cinnamaldehyde aerosol (mean ± s.e.m., n = 16); *P < 0.05 compared to vehicle; **P < 0.01 compared to vehicle; #P < 0.05 compared to baseline.
Discussion
The data presented here support the hypothesis that TRPA1 agonists can directly activate vagal C-fibres innervating mouse airways. Whereas it is well known that TRPV1 stimuli activate vagal bronchopulmonary C-fibres in mammals (Coleridge & Coleridge, 1977; Fuller et al. 1985; Kollarik et al. 2003), these results are among the first to show that TRPA1-selective stimuli can also evoke action potential discharge from bronchopulmonary C-fibres and consequent defensive reflexes. Although menthol induced an action potential discharge of mouse bronchopulmonary C-fibres, data obtained by single-cell RT-PCR, calcium measurement and patch-clamp recording fail to support an important role for TRPM8 in lung-labelled sensory nerves.
Cinnamaldehyde, a TRPA1 agonist, evoked a prolongation in the time of braking, a stereotypical nocifensor reflex in several species, including the mouse (Remmers et al. 1986; Alarie, 1998). We argue that this is most likely to be due to activation of sensory C-fibres in the airways via direct TRPA1 stimulation. This prediction is supported by several lines of evidence. First, lung-specific neurons that expressed TRPV1 mRNA also expressed TRPA1 mRNA based on single neuron RT-PCR analysis. Second, capsaicin-sensitive lung-specific vagal sensory neurons uniformly responded to the TRPA1 agonist cinnamaldehyde with a rapid increase in intracellular calcium. Third, whole cell patch clamp recordings revealed that these lung-specific neurons also responded to cinnamaldehyde with large inward currents typical of TRPA1 gating. Finally, cinnamaldehyde evoked action potential discharge from the C-fibre nerve ending in the lungs, by a mechanism that was blocked by ruthenium red, a non-selective TRPA1 antagonist. Ruthenium red also blocks TRPV1, but cinnamaldehyde does not stimulate TRPV1 (we noted several vagal sensory neurons that were not labelled from the lungs that responded strongly to capsaicin, but showed no response to cinnamaldehyde in our calcium imaging studies).
Our data, at the level of both gene expression and function, indicate that vagal sensory neurons innervating mouse lungs coexpress TRPV1 and TRPA1, with little evidence for a population of pulmonary afferent neurons expressing either channel alone. This may not be the case for vagal sensory neurons innervating other tissues in the mouse as approximately 40% of the capsaicin-sensitive neurons not labelled from the lungs failed to respond to cinnamaldehyde. This supports the hypothesis that there is a subpopulation of TRPV1 expressing vagal sensory neurons that innervate non-pulmonary tissue which does not express TRPA1, or at least fail to express the channel sufficiently to evoke functional responses. This hypothesis should be considered tentative until studies specifically addressing this issue are carried out. Neurons coexpressing TRPA1 and TRPV1 have also been identified in nodose ganglia labelled from the peritoneal cavity (Peeters et al. 2006), trigeminal neurons (Kobayashi et al. 2005) and DRG neurons (Elitt et al. 2006; Hjerling-Leffler et al. 2007).
TRPV1 is well recognized to provide a mechanism through which disparate stimuli (arachidonic acid metabolites, certain autacoids, acid, increases in temperature) can lead to nociceptor activation (Jia & Lee, 2007). This supports the idea that developing TRPV1 antagonists would decrease nociceptors activity in the face of various stimuli. The observation that virtually all TRPV1 nerves innervating the mouse lung also express TRPA1 indicates that these nerves may also be activated by a large array of potential stimuli, even in the presence of a TRPV1 antagonist. Both TRPA1 and TRPV1 may be regulated by neurotrophic factors, proteases, and certain autacoids such as bradykinin (Shu & Mendell, 1999; Kollarik et al. 2003; Amadesi et al. 2004; Bandell et al. 2004; Diogenes et al. 2007; Dai et al. 2007).
Both TRPA1 and TRPV1 may contribute to the development of hypersensitivity to noxious stimulations (i.e. hyperalgesia) (LaMotte et al. 1992; Obata et al. 2005; Bautista et al. 2006; Kwan et al. 2006; Dhaka et al. 2006). In addition, TRPA1 is activated by certain environmental irritants such as acrolein (Bautista et al. 2006) that fail to activate TRPV1. TRPA1 may be activated by cold temperature (Story et al. 2003) associated with inhalation of cold dry air, a stimulus known to provoke asthma attacks in susceptible individuals (Giesbrecht & Younes, 1995). Parenthetically, it is interesting that in the present model, TRPA1 may be more relevant in this regard than TRPM8. Large concentrations of menthol activated action potential discharge in 3 of 4 lung C-fibres. Considered with the other data presented, however, it would seem unlikely that this effect was a consequence of stimulation of neuronal TRPM8. Menthol may have stimulated the C-fibres by mechanisms other than TRPM8 (Schafer et al. 1986), or alternatively it remains possible that menthol acts on C-fibres in the lungs indirectly by stimulating TRPM8 in non-neural tissue (Yang et al. 2006).
Considered together, the present findings support the hypothesis that TRPA1 may be another important conduit for bronchopulmonary C-fibre activation. This would indicate that it may be more rational to find mechanisms that non-selectively inhibit both TRPV1 and TRPA1 when trying to decrease airway nociceptor activity than to attempt to selectively block either channel alone.
Acknowledgments
This research was funded by grants from the National Institutes of Health, Bethesda, MD, USA, the German Research Foundation (SFB587/B4) and the German Academic Exchange Service (DAAD). The authors thank Mrs Sonya Meeker, Mrs Emma Spies and Ms Birthe Ellinghusen for excellent technical assistance.
References
- Alarie Y. Computer-based bioassay for evaluation of sensory irritation of airborne chemicals and its limit of detection. Arch Toxicol. 1998;72:277–282. doi: 10.1007/s002040050502. [DOI] [PubMed] [Google Scholar]
- Amadesi S, Nie J, Vergnolle N, Cottrell GS, Grady EF, Trevisani M, Manni C, Geppetti P, McRoberts JA, Ennes H, Davis JB, Mayer EA, Bunnett NW. Protease-activated receptor 2 sensitizes the capsaicin receptor transient receptor potential vanilloid receptor 1 to induce hyperalgesia. J Neurosci. 2004;24:4300–4312. doi: 10.1523/JNEUROSCI.5679-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41:849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124:1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Coleridge HM, Coleridge JC. Impulse activity in afferent vagal C-fibres with endings in the intrapulmonary airways of dogs. Respir Physiol. 1977;29:125–142. doi: 10.1016/0034-5687(77)90086-x. [DOI] [PubMed] [Google Scholar]
- Coleridge JC, Coleridge HM. Afferent vagal C fibre innervation of the lungs and airways and its functional significance. Rev Physiol Biochem Pharmacol. 1984;99:1–110. doi: 10.1007/BFb0027715. [DOI] [PubMed] [Google Scholar]
- Dai Y, Wang S, Tominaga M, Yamamoto S, Fukuoka T, Higashi T, Kobayashi K, Obata K, Yamanaka H, Noguchi K. Sensitization of TRPA1 by PAR2 contributes to the sensation of inflammatory pain. J Clin Invest. 2007;117:1979–1887. doi: 10.1172/JCI30951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhaka A, Viswanath V, Patapoutian A. Trp ion channels and temperature sensation. Annu Rev Neurosci. 2006;29:135–161. doi: 10.1146/annurev.neuro.29.051605.112958. [DOI] [PubMed] [Google Scholar]
- Diogenes A, Akopian AN, Hargreaves KM. NGF up-regulates TRPA1: implications for orofacial pain. J Dent Res. 2007;86:550–555. doi: 10.1177/154405910708600612. [DOI] [PubMed] [Google Scholar]
- Doerner JF, Gisselmann G, Hatt H, Wetzel CH. Transient receptor potential channel A1 is directly gated by calcium ions. J Biol Chem. 2007;282:13180–13189. doi: 10.1074/jbc.M607849200. [DOI] [PubMed] [Google Scholar]
- Elitt CM, McIlwrath SL, Lawson JJ, Malin SA, Molliver DC, Cornuet PK, Koerber HR, Davis BM, Albers KM. Artemin overexpression in skin enhances expression of TRPV1 and TRPA1 in cutaneous sensory neurons and leads to behavioral sensitivity to heat and cold. J Neurosci. 2006;26:8578–8587. doi: 10.1523/JNEUROSCI.2185-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller RW, Dixon CM, Barnes PJ. Bronchoconstrictor response to inhaled capsaicin in humans. J Appl Physiol. 1985;58:1080–1084. doi: 10.1152/jappl.1985.58.4.1080. [DOI] [PubMed] [Google Scholar]
- Giesbrecht GG, Younes M. Exercise- and cold-induced asthma. Can J Appl Physiol. 1995;20:300–314. doi: 10.1139/h95-023. [DOI] [PubMed] [Google Scholar]
- Hales CA, Barkin PW, Jung W, Trautman E, Lamborghini D, Herrig N, Burke J. Synthetic smoke with acrolein but not HCl produces pulmonary edema. J Appl Physiol. 1988;64:1121–1133. doi: 10.1152/jappl.1988.64.3.1121. [DOI] [PubMed] [Google Scholar]
- Hales CA, Musto SW, Janssens S, Jung W, Quinn DA, Witten M. Smoke aldehyde component influences pulmonary edema. J Appl Physiol. 1992;72:555–561. doi: 10.1152/jappl.1992.72.2.555. [DOI] [PubMed] [Google Scholar]
- Hjerling-Leffler J, Alqatari M, Ernfors P, Koltzenburg M. Emergence of functional sensory subtypes as defined by transient receptor potential channel expression. J Neurosci. 2007;27:2435–2443. doi: 10.1523/JNEUROSCI.5614-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Lee LY. Role of TRPV receptors in respiratory diseases. Biochim Biophys Acta. 2007;1772:915–927. doi: 10.1016/j.bbadis.2007.01.013. [DOI] [PubMed] [Google Scholar]
- Jordt SE, Bautista DM, Chuang HH, McKemy DD, Zygmunt PM, Hogestatt ED, Meng ID, Julius D. Mustard oils and cannabinoids excite sensory nerve fibres through the TRP channel ANKTM1. Nature. 2004;427:260–265. doi: 10.1038/nature02282. [DOI] [PubMed] [Google Scholar]
- Kobayashi K, Fukuoka T, Obata K, Yamanaka H, Dai Y, Tokunaga A, Noguchi K. Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with aδ/c-fibers and colocalization with trk receptors. J Comp Neurol. 2005;493:596–606. doi: 10.1002/cne.20794. [DOI] [PubMed] [Google Scholar]
- Kollarik M, Dinh QT, Fischer A, Undem BJ. Capsaicin-sensitive and -insensitive vagal bronchopulmonary C-fibres in the mouse. J Physiol. 2003;551:869–879. doi: 10.1113/jphysiol.2003.042028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50:277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- Lamb JP, Sparrow MP. Three-dimensional mapping of sensory innervation with substance P in porcine bronchial mucosa: comparison with human airways. Am J Respir Crit Care Med. 2002;166:1269–1281. doi: 10.1164/rccm.2112018. [DOI] [PubMed] [Google Scholar]
- LaMotte RH, Lundberg LE, Torebjork HE. Pain, hyperalgesia and activity in nociceptive C units in humans after intradermal injection of capsaicin. J Physiol. 1992;448:749–764. doi: 10.1113/jphysiol.1992.sp019068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee LY. Reflexes of the lung and airways. J Appl Physiol. 2006;101:1–2. doi: 10.1152/japplphysiol.00388.2006. [DOI] [PubMed] [Google Scholar]
- MacGlashan D., Jr Single-cell analysis of Ca++ changes in human lung mast cells: graded vs. all-or-nothing elevations after IgE-mediated stimulation. J Cell Biol. 1989;109:123–134. doi: 10.1083/jcb.109.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416:52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- Nagata K, Duggan A, Kumar G, Garcia-Anoveros J. Nociceptor and hair cell transducer properties of TRPA1, a channel for pain and hearing. J Neurosci. 2005;25:4052–4061. doi: 10.1523/JNEUROSCI.0013-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nassenstein C, Dawbarn D, Pollock K, Allen SJ, Erpenbeck VJ, Spies E, Krug N, Braun A. Pulmonary distribution, regulation, and functional role of Trk receptors in a murine model of asthma. J Allergy Clin Immunol. 2006;118:597–605. doi: 10.1016/j.jaci.2006.04.052. [DOI] [PubMed] [Google Scholar]
- Obata K, Katsura H, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Tominaga M, Noguchi K. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest. 2005;115:2393–2401. doi: 10.1172/JCI25437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen SF, Owsianik G, Nilius B. TRP channels: an overview. Cell Calcium. 2005;38:233–252. doi: 10.1016/j.ceca.2005.06.028. [DOI] [PubMed] [Google Scholar]
- Peeters PJ, Aerssens J, de Hoogt R, Stanisz A, Gohlmann HW, Hillsley K, Meulemans A, Grundy D, Stead RH, Coulie B. Molecular profiling of murine sensory neurons in the nodose and dorsal root ganglia labeled from the peritoneal cavity. Physiol Genomics. 2006;24:252–263. doi: 10.1152/physiolgenomics.00169.2005. [DOI] [PubMed] [Google Scholar]
- Remmers JE, Richter DW, Ballantyne D, Bainton CR, Klein JP. Reflex prolongation of stage I of expiration. Pflugers Arch. 1986;407:190–198. doi: 10.1007/BF00580675. [DOI] [PubMed] [Google Scholar]
- Riccio MM, Myers AC, Undem BJ. Immunomodulation of afferent neurons in guinea-pig isolated airway. J Physiol. 1996;491:499–509. doi: 10.1113/jphysiol.1996.sp021234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer K, Braun HA, Isenberg C. Effect of menthol on cold receptor activity. Analysis of receptor processes. J Gen Physiol. 1986;88:757–776. doi: 10.1085/jgp.88.6.757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu X, Mendell LM. Nerve growth factor acutely sensitizes the response of adult rat sensory neurons to capsaicin. Neurosci Lett. 1999;274:159–162. doi: 10.1016/s0304-3940(99)00701-6. [DOI] [PubMed] [Google Scholar]
- Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112:819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- Taylor-Clark TE, Undem BJ, MacGlashan DW, Ghatta S, Carr MJ, McAlexander MA. Prostaglandin-induced activation of nociceptive neurons via direct interaction with transient receptor potential A1 (TRPA1) Mol Pharmacol. 2008;73:274–281. doi: 10.1124/mol.107.040832. [DOI] [PubMed] [Google Scholar]
- Trevisani M, Siemens J, Materazzi S, Bautista DM, Nassini R, Campi B, Imamachi N, Andre E, Patacchini R, Cottrell GS, Gatti R, Basbaum AI, Bunnett NW, Julius D, Geppetti P. 4-Hydroxynonenal, an endogenous aldehyde, causes pain and neurogenic inflammation through activation of the irritant receptor TRPA1. Proc Natl Acad Sci U S A. 2007;104:13519–13524. doi: 10.1073/pnas.0705923104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayaraghavan R, Schaper M, Thompson R, Stock MF, Boylstein LA, Luo JE, Alarie Y. Computer assisted recognition and quantitation of the effects of airborne chemicals acting at different areas of the respiratory tract in mice. Arch Toxicol. 1994;68:490–499. doi: 10.1007/s002040050101. [DOI] [PubMed] [Google Scholar]
- Yang XR, Lin MJ, McIntosh LS, Sham JS. Functional expression of transient receptor potential melastatin- and vanilloid-related channels in pulmonary arterial and aortic smooth muscle. Am J Physiol Lung Cell Mol Physiol. 2006;290:L1267–L1276. doi: 10.1152/ajplung.00515.2005. [DOI] [PubMed] [Google Scholar]
- Zurborg S, Yurgionas B, Jira JA, Caspani O, Heppenstall PA. Direct activation of the ion channel TRPA1 by Ca2+ Nat Neurosci. 2007;10:277–279. doi: 10.1038/nn1843. [DOI] [PubMed] [Google Scholar]