The compelling evidence that associates small size at birth with later cardiovascular disease in humans (Barker, 1998) encourages research into the determinants of fetal growth. It is generally accepted that both nutrient and oxygen supply to the fetus are important factors that modulate whether the genetic potential for fetal growth is achieved. In humans, fetal hypoxia occurs during pregnancy at high altitude (Moore, 1990) or under pathological pregnancies at sea level complicated by placental insufficiency (Baschat, 2004). In both cases, it has been shown that birth weight is reduced (Moore et al. 2004; Barker, 1998, respectively). However, in the former there may be confounding factors of altitudinal impoverishment, hypothermia and hypobaria, and in the latter, decreases in fetal nutrient and oxygen transfer occur simultaneously, such that the cause of the reduced birth weight cannot be discerned. Previous studies in mammalian animals have suffered from the same problems. A recent paper in The Journal of Physiology by Giussani et al. (2007) circumvents these problems by using a chick model, which has the dual advantages of external embryonic development and a cardiovascular system that resembles that found in humans (Ruijtenbeek et al. 2002). Such a model permitted the novel isolation of the effects of alterations in oxygenation, independent of factors owing to uncontrolled placental physiology or maternal nutritional changes during embryonic development.
Thus, Giussani et al. (2007) elegantly used the natural altitudinal demarcation of the Andean Corillera in Bolivia to rear at least six generations of hens either in the sea level city of Santa Cruz or at high altitude in La Paz. The eggs from such hens were incubated at either the same altitude of their familial residence, or at an altitude drastically different from their familial residence, whilst having appropriate egg rotation and keeping temperature and humidity constant during incubation. Thus, incubation optimized to maximize embryonic development yielded five experimental groups of fertilized eggs: controls laid by sea level hens incubated at sea level (SLSL), those laid by sea level hens incubated at high altitude (SLHA), those laid by high altitude hens incubated at high altitude (HAHA), those laid by high altitude hens incubated at sea level (HASL) and finally, since high altitude conditions are hypobaric and hypoxic, eggs laid by sea level hens were incubated at high altitude with exogenous oxygen delivery at partial pressures comparable to sea level (SLHA + oxygen), to negate the hypoxia of high altitude. This latter control group thereby allowed the influence of oxygenation on birth weight to be assessed independently of atmospheric pressure.
The paper shows the results of seven important measured parameters, which have been here denoted as: Me0 (mass of egg before incubation period at day 0); Me20 (mass of egg after incubation period at day 20); 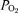 and HCT (partial pressure of oxygen and percentage of red-cell haematocrit, respectively, of chorio-allantoic blood at the end of the incubation period); Mf20 (mass of fetus at day 20), Me20–f20 (mass of egg without fetus at day 20) and HD (fetal head diameter at day 20). Giussani et al. (2007) calculated two ratios from these parameters. They defined the ‘growth efficiency’ as Mf20/(Me20–Me20–f20) in order to determine whether hypoxia at high altitude affects how well the yolk sac ‘resource’ is converted into fetal body mass. However, this hypoxia might not affect the conversion of ‘resource’ into fetal mass, but instead the uptake of ‘resource’ such that it can then be converted. Thus, ‘partitioning of the resource’ was calculated by Mf20/Me20 and (Me20–Mf20)/Me20, expressed as percentages, defining the proportion of fetal mass and proportion of unused yolk sac ‘resource’ left in the egg, respectively, relative to the mass of the egg at day 20.
and HCT (partial pressure of oxygen and percentage of red-cell haematocrit, respectively, of chorio-allantoic blood at the end of the incubation period); Mf20 (mass of fetus at day 20), Me20–f20 (mass of egg without fetus at day 20) and HD (fetal head diameter at day 20). Giussani et al. (2007) calculated two ratios from these parameters. They defined the ‘growth efficiency’ as Mf20/(Me20–Me20–f20) in order to determine whether hypoxia at high altitude affects how well the yolk sac ‘resource’ is converted into fetal body mass. However, this hypoxia might not affect the conversion of ‘resource’ into fetal mass, but instead the uptake of ‘resource’ such that it can then be converted. Thus, ‘partitioning of the resource’ was calculated by Mf20/Me20 and (Me20–Mf20)/Me20, expressed as percentages, defining the proportion of fetal mass and proportion of unused yolk sac ‘resource’ left in the egg, respectively, relative to the mass of the egg at day 20.
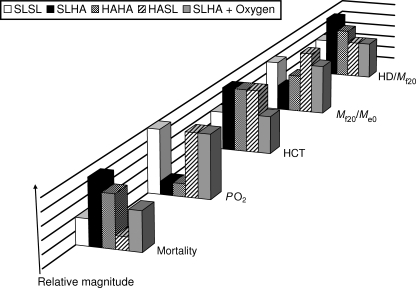
The important results from this study by Giussani et al. (2007) are schematically summarized in Fig. 1. We highlight six key findings. Firstly, embryonic mortality was shown to be exquisitely sensitive to the hypoxia during the incubation period at high altitude and this was suppressed by oxygen supplementation (SLHA + oxygen). However, this effect of hypoxia was dampened in extent in those embryos laid by hens at high altitude (HAHA).
Figure 1. Schematic summary chart of key results of Giussani et al. (2007).
Heights of bars are shown on an arbitrary relative scale to permit graphical comparison of statistically different or similar values from five experimental groups: (i) sea level chick embryos incubated at sea level (SLSL, open bars) or (ii) high altitude (SLHA, black bars), (iii) high altitude embryos incubated at high altitude (HAHA, stippled bars) or (iv) sea level (HASL, hatched bars), and (v) sea level chick embryos incubated at high altitude with oxygen supplementation (SLHA + oxygen, grey bars). Mortality: percentage embryonic mortality at day 20 of incubation period; 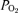 : partial pressure of oxygen in chorio-allantoic blood sample, measured in mmHg in the study; HCT: percentage haematocrit in chorio-allantoic blood sample; Mf20/Me0: fetal mass at day 20 relative to initial egg mass at day 0; HD/Mf20: ratio of fetal head diameter to fetal body mass at day 20. Thus, for example, the HCT results show that the value of HCT remained low for SLSL and SLHA + oxygen and that SLHA, HAHA and HASL all showed raised HCT values and that each of the increases was statistically indistinguishable from one another, denoted in the chart by identical bar heights.
: partial pressure of oxygen in chorio-allantoic blood sample, measured in mmHg in the study; HCT: percentage haematocrit in chorio-allantoic blood sample; Mf20/Me0: fetal mass at day 20 relative to initial egg mass at day 0; HD/Mf20: ratio of fetal head diameter to fetal body mass at day 20. Thus, for example, the HCT results show that the value of HCT remained low for SLSL and SLHA + oxygen and that SLHA, HAHA and HASL all showed raised HCT values and that each of the increases was statistically indistinguishable from one another, denoted in the chart by identical bar heights.
Secondly, this paper shows, for the first time, that high altitude induces chorio-allantoic hypoxaemia in surviving chicks. Interestingly, the results show a corresponding increase in packed red-cell mass for eggs with either a familial residential link with high altitude (HASL) or an experimentally imposed link with high altitude owing to the incubation (SLHA), or both links (HAHA). This effect on HCT was not seen when oxygen supplementation was given (SLHA + oxygen), suggesting a causal relationship between oxygen partial pressure and packed red-cell mass.
Thirdly, the raw data show differences in pre-incubation egg mass. Thus, eggs laid by hens at high altitude were already at a weight disadvantage when compared with eggs laid by sea level hens.
Fourthly, when the initial differences in egg mass were taken into account with the absolute masses of the fetuses (Mf20/Me0), they showed that development at high altitude (SLHA, HAHA) led to a weight restriction compared with development at sea level, and that this difference was not seen when development at high altitude was supplemented with oxygen (SLHA + oxygen). Under such a scenario, fetal birth weight was shown to be statistically indistinguishable from SLSL fetuses, suggesting that oxygen supplementation prevents weight restriction.
Fifthly, calculation of Mf20/Me0 further showed that relative fetal mass was reduced by hypoxia at high altitude by 45.2% in SLHA and 22.2% in HAHA, relative to SLSL controls. Comparing these two percentages shows that maternal influences of residential location help to reduce the deleterious effects of hypoxia on fetal birth weight. Interestingly, HASL showed restored growth and these fetuses grew heavier than the SLSL control group. This suggests that location of maternal residence modulates fetal growth and that these maternal factors remain active when prevailing embryonic developmental conditions are not as predicted by that of the mother's residential location.
Sixthly, growth restriction was shown to spare vital organs such as the brain at the expense of peripheral vascular beds, and the ratio of head diameter to fetal body mass was increased for incubations at high altitude (SLHA and HAHA) but by a lesser amount in the case of HAHA. Oxygen supplementation removed this effect on the SLHA group, confirming a causal relationship between oxygen deprivation during embryonic development and asymmetrical growth restriction.
Finally, by using the calculated values of ‘growth efficiency’ and ‘partition of the resource’, Giussani et al. (2007) asked two insightful questions of how hypoxia caused by high altitude suppresses fetal growth, and how the effects of such mechanisms are prevented by the demonstrated protection in highland chickens. In answer to the first question, the authors show that hypoxia at high altitude does not affect the proficiency with which the yolk sac ‘resource’ is converted into embryonic tissue (growth efficiency) but it has a significant effect on fetal resource uptake, such that eggs with lighter fetuses (SLHA, HAHA) have proportionately more unusable ‘resource’.
The data overall resemble those presented in an epidemiological study of human populations in the same area of Bolivia by the same research group (Giussani et al. 2001). Thus, the hypoxia at high altitude, asymmetric growth restriction and maternal influences shown in Giussani et al. (2007) suggest that the chick embryo model is likely to be a good candidate in the future for the investigation of clinically relevant human conditions, such as placental insufficiency and pre-eclampsia.
By showing that oxygen deprivation, independent of maternal nutritional status during embryonic development, has a clear and significant role in the control of fetal growth and that prolonged residence at high altitude confers significant protection against the effects of hypoxia on fetal mortality and growth, the paper by Giussani et al. (2007) stimulates and focuses future work. This further work should be aimed at unravelling the mechanisms underlying the protection due to prolonged maternal residence at high altitude shown by Giussani et al. (2007), which are likely to reside within the feto-placental unit rather than via genetic influences on maternal oxygen delivery (Zamudio et al. 2007). It is an open question whether the period of six generations for rearing hens in Giussani et al. (2007) was long enough for adaptations to high altitude to become genetically determined, but as these authors point out, heritable genomic imprinting may be involved, precluding the requirement of a change in DNA sequence. This possibility must be explored in the chick embryo model. Moreover, the mechanisms by which hypoxia reduces fetal ‘resource’ uptake remain to be elucidated and this is very amenable to study using the chick embryo model, rather than using mammalian ones, because of the technical challenges of calculating maternal glucose delivery and umbilical glucose uptake. Thus, not only have Giussani et al. (2007) shown a dominant role of oxygenation for fetal birth weight, they have also emphasized the experimental benefits of using the chick embryo model to help in the quest to understand the causes of fetal pathology.
References
- Barker DJP. Mothers, Babies and Health in Later Life. Edinburgh: Churchill Livingstone; 1998. [Google Scholar]
- Baschat AA. Fetal responses to placental insufficiency: an update. BJOG. 2004;111:1031–1041. doi: 10.1111/j.1471-0528.2004.00273.x. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Phillips PS, Anstee S, Barker DJP. Effects of altitude versus economic status on birth weight and body shape at birth. Ped Res. 2001;49:490–494. doi: 10.1203/00006450-200104000-00009. [DOI] [PubMed] [Google Scholar]
- Giussani DA, Salinas CE, Villena M, Blanco CE. The role of oxygen in prenatal growth: studies in the chick embryo. J Physiol. 2007;585:911–917. doi: 10.1113/jphysiol.2007.141572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore LG. Maternal O2 transport and fetal growth in Colorado, Peru and Tibet high-altitude residents. Am J Hum Biol. 1990;2:627–637. doi: 10.1002/ajhb.1310020606. [DOI] [PubMed] [Google Scholar]
- Moore LG, Shriver M, Bemis L, Hickler B, Wilson M, Brutsaert T, Parra E, Vargas E. Maternal adaptation to high-altitude pregnancy: an experiment of nature – a review. Placenta. 2004;25(Suppl. A):S60–S71. doi: 10.1016/j.placenta.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Ruijtenbeek K, De Mey JG, Blanco CE. The chicken embryo in developmental physiology of the cardiovascular system: a traditional model with new possibilities. Am J Physiol Regul Integr Comp Physiol. 2002;283:R549–R550. doi: 10.1152/ajpregu.00107.2002. [DOI] [PubMed] [Google Scholar]
- Zamudio S, Postigo L, Illsley NP, Rodriguez C, Heredia G, Brimacombe M, Echalar L, Torricos T, Tellez W, Maldonado I, Balanza E, Alvarez T, Ameller J, Vargas E. Maternal oxygen delivery is not related to altitude- and ancestry-associated differences in human fetal growth. J Physiol. 2007;582:883–895. doi: 10.1113/jphysiol.2007.130708. [DOI] [PMC free article] [PubMed] [Google Scholar]