Abstract
One of the mechanisms by which the experience-dependent reorganization of neural circuitry can occur is through changes in synaptic strength. Almost every excitatory synapse in the mammalian brain exhibits LTP (long-term potentiation) or LTD (long-term depression), two cellular mechanisms of synaptic plasticity. However, LTP and LTD have been reported much more rarely at fast inhibitory GABAA receptor synapses. Our recent study suggests that in vivo morphine initiates a long-lasting alteration of GABAergic synapses in the ventral tegmental area (VTA) by blocking the mechanisms required for LTP of GABAergic synapses. Here we put this work into the context of other examples of synaptic plasticity at GABAergic synapses.
The most widely studied candidate mechanisms for changing synaptic strength are LTP and LTD, hypothesized to play critical roles in the establishment of many forms of experience-dependent plasticity, including learning and memory. Recent studies have begun to show that excitatory synapses in brain regions important in addiction can express LTP- and LTD-like changes in response to administration of addictive drugs, and the molecular mechanisms underlying these synaptic modifications share those observed in other forms of plasticity. These findings support the idea that the development of drug addiction, an example of an experience-dependent neuroadaptation, involves usurping or disrupting synaptic plasticity mechanisms (Hyman & Malenka, 2001; Hyman et al. 2006; Kauer & Malenka, 2007).
GABAergic synapses exhibit plasticity
By releasing GABA onto GABAA receptors, inhibitory interneurons control the output of their target neurons by opposing synaptic excitation and limiting the spread of neural activity (Farrant & Nusser, 2005; Akerman & Cline, 2007). As an illustration of this point, blocking GABAA receptors in the mature CNS increases principal neuron firing rates, and in cortical structures can promote epileptiform bursting. Thus, modifications in the strength of GABAergic synapses will alter the patterns of activity generated by a neuronal network, leading to downstream behavioural changes (Gaiarsa et al. 2002). As with plasticity at excitatory synapses, much of the work on LTP and LTD of GABAergic synapses has been carried out in the hippocampus (Stelzer et al. 1987, 1994; Grunze et al. 1996; McLean et al. 1996; Kang et al. 1998; Caillard et al. 1999a,b; Lu et al. 2000; Gubellini et al. 2001, 2005; Chevaleyre & Castillo, 2003; Patenaude et al. 2003; Chevaleyre et al. 2007). However, synaptic plasticity of GABAergic synapses has also been reported in other brain regions, including the neocortex (Komatsu, 1994; Komatsu & Yoshimura, 2000; Lien et al. 2006; Maffei et al. 2006), the cerebellum (Kano, 1994; Mitoma et al. 1994; Mitoma & Konishi, 1996; Kawaguchi & Hirano, 2002; Saitow et al. 2005; Kawaguchi & Hirano, 2007), the deep cerebellar nuclei (DCN) (Morishita & Sastry, 1993, 1996; Aizenman et al. 1998; Ouardouz et al. 2000), and the brain stem (Glaum & Brooks, 1996; Grabauskas & Bradley, 1999). Our recent work explored the mechanisms underlying synaptic plasticity at GABAergic synapses on midbrain dopamine neurons (Nugent et al. 2007).
It is clear that LTP and LTD at GABAergic synapses utilize diverse mechanisms depending on the cell type and developmental stage. As for the majority of forms of excitatory synapse LTP/LTD (Malenka & Bear, 2004), at many GABAergic synapses a rise in postsynaptic Ca2+ is necessary to induce plasticity, although the source of Ca2+ and the downstream intracellular signalling cascades differ from one brain area to another (Kano, 1995; Gaiarsa et al. 2002). Considerable effort has been directed toward explaining how activity of inhibitory synapses can generate a rise in intracellular Ca2+ when GABAA receptors are Cl− channels impermeant to Ca2+.
GABAA synapses in developing brain
A number of studies in various brain regions have found that Ca2+ enters the postsynaptic cell through NMDARs or voltage-gated Ca2+ channels (Kano, 1994; Glaum & Brooks, 1996; Grunze et al. 1996; McLean et al. 1996; Caillard et al. 1999a,b; Lu et al. 2000; Ouardouz et al. 2000; Nugent et al. 2007). For example, early in postnatal development, hippocampal GABAergic synapses can exhibit bi-directional plasticity. In neonates, at this and many synapses, GABAA receptor-mediated currents are depolarizing, because the expression of specific Cl− transporters promotes high levels of intracellular Cl− (Cherubini et al. 1991; Akerman & Cline, 2007). The activation of these receptors therefore can provide the initial membrane depolarization required to open NMDAR channels resulting in LTD (Kano, 1994; McLean et al. 1996; Caillard et al. 1999b), or to open voltage-gated calcium channels resulting in LTP at these synapses (McLean et al. 1996). This form of LTP at hippocampal synapses is restricted to the first postnatal week, a period when the GABAergic synapses are reaching maturity. At other GABAA synapses, plasticity is dependent on intracellular sources of Ca2+ such as InsP3-sensitive stores, or Ca2+-induced Ca2+ release stores (Hashimoto et al. 1996; Komatsu, 1996). Several of these examples of GABAA synapse LTP result from postsynaptic increases in GABAA receptor number or sensitivity to GABA (Kano, 1994; Ouardouz et al. 2000; Maffei et al. 2006), although the cellular mechanisms that underlie these changes remain poorly understood.
Postsynaptic electrical activity can trigger LTP of GABAergic synapses
Aizenman et al. (1998) studied the cerebellar Purkinje cell-deep cerebellar nuclei synapse and first proposed a novel model to explain how LTP and LTD can be triggered solely by the activity of inhibitory synapses. Here LTP requires a rise in postsynaptic intracellular calcium that can be achieved through rebound depolarization after hyperpolarizing IPSPs. Following a burst of IPSPs, the membrane rapidly depolarizes, reaching a potential at which voltage-gated calcium channels are activated, allowing an essential increase in intracellular Ca2+ (Aizenman et al. 1998; Ouardouz et al. 2000). Again, LTP is apparently maintained by an increase in postsynaptic GABAA receptor number/conductance, as exogenously applied GABAA agonists elicit a larger postsynaptic response following LTP induction, but the mechanisms have not been explored further (Ouardouz et al. 2000). A form of LTP at GABAergic synapses, recently described in developing visual cortex, is also induced by a novel, postsynaptic activity-dependent mechanism (Maffei et al. 2006). This LTP is triggered at GABAergic synapses on star pyramidal cells by subthreshold depolarization of the pyramidal cells during presynaptic firing, but not during coincident presynaptic and postsynaptic firing. The involvement of postsynaptic Ca2+ has not been tested as yet, but the rather precise requirements for LTP induction suggest that the signalling molecule involved must operate in a narrow concentration or time window. This form of LTP is also apparently maintained by postsynaptic changes. LTP at these GABAergic synapses may play an important role in the critical period for visual cortex development.
Endocannabinoids mediate LTD of GABAergic synapses
Endocannabinoid-mediated LTD (ec-LTD) at GABAA synapses in the hippocampus occurs entirely independently of postsynaptic Ca2+ (Chevaleyre et al. 2006). Ec-LTD is initiated by glutamate release onto the metabotropic glutamate receptors on the postsynaptic cell. Activation of the mGluRs then leads to production of an endocannabinoid, most probably 2-arachidonylglycerol, which acts as a retrograde messenger that binds to CB1 receptors on neighbouring presynaptic GABAergic nerve terminals. CB1 receptors cause a long-lasting depression of GABA release involving PKA signalling and the active zone protein RIM1α (Chevaleyre et al. 2007). Thus, unlike many other examples of GABAA synapse plasticity, ec-LTD is mediated by a change in presynaptic function. Endocannabinoid-mediated LTD has also been observed at other GABAergic and glutamatergic synapses, with some mechanistic variations on the theme (Chevaleyre et al. 2006).
GABAA synapses in the VTA exhibit NO-dependent LTP
Drugs of abuse share one important feature: the activation of the mesolimbic dopamine system. This involves increased firing of dopamine neurons in the VTA and a subsequent increase of dopamine released into the nucleus accumbens and other regions of the limbic forebrain (Di Chiara & Imperato, 1988; Nestler, 2001; Hyman et al. 2006). We became interested in the question of whether GABAergic synapses in the VTA could undergo LTP because μ-opioid receptors (the targets of morphine) are concentrated on GABAergic cells, and because GABAergic drugs delivered to the VTA are reinforcing. Using whole-cell recording in rat brain slices, we showed that high-frequency stimulation (HFS) induces LTP of GABAA-mediated synaptic transmission (LTPGABA) onto dopamine neurons of the VTA (Fig. 1) (Nugent et al. 2007). LTPGABA required an increase in postsynaptic Ca2+ concentration. We found that LTPGABA is heterosynaptic, i.e. it is triggered when glutamate activates NMDA receptors but potentiates neighbouring GABAergic synapses. Importantly, LTPGABA did not require active GABAA synapses, as the potentiation could be triggered by NMDAR activation in the presence of GABAA receptor antagonists. LTPGABA was associated with modifications in the coefficient of variation and paired pulse ratio of evoked GABAA IPSCs, suggesting that it is maintained by persistently increased GABA release. Similarly to ec-LTD, if the LTP induction occurs postsynaptically while the locus of expression is presynaptic, then a retrograde messenger is required that must travel backward from the postsynaptic dopamine neuron to increase GABA release from presynaptic terminals. Several lines of evidence supported nitric oxide (NO) as the retrograde signal maintaining LTPGABA. Inhibition of NO production or bathing the brain slice in NO scavengers blocked LTPGABA. Furthermore, increasing NO levels enhanced GABAA IPSCs. We also found that a guanylate cyclase inhibitor blocked LTPGABA, whereas a cGMP analogue mimicked it, indicating that NO facilitates GABA release by activation of presynaptic guanylate cyclase (Figs 2 and 4). This was the first demonstration of a presynaptically maintained LTP at GABAergic synapses requiring NO as a retrograde messenger.
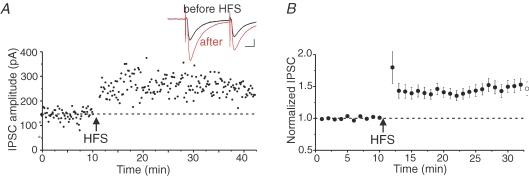
Figure 1. GABAergic synapses on dopamine neurons are potentiated after high-frequency stimulation.
A, single experiment showing LTPGABA recorded in a dopamine neuron while whole-cell voltage-clamped at −70 mV. At the arrow (HFS), the afferents were stimulated using a 100 Hz, 1-s-long train, repeated twice 20 s apart, while holding the dopamine neuron in current-clamp. Inset, averaged IPSCs before (black) and 25 min after HFS (red). In this and all figures, 10 consecutive IPSCs from each condition were averaged for illustration. Calibration: 10 ms, 50 pA. In this and all IPSC experiments, 10 μm DNQX and 1 μm strychnine were present to block AMPARs and glycine receptors, respectively. The internal solution was K+-based, so that IPSCs are seen as inward synaptic currents. B, average of 71 experiments from dopamine cells. LTPGABA was not triggered in all cells, but data from all cells are included in this and subsequent graphs. (Adapted from Nugent et al. 2007.)
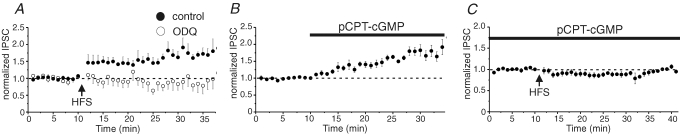
Figure 2. LTPGABA requires NO–cGMP signalling.
A, the guanylate cyclase inhibitor ODQ blocks LTPGABA. 10 μm ODQ (1H-[1,2,4] oxadiazolo [4,3-a] quinoxalin-1-dione) was bath-applied beginning at least 10 min prior to HFS (arrow). LTPGABA was blocked, implicating the NO–cGMP signalling cascade in LTPGABA (control LTP: •, 160 ± 5.7% of pre-HFS values, n = 6; ODQ-treated cells: ○, 81 ± 4% of pre-drug HFS, n = 8). B, pCPT-cGMP (100 μm), a cGMP analogue, potentiated IPSCs without HFS (169 ± 5% of pre-drug values, n = 11). C, in experiments like those in B, after the IPSCs reached a new, potentiated level in 100 μm pCPT-cGMP, HFS was delivered (arrow). pCPT-cGMP occludes potentiation of IPSCs by HFS (89 ± 0.5 of pre-HFS values, n = 6). (Adapted from Nugent et al. 2007.)
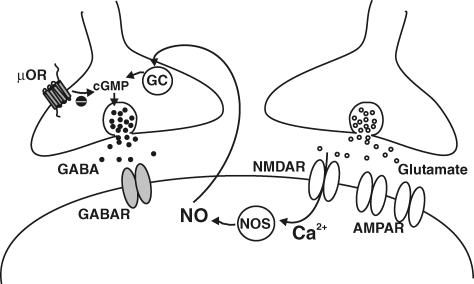
Figure 4. Model of the signalling molecules involved in induction of LTPGABA.
LTP of GABAergic synapses is heterosynaptic, triggered by NMDA receptor activation at glutamate synapses and requires NO–cGMP signalling. An in vivo injection of morphine prevents LTPGABA through the presynaptic interaction between opioid signalling pathways and NO targets.
NMDA receptor activation on VTA dopamine cells also leads to long-term potentiation of excitatory synapses (Bonci & Malenka, 1999; Overton et al. 1999). Our work suggests that NMDAR activation is likely to trigger parallel long-term plasticity at inhibitory synapses. The balance between excitatory and inhibitory synaptic input regulates neuronal cell firing, and therefore activity-dependent simultaneous adjustment of the strengths of inhibitory and excitatory synapses can stabilize the circuit, preventing saturation of neuronal firing (Galarreta & Hestrin, 1998; Varela et al. 1999; Abbott & Chance, 2005). Our data suggest that in the VTA, NMDA receptor activation could normally act as a ‘gain modulator’, with LTP at excitatory synapses balanced by LTPGABA, stabilizing the firing rate of dopamine neurons (Abbott & Chance, 2005). Similarly, in the hippocampus, activity in excitatory afferents can also trigger simultaneous NMDAR-dependent LTP at excitatory synapses and retrograde messenger-induced plasticity at neighbouring inhibitory synapses. However, in the case of endocannabinoid-triggered plasticity, the GABAergic terminals undergo LTD, not LTP. This coincident activation of excitatory and inhibitory synapses in the hippocampus would therefore be expected to do just the opposite of that in the VTA – to synergize, promoting excitability rather than maintaining stability, at least at the local dendritic level. Both ec-LTD in the hippocampus and NO-triggered LTPGABA in the VTA share common features – each is triggered by postsynaptic glutamate receptors, requires a retrograde messenger, and is maintained by persistent presynaptic alteration of GABA release at nearby synaptic terminals – suggesting that these may be common themes in CNS circuit modifications. The precise circuits and circumstances in which synaptic plasticity at excitatory and inhibitory synapses occurs simultaneously will be an exciting avenue for future research.
LTPGABA is blocked by in vivo exposure to morphine
NMDA receptor-dependent LTP has been demonstrated at excitatory synapses on midbrain dopamine neurons and can also be induced by addictive drugs (Bonci & Malenka, 1999; Overton et al. 1999; Jones et al. 2000; Mansvelder & McGehee, 2000; Ungless et al. 2001; Saal et al. 2003; Faleiro et al. 2004; Liu et al. 2005). Several lines of evidence support the involvement of synaptic plasticity at excitatory synapses of the mesolimbic dopaminergic system in the development of addiction (Hyman & Malenka, 2001; Carlezon & Nestler, 2002; Thomas & Malenka, 2003; Jones & Bonci, 2005; Kauer & Malenka, 2007). When we found that LTPGABA accompanies NMDAR activation at VTA synapses, we therefore investigated whether or not morphine, which modulates inhibitory function in the VTA, can modulate LTPGABA. Intriguingly, we found that in vivo morphine administration entirely blocked LTPGABA (Nugent et al. 2007). GABAA synapses in VTA slices from rats that had received morphine 24 h earlier did not exhibit LTP (Fig. 3). We further investigated the mechanism by which morphine blocked LTPGABA. Increasing NO levels exogenously had no effect on GABAA synapses in morphine-treated animals, whereas application of a cGMP analogue still potentiated the synapses. These data suggest a model in which in vivo morphine interrupts the signalling between NO and cGMP generation, perhaps at the level of guanylate cyclase (Fig. 4). This key finding may inform the development of novel drugs to prevent or treat addiction.
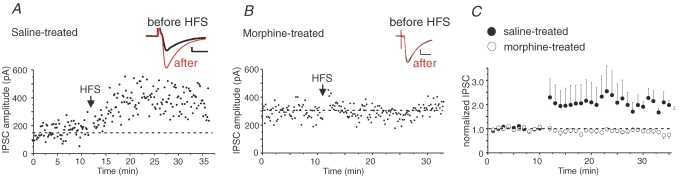
Figure 3. Opioids block LTPGABAin vivo.
A and B, in vivo exposure to morphine prevents LTPGABA in slices prepared 24 h later. Single experiments and sample IPSCs (insets) from slices from a saline-injected animal (A) and a morphine-injected animal (B). Calibration: 10 ms, 50 pA. C, averaged experiments from slices prepared 24 h after either saline or 10 mg kg−1 morphine injection delivered i.p. (saline cells, •, 196 ± 20% of pre-HFS values, n = 10; morphine-treated cells, ○, 91 ± 4% of pre-HFS values, n = 11). (Adapted from Nugent et al. 2007.)
One question raised by our work is whether other drugs of abuse may also block LTPGABA. Although as yet we have not answered this question directly, intriguing experimental evidence suggests that repeated daily exposure to cocaine in vivo reduces GABAA receptor-mediated inhibition of dopamine neurons (Liu et al. 2005). The relationship between LTPGABA and this phenomenon has not yet been tested, but these data suggest that attenuation of local GABAA-mediated inhibition in the VTA may represent a common target of addictive drugs.
Pharmacological blockade of GABAergic transmission, presumably by enhancing Ca2+ influx by depolarization or NMDA receptor activation, facilitates the induction of LTP at excitatory synapses. On the basis of this observation, changes in GABAergic synaptic transmission will have important consequences for glutamatergic synaptic plasticity (Chevaleyre et al. 2006). The modulation of dopamine transmission in the VTA as a result of the loss of LTPGABA will therefore contribute not only to increased dopamine cell firing and dopamine release, but also to LTP at excitatory synapses, which has been reported after either morphine and cocaine exposure (Ungless et al. 2001; Saal et al. 2003; Borgland et al. 2004; Liu et al. 2005). LTPGABA can thus be regarded as contributing to metaplasticity, in which an existing form of synaptic plasticity (LTP at excitatory synapses) is modulated (Abraham & Tate, 2007). After morphine administration the loss of normal inhibitory control coupled with metaplastic potentiation of excitatory synapses may represent neuroadaptations that increase the incentive properties of these addicting drugs.
Conclusions and future directions
Understanding the mechanisms that control plasticity at GABAergic synapses is essential to assessing their critical role in CNS function. Our recent work provides an example of drug-induced modification of GABAergic synapses in response to in vivo drug exposure. These findings strengthen the idea that changes in synaptic plasticity may contribute to the development of addictive behaviour. Understanding the cellular mechanisms involved in the particular forms of synaptic plasticity in addiction-related brain areas could provide new insights into the molecular pathology of drug addiction and new therapeutic approaches. Many questions remain to be elucidated regarding how LTPGABA is expressed and maintained at VTA synapses, and whether other drugs of abuse modulate plasticity in the same way that morphine does. Given the importance of NO signalling in the brain, it will also be of great interest to determine whether NO-mediated LTP is a property of GABAergic synapses in other brain areas. Compared with our understanding of synaptic plasticity at excitatory synapses, many questions are unanswered regarding GABAA synapse plasticity. How are GABAA receptors trafficked and stabilized during postsynaptically maintained forms of LTP and LTD? How precisely do retrograde messenger molecules persistently modulate GABA release from presynaptic terminals? What are the behavioural correlates of synaptic plasticity at GABAergic synapses, and how long does it last? Why is it that some GABAergic synapses can undergo LTP or LTD while others appear unmodifiable? As patch-clamp recordings have become the standard approach in the study of synaptic function, the use of slices from immature animals has become more common; another important question therefore is to what extent GABAA synapse plasticity is a feature of immature versus mature brain. It is clear that GABAergic inhibition is a key element of essentially every brain circuit, and the control of GABAergic synaptic strength is an important and growing area of interest.
Acknowledgments
This work was supported by NIH grants DA11289 and NS050570 to J.A.K. and 1 F32 DA021973-01A1 to F.S.N.
References
- Abbott LF, Chance FS. Drivers and modulators from push-pull and balanced synaptic input. Prog Brain Res. 2005;149:147–155. doi: 10.1016/S0079-6123(05)49011-1. [DOI] [PubMed] [Google Scholar]
- Abraham WC, Tate WP. Metaplasticity: a new vista across the field of synaptic plasticity. Prog Neurobiol. 2007;52:303–323. doi: 10.1016/s0301-0082(97)00018-x. [DOI] [PubMed] [Google Scholar]
- Aizenman CD, Manis PB, Linden DJ. Polarity of long-term synaptic gain change is related to postsynaptic spike firing at a cerebellar inhibitory synapse. Neuron. 1998;21:827–835. doi: 10.1016/s0896-6273(00)80598-x. [DOI] [PubMed] [Google Scholar]
- Akerman CJ, Cline HT. Refining the roles of GABAergic signaling during neural circuit formation. Trends Neurosci. 2007;30:382–389. doi: 10.1016/j.tins.2007.06.002. [DOI] [PubMed] [Google Scholar]
- Bonci A, Malenka RC. Properties and plasticity of excitatory synapses on dopaminergic and GABAergic cells in the ventral tegmental area. J Neurosci. 1999;19:3723–3730. doi: 10.1523/JNEUROSCI.19-10-03723.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Malenka RC, Bonci A. Acute and chronic cocaine-induced potentiation of synaptic strength in the ventral tegmental area: electrophysiological and behavioral correlates in individual rats. J Neurosci. 2004;24:7482–7490. doi: 10.1523/JNEUROSCI.1312-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillard O, Ben-Ari Y, Gaiarsa JL. Long-term potentiation of GABAergic synaptic transmission in neonatal rat hippocampus. J Physiol. 1999a;518:109–119. doi: 10.1111/j.1469-7793.1999.0109r.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caillard O, Ben-Ari Y, Gaiarsa JL. Mechanisms of induction and expression of long-term depression at GABAergic synapses in the neonatal rat hippocampus. J Neurosci. 1999b;19:7568–7577. doi: 10.1523/JNEUROSCI.19-17-07568.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlezon WA, Jr, Nestler EJ. Elevated levels of GluR1 in the midbrain: a trigger for sensitization to drugs of abuse? Trends Neurosci. 2002;25:610–615. doi: 10.1016/s0166-2236(02)02289-0. [DOI] [PubMed] [Google Scholar]
- Cherubini E, Gaiarsa JL, Ben-Ari Y. GABA: an excitatory transmitter in early postnatal life. Trends Neurosci. 1991;14:515–519. doi: 10.1016/0166-2236(91)90003-d. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Castillo PE. Heterosynaptic LTD of hippocampal GABAergic synapses: a novel role of endocannabinoids in regulating excitability. Neuron. 2003;38:461–472. doi: 10.1016/s0896-6273(03)00235-6. [DOI] [PubMed] [Google Scholar]
- Chevaleyre V, Heifets BD, Kaeser PS, Sudhof TC, Castillo PE. Endocannabinoid-mediated long-term plasticity requires cAMP/PKA signaling and RIM1α. Neuron. 2007;54:801–812. doi: 10.1016/j.neuron.2007.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chevaleyre V, Takahashi KA, Castillo PE. Endocannabinoid-mediated synaptic plasticity in the CNS. Annu Rev Neurosci. 2006;29:37–76. doi: 10.1146/annurev.neuro.29.051605.112834. [DOI] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faleiro LJ, Jones S, Kauer JA. Rapid synaptic plasticity of glutamatergic synapses on dopamine neurons in the ventral tegmental area in response to acute amphetamine injection. Neuropsychopharmacol. 2004;29:2115–2125. doi: 10.1038/sj.npp.1300495. [DOI] [PubMed] [Google Scholar]
- Farrant M, Nusser Z. Variations on an inhibitory theme: phasic and tonic activation of GABAA receptors. Nat Rev Neurosci. 2005;6:215–229. doi: 10.1038/nrn1625. [DOI] [PubMed] [Google Scholar]
- Gaiarsa JL, Caillard O, Ben-Ari Y. Long-term plasticity at GABAergic and glycinergic synapses: mechanisms and functional significance. Trends Neurosci. 2002;25:564–570. doi: 10.1016/s0166-2236(02)02269-5. [DOI] [PubMed] [Google Scholar]
- Galarreta M, Hestrin S. Frequency-dependent synaptic depression and the balance of excitation and inhibition in the neocortex. Nat Neurosci. 1998;1:587–594. doi: 10.1038/2822. [DOI] [PubMed] [Google Scholar]
- Glaum SR, Brooks PA. Tetanus-induced sustained potentiation of monosynaptic inhibitory transmission in the rat medulla: evidence for a presynaptic locus. J Neurophysiol. 1996;76:30–38. doi: 10.1152/jn.1996.76.1.30. [DOI] [PubMed] [Google Scholar]
- Grabauskas G, Bradley RM. Potentiation of GABAergic synaptic transmission in the rostral nucleus of the solitary tract. Neurosci. 1999;94:1173–1182. doi: 10.1016/s0306-4522(99)00379-6. [DOI] [PubMed] [Google Scholar]
- Grunze HC, Rainnie DG, Hasselmo ME, Barkai E, Hearn EF, McCarley RW, Greene RW. NMDA-dependent modulation of CA1 local circuit inhibition. J Neurosci. 1996;16:2034–2043. doi: 10.1523/JNEUROSCI.16-06-02034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gubellini P, Ben-Ari Y, Gaiarsa JL. Activity- and age-dependent GABAergic synaptic plasticity in the developing rat hippocampus. Eur J Neurosci. 2001;14:1937–1946. doi: 10.1046/j.0953-816x.2001.01823.x. [DOI] [PubMed] [Google Scholar]
- Gubellini P, Ben-Ari Y, Gaiarsa JL. Endogenous neurotrophins are required for the induction of GABAergic long-term potentiation in the neonatal rat hippocampus. J Neurosci. 2005;25:5796–5802. doi: 10.1523/JNEUROSCI.0824-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto T, Ishii T, Ohmori H. Release of Ca2+ is the crucial step for the potentiation of IPSCs in the cultured cerebellar Purkinje cells of the rat. J Physiol. 1996;497:611–627. doi: 10.1113/jphysiol.1996.sp021794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC. Addiction and the brain: the neurobiology of compulsion and its persistence. Nat Rev Neurosci. 2001;2:695–703. doi: 10.1038/35094560. [DOI] [PubMed] [Google Scholar]
- Hyman SE, Malenka RC, Nestler EJ. Neural mechanisms of addiction: the role of reward-related learning and memory. Annu Rev Neurosci. 2006;29:565–598. doi: 10.1146/annurev.neuro.29.051605.113009. [DOI] [PubMed] [Google Scholar]
- Jones S, Bonci A. Synaptic plasticity and drug addiction. Curr Opin Pharmacol. 2005;5:20–25. doi: 10.1016/j.coph.2004.08.011. [DOI] [PubMed] [Google Scholar]
- Jones S, Kornblum JL, Kauer JA. Amphetamine blocks long-term synaptic depression in the ventral tegmental area. J Neurosci. 2000;20:5575–5580. doi: 10.1523/JNEUROSCI.20-15-05575.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Jiang L, Goldman SA, Nedergaard M. Astrocyte-mediated potentiation of inhibitory synaptic transmission. Nat Neurosci. 1998;1:683–692. doi: 10.1038/3684. [DOI] [PubMed] [Google Scholar]
- Kano M. Calcium-induced long-lasting potentiation of GABAergic currents in cerebellar Purkinje cells. Jpn J Physiol. 1994;44(Suppl. 2):S131–S136. [PubMed] [Google Scholar]
- Kano M. Plasticity of inhibitory synapses in the brain: a possible memory mechanism that has been overlooked. Neurosci Res. 1995;21:177–182. doi: 10.1016/0168-0102(94)00860-i. [DOI] [PubMed] [Google Scholar]
- Kauer JA, Malenka RC. Synaptic plasticity and addiction. Nat Rev Neurosci. 2007;8:844–858. doi: 10.1038/nrn2234. [DOI] [PubMed] [Google Scholar]
- Kawaguchi SY, Hirano T. Signaling cascade regulating long-term potentiation of GABAA receptor responsiveness in cerebellar Purkinje neurons. J Neurosci. 2002;22:3969–3976. doi: 10.1523/JNEUROSCI.22-10-03969.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi SY, Hirano T. Sustained structural change of GABAA receptor-associated protein underlies long-term potentiation at inhibitory synapses on a cerebellar Purkinje neuron. J Neurosci. 2007;27:6788–6799. doi: 10.1523/JNEUROSCI.1981-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu Y. Age-dependent long-term potentiation of inhibitory synaptic transmission in rat visual cortex. J Neurosci. 1994;14:6488–6499. doi: 10.1523/JNEUROSCI.14-11-06488.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu Y. GABAB receptors, monoamine receptors, and postsynaptic inositol trisphosphate-induced Ca2+ release are involved in the induction of long-term potentiation at visual cortical inhibitory synapses. J Neurosci. 1996;16:6342–6352. doi: 10.1523/JNEUROSCI.16-20-06342.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komatsu Y, Yoshimura Y. Activity-dependent maintenance of long-term potentiation at visual cortical inhibitory synapses. J Neurosci. 2000;20:7539–7546. doi: 10.1523/JNEUROSCI.20-20-07539.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien CC, Mu Y, Vargas-Caballero M, Poo MM. Visual stimuli-induced LTD of GABAergic synapses mediated by presynaptic NMDA receptors. Nat Neurosci. 2006;9:372–380. doi: 10.1038/nn1649. [DOI] [PubMed] [Google Scholar]
- Liu QS, Pu L, Poo MM. Repeated cocaine exposure in vivo facilitates LTP induction in midbrain dopamine neurons. Nature. 2005;437:1027–1031. doi: 10.1038/nature04050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu YM, Mansuy IM, Kandel ER, Roder J. Calcineurin-mediated LTD of GABAergic inhibition underlies the increased excitability of CA1 neurons associated with LTP. Neuron. 2000;26:197–205. doi: 10.1016/s0896-6273(00)81150-2. [DOI] [PubMed] [Google Scholar]
- McLean HA, Caillard O, Ben-Ari Y, Gaiarsa JL. Bidirectional plasticity expressed by GABAergic synapses in the neonatal rat hippocampus. J Physiol. 1996;496:471–477. doi: 10.1113/jphysiol.1996.sp021699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffei A, Nataraj K, Nelson SB, Turrigiano GG. Potentiation of cortical inhibition by visual deprivation. Nature. 2006;443:81–84. doi: 10.1038/nature05079. [DOI] [PubMed] [Google Scholar]
- Malenka RC, Bear MF. LTP and LTD: an embarrassment of riches. Neuron. 2004;44:5–21. doi: 10.1016/j.neuron.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Mansvelder HD, McGehee DS. Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron. 2000;27:349–357. doi: 10.1016/s0896-6273(00)00042-8. [DOI] [PubMed] [Google Scholar]
- Mitoma H, Kobayashi T, Song SY, Konishi S. Enhancement by serotonin of GABA-mediated inhibitory synaptic currents in rat cerebellar Purkinje cells. Neurosci Lett. 1994;173:127–130. doi: 10.1016/0304-3940(94)90165-1. [DOI] [PubMed] [Google Scholar]
- Mitoma H, Konishi S. Long-lasting facilitation of inhibitory transmission by monoaminergic and cAMP-dependent mechanism in rat cerebellar GABAergic synapses. Neurosci Lett. 1996;217:141–144. [PubMed] [Google Scholar]
- Morishita W, Sastry BR. Long-term depression of IPSPs in rat deep cerebellar nuclei. Neuroreport. 1993;4:719–722. doi: 10.1097/00001756-199306000-00030. [DOI] [PubMed] [Google Scholar]
- Morishita W, Sastry BR. Postsynaptic mechanisms underlying long-term depression of GABAergic transmission in neurons of the deep cerebellar nuclei. J Neurophysiol. 1996;76:59–68. doi: 10.1152/jn.1996.76.1.59. [DOI] [PubMed] [Google Scholar]
- Nestler EJ. Molecular neurobiology of addiction. Am J Addiction. 2001;10:201–217. doi: 10.1080/105504901750532094. [DOI] [PubMed] [Google Scholar]
- Nugent FS, Penick EC, Kauer JA. Opioids block long-term potentiation of inhibitory synapses. Nature. 2007;446:1086–1090. doi: 10.1038/nature05726. [DOI] [PubMed] [Google Scholar]
- Ouardouz M, Ouardouz M, Sastry BR. Mechanisms underlying LTP of inhibitory synaptic transmission in the deep cerebellar nuclei. J Neurophysiol. 2000;84:1414–1421. doi: 10.1152/jn.2000.84.3.1414. [DOI] [PubMed] [Google Scholar]
- Overton PG, Richards CD, Berry MS, Clark D. Long-term potentiation at excitatory amino acid synapses on midbrain dopamine neurons. Neuroreport. 1999;10:221–226. doi: 10.1097/00001756-199902050-00004. [DOI] [PubMed] [Google Scholar]
- Patenaude C, Chapman CA, Bertrand S, Congar P, Lacaille JC. GABAB receptor- and metabotropic glutamate receptor-dependent cooperative long-term potentiation of rat hippocampal GABAA synaptic transmission. J Physiol. 2003;553:155–167. doi: 10.1113/jphysiol.2003.049015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saal D, Dong Y, Bonci A, Malenka RC. Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron. 2003;37:577–582. doi: 10.1016/s0896-6273(03)00021-7. [DOI] [PubMed] [Google Scholar]
- Saitow F, Suzuki H, Konishi S. β-Adrenoceptor-mediated long-term up-regulation of the release machinery at rat cerebellar GABAergic synapses. J Physiol. 2005;565:487–502. doi: 10.1113/jphysiol.2005.084384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer A, Simon G, Kovacs G, Rai R. Synaptic disinhibition during maintenance of long-term potentiation in the CA1 hippocampal subfield. Proc Natl Acad Sci U S A. 1994;91:3058–3062. doi: 10.1073/pnas.91.8.3058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelzer A, Slater NT, ten Bruggencate G. Activation of NMDA receptors blocks GABAergic inhibition in an in vitro model of epilepsy. Nature. 1987;326:698–701. doi: 10.1038/326698a0. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Malenka RC. Synaptic plasticity in the mesolimbic dopamine system. Philos Trans R Soc Lond B Biol Sci. 2003;358:815–819. doi: 10.1098/rstb.2002.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungless MA, Whistler JL, Malenka RC, Bonci A. Single cocaine exposure in vivo induces long-term potentiation in dopamine neurons. Nature. 2001;411:583–587. doi: 10.1038/35079077. [DOI] [PubMed] [Google Scholar]
- Varela JA, Song S, Turrigiano GG, Nelson SB. Differential depression at excitatory and inhibitory synapses in visual cortex. J Neurosci. 1999;19:4293–4304. doi: 10.1523/JNEUROSCI.19-11-04293.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]