Abstract
We recently showed that propriospinal neurons contribute to bulbospinal activation of locomotor networks in the in vitro neonatal rat brainstem–spinal cord preparation. In the present study, we examined whether propriospinal neurons alone, in the absence of long direct bulbospinal transmission to the lumbar cord, can successfully mediate brainstem activation of the locomotor network. In the presence of staggered bilateral spinal cord hemisections, the brainstem was stimulated electrically while recording from lumbar ventral roots. The rostral hemisection was located between C1 and T3 and the contralateral caudal hemisection was located between T5 and mid-L1. Locomotor-like activity was evoked in 27% of the preparations, which included experiments with staggered hemisections placed only two segments apart. There was no relation between the likelihood of developing locomotor-like activity and the distance separating the two hemisections or specific level of the hemisections. In some experiments, where brainstem stimulation alone was ineffective, neurochemical excitation of propriospinal neurons (using 5-HT and NMDA) at concentrations subthreshold for producing locomotor-like activity, promoted locomotor-like activity in conjunction with brainstem stimulation. In other experiments, involving neither brainstem stimulation nor cord hemisections, the excitability of propriospinal neurons in the cervical and/or thoracic region was selectively enhanced by bath application of 5-HT and NMDA or elevation of bath K+ concentration. These manipulations produced locomotor-like activity in the lumbar region. In total, the results suggest that propriospinal neurons are sufficient for transmission of descending locomotor command signals. This observation has implications for regeneration strategies aimed at restoration of locomotor function after spinal cord injury.
During locomotion, propriospinal neurons mediate forelimb–hindlimb coordination in quadrupeds (e.g. Miller et al. 1975; Halbertsma et al. 1976; Ballion et al. 2001; Juvin et al. 2005) and orchestrate intersegmental phase-lags in the lamprey (e.g. Matsushima & Grillner, 1990; Buchanan, 1992; Cohen et al. 1992; Miller & Sigvardt, 2000). Propriospinal neurons are also involved in selecting appropriate rhythmic motor patterns in the turtle (Berkowitz & Stein, 1994) and appear to have a role in coordinating arm–leg movements during walking in humans (for review see Dietz, 2002).
We recently showed that a propriospinal system of neurons also contributes to the descending activation of locomotor activity in response to brainstem stimulation (Zaporozhets et al. 2006a). This raises the possibility that regeneration of relatively short axonal connections belonging to this propriospinal system may restore propagation of the locomotor command signal across the site of spinal cord injury. Short propriospinal neurons are more vulnerable to contusion insults than long propriospinal projections; however, cell bodies of short propriospinal neurons are also relatively resistant to retrograde death after axotomy (Conta & Stelzner, 2004). Thus, if successful, re-establishing propriospinal connections across the lesion site may circumvent the need to develop potentially more difficult repair strategies aimed at regenerating direct bulbospinal projections over long distances.
However, in addition to propriospinal pathways, long direct pathways may have an essential role in transmitting the brainstem locomotor signal. For instance, long direct reticulospinal projections travelling in the ventral and ventrolateral fasciculi are thought to mediate bulbospinal activation of locomotion in the cat and rat (e.g. Steeves & Jordan, 1980, 1984; Eidelberg et al. 1981; Iwahara et al. 1991; Noga et al. 1991, 2003; Stelzner & Cullen, 1991; Magnuson & Trinder, 1997; Schucht et al. 2002; Matsuyama et al. 2004). Lloyd (1941) proposed a bulbospinal correlation system wherein brainstem motor impulses travel through long-projecting reticulospinal axons and mediate their influence on motoneurons via a propriospinal system. He suggested the propriospinal system was as an extension of the brainstem reticular nuclei, received input from vestibulospinal, corticospinal and primary afferent input, and set the state of the animal appropriate for initiation of voluntary movement. Thus, a critical issue with respect to functional recovery is whether re-establishing the propriospinal system alone, in the absence of regenerated long bulbospinal fibres, is sufficient to mediate descending activation of the locomotor network.
In order to examine whether the propriospinal pathway alone is capable of transmitting a functionally effective locomotor command signal, long direct pathways must be selectively blocked. Therefore, in the present study we tested the effect of staggered contralateral hemisections of the spinal cord on the capacity to induce locomotion in the lumbar region in response to electrical stimulation of the brainstem. In addition we examined whether neurochemical excitation of propriospinal neurons at the cervical and/or thoracic cord levels promoted locomotor activity in the lumbar region. Preliminary results were presented previously in abstract form (Zaporozhets et al. 2006b).
Methods
Experimental protocols used in this study were in compliance with the guidelines set by the Canadian Council on Animal Care and the University of Manitoba. Experiments were performed on 203 brainstem–spinal cord preparations isolated from Sprague–Dawley rats (1–5 days old). Isolation of the spinal cord, as well as methods of extracellular recording, were previously described (e.g. Cowley & Schmidt, 1995). In brief, animals were anaesthetized with isofluorane, decerebrated at the mid-collicular level, eviscerated, and placed in a bath chamber containing artificial cerebrospinal fluid (ACSF) composed as follows (mm): NaCl 128, KCl 4.0, NaH2PO4 0.5, CaCl2 1.5, NaHCO3 21, MgSO4 1.0 and glucose 30, equilibrated to pH 7.4 with 95% O2–5% CO2. Experiments were conducted at room temperature (ACSF approximately 22°C). In some experiments the spinal cord bath was partitioned using a barrier made of plastic strips sealed at cord contact edges with petroleum jelly.
Ventral root recordings were obtained using glass suction electrodes. The records were band-pass filtered (30–3000 Hz), digitized and captured using Axoscope (v. 9.0 Axon Instruments) software. Axoscope files were converted to an appropriate binary format for further analysis using special-purpose software (developed by the Spinal Cord Research Centre, University of Manitoba). Sigma-Stat was used for the logistic regression analysis.
Induction of locomotor-like activity
Electrical stimulation of the brainstem was performed according to our previously reported method (Zaporozhets et al. 2004). In brief, an ACSF-filled glass electrode, with a tip diameter of 200–300 μm, was placed in contact with the ventral surface of the brainstem. Monophasic rectangular current pulses (4–20 ms, 0.5–10 mA, 0.8–2.0 Hz) were delivered using bipolar stimulation. Stimulation was applied for a maximum of 2–3 min. If initial attempts to induce locomotion were unsuccessful, brainstem stimulation was periodically administered over the course of several hours. Some preparations developed locomotor-like activity soon after completion of the hemisections and placement of the recording electrodes. Other preparations initially failed to respond to brainstem stimulation but became responsive after several hours.
Combinations of N-methyl-d-aspartate (NMDA), 5-hydroxytryptamine (5-HT) and bicuculline (BIC) are capable of inducing lumbar rhythmic activity when applied to the brainstem (e.g. Smith & Feldman, 1987; Zaporozhets et al. 2006a). Thus, in some experiments, these neurochemicals were applied to selected spinal cord regions in an effort to excite cervical and/or thoracic propriospinal neurons and thereby induce locomotor-like activity in the lumbar cord. Neurochemicals were applied from concentrated stock solutions (1–10 mm) and all concentrations refer to final bath concentrations which ranged as follows: NMDA, 2–10 μm; 5-HT, 15–50 μm; and BIC, 10–20 μm.
Criteria for locomotor-like activity
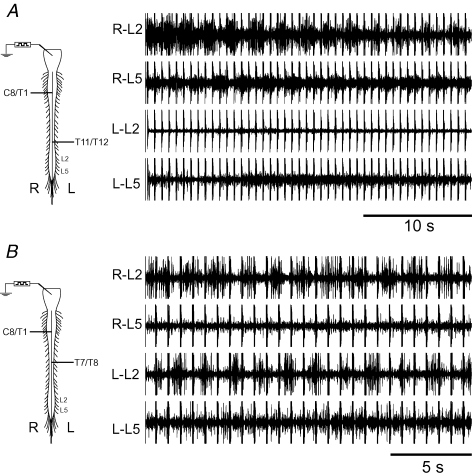
We recorded from L2 and L5 ventral roots, bilaterally, in all preparations. Phasic lumbar ventral root discharge in the in vitro neonatal rat varies in pattern and quality depending on the preparation and experimental conditions. Ventral root records were classified as locomotor-like if the activity was rhythmic, featured appropriate side-to-side and flexor–extensor alternation pattern, and contained at least five successive ventral root bursts. Phasic ventral root discharge was considered rhythmic if the coefficient of variation (expressed as CV ± standard deviation) of the cycle period was ≤ 25%. The latter criterion is based on previous observations of cycle period CV in the in vitro neonatal rat preparation during locomotor-like activity (Cowley et al. 2005). The pattern was deemed consistent with locomotion if (a) contralateral alternation was observed between the left and right sides at the L2 level and/or between left and right sides at the L5 level, and (b) ipsilateral alternating activity between the L2 (predominantly flexor-related activity) and L5 (predominantly extensor-related activity) roots was present on at least one side. Thus, for example, alternating activity between the right L2 and L5 ventral roots is observed in the bilaterally hemisected preparation shown in Fig. 1A; however, this recording was rejected because of the lack of left–right alternation. It is acknowledged that the presence of rhythmic alternation between L2 and L5 discharge, without rhythmic activity on the contralateral side, may reflect locomotor network operation recruited on one side of the cord. However, for the purpose of this series, we used stricter criteria requiring that lumbar discharge be present bilaterally. There was no evidence in this series that lumbar locomotor-like activity was consistently lost on one side or the other in relation to the side with the caudal hemisection. Figure 1B shows another example of a rejected record. In this case left–right alternation was present, but no evidence of L2–L5 alternation was seen.
Figure 1. Two examples of lumbar ventral root rhythmic activity that did not meet criteria for locomotor-like activity.
A, rhythmic alternating right (R) L2 and L5 was evoked in response to electrical stimulation of the brainstem, consistent with an alternating pattern of flexor and extensor activity, respectively. However, rhythmic activity was absent on the left (L) side and therefore side-to-side alternating activity could not be documented. B, in this example, left–right alternation is present at the L2 segmental level. However, the recording was not considered locomotor-like for the purposes of this series because of the absence of flexor (L2)–extensor (L5) alternation. Horizontal lines in spinal cord diagrams indicate hemisection levels. Regular occurring spikes in ventral root recordings are artifacts related to brainstem electrical stimulation.
Hemisections and histological processing
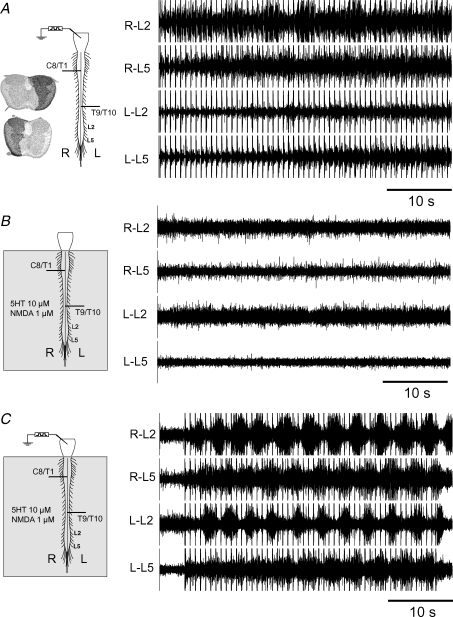
Hemisections of the spinal cord were made using iridectomy scissors. The completeness of hemisections was verified by separating the sectioned tissue such that an unobstructed view of the bottom of the bath chamber was seen, using a surgical dissection microscope. After the in vitro experiment, spinal cords were stored in 4% paraformaldehyde. The cord was transferred to 15% sucrose in 0.1 m phosphate buffer 48 h before slicing the tissue on a cryostat. The tissue was stabilized with agar before sectioning. Serial axial sections (30 μm thick) were prepared starting in the segment immediately rostral to the hemisection and continuing through the hemisected region until bilaterally intact spinal cord was again encountered caudal to the lesion. Sections were stained with cresyl violet and a digital image of each section was captured. In order to illustrate the lesion extent, the image of a section from the hemisected region was superimposed on an image of bilaterally intact spinal cord tissue obtained immediately rostral or caudal to the lesion. Thus, the dark overlap region illustrates the extent of contralaterally preserved tissue at the hemisection site (see Figs 3, 4, 5 and 7A).
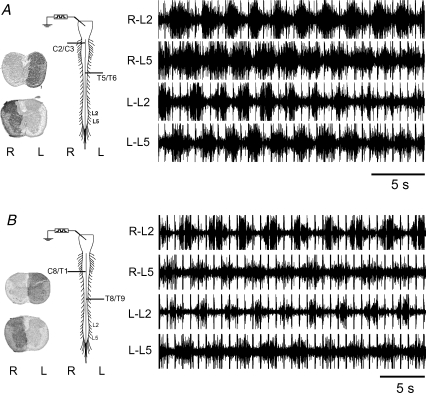
Figure 3. Locomotor-like activity evoked in response to electrical stimulation of the brainstem in the presence of staggered hemisections.
A, locomotor-like activity, consisting of rhythmic alternation of ipsilateral L2 and L5 ventral roots and left–right alternation, was well developed (CV = 12%) in the presence of spinal cord hemisections located at right C2/3 and left T5/6. Note the hemisections extended beyond the midline ensuring interruption of all ipsilateral projections on the corresponding side. B, locomotor-like activity in this preparation was well developed (CV = 13%) despite hemisections located below the cervical enlargement (right C8/T1) and at left T8/9. Note, the dark region of the axial tissue sections indicates the extent of residual intact spinal cord at the lesion site, superimposed on the nearest section of non-lesioned bilaterally intact spinal cord.
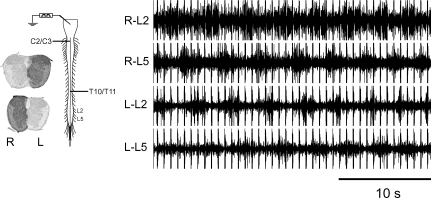
Figure 4. Locomotor-like activity (CV = 11%) evoked in response to electrical stimulation of the brainstem in the presence of staggered hemisections, one of which is located in the caudal thoracic cord (left T10/11).
The rostral right hemisection was made at C2/3. Thus, bilateral lumbar locomotor-like activity was recruited via descending excitation travelling in the right thoracic hemi-cord.
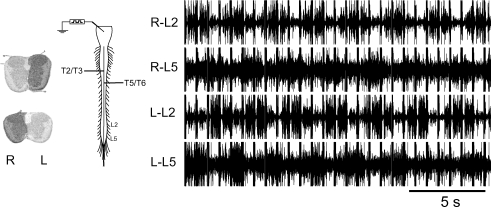
Figure 5. Locomotor-like activity (CV = 9%) evoked in response to electrical stimulation of the brainstem in the presence of closely spaced staggered hemisections.
Only three segments were available, between the right hemisection at T2/3 and the left hemisection at T5/6, for left-to-right cross-over of descending transmission of locomotor excitation.
Figure 7. Neurochemical stimulation of the spinal cord facilitated propagation of the bulbospinal locomotor command signal.
A, electrical stimulation of the brainstem failed to evoke a locomotor-like pattern in this preparation. B, whole cord application of 5-HT (10 μm) and NMDA (1 μm) was subthreshold for the induction of locomotor-like activity. C, electrical stimulation of the brainstem, in combination with subthreshold concentrations of 5-HT (10 μm) and NMDA (1 μm), evoked locomotor-like activity.
Results
In order to examine whether descending propriospinal transmission, in the absence of conduction in long direct bulbospinal pathways, was capable of activating locomotor activity in the lumbar cord, two approaches were used. Either the brainstem was stimulated electrically (in preparations with staggered contralateral hemisections) or propriospinal neurons in the rostral spinal cord were stimulated chemically (in unlesioned cords) in an attempt to induce locomotor-like activity in the lumbar region.
Staggered contralateral hemisections
Hemisection of the cervical or thoracic spinal cord disrupts bulbospinal axons projecting directly to the ipsilateral lumbar cord. In 174 preparations double hemisections, applied to opposite sides of the cord at various rostrocaudal levels, were used to disrupt all direct-projecting uncrossed bulbospinal projections. Using this protocol, ipsilaterally projecting propriospinal pathways were also disrupted. However, propriospinal axons that cross the midline in segments located between the two staggered contralateral hemisections should remain available for propagation of neural activity.
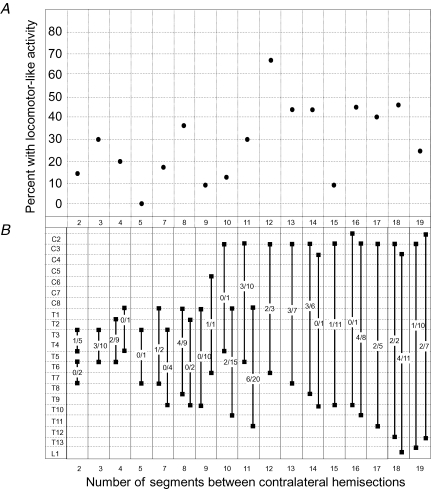
Stimulation of the brainstem evoked responses that met criteria for locomotor-like activity (see Methods) in 27% (n = 47) of the preparations. In contrast, electrical stimulation of the brainstem evokes locomotor-like activity in 93% of preparations without spinal cord lesions (Zaporozhets et al. 2004). The average number of ventral root bursts observed per brainstem stimulation episode was 13. Among successful attempts, the rostral hemisection was placed above the cervical enlargement (C1/C2, n = 2; C2/C3, n = 21; or C3/C4, n = 4), within the cervical enlargement (C5/6, n = 1), or below the cervical enlargement (C8/T1, n = 13; T1/2, n = 2; or T2/3, n = 4) as shown in Fig. 2B. The contralateral caudal hemisection in successful preparations was located at T4/5 (n = 1), T5/6 (n = 8), T6/7 (n = 3), T7/8 (n = 4), T8/9 (n = 7), T9/10 (n = 1), T10/11 (n = 6), T11/12 (n = 8), T12/13 (n = 4), T13/L1 (n = 1) and at the mid L1 level (n = 4). The average cycle period CV was 14 ± 6% (range 2–25%). The average side-to-side phase lag of the left versus right ventral root burst at the same segmental level was 0.46 ± 0.11. The number of segments between the rostral and caudal hemisections was not correlated with the rate of success inducing locomotor-like activity (Fig. 2A, logistic regression P = 0.261).
Figure 2. Segmental location of spinal cord hemisections.
A, the percentage success rate of inducing locomotor-like activity is plotted against the number of segments located between staggered contralateral hemisections. Logistic regression analysis indicated no statistically significant correlation, P = 0.261. B, the segmental location of rostral and corresponding caudal hemisections for all preparations is illustrated. Fractions indicate the number of preparations displaying locomotor-like rhythm versus the total number of preparations tested with the specified lesion combination. A preparation with six segments between the two hemisections was not used.
An example of locomotor-like activity, with ipsilateral flexor (L2) and extensor (L5) alternation, as well as left–right alternation at the L2 and L5 levels, despite the presence of over-hemisection at right C2/3 and left T5/6, is shown in Fig. 3A. The rostral hemisection interrupted direct brainstem projections travelling in the right half of the spinal cord, including projections to the cervical enlargement; the lesion at T5/T6 blocked direct descending projections in the left side of the cord. Thus, descending signal cross-over occurred in the cervical enlargement and/or upper thoracic region. If, in this example, brainstem projections from the left side recruited propriospinal neurons participating in forelimb rhythm-generating centres bilaterally, then the question is raised whether preservation of cervico-lumbar ipsilateral connections in particular, at least on one side, is critical for the propagation of the locomotor command signal. Additional observations suggest this is not the case. The rostral hemisection was placed at the caudal end of the cervical enlargement in some experiments (e.g. C8/T1, Fig. 3B). In combination with a contralateral thoracic hemisection (e.g. T8/9, Fig. 3B) all direct ipsilateral connections between the cervical and lumbar enlargements would be blocked. Despite these lesions, the locomotor command signal successfully crossed-over in the thoracic region.
Locomotor-like activity was recruited bilaterally in the lumbar region, even if one of the hemisections was located in the caudal thoracic or upper lumbar region (Fig. 4). Thus bilateral lumbar locomotor network activity can be recruited by descending excitatory signals travelling unilaterally throughout the length of the thoracic cord.
Propagation of the brainstem locomotor command signal was still possible when only two or three bilaterally intact segments remained available for signal cross-over between the rostral and caudal hemisections (Fig. 5).
Neurochemical stimulation of propriospinal neuron cell bodies
Neurochemical manipulations were used to selectively activate neuronal cell bodies and not axons of passage (i.e. long direct bulbospinal projections). Thus 5-HT and NMDA, with or without bicuculline, were added to the cervical and/or thoracic bath in order to excite neurons in these regions. Two types of experiment were performed.
Bilaterally intact spinal cord preparations
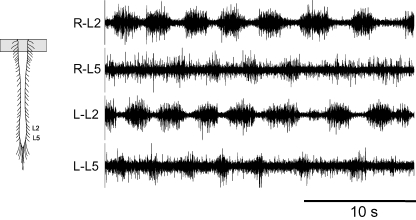
In this group of experiments complete transections were made at the cervico-medullary junction and no cord hemisections were made. Application of 5-HT and NMDA to the C1–C4 bath compartment, with or without bicuculline, evoked rhythmic lumbar ventral root discharge in 27/27 preparations. The discharge pattern was considered locomotor-like in 8/27 preparations (Fig. 6, mean CV = 19 ± 4%). Similarly, application of neurochemicals to the C5–T5 or T4–T10 bath compartments produced locomotor-like activity in the lumbar region in 7/18 (mean CV = 17 ± 6%) and 3/6 (mean CV = 11 ± 2%) preparations, respectively. Locomotor-like activity was also evoked when neuron excitability was increased in 1/2 preparations by raising the K+ ion concentration from 3.5 to 10 mm in the C5–T2 (CV = 15%) bath compartment. These results are compatible with previous studies reporting lumbar rhythmic activity in response to the application of 5-HT and NMDA to the cervico-thoracic cord (Cowley & Schmidt, 1997; Ballion et al. 2001).
Figure 6. Locomotor-like activity (CV = 19%) was evoked in the lumbar region by chemical stimulation of propriospinal neurons in the rostral cervical region.
Bath application of 5-HT (50 μm) and NMDA (15 μm) to spinal cord segments C1–C4 produced rhythmic alternating activity of left and right sides as well as ipsilateral L2 and L5 alternation.
Staggered hemisection preparations
In this group of experiments we examined whether brainstem stimulation of double hemisected cords, combined with enhanced excitation of propriospinal neurons, facilitated locomotor command signal propagation in preparations that otherwise failed to display locomotor activity during brainstem stimulation alone (e.g. Fig. 7A). Because locomotor-like activity can be directly induced by whole cord neurotransmitter application, 5-HT and NMDA were applied at concentrations subthreshold for rhythmogenesis (e.g. Fig. 7B). Bicuculline was not used in these experiments. In 10/42 such preparations the combination of 5-HT, NMDA and electrical stimulation of the brainstem elicited locomotor-like activity (CV 11 ± 3%, e.g. Fig. 7C).
Discussion
The results demonstrate that a locomotor command signal originating in the brainstem propagates caudally despite disruption of long direct axonal projections to the lumbar cord. In addition, because fibres lesioned by staggered hemisections also include part of the propriospinal system, the observations show that even partial preservation of propriospinal pathways is sufficient for transmission of the locomotor signal in the neonatal rat preparation.
Do bulbospinal projections cross the midline in the spinal cord?
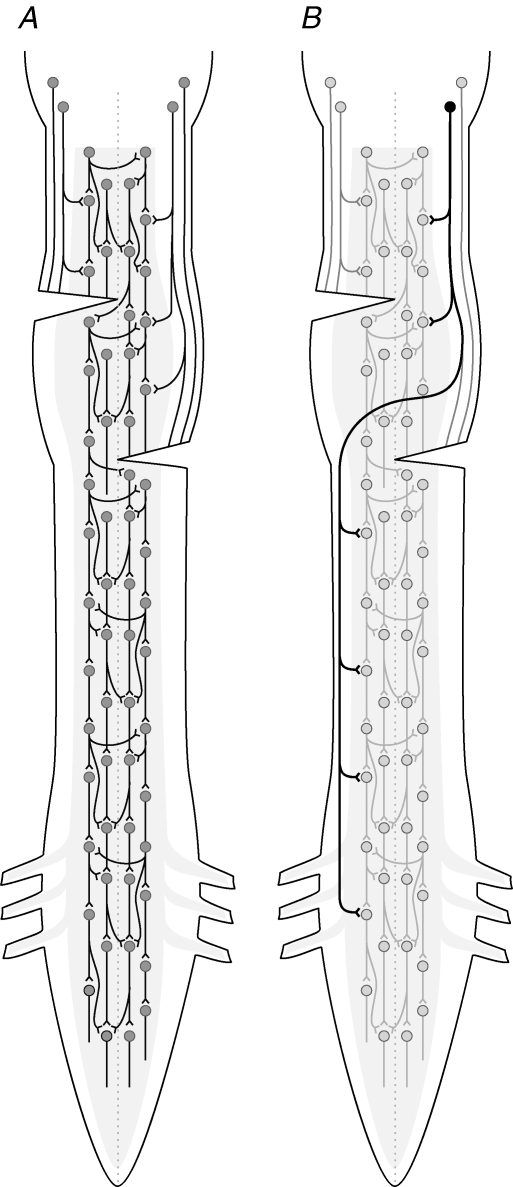
Our interpretation of the results assumes propriospinal, but not long direct bulbospinal, projections underlie the propagation of the locomotor command signal that crosses the midline to the opposite side of the cord and continues caudally to the lumbar region (Fig. 8A). It is well established that propriospinal neurons may be ascending or descending, short or long, and project ispilaterally or contralaterally (e.g. Sherrington & Laslett, 1903; Lloyd, 1941; Barilari & Kuypers, 1969; Jankowska et al. 1973; Vasilenko, 1975; Matsushita et al. 1979; Skinner et al. 1979; Menetry et al. 1985; Alstermark et al. 1987; Sherriff & Henderson, 1994; Eide et al. 1999; Butt & Kiehn, 2003; Strauss & Lev-Tov, 2003; Conta & Stelzner, 2004; Reed et al. 2006). It is also known that bulbospinal projections can mediate contralateral spinal effects via ipsilateral synaptic contact with spinal neurons, which then project across the midline (e.g. Scheibel & Scheibel, 1966). However, less is known about whether any long bulbospinal axons are capable of decussating in the cervical or thoracic region and then descending on the contralateral side (without an intervening synapse) as direct projections to the lumbar region (Fig. 8B). If such a decussating bulbospinal system exists, it could in theory contribute to descending propagation of the locomotor signal, in addition to crossed propriospinal pathways, in the presence of staggered contralateral hemisections.
Figure 8. Hypothetical representation of propriospinal and direct bulbospinal systems involved in descending transmission of the locomotor command signal.
A, the present data suggest that propriospinal neurons alone may be sufficient to mediate descending activation of locomotor networks. The range of projection length of individual locomotor-related propriospinal neurons is unknown. In addition to the propriospinal system, some bulbospinal axons or their collaterals may activate contralateral locomotor-related neurons via commissural projections. B, there is no direct evidence that locomotor-related bulbospinal projections can cross the midline in the spinal cord and continue descending long distances, without an intervening synapse, on the contralateral side; however, this possibility is not absolutely excluded.
Stelzner & Cullen (1991) injected [3H]proline and horseradish peroxidase into the lumbar cord of newborn rats subjected to bilateral staggered hemisections in the mid-thoracic region. Retrogradely labelled cells were found in the intermediate grey matter of the interlesion zone bilaterally, consistent with propriospinal neurons, and no evidence of cell labelling was found in the brainstem (Stelzner & Cullen, 1991). However, these authors point out several limitations of the labelling technique. In addition, the short interlesion length of thoracic cord (1–3 segments) may have contributed to a failure to detect any bulbospinal fibres that traversed the interlesion zone.
Considerable evidence implicates an important role for reticulospinal projections in the control of locomotion in mammals (e.g. Orlovsky, 1970; Mori et al. 1983; Shefchyk et al. 1984; Garcia-Rill & Skinner, 1987; Noga et al. 1988; Rossignol et al. 2006) and other vertebrates (e.g. Deliagina et al. 2000). For instance some descending reticulospinal axons terminate in the ventromedial grey region, which contains neurons that are rhythmically active during locomotion, including commissural neurons (Harrison et al. 1986; Huang et al. 2000; Antonino-Green et al. 2002; Butt et al. 2002; Lanuza et al. 2004; Hinckley et al. 2005; Wilson et al. 2005; Matsuyama et al. 2006). Electrophysiological studies (Jankowska et al. 2003) have shown that reticulospinal axons originating on one side of the cat brainstem terminate bilaterally in the mid to lower lumbar and sacral regions. Of note, however, reticulospinal axon collaterals which cross the midline in the lumbosacral region terminate at the same segmental level (Kausz, 1991). Thus, in the present study, these particular long direct projections are not candidates to mediate the descending locomotor command because they would have been severed by the staggered hemisections made in the cervical and contralateral thoracic regions. Peterson et al. (1975) used microelectrode stimulation and recording techniques to show that some reticulospinal axon collaterals cross the midline in the cervical cord. More recently, Matsuyama et al. (1997, 2004) employed Phaseolus vulgaris leucoagglutinin to determine the termination patterns of reticulospinal axon collaterals originating from gigantocellular tegmental field (FTG) neurons in the cat. Approximately 20% of FTG collaterals innervated the contralateral (as well as ipsilateral) grey matter in the cervico-thoracic region. However, similar to the observations of Kausz (1991), the rostro-caudal extent of the collateral terminations was less than 1 mm. Therefore, if these bulbospinal axons transmit excitation to the contralateral lumbar region an interposed propriospinal neuron in the contralateral cervico-thoracic grey matter would be required.
Some corticospinal tract fibres re-cross the midline within the spinal cord of dogs and monkeys (e.g. Sherrington, 1889; Liu & Chambers, 1964; Kuypers & Brinkman, 1970) and cats (Satomi et al. 1988: Li & Martin, 2002). However, the corticospinal tract is not a likely critical pathway for the activation of locomotor activity (Steeves & Jordan, 1980; Loy et al. 2002; Schucht et al. 2002), although it does have a role in skilled limb movements during locomotion (e.g. Metz et al. 1998; Muir & Whishaw, 1999; Drew et al. 2002; Kanagal & Muir, 2007). Moreover, with respect to the present study, although most supraspinal inputs to the lower spinal cord are present at birth (Leong et al. 1984; Kudo et al. 1993), corticospinal tract axons are not found in the lumbar grey matter until postnatal day 9 (Donatelle, 1977).
Lateral pontine noradrenergic projections to the spinal cord have also been studied. Fluorescent and biochemical examination of rat coerulospinal projections indicate these fibres decussate throughout the spinal cord; more specifically, approximately 50% of the coerulospinal noradrenaline content on one side of the cord derives from the contralateral locus coeruleus (Karoum et al. 1980; Commissiong, 1981; also see Davies et al. 1983). However, these studies do not indicate whether coerulospinal axons continue to course caudally after crossing the midline. In the monkey, most descending projections from the lateral pontine region travel through the ipsilateral spinal cord, although a small number of subcoeruleus/parabrachial axons descend in the contralateral cervical, thoracic and lumbosacral lateral funiculus (Westlund & Coulter, 1980). The level at which subcoeruleus and parabrachial projections cross (i.e. brainstem versus spinal cord) is unclear, as is the extent to which these axons travel caudally after crossing the midline (Westlund & Coulter, 1980). Locus coeruleus projections cross in the lower lumbar and sacral regions (Westlund & Coulter, 1980). A study of cat lateral pontine neurons (locus coeruleus, subcoeruleus, Kolliker-Fuse, and lateral parabrachial) showed that axons project ipsilaterally to thoracic segments, decussate starting in the lower lumbar segments, and display extensive bilateral representation in the sacral cord (Kausz, 1986). These long descending projections, which cross in lumbosacral spinal cord regions, would have been lesioned by the staggered hemisections used in the present series. Experiments involving unilateral Evans blue injection into the cat lumbar cord suggested that lateral pontine neurons innervating the lumbar cord crossed above and below mid-thoracic hemisections (Stevens et al. 1985). Thus, the literature on descending projections from the lateral pontine region suggests that long direct crossed pathways reaching the lumbar cord may exist. If this is the case in the neonatal rat, the staggered contralateral hemisections used in the present experiments may not have abolished all direct bulbospinal projections to the lumbar cord. Nevertheless, even if such pathways exist our observation that neurochemical stimulation of the rostral cervical cord, which does not activate bulbospinal axons of passage, induced locomotor-like rhythm in the lumbar segments supports the idea that a propriospinal system, in isolation, has the capacity to mediate descending activation of the locomotor network.
Implications for functional recovery
A consistent observation in rodents (Feringa et al. 1976; Malmsten, 1983; Little et al. 1988; Ballerman & Fouad, 2006), cats (Jane et al. 1964; Kato et al. 1985; Eidelberg et al. 1986), monkeys (Mettler & Liss, 1959; Lassek & Anderson, 1961) and even humans (Nathan & Smith, 1973) is that spinal cord hemisection produces ipsilateral motor deficits which can spontaneously recover, starting after several days and continuing for several weeks (or months, in the case of humans). Bilateral staggered hemisection, on the other hand, is associated with permanent paraplegia in monkeys (Lassek & Anderson, 1961) and humans (Nathan & Smith, 1973). Adult rats display bilateral lower limb paralysis after a second (contralateral) hemisection and subsequent recovery was not observed; however, animals in this study were kept alive for only 2 days after the second lesion (Harris et al. 1994). In the presence of contralateral cord hemisections cats recover locomotor ability over the course of several weeks, after an initial phase of paraplegia, if the ventral column is spared on at least one side (Jane et al. 1964). Recovery was postulated to be due to a propriospinal system travelling in the preserved ventral column(s) (Jane et al. 1964). In contrast, the present results demonstrate that the in vitro neonatal rat preparation retains the capacity to generate lumbar locomotor-like activity in response to brainstem stimulation, even in the acutely lesioned state and in the presence of complete hemisections. Whether or not the propriospinal system demonstrated in the present acute neonatal rat preparation is recruited by long-term recovery processes after lesions in adult animals remains to be determined.
Stelzner & Cullen reported that one-month-old rats subjected to bilateral staggered mid-thoracic hemisections show little or no recovery of lower limb motor function over the course of 6 months (1991). They also showed that locomotor responses did recover in similarly lesioned newborn rats. Subsequent complete cord transection rostral to the region of double hemisections had no effect on the locomotor responses, leading to the conclusion that intrinsic cord mechanisms, rather than propriospinal connections, mediated the recovery (Stelzner & Cullen, 1991). However, the small interlesion region (1–3 segments) in these experiments may have limited the capacity of descending systems to influence the lumbar region. Whether locomotor recovery would occur in adult rats after staggered hemisections with larger interlesion regions (5–10 segments), remains to be tested.
The present results, in combination with our previous observations (Zaporozhets et al. 2006a), provide strong evidence that a propriospinal relay system is an important and probably sufficient conduit for descending activation of the locomotor network in the neonatal rat. In addition, one might speculate that the propriospinal system may be more than a passive conduit for descending transmission. It may serve as an active component of locomotor circuitry, consistent with the concept that locomotor networks are distributed rostro-caudally throughout the spinal cord in rats (Cowley & Schmidt, 1997) and humans (Dietz et al. 1999).
Most spinal cord injuries in humans are partial rather than complete and it is highly improbable that partial spinal cord injury would completely abolish all long-projecting bulbospinal fibres while selectively sparing propriospinal fibres. Thus, even if spinal relay connections alone should prove insufficient for transmission of the locomotor command signal in adult mammals, the propriospinal system remains a logical target for functional recovery strategies involving regeneration and direct pharmacological and/or electrical excitation (e.g. Yakovenko et al. 2007). In addition, descending propriospinal neurons may be able to serve as an ‘alternative route’ for transmitting information normally carried by long bulbospinal projections. Indeed there is good evidence that this type of re-routing occurs spontaneously during recovery of locomotor activity in the lamprey (for review see McClellan, 1998) and embryonic chick (Sholomenko & Delaney, 1998) and during the re-establishment of cortical influence on lumbar neurons (Bareyre et al. 2004; Vavrek et al. 2006).
Acknowledgments
This work was supported by the Canadian Institutes of Health Research. K.C.C. was supported by the Will-to-Win Scholar Fund. The authors would like to thank Maria Setterbom for technical assistance with figure preparation.
References
- Alstermark B, Lundberg A, Pinter M, Sasaki S. Long C3–C5 propriospinal neurones in the cat. Brain Res. 1987;404:382–388. doi: 10.1016/0006-8993(87)91400-4. [DOI] [PubMed] [Google Scholar]
- Antonino-Green DM, Cheng J, Magnuson DS. Neurons labeled from locomotor-related ventrolateral funiculus stimulus sites in the neonatal rat spinal cord. J Comp Neurol. 2002;442:226–238. doi: 10.1002/cne.10081. [DOI] [PubMed] [Google Scholar]
- Ballerman M, Fouad K. Spontaneous locomotor recovery in spinal cord injured rats is accompanied by anatomical plasticity of reticulospinal fibres. Eur J Neurosci. 2006;23:1988–1996. doi: 10.1111/j.1460-9568.2006.04726.x. [DOI] [PubMed] [Google Scholar]
- Ballion B, Morin D, Viala D. Forelimb locomotor generators and quadrupedal locomotion in the neonatal rat. Eur J Neurosci. 2001;14:1727–1738. doi: 10.1046/j.0953-816x.2001.01794.x. [DOI] [PubMed] [Google Scholar]
- Bareyre FM, Kerschensteiner M, Raineteau O, Mettenleiter TC, Weinmann O, Schwab ME. The injured spinal cord spontaneously forms a new intraspinal circuit in adult rats. Nat Neurosci. 2004;7:269–277. doi: 10.1038/nn1195. [DOI] [PubMed] [Google Scholar]
- Barilari MG, Kuypers HGJM. Propriospinal fibers interconnecting the spinal enlargments in the cat. Brain Res. 1969;14:321–330. doi: 10.1016/0006-8993(69)90113-9. [DOI] [PubMed] [Google Scholar]
- Berkowitz A, Stein PSG. Activity of descending propriospinal axons in the turtle hindlimb enlargement during two forms of fictive scratching: Broad tuning to regions of the body surface. J Neurosci. 1994;14:5089–5104. doi: 10.1523/JNEUROSCI.14-08-05089.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchanan JT. Neural network simulations of coupled locomotor oscillators in the lamprey spinal cord. Biol Cybern. 1992;66:367–374. doi: 10.1007/BF00203673. [DOI] [PubMed] [Google Scholar]
- Butt SJB, Harris-Warrick RM, Kiehn O. Firing properties of identified interneuron populations in the mammalian hindlimb central pattern generator. J Neurosci. 2002;22:9961–9971. doi: 10.1523/JNEUROSCI.22-22-09961.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt SJB, Kiehn O. Functional identification of interneurons responsible for left-right coordination of hindlimbs in mammals. Neuron. 2003;38:953–963. doi: 10.1016/s0896-6273(03)00353-2. [DOI] [PubMed] [Google Scholar]
- Cohen AH, Ermentrout GB, Kiemel T, Kopell N, Sigvardt KA, Williams TL. Modelling of intersegmental coordination in the lamprey central pattern generator for locomotion. Trends Neurosci. 1992;15:434–438. doi: 10.1016/0166-2236(92)90006-t. [DOI] [PubMed] [Google Scholar]
- Commissiong JW. Evidence that the noradrenergic coerulospinal projections decussate at the spinal level. Brain Res. 1981;212:145–151. doi: 10.1016/0006-8993(81)90042-1. [DOI] [PubMed] [Google Scholar]
- Conta AC, Stelzner DJ. Differential vulnerability of propriospinal tract neurons to spinal cord contusion injury. J Comp Neurol. 2004;479:347–359. doi: 10.1002/cne.20319. [DOI] [PubMed] [Google Scholar]
- Cowley KC, Schmidt BJ. Effects of inhibitory amino acid antagonists on reciprocal inhibitory interactions during rhythmic motor activity in the in vitro neonatal rat spinal cord. J Neurophysiol. 1995;74:1109–1117. doi: 10.1152/jn.1995.74.3.1109. [DOI] [PubMed] [Google Scholar]
- Cowley KC, Schmidt BJ. Regional distribution of the locomotor pattern-generating network in the neonatal rat spinal cord. J Neurophysiol. 1997;77:247–259. doi: 10.1152/jn.1997.77.1.247. [DOI] [PubMed] [Google Scholar]
- Cowley KC, Zaporozhets E, MacLean JN, Schmidt BJ. Is NMDA receptor activation essential for the production of locomotor-like activity in the neonatal rat spinal cord? J Neurophysiol. 2005;94:3805–3814. doi: 10.1152/jn.00016.2005. [DOI] [PubMed] [Google Scholar]
- Davies JE, Marsden CA, Roberts MHT. Hyperalgesia and the reduction of monoamines resulting from lesions of the dorsolateral funiculus. Brain Res. 1983;261:59–68. doi: 10.1016/0006-8993(83)91283-0. [DOI] [PubMed] [Google Scholar]
- Deliagina TG, Zelenin PV, Fagerstedt P, Grillner S, Orlovsky GN. Activity of reticulospinal neurons during locomotion in the freely behaving lamprey. J Neurophysiol. 2000;83:853–863. doi: 10.1152/jn.2000.83.2.853. [DOI] [PubMed] [Google Scholar]
- Dietz V. Do human bipeds use quadrupedal coordination? Trends Neurosci. 2002;25:462–467. doi: 10.1016/s0166-2236(02)02229-4. [DOI] [PubMed] [Google Scholar]
- Dietz V, Nakazawa K, Wirz M, Erni T. Level of spinal cord lesion determines locomotor activity in spinal man. Exp Brain Res. 1999;128:405–409. doi: 10.1007/s002210050861. [DOI] [PubMed] [Google Scholar]
- Donatelle JM. Growth of the corticospinal tract and the development of placing reactions in the postnatal rat. J Comp Neurol. 1977;175:207–232. doi: 10.1002/cne.901750205. [DOI] [PubMed] [Google Scholar]
- Drew T, Jiang W, Widajewicz W. Contributions of the motor cortex to the control of the hindlimbs during locomotion in the cat. Brain Res Rev. 2002;40:178–191. doi: 10.1016/s0165-0173(02)00200-x. [DOI] [PubMed] [Google Scholar]
- Eide A-L, Glover J, Kjaerulff O, Kiehn O. Characterization of commissural interneurons in the lumbar region of the neonatal rat spinal cord. J Comp Neurol. 1999;403:332–345. [PubMed] [Google Scholar]
- Eidelberg E, Nguyen LH, Deza LD. Recovery of locomotor function after hemisection of the spinal cord in cats. Brain Res Bull. 1986;16:507–515. doi: 10.1016/0361-9230(86)90180-2. [DOI] [PubMed] [Google Scholar]
- Eidelberg E, Story JL, Walden JG, Meyer BL. Anatomical correlates of return of locomotor function after partial spinal cord lesions in cats. Exp Brain Res. 1981;42:81–88. doi: 10.1007/BF00235732. [DOI] [PubMed] [Google Scholar]
- Feringa ER, Kinning WK, Britten AG, Vahlsing HL. Recovery in rats after spinal cord injury. Neurology. 1976;26:839–843. doi: 10.1212/wnl.26.9.839. [DOI] [PubMed] [Google Scholar]
- Garcia-Rill E, Skinner RD. The mesencephalic locomotor region. II. Projections to reticulospinal neurons. Brain Res. 1987;411:13–20. doi: 10.1016/0006-8993(87)90676-7. [DOI] [PubMed] [Google Scholar]
- Halbertsma JM, Miller S, van der Meche FGA. Basic programs for the phasing of. flexion and extension movements of the limbs during locomotion. In: Herman RM, Grillner S, Stein P, Stuart DG, editors. Neural Control of Locomotion. New York: Plenum; 1976. pp. 489–517. [Google Scholar]
- Harris RM, Little JW, Goldstein B. Spared descending pathways mediate locomotor recovery after subtotal spinal cord injury. Neurosci Lett. 1994;180:37–40. doi: 10.1016/0304-3940(94)90908-3. [DOI] [PubMed] [Google Scholar]
- Harrison PJ, Jankowska E, Zytnicki D. Lamina VIII interneurons interposed in cross reflex pathways in the cat. J Physiol. 1986;371:147–166. doi: 10.1113/jphysiol.1986.sp015965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinckley CA, Hartley R, Wu L, Todd A, Ziskind-Conhaim L. Locomotor-like rhythms in a genetically distinct cluster of interneurons in the mammalian spinal cord. J Neurophysiol. 2005;93:1439–1449. doi: 10.1152/jn.00647.2004. [DOI] [PubMed] [Google Scholar]
- Huang A, Noga BR, Carr PA, Fedirchuk B, Jordan LM. Spinal cholinergic neurons activated during locomotion: localization and electrophysiological characterization. J Neurophysiol. 2000;83:3537–3547. doi: 10.1152/jn.2000.83.6.3537. [DOI] [PubMed] [Google Scholar]
- Iwahara T, Atsuta Y, Garcia-Rill E, Skinner RD. Locomotion induced by spinal cord stimulation in the neonate rat in vitro. Somatosens Mot Res. 1991;8:281–287. doi: 10.3109/08990229109144751. [DOI] [PubMed] [Google Scholar]
- Jane JA, Evans JP, Fisher LE. An investigation concerning the restitution of motor function following injury to the spinal cord. J Neurosurg. 1964;21:167–171. doi: 10.3171/jns.1964.21.3.0167. [DOI] [PubMed] [Google Scholar]
- Jankowska E, Hammar I, Slawinska U, Maleszak K, Edgley SA. Neuronal basis of crossed actions from the reticular formation on feline hindlimb motoneurons. J Neurosci. 2003;23:1867–1878. doi: 10.1523/JNEUROSCI.23-05-01867.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowska E, Lundberg A, Stuart D. Propriospinal control of last order interneurones of spinal reflex pathways in the cat. Brain Res. 1973;53:227–231. doi: 10.1016/0006-8993(73)90786-5. [DOI] [PubMed] [Google Scholar]
- Juvin L, Simmers J, Morin D. Propriospinal circuitry underlying interlimb coordination in mammalian quadrupedal locomotion. J Neurosci. 2005;25:6025–6035. doi: 10.1523/JNEUROSCI.0696-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanagal SG, Muir GD. Bilateral dorsal funicular lesions alter sensorimotor behaviour in rats. Exp Neurol. 2007;205:513–524. doi: 10.1016/j.expneurol.2007.03.014. [DOI] [PubMed] [Google Scholar]
- Karoum F, Commissiong JW, Neff NH, Wyatt RJ. Biochemical evidence for uncrossed and crossed locus coeruleus projections to the spinal cord. Brain Res. 1980;196:237–241. doi: 10.1016/0006-8993(80)90730-1. [DOI] [PubMed] [Google Scholar]
- Kato M, Murakami S, Hirayama H, Hikino K. Recovery of postural control following chronic bilateral hemisections at different spinal cord levels in adult cats. Exp Neurol. 1985;90:350–364. doi: 10.1016/0014-4886(85)90024-x. [DOI] [PubMed] [Google Scholar]
- Kausz M. Distribution of neurons in the lateral pontine tegmentum projecting to thoracic, lumbar and sacral spinal segments in the cat. J Hirnforsch. 1986;27:485–493. [PubMed] [Google Scholar]
- Kausz M. Arrangement of neurons in the medullary reticular formation and raphe nuclei projecting to thoracic, lumbar and sacral segments of the spinal cord in the cat. Anat Embryol. 1991;183:151–163. doi: 10.1007/BF00174396. [DOI] [PubMed] [Google Scholar]
- Kudo N, Furukawa F, Okado N. Development of descending fibres to the rat embryonic spinal cord. Neurosci Res. 1993;16:131–141. doi: 10.1016/0168-0102(93)90080-a. [DOI] [PubMed] [Google Scholar]
- Kuypers HG, Brinkman J. Precentral projections to different parts of the spinal intermediate zone in the rhesus monkey. Brain Res. 1970;24:29–48. doi: 10.1016/0006-8993(70)90272-6. [DOI] [PubMed] [Google Scholar]
- Lanuza GM, Gosgnach S, Pierani A, Jessel TM, Goulding M. Genetic identification of spinal interneurons that coordinate left-right locomotor activity necessary for walking movements. Neuron. 2004;42:375–386. doi: 10.1016/s0896-6273(04)00249-1. [DOI] [PubMed] [Google Scholar]
- Lassek AM, Anderson PA. Motor function after spaced contralateral hemisections in the spinal cord. Neurology. 1961;11:362–365. doi: 10.1212/wnl.11.4.362. [DOI] [PubMed] [Google Scholar]
- Leong SK, Shieh J, Wong WC. Localizing spinal-cord-projecting neurons in neonatal and immature albino rats. J Comp Neurol. 1984;228:18–23. doi: 10.1002/cne.902280104. [DOI] [PubMed] [Google Scholar]
- Li Q, Martin JH. Postnatal development of connectional specificity of corticospinal terminals in the cat. J Comp Neurol. 2002;447:57–71. doi: 10.1002/cne.10203. [DOI] [PubMed] [Google Scholar]
- Little JW, Harris RM, Sohlberg RC. Locomotor recovery following subtotal spinal cord lesions in a rat model. Neurosci Lett. 1988;87:189–194. doi: 10.1016/0304-3940(88)90168-1. [DOI] [PubMed] [Google Scholar]
- Liu CN, Chambers WW. An experimental study of the cortico-spinal system in the monkey (Macaca mulatta). The spinal pathways and preterminal distribution of degenerating fibers following discrete lesions of the pre- and postcentral gyri and bulbar pyramid. J Comp Neurol. 1964;123:257–283. doi: 10.1002/cne.901230209. [DOI] [PubMed] [Google Scholar]
- Lloyd DPC. Activity in neurons of the bulbospinal correlation system. J Neurophysiol. 1941;4:115–134. [Google Scholar]
- Loy DN, Talbott JF, Onifer SM, Mills MD, Burke DA, Dennison JB, Fajardo LC, Magnuson DSK, Whittemore SR. Both dorsal and ventral spinal cord pathways contribute to overground locomotion in the adult rat. Exp Neurol. 2002;177:575–580. doi: 10.1006/exnr.2002.7959. [DOI] [PubMed] [Google Scholar]
- McClellan AD. Spinal cord injury: Lessons from locomotor recovery and axonal regeneration in lower vertebrates. Neuroscientist. 1998;4:250–263. [Google Scholar]
- Magnuson DSK, Trinder TC. Locomotor rhythm evoked by ventrolateral funiculus stimulation in the neonatal rat spinal cord in vitro. J Neurophysiol. 1997;77:200–206. doi: 10.1152/jn.1997.77.1.200. [DOI] [PubMed] [Google Scholar]
- Malmsten J. Time course of segmental reflex changes after chronic spinal cord hemisection in the rat. Acta Physiol Scand. 1983;119:435–443. doi: 10.1111/j.1748-1716.1983.tb07359.x. [DOI] [PubMed] [Google Scholar]
- Matsushima T, Grillner S. Intersegmental co-ordination of undulatory movements – a ‘trailing oscillator’ hypothesis. Neuroreport. 1990;1:97–100. doi: 10.1097/00001756-199010000-00003. [DOI] [PubMed] [Google Scholar]
- Matsushita M, Ikeda M, Hosoya Y. The location of spinal neurons with long descending axons (long descending propriospinal tract neurons) in the cat: a study with the horseradish peroxidase technique. J Comp Neurol. 1979;184:63–80. doi: 10.1002/cne.901840105. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Kobayashi S, Aoki M. Projection patterns of lamina VIII commissural neurons in the lumbar spinal cord of the adult cat: an anterograde neural tracing study. Neuroscience. 2006;140:203–218. doi: 10.1016/j.neuroscience.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Mori F, Nakajima K, Drew T, Aoki M, Moro S. Locomotor role of the corticoreticular-reticulospinal-spinal interneuronal system. Prog Brain Res. 2004;143:239–249. doi: 10.1016/S0079-6123(03)43024-0. [DOI] [PubMed] [Google Scholar]
- Matsuyama K, Takakusaki K, Katsumi K, Mori S. Multi-segmental innervation of single pontine reticulospinal axons in the cervico-thoracic region of the cat: Anterograde PHA-1 tracing study. J Comp Neurol. 1997;377:234–250. [PubMed] [Google Scholar]
- Menetry D, De Pommery J, Roudier F. Propriospinal fibers reaching the lumbar enlargement in the rat. Neurosci Lett. 1985;58:257–261. doi: 10.1016/0304-3940(85)90174-0. [DOI] [PubMed] [Google Scholar]
- Mettler FA, Liss H. Functional recovery in primates after large subtotal spinal cord lesions. J Neuropathol Exp Neurol. 1959;18:509–516. [Google Scholar]
- Metz GAS, Dietz V, Schwab ME, van de Meent H. The effects of unilateral pyramidal tract section on hindlimb motor performance in the rat. Behav Brain Res. 1998;96:37–46. doi: 10.1016/s0166-4328(97)00195-2. [DOI] [PubMed] [Google Scholar]
- Miller WL, Sigvardt KA. Extent and role of multisegmental coupling in the lamprey spinal locomotor pattern generator. J Neurophysiol. 2000;83:465–476. doi: 10.1152/jn.2000.83.1.465. [DOI] [PubMed] [Google Scholar]
- Miller S, van der Burg J, van der Meche FGA. Coordination of movements of the hindlimbs and forelimbs in different forms of locomotion in normal and decerebrate cats. Brain Res. 1975;91:217–237. doi: 10.1016/0006-8993(75)90544-2. [DOI] [PubMed] [Google Scholar]
- Mori S, Kawahara K, Sakamoto T. Supraspinal aspects of locomotion in the mesencephalic cat. Symp Soc Exp Biol. 1983;37:445–468. [PubMed] [Google Scholar]
- Muir GD, Whishaw IQ. Complete locomotor recovery following corticospinal tract lesions: measurement of ground reaction forces during overground locomotion in rats. Behav Brain Res. 1999;103:45–53. doi: 10.1016/s0166-4328(99)00018-2. [DOI] [PubMed] [Google Scholar]
- Nathan PW, Smith MC. Effects of two unilateral cordotomies on the motility of the lower limbs. Brain. 1973;96:471–494. doi: 10.1093/brain/96.3.471. [DOI] [PubMed] [Google Scholar]
- Noga BR, Kettler J, Jordan LM. Locomotion produced in mesencephalic cats by injection of putative transmitter substances and antagonists into the medial reticular formation and the pontomedullary locomotor strip. J Neurosci. 1988;8:2074–2086. doi: 10.1523/JNEUROSCI.08-06-02074.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noga BR, Kriellaars DJ, Brownstone RM, Jordan LM. Mechanism for activation of locomotor centers in the spinal cord by stimulation of the mesencephalic locomotor region. J Neurophysiol. 2003;90:1464–1478. doi: 10.1152/jn.00034.2003. [DOI] [PubMed] [Google Scholar]
- Noga BR, Kriellaars DJ, Jordan LM. The effect of selective brainstem or spinal cord lesions on treadmill locomotion evoked by stimulation of the mesencephalic or pontomedullary locomotor regions. J Neurosci. 1991;11:1691–1700. doi: 10.1523/JNEUROSCI.11-06-01691.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlovsky GN. Connexions of the reticulo-spinal neurons with the ‘locomotor sections’ of the brainstem. Biophysics. 1970;15:178–186. [Google Scholar]
- Peterson BW, Maunz RA, Pitts NG, Mackel RG. Patterns of projection and branching of reticulospinal neurons. Exp Brain Res. 1975;23:333–351. doi: 10.1007/BF00238019. [DOI] [PubMed] [Google Scholar]
- Reed WR, Shum-Siu A, Onifer SM, Magnuson DS. Inter-enlargement pathways in the ventrolateral funiculus of the adult rat spinal cord. Neuroscience. 2006;142:1195–1207. doi: 10.1016/j.neuroscience.2006.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol S, Dubuc R, Gossard JP. Dynamic sensorimotor interactions in locomotion. Physiol Rev. 2006;86:89–154. doi: 10.1152/physrev.00028.2005. [DOI] [PubMed] [Google Scholar]
- Satomi H, Takahashi K, Aoki M, Kosaka I. Anatomical evidence for the re-crossing of lateral corticospinal fibres via the posterior gray commissure in the cat spinal cord. Neurosci Lett. 1988;88:157–160. doi: 10.1016/0304-3940(88)90118-8. [DOI] [PubMed] [Google Scholar]
- Scheibel ME, Scheibel AB. Terminal axonal patterns in cat spinal cord. I. The lateral corticospinal tract. Brain Res. 1966;2:333–350. doi: 10.1016/0006-8993(66)90003-5. [DOI] [PubMed] [Google Scholar]
- Schucht P, Raineteau O, Schwab ME, Fouad K. Anatomical correlates of locomotor recovery following dorsal and ventral lesions of the rat spinal cord. Exp Neurol. 2002;176:143–153. doi: 10.1006/exnr.2002.7909. [DOI] [PubMed] [Google Scholar]
- Shefchyk SJ, Jell RM, Jordan LM. Reversible cooling of the brainstem reveals areas required for mesencephalic locomotor region evoked treadmill locomotion. Exp Brain Res. 1984;56:257–262. doi: 10.1007/BF00236281. [DOI] [PubMed] [Google Scholar]
- Sherriff FE, Henderson Z. A cholinergic propriospinal innervation of the rat spinal cord. Brain Res. 1994;634:150–154. doi: 10.1016/0006-8993(94)90268-2. [DOI] [PubMed] [Google Scholar]
- Sherrington CS. On nerve-tracts degenerating secondarily to lesions of the cortex cerebri. J Physiol. 1889;10:429–432. doi: 10.1113/jphysiol.1889.sp000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherrington CS, Laslett EE. Observations on some spinal reflexes and the interconnections of spinal segments. J Physiol. 1903;29:58–96. doi: 10.1113/jphysiol.1903.sp000946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholomenko GN, Delaney KR. Restitution of functional neural connections in chick embryos assessed in vitro after spinal cord transection in ovo. Exp Neurol. 1998;154:430–451. doi: 10.1006/exnr.1998.6944. [DOI] [PubMed] [Google Scholar]
- Skinner RD, Coulter JD, Adams RJ, Remmel RS. Cells of origin of long descending propriospinal fibers connecting the spinal enlargements in cat and monkey determined by horseradish peroxidase and electrophysiological techniques. J Comp Neurol. 1979;188:443–454. doi: 10.1002/cne.901880307. [DOI] [PubMed] [Google Scholar]
- Smith JC, Feldman JL. In vitro brainstem-spinal cord preparations for study of motor systems for mammalian respiration and locomotion. J Neurosci Methods. 1987;21:321–333. doi: 10.1016/0165-0270(87)90126-9. [DOI] [PubMed] [Google Scholar]
- Steeves JD, Jordan LM. Localization of a descending pathway in the spinal cord which is necessary for controlled treadmill locomotion. Neurosci Lett. 1980;20:283–288. doi: 10.1016/0304-3940(80)90161-5. [DOI] [PubMed] [Google Scholar]
- Steeves JD, Jordan LM. Autoradiographic demonstration of the projections from the mesencephalic locomotor region. Brain Res. 1984;307:263–276. doi: 10.1016/0006-8993(84)90480-3. [DOI] [PubMed] [Google Scholar]
- Stelzner DJ, Cullen JM. Do propriospinal projections contribute to hindlimb recovery when all long tracts are cut in neonatal or weanling rats? Exp Neurol. 1991;114:193–205. doi: 10.1016/0014-4886(91)90036-c. [DOI] [PubMed] [Google Scholar]
- Stevens RT, Apkarian AV, Hidge CJ. Funicular course of catecholamine fibers innervating the lumbar spinal cord of the cat. Brain Res. 1985;336:243–251. doi: 10.1016/0006-8993(85)90651-1. [DOI] [PubMed] [Google Scholar]
- Strauss I, Lev-Tov A. Neural pathways between sacrocaudal afferents and lumbar pattern generators in neonatal rats. J Neurophysiol. 2003;89:773–784. doi: 10.1152/jn.00716.2002. [DOI] [PubMed] [Google Scholar]
- Vasilenko DA. Propriospinal pathways in the ventral funicles of the cat spinal cord: their effects on lumbosacral motoneurones. Brain Res. 1975;93:502–506. doi: 10.1016/0006-8993(75)90189-4. [DOI] [PubMed] [Google Scholar]
- Vavrek R, Girgis J, Tetzlaff W, Hiebert GW, Fouad K. BDNF promotes connections of corticospinal neurons onto spared descending interneurons in spinal cord injured rats. Brain. 2006;129:1534–1545. doi: 10.1093/brain/awl087. [DOI] [PubMed] [Google Scholar]
- Westlund KN, Coulter JD. Descending projections of the locus coeruleus and subcoeruleus/medial parabrachial nuclei in monkey: Axonal transport studies and dopamine-beta-hydroxylase immunocytochemistry. Brain Res. 1980;2:235–264. doi: 10.1016/0165-0173(80)90009-0. [DOI] [PubMed] [Google Scholar]
- Wilson JM, Hartley R, Maxwell DJ, Todd AJ, Lieberam I, Kaltschmidt JA, Yoshida Y, Jessel TM, Brownstone RM. Conditional rhythmicity of ventral spinal interneurons defined by expression of the Hb9 homeodomain protein. J Neurosci. 2005;25:5710–5719. doi: 10.1523/JNEUROSCI.0274-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakovenko S, Kowalczewski J, Prochazka A. Intraspinal stimulation caudal to spinal cord transections in rats. Testing the propriospinal hypothesis. J Neurophysiol. 2007;97:2570–2574. doi: 10.1152/jn.00814.2006. [DOI] [PubMed] [Google Scholar]
- Zaporozhets E, Cowley KC, Schmidt BJ. A reliable technique for the induction of locomotor-like activity in the in vitro neonatal rat spinal cord using brainstem electrical stimulation. J Neurosci Methods. 2004;139:33–41. doi: 10.1016/j.jneumeth.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Zaporozhets E, Cowley KC, Schmidt BJ. Propriospinal neurons contribute to bulbospinal transmission of the locomotor command signal in the neonatal rat spinal cord. J Physiol. 2006a;572:443–458. doi: 10.1113/jphysiol.2005.102376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaporozhets E, Cowley KC, Schmidt BJ. Effect of selected spinal cord lesions on locomotor-like activity evoked by electrical stimulation of the neonatal rat brainstem in vitro. Soc Abstr Neurosci. 2006b;36:448.11. [Google Scholar]