Abstract
Kv7 channels (KCNQ) represent a family of voltage-gated K+ channels which plays a prominent role in brain and cardiac excitability. Their physiological importance is underscored by the existence of mutations in human Kv7 genes, leading to severe cardiovascular and neurological disorders such as the cardiac long QT syndrome and neonatal epilepsy. Kv7 channels exhibit some structural and functional features that are distinct from other Kv channels. Notably, the Kv7 C-terminus is long compared to other K+ channels and is endowed with characteristic structural domains, including coiled-coils, amphipatic α helices containing calmodulin-binding motifs and basic amino acid clusters. Here we provide a brief overview of current insights and as yet unsettled issues about the structural and functional attributes of the C-terminus of Kv7 channels. Recent data indicate that the proximal half of the Kv7 C-terminus associates with one calmodulin constitutively bound to each subunit. Epilepsy and long QT mutations located in this proximal region impair calmodulin binding and can affect channel gating, folding and trafficking. The distal half of the Kv7 C-terminus directs tetramerization, employing tandem coiled-coils. Together, the data indicate that the Kv7 C-terminal domain is a multimodular structure playing a crucial role in channel gating, assembly and trafficking as well as in scaffolding the channel complex with signalling proteins.
Kv7 channels (Kv7.1–5 or KCNQ1–5) comprise a subfamily of voltage-gated K+ channels (Kv), that play important functions in various tissues including epithelia, brain, heart and inner ear organs (Jentsch, 2000; Robbins, 2001). While in the kidney and gastro-intestinal tract, they are crucial for the trans-epithelial K+ transport; in neuronal and cardiac cells they play a key role in modulating excitability. In the brain, the complex formed by Kv7.2/3 and Kv7.5/3 α subunits produce the so called ‘M-current’, a slowly activating, non-inactivating K+ current (Jentsch, 2000; Rogawski, 2000; Delmas & Brown, 2005). The M-current has profound effects on neuronal excitability as its low-threshold gating and slow activation act as a brake for repetitive firing. In the heart, the assembly of Kv7.1 and KCNE1 produces the IKS potassium current that is crucial for the late repolarization of the cardiac action potential (Barhanin et al. 1996; Sanguinetti et al. 1996). Kv7 channels have a prominent role in human diseases and harbour numerous mutations that produce severe cardiovascular and neurological disorders such as the cardiac long QT syndrome (LQT) or benign familial neonatal convulsions (BFNC), a neonatal form of epilepsy (Jentsch, 2000; Rogawski, 2000; Robbins, 2001).
Like all Kv channels, the Kv7 α subunits share a common core structure of six transmembrane segments with a voltage-sensing domain (S1–S4) and a pore domain (S5–S6). In Shaker-related Kv channels, the intracellular N-terminus is endowed with a T1 tetramerization domain which determines the specificity of subunit assembly and serves as a platform for attachment of the auxiliary β subunit Kvβ (Gulbis et al. 2000). The T1 domain was also suggested to play an additional role in channel gating (Cushman et al. 2000; Minor et al. 2000). In contrast, Kv7 channels do not possess an N-terminal T1 tetramerization domain, but exhibit a large C-terminus (300–500 residues), which was recently suggested to be important for channel gating, assembly and trafficking (Schmitt et al. 2000; Maljevic et al. 2003; Schwake et al. 2003, 2006; Ghosh et al. 2006; Shamgar et al. 2006; Etxeberria et al. 2007; Howard et al. 2007; Wiener et al. 2007). In this report, we briefly depict the structural and functional features of the C-terminus of Kv7 channels and discuss its multimodular nature.
Calmodulin-binding module
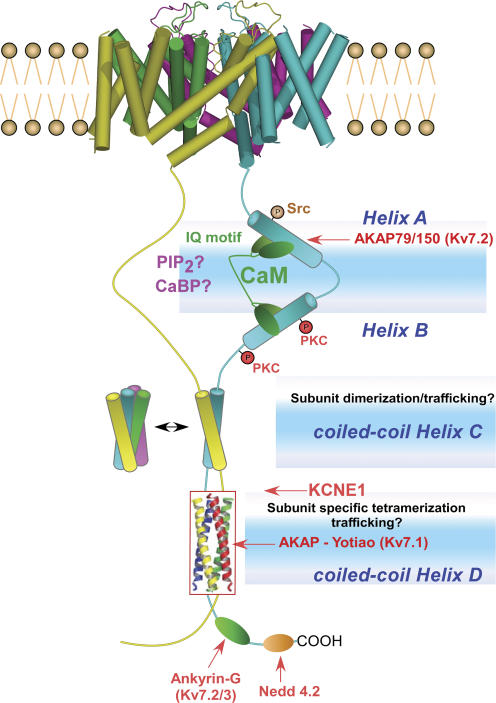
Initial yeast two-hybrid screens and biochemical experiments revealed that calmodulin (CaM) interacts with the C-terminus of Kv7.1–Kv7.5 channel proteins (Wen & Levitan, 2002; Yus-Najera et al. 2002; Gamper & Shapiro, 2003). CaM is found to be tethered constitutively to Kv7.2/3 channels, in the absence or presence of Ca2+ (Wen & Levitan, 2002). The structural elements critical for CaM binding to Kv7 channels appear to lie in two conserved motifs of the proximal half of the Kv7 C-terminus (Fig. 1). Secondary structure analysis of the Kv7 C-terminus predicts four helical regions (helices A–D) conserved in all family members (Yus-Najera et al. 2002). While helices C and D are thought to form coiled-coil assemblies, helices A and B encode the binding module for CaM (Wen & Levitan, 2002; Yus-Najera et al. 2002; Gamper & Shapiro, 2003). Helix A has a CaM-binding sequence that partly conforms to the consensus IQ motif and helix B displays two overlapping consensus 1-5-10 CaM-binding motifs (Yus-Najera et al. 2002). CaM appears to be an essential auxiliary subunit of all Kv7 channels. Thus, long QT mutations located close to the IQ motif of the Kv7.1 C-terminus impair CaM binding and markedly decrease current density (Ghosh et al. 2006; Shamgar et al. 2006). Similarly, BFNC mutations in helices A and B of Kv7.2 subunits exhibit weaker CaM binding and produce smaller Kv7.2 K+ currents (Richards et al. 2004; Etxeberria et al. 2007).
Figure 1. Structure of Kv7 channels and interaction sites on the carboxy-terminal tail.
The C-termini of only two subunits are depicted for clarity. The Kv7 C-terminus exhibits conserved interaction sites with CaM at helices A and B, with PIP2, as well as specific interaction sites (red arrows) with the AKAP79/150 in Kv7.2 at helix A-linker A–B, with the AKAP-yotiao in Kv7.1 at helix D, with ankyrin-G in Kv7.2/3 and with Nedd4-2 in Kv7.1 and Kv7.2/3. Helix B is endowed with PKC phosphorylation sites. Helix A contains a tyrosine kinase phosphorylation site. In addition to a potential role in channel trafficking, the coiled-coils C and D may correspond to subunit dimerization and tetramerization modules, respectively. Neighbouring subunits would form a dimer at helix C. The helix C complex may undergo dimerization of its dimeric coiled-coil while the helix D complex is depicted as a stable, tetrameric parallel coiled-coil, as seen in the crystal structure.
While it is clear that proper CaM binding to Kv7 C-terminus is required to produce functional channels, the role of CaM in Kv7 channel function is not well understood and remains controversial. One study showed that Kv7.2 mutants deficient in CaM binding produce undetectable currents when coexpressed with Kv7.3 in CHO cells, although they are expressed and targeted to the cell membrane and retain the ability to assemble with Kv7.3 (Wen & Levitan, 2002). In contrast, a recent study found that a BFNC mutation that weakens CaM binding leads to endoplasmic reticulum (ER) retention of Kv7.2, reducing the number of channels that reach the plasma membrane (Etxeberria et al. 2007). We and others showed that long QT mutations impairing CaM binding to Kv7.1 C-terminus affect channel gating, folding and trafficking (Ghosh et al. 2006; Shamgar et al. 2006). The long QT mutations produce a right-shift in channel activation and lead to macroscopic inactivation. Interestingly, the data indicate that CaM binding is essential for correct folding of the Kv7.1 C-terminus. Hence, recombinant production of a soluble Kv7.1 C-terminus requires bacterial coexpression of CaM. In addition, CaM binding to Kv7.1 C-terminus is also found to be necessary for correct channel trafficking to the plasma membrane, as revealed by cell surface biotinylation experiments. Also, CaM overexpression greatly improves the membrane targeting of wild-type Kv7.1 and even more dramatically that of long QT mutant channels which exhibit weaker CaM binding (Shamgar et al. 2006).
There are prominent differences in the role played by Ca2+–CaM as a Ca2+ sensor in the signalling of the different Kv7 channel subtypes. Overexpression of CaM in CHO cells is found to robustly reduce currents of Kv7.2, Kv7.4 and Kv7.5, but not those of Kv7.1 and Kv7.3 (Gamper et al. 2005). In sympathetic neurons, it was shown that bradykinin-induced rise in intracellular Ca2+ leads to suppression of the M-current via Ca2+–CaM. Overexpression of a Ca2+-insensitive mutant of CaM blunts bradykinin-induced M-current suppression (Gamper & Shapiro, 2003). However, channel inhibition by Ca2+–CaM is subunit specific. We showed in Xenopus oocytes that Kv7.1 and IKS currents (Kv7.1/KCNE1) are stimulated by increases in intracellular Ca2+ and are markedly inhibited by CaM antagonists. Remarkably, a rise in intracellular Ca2+ produces a left-shift in the voltage dependence of activation of IKS channels (Shamgar et al. 2006). These data are consistent with a recent study performed in guinea pig cardiomyocytes showing that at high stimulation rates, there is a Ca2+-induced increase in IKS currents that is sensitive to a CaM antagonist (Bai et al. 2005). This suggests that Ca2+–CaM is the Ca2+ sensor that stimulates IKS channels during cardiac activity, via an exquisite Ca2+ sensitivity. In all, it appears that the CaM-binding module at the Kv7 C-terminus performs a dual function: (1) a Ca2+ sensor function which affects channel gating in a subtype-specific manner, thereby suppressing Kv7.2, Kv7.4 and Kv7.5 currents and stimulating Kv7.1 and IKS channel activity; (2) a role in channel folding and trafficking. This dual feature implies that Kv7 channels may have two distinct CaM-binding sites, one of very high affinity acting as a constitutive tether at the Kv7 C-terminus to perform the folding and trafficking function and one of lower affinity to mediate Ca2+-sensing function.
Although CaM is ubiquitously expressed in all eukaryotic cells, emerging evidence supports a role for Ca2+-binding proteins (CaBPs) other than CaM in the regulation of various effectors, including ion channels (Burgoyne & Weiss, 2001; Haeseleer et al. 2002). For example, the respective interaction of Ca2+-binding protein-1 (CaBP1) and visinin-like protein-2 (VILIP-2) with Cav1.2 and Cav2.1 channels, slows Ca2+-dependent inactivation and causes Ca2+-dependent facilitation (Zhou et al. 2004; Lautermilch et al. 2005). These effects are in sharp contrast with those of CaM which promotes strong Ca2+-dependent inactivation. Thus, as with Ca2+ channels, there is a possibility that different CaBPs could interact with the same CaM-binding site at the Kv7 C-terminus to mediate distinct kinds of channel regulation (Fig. 1). This scenario may be especially relevant for the neuronally expressed Kv7.2–5 subunits where brain CaBPs may serve as Ca2+ sensors to modulate channel activity in a different way to ubiquitous CaM.
Phosphoinositide-binding module
Although primarily gated by voltage, many lines of evidence indicate that Kv7 channels are modulated by plasma membrane levels of phosphatidylinositol 4,5-bisphosphate (PIP2) (Delmas & Brown, 2005; Suh & Hille, 2005; Gamper & Shapiro, 2007). Heterologously expressed Kv7.2/3 channels quickly rundown upon patch excision but are restored upon cytoplasmic addition of PIP2 and are suppressed by PIP2 scavengers (Suh & Hille, 2002; Ford et al. 2003; Zhang et al. 2003). Furthermore, native M-currents rundown and inclusion of cytosolic PIP2 hastens their recovery (Ford et al. 2003). Maximal open probabilities (Po) of Kv7.2–Kv7.4 homomultimers and of Kv7.2/7.3 heteromultimers are strongly dependent on cytoplasmic application of PIP2 to inside-out patches (Li et al. 2005). While Kv7.3 has a maximal Po near unity, Kv7.2, Kv7.4 and Kv7.5 have much lower maximal Po values. Raising tonic PIP2 by coexpression of phosphatidylinositol (4)5-kinase increases the maximal Po of both Kv7.2 and Kv7.2/7.3 channels in on-cell patches (Li et al. 2005). Thus, it is suggested that PIP2 acts to stabilize the open state of Kv7.2, Kv7.4, and Kv7.5 channels, such that Po is increased at all voltages. According to this hypothesis, Kv7.2, Kv7.4, and Kv7.5 exhibit lower affinity for PIP2 compared to Kv7.3 (Li et al. 2005; Gamper & Shapiro, 2007). Kv7.1 subunits coexpressed with KCNE1 produce IKS currents that spontaneously rundown in excised patch-clamp recordings. This rundown is markedly slowed by cytosolic application of PIP2 and is fully prevented by application of PIP2 plus MgATP (Loussouarn et al. 2003). However, cytosolic application of PIP2 slows deactivation kinetics and shifts the voltage dependence of channel activation towards negative potentials. Thus, for Kv7.1, PIP2 may affect channel gating by modulating voltage sensitivity rather than maximum Po (Delmas & Brown, 2005).
The site of PIP2 binding on Kv7 channels has been previously suggested to be located in their proximal C-termini, though the exact position remains elusive (Loussouarn et al. 2003; Zhang et al. 2003; Park et al. 2005; Robbins et al. 2006). However, direct PIP2-binding experiments have not been performed and a more precise mapping remains to be established. Interestingly, a recent study characterizing the interaction of CaM- and phosphoinositide-mediated regulation of TRPC6 channels also showed that PIP2 and PIP3 directly bind to the Kv7.1 C-terminus and disrupt CaM binding to helices A and B (Kwon et al. 2007). To test whether CaM binding to Kv7.1 was dissociated by phosphoinositides, they fused each of the two C-terminal CaM-binding regions (amino acids (AAs) 345–400 and 504–565) to a maltose-binding protein and performed competition assays. They found that CaM binding to both CaM-binding regions was reduced by PIP2 and PIP3. Notably, this property extends to other ion channels, including TRPC channels and the voltage-gated Ca2+ channel Cav1.2 (Kwon et al. 2007). The CaM- and PIP2-binding modules in Kv7 C-termini probably overlap physically and functionally. Thus, increase in phospholipase C activity would decrease the PIP2 concentration and potentially lead to enhanced CaM regulation as a result of increased CaM–Kv7 interaction. Along with this idea, an attractive hypothesis has recently suggested a spatial sequestration of PIP2 by electrostatic interactions with natively unfolded proteins such as MARCKS or with ion channels like the NR1 glutamate channel subunit (McLaughlin & Murray, 2005). The NR1 subunits bind PIP2 and Ca2+–CaM within a common juxtamembranal basic/hydrophobic cluster. It is suggested that NR1 possibly functions as ‘PIPmodulin’ and releases PIP2 locally in response to certain stimuli such as Ca2+–CaM interaction. This concerted reciprocal modulation is particularly relevant for M-channels (Kv7.2/3) whose activity is promoted by PIP2 and suppressed by Ca2+–CaM. In contrast, both PIP2 and Ca2+–CaM are shown to activate Kv7.1 and IKS channels by producing a left-shift in the voltage dependence of activation (Loussouarn et al. 2003; Shamgar et al. 2006). Are these activating effects mediated by PIP2 and Ca2+–CaM, additive, mutually exclusive or cooperative? This issue remains to be addressed. The dual mode of regulation by PIP2 and CaM also has implications for the molecular mechanisms of channelopathies resulting from mutations in PIP2- and CaM-binding sites. BFNC mutations in helices A and B of Kv7.2 exhibit weaker CaM binding and lead to neonatal epilepsy (Richards et al. 2004; Etxeberria et al. 2007). Similarly, mutations that affect PIP2 or CaM binding to Kv7.1 underlie certain forms of the cardiac LQT syndrome (Park et al. 2005; Ghosh et al. 2006; Shamgar et al. 2006).
Coiled-coil modules
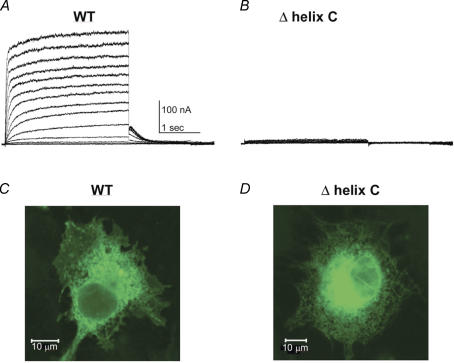
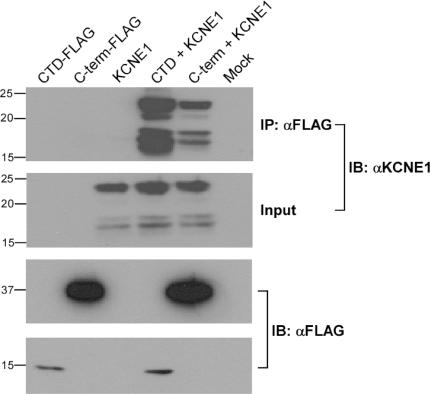
In the conserved regions of Kv7 C-termini, the proximal half binds CaM (helices A and B) while the distal half is endowed with two tandemly arranged coiled-coils, corresponding to helices C and D or the Head and the Tail, respectively (Yus-Najera et al. 2002; Jenke et al. 2003). This distal C-terminal region that is also called the A-domain is suggested to function as an assembly domain that directs subtype-specific assembly (Schmitt et al. 2000; Maljevic et al. 2003; Schwake et al. 2003, 2006). Helix D shows significant sequence difference among Kv7 channel subtypes, therefore this domain was suggested to be a primary determinant of assembly specificity (Maljevic et al. 2003; Schwake et al. 2003, 2006). A recent study has provided the high-resolution structure (2 Å) of the Kv7.4 helix D or Tail domain (Howard et al. 2007). Results show that the helix D (Tail domain) is a self-assembling, parallel, four-stranded coiled-coil that is conserved among Kv7 channel subtypes, thereby providing a critical module for Kv7 tetrameric assembly. Structural and biochemical data indicate that sequence variations among helix D at both the hydrophobic core of the coil and the polar network at the subunit interfaces appear to encode assembly-specificity determinants of Kv7 channel subtypes (Howard et al. 2007). We also probed the structural and functional characteristics of helices C and D in Kv7.1 subunits. Both analytical size-exclusion chromatography and sedimentation studies indicate that the helix C coiled-coil is dimeric and undergoes concentration-dependent self association to form a dimer of dimers (Fig. 1) (Wiener et al. 2007). To probe the function of helix C, currents were recorded from CHO cells expressing a helix C deletion channel construct. Deletion of helix C in Kv7.1 does not result in detectable K+ current (Fig. 2). The expressed mutant protein appears to be retained in the ER–Golgi complex, as shown by confocal immunocytochemical labelling, suggesting also a role for helix C in channel trafficking (Fig. 2). The helix C module is strongly conserved within the Kv7 subfamily, at both buried and surface-accessible residues. The buried residue conservation suggests a general role in promoting oligomerization, while the conservation of surface residues hints at the role of platform for association with proteins involved in trafficking and/or regulation. The helix D module differs in its conservation characteristics, exhibiting markedly lower overall levels within the Kv7 subfamily. In line with the recently published work on Kv7.4 (Howard et al. 2007), the crystallographic studies on Kv7.1 reveal that helix D is also a tetrameric parallel-orientated coiled-coil quaternary structure (Wiener et al. 2007). Kv7.1 subunits which do not coassemble with any of the other Kv7 subtypes, display significant differences relative to Kv7.4 at both the core positions and in the polar network of the helix D coiled-coil structure (Howard et al. 2007; Wiener et al. 2007). In addition, helix D of Kv7.1 should be about two helical turns longer than the other family members. This additional heptad repeat will favour assembly and folding with a subunit partner of equal length and sequence. Interestingly, LQT mutations located in helix D of Kv7.1 involve non-a or d coiled-coil position residues. One mutation, G589D has been shown to abolish sympathetic regulation of the cardiac IKS current by disrupting the interaction of the IKS channel complex (Kv7.1/KCNE1) with the A-kinase anchoring protein (AKAP) yotiao (Marx et al. 2002). Notably, all of the known helix D LQT mutations cluster to a single hot spot on the coiled-coil surface, which suggests that this site may interact with the protein yotiao (Fig. 1) (Howard et al. 2007; Wiener et al. 2007). Thus, the distal Kv7 C-terminus endowed with two coiled-coils appears to act as a module for proper channel assembly and trafficking as well as a platform for interaction with other signalling proteins. Consistent with this complex function, we found that the C-terminal domain (CTD) of Kv7.1, including helices C and D, interacts with the C-terminus of the auxiliary subunit KCNE1 (Fig. 3). Both pulldown and immunoprecipitation experiments demonstrate a physical interaction between the two C-terminal domains of the IKS channel complex. Hence, beyond channel assembly, the two tandemly arranged coiled-coils may be an important site for nucleation of macromolecular protein complexes that regulate channel activity. Mutations affecting this module may generate significant disruption of protein–protein interaction networks and lead to channelopathies.
Figure 2. Functional properties of Kv7.1 Δhelix C mutant.
Representative current traces of WT KCNQ1 (A) and Kv7.1 Δhelix C mutant (deletion AAs 548–562) (B). From a holding potential of −90 mV, CHO cells were stepped for 3 s from −70 mV to +60 mV in 10 mV increments and repolarized for 1 s at −60 mV. Deletion of helix C does not result in any detectable K+ currents. Immunocytochemical imaging of COS cells expressing WT Kv7.1 (C) and Kv7.1 Δhelix C mutant (D), detected with rabbit anti-Kv7.1 antibodies. While the Kv7.1 Δhelix C mutant exhibits essentially intracellular localization with strong accumulation in the ER, the WT channel protein displays both intracellular and significant plasma membrane distribution. Images were taken using a Zeiss 510 meta confocal microscope with a 458 nm excitation argon laser line.
Figure 3. KCNE1 interacts with the Kv7.1 C-terminal domain.
The Kv7.1 FLAG-tagged C-terminal domain (CTD) (AAs 510–620) or the full length C-terminus (C-term) (AAs 354–676) were co-expressed with KCNE1 in HEK 293T cells. After standard lysis procedure, immunoprecipitation experiments were carried out using anti-αM2 (FLAG) antibodies. KCNE1 is clearly able to co-immunoprecipitate with both Kv7.1 CTD and Kv7.1 C-term (upper panel).
Other interactions with the Kv7 C-terminus
The Kv7 C-terminus is a target site for various protein kinase phosphorylations. Kv7.2 C-terminus binds the AKAP79/150 protein which forms a trimeric complex with protein kinase C (PKC) (Hoshi et al. 2003). Activation of PKC leads to phosphorylation of serine residues located in helix B and its C-terminal boundary, which depresses Kv7.2 currents. These data suggest a major role for PKC activation in transmitter-mediated inhibition of M-currents (Hoshi et al. 2003; Delmas & Brown, 2005). Given that AKAP79, like Kv7.2, is also known to interact with CaM (Higashida et al. 2005), it remains to determine how Ca2+-mediated signals are integrated within this macromolecular scaffolding complex. The Kv7 C-terminus is also a substrate for tyrosine phosphorylation, via receptor- and non-receptor-tyrosine-kinases. The non-receptor tyrosine kinase Src was shown to suppress native M-currents as well as Kv7 channels in a subunit-specific manner. Kv7.3, Kv7.4 and Kv7.5 subunits are phosphorylated by Src kinase, which leads to depression of the currents (Gamper et al. 2003). However, in contrast to PKC, Src phosphorylation does not appear to contribute to muscarinic receptor-mediated inhibition. In Kv7.3 subunits, Src kinase phosphorylates two tyrosine residues, one in the N-terminus and the other in the C-terminus at helix A (Fig. 1) (Li et al. 2004). Biochemical experiments showed that Src kinase physically interacts with the Kv7 subunits, though the site of interaction remains to be determined. Data also indicate that Src kinase does not alter channel density at the plasma membrane but rather depresses open probability (Li et al. 2004). Recently, the receptor tyrosine kinase EGF was found to produce a biphasic inhibition of both recombinant Kv7.2/3 channels and native M-currents. The late EGF receptor-mediated inhibition involves phosphorylation of similar tyrosine residues to those which were phosphorylated by Src kinase in Kv7.3 subunits (Jia et al. 2007). Thus, both receptor- and non-receptor-tyrosine-kinase signalling may affect neuronal excitability via modulation of M-channels.
The Kv7 C-terminus appears to play a crucial role in the regulation of channel cell surface expression via interaction with Nedd4-2 ubiquitin ligase. Recent studies showed that the current amplitude of Kv7.1/KCNE1, Kv7.2/3 and Kv7.3/5 channels is reduced by Nedd4-2 ubiquitin ligase. GST-fusion pulldowns and co-immunoprecipitations demonstrated a direct interaction between the Kv7 subunits and Nedd4-2 (Ekberg et al. 2007; Jespersen et al. 2007). In transfected cells, Nedd4-2 could ubiquitinate Kv7.1 and Kv7.2/3 channels. The Nedd4-mediated regulation of Kv7.1 channels is strictly dependent on a PY motif located in the very distal part of the C-terminus, downstream helix D (Fig. 1) (Jespersen et al. 2007). When this motif is mutated, the currents and ubiquitination levels are unaffected by Nedd4-2 and the interaction between the ubiquitin ligase and Kv7.1 subunits is suppressed (Jespersen et al. 2007). Deletion experiments showed that the C-terminus of Kv7.3 subunits is required for Nedd4-2-mediated regulation of heteromeric Kv7.2/3 channels; however, it is not clear yet whether typical or atypical PY motifs are involved in ubiquitin ligase–channel interaction (Ekberg et al. 2007). This Nedd4-2-mediated regulation could be important for the control of Kv7 channel density at the plasma membrane and hence may play a role in cell excitability under particular pathophysiological conditions such as cardiac ischaemia, epilepsy, Alzheimer's disease or chronic pain.
The C-terminus of Kv7.2 and Kv7.3 subunits but not of other Kv7 channel subtypes was found to contain an interacting domain for ankyrin-G (Pan et al. 2006). This domain, also called C3 motif, is about 10 amino acids long and maps to the distal C-terminus, downstream helix D (Fig. 1) (Pan et al. 2006). Ankyrin-G is a large adaptor protein that binds multiple integral membrane proteins and mediates their interactions with the actin–spectrin cortical cytoskeleton. A similar ankyrin-G interaction motif was also identified as necessary and sufficient for targeting voltage-gated Na+ channels at the axon initial segment (AIS) (Garrido et al. 2003; Lemaillet et al. 2003). Kv7.2 and Kv7.3 subunits are found to be expressed at nodes of Ranvier and at AISs of several central and peripheral neurons (Devaux et al. 2004; Chung et al. 2006; Pan et al. 2006). As with voltage-gated sodium channels, targeting of Kv7.2 and Kv7.3 at AIS is abolished in ankyrin-G knock-out mice. Deletion of the ankyrin-G-binding motif in Kv7.2 alone does not alter the AIS localization of Kv7.2/3 heteromers. In contrast, deletion of the ankyrin-G-binding motif in Kv7.3 markedly reduces AIS targeting of the complex, implicating Kv7.3 as a major determinant of M-channel localization to the AIS (Rasmussen et al. 2007). Interestingly, all identified voltage-gated sodium channel (Nav) and Kv7 genes of worms, insects and molluscs lack the ankyrin-G-binding motif, while their vertebrate orthologues possess it (Pan et al. 2006). It is thus suggested that there is an exquisite coincidence between the emergence of myelination and a mechanism for synchronized nodal and AIS retention of Kv7 and Nav channels at a similar period of evolution (Pan et al. 2006).
Prospective
The Kv7 C-terminus appears to share some functional properties of the T1 domain of Shaker-related Kv channels. Like the T1 domain, the Kv7 C-terminus serves as a channel tetramerization domain. It was suggested that T1 enhances channel assembly by increasing the local concentration of subunits in a multistep process, thereby implying that another oligomerization component probably exists in the membrane core of the protein (Zerangue et al. 2000). Then, T1 is not required for subsequent steps in channel assembly and folding. In contrast, Kv7 channel formation cannot occur without its C-terminus which must provide the necessary free energy deficit for assembly. Like the T1 domain, the Kv7 C-terminus plays a role in channel gating via its CaM- and PIP2-binding modules. However, in Kv7 channels these gating modules are located C-terminal to the voltage sensor and the pore domains, which may imply differences in the coupling mechanisms to the channel gates. Like the T1 domain, the Kv7 C-terminus interacts with an auxiliary subunit, but so far this feature is restricted to the Kv7.1 subtype via its interaction with KCNE1. In all, the data indicate that the Kv7 C-terminus is a multifunctional module playing a crucial role in channel gating, assembly and trafficking as well as in scaffolding the channel complex with signalling proteins. Yet, there are several unsettled issues. How does PIP2 affect the function of Ca2+–CaM? By which molecular mechanisms do they affect Kv7 channel gating? Are there other Ca2+-binding proteins (CaBPs) to mediate distinct kinds of channel regulation? What are the differential structural and functional attributes of the two coiled-coil modules (helices C and D) and to what scaffolding/signalling proteins do they specifically interact? Are the linkers connecting the α helices A–D structured or disordered and do they have functional significance? Needless to say, high-resolution structures of the Kv7 C-termini will be helpful to tackle these questions.
Acknowledgments
This work is supported by the Israel Science Foundation (ISF 672/05), the Ministry of Science and Technology (Tachtiot 2007-09) and the Keren Wolfson Family funds to B.A.
References
- Bai CX, Namekata I, Kurokawa J, Tanaka H, Shigenobu K, Furukawa T. Role of nitric oxide in Ca2+ sensitivity of the slowly activating delayed rectifier K+ current in cardiac myocytes. Circ Res. 2005;96:64–72. doi: 10.1161/01.RES.0000151846.19788.E0. [DOI] [PubMed] [Google Scholar]
- Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. KvLQT1 and lsK (minK) proteins associate to form the IKs cardiac potassium current. [See comments] Nature. 1996;384:78–80. doi: 10.1038/384078a0. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD, Weiss JL. The neuronal calcium sensor family of Ca2+-binding proteins. Biochem J. 2001;353:1–12. [PMC free article] [PubMed] [Google Scholar]
- Chung HJ, Jan YN, Jan LY. Polarized axonal surface expression of neuronal KCNQ channels is mediated by multiple signals in the KCNQ2 and KCNQ3 C-terminal domains. Proc Natl Acad Sci U S A. 2006;103:8870–8875. doi: 10.1073/pnas.0603376103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cushman SJ, Nanao MH, Jahng AW, DeRubeis D, Choe S, Pfaffinger PJ. Voltage dependent activation of potassium channels is coupled to T1 domain structure. Nat Struct Biol. 2000;7:403–407. doi: 10.1038/75185. [DOI] [PubMed] [Google Scholar]
- Delmas P, Brown DA. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci. 2005;6:850–862. doi: 10.1038/nrn1785. [DOI] [PubMed] [Google Scholar]
- Devaux JJ, Kleopa KA, Cooper EC, Scherer SS. KCNQ2 is a nodal K+ channel. J Neurosci. 2004;24:1236–1244. doi: 10.1523/JNEUROSCI.4512-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekberg J, Schuetz F, Boase NA, Conroy SJ, Manning J, Kumar S, Poronnik P, Adams DJ. Regulation of the voltage-gated K+ channels KCNQ2/3 and KCNQ3/5 by ubiquitination. Novel role for Nedd4-2. J Biol Chem. 2007;282:12135–12142. doi: 10.1074/jbc.M609385200. [DOI] [PubMed] [Google Scholar]
- Etxeberria A, Aivar P, Rodriguez-Alfaro JA, Alaimo A, Villace P, Gomez-Posada JC, Areso P, Villarroel A. Calmodulin regulates the trafficking of KCNQ2 potassium channels. FASEB J. 2007 doi: 10.1096/fj.07-9712com. DOI 10.1096/fj.07-9712com. [DOI] [PubMed] [Google Scholar]
- Ford CP, Stemkowski PL, Light PE, Smith PA. Experiments to test the role of phosphatidylinositol 4,5-bisphosphate in neurotransmitter-induced M-channel closure in bullfrog sympathetic neurons. J Neurosci. 2003;23:4931–4941. doi: 10.1523/JNEUROSCI.23-12-04931.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamper N, Li Y, Shapiro MS. Structural requirements for differential sensitivity of KCNQ K+ channels to modulation by Ca2+/calmodulin. Mol Biol Cell. 2005;16:3538–3551. doi: 10.1091/mbc.E04-09-0849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamper N, Shapiro MS. Calmodulin mediates Ca2+-dependent modulation of M-type K+ channels. J Gen Physiol. 2003;122:17–31. doi: 10.1085/jgp.200208783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamper N, Shapiro MS. Regulation of ion transport proteins by membrane phosphoinositides. Nat Rev Neurosci. 2007;8:921–934. doi: 10.1038/nrn2257. [DOI] [PubMed] [Google Scholar]
- Gamper N, Stockand JD, Shapiro MS. Subunit-specific modulation of KCNQ potassium channels by Src tyrosine kinase. J Neurosci. 2003;23:84–95. doi: 10.1523/JNEUROSCI.23-01-00084.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido JJ, Giraud P, Carlier E, Fernandes F, Moussif A, Fache MP, Debanne D, Dargent B. A targeting motif involved in sodium channel clustering at the axonal initial segment. Science. 2003;300:2091–2094. doi: 10.1126/science.1085167. [DOI] [PubMed] [Google Scholar]
- Ghosh S, Nunziato DA, Pitt GS. KCNQ1 assembly and function is blocked by long-QT syndrome mutations that disrupt interaction with calmodulin. Circ Res. 2006;98:1048–1054. doi: 10.1161/01.RES.0000218863.44140.f2. [DOI] [PubMed] [Google Scholar]
- Gulbis JM, Zhou M, Mann S, MacKinnon R. Structure of the cytoplasmic β subunit-T1 assembly of voltage-dependent K+ channels. Science. 2000;289:123–127. doi: 10.1126/science.289.5476.123. [DOI] [PubMed] [Google Scholar]
- Haeseleer F, Imanishi Y, Sokal I, Filipek S, Palczewski K. Calcium-binding proteins: intracellular sensors from the calmodulin superfamily. Biochem Biophys Res Commun. 2002;290:615–623. doi: 10.1006/bbrc.2001.6228. [DOI] [PubMed] [Google Scholar]
- Higashida H, Hoshi N, Zhang JS, Yokoyama S, Hashii M, Jin D, Noda M, Robbins J. Protein kinase C bound with A-kinase anchoring protein is involved in muscarinic receptor-activated modulation of M-type KCNQ potassium channels. Neurosci Res. 2005;51:231–234. doi: 10.1016/j.neures.2004.11.009. [DOI] [PubMed] [Google Scholar]
- Hoshi N, Zhang JS, Omaki M, Takeuchi T, Yokoyama S, Wanaverbecq N, Langeberg LK, Yoneda Y, Scott JD, Brown DA, Higashida H. AKAP150 signaling complex promotes suppression of the M-current by muscarinic agonists. Nat Neurosci. 2003;6:564–571. doi: 10.1038/nn1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard RJ, Clark KA, Holton JM, Minor DL., Jr Structural insight into KCNQ (Kv7) channel assembly and channelopathy. Neuron. 2007;53:663–675. doi: 10.1016/j.neuron.2007.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenke M, Sanchez A, Monje F, Stuhmer W, Weseloh RM, Pardo LA. C-terminal domains implicated in the functional surface expression of potassium channels. EMBO J. 2003;22:395–403. doi: 10.1093/emboj/cdg035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch TJ. Neuronal KCNQ potassium channels: physiology and role in diseases. Nat Neurosci. 2000;1:21–30. doi: 10.1038/35036198. [DOI] [PubMed] [Google Scholar]
- Jespersen T, Membrez M, Nicolas CS, Pitard B, Staub O, Olesen SP, Baro I, Abriel H. The KCNQ1 potassium channel is down-regulated by ubiquitylating enzymes of the Nedd4/Nedd4-like family. Cardiovasc Res. 2007;74:64–74. doi: 10.1016/j.cardiores.2007.01.008. [DOI] [PubMed] [Google Scholar]
- Jia Q, Jia Z, Zhao Z, Liu B, Liang H, Zhang H. Activation of epidermal growth factor receptor inhibits KCNQ2/3 current through two distinct pathways: membrane PtdIns(4,5)P2 hydrolysis and channel phosphorylation. J Neurosci. 2007;27:2503–2512. doi: 10.1523/JNEUROSCI.2911-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon Y, Hofmann T, Montell C. Integration of phosphoinositide- and calmodulin-mediated regulation of TRPC6. Mol Cell. 2007;25:491–503. doi: 10.1016/j.molcel.2007.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautermilch NJ, Few AP, Scheuer T, Catterall WA. Modulation of CaV2.1 channels by the neuronal calcium-binding protein visinin-like protein-2. J Neurosci. 2005;25:7062–7070. doi: 10.1523/JNEUROSCI.0447-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaillet G, Walker B, Lambert S. Identification of a conserved ankyrin-binding motif in the family of sodium channel a subunits. J Biol Chem. 2003;278:27333–27339. doi: 10.1074/jbc.M303327200. [DOI] [PubMed] [Google Scholar]
- Li Y, Gamper N, Hilgemann DW, Shapiro MS. Regulation of Kv7 (KCNQ) K+ channel open probability by phosphatidylinositol 4,5-bisphosphate. J Neurosci. 2005;25:9825–9835. doi: 10.1523/JNEUROSCI.2597-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Langlais P, Gamper N, Liu F, Shapiro MS. Dual phosphorylations underlie modulation of unitary KCNQ K+ channels by Src tyrosine kinase. J Biol Chem. 2004;279:45399–45407. doi: 10.1074/jbc.M408410200. [DOI] [PubMed] [Google Scholar]
- Loussouarn G, Park KH, Bellocq C, Baro I, Charpentier F, Escande D. Phosphatidylinositol-4,5-bisphosphate, PIP2, controls KCNQ1/KCNE1 voltage-gated potassium channels: a functional homology between voltage-gated and inward rectifier K+ channels. EMBO J. 2003;22:5412–5421. doi: 10.1093/emboj/cdg526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLaughlin S, Murray D. Plasma membrane phosphoinositide organization by protein electrostatics. Nature. 2005;438:605–611. doi: 10.1038/nature04398. [DOI] [PubMed] [Google Scholar]
- Maljevic S, Lerche H, Seebohm G, Alekov AK, Busch AE, Lerche H. C-terminal interaction of KCNQ2 and KCNQ3 K+ channels. J Physiol. 2003;548:353–360. doi: 10.1113/jphysiol.2003.040980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marx SO, Kurokawa J, Reiken S, Motoike H, D'Armiento J, Marks AR, Kass RS. Requirement of a macromolcular signaling complex for β adrenergic receptor modulation of the KCNQ1-KCNE1 potassium channel. Science. 2002;295:496–498. doi: 10.1126/science.1066843. [DOI] [PubMed] [Google Scholar]
- Minor DL, Lin YF, Mobley BC, Avelar A, Jan YN, Jan LY, Berger JM. The polar T1 interface is linked to conformational changes that open the voltage-gated potassium channel. Cell. 2000;102:657–670. doi: 10.1016/s0092-8674(00)00088-x. [DOI] [PubMed] [Google Scholar]
- Pan Z, Kao T, Horvath Z, Lemos J, Sul J-Y, Cranstoun SD, Bennett V, Scherer SS, Cooper EC. A common ankyrin-G-based mechanism retains KCNQ and NaV channels at electrically active domains of the axon. J Neurosci. 2006;26:2599–2613. doi: 10.1523/JNEUROSCI.4314-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KH, Piron J, Dahimene S, Merot J, Baro I, Escande D, Loussouarn G. Impaired KCNQ1-KCNE1 and phosphatidylinositol-4,5-bisphosphate interaction underlies the long QT syndrome. Circ Res. 2005;96:730–739. doi: 10.1161/01.RES.0000161451.04649.a8. [DOI] [PubMed] [Google Scholar]
- Rasmussen HB, Frokjaer-Jensen C, Jensen CS, Jensen HS, Jorgensen NK, Misonou H, Trimmer JS, Olesen SP, Schmitt N. Requirement of subunit co-assembly and ankyrin-G for M-channel localization at the axon initial segment. J Cell Sci. 2007;120:953–963. doi: 10.1242/jcs.03396. [DOI] [PubMed] [Google Scholar]
- Richards MC, Heron SE, Spendlove HE, Scheffer IE, Grinton B, Berkovic SF, Mulley JC, Davy A. Novel mutations in the KCNQ2 gene link epilepsy to a dysfunction of the KCNQ2–calmodulin interaction. J Med Genet. 2004;41:e35. doi: 10.1136/jmg.2003.013938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins J. KCNQ potassium channels: physiology, pathophysiology, and pharmacology. Pharmacol Ther. 2001;90:1–19. doi: 10.1016/s0163-7258(01)00116-4. [DOI] [PubMed] [Google Scholar]
- Robbins J, Marsh SJ, Brown DA. Probing the regulation of M (Kv7) potassium channels in intact neurons with membrane-targeted peptides. J Neurosci. 2006;26:7950–7961. doi: 10.1523/JNEUROSCI.2138-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogawski MA. KCNQ2/KCNQ3 K+ channels and the molecular pathogenesis of epilepsy: implications for therapy. Trends Neurosci. 2000;23:393–398. doi: 10.1016/s0166-2236(00)01629-5. [DOI] [PubMed] [Google Scholar]
- Sanguinetti MC, Curran ME, Zou A, Shen J, Spector PS, Atkinson DL, Keating MT. Coassembly of KvLQT1 and minK (IsK) proteins to form cardiac IKs potassium channel. Nature. 1996;384:80–83. doi: 10.1038/384080a0. [DOI] [PubMed] [Google Scholar]
- Schmitt N, Schwarz M, Peretz A, Abitbol I, Attali B, Pongs O. A recessive C-terminal Jervell and Lange-Nielsen mutation of the KCNQ1 channel impairs subunit assembly. EMBO J. 2000;19:332–340. doi: 10.1093/emboj/19.3.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwake M, Athanasiadu D, Beimgraben C, Blanz J, Beck C, Jentsch TJ, Saftig P, Friedrich T. Structural determinants of M-type KCNQ (Kv7) K+ channel assembly. J Neurosci. 2006;26:3757–3766. doi: 10.1523/JNEUROSCI.5017-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwake M, Jentsch TJ, Friedrich T. A carboxy-terminal domain determines the subunit specificity of KCNQ K+ channel assembly. EMBO Rep. 2003;4:76–81. doi: 10.1038/sj.embor.embor715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamgar L, Ma L, Schmitt N, Haitin Y, Peretz A, Wiener R, Hirsch J, Pongs O, Attali B. Calmodulin is essential for cardiac IKS channel gating and assembly: impaired function in long-QT mutations. Circ Res. 2006;98:1055–1063. doi: 10.1161/01.RES.0000218979.40770.69. [DOI] [PubMed] [Google Scholar]
- Suh BC, Hille B. Recovery from muscarinic modulation of M current channels requires phosphatidylinositol 4,5-bisphosphate synthesis. Neuron. 2002;35:507–520. doi: 10.1016/s0896-6273(02)00790-0. [DOI] [PubMed] [Google Scholar]
- Suh BC, Hille B. Regulation of ion channels by phosphatidylinositol 4,5-bisphosphate. Curr Opin Neurobiol. 2005;15:370–378. doi: 10.1016/j.conb.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Wen H, Levitan IB. Calmodulin is an auxiliary subunit of KCNQ2/3 potassium channels. J Neurosci. 2002;22:7991–8001. doi: 10.1523/JNEUROSCI.22-18-07991.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiener R, Haitin Y, Shamgar L, Fernandez-Alonso MC, Martos A, Chomsky-Hecht O, Rivas G, Attali B, Hirsch JA. The KCNQ1 (Kv7.1) C-terminus, a multi-tiered scaffold for subunit assembly and protein interaction. J Biol Chem. 2007 doi: 10.1074/jbc.M707541200. DOI 10.1074/jbc.M707541200. [DOI] [PubMed] [Google Scholar]
- Yus-Najera E, Santana-Castro I, Villarroel A. The identification and characterization of a noncontinuous calmodulin-binding site in noninactivating voltage-dependent KCNQ potassium channels. J Biol Chem. 2002;277:28545–28553. doi: 10.1074/jbc.M204130200. [DOI] [PubMed] [Google Scholar]
- Zerangue N, Jan YN, Jan LY. An artificial tetramerization domain restores efficient assembly of functional Shaker channels lacking T1. Proc Natl Acad Sci U S A. 2000;97:3591–3595. doi: 10.1073/pnas.060016797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Craciun LC, Mirshahi T, Rohacs T, Lopes CM, Jin T, Logothetis DE. PIP2 activates KCNQ channels, and its hydrolysis underlies receptor-mediated inhibition of M currents. Neuron. 2003;37:963–975. doi: 10.1016/s0896-6273(03)00125-9. [DOI] [PubMed] [Google Scholar]
- Zhou H, Kim SA, Kirk EA, Tippens AL, Sun H, Haeseleer F, Lee A. Ca2+-binding protein-1 facilitates and forms a postsynaptic complex with Cav1.2 (L-type) Ca2+ channels. J Neurosci. 2004;24:4698–4708. doi: 10.1523/JNEUROSCI.5523-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]