Abstract
We measured a low-threshold, inactivating K+ current, i.e. A-current (IA), in respiratory neurons of the preBötzinger complex (preBötC) in rhythmically active slice preparations from neonatal C57BL/6 mice. The majority of inspiratory neurons (21/34 = 61.8%), but not expiratory neurons (1/8 = 12.5%), expressed IA. In whole-cell and somatic outside-out patches IA activated at −60 mV (half-activation voltage measured −16.3 mV) and only fully inactivated above −40 mV (half-inactivation voltage measured −85.6 mV), indicating that IA can influence membrane trajectory at baseline voltages during respiratory rhythm generation in vitro. 4-Aminopyridine (4-AP, 2 mm) attenuated IA in both whole-cell and somatic outside-out patches. In the context of rhythmic network activity, 4-AP caused irregular respiratory-related motor output on XII nerves and disrupted rhythmogenesis as detected with whole-cell and field recordings in the preBötC. Whole-cell current-clamp recordings showed that 4-AP changed the envelope of depolarization underlying inspiratory bursts (i.e. inspiratory drive potentials) from an incrementing pattern to a decrementing pattern during rhythm generation and abolished current pulse-induced delayed excitation. These data suggest that IA opposes excitatory synaptic depolarizations at baseline voltages of approximately −60 mV and influences the inspiratory burst pattern. We propose that IA promotes orderly recruitment of constituent rhythmogenic neurons by minimizing the activity of these neurons until they receive massive coincident synaptic input, which reduces the periodic fluctuations of inspiratory activity.
Rhythmic motor behaviours originate from central pattern generator (CPG) networks in the brainstem and spinal cord (Marder, 2001). A key issue is to what degree proper network function (i.e. rhythmogenesis) depends on specific ion channels in constituent rhythm-generating neurons (Stein, 1997). The respiratory CPG is an excellent model for examining this question because its constituent rhythmogenic neurons are contained within the preBötzinger complex (preBötC) (Smith et al. 1991; Feldman & Del Negro, 2006) and the network output is measurable in vitro. Transverse medullary slices containing the preBötC spontaneously generate behaviourally relevant motor activity that can be monitored via the hypoglossal nerve (XII).
Most studies of respiratory rhythm generation have focused on the role of voltage-dependent inward currents (Mironov & Richter, 1998; Pierrefiche et al. 1999; Mironov et al. 2000; Thoby-Brisson et al. 2000; Del Negro et al. 2001, 2002a,b, 2005; Onimaru et al. 2003; Pena et al. 2004; Ptak et al. 2005; Pace et al. 2007b), neuromodulation (Johnson et al. 1996; Rekling et al. 1996b; Onimaru et al. 1998; Shao & Feldman, 2000; Pena & Ramirez, 2002, 2004; Ruangkittisakul et al. 2006), as well as excitatory and inhibitory synaptic currents (Greer et al. 1991; Funk et al. 1993, 1995; Brockhaus & Ballanyi, 1998; Pierrefiche et al. 1998; Shao et al. 2003; Paarmann et al. 2005; Pace et al. 2007a). Apart from an ATP-inhibited K+ current primarily activated during hypoxia (Pierrefiche et al. 1996; Mironov et al. 1998, 1999; Mironov & Richter, 2000, 2001; Haller et al. 2001a,b), K+ currents have not been well-characterized in the preBötC of neonatal rodents, nor have their contributions to rhythmogenesis been analysed.
Transient K+ currents are important in invertebrate CPGs and the primitive vertebrate lamprey CPG for swimming, where they control the sequence of cell activation during the behavioural patterns and influence spike timing and frequency (Getting, 1983; Tierney & Harris-Warrick, 1992; Hess & El Manira, 2001).
In the respiratory CPG, Rekling et al. (1996a) described a subset of inspiratory neurons that depolarized with a ramp-like trajectory and started spiking ∼400 ms prior to XII output, dubbed type 1 neurons, which are putatively rhythmogenic (Rekling & Feldman, 1998; Gray et al. 1999). Type 1 neurons held at hyperpolarized membrane potentials exhibited delayed excitation in response to 400 ms step pulses of depolarizing current (Rekling & Feldman, 1998). Delayed excitation is often attributed to transient K+ currents (i.e. A-currents, IA) (Hagiwara et al. 1961; Getting, 1983; Dekin & Getting, 1987; Dekin et al. 1987; Nisenbaum et al. 1994), thus Rekling and colleagues proposed that rhythmogenic preBötC neurons expressed IA, although they did not measure it in voltage clamp nor speculate on its role in rhythm generation (Rekling et al. 1996a; Rekling & Feldman, 1998). Inyushkin (2005) confirmed that preBötC neurons expressed IA using voltage clamp, but stopped short of analysing its contributions to rhythmogenesis.
Here we studied preBötC neurons that became active preceding inspiratory bursts, which we refer to as ‘early inspiratory’ to distinguish them from more rostral ‘pre-inspiratory’ neurons (Onimaru et al. 2006), and sought to measure IA and evaluate its role in rhythm generation.
Methods
The Institutional Animal Care and Use Committee at The College of William and Mary approved all protocols. Neonatal (P0–7) C57BL/6 mice were anaesthetized via hypothermia until mice lacked a tail-pinch response. Mice were rapidly decerebrated and then dissected. Transverse slices (550 μm thick) containing the preBötC and hypoglossal (XII) nerves were sectioned with a vibrating microtome from the medulla oblongata. The rostral cut captured the rostral-most XII nerves, the dorsomedial cell column and principal lateral loop of the inferior olivary nucleus while the caudal cut captured the obex.
Slices were perfused at 26–28°C with an artificial cerebrospinal fluid (ACSF) containing (mm): 124 NaCl, 9 KCl, 0.5 NaH2PO4, 25 NaHCO3, 30 d-glucose, 1.5 CaCl2.2H2O, and 1 MgSO4. We identified putatively rhythmogenic inspiratory preBötC neurons (see first section in Results, below) with 9 mm K+ in the ACSF to maintain rhythmic network function, and then switched to 3 mm K+ to isolate and measure the properties of IA because the lower [K+] more closely matches the in vivo milieu. We acquired data from a total of 65 inspiratory neurons, 8 expiratory neurons, and 4 field-recordings in a total of 77 slices and 77 animals.
Most voltage- and current-clamp experiments were performed with a HEKA EPC-10 patch-clamp amplifier and Patchmaster software (Lambrecht, Germany). Dose–response experiments were performed with a Model 2400 patch-clamp amplifier (A-M Systems, Sequim, WA, USA) using Chart 5 software and a Powerlab 8/30 (AD Instruments, Colorado Springs, CO, USA) for stimulation. The remaining voltage-clamp experiments utilized a LabJack U3 (LabJack Corporation, Lakewood, CO, USA) as a waveform generator commanding the voltage-clamp amplifier controlled with custom C/C++ software written for a G4 Powerbook (Apple Inc., Cupertino, CA, USA). Respiratory-related motor output was monitored from XII nerves with extracellular suction electrodes and a high-gain differential amplifier with band-pass filtering (0.3–1 kHz) (Dagan Instruments, Minneapolis, MN, USA). Raw XII activity was conditioned using a true RMS-to-DC converter (Analog Devices, One Technology Way, Norwood, MA, USA) to provide a full-wave rectified and smoothed XII waveform. Data were acquired digitally and analysed using Chart 5, Igor Pro 5 (WaveMetrics, Lake Oswego, OR, USA), Excel (Microsoft, Redmond, WA, USA) and custom software. An 8 mV liquid junction potential was corrected online in both current- and voltage clamp.
Whole-cell capacitance (CM) was measured using 50 ms voltage steps from −60 mV to command potentials from −75 mV to −65 mV in a 10-step sequence. Charge (Q) was computed by integrating leak-subtracted capacitative current (ΔQ =∫IC) and CM was calculated from CM=ΔQ/ΔV. Series (access) resistance (RS) was monitored throughout voltage-clamp recordings according to the Thevenin equivalent circuit, which allows RS to be calculated from the decay time constant (τm) in response to small voltage steps with RS=τm/Cm as long as RS was much less than the input resistance (RN). We monitored RN via P/N online leak protocols. To avoid voltage-clamp errors we discarded experiments in which RS > 0.1 RN. We compensated for RS in whole-cell using analog feedback circuitry within the EPC-10 as much as possible without causing clamp oscillations that jeopardize stable recording. We rechecked RS and RN to assess voltage-clamp viability before running sequences of episodic protocols, ensuring the reliability of the acquired data.
We used the following standard patch solution containing (mm): 140 potassium gluconate, 5 NaCl, 0.1 EGTA, 10 Hepes, 2 Mg-ATP, and 0.3 Na(3)-GTP. KOH was used to equilibrate pH at 7.2. To isolate IA in voltage clamp (Figs 1B, 2B, 3, 5 and 7A) and test for delayed excitation as in Figs 1C and 2C, we used a low Ca2+–high Mg2+ extracellular ACSF containing (mm): 124 NaCl, 3 KCl, 25 NaHCO3, 30 d-glucose, 0.5 CaCl2·2H2O, 2 MgSO4, 0.001 TTX, and 0.2 CdCl2.
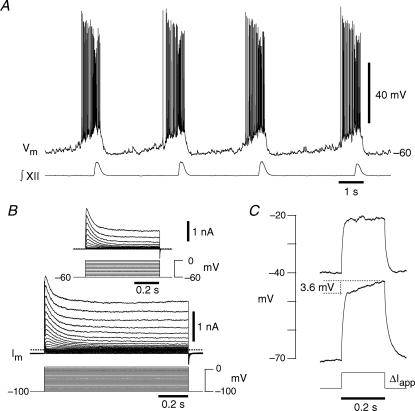
Figure 1. Phenotypic behaviours of inspiratory neurons located in the preBötC.
A, current-clamp recording showing early inspiratory activity that precedes the integrated XII nerve (∫XII) output in 9 mm[K+]o, a hallmark property of rhythmogenic neurons. B, voltage-clamp recording from a holding potential of −100 mV illustrating the transient K+ currents evoked at depolarized membrane potentials. Inset, a different neuron showing transient K+ currents evoked from a holding potential of −60 mV. C, the same neuron as in A and B illustrating voltage-dependent delayed excitation where ΔIapp was 95 pA.
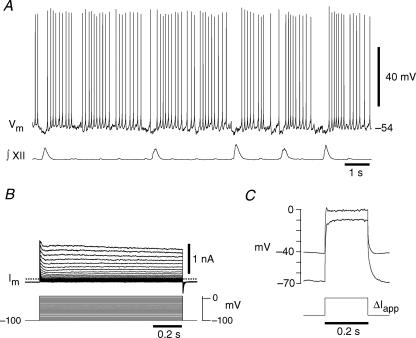
Figure 2. Phenotypic behaviours of expiratory neurons located in the preBötC.
A, current clamp showing tonic spiking during the expiratory phase that is inhibited during ∫XII output in 9 mm[K+]o, a hallmark property of expiratory neurons. B, the corresponding voltage-clamp recording shows only minimal transient K+ currents. C, delayed excitation is not exhibited by the expiratory neuron where ΔIapp was 379 pA.
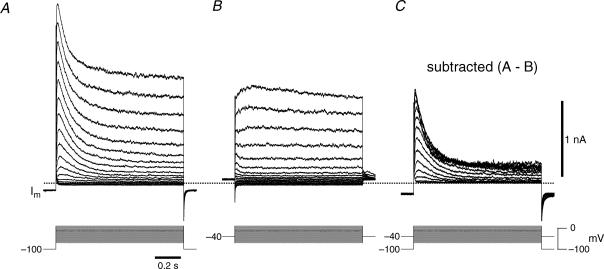
Figure 3. Isolation of IA in inspiratory neurons.
A, voltage-clamp recording from a holding potential of −100 mV illustrating the transient K+ currents evoked at depolarized membrane potentials. B, voltage-clamp recording from a holding potential of −40 mV illustrating the lack of significant transient K+ currents evoked at depolarized membrane potentials. C, IA was isolated by subtracting the current traces in B from A while the voltage-clamp protocols are shown superimposed.
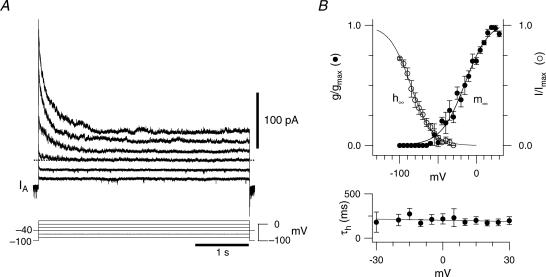
Figure 5. Biophysics of IA in inspiratory neurons.
A, the voltage dependence of activation measured from difference currents evoked by depolarizing step commands delivered from −100 mV and −40 mV in an outside-out patch. The voltage-clamp protocols are shown superimposed and alternating traces were eliminated to facilitate illustration. B, steady-state activation curve (m∞) and inactivation curve (h∞) from outside-out patches (top). The time constant of inactivation (τh) as a function of voltage (bottom).
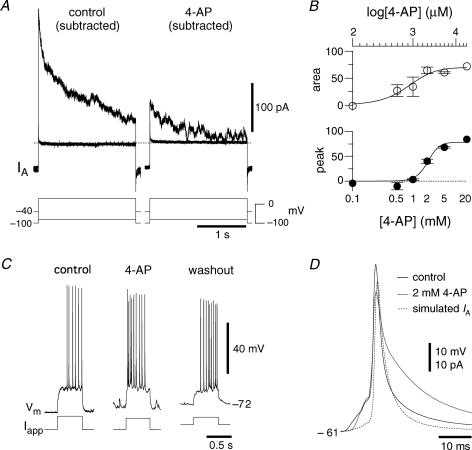
Figure 7. 4-Aminopyridine attenuates IA and blocks delayed excitation.
A, difference currents evoked from −100 and −40 mV in somatic outside-out patches in the presence of 1 μm TTX, 200 μm Cd2+ and 3 mm extracellular [K+] before and after application of 2 mm 4-AP. Two activation steps are shown: −80 mV and +30 mV. B, dose–response curve of IA in outside-out patches. C, in a different cell from A, 2 mm 4-AP abolished delayed excitation in current clamp. This neuron is also analysed in Fig. 9. D, the effects of 4-AP on pulse-induced spikes averaged over 4 neurons.
We measured the voltage dependence and kinetics of IA using Fitmaster software by HEKA (Lambrecht, Germany) and Igor Pro. Activation and inactivation functions took the form:
where x∞ reflects voltage-dependent steady-state activation (m∞) or inactivation (h∞), θx is the membrane potential of half-activation (θm) or half-inactivation (θh), and σx is the slope factor.
We computed the time course of IA using recorded voltage trajectories from current clamp (Fig. 6) and the chord conductance equation IA=gAmA(∞)hA (V–EK). EK was −71 mV to simulate in vitro conditions, the voltage-dependent parameters matched the values from Results, and dhA/dt = (hA(∞)–hA)/τh(V), with τh(V) = (202–0.42·V) (fitted empirically, see Fig. 5B). The differential equation was integrated using the 4th order Runge–Kutta method in custom C/C++ software run on Apple Macintosh G5 computers under OS 10.4. Integration step size was 0.25 ms to match the 4 kHz experimental sampling rate.
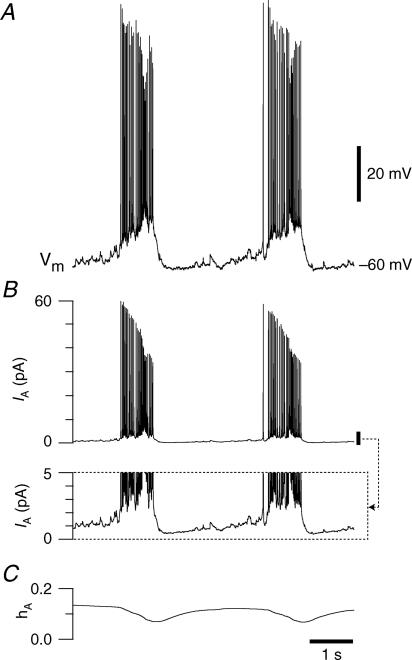
Figure 6. Simulating the role of IA.
A, the membrane trajectory from Fig. 1A. B, the time course of IA as computed from Hodgkin–Huxley-style equations (see Methods). Inset, a detail of the calculated IA with expanded y-axis emphasizing the low magnitude (1–2 pA) of the current during the interburst interval. C, the time course of the computed hA.
Inspiratory drive potentials were analysed using the Peak Parameters extension in Chart software. Leading and trailing slopes of the drive potential were calculated from digitally smoothed traces that minimize spikes but preserve the underlying drive potential characteristics (Pace et al. 2007b). Peak amplitude and baseline were automatically detected and the leading slope was computed from 20% of peak amplitude to 80% peak amplitude and trailing slope is calculated from 80% to 20%. Cycle-triggered averages were generated with custom software written in the Python programming language.
Sample means were generally compared using t test, or Fisher Exact test where indicated. Mean values are reported with standard error (mean ±s.e.m.) and significance was set at a P value of 0.05.
Results
Inspiratory preBötC neurons express IA
Rhythmogenic neurons can be distinguished on the basis of small soma size (measurable via CM) and an incremental pattern of depolarization followed by repetitive spike discharge several hundred milliseconds prior to inspiratory-related XII (or C4) motor activity (Fig. 1A) (Bianchi et al. 1995; Rekling et al. 1996a; Rekling & Feldman, 1998; Ballanyi et al. 1999; Richter & Spyer, 2001). In a prior study we showed that these intrinsic properties are a reliable means to identify rhythmogenic preBötC neurons (Hayes & Del Negro, 2007).
We isolated K+ currents in whole-cell voltage clamp using low Ca2+ ACSF containing 3 mm extracellular [K+], 1 μm TTX and 200 μm Cd2+. Depolarizing step commands from −100 mV (up to +10 mV) evoked sustained K+ currents in addition to transient K+ currents, i.e. IA (Fig. 1B). IA could also be evoked by depolarizing step commands from a −60 mV holding potential (Fig. 1B, inset), suggesting that IA does not completely inactivate at baseline membrane potentials observed during normal inspiratory activity in vitro (e.g. Fig. 1A).
In current clamp, depolarizing current steps from a holding potential of −70 mV evoked a ramping depolarization (ΔV/Δt = 18 mV s-1), whereas steps from −40 mV resulted in passive responses that quickly achieved steady state (Fig. 1C), which indicates that IA is de-inactivated at hyperpolarized potentials, but steady-state inactivated at voltages above spike threshold.
Expiratory neurons in the preBötC are inhibited during XII motor activity but otherwise spike tonically (Fig. 2A). Their K+ currents were typically smaller overall, and only one expressed IA (compare Figs 1B and 2B, note scale bars are the same, n = 8). In current clamp, depolarizing step commands did not generally evoke a ramping depolarization from any holding potential (Fig. 2C), which is consistent with the lack of IA.
Biophysical properties of IA
We separated IA from non-inactivating K+ currents by subtraction. Using the same conditions as Figs 1B and 2B, we applied a sequence of 1 s step commands from −70 to +10 mV from a holding potential of −100 mV (Fig. 3A) and then repeated these steps from −40 mV (Fig. 3B). The difference current was defined as IA, which activated at −60 mV and its maximum amplitude exceeded 1 nA at voltages greater than 0 mV (Fig. 3C).
Twenty-one of 34 (61.7%) inspiratory neurons expressed a peak transient outward current that exceeded the steady-state outward current and decayed exponentially with a time constant greater than 15 ms, which we defined as measurable IA (Fig. 4A). This fraction of expression was significantly different from the 1/8 (12.5%) expiratory neurons found to express IA (Fisher Exact test: P = 0.015).
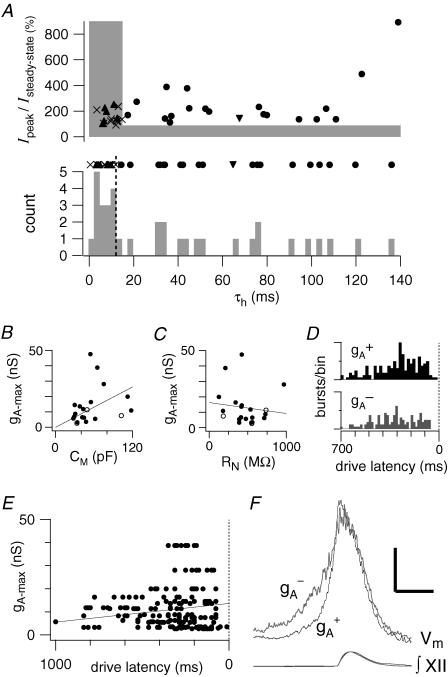
Figure 4. Whole-cell characteristics of IA in preBötC neurons.
A (top), the peak transient current normalized to the steady-state outward current plotted versus the measured whole-cell time constant of inactivation of outward currents (τh). The shaded area represents the boundary of measurements that were classified as not expressing IA. Filled circles represent measured IA-expressing inspiratory neurons, the downward pointed triangle represents the single IA-expressing expiratory neuron, the crosses represent the inspiratory neurons that did not express IA, and the upward pointing triangles represent non-IA-expressing expiratory neurons. A (middle), the data points from top panel collapsed onto the τh axis. A (bottom), a histogram of the samples from middle panel in 2.5 ms bins. Points to the right of the vertical dashed line were called IA-expressing neurons. B, the measured maximum conductance of IA (gA-max) versus CM. The diagonal line represents a fit to the data with the y-intercept at the origin. Filled circles represent data points acquired using the subtraction protocol described in the text, while open circles represent data points acquired from a holding potential of −60 mV which may underestimate gA-max. C, the gA-maxversus RN where the diagonal line is a linear fit to the data with no constraints. D, a pooled histogram showing the drive latencies of inspiratory neurons that expressed IA(gA+) or did not not (gA-) with up to 10 cycles per neuron. The maximum ordinate on each histogram is 16 bursts bin-1. E, the drive latencies from each neuron with a given gA. The line is fitted to the mean drive latencies of the pool of neurons. F, cycle-triggered averages of the membrane trajectory of inspiratory neurons with their associated XII recorded below. The vertical scale bar is 5 mV while the horizontal scale bar is 400 ms.
For inspiratory neurons expressing IA, CM measured 53.6 ± 5.9 pF (n = 21) and the difference between the onset of inspiratory-related EPSPs and the upstroke of XII activity (i.e. the drive latency) measured 305.2 ± 14.7 ms, which suggests these cells are putatively rhythmogenic (Rekling et al. 1996a; Rekling & Feldman, 1998; Feldman & Del Negro, 2006; Hayes & Del Negro, 2007). The CM of neurons expressing IA was directly related to gA (Fig. 4B). Fitting the whole-cell conductance for IA (gA) linearly with CM, with a y-intercept at zero, resulted in a slope of 0.219 ± 0.09 nS pF-1. There was no obvious relationship between RN of neurons expressing IA and gA (Fig. 4C).
The 13 of 34 (38.2%) preBötC neurons without measurable IA exhibited drive latencies of 321.7 ± 14.6 ms and CM of 44.4 ± 3.1 pF, which were indistinguishable from IA-expressing neurons (latencies: P = 0.428; CM: P = 0.181). Histograms of drive latencies for neurons with and without IA are depicted in Fig. 4D showing the substantial overlap of the variability of burst activation. The gA for neurons with IA tends to increase as the drive latency decreases (Fig. 4E, latency =–0.008 gA+ 13.6). Cycle-triggered averages (Fig. 4F) indicate that in neurons with IA (n = 16) the voltage trajectory prior to the inspiratory phase shows a more abrupt rise that is statistically different from neurons without IA (n = 8) (79.1 ± 11.6 ms versus 120.4 ± 14.4 ms, P = 0.037).
Detailed voltage-clamp analysis was precluded in whole-cell recordings because of inherent space-clamp limitations and series resistance errors attributable to large magnitude membrane currents (Armstrong et al. 1992). Therefore we studied IA in somatic outside-out patches, repeating the subtraction protocol described above with step commands that reached +30 mV (Fig. 5A). The IA activation function (see Methods) was fitted with the parameters θm=–16.3 mV and σm= 14.9 mV. Even in patches, IA generally exceeded 200 pA with a maximum conductance of 1.14 ± 0.36 nS (n = 6).
We measured the steady-state inactivation of IA at +10 mV for 500 ms following 1 s conditioning prepulses from −100 to +10 mV. The inactivation curve reaches its minimum above −40 mV and was fitted with the parameters θh = –85.6 mV and σh = –13.8 mV. These data explain why transient K+ currents can be evoked from a holding potential of −60 mV (e.g. Fig. 1B, inset); IA is not fully inactivated at that potential, h∞(–60) = 0.135 (Fig. 5B).
Over the range −30 to +30 mV, the inactivation time constant for IA was 200–300 ms and could be empirically fitted with a line in the form, τh(V) = 202–0.42 V (Fig. 5B, bottom). Interestingly, τh(V) of ∼200 ms is commensurate with both the ramping depolarization responses observed in current clamp from baseline voltages of −70 mV (e.g. Fig.1C) and the transient ramp-like depolarization seen during endogenous network activity (e.g. Fig. 1A), suggesting the involvement of IA in these membrane behaviours.
We computed the expected time course of IA for the neuron in Fig. 1A to ascertain if and when the current would be active during the respiratory cycle (Fig. 6A). We used gA of 11.7 nS, commensurate with the average maximum conductance, the chord conductance equation IA=gA*mA(∞)*hA*(V – EK), the equation for inactivation gating (dhA/dt), and empirically determined voltage dependence and kinetics (i.e. Fig. 5B). As the neuron begins to initiate the inspiratory burst, IA rapidly achieves ∼60 pA, then diminishes throughout the burst and measures ∼40 pA at burst termination (Fig. 6B). This illustrates that IA provides hyperpolarizing current during the burst with a particularly large influence at burst onset.
IA exhibits window current between −60 and −40 mV that peaks at −52.2 mV with 0.6% of the maximum current active at steady state. This suggests that IA does not substantially influence the baseline membrane potential during the majority of the quiescent phase of network activity but nonetheless resides in a sufficiently de-inactivated state that it can be rapidly evoked by depolarization; hA(∞) is less than 0.2 through the expiratory phase (Fig. 6C). This is supported by the simulation where only 1–2 pA of IA flows during the inter-inspiratory burst interval, yet IA rapidly exceeds 50 pA at burst onset (Fig. 6B). Since voltage-clamp measurements may be subject to error, we examined whether disparities in the activation and inactivation curves could influence IA. We shifted the half-activation and half-inactivation 10 mV in both the positive and negative direction and re-ran the simulations. When both curves were shifted, peak current was linearly related to the magnitude of the shift with a 60% increase for a +10 mV shift and a 42% decrease for a −10 mV shift, while the time course of IA did not change. Regardless of shifts in voltage dependence, less than 2 pA of IA flowed during the inter-inspiratory burst interval. These data suggest that IA may play a role in inspiratory burst dynamics primarily as a result of the large conductance and its availability for activation, but IA has little influence during the interburst membrane trajectory.
To test the role of IA in the preBötC we first had to understand its pharmacology. We performed dose–response experiments with 4-aminopyridine (4-AP) in outside-out patches (Fig. 7B, n = 13). We measured the change in peak transient outward current due to 4-AP and the change in the total area of the transient outward component. The IC50 for the peak response was 2.0 mm, which is close to the IC50 of most A-currents (Rogawski, 1985). The IC50 for the total area was 0.8 mm, while approximately 20% of the transient outward current could not be blocked by even saturating doses of 4-AP.
4-AP (2 mm) substantially attenuated IA in outside-out patches (Fig. 7A); 4-AP likewise attenuated IA in whole-cell recordings (n = 4), as previously shown (Inyushkin, 2005). Interestingly, non-inactivating outward currents evoked from the holding potential −40 mV were unaffected by 4-AP. We computed the 4-AP-sensitive current not attributable to IA by subtracting the current evoked at +30 mV (from a holding potential of −40 mV) in control and 4-AP conditions, which measured < 25 pA. In contrast, the 4-AP-sensitive IA regularly exceeded 200 pA (Fig. 7A), where IA was defined as the subtracted current at +30 mV following prepulse holding potentials of −100 mV and −40 mV (Fig. 3). These data indicate that 4-AP attenuates IA but does not affect sustained outward currents at baseline membrane potentials (e.g. channels related to the KCNQ subfamily). Consistent with blockade of IA, delayed excitation in whole-cell current clamp, evoked by 500 ms current steps in the interval between XII discharge from a holding potential less than −70 mV, was also abolished by 4-AP (Fig. 7C). We also analysed the effects of 4-AP on pulse-evoked spikes (Fig. 7D). In 189 spikes of 4 neurons tested at rheobase, 2 mm 4-AP significantly increased the width at half-maximum of spikes 177 ± 26% from 2.7 ± 0.1 to 4.8 ± 0.6 ms (P = 0.031) and the area of the spike to 137 ± 13% from 304.7 ± 18.8 to 416.9 ± 23.2 mV·ms (P = 0.023). Some of this effect may have been attributable to attenuation of IA (see simulated time-course and magnitude of IA in Fig. 7D), but is more likely due to 4-AP effects on other channels in the Kv subfamily, which give rise to currents with delayed rectifier-like properties in addition to IA.
Since IA is expressed in more than half of the inspiratory preBötC neurons we recorded, in which it is available at typical baseline membrane potentials and generates large magnitude outward currents lasting several hundred milliseconds (Figs 1 and 3–7), we posited that IA could influence rhythmogenesis.
4-AP affects rhythmic activity in the preBötC
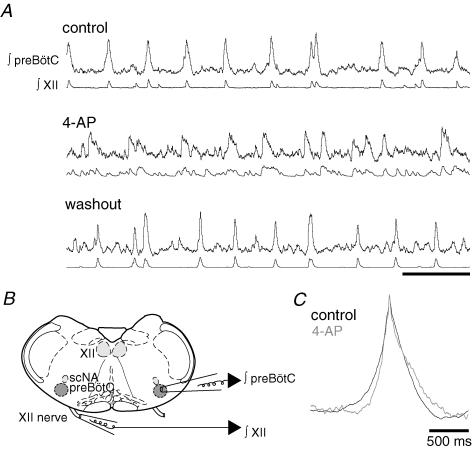
We used 4-AP sensitivity to test the role of IA in rhythm generation. In the context of respiratory network activity 4-AP caused erratic XII output that could not be straightforwardly interpreted. Therefore, we sought to determine whether the disorganized XII activity reflected a breakdown in rhythmogenesis by performing field recordings within the preBötC while recording the contralateral XII activity (Fig. 8B). The preBötC and XII activity patterns were coherent and recognizably rhythmic in control and washout, whereas 4-AP caused noisy preBötC rhythms that fluctuated in amplitude and period, and did not always match the output of the XII channel (Fig. 8A, n = 4). Under these conditions, the average rise time of the inspiratory activity within the preBötC decreased significantly in the presence of 4-AP from 141.3 ± 5.14 to 86.3 ± 8.8 ms (P = 0.016), while the falling slope did not change (P = 0.760, Fig. 8C).
Figure 8. Effects of 4-AP on the preBötC network.
A, ∫preBötC (top traces) and ∫XII (bottom traces) under control conditions, in the presence of 2 mm 4-AP and washout. Scale bar is 5 s. B, cartoon showing the configuration of the preBötC field-recording pipette (∫preBötC) and ∫XII suction electrode in A and C. The subcompact division of the nucleus ambiguus is indicated by scNA, and the hypoglossal motonucleus is indicated by XII. C, cycle-triggered averages of the field preBötC recordings under control conditions and in 2 mm 4-AP (n = 4).
As 4-AP perturbed rhythmic activity in preBötC field recordings we sought to determine the cellular basis for population-level fluctuations via whole-cell recordings and analyses (Figs 9 and 10).
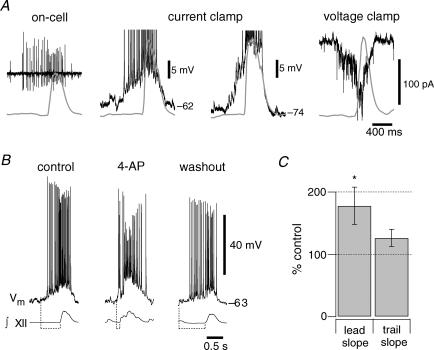
Figure 9. IA affects the discharge pattern of preBötC neurons.
A, on-cell activity of a preBötC neuron with early inspiratory spiking relative to the ∫XII. Current-clamp recording at 0 pA holding current illustrating similar activity as observed on-cell, i.e. early inspiratory spiking relative to XII output. Current-clamp recording at −40 pA holding current reveals temporal summation of EPSPs, beginning 400 ms prior to XII output. Voltage-clamp recording at a holding potential of −60 mV shows temporal summation of EPSCs prior to XII output. Baseline current was −25 pA. B, the burst discharge pattern changes from predominantly incremental (left) to decremental (middle) and back to incremental in washout (right). All recordings were at 0 pA bias current. Dashed lines at the bottom indicate the change in drive latency for each inspiratory burst illustrated. Traces in A, B and 7C were recorded in the same neuron. C, summary of the effects of 4-AP on the leading and trailing slope of inspiratory bursts (n = 8). Significance at P < 0.05 is shown with an asterisk.
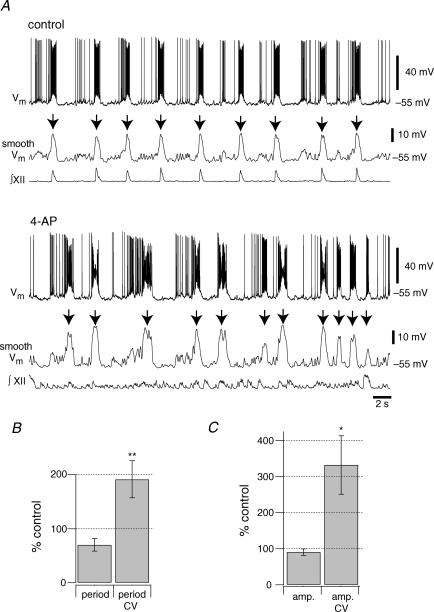
Figure 10. Effects of 4-AP on the period and amplitude of inspiratory bursts.
A, current-clamp recordings of an inspiratory neuron in control and 2 mm 4-AP. All recordings were at 0 pA bias current. Smooth Vm denotes traces conditioned by a 5 Hz low-pass filter subsequently used to select inspiratory burst events (indicated by down arrows) and to measure their period and amplitude for the analyses in B and C. B, the mean change in burst period and coefficient of variation (n = 8). C, the mean change in burst amplitude and coefficient of variation (n = 8). Significance at P < 0.05 and P < 0.01 are shown with single and double asterisks, respectively.
The depolarizing activity occurring 400 ms (or more) prior to the inspiratory burst is largely synaptically driven, as we showed previously (Hayes & Del Negro, 2007). This early inspiratory activity can be observed in the on-cell recording configuration prior to whole-cell (Fig. 9A). In current clamp at baseline membrane potential (approximately −60 mV) the ascending ramp-like trajectory is accompanied by vigorous spiking. Hyperpolarizing the membrane potential reveals the temporal summation of EPSPs in the early inspiratory phase, which are also well resolved as summating EPSCs in voltage clamp (Fig. 9A, left to right). These data emphasize that a rapidly activating K+ current, which is de-inactivated at baseline voltages and subject to temporally summating synaptic inputs, may be uniquely poised to influence the dynamics of the inspiratory burst pattern.
We tested this idea by applying 2 mm 4-AP and measuring its effects on inspiratory bursts (Fig. 9B and C). The pattern changed from incrementing in control to decrementing in the presence of 4-AP (Fig. 9B). The leading slope of the inspiratory burst changed significantly in 4-AP from 53.1 ± 7.4 to 80.2 ± 5.3 mV · s-1 (P = 0.032, n = 8, Fig. 9C). However, the trailing slope did not change significantly: −52.7 ± 2.5 mV s-1 in control versus−65.5 ± 6.0 mV s-1 in 4-AP (P = 0.095, n = 8, Fig. 9C). These general features are consistent with the average field-recording data analysed in Fig. 8C.
These data suggest that 4-AP removed a hyperpolarizing current that normally influenced the onset of the inspiratory burst, but its influence diminished during the inspiratory burst, which is consistent with the role of IA we predicted (Fig. 6). Moreover, 4-AP did not change the baseline membrane (bias current is 0 pA in Figs 9B and 10A), which is consistent with the lack of significant window current measured in voltage clamp (see Fig. 5B) as well as the predictions of our simulation (Fig. 6B).
We also analysed the effect of 4-AP on drive potential amplitude and its coefficient of variation (CV) as well as respiratory cycle period and its CV. A representative experiment illustrates that burst sizes became more variable and that the timing of the neuronal activity became more variable (Fig. 10A). We applied a 5 Hz low-pass filter to the voltage trajectory, which filters out spikes and facilitates measurements of the underlying inspiratory drive potentials (Pace et al. 2007b). The amplitude and period of inspiratory activity fluctuated in 4-AP. The average period of drive potentials did not change significantly between control and 4-AP application (P = 0.052) while the CV for period approximately doubled from 0.32 ± 0.05 to 0.52 ± 0.03, which was statistically significant (P = 0.006, n = 8, Fig. 10B). The average amplitude of the drive potential did not significantly change (P = 0.408) but the CV for amplitude changed significantly from 0.26 ± 0.06 to 0.58 0.08 (P = 0.015, n = 8, Fig. 10C), which suggests that IA influences the regularity of both the cycle period and the drive potential magnitude in preBötC neurons.
Discussion
We studied biophysical properties of IA in preBötC neurons and analysed its role in respiratory rhythm generation. IA recovers from inactivation at membrane potentials traversed during the interburst interval and remains relatively de-inactivated at baseline voltages, and thus available to be readily activated at the onset of the inspiratory phase of each respiratory cycle. IA is prevalent among inspiratory neurons while being sparse in expiratory neurons. We found that inspiratory neurons expressing IA received synaptic input at approximately the same time on average as inspiratory neurons that lack significant IA (Fig. 4D), but that the rate at which these neurons responded to network activity was slower (Fig. 4E). Blockade of IA increased the rate at which peak activity was achieved at both the network (Fig. 8C) and neuronal level (Fig. 9) which was correlated with an increase in the variability of both burst amplitude and period. Our data suggest that IA may normally slow the onset of network inspiratory activity by counteracting excitatory synaptic input until there is massive excitatory drive to overcome IA. We suggest this thereby promotes regular burst size and frequency throughout the network by suppressing spurious inputs.
The rhythm and pattern for breathing are generated in the brainstem. Neurons in the dorsal respiratory group (DRG), including the nucleus tractus solitarius (NTS) where IA has been characterized (Champagnat et al. 1986; Dekin & Getting, 1987; Dekin et al. 1987), participate in afferent feedback and autonomic regulation. The ventral respiratory group (VRG) contains a bilaterally distributed column of neurons including rhythmogenic interneurons concentrated in the preBötC as well as premotoneurons that project to cranial and spinal motoneurons to carry out breathing movements (Bianchi et al. 1995; Onimaru et al. 1997; Ballanyi et al. 1999).
The role of IA has been studied in mathematical models of respiratory networks that include the VRG and DRG, which predicted that IA could influence the initial ramping trajectory of inspiratory neurons if it were sufficiently de-inactivated during the interburst phase of the respiratory cycle (Rybak et al. 1997). However, one caveat is that this network model did not explicitly consider the dynamics of preBötC neurons as a centre of rhythm generation and subsequent models of the preBötC have not analysed the role of IA (Butera et al. 1999a,b; Del Negro et al. 2001; Rybak et al. 2003, 2004; Kosmidis et al. 2004). Our study is the first detailed characterization of IA from putatively rhythmogenic preBötC neurons, as well as the first analysis of the role of IA during endogenous respiratory network activity in vitro.
Role of IA in the preBötC in vitro
We measured the voltage dependence and kinetics of IA in somatic outside-out patches to minimize space-clamp limitations and series-resistance errors. IA activates near −60 mV and is not fully inactivated until approximately −30 mV. Because these activation and inactivation functions encompass the range of membrane potentials visited during the interval between inspiratory bursts, IA resides in a partially de-inactivated state at typical baseline membrane potentials in vitro. However, IA has little window current, and consequently 4-AP application did not depolarize preBötC neurons. These properties indicate that IA contributes little to the baseline membrane potential but can be rapidly recruited by synaptic depolarization at the onset of the inspiratory phase of the respiratory cycle (see Fig. 6).
IA often causes delayed excitation, wherein the depolarization evoked by current pulses evolves with a ramp-like trajectory lasting several hundred milliseconds (or longer) because IA activates rapidly and inactivates slowly (Hagiwara et al. 1961; Getting, 1983, 2989; Dekin & Getting, 1987; Gabel & Nisenbaum, 1998). Delayed excitation affects synaptic integration (Storm, 1988; Hoffman et al. 1997; Gulledge et al. 2005). This is particularly relevant in preBötC neurons with small CM and early drive latency that are probably rhythmogenic, in which synaptic excitation builds up over several hundred milliseconds prior to respiratory-related motor output (Rekling et al. 1996a; Rekling & Feldman, 1998; also see Fig. 1A and 9; Hayes & Del Negro, 2007). Since IA produces delayed excitation in preBötC neurons, is de-inactivated at baseline membrane potentials (Fig. 1B inset and 5B), and has a 200 ms inactivation time constant, we conclude that IA plays a major role in shaping the ramp-like incremental discharge pattern characteristic of rhythmogenic neurons. Supporting evidence for this role is the dramatic increase in the leading slope of inspiratory activity following 4-AP application (Figs 8C and 9) as well as our comparison of membrane trajectories between neurons that express IA and those that do not (Fig. 4F).
In invertebrate CPGs, IA regulates the order in which rhythmogenic neurons discharge (Getting, 1983; Tierney & Harris-Warrick, 1992). In the stomatogastric ganglion of lobster Panulirus interruptus, IA slows the frequency of anterior burster and pyloric dilator cells and delays the response to inhibition of follower lateral, early, and late pyloric cells (Tierney & Harris-Warrick, 1992). Likewise in the opistobranch mollusk Tritonia diomedea, IA causes the appropriate tail escape–swim pattern to be generated by delaying the activation of ventral swimming interneurons that receive synaptic input from cerebral cell 2 (Getting, 1983). Additionally, in the inking behavioural circuits of Aplysia californica, IA impedes ink release in the absence of substantial synaptic input to L14 neurons (Byrne et al. 1979; Byrne, 1980).
In the mammalian preBötC, network rhythms continued in the presence of 4-AP and could be measured in whole-cell and field recordings. 4-AP did not affect the mean period or amplitude of inspiratory burst-like discharges, but it did significantly increase period and amplitude variability (Fig. 10B and C). The increase in variability was correlated with the diminished ramp-like incremental discharge pattern in 4-AP. One possible explanation for the destabilization of periodic preBötC activity is that, like in Aplysia, IA delays neuronal activation until the temporal summation of excitatory synaptic input builds up sufficiently to overcome and outlast the transient K+ current. We found that the more IA is expressed in a neuron, the less the neuron is responsive to synaptic input preceding a burst (Fig. 4E and F) which is consistent with this hypothesis. However, the neurons lacking IA are not expected to reach their peak activity until the neurons expressing some degree of IA, which make up the majority of early inspiratory neurons, are also active. Thus IA would influence the orderly recruitment of rhythm-generating neurons in the build-up to the inspiratory burst and ensure that the inspiratory phase does not begin until and unless a substantial fraction of the rhythmogenic population was involved. We speculate that this role for IA would promote regularity in respiratory network behaviour, since the pharmacological attenuation of IA caused substantial fluctuations in the amplitude and period of inspiratory burst activity.
An important caveat to this assertion is that 2 mm 4-AP extends spike duration significantly (Fig. 7D), which suggests that other outward currents may be affected by 4-AP such as Ca2+-activated K+ currents (Andreasen, 2002) or delayed-rectifier currents (Rusch & Eatock, 1996). However, the trailing slope of bursts did not change significantly at the network level (Fig. 8C) or neuronal level (Fig. 9C) suggesting the contribution of any outward currents active at the end of the burst were not significantly affected by 4-AP.
The above interpretation is further complicated by the fact that other neurons contained in slice preparations are also sensitive to 4-AP. Both raphé neurons (Aghajanian, 1985) and NTS neurons (Haddad & Getting, 1989) express IA and project to preBötC neurons (Al-Zubaidy et al. 1996; Blessing, 1997; Pace et al. 2007b). These neurons may influence the preBötC by providing spurious excitatory or inhibitory input in 4-AP, but given that the slope of inspiratory activity changes in field recordings and in intracellular recordings, which is expected with a blockade of IA, we are confident that at least some of this modulation is due to direct effects on rhythmogenic preBötC neurons. It is also unlikely that expiratory neurons in the preBötC that project to inspiratory cells can explain the effects of 4-AP on network activity because they did not express significant IA.
Raphé and NTS neurons also project to hypoglossal motoneurons (Rekling et al. 2000). If these projection neurons are the same ones that have been shown to express IA (Champagnat et al. 1986; Dekin & Getting, 1987; Dekin et al. 1987) then their response to 4-AP could further contribute to the very erratic XII discharge we observed. Evidence in support of this notion is that 4-AP is known to substantially increase spontaneous synaptic input to XII motoneurons and that IA in XII motoneurons is relatively 4-AP-insensitive, fast-inactivating and mainly involved in spike repolarization (Haddad et al. 1990; Viana et al. 1993; Lape & Nistri, 1999).
Molecular identity of preBötC IA
IA in preBötC neurons shares similar voltage sensitivity and kinetic properties as members of the Shal-family (Kv4) K+ channels (Pak et al. 1991). In particular, mammalian Kv4.1 and Kv4.3 channels expressed in Xenopus oocytes (Serodio et al. 1994, 1996) closely match the IA that we recorded in the preBötC. Kv4.1 subunits are sparsely expressed in mammalian brain tissue while Kv4.3 is widespread (Trimmer & Rhodes, 2004) and has voltage-independent kinetics (Serodio et al. 1996). This suggests that Kv4.3 expression may give rise to at least some of the IA in preBötC neurons. Kv4.2 is also a good candidate because it is present in the nearby raphé neurons (Serodio & Rudy, 1998). Any of these, or other, Kv subunits could potentially make up preBötC IA because the voltage dependence and kinetics could be influenced by Kv channel interacting proteins (An et al. 2000; Rhodes et al. 2004) or neuromodulation (Birnbaum et al. 2004). However, definitive molecular level identification will require single-cell reverse transcriptase polymerase chain reaction experiments or neuroanatomical approaches.
In addition to IA, some of the K+ current affected by 4-AP probably includes voltage-gated K+ channels with delayed rectifier-like properties that derive from the Kv subfamily, such as Kv2.x and Kv3.x subtypes, because 4-AP extended action potential duration. However, the 4-AP-sensitive current is unlikely to include non-inactivating K+ currents from the KCNQ subfamily, since we found no 4-AP-sensitive sustained currents from the relatively depolarized holding potential of −40 mV.
The prevalence of IA in rhythmogenic preBötC neurons
To identify inspiratory neurons in the preBötC that may be important for rhythm generation Rekling et al. (1996a) measured the drive latency and proposed that the earliest neurons to activate during the respiratory cycle are important for rhythmogenesis. Furthermore, we recently showed that membrane capacitance (CM) of 30–65 pF is correlated with early drive latency in preBötC neurons that most likely play the foremost role in rhythmogenesis (Hayes & Del Negro, 2007). Since we found that the majority, but not all, putatively rhythmogenic neurons express somatic IA based on these criteria, our data suggest that rhythmogenic neurons do not uniformly express IA as originally suggested (Rekling et al. 1996a). However, we cannot exclude the possibility that many of the neurons we recorded may have expressed unrecognized dendritically localized IA as our measurements strictly depend on the quality of our somatic voltage clamp.
Since an A-current as we have described is generally lacking in expiratory neurons, but prevalent in inspiratory neurons, we hypothesize that the molecular identity of channels that give rise to IA may be a unique functional marker for putatively rhythmogenic neurons within the preBötC.
Role of IAin vivo
In the context of endogenous network activity in vitro EK is approximately −71 mV, while in vivo it is probably closer to −98 mV – assuming 3.2 mm[K+] in the cerebrospinal fluid (Melton et al. 1991; cf. Richter et al. 1978). We therefore expect higher driving force for IAin vivo, and thus the window current would have a larger hyperpolarizing influence on baseline membrane potential. This could further de-inactivate IA between inspiratory bursts, compared to what we observe in vitro. In this environment, where inspiratory neurons are expected to be under intensive bombardment of excitatory and inhibitory input, IA may play an even more substantial role in properly synchronizing respiratory rhythmic activity through its ability to rapidly activate with a large outward current and quench spurious depolarizations.
Acknowledgments
This work was supported by the National Science Foundation, Integrative and Organismal Biology Award no. 0616099 (Arlington, VA, USA), The Suzann Wilson Matthews Faculty Research Award (The College of William and Mary), and The Jeffress Memorial Trust (Richmond, Virginia, USA). J.A.H. and B.R.B. were funded (in part) by the postdoctoral and undergraduate biological sciences education program grant awarded to The College of William and Mary by the Howard Hughes Medical Institute.
References
- Aghajanian GK. Modulation of a transient outward current in serotonergic neurones by α1-adrenoceptors. Nature. 1985;315:501–503. doi: 10.1038/315501a0. [DOI] [PubMed] [Google Scholar]
- Al-Zubaidy ZA, Erickson RL, Greer JJ. Serotonergic and noradrenergic effects on respiratory neural discharge in the medullary slice preparation of neonatal rats. Pflugers Arch. 1996;431:942–949. doi: 10.1007/s004240050089. [DOI] [PubMed] [Google Scholar]
- An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, Hinson JW, Mattsson KI, Strassle BW, Trimmer JS, Rhodes KJ. Modulation of A-type potassium channels by a family of calcium sensors. Nature. 2000;403:553–556. doi: 10.1038/35000592. [DOI] [PubMed] [Google Scholar]
- Andreasen M. Inhibition of slow Ca2+-activated K+ current by 4-aminopyridine in rat hippocampal CA1 pyramidal neurones. Br J Pharmacol. 2002;135:1013–1025. doi: 10.1038/sj.bjp.0704533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong CM, Gilly WF, Bernardo R. Methods in Enzymology. New York: Academic Press; 1992. Access resistance and space clamp problems associated with whole-cell patch clamping; pp. 100–122. [DOI] [PubMed] [Google Scholar]
- Ballanyi K, Onimaru H, Homma I. Respiratory network function in the isolated brainstem-spinal cord of newborn rats. Prog Neurobiol. 1999;59:583–634. doi: 10.1016/s0301-0082(99)00009-x. [DOI] [PubMed] [Google Scholar]
- Bianchi AL, Denavit-Saubie M, Champagnat J. Central control of breathing in mammals: neuronal circuitry, membrane properties, and neurotransmitters. Physiol Rev. 1995;75:1–45. doi: 10.1152/physrev.1995.75.1.1. [DOI] [PubMed] [Google Scholar]
- Birnbaum SG, Varga AW, Yuan LL, Anderson AE, Sweatt JD, Schrader LA. Structure and function of Kv4-family transient potassium channels. Physiol Rev. 2004;84:803–833. doi: 10.1152/physrev.00039.2003. [DOI] [PubMed] [Google Scholar]
- Blessing WW. The Lower Brainstem and Bodily Homeostasis. New York: Oxford University Press; 1997. [Google Scholar]
- Brockhaus J, Ballanyi K. Synaptic inhibition in the isolated respiratory network of neonatal rats. Eur J Neurosci. 1998;10:3823–3839. doi: 10.1046/j.1460-9568.1998.00396.x. [DOI] [PubMed] [Google Scholar]
- Butera RJ, Jr, Rinzel J, Smith JC. Models of respiratory rhythm generation in the pre-Bötzinger complex. I. Bursting pacemaker neurons. J Neurophysiol. 1999a;82:382–397. doi: 10.1152/jn.1999.82.1.382. [DOI] [PubMed] [Google Scholar]
- Butera RJ, Jr, Rinzel J, Smith JC. Models of respiratory rhythm generation in the pre-Bötzinger complex. II. Populations of coupled pacemaker neurons. J Neurophysiol. 1999b;82:398–415. doi: 10.1152/jn.1999.82.1.398. [DOI] [PubMed] [Google Scholar]
- Byrne JH. Quantitative aspects of ionic conductance mechanisms contributing to firing pattern of motor cells mediating inking behavior in Aplysia californica. J Neurophysiol. 1980;43:651–668. doi: 10.1152/jn.1980.43.3.651. [DOI] [PubMed] [Google Scholar]
- Byrne JH, Shapiro E, Dieringer N, Koester J. Biophysical mechanisms contributing to inking behavior in Aplysia. J Neurophysiol. 1979;42:1233–1250. doi: 10.1152/jn.1979.42.5.1233. [DOI] [PubMed] [Google Scholar]
- Champagnat J, Jacquin T, Richter DW. Voltage-dependent currents in neurones of the nuclei of the solitary tract of rat brainstem slices. Pflugers Arch. 1986;406:372–379. doi: 10.1007/BF00590939. [DOI] [PubMed] [Google Scholar]
- Dekin MS, Getting PA. In vitro characterization of neurons in the ventral part of the nucleus tractus solitarius. II. Ionic basis for repetitive firing patterns. J Neurophysiol. 1987;58:215–229. doi: 10.1152/jn.1987.58.1.215. [DOI] [PubMed] [Google Scholar]
- Dekin MS, Getting PA, Johnson SM. In vitro characterization of neurons in the ventral part of the nucleus tractus solitarius. I. Identification of neuronal types and repetitive firing properties. J Neurophysiol. 1987;58:195–214. doi: 10.1152/jn.1987.58.1.195. [DOI] [PubMed] [Google Scholar]
- Del Negro CA, Johnson SM, Butera RJ, Smith JC. Models of respiratory rhythm generation in the pre-Bötzinger complex. III. Experimental tests of model predictions. J Neurophysiol. 2001;86:59–74. doi: 10.1152/jn.2001.86.1.59. [DOI] [PubMed] [Google Scholar]
- Del Negro CA, Koshiya N, Butera RJ, Jr, Smith JC. Persistent sodium current, membrane properties and bursting behavior of pre-Bötzinger complex inspiratory neurons in vitro. J Neurophysiol. 2002a;88:2242–2250. doi: 10.1152/jn.00081.2002. [DOI] [PubMed] [Google Scholar]
- Del Negro CA, Morgado-Valle C, Feldman JL. Respiratory rhythm: an emergent network property? Neuron. 2002b;34:821–830. doi: 10.1016/s0896-6273(02)00712-2. [DOI] [PubMed] [Google Scholar]
- Del Negro CA, Morgado-Valle C, Hayes JA, Mackay DD, Pace RW, Crowder EA, Feldman JL. Sodium and calcium current-mediated pacemaker neurons and respiratory rhythm generation. J Neurosci. 2005;25:446–453. doi: 10.1523/JNEUROSCI.2237-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman JL, Del Negro CA. Looking for inspiration: new perspectives on respiratory rhythm. Nat Rev Neurosci. 2006;7:232. doi: 10.1038/nrn1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk GD, Smith JC, Feldman JL. Generation and transmission of respiratory oscillations in medullary slices: role of excitatory amino acids. J Neurophysiol. 1993;70:1497–1515. doi: 10.1152/jn.1993.70.4.1497. [DOI] [PubMed] [Google Scholar]
- Funk GD, Smith JC, Feldman JL. Modulation of neural network activity in vitro by cyclothiazide, a drug that blocks desensitization of AMPA receptors. J Neurosci. 1995;15:4046–4056. doi: 10.1523/JNEUROSCI.15-05-04046.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabel LA, Nisenbaum ES. Biophysical characterization and functional consequences of a slowly inactivating potassium current in neostriatal neurons. J Neurophysiol. 1998;79:1989–2002. doi: 10.1152/jn.1998.79.4.1989. [DOI] [PubMed] [Google Scholar]
- Getting PA. Mechanisms of pattern generation underlying swimming in Tritonia. III. Intrinsic and synaptic mechanisms for delayed excitation. J Neurophysiol. 1983;49:1036–1050. doi: 10.1152/jn.1983.49.4.1036. [DOI] [PubMed] [Google Scholar]
- Getting PA. Emerging principles governing the operation of neural networks. Annu Rev Neurosci. 1989;12:185–204. doi: 10.1146/annurev.ne.12.030189.001153. [DOI] [PubMed] [Google Scholar]
- Gray PA, Rekling JC, Bocchiaro CM, Feldman JL. Modulation of respiratory frequency by peptidergic input to rhythmogenic neurons in the preBötzinger complex. Science. 1999;286:1566–1568. doi: 10.1126/science.286.5444.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greer JJ, Smith JC, Feldman JL. Role of excitatory amino acids in the generation and transmission of respiratory drive in neonatal rat. J Physiol. 1991;437:727–749. doi: 10.1113/jphysiol.1991.sp018622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gulledge AT, Kampa BM, Stuart GJ. Synaptic integration in dendritic trees. J Neurobiol. 2005;64:75–90. doi: 10.1002/neu.20144. [DOI] [PubMed] [Google Scholar]
- Haddad GG, Donnelly DF, Getting PA. Biophysical properties of hypoglossal neurons in vitro: intracellular studies in adult and neonatal rats. J Appl Physiol. 1990;69:1509–1517. doi: 10.1152/jappl.1990.69.4.1509. [DOI] [PubMed] [Google Scholar]
- Haddad GG, Getting PA. Repetitive firing properties of neurons in the ventral region of nucleus tractus solitarius. In vitro studies in adult and neonatal rat. J Neurophysiol. 1989;62:1213–1224. doi: 10.1152/jn.1989.62.6.1213. [DOI] [PubMed] [Google Scholar]
- Hagiwara S, Kusano K, Saito N. Membrane changes of Onchidium nerve cell in potassium-rich media. J Physiol. 1961;155:470–489. doi: 10.1113/jphysiol.1961.sp006640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller M, Mironov SL, Karschin A, Richter DW. Dynamic activation of KATP channels in rhythmically active neurons. J Physiol. 2001a;537:69–81. doi: 10.1111/j.1469-7793.2001.0069k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haller M, Mironov SL, Richter DW. Intrinsic optical signals in respiratory brain stem regions of mice: neurotransmitters, neuromodulators, and metabolic stress. J Neurophysiol. 2001b;86:412–421. doi: 10.1152/jn.2001.86.1.412. [DOI] [PubMed] [Google Scholar]
- Hayes JA, Del Negro CA. Neurokinin receptor-expressing pre-Bötzinger complex neurons in neonatal mice studied in vitro. J Neurophysiol. 2007;97:4215–4224. doi: 10.1152/jn.00228.2007. [DOI] [PubMed] [Google Scholar]
- Hess D, El Manira A. Characterization of a high-voltage-activated IA current with a role in spike timing and locomotor pattern generation. Proc Natl Acad Sci U S A. 2001;98:5276–5281. doi: 10.1073/pnas.091096198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman DA, Magee JC, Colbert CM, Johnston D. K+ channel regulation of signal propagation in dendrites of hippocampal pyramidal neurons. Nature. 1997;387:869–875. doi: 10.1038/43119. [DOI] [PubMed] [Google Scholar]
- Inyushkin AN. Thyroliberin blocks the potassium A-current in neurons in the respiratory center of adult rats in vitro. Neurosci Behav Physiol. 2005;35:549–554. doi: 10.1007/s11055-005-0091-4. [DOI] [PubMed] [Google Scholar]
- Johnson SM, Smith JC, Feldman JL. Modulation of respiratory rhythm in vitro: role of Gi/o protein-mediated mechanisms. J Appl Physiol. 1996;80:2120–2133. doi: 10.1152/jappl.1996.80.6.2120. [DOI] [PubMed] [Google Scholar]
- Kosmidis EK, Pierrefiche O, Vibert JF. Respiratory-like rhythmic activity can be produced by an excitatory network of non-pacemaker neuron models. J Neurophysiol. 2004;92:686–699. doi: 10.1152/jn.00046.2004. [DOI] [PubMed] [Google Scholar]
- Lape R, Nistri A. Voltage-activated K+ currents of hypoglossal motoneurons in a brain stem slice preparation from the neonatal rat. J Neurophysiol. 1999;81:140–148. doi: 10.1152/jn.1999.81.1.140. [DOI] [PubMed] [Google Scholar]
- Marder E. Moving rhythms. Nature. 2001;410:755. doi: 10.1038/35071196. [DOI] [PubMed] [Google Scholar]
- Melton JE, Chae LO, Neubauer JA, Edelman NH. Extracellular potassium homeostasis in the cat medulla during progressive brain hypoxia. J Appl Physiol. 1991;70:1477–1482. doi: 10.1152/jappl.1991.70.4.1477. [DOI] [PubMed] [Google Scholar]
- Mironov SL, Langohr K, Haller M, Richter DW. Hypoxia activates ATP-dependent potassium channels in inspiratory neurones of neonatal mice. J Physiol. 1998;509:755–766. doi: 10.1111/j.1469-7793.1998.755bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironov SL, Langohr K, Richter DW. A1 adenosine receptors modulate respiratory activity of the neonatal mouse via the cAMP-mediated signaling pathway. J Neurophysiol. 1999;81:247–255. doi: 10.1152/jn.1999.81.1.247. [DOI] [PubMed] [Google Scholar]
- Mironov SL, Langohr K, Richter DW. Hyperpolarization-activated current, Ih, in inspiratory brainstem neurons and its inhibition by hypoxia. Eur J Neurosci. 2000;12:520–526. doi: 10.1046/j.1460-9568.2000.00928.x. [DOI] [PubMed] [Google Scholar]
- Mironov SL, Richter DW. L-type Ca2+ channels in inspiratory neurones of mice and their modulation by hypoxia. J Physiol. 1998;512:75–87. doi: 10.1111/j.1469-7793.1998.075bf.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mironov SL, Richter DW. Intracellular signalling pathways modulate KATP channels in inspiratory brainstem neurones and their hypoxic activation: involvement of metabotropic receptors, G-proteins and cytoskeleton. Brain Res. 2000;853:60–67. doi: 10.1016/s0006-8993(99)02234-9. [DOI] [PubMed] [Google Scholar]
- Mironov SL, Richter DW. Oscillations and hypoxic changes of mitochondrial variables in neurons of the brainstem respiratory centre of mice. J Physiol. 2001;533:227–236. doi: 10.1111/j.1469-7793.2001.0227b.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisenbaum ES, Xu ZC, Wilson CJ. Contribution of a slowly inactivating potassium current to the transition to firing of neostriatal spiny projection neurons. J Neurophysiol. 1994;71:1174–1189. doi: 10.1152/jn.1994.71.3.1174. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Arata A, Homma I. Neuronal mechanisms of respiratory rhythm generation: an approach using in vitro preparation. Jpn J Physiol. 1997;47:385–403. doi: 10.2170/jjphysiol.47.385. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Ballanyi K, Homma I. Contribution of Ca2+-dependent conductances to membrane potential fluctuations of medullary respiratory neurons of newborn rats in vitro. J Physiol. 2003;552:727–741. doi: 10.1113/jphysiol.2003.049312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onimaru H, Kumagawa Y, Homma I. Respiration-related rhythmic activity in the rostral medulla of newborn rats. J Neurophysiol. 2006;96:55–61. doi: 10.1152/jn.01175.2005. [DOI] [PubMed] [Google Scholar]
- Onimaru H, Shamoto A, Homma I. Modulation of respiratory rhythm by 5-HT in the brainstem-spinal cord preparation from newborn rat. Pflugers Arch. 1998;435:485–494. doi: 10.1007/s004240050543. [DOI] [PubMed] [Google Scholar]
- Paarmann I, Frermann D, Keller BU, Villmann C, Breitinger HG, Hollmann M. Kinetics and subunit composition of NMDA receptors in respiratory-related neurons. J Neurochem. 2005;93:812–824. doi: 10.1111/j.1471-4159.2005.03027.x. [DOI] [PubMed] [Google Scholar]
- Pace RW, Mackay DD, Feldman JL, Del Negro CA. Inspiratory bursts in the preBötzinger complex depend on a calcium-activated non-specific cation current linked to glutamate receptors in neonatal mice. J Physiol. 2007a;582:113–125. doi: 10.1113/jphysiol.2007.133660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pace RW, Mackay DD, Feldman JL, Del Negro CA. Role of persistent sodium current in mouse preBötzinger complex neurons and respiratory rhythm generation. J Physiol. 2007b;580:485–496. doi: 10.1113/jphysiol.2006.124602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pak MD, Baker K, Covarrubias M, Butler A, Ratcliffe A, Salkoff L. mShal, a subfamily of A-type K+ channel cloned from mammalian brain. Proc Natl Acad Sci U S A. 1991;88:4386–4390. doi: 10.1073/pnas.88.10.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena F, Parkis MA, Tryba AK, Ramirez J-M. Differential contribution of pacemaker properties to the generation of respiratory rhythms during normoxia and hypoxia. Neuron. 2004;43:105. doi: 10.1016/j.neuron.2004.06.023. [DOI] [PubMed] [Google Scholar]
- Pena F, Ramirez JM. Endogenous activation of serotonin-2A receptors is required for respiratory rhythm generation in vitro. J Neurosci. 2002;22:11055–11064. doi: 10.1523/JNEUROSCI.22-24-11055.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pena F, Ramirez JM. Substance P-mediated modulation of pacemaker properties in the mammalian respiratory network. J Neurosci. 2004;24:7549–7556. doi: 10.1523/JNEUROSCI.1871-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrefiche O, Bischoff AM, Richter DW. ATP-sensitive K+ channels are functional in expiratory neurones of normoxic cats. J Physiol. 1996;494:399–409. doi: 10.1113/jphysiol.1996.sp021501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierrefiche O, Haji A, Bischoff A, Richter DW. Calcium currents in respiratory neurons of the cat in vivo. Pflugers Arch. 1999;438:817–826. doi: 10.1007/s004249900090. [DOI] [PubMed] [Google Scholar]
- Pierrefiche O, Schwarzacher SW, Bischoff AM, Richter DW. Blockade of synaptic inhibition within the pre-Bötzinger complex in the cat suppresses respiratory rhythm generation in vivo. J Physiol. 1998;509:245–254. doi: 10.1111/j.1469-7793.1998.245bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ptak K, Zummo GG, Alheid GF, Tkatch T, Surmeier DJ, McCrimmon DR. Sodium currents in medullary neurons isolated from the pre-Bötzinger complex region. J Neurosci. 2005;25:5159–5170. doi: 10.1523/JNEUROSCI.4238-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rekling JC, Champagnat J, Denavit-Saubie M. Electroresponsive properties and membrane potential trajectories of three types of inspiratory neurons in the newborn mouse brain stem in vitro. J Neurophysiol. 1996a;75:795–810. doi: 10.1152/jn.1996.75.2.795. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Champagnat J, Denavit-Saubie M. Thyrotropin-releasing hormone (TRH) depolarizes a subset of inspiratory neurons in the newborn mouse brain stem in vitro. J Neurophysiol. 1996b;75:811–819. doi: 10.1152/jn.1996.75.2.811. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Feldman JL. PreBötzinger complex and pacemaker neurons: hypothesized site and kernel for respiratory rhythm generation. Annu Rev Physiol. 1998;60:385–405. doi: 10.1146/annurev.physiol.60.1.385. [DOI] [PubMed] [Google Scholar]
- Rekling JC, Funk GD, Bayliss DA, Dong XW, Feldman JL. Synaptic control of motoneuronal excitability. Physiol Rev. 2000;80:767–852. doi: 10.1152/physrev.2000.80.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes KJ, Carroll KI, Sung MA, Doliveira LC, Monaghan MM, Burke SL, Strassle BW, Buchwalder L, Menegola M, Cao J, An WF, Trimmer JS. KChIPs and Kv4 α subunits as integral components of A-type potassium channels in mammalian brain. J Neurosci. 2004;24:7903–7915. doi: 10.1523/JNEUROSCI.0776-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter DW, Camerer H, Sonnhof U. Changes in extracellular potassium during the spontaneous activity of medullary respiratory neurones. Pflugers Arch. 1978;376:139–149. doi: 10.1007/BF00581577. [DOI] [PubMed] [Google Scholar]
- Richter DW, Spyer KM. Studying rhythmogenesis of breathing: comparison of in vivo and in vitro models. Trends Neurosci. 2001;24:464–472. doi: 10.1016/s0166-2236(00)01867-1. [DOI] [PubMed] [Google Scholar]
- Rogawski MA. The A-current: how ubiquitous a feature of excitable cells is it? Trends Neurosci. 1985;8:214–219. [Google Scholar]
- Ruangkittisakul A, Schwarzacher SW, Secchia L, Poon BY, Ma Y, Funk GD, Ballanyi K. High sensitivity to neuromodulator-activated signaling pathways at physiological [K+] of confocally imaged respiratory center neurons in on-line-calibrated newborn rat brainstem slices. J Neurosci. 2006;26:11870–11880. doi: 10.1523/JNEUROSCI.3357-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusch A, Eatock RA. A delayed rectifier conductance in type I hair cells of the mouse utricle. J Neurophysiol. 1996;76:995–1004. doi: 10.1152/jn.1996.76.2.995. [DOI] [PubMed] [Google Scholar]
- Rybak IA, Paton JF, Schwaber JS. Modeling neural mechanisms for genesis of respiratory rhythm and pattern. I. Models of respiratory neurons. J Neurophysiol. 1997;77:1994–2006. doi: 10.1152/jn.1997.77.4.1994. [DOI] [PubMed] [Google Scholar]
- Rybak IA, Shevtsova NA, Ptak K, McCrimmon DR. Intrinsic bursting activity in the pre-Bötzinger complex: role of persistent sodium and potassium currents. Biol Cybern. 2004;90:59–74. doi: 10.1007/s00422-003-0447-1. [DOI] [PubMed] [Google Scholar]
- Rybak IA, Shevtsova NA, St-John WM, Paton JF, Pierrefiche O. Endogenous rhythm generation in the pre-Bötzinger complex and ionic currents: modelling and in vitro studies. Eur J Neurosci. 2003;18:239–257. doi: 10.1046/j.1460-9568.2003.02739.x. [DOI] [PubMed] [Google Scholar]
- Serodio P, Kentros C, Rudy B. Identification of molecular components of A-type channels activating at subthreshold potentials. J Neurophysiol. 1994;72:1516–1529. doi: 10.1152/jn.1994.72.4.1516. [DOI] [PubMed] [Google Scholar]
- Serodio P, Rudy B. Differential expression of Kv4 K+ channel subunits mediating subthreshold transient K+ (A-type) currents in rat brain. J Neurophysiol. 1998;79:1081–1091. doi: 10.1152/jn.1998.79.2.1081. [DOI] [PubMed] [Google Scholar]
- Serodio P, Vega-Saenz de Miera E, Rudy B. Cloning of a novel component of A-type K+ channels operating at subthreshold potentials with unique expression in heart and brain. J Neurophysiol. 1996;75:2174–2179. doi: 10.1152/jn.1996.75.5.2174. [DOI] [PubMed] [Google Scholar]
- Shao XM, Feldman JL. Acetylcholine modulates respiratory pattern: effects mediated by M3-like receptors in preBötzinger complex inspiratory neurons. J Neurophysiol. 2000;83:1243–1252. doi: 10.1152/jn.2000.83.3.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao XM, Ge Q, Feldman JL. Modulation of AMPA receptors by cAMP-dependent protein kinase in preBötzinger complex inspiratory neurons regulates respiratory rhythm in the rat. J Physiol. 2003;547:543–553. doi: 10.1113/jphysiol.2002.031005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JC, Ellenberger HH, Ballanyi K, Richter DW, Feldman JL. Pre-Bötzinger complex: a brainstem region that may generate respiratory rhythm in mammals. Science. 1991;254:726–729. doi: 10.1126/science.1683005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein PSG. Neurons, Networks, and Motor Behavior. Cambridge: MIT Press; 1997. [Google Scholar]
- Storm JF. Temporal integration by a slowly inactivating K+ current in hippocampal neurons. Nature. 1988;336:379–381. doi: 10.1038/336379a0. [DOI] [PubMed] [Google Scholar]
- Thoby-Brisson M, Telgkamp P, Ramirez JM. The role of the hyperpolarization-activated current in modulating rhythmic activity in the isolated respiratory network of mice. J Neurosci. 2000;20:2994–3005. doi: 10.1523/JNEUROSCI.20-08-02994.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tierney AJ, Harris-Warrick RM. Physiological role of the transient potassium current in the pyloric circuit of the lobster stomatogastric ganglion. J Neurophysiol. 1992;67:599–609. doi: 10.1152/jn.1992.67.3.599. [DOI] [PubMed] [Google Scholar]
- Trimmer JS, Rhodes KJ. Localization of voltage-gated ion channels in mammalian brain. Annu Rev Physiol. 2004;66:477–519. doi: 10.1146/annurev.physiol.66.032102.113328. [DOI] [PubMed] [Google Scholar]
- Viana F, Bayliss DA, Berger AJ. Multiple potassium conductances and their role in action potential repolarization and repetitive firing behavior of neonatal rat hypoglossal motoneurons. J Neurophysiol. 1993;69:2150–2163. doi: 10.1152/jn.1993.69.6.2150. [DOI] [PubMed] [Google Scholar]