Abstract
Duchenne muscular dystrophy (DMD) is a severe degenerative muscle disease caused by a mutation in the gene encoding dystrophin, a protein linking the cytoskeleton to the extracellular matrix. In this study we investigated whether the antioxidant N-acetylcysteine (NAC) provided protection against dystrophic muscle damage in the mdx mouse, an animal model of DMD. In isolated mdx muscles, NAC prevented the increased membrane permeability and reduced the force deficit associated with stretch-induced muscle damage. Three-week-old mdx mice were treated with NAC in the drinking water for 6 weeks. Dihydroethidium staining showed that NAC treatment reduced the concentration of reactive oxygen species (ROS) in mdx muscles. This was accompanied by a significant decrease in centrally nucleated fibres in muscles from NAC-treated mdx mice. Immunoblotting showed that NAC treatment decreased the nuclear protein expression of NF-κB, a transcription factor involved in pro-inflammatory cytokine expression. Finally, we show that NAC treatment reduced caveolin-3 protein levels and increased the sarcolemmal expression of β-dystroglycan and the dystrophin homologue, utrophin. Taken together, our findings suggest that ROS play an important role in the dystrophic pathogenesis, both in terms of activating damage pathways and in regulating the expression of some dystrophin-associated membrane proteins. These results offer the prospect that antioxidants such as NAC could have therapeutic potential for DMD patients.
Duchenne muscular dystrophy (DMD) is the most common and devastating form of muscular dystrophy, with an incidence of 1 in every 3500 male births. It is characterized by progressive muscle degeneration as a result of ongoing muscle damage and incomplete regeneration. This causes profound muscle weakness and thus affected individuals are often wheelchair bound by the age of 10–12 and usually die as a result of respiratory and/or cardiac complications by about the age of 20. It has been known for 20 years that DMD is caused by an X-chromosome mutation leading to the absence of the protein dystrophin from muscle (Hoffman et al. 1987). Dystrophin is a large protein (427 kDa), which links the cytoskeleton to a group of membrane-bound proteins, the dystrophin-associated glycoprotein (DAG) complex. The DAG, in turn, binds to the extracellular matrix and therefore dystrophin provides a structural link between the cytoskeleton and the extracellular matrix.
The mdx mouse also lacks dystrophin and is the most widely used animal model of DMD. Muscles from mdx mice undergo periods of muscle degeneration and regeneration, which is most extensive from about 3–8 weeks of age. Although it has a milder phenotype than DMD patients, the mdx mouse has provided researchers with a much greater understanding of the mechanisms that cause muscle degeneration in DMD and has also played a pivotal role in testing potential therapeutic strategies (Khurana & Davies, 2003).
In recent years, our laboratory has focused on a class of ion channels, known as mechanosensitive or stretch-activated channels (SACs), which we have shown contributes to muscle damage in mdx mice (Yeung et al. 2005; Whitehead et al. 2006a). It is well established that mdx muscles are more susceptible to damage from stretched (eccentric) contractions than normal muscles (Petrof et al. 1993). We have found that calcium entry through SACs is an important cause of muscle damage, since three SAC blockers prevented the rise in intracellular Ca2+ and significantly improved force after stretched contractions in mdx muscle (Yeung et al. 2005). More recently, we have also shown that the SAC blocker streptomycin reduced membrane permeability and improved muscle force in mdx mice following stretched contractions, both in isolated muscles and in intact mice subjected to downhill treadmill exercise (Whitehead et al. 2006a). From a mechanistic point of view, these experiments suggested that the stretch-induced increased membrane permeability in dystrophic muscle was not due to mechanical tears but rather the result of a damage pathway dependent on Ca2+ entry through SACs.
In skeletal muscle, increased intracellular Ca2+ can enhance the production of reactive oxygen species (ROS) by mitochondria (Nethery et al. 2000) and NADPH oxidase (Martins et al. 2008). ROS such as the hydroxyl radical have also been shown to increase membrane permeability in muscle fibres due to elevated intracellular Ca2+, most likely through lipid peroxidation (Howl & Publicover, 1990). Therefore, stretch-induced Ca2+ influx through SACs might enhance ROS generation and increase membrane permeability in mdx muscle. ROS have been postulated as a possible cause of dystrophic muscle damage for a number of years (Rando, 2002; Whitehead et al. 2006b; Tidball & Wehling-Henricks, 2007). Work carried out by Rando and colleagues has shown that myotubes from mdx muscles are much more susceptible to ROS-induced cell death than wild-type muscles (Rando et al. 1998). This group has also shown that in 3-week-old mdx mice, an age where there are no overt signs of muscle damage, there is evidence of oxidative stress, as shown by increased lipid oxidation products and increased production of antioxidant enzymes (Disatnik et al. 1998). This important finding suggested that increased ROS production was a primary feature of dystrophic muscle damage and not a secondary effect of muscle degeneration caused by other mechanisms. Another ROS-mediated effect on dystrophic muscles is increased protein oxidation, both in mdx mice (Hauser et al. 1995) and DMD patients (Haycock et al. 1996), which can cause a wide range of deleterious effects on muscle contractile function (Smith & Reid, 2006).
Further evidence in support of oxidative stress as a cause of the dystrophic pathophysiology comes from in vivo studies in which mdx mice fed antioxidants derived from green tea (Buetler et al. 2002) or a low iron diet, which reduces hydroxyl radicals (Bornman et al. 1998), showed reduced signs of muscle damage. A recent study has also shown that a synthetic vitamin E analogue, IRFI-042, which has strong antioxidant properties, improved mdx muscle function and reduced the activation of the transcription factor nuclear factor-κB (NF-κB), which can be activated by ROS (Messina et al. 2006). NF-κB regulates transcription of a plethora of genes but of particular relevance in dystrophic muscles are the pro-inflammatory cytokines such as TNF-α (Hodgetts et al. 2006) and matrix metalloproteinases (Hnia et al. 2007), which have been shown to contribute to muscle damage in mdx muscles. Moreover Kumar & Boriek (2003) showed that cyclic, passive stretch of mdx diaphragm increased activation of NF-kB, which was attenuated by the antioxidant N-acetylcysteine (NAC).
In this paper, we use NAC to investigate the effects of ROS on muscle damage in mdx mice. NAC was chosen because it is an effective antioxidant in skeletal muscle. In isolated muscle preparations it protects against contraction-induced oxidative stress (Sandstrom et al. 2006) and reduces muscle fatigue (Smith & Reid, 2006). Furthermore, following oral administration, NAC has been shown to inhibit NF-κB activation in unloaded mouse muscle (Farid et al. 2005) and to delay muscle fatigue in humans (McKenna et al. 2006). Here, we used two experimental approaches: (1) stretched contractions of isolated mdx muscles with or without NAC and (2) young mdx mice given NAC orally for 6 weeks. Specifically, we tested three main hypotheses through these experiments. (1) Do ROS contribute to the decreased muscle force and increased membrane permeability following stretched contractions in isolated mdx muscle? (2) Can NAC reduce intracellular ROS levels and offer protection against mdx muscle damage in vivo? (3) Can NAC treatment reduce NF-κB activation and regulate the sarcolemmal expression of key proteins associated with the dystrophin complex?
Methods
Animals
Male mdx and wild-type (C57 BL/10 ScSn) mice at either 3 or 8 weeks of age were supplied by Animal Resources Centre, Perth, WA, Australia. The experiments were approved by the Animal Ethics Committee of the University of Sydney.
Isolated muscle experiments
Mice were killed with an i.p. injection of pentobarbitone sodium (163 mg kg− 1). The extensor digitorum longus (EDL) muscles were dissected from 8- to 10-week-old mdx and wild-type mice. Aluminium foil clips were attached to the tendons at both ends of the muscle. The muscle was then placed in a Perspex chamber and the aluminium clips were positioned on metal hooks that were attached to a force transducer and the lever of a motor (high speed length controller, model 300B-LC, Aurora Scientific Inc., Ontario, Canada). Two platinum electrodes, parallel to the muscle, provided stimulation. The muscle was perfused with a standard solution containing (mm): NaCl (121), KCl (5), CaCl2 (1.8), MgCl2 (0.5), NaH2PO4 (0.4), NaHCO3 (24) and glucose (5.5). The solution was continually bubbled with 95% O2–5% CO2 (pH 7.4). Experiments were carried out at both room temperature (∼22°C) and 35°C, as described below.
Stretched contractions on isolated muscles
In these experiments, the muscle was perfused with either standard control solution (con) or standard solution containing 20 mmN-acetylcysteine (NAC). This concentration of NAC has previously been shown to have little effect on the isometric force produced by skeletal muscle (Khawli & Reid, 1994). Initially, the muscle was stimulated two or three times at 120 Hz to determine the supramaximal stimulus voltage and was then rested for 30 min at room temperature. The length–tension relationship for maximal tetani was determined by stimulating the muscle (120 Hz, 300 ms) at 60 s intervals over a range of muscle lengths. The muscle was then set at its optimum length (Lo). At this time, 0.02% (w/v) Evans Blue Dye (EBD) and 0.5% bovine serum albumin (BSA) were added to the perfusate, either with or without NAC, for the remainder of the experiment. EBD is a membrane-impermeant fluorescent dye, and is a commonly used marker of membrane disruptions in skeletal muscle (Hamer et al. 2002). BSA was added to the solution, since EBD is known to bind to serum albumin in vivo and we have shown that it prevents drug interactions with a similar membrane-impermeant dye, procion orange (Whitehead et al. 2006a).
A single tetanic contraction was performed 30 min after the length–tension measurements, in order to confirm that the preparation was stable and to establish the baseline force before the stretched contractions. The muscle chamber temperature was then switched to 35°C. This was achieved by heating the solution in a water bath and directing it through a custom-designed aluminium heat exchanger, which provided a constant temperature in the chamber. After 1 min at 35°C, the muscle was subjected to three stretched contractions, 30 s apart. Each stretched contraction involved a 300 ms tetani with a stretch from Lo to Lo+ 30%, beginning 150 ms after the start of stimulation and lasting for 100 ms (stretching velocity, 3 ×Lo s−1). At 60 min after the stretched contractions, the length–tension relationship was again measured and maximum isometric force was determined. The muscle was frozen in isopentane cooled in liquid nitrogen and stored at −80°C. Frozen EDL cross-sections (10 μm) were placed on poly l-lysine coated slides, fixed in ice-cold methanol (−22°C) for 5 min and then air-dried at room temperature (∼22–25°C). To quantify the uptake of EBD into muscle fibres, sections were viewed under a fluorescence microscope (Zeiss Axioplan 2), with an excitation range of 545/30 nm and 570 nm long pass filter. Digital images were captured with a CCD camera attached to the microscope (Zeiss AxioCam HRm). All sections were imaged using identical optical settings. EBD positive muscle fibres with a fluorescence intensity that exceeded a specified threshold were included in the analysis. The area of EBD positive fibres was calculated as a percentage of the entire muscle cross-sectional area.
For in vivo experiments mdx mice at 3 weeks of age were divided into two groups, control mice receiving normal drinking water (con) and the treated mice receiving drinking water containing 1% (∼60 mm) N-acetylcysteine (NAC) (Farid et al. 2005). After 6 weeks of treatment (9 weeks of age), mice were killed and the EDL and tibialis anterior (TA) muscles were removed. The EDL muscles were mounted in tissue-tek and frozen in isopentane cooled in liquid nitrogen. The TA muscles were snap frozen in liquid nitrogen.
Immunostaining and central nuclei detection
EDL cross-sections (10 μm) from control and NAC treated mdx mice, and age-matched wild-type mice, were mounted on slides and were used for both immunostaining of dystrophin-associated proteins and visualization of central nuclei. Details of the immunostaining procedures have been previously described (Yeung et al. 2005). Firstly, cross-sections were incubated with AffiniPure Fab and Fc fragments (Jackson ImmunoResearch Laboratories, West Grove, PA, USA) at a concentration of 60 μg l−1 in 2% BSA for 30 min, washed in phosphate-buffered saline (PBS) and then blocked in 2% BSA for 15 min. We have previously shown that this procedure prevents non-specific binding of antimouse secondary antibodies to endogenous mouse immunoglobulins in the tissue section. The primary antibodies used were mouse monoclonal antibodies against: caveolin-3, diluted 1: 50 (BD Transduction Laboratories, USA), β-dystroglycan, diluted 1: 20 (NCL-b-DG, Novocastra Laboratories, Newcastle upon Tyne, UK) and Utrophin, diluted 1: 20 (NCL-DRP2, Novocastra Laboratories). The secondary antibody was a goat antimouse conjugated with Cy3, diluted 1: 200 (Jackson ImmunoResearch Laboratories). For detecting nuclei, the secondary antibody solution also contained the fluorescent nuclear stain 4′,6-diamidino-2-phenylindole (DAPI; Sigma, St Louis, MO, USA), which was diluted 1: 100. Fluorescence images of cross-sections were taken with 5 × or 20 × (Zeiss Plan Neola) objectives and the number of muscle fibres with central nuclei was calculated as a percentage of the total number of fibres with visible nuclei (i.e. both central and peripheral).
Dihydroethidium staining for ROS detection
Dihydroethidium (DHE) is a commonly used indicator of ROS production both in vitro (Benov et al. 1998) and in vivo (Robinson et al. 2006). It is oxidized by ROS, particularly superoxide (O2−), forming ethidium bromide, which fluoresces red when intercalated with DNA (Benov et al. 1998). Therefore the intensity of fluorescence localized in cell nuclei is a measure of the ROS production in that cell. ROS production was measured by incubating EDL cross-sections with 5 μm DHE in PBS at 37°C for 30 min. DHE intensity was quantified by counting the number of pixels exceeding a specified threshold, which was set in order to eliminate interference from any background fluorescence.
Western blotting techniques
Frozen TA muscles were lysed with a polytron PT 1200 homogenizer (Kinematica, Littau/Lucerne, Switzerland) using ice cold lysis buffers for either total lysate or nuclear fractions. For total lysate, muscles were lysed in a buffer containing: 50 mm Tris pH 7.5, 150 mm NaCl, 25 mm EDTA, 25 mm EGTA, 1% Triton X-100, protease inhibitor cocktail, calpain 1 inhibitor and phosphatase inhibitor (Sigma). After 30 min of incubation on ice, homogenates were centrifuged at 15 800g for 30 min at 4°C and the supernatant was removed. For nuclear fractions, two buffers were used. Firstly, muscles were lysed in a buffer containing 10 mm Hepes, 1.5 mm MgCl2, 10 mm KCl, 0.5 mm DTT, 0.05% NP40, protease, calpain 1 and phospatase inhibitors. Muscle lysates were kept on ice for 10 min and then centrifuged at 800g for 10 min at 4°C. The supernatant was removed and the pellet, containing the nuclear proteins, was then re-suspended in buffer used for the total lysate. After 30 min, homogenates were centrifuged at 15 800g for 30 min at 4°C and the supernatant removed. Protein concentration in the supernatant was determined using the Bradford assay (Bio-Rad, Hercules, CA, USA).
A total of 20 μg protein per well was loaded into a 4% (stacking)–12% (resolving) polyacrylamide gel. Proteins were separated by SDS-PAGE and transferred to a nitrocellulose membrane using a Mini Trans-Blot Transfer Cell (Bio-Rad). Membranes were blocked for 30 min with 5% skim milk powder in PBS-Tween20 at room temperature. For total lysate, membranes were incubated with a primary antibody against caveolin-3 diluted 1: 1000 in blocking buffer for 1 h at room temperature. For nuclear fractions, membranes were incubated with a primary antibody for NF-κB (p65 phospho S276, Abcam, Cambridge, UK), which was diluted 1: 500 in blocking buffer overnight at 4°C. After rinsing with PBS-T, membranes were probed for 1 h at room temperature with an HRP-conjugated anti-mouse (for caveolin-3) or anti-rabbit (for NF-κB) secondary antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA) at a dilution of 1: 1000. A mouse monoclonal β-actin antibody (Sigma) was used as a control to ensure equal loading between wells. The protein bands were developed using an ECL Plus detection kit (Amersham Pharmacia Biotech, UK) and visualized with an Alpha Innotech (San Leandro, CA, USA) FluoChem SP Imaging System. Protein band quantification was obtained by multiplying the area and intensity of each band using Image J (National Institutes of Health, USA). mdx values were normalized to wild-type in each gel.
Statistics
All data are presented as the mean ±s.e.m. A significant difference between groups was determined through the use of Student's t test for unpaired data or two-factor ANOVA. The significance level was set at P < 0.05.
Results
NAC improves mdx muscle force after stretched contractions
Stretched contractions were performed at 35°C rather than at room temperature, since ROS production in isolated contracting skeletal muscle is known to increase significantly over this temperature range (Arbogast & Reid, 2004). In preliminary experiments, we found that the force reduction in mdx muscles from three stretched contractions at 35°C was comparable (∼35% of prestretch values) to that measured after 10 stretched contractions at room temperature (Whitehead et al. 2006a). Therefore, we decided to use three stretched contractions in this current series of experiments.
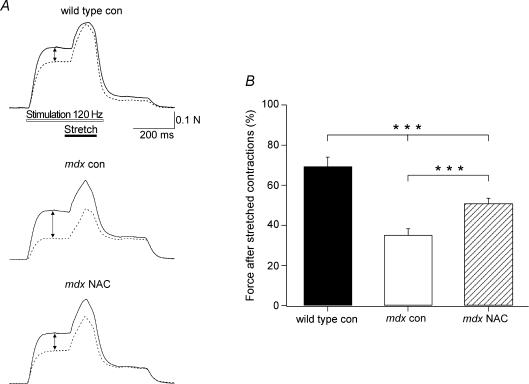
As shown in Fig. 1A, the force reduction caused by stretched contractions was considerably greater in mdx muscles than wild-type. In comparison, NAC treated muscles showed significantly less force loss than mdx control (con) muscles. At 60 min after the stretched contractions (Fig. 1B), the mean force was 35 ± 3% (of prestretch values) for mdx control muscles (n = 9), which was significantly less than that of mdx NAC muscles, where the value was 51 ± 2% (P < 0.001, n = 11). Wild-type muscles had a mean force of 69 ± 4% (n = 4), significantly greater than both mdx con and mdx NAC (P < 0.001). In order to establish that the NAC-induced protection against the stretch-induced force loss was specific to mdx muscle, we also carried out some experiments with NAC treated wild-type muscles. NAC had no significant effect on force reduction in wild-type mice (72 ± 1%, n = 6).
Figure 1. The effect of NAC on mdx muscle force after stretched contractions.
A, force records measured during the 1st (continuous line) and 3rd (dotted line) stretched contractions for wild-type, mdx con and mdx NAC muscles. Note the smaller drop in isometric force (arrow) for mdx NAC compared with mdx con. B, the mean ±s.e.m. isometric force measured 60 min after stretched contractions for wild-type and mdx muscles with NAC or without (con). ***Significant difference at P < 0.001.
NAC prevents EBD uptake in mdx muscle after stretched contractions
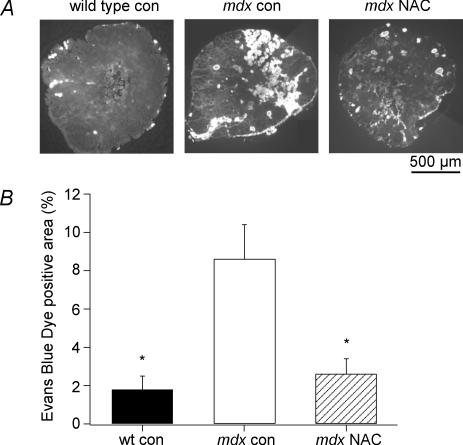
EBD uptake into muscle fibres was used to assess the increased membrane permeability in mdx and wild-type muscles following stretched contractions. EDL muscles were frozen 60 min after the stretched contractions and cross-sections taken from the middle of the muscle belly were used for EBD quantification. In Fig. 2A, representative cross-sections show that mdx con muscles had significantly more dye-positive fibres than wild-type, while NAC largely prevented the increased dye uptake in mdx muscles. When the data were pooled (Fig. 2B), mdx control muscles had 8.6 ± 1.8% (n = 8) EBD positive fibres compared to 1.8 ± 0.7% for wild-type muscles (n = 5; P < 0.05) and to 2.6 ± 0.8% for mdx NAC muscles (n = 7; P < 0.05). These data suggest that ROS contribute to most of the increased stretch-induced membrane permeability in mdx muscle at 35°C.
Figure 2. The effect of NAC on membrane permeability after stretched contractions.
A, EDL cross-sections showing the uptake of Evans Blue Dye (EBD) into muscle fibres, as measured 60 min after stretched contractions for wild-type, mdx con and mdx NAC. B, the mean ±s.e.m. area of EBD positive fibres after stretched contractions, expressed as a percentage of the entire muscle cross-section, as shown in A.
NAC treatment reduces central nuclei in intact mdx mice
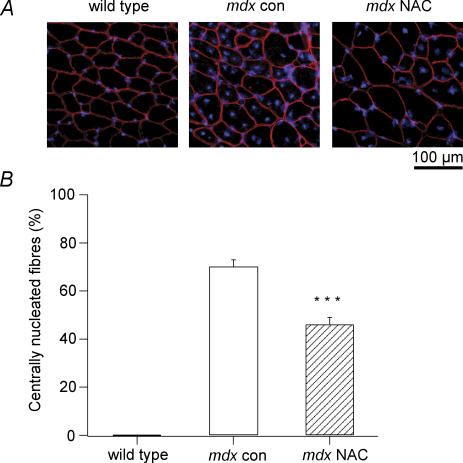
Muscle fibre regeneration following a period of necrosis can be quantified by the presence of central nuclei in muscle cross-sections. In these experiments, mdx mice were treated with 1% NAC in their drinking water from 3 to 9 weeks of age, which is the period of most extensive damage and regeneration. Muscle cross-sections from mdx con mice at 9 weeks of age had a large number of fibres that contained central nuclei, and there were noticeably less in NAC treated mdx muscles (Fig. 3A). The mean number of centrally nucleated fibres was 70 ± 3% for mdx con muscles (n = 6) and this was significantly reduced to 46 ± 3% in mdx NAC treated muscles (n = 6, P < 0.001, Fig. 3B). Given that wild-type muscles had no central nuclei (Fig. 3), this result indicates that muscles from NAC treated mdx mice had approximately one-third less damaged fibres than mdx con mice.
Figure 3. Central nuclei in NAC treated and control mdx mice.
A, a region of EDL cross-sections from a wild-type, mdx con and mdx NAC treated mouse, which are immunostained with a caveolin-3 antibody (surface membrane) and counterstained with DAPI (nuclei). B, the mean ±s.e.m. percentage of centrally nucleated muscle fibres in cross-sections from wild-type, mdx con and mdx NAC treated mice. ***Significant difference between mdx con and mdx NAC (P < 0.001).
NAC treatment protects against force loss from stretched contractions
In the isolated muscle experiments (as described earlier), NAC provided protection against the force reduction in mdx muscles. Therefore, we wanted to investigate if in vivo treatment of NAC in mdx mice also protected against stretch-induced damage. The first series of experiments were carried out at room temperature. EDL muscles isolated from control or NAC treated mice underwent a series of four stretched contractions, and muscle force was measured 60 min later. At this time, the mean force for the mdx con muscles was 53 ± 2% of prestretch values (n = 6) whereas muscles from NAC treated mice had a significantly greater force value of 60 ± 1% (n = 4; P < 0.05). In a second series of experiments at 35°C, force following the stretched contractions was not significantly different between mdx con and mdx NAC treated mice (data not shown). These results suggest that the concentration of NAC in muscles of treated mice was sufficient to protect against stretch-induced ROS at room temperature but not at 35°C, where ROS production is much greater (see Discussion).
NAC treatment prevents excessive ROS production in muscles from mdx mice
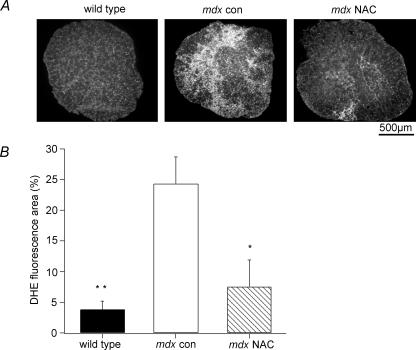
In order to test whether the NAC treatment was reducing levels of ROS in mdx muscles, we used the ROS-sensitive dye DHE. EDL cross-sections were incubated with 5 μm DHE at 37°C and the fluorescence intensity above a specified threshold was measured from fluorescence images. As shown in Fig. 4A, mdx con muscles had much more widespread and intense nuclear staining than either mdx NAC treated or wild-type muscles. It can also be seen that the brightest staining in mdx con muscle occurs in densely packed areas, which would be consistent with infiltrating inflammatory cells since these cells can produce significant amounts of ROS (Nguyen & Tidball, 2003). As shown in Fig. 4B, the area of DHE staining in mdx con muscles was 24.3 ± 4.4% (n = 9), which was significantly less (P < 0.05) in mdx NAC muscles (7.5 ± 4.4%; n = 6; P < 0.05) and wild-type muscles (3.8 ± 1.4%; n = 5; P < 0.01).
Figure 4. ROS production in muscles from NAC-treated and control mdx mice.
A, EDL cross-sections showing DHE fluorescence from wild-type, mdx con and mdx NAC treated mice. Intense staining in the mdx con section is possibly due to ROS production by inflammatory cells. B, the mean ±s.e.m. DHE fluorescence, as measured for wild-type, mdx con and mdx NAC treated mice. Values represent the area of fluorescence, above a specified threshold, expressed as a percentage of the total area of the muscle cross-section. **Significant difference between wild-type and mdx con (P < 0.01); *significant difference between mdx con and mdx NAC (P < 0.05).
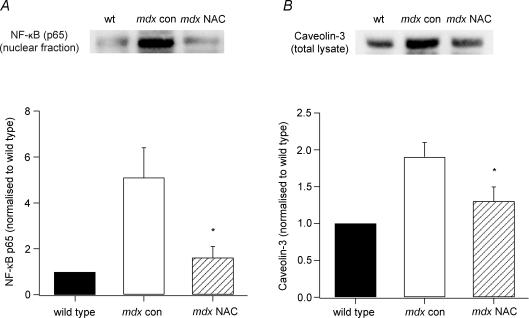
NAC treatment reduces nuclear NF-κB protein expression in mdx mice
NF-κB normally resides in the cytosol but when activated by certain stimuli, including ROS, it translocates to the nucleus and binds to DNA to regulate gene expression. Therefore, we were interested to see if NAC could reduce the activation of NF-κB in mdx muscles. In these experiments, we used Western blotting to quantify the nuclear translocation of the NF-κB protein subunit p65. As shown in Fig. 5A, p65 levels were much greater for mdx con muscles than for wild-type, while NAC considerably reduced protein expression in mdx muscles. This was confirmed by the pooled data in which, normalized to wild-type, the p65 protein levels in mdx con muscles were 5.1 ± 1.3 times higher (n = 5), while NAC treatment significantly reduced this to 1.6 ± 0.5 (P < 0.05; n = 5).
Figure 5. Protein expression of NF-κB and caveolin-3 in NAC-treated and control mdx mice.
Upper panels, Western blots showing muscle protein levels of NF-κB p65 (nuclear fraction) (A) and caveolin-3 (total lysate) (B) for wild-type, mdx con and mdx NAC mice. Lower panels, pooled data showing the mean ±s.e.m. protein levels for NF-κB p65 and caveolin-3. *Significant difference between mdx con and mdx NAC (P < 0.05).
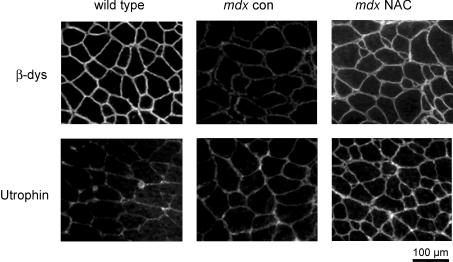
NAC treatment reduces caveolin-3 and increases β-dystroglycan and utrophin sarcolemmal expression in mdx mice
In these experiments, we wanted to determine if ROS were involved in regulating the expression of dystrophin-associated proteins. Western blotting showed that caveolin-3 protein levels in mdx con mice were about double that of wild-type, and NAC treatment reduced levels in mdx mice (Fig. 5B). When the pooled data were normalized to wild-type, mdx con muscles were 1.9 ± 0.2 times greater (n = 6) and this value was significantly reduced by NAC treatment to 1.3 ± 0.2 (P < 0.05; n = 5). EDL cross-sections from wild-type, mdx con and mdx NAC-treated mice were immunostained with antibodies for caveolin-3, β-dystroglycan and utrophin. Confirming the Western blotting results, muscle sections from NAC-treated mdx mice showed less intense caveolin-3 staining at the membrane compared to mdx con mice (see Fig. 3A). We also found that the sarcolemmal staining of both β-dystroglycan and utrophin was increased for muscles from NAC-treated mdx mice compared to mdx con mice (Fig. 6). As expected, utrophin staining was negligible in wild-type muscles.
Figure 6. Immunostaining of β-dystroglycan and utrophin in NAC-treated and control mdx mice.
Immunostaining of muscle cross-sections from wild-type, mdx con and mdx NAC mice, showing the sarcolemmal expression of β-dystroglycan (β-dys), and utrophin.
Discussion
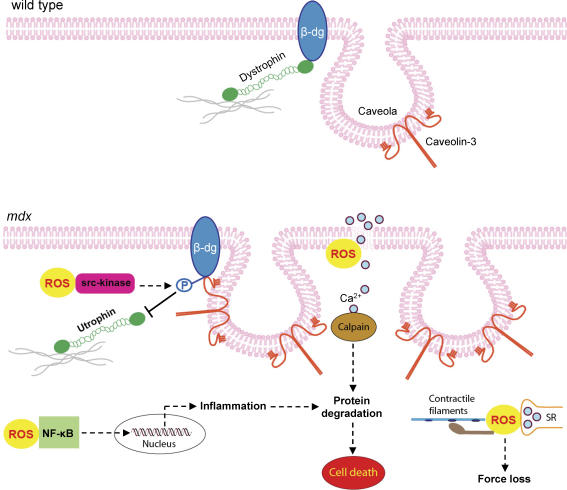
There is considerable evidence of oxidative damage to dystrophic muscles as well as positive benefits provided by antioxidant treatments (Messina et al. 2006; Hnia et al. 2007). Dystrophin-deficient muscle cells are more sensitive to ROS-induced damage (Rando et al. 1998) and there is evidence of up-regulation of endogenous antioxidant defence systems, suggestive of increased ROS production (Tidball & Wehling-Henricks, 2007). In the current study, we used the antioxidant NAC to provide novel data that increased ROS production contributes to both acute muscle damage, caused by stretched contractions, and to the ongoing degenerative process that occurs naturally in muscles of intact mdx mice. We also found that ROS play a role in the dysregulation of dystrophin-associated proteins, which could be reversed by NAC treatment. Our interpretation of these findings is summarized in Fig. 7. The data presented in this paper are consistent with recent results from our laboratory, in which NAC was shown to improve left ventricular muscle function and reduce signs of cardiac damage and degeneration in mdx mice (Williams & Allen, 2007).
Figure 7. Schematic diagram showing the deleterious effects of ROS on mdx muscle, which were ameliorated by NAC.
The figure also highlights the increased number of caveolae and caveolin-3 in mdx muscle compared to wild-type. Abbreviations: β-dg, β-dystroglycan; P, phosphorylation; SR, sarcoplasmic reticulum.
NAC prevents the stretch-induced membrane permeability in mdx muscle
It is widely thought that the surface membrane of dystrophic muscles is more fragile than that of normal muscles, thereby making it more susceptible to developing micro tears during muscle contraction, particularly when stretched contractions are involved (Moens et al. 1993; Petrof et al. 1993). However, we recently reported that most of the increased membrane permeability following stretched contractions of mdx muscle at room temperature could be prevented by the SAC blockers streptomycin and GsMTx4 (Whitehead et al. 2006a). Given that these drugs also prevent the stretch-induced rise in intracellular Ca2+ in mdx fibres (Yeung et al. 2005), we postulated that a Ca2+-dependent damage pathway was the most likely cause of the increased membrane permeability. In the current study, the results from the isolated muscle experiments indicate that NAC prevents the increased membrane permeability associated with stretched contractions in mdx muscle at 35°C. Therefore, this provides further evidence showing that the stretch-induced increase in membrane permeability in mdx muscle is not a primary consequence of mechanical tears but rather due to the effects of ROS and/or Ca2+ influx through SACs (Whitehead et al. 2006a). While the number of EBD positive fibres in the current study is relatively small compared to the large force deficit, they are both reliable indicators of damage. EBD uptake reflects a large increase in membrane permeability by a small population of severely damaged, prenecrotic fibres (Hamer et al. 2002; Whitehead et al. 2006a), whereas most fibres can exclude EBD and remain excitable but are still damaged and have a large force deficit (Yeung et al. 2005).
ROS contribute to the stretch-induced force reduction in mdx muscle
A distinguishing feature of the damage associated with stretched contractions in normal muscles is that the reduction in force persists for several days (Morgan & Allen, 1999). As expected, the reduction in force was considerably greater for mdx muscles compared to wild-type (Moens et al. 1993; Whitehead et al. 2006a). Importantly, NAC provided significant protection against the force reduction in mdx muscles but not wild-type. This suggests that excessive ROS production and oxidative damage during stretched contractions is not an inherent feature of normal skeletal muscle but rather an abnormality associated with dystrophic muscle. While it is unclear how the increased ROS caused a decrease in muscle force in our experiments, it is well known that ROS can oxidize contractile and excitation–contraction (E-C) coupling proteins and that skeletal muscle redox balance affects muscle force production (Reid, 2001). In addition, the ROS mediated increase in membrane permeability could also contribute to the loss of force in mdx muscle via an influx of Na+, which would cause membrane depolarization and reduce muscle fibre excitability.
NAC treatment reduces mdx muscle damage in vivo
After 6 weeks of oral NAC administration, muscles from mdx mice showed a marked reduction in muscle damage. The presence of central nuclei in muscle fibres is a well-established indicator of regeneration following muscle damage (McGeachie et al. 1993). NAC treatment significantly reduced the number of mdx muscle fibres with central nuclei, implying that the progression of muscle damage was slowed. ROS levels were substantially increased in mdx muscles and greatly attenuated by NAC treatment (see Fig. 4). This suggests that increased oxidative damage is an important factor during this extensive period (3–9 weeks of age) of muscle degeneration in mdx mice. The DHE-stained muscle sections from mdx control mice also revealed areas of densely packed, intensely stained cells, which are possibly inflammatory cells such as macrophages and neutrophils. These cells are known to produce substantial concentrations of ROS from NADPH oxidase, which can cause muscle cell membrane lysis during inflammatory cascades (Nguyen & Tidball, 2003). Therefore, inflammatory cells might provide an additional source of ROS in mdx muscles, which serves to exacerbate the pre-existing damage caused by intracellular ROS-mediated pathways.
NAC treatment inhibits NF-κB nuclear expression in mdx mice
NF-κB activation is caused by a variety of cellular stimuli, including ROS. On activation, NF-κB translocates to the nucleus and regulates transcription of many genes (Kumar et al. 2004). NF-κB DNA binding has been shown to be increased many fold in muscles from DMD patients (Monici et al. 2003) and mdx mice (Messina et al. 2006), even at a young age (15 days), well before the onset of measurable muscle damage (Kumar & Boriek, 2003). This is consistent with the increased oxidative stress in young mdx muscles (Disatnik et al. 1998) and therefore ROS-induced activation of NF-κB is likely to be a contributing factor to the extensive wave of muscle damage which follows this dormant stage. Amongst the genes regulated by NF-κB, a number have been shown to be involved in the dystrophic disease process including pro-inflammatory cytokines such as TNFα (Grounds & Torrisi, 2004; Hodgetts et al. 2006), and matrix metalloproteinases, which cause cleavage of β-dystroglycan (Hnia et al. 2007). Our results showing that NAC treatment significantly reduced nuclear NF-κB p65 protein expression suggests that ROS are an important mediator of NF-κB activation and translocation to the nucleus. Our results are also consistent with recent papers showing that NF-κB DNA binding activity is significantly reduced in mdx mice receiving antioxidant treatments (Messina et al. 2006; Hnia et al. 2007) and in normal mice receiving NAC (Farid et al. 2005).
NAC treatment regulates expression of dystrophin-associated proteins
While most of the proteins of the DAG complex are down-regulated in dystrophic muscles, caveolin-3 is a notable exception, with levels approximately 2- to 3-fold greater in both mdx (Vaghy et al. 1998) and DMD muscles (Repetto et al. 1999). In our experiments, Western blotting and immunostaining showed that caveolin-3 expression was decreased in NAC-treated mdx mice and this was accompanied by an increased sarcolemmal expression of β-dystroglycan and the dystrophin homologue, utrophin (Fig. 6). This suggests that ROS-mediated pathways contribute to the dysregulation of these dystrophin-associated proteins in mdx muscle. Our findings are consistent with a recent publication showing increased β-dystroglycan protein levels in mdx mice receiving an antioxidant treatment (Hnia et al. 2007). We hypothesize that caveolin-3 might play an important role in regulating this remodelling of the DAG complex in dystrophic muscles. Transgenic overexpression of caveolin-3 in mice leads to a down-regulation of β-dystroglycan and dystrophin, and produces a DMD-like muscle disease (Galbiati et al. 2000). Moreover caveolin-3 competes with dystrophin and utrophin for the same binding site (C-terminal tail) on β-dystroglycan (Sotgia et al. 2000), suggesting that a normal balance between caveolin-3 and dystrophin/utophin is necessary for preventing muscle degeneration. Tyrosine phosphorylation of β-dystroglycan inhibits the binding of dystrophin and utrophin, while caveolin-3 binding is unaffected (Ilsley et al. 2002). This tyrosine phosphorylation, mediated by src kinase, also leads to internalization of β-dystroglycan from the membrane to intracellular vesicles (Sotgia et al. 2003). Thus, given that src kinase is activated by ROS (Chen et al. 2005), we hypothesize that NAC inhibited ROS-induced src kinase activation, which both increased β-dystroglycan membrane expression and utrophin binding to β-dystroglycan (see Fig. 7). This is an important finding since transgenic overexpression of utrophin can prevent dystrophy equally as well as dystrophin (Khurana & Davies, 2003). One implication is that the effectiveness of pharmacological agents designed to up-regulate endogenous utrophin would be enhanced by antioxidant treatments.
Possible sources of increased ROS production in stretched mdx muscles
Given the increased oxidative damage to mdx muscle it is important to elucidate the source(s) of excessive ROS production, particularly following stretched contractions. The two most likely candidates are the mitochondria and NADPH oxidase. It is known that mitochondrial Ca2+ overload can accelerate ROS production in muscle (Brookes et al. 2004), possibly via increased calcium-dependent phospholipase activity (Nethery et al. 2000). This would seem to be a likely source of ROS in dystrophic muscle given that resting intracellular Ca2+ is elevated in mdx fibres following stretched contractions (Yeung et al. 2005) and mitochondrial calcium uptake is greater in mdx myotubes than wild-type (Robert et al. 2001). However, it has been shown that activation of NF-κB by cyclic stretch in mdx diaphragm muscle could be prevented by NAC but not by the SAC blocker gadolinium (Kumar & Boriek, 2003). Therefore, it would seem that under these conditions, the stretch-induced increase in ROS occurred independently of Ca2+ influx through SACs and subsequent mitochondrial Ca2+ overload. NADPH oxidase contributes to the increased ROS production following osmotic swelling in mdx ventricular myocytes (Jung et al. 2008) and is activated by both Ca2+-dependent and -independent pathways in osmotically stretched skeletal muscle (Martins et al. 2008). Therefore, NADPH oxidase is a likely source of stretch-induced ROS production in mdx muscle.
Antioxidants as a potential therapeutic approach for DMD
Clinical trials of antioxidants in DMD patients, undertaken two decades ago, proved to be disappointing (Rando, 2002). However, there are a number of factors which could account for the negative outcome. Firstly, these trials commenced at an advanced stage of the disease, when significant muscle fibre loss had already occurred. Antioxidants would be expected to reduce or prevent muscle damage and degeneration but not to replace lost fibres. Secondly, the antioxidants used in these trials (SOD, vitamin E and selenium) are not membrane permeant and would be ineffective in scavenging intracellular ROS. NAC, on the other hand, is membrane permeant and can both scavenge intracellular ROS as well as boost levels of endogenous antioxidants such as GSH (Sandstrom et al. 2006) and increase Mg-SOD activity in septic diaphragm muscle (Barreiro et al. 2005). Thus, based on our current results with NAC and recent papers showing positive effects of other antioxidants on mdx muscle, it is worth reconsidering antioxidants as a therapeutic option for DMD. Importantly, from a clinical point of view, NAC has been approved for use in humans and has shown benefits in a wide range of diseases (Arakawa & Ito, 2007). It is clear that the dystrophic disease process is complex and multifactorial, as evidenced by the ever-increasing number of pharmacological approaches which provide varying degrees of protection against muscle damage in mdx mice (Khurana & Davies, 2003). Our data show that NAC can provide considerable protection against the ongoing muscle degeneration in intact mdx mice and against damage resulting from stretched contractions. The logical extension of these findings is to combine NAC, or other antioxidants, with blockers of parallel damage pathways in mdx muscle, in order to provide a more effective therapeutic approach for DMD.
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia.
References
- Arakawa M, Ito Y. N-acetylcysteine and neurodegenerative diseases: Basic and clinical pharmacology. Cerebellum. 2007;6:308–314. doi: 10.1080/14734220601142878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbogast S, Reid MB. Oxidant activity in skeletal muscle fibers is influenced by temperature, CO2 level and muscle-derived nitric oxide. Am J Physiol Regul Integr Comp Physiol. 2004;287:R698–R705. doi: 10.1152/ajpregu.00072.2004. [DOI] [PubMed] [Google Scholar]
- Barreiro E, Sanchez D, Galdiz JB, Hussain SN, Gea J. N-acetylcysteine increases manganese superoxide dismutase activity in septic rat diaphragms. Eur Respir J. 2005;26:1032–1039. doi: 10.1183/09031936.05.00003705. [DOI] [PubMed] [Google Scholar]
- Benov L, Sztejnberg L, Fridovich I. Critical evaluation of the use of hydroethidine as a measure of superoxide anion radical. Free Radic Biol Med. 1998;25:826–831. doi: 10.1016/s0891-5849(98)00163-4. [DOI] [PubMed] [Google Scholar]
- Bornman L, Rossouw H, Gericke GS, Polla BS. Effects of iron deprivation on the pathology and stress protein expression in murine X-linked muscular dystrophy. Biochem Pharmacol. 1998;56:751–757. doi: 10.1016/s0006-2952(98)00055-0. [DOI] [PubMed] [Google Scholar]
- Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol. 2004;287:C817–C833. doi: 10.1152/ajpcell.00139.2004. [DOI] [PubMed] [Google Scholar]
- Buetler TM, Renard M, Offord EA, Schneider H, Ruegg UT. Green tea extract decreases muscle necrosis in mdx mice and protects against reactive oxygen species. Am J Clin Nutr. 2002;75:749–753. doi: 10.1093/ajcn/75.4.749. [DOI] [PubMed] [Google Scholar]
- Chen DB, Li SM, Qian XX, Moon C, Zheng J. Tyrosine phosphorylation of caveolin 1 by oxidative stress is reversible and dependent on the c-src tyrosine kinase but not mitogen-activated protein kinase pathways in placental artery endothelial cells. Biol Reprod. 2005;73:761–772. doi: 10.1095/biolreprod.105.040881. [DOI] [PubMed] [Google Scholar]
- Disatnik MH, Dhawan J, Yu Y, Beal MF, Whirl MM, Franco AA, Rando TA. Evidence of oxidative stress in mdx mouse muscle: studies of the pre-necrotic state. J Neurol Sci. 1998;161:77–84. doi: 10.1016/s0022-510x(98)00258-5. [DOI] [PubMed] [Google Scholar]
- Farid M, Reid MB, Li YP, Gerken E, Durham WJ. Effects of dietary curcumin or N-acetylcysteine on NF-κB activity and contractile performance in ambulatory and unloaded murine soleus. Nutr Metab (Lond) 2005;2:20. doi: 10.1186/1743-7075-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galbiati F, Volonte D, Chu JB, Li M, Fine SW, Fu M, Bermudez J, Pedemonte M, Weidenheim KM, Pestell RG, Minetti C, Lisanti MP. Transgenic overexpression of caveolin-3 in skeletal muscle fibers induces a Duchenne-like muscular dystrophy phenotype. Proc Natl Acad Sci U S A. 2000;97:9689–9694. doi: 10.1073/pnas.160249097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grounds MD, Torrisi J. Anti-TNFα (Remicade) therapy protects dystrophic skeletal muscle from necrosis. FASEB J. 2004;18:676–682. doi: 10.1096/fj.03-1024com. [DOI] [PubMed] [Google Scholar]
- Hamer PW, McGeachie JM, Davies MJ, Grounds MD. Evans Blue Dye as an in vivo marker of myofibre damage: optimising parameters for detecting initial myofibre membrane permeability. J Anat. 2002;200:69–79. doi: 10.1046/j.0021-8782.2001.00008.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser E, Hoger H, Bittner R, Widhalm K, Herkner K, Lubec G. Oxyradical damage and mitochondrial enzyme activities in the mdx mouse. Neuropediatrics. 1995;26:260–262. doi: 10.1055/s-2007-979768. [DOI] [PubMed] [Google Scholar]
- Haycock JW, MacNeil S, Jones P, Harris JB, Mantle D. Oxidative damage to muscle protein in Duchenne muscular dystrophy. Neuroreport. 1996;8:357–361. doi: 10.1097/00001756-199612200-00070. [DOI] [PubMed] [Google Scholar]
- Hnia K, Hugon G, Rivier F, Masmoudi A, Mercier J, Mornet D. Modulation of p38 mitogen-activated protein kinase cascade and metalloproteinase activity in diaphragm muscle in response to free radical scavenger administration in dystrophin-deficient Mdx mice. Am J Pathol. 2007;170:633–643. doi: 10.2353/ajpath.2007.060344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgetts S, Radley H, Davies M, Grounds MD. Reduced necrosis of dystrophic muscle by depletion of host neutrophils, or blocking TNFα function with Etanercept in mdx mice. Neuromuscul Disord. 2006;16:591–602. doi: 10.1016/j.nmd.2006.06.011. [DOI] [PubMed] [Google Scholar]
- Hoffman EP, Brown RH, Jr, Kunkel LM. Dystrophin: the protein product of the Duchenne muscular dystrophy locus. Cell. 1987;51:919–928. doi: 10.1016/0092-8674(87)90579-4. [DOI] [PubMed] [Google Scholar]
- Howl JD, Publicover SJ. Permeabilisation of the sarcolemma in mouse diaphragm exposed to Bay K 8644 in vitro: time course, dependence on Ca2+ and effects of enzyme inhibitors. Acta Neuropathol. 1990;79:438–443. doi: 10.1007/BF00308721. [DOI] [PubMed] [Google Scholar]
- Ilsley JL, Sudol M, Winder SJ. The WW domain: linking cell signalling to the membrane cytoskeleton. Cell Signal. 2002;14:183–189. doi: 10.1016/s0898-6568(01)00236-4. [DOI] [PubMed] [Google Scholar]
- Jung C, Martins AS, Niggli E, Shirokova N. Dystrophic cardiomyopathy: amplification of cellular damage by Ca2+ signaling and reactive oxygen species-generating pathways. Cardiovasc Res. 2008 doi: 10.1093/cvr/cvm089. in press. [DOI] [PubMed] [Google Scholar]
- Khawli FA, Reid MB. N-acetylcysteine depresses contractile function and inhibits fatigue of diaphragm in vitro. J Appl Physiol. 1994;77:317–324. doi: 10.1152/jappl.1994.77.1.317. [DOI] [PubMed] [Google Scholar]
- Khurana TS, Davies KE. Pharmacological strategies for muscular dystrophy. Nat Rev Drug Discov. 2003;2:379–390. doi: 10.1038/nrd1085. [DOI] [PubMed] [Google Scholar]
- Kumar A, Boriek AM. Mechanical stress activates the nuclear factor-κB pathway in skeletal muscle fibers: a possible role in Duchenne muscular dystrophy. FASEB J. 2003;17:386–396. doi: 10.1096/fj.02-0542com. [DOI] [PubMed] [Google Scholar]
- Kumar A, Takada Y, Boriek AM, Aggarwal BB. Nuclear factor-κB: its role in health and disease. J Mol Med. 2004;82:434–448. doi: 10.1007/s00109-004-0555-y. [DOI] [PubMed] [Google Scholar]
- Martins AS, Shkryl VM, Nowycky MC, Shirokova N. Reactive oxygen species contribute to Ca2+ signals produced by osmotic stress in mouse skeletal muscle fibres. J Physiol. 2008;586:197–210. doi: 10.1113/jphysiol.2007.146571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeachie JK, Grounds MD, Partridge TA, Morgan JE. Age-related changes in replication of myogenic cells in mdx mice: quantitative autoradiographic studies. J Neurol Sci. 1993;119:169–179. doi: 10.1016/0022-510x(93)90130-q. [DOI] [PubMed] [Google Scholar]
- McKenna MJ, Medved I, Goodman CA, Brown MJ, Bjorksten AR, Murphy KT, Petersen AC, Sostaric S, Gong X. N-acetylcysteine attenuates the decline in muscle Na+,K+-pump activity and delays fatigue during prolonged exercise in humans. J Physiol. 2006;576:279–288. doi: 10.1113/jphysiol.2006.115352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messina S, Altavilla D, Aguennouz M, Seminara P, Minutoli L, Monici MC, Bitto A, Mazzeo A, Marini H, Squadrito F, Vita G. Lipid peroxidation inhibition blunts nuclear factor-κB activation, reduces skeletal muscle degeneration, and enhances muscle function in mdx mice. Am J Pathol. 2006;168:918–926. doi: 10.2353/ajpath.2006.050673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens P, Baatsen PH, Marechal G. Increased susceptibility of EDL muscles from mdx mice to damage induced by contractions with stretch. J Muscle Res Cell Motil. 1993;14:446–451. doi: 10.1007/BF00121296. [DOI] [PubMed] [Google Scholar]
- Monici MC, Aguennouz M, Mazzeo A, Messina C, Vita G. Activation of nuclear factor-κB in inflammatory myopathies and Duchenne muscular dystrophy. Neurology. 2003;60:993–997. doi: 10.1212/01.wnl.0000049913.27181.51. [DOI] [PubMed] [Google Scholar]
- Morgan DL, Allen DG. Early events in stretch-induced muscle damage. J Appl Physiol. 1999;87:2007–2015. doi: 10.1152/jappl.1999.87.6.2007. [DOI] [PubMed] [Google Scholar]
- Nethery D, Callahan LA, Stofan D, Mattera R, DiMarco A, Supinski G. PLA2 dependence of diaphragm mitochondrial formation of reactive oxygen species. J Appl Physiol. 2000;89:72–80. doi: 10.1152/jappl.2000.89.1.72. [DOI] [PubMed] [Google Scholar]
- Nguyen HX, Tidball JG. Null mutation of gp91phox reduces muscle membrane lysis during muscle inflammation in mice. J Physiol. 2003;553:833–841. doi: 10.1113/jphysiol.2003.051912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrof BJ, Shrager JB, Stedman HH, Kelly AM, Sweeney HL. Dystrophin protects the sarcolemma from stresses developed during muscle contraction. Proc Natl Acad Sci U S A. 1993;90:3710–3714. doi: 10.1073/pnas.90.8.3710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando TA. Oxidative stress and the pathogenesis of muscular dystrophies. Am J Phys Med Rehabil. 2002;81:S175–S186. doi: 10.1097/00002060-200211001-00018. [DOI] [PubMed] [Google Scholar]
- Rando TA, Disatnik MH, Yu Y, Franco A. Muscle cells from mdx mice have an increased susceptibility to oxidative stress. Neuromuscul Disord. 1998;8:14–21. doi: 10.1016/s0960-8966(97)00124-7. [DOI] [PubMed] [Google Scholar]
- Reid MB. Invited Review: redox modulation of skeletal muscle contraction: what we know and what we don't. J Appl Physiol. 2001;90:724–731. doi: 10.1152/jappl.2001.90.2.724. [DOI] [PubMed] [Google Scholar]
- Repetto S, Bado M, Broda P, Lucania G, Masetti E, Sotgia F, Carbone I, Pavan A, Bonilla E, Cordone G, Lisanti MP, Minetti C. Increased number of caveolae and caveolin-3 overexpression in Duchenne muscular dystrophy. Biochem Biophys Res Commun. 1999;261:547–550. doi: 10.1006/bbrc.1999.1055. [DOI] [PubMed] [Google Scholar]
- Robert V, Massimino ML, Tosello V, Marsault R, Cantini M, Sorrentino V, Pozzan T. Alteration in calcium handling at the subcellular level in mdx myotubes. J Biol Chem. 2001;276:4647–4651. doi: 10.1074/jbc.M006337200. [DOI] [PubMed] [Google Scholar]
- Robinson KM, Janes MS, Pehar M, Monette JS, Ross MF, Hagen TM, Murphy MP, Beckman JS. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc Natl Acad Sci U S A. 2006;103:15038–15043. doi: 10.1073/pnas.0601945103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandstrom ME, Zhang SJ, Bruton J, Silva JP, Reid MB, Westerblad H, Katz A. Role of reactive oxygen species in contraction-mediated glucose transport in mouse skeletal muscle. J Physiol. 2006;575:251–262. doi: 10.1113/jphysiol.2006.110601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith MA, Reid MB. Redox modulation of contractile function in respiratory and limb skeletal muscle. Respir Physiol Neurobiol. 2006;151:229–241. doi: 10.1016/j.resp.2005.12.011. [DOI] [PubMed] [Google Scholar]
- Sotgia F, Bonuccelli G, Bedford M, Brancaccio A, Mayer U, Wilson MT, Campos-Gonzalez R, Brooks JW, Sudol M, Lisanti MP. Localization of phospho-β-dystroglycan (pY892) to an intracellular vesicular compartment in cultured cells and skeletal muscle fibers in vivo. Biochem. 2003;42:7110–7123. doi: 10.1021/bi0271289. [DOI] [PubMed] [Google Scholar]
- Sotgia F, Lee JK, Das K, Bedford M, Petrucci TC, Macioce P, Sargiacomo M, Bricarelli FD, Minetti C, Sudol M, Lisanti MP. Caveolin-3 directly interacts with the C-terminal tail of β-dystroglycan. Identification of a central WW-like domain within caveolin family members. J Biol Chem. 2000;275:38048–38058. doi: 10.1074/jbc.M005321200. [DOI] [PubMed] [Google Scholar]
- Tidball JG, Wehling-Henricks M. The role of free radicals in the pathophysiology of muscular dystrophy. J Appl Physiol. 2007;102:1677–1686. doi: 10.1152/japplphysiol.01145.2006. [DOI] [PubMed] [Google Scholar]
- Vaghy PL, Fang J, Wu W, Vaghy LP. Increased caveolin-3 levels in mdx mouse muscles. FEBS Lett. 1998;431:125–127. doi: 10.1016/s0014-5793(98)00738-8. [DOI] [PubMed] [Google Scholar]
- Whitehead NP, Streamer M, Lusambili LI, Sachs F, Allen DG. Streptomycin reduces stretch-induced membrane permeability in muscles from mdx mice. Neuromuscul Disord. 2006a;16:845–854. doi: 10.1016/j.nmd.2006.07.024. [DOI] [PubMed] [Google Scholar]
- Whitehead NP, Yeung EW, Allen DG. Muscle damage in mdx (dystrophic) mice: role of calcium and reactive oxygen species. Clin Exp Pharmacol Physiol. 2006b;33:657–662. doi: 10.1111/j.1440-1681.2006.04394.x. [DOI] [PubMed] [Google Scholar]
- Williams IA, Allen DG. The role of reactive oxygen species in the hearts of dystrophin-deficient mdx mice. Am J Physiol Heart Circ Physiol. 2007;293:H1969–H1977. doi: 10.1152/ajpheart.00489.2007. [DOI] [PubMed] [Google Scholar]
- Yeung EW, Whitehead NP, Suchyna TM, Gottlieb PA, Sachs F, Allen DG. Effects of stretch-activated channel blockers on [Ca2+]i and muscle damage in the mdx mouse. J Physiol. 2005;562:367–380. doi: 10.1113/jphysiol.2004.075275. [DOI] [PMC free article] [PubMed] [Google Scholar]