Abstract
The regulation of neuromediator expression by neuronal activity in the enteric nervous system (ENS) is currently unknown. Using primary cultures of ENS derived from rat embryonic intestine, we have characterized the regulation of tyrosine hydroxylase (TH), a key enzyme involved in the synthesis of dopamine. Depolarization induced either by 40 mm KCl, veratridine or by electrical field stimulation produced a robust and significant increase in the proportion of TH immunoreactive (TH-IR) neurons (total neuronal population was identified with PGP9.5 or Hu) compared to control. This increase in the proportion of TH-IR neurons was significantly reduced by the sodium channel blocker tetrodotoxin (0.5 μm), demonstrating that neuronal activity was critically involved in the effects of these depolarizing stimuli. KCl also increased the proportion of VIP-IR but not nNOS-IR enteric neurons. The KCl-induced increase in TH expression was partly reduced in the presence of the nicotinic receptor antagonist hexamethonium (100 μm), of noradrenaline (1 μm) and of the α2-adrenoreceptor agonist clonidine (1 μm). Combining pharmacological and calcium imaging studies, we have further shown that L-type calcium channels were involved in the increase of TH expression induced by KCl. Finally, using specific inhibitors, we have shown that both protein kinases A and C as well as the extracellular signal-regulated kinases were required for the increase in the proportion of TH-IR neurons induced by KCl. These results are the first demonstration that TH phenotype of enteric neurons can be regulated by neuronal activity. They could also set the basis for the study of the pathways and mechanisms involved in the neurochemical plasticity observed both during ENS development and in inflammatory enteric neuropathies.
The enteric nervous system (ENS) is a complex network of neurons and glial cells located within the gastrointestinal tract, also named the ‘brain of the gut’, which can function independently of the central nervous system (CNS). This system controls gastrointestinal (GI) motility, exocrine and endocrine secretions as well as microcirculation (Goyal & Hirano, 1996). Overall, more than 20 candidate neurotransmitters regulating GI functions have now been identified in enteric neurons, including acetylcholine, vasoactive intestinal polypeptide and nitric oxide (Schemann & Neunlist, 2004).
Although the GI tract contains dopamine (Eaker et al. 1988), it has been difficult to determine whether dopamine is present either in the enteric neurons themselves or in the sympathetic innervation where dopamine is the precursor of noradrenaline. However, recent reports demonstrated that dopamine is an enteric neurotransmitter in the human (Anlauf et al. 2003) as well as in the mouse ENS (Li et al. 2004; Li et al. 2006). Tyrosine hydroxylase immunoreactive (TH-IR) neurons have been identified in the stomach of adult ferrets and guinea pigs as well as in the whole digestive tract of adult mice (Schemann et al. 1995; Sann et al. 1998; Li et al. 2004). In adult humans, a recent report showed that TH-IR neurons constituted around 20% of all enteric neurons in the upper gastrointestinal tract (Anlauf et al. 2003). These neurons were considered to be dopaminergic since they were not stained with antibodies against dopamine β-hydroxylase (DBH) (Anlauf et al. 2003). Although it has been suggested that endogenous dopamine inhibits colonic motility (Walker et al. 2000) and modulate secretion (Zhang et al. 2007), the precise function of enteric dopaminergic neurons remains unclear.
In the CNS, an up-regulation of TH expression can be induced by various stimuli such as depolarization and nicotine. Furthermore, several reports have demonstrated that the extracellular signal-regulated kinases (ERKs) pathway plays a critical role in the increase of TH expression (Du et al. 1998; Shah et al. 2006). In contrast, little is known about the regulation of TH expression in the ENS. It has been shown recently that sympathetic denervation up-regulates TH expression by increasing both the abundance of transcripts encoding TH and the numbers of TH-IR neurons in the bowel of mice (Li et al. 2004). Some GI pathologies are also associated with a change in TH expression. In particular, an increase in TH-IR neurons is observed in the myenteric plexus of patients with Crohn's disease (Belai et al. 1997) while a loss of dopaminergic neurons has been described in the myenteric plexus of patients with Parkinson's disease (Singaram et al. 1995).
The present study was therefore aimed at characterizing the regulation of TH phenotype in the enteric neurons. Primary cultures of ENS derived from rat embryonic intestine were used to study the regulation of TH expression by neuronal activity. In the first part of the study, addition of extracellular KCl and veratridine and electrical field stimulation (EFS) were used to study the regulation of TH expression in our primary culture of enteric neurons. In a second set of experiments, the signalling pathways involved in the regulation of TH expression by neuronal activity were studied by using KCl-induced depolarization as a stimulus (Kilbourne et al. 1992; Brosenitsch et al. 1998; Cigola et al. 1998). We showed that KCl, veratridine and EFS increased the proportion of TH-IR enteric neurons and that these effects were dependent on neuronal activity. The increase of TH-IR neurons induced by KCl was mediated in part via nicotinergic and noradrenergic pathways and required calcium (Ca2+) influx through L-type Ca2+channels. Finally, we showed that protein kinase A (PKA), protein kinase C (PKC) and ERKs were involved in this activity-dependent regulation of TH expression.
Methods
Cell culture
Pregnant Sprague–Dawley rats were purchased (CERJ, Le Genest St Isle, France and Janvier-Breeding Center, Belgium) and manipulated in compliance with the French and Belgian institutional guidelines. These procedures were approved by the local institutional animal research committee (Agreement E. 44011; Inserm, Nantes, France) and by the Ethical Committee for Animal Experiments of the KU Leuven (Agreement P06077), Belgium. Every effort was made to minimize animal suffering and the number of animals used.
Pregnant rats were killed by an overdose of CO2 followed by severing the carotid arteries. The embryos (E15; 35–45 per isolation from 3 pregnant rats) were removed and killed by decapitation. Then, the small intestines of embryos were removed and finely diced in HBSS (Sigma, Saint Quentin Fallavier, France). Tissue fragments were collected in 5 ml of medium (DMEM–F12 1: 1 medium) and digested at 37°C for 15 min in 0.1% trypsin (Sigma). The trypsin reaction was stopped by adding 10 ml of medium containing 10% fetal calf serum and then treated by DNAse I (0.01%; Sigma) for 10 min at 37°C. After triturating with a 10 ml pipette, cells were centrifuged at 750 r.p.m. for 10 min. Cells were counted and then seeded at a density of 2.4×105 cells cm−2 on 24-well plates previously coated for 6 h with a solution of gelatin (0.5%; Sigma) in sterile phosphate buffered saline (PBS). After 24 h, the medium was replaced with a serum-free medium (DMEM–F12 1: 1 containing 1% of N-2 supplement (Life Technologies, Cergy Pontoise, France)). Cells were maintained in culture for 14 days. Half of the medium was replaced every other day.
Electrical activation of enteric neurons and pharmacological experiments
To study the effect of neuronal activity upon the neurochemical phenotype, enteric neurons were electrically stimulated in 24-well plates fitted with a pair of platinum electrodes connected to an electrical stimulator (Dual Impedance Research Stimulator, Harvard Apparatus Ltd, Edenbridge, UK). The electrical field stimulation (EFS) was achieved with trains of constant current pulses with the following parameters: pulse duration: 200 μs; amplitude: 8 V; frequency: 15 Hz, applied during 8 h. Electrode polarity was changed every 15 min and half of the medium was changed at the end of the stimulation protocol. Following EFS, cultures were left unstimulated for an additional 15 h.
Immunohistochemistry and identification of neuronal cell populations
After fixation (in 0.1 m PBS containing 4% paraformaldehyde for 1 h at room temperature), cells were washed 3 times in PBS. Cells were permeabilized for 30 min in PBS/NaN3 containing 0.5% Triton X-100 and 4% horse serum before being incubated with rabbit anti-tyrosine hydroxylase (anti-TH; 1: 1000; Pel-Freez, Rogers, AR, USA), with mouse anti-dopamine β-hydroxylase (anti-DBH; 1: 200; Fitzgerald Industries International, Concord, MA, USA), with mouse anti-vasoactive intestinal peptide (anti-VIP; 1: 800; US biological, MA, USA), with anti-neuronal nitric oxide synthase (anti-nNOS; 1: 2000; Alexis, CA, USA) or anti-caspase 3 active (1: 2000; Sigma, Saint Quentin Fallavier, France) diluted in PBS/NaN3, 0.5% Triton X-100 and 4% horse serum for 1.5 h at room temperature. After incubation with primary antisera, cells were washed 3 times with PBS and incubated for 30 min with the following secondary antibodies coupled to fluorophores: donkey anti-rabbit or anti-mouse IgG conjugated to carboxymethylindocyanine (1: 500; Jackson Laboratories, purchased from Immunotech, Marseille, France). In the following step, the cells were labelled with rabbit anti-PGP9.5 (1: 2000; Ultraclone Limited, Isle of Wight, UK) or with mouse anti-Hu (1: 200; Molecular Probes, Eugene, OR, USA) (general neuronal markers) for 1.5 h. Following incubation with primary antisera, cells were washed with PBS and incubated for 30 min with donkey anti-rabbit IgG or donkey anti-mouse conjugated to FluoProbes®488 (1: 200; Interchim, Montluçon, France).
Cell counting procedure
The number of TH-IR cells and PGP9.5-IR or Hu-IR cells was counted in at least 20 ganglia (649 ± 19 neurons per preparation, n = 12) per well and per condition. The data were expressed as a percentage of TH-IR neurons normalized to the total PGP9.5-IR or Hu-IR neurons.
Quantitative PCR analysis
Total RNA extraction from cells was performed with RNAeasy Minikit (Qiagen S.A., Courtaboeuf, France) according to the manufacturer's instructions. For reverse transcription, 2 μg RNA was combined with 0.5 μg of random hexamers (Amersham, Piscataway, NJ, USA), transcription buffer (50 mm Tris-HCl, pH 8.3, 75 mm KCl, 3 mm MgCl2, 10 mm DTT), dNTPs (10 mm each), RNasin (20 units; Promega, Madison, WI, USA), and RNaseH Maloney murine leukaemia virus reverse transcriptase (200 units; Promega) in a total volume of 25 μl. Incubation was performed at 42°C for 60 min. The following primers were used: TH forward: 5′-GTG AAC CAA TTC CCC ATG-3′, TH reverse: 5′-GGT CGC AGC TGG AAG C-3′ and hypoxanthine-guanine phosphoribosyltransferase (HPRT) forward: 5′-CCT TGG TCA AGC AGT ACA GCC-3′ and HPRT reverse: 5′-TTC GCT GAT GAC ACA AAC ATG A-3′. Real-time PCR procedure was used with custom TaqMan® gene expression assays (PE Applied Biosystems, Foster City, CA, USA) and with gene expression assay (PE Applied Biosystems) for the HPRT. TaqMan probes were chosen to avoid alternative splicing in the target gene. All samples were analysed in duplicate in a total volume of 25 μl with 1 μl of cDNA, 1.25 μl probes and 12.75 μl TaqMan Universal PCR Master Mix 2X (PE Applied Biosystems) and processed in the ABI PRISM 7900HT Sequence Detection System using universal cycling conditions (40 cycles of 95°C for 15 s; 60°C for 1 min). The mRNA level of expression was determined using the formula of the comparative cycle threshold (Ct): 2−ΔΔCt, where
as previously described (Livak & Schmittgen, 2001).
Intracellular [Ca2+] measurements
For intracellular Ca2+ measurements ([Ca2+]i), primary cultures of ENS were seeded on 18 and 13 mm coverslips. After 13 days of culture, experiments were performed at room temperature in a modified Krebs solution containing (mm) 148 NaCl, 5 KCl, 2 CaCl2, 1 MgCl2, 10 glucose, 10 Hepes, with pH adjusted to 7.38 with NaOH (5 m). The high-KCl buffer used for stimulating and identifying neurons contained 75 mm KCl and Na+ was reduced to 78 mm. The [Ca2+]i changes were assayed by Fluo-4 fluorescence imaging. In brief, cultures seeded on coverslips were loaded for 30 min with 10 μm Fluo-4 AM (F14217, Invitrogen, Merelbeke, Belgium) in modified Krebs solution and subsequently rinsed with fresh modified Krebs solution. After loading with the dye, the coverslips with cells were transferred to a recording chamber and mounted on a Zeiss Axiovert 200m microscope equipped with a monochromator (Poly V) and a cooled CCD camera (Imago QE) both from TILL Photonics (Gräfelfing, Germany). Fluo-4 was excited at 488 nm, and its fluorescence emission was collected at 525/50 nm. Recordings were made using a 20X objective under constant perfusion (1 ml minute−1) with modified Krebs solution. The high-KCl medium (75 mm) used to identify neurons and the 10 mm KCl modified Krebs solution (containing Bay-K8644 (1 μm) or not) was applied onto the neurons for 5 s.
Images were collected using TiLLVision software (TILL Photonics, Gräfelfing, Germany) and stored on a personal computer. Further analysis was done using custom written macros in IGOR PRO (Wavemetrics, Lake Oswego, OR, USA). Regions of interest (ROIs) were drawn over each cell, fluorescence intensity was normalized to the basal fluorescence at the onset of the recording for each ROI, and peaks were analysed. A peak was considered if the signal rose above baseline + 5 times the intrinsic noise level. The percentage of responsive cells (%RC) and the maximum [Ca2+]i peak amplitude were determined.
Pharmacological experiments
To study the effect of neuronal activity using depolarizing agents, cells were incubated with KCl 40 mm (Sigma) or 30 μm veratridine (Sigma). An equimolar concentration of NaCl (40 mm) was used to test for any osmotic effect. Tetrodotoxin (0.5 μm, Sigma) was added 30 min prior to the addition of 40 mm KCl or 30 μm veratridine or EFS. Hexamethonium (100 μm), noradrenaline (1 μm), clonidine (1 μm) and nifedipine (1 μm) were added 30 min prior to the addition of 40 mm KCl (all drugs from Sigma). Bay-K8644 (1 μm, Sigma) was added in the presence of 10 mm KCl, as a low concentration of KCl was necessary to unravel the effects of Bay-K8644 in enteric neurons similarly to what was reported in primary sensory neurons (Brosenitsch et al. 1998). To study the signalling pathway involved in the effects of KCl on TH expression, the following drugs (all purchased from Calbiochem) were added 30 min prior to the addition of 40 mm KCl: PD98059, a MAP/ERK (MEK) inhibitor (50 μm) (Alessi et al. 1995), SB203580, a p38 mitogen activated protein kinase (p38 MAPK) inhibitor (10 μm) (Cuenda et al. 1995), H89, a PKA inhibitor (2 μm) (Chijiwa et al. 1990), SQ 22536, an inhibitor of adenylyl-cyclase (100 μm) (Haslam et al. 1978), GF109203X, a PKC inhibitor (1 μm) (Toullec et al. 1991), and PP2, a Src family kinase inhibitor (1 μm) (Hanke et al. 1996).
Statistical analysis
All data are given as the mean ± standard error of the mean (s.e.m.). Comparisons of means between groups were performed by Student's t test for unpaired data or by analysis of variance followed by Turkey's test. When data were not normally distributed, a Mann–Whitney U test was performed. Differences were considered statistically significant if P < 0.05. n indicates the number of experiments.
Results
Primary culture of rat enteric nervous system
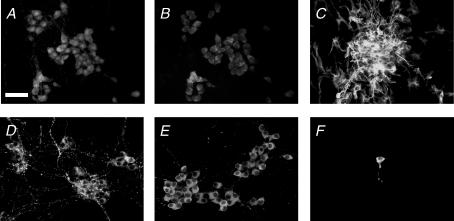
Dissociated cells of the embryonic gut were seeded at a density of 2.4×105 cells cm−2. Following 14 days of culture, enteric neurons are organized in ganglia connected to each other by interganglionic fibre strands. Ganglia contained 29 ± 2 neurons identified with PGP9.5 (Fig. 1A) and an identical number of neurons identified with Hu (30 ± 1; n = 4; P = 1) (Fig. 1B). In addition, glial fibrillary acidic protein (GFAP) positive enteric glial cells were also present in enteric ganglia and along interganglionic fibre strands (Fig. 1C). Analysis of the neurochemically identified population revealed that 16 ± 0% of PGP9.5-IR neurons were VIP–IR (Fig. 1D) and 68 ± 6% were nNOS-IR (Fig. 1E). In contrast, only 1% of neurons were TH- IR (Fig. 1F).
Figure 1. Characterization of primary culture of ENS.
After 14 days in culture, the presence of enteric neurons in primary culture of ENS was assessed by PGP9.5 (A) and Hu immunostaining (B). Enteric glial cells were identified by immunostaining with glial fibrillary acid protein antibodies (C). The neurochemical phenotype was determined by immunostaining using antibodies specific for VIP (D), nNOS (E) and TH (F). Scales bar represents 25 μm.
KCl modulates the neurochemcial coding in primary cultures of enteric neurons
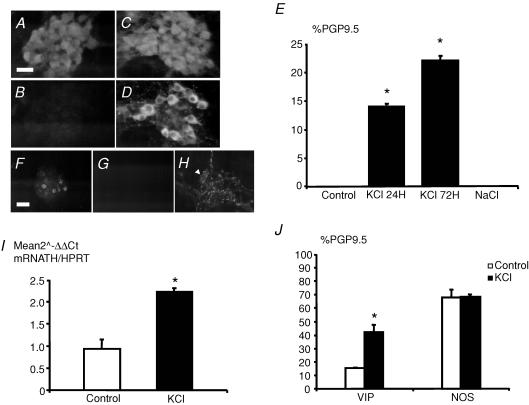
Treatment of primary culture of rat ENS with 40 mm KCl induced a significant and time-dependent increase in the proportion of TH-IR neurons. The KCl-induced increase in TH-IR neurons was observed as early as 24 h of treatment (14 ± 0%versus 0 ± 0% of PGP9.5 IR neurons, in the presence or in the absence of KCl, respectively, n = 12; P < 0.001) and reached about 20% after 72 h (22 ± 1 versus 0 ± 0% of PGP9.5 IR neurons, respectively, n = 8; P < 0.001) (Fig. 2A–E). No cell death as assessed by active caspase 3-IR was observed even following 72 h of treatment with KCl (data not shown). Addition of NaCl of equimolar concentration (40 mm) did not induce TH-IR neurons (Fig. 2E). In addition, these TH-IR neurons were not dopamine β-hydroxylase (DBH)-IR (Fig. 2F–H), suggesting that they are not noradrenergic. The KCl-induced increase in TH-IR was associated with a significant increase by 2.4-fold of transcripts encoding TH compared to control (n = 8; P≤ 0.001) (Fig. 2I).
Figure 2. KCl induces a significant increase in TH and VIP but not nNOS expression in enteric neurons.
After 14 days in culture, enteric neurons identified with PGP9.5 (A) were not immunoreactive (IR) for TH (B). Treatment with 40 mm KCl induced a robust increase in TH-IR (D) in PGP9.5-IR enteric neurons (C). The proportion of TH-IR neurons normalized to the total neuronal population identified with PGP9.5 was significantly increased following KCl treatment for 24 and 72 h (E; n = 12; P < 0.001 and n = 8, P < 0.001, respectively, t test). In contrast, incubation of equimolar concentration of NaCl (40 mm) did not modify the proportion of TH-IR neurons (E; n = 8). TH-IR neurons (F) were not dopamine β-hydroxylase (DBH)-IR (G), although the antibody labelled neurons in adult rat ileum (H; arrow). KCl treatment increased TH mRNA transcript expression normalized to HPRT as compared to control (I; n = 8; P < 0.001, t test). Treatment with KCl for 72 h significantly increased the proportion of VIP-IR neurons (J; n = 4; P = 0.003, t test) but not of nNOS-IR neurons (J; n = 4; P = 1, t test). Scale bar represents 25 μm for A–D and H and 50 μm for F and G. Data are presented as the mean ±s.e.m.
The specificity of the effects of KCl upon other neuromediators was further studied by assessing the proportion of two other neurochemical markers: VIP and NOS. Treatment by KCl induced a significant increase in the proportion of VIP-IR neurons (42 ± 5%versus 16 ± 0% of PGP9.5-IR neurons, in the presence or in the absence of KCl, respectively, n = 4; P = 0.003) but not of NOS-IR neurons as compared to control (68 ± 2%versus 68 ± 6% of PGP9.5 IR neurons, in the presence or in the absence of KCl, respectively, n = 4; P = 1) (Fig. 2J). These changes were also paralleled at the transcriptional levels (data not shown).
Neuronal activity mediates changes in TH expression
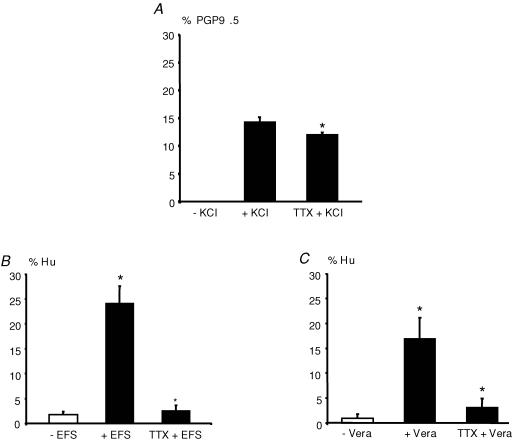
In order to determine whether neuronal activity was involved in the regulation of TH expression, we first studied the effects of the sodium channel blocker tetrodotoxin (TTX) on the KCl-induced increase in TH expression. The KCl-induced increase in the proportion of TH-IR neurons was significantly reduced by 16% (n = 5; P = 0.036) in the presence of 0.5 μm TTX (Fig. 3A). Next, we showed that electrical field stimulation (EFS) of enteric neurons for 8 h induced a significant increase in TH-IR neurons as compared to control (26 ± 3%versus 4 ± 1% of Hu-IR neurons, respectively, n = 3; P < 0.001) (Fig. 3B). This effect was completely prevented in the presence of TTX (Fig. 3B). Further reinforcing the role of neuronal activity in the control of TH expression, we showed that treatment of primary culture of ENS with 30 μm veratridine, a potent activator of sodium channels, induced a significant increase in TH expression in enteric neurons as compared to control (17 ± 4 versus 1 ± 1% of Hu-IR neurons, respectively, n = 4; P = 0.022) (Fig. 3C). Again, this effect was blocked by TTX (Fig. 3C). Throughout the rest of the study, experiments were conducted using KCl as a model of activity-dependent stimuli.
Figure 3. Neuronal activity is involved in the regulation of TH expression.
The increase in the proportion of TH-IR neurons induced by KCl (normalized to the number of PGP9.5-IR neurons) was significantly reduced by 0.5 μm tetrodotoxin (TTX + KCl) (A; n = 5; P = 0.036 one-way ANOVA followed by Turkey's test). EFS (8 h of electrical stimulation) significantly increased the proportion of TH-IR neurons normalized to the number of Hu-IR neurons (+ EFS) as compared to control (− EFS) (B; n = 3; P < 0.001, one-way ANOVA). This effect was blocked following EFS in the presence of 0.5 μm tetrodotoxin (TTX + EFS) (B). Treatment of primary culture of enteric neurons with 30 μm veratridine (24 h) (+ Vera) significantly increased the proportion of TH-IR neurons (normalized to the number of Hu-IR neurons) as compared to control (− Vera) (C; n = 4; P = 0.022, one-way ANOVA). These effects were also blocked in the presence of 0.5 μm tetrodotoxin (TTX + Vera) (C).
Cholinergic and adrenergic pathways modulate the proprotion of TH-IR neurons
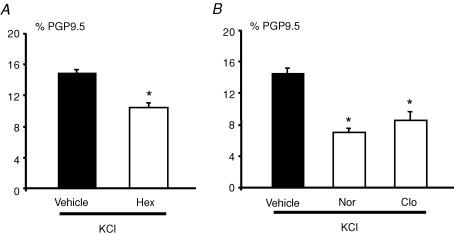
KCl-induced changes in TH expression were significantly reduced down to 70% (n = 9; P < 0.001) by hexamethonium (100 μm), an antagonist of nicotinic receptors (Fig. 4A). In order to ‘physiologically’ decrease cholinergic activity, we used noradrenaline, which is known to inhibit acetylcholine release from excitatory cholinergic nerve terminals via α2-adrenoceptors (Scheibner et al. 2002). Treatment of ENS with KCl in the presence of noradrenaline (1 μm) significantly reduced the proportion of TH-IR neurons compared to control cultures (n = 4; P = 0.005) (Fig. 4B). A similar decrease in the proportion of TH-IR neurons was observed when clonidine (1 μm), a specific α2-adrenoreceptor agonist, was used (n = 4; P = 0.005) (Fig. 4B).
Figure 4. Cholinergic and noradrenergic pathways regulate the KCl-induced increase in the proportion of TH-immunoreactive neurons.
The increase in the proportion of TH-IR neurons induced by KCl was significantly reduced by hexamethonium (Hex, 100 μm) as compared to control (Vehicle) (A; n = 9; P < 0.001, t test). The KCl-induced increase in the proportion of TH-IR neurons was also significantly reduced by noradrenaline (Nor, 1 μm) and clonidine (Clo, 1 μm) as compared to control (Vehicle) (B; n = 4; P≤ 0.001 and n = 4; P = 0.005, respectively, t test). Data are presented as the mean ±s.e.m.
KCl-induced increase in the proportion of TH-IR neurons involves L-type Ca2+ channels
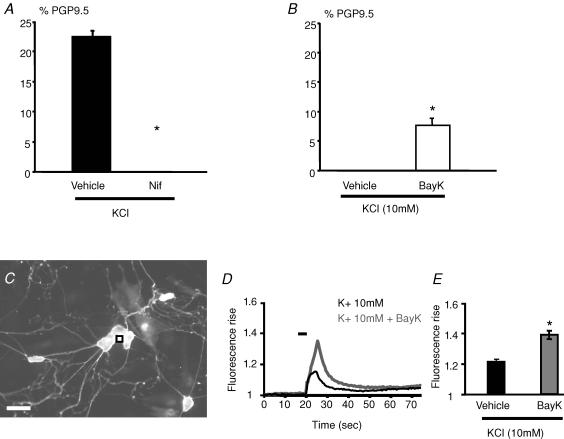
Previous studies performed in olfactory and sensory neurons showed that the KCl-induced increase of TH expression resulted at least in part from an increase in intracellular Ca2+ through L-type Ca2+ channels (Brosenitsch et al. 1998; Cigola et al. 1998). The involvement of L-type Ca2+ channels in KCl-induced increase in the proportion of TH-IR neurons was therefore first studied in enteric neurons with nifedipine, a specific antagonist of these channels. Treatment of primary culture of ENS with nifedipine (1 μm) completely prevented the KCl-induced increase in the proportion of TH-IR neurons (Fig. 5A). Conversely, incubation of primary cultures of ENS with the selective L-type Ca2+ channels agonist BayK-8644 (in the presence of 10 mm KCl, as previously described; Cigola et al. 1998) significantly increased the proportion of TH-IR neurons as compared to control (10 mm KCl) (7.7 ± 1%versus 0 ± 0%, n = 6; P = 0.002) (Fig. 5B). Addition of 10 mm KCl per se did not modify the proportion of TH-IR neurons (Fig. 5B). Using Ca2+ imaging studies, we have further shown that microapplication of 1 μm BayK-8644 in the presence of 10 mm KCl to enteric neurons induced a significant increase in the transient Ca2+ rise as compared to application of 10 mm KCl alone (n = 93 neurons, P < 0.0001) (Fig. 5D and E). In addition, the percentage of KCl-responsive cells showed a slight increase in the presence of BayK-8644 (97.3%versus 84.5% in 10 mm KCl alone). After performing the experiments, neurons were identified by exposure to 75 mm KCl (Fig. 5C).
Figure 5. L-type calcium channels are involved in the KCl-induced increase in the proportion of TH-immunoreactive neurons.
The increase in the proportion of TH-IR neurons induced by KCl was completely prevented by nifedipine (Nif, 1 μm) (A; n = 6, P = 0.002, Mann–Whitney rank sum test). Treatment with BayK-8644 (BayK, 1 μm) in the presence of 10 mm KCl significantly increased the proportion of TH-IR neurons as compared to control (Vehicle) (B; n = 6, P = 0.002, Mann–Whitney rank sum test). Intracellular calcium measurements were performed on cells loaded with Fluo-4 AM and identification of neurons was performed by application of 75 mm KCl (C) and Ca2+ signals were calculated from individual neurons (e.g. as indicated with a black square). Microejection of 10 mm KCl (5 s ejection duration) onto neurons induced a transient relative fluorescence rise (F1/F0) of the Ca2+ indicator Fluo-4 over time (D). Microejection of 10 mm KCl in the presence of BayK-8644 (dark grey line, 1 μm) induced a larger transient fluorescence rise than with 10 mm KCl (D). The traces in this example are from the neuron marked (black square) in C. The amplitude of the transient relative fluorescence rise induced by 10 mm KCl is significantly larger in the presence of BayK-8644 (E; P < 0.0001; n = 93 neurons). Scale bar represents 50 μm. Data are presented as the mean ±s.e.m.
ERKs but not p38 are involved in KCl-induced increase in the proportion TH-IR neurons
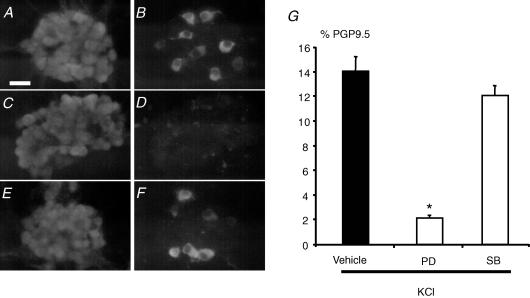
The involvement of the ERKs pathway in the regulation of KCl-induced increase in TH-IR in enteric neurons was studied with pharmacological tools. Pretreatment of enteric neurons with PD98059 (MEK inhibitor; 50 μm) significantly reduced by 85% the KCl-induced increase in the proportion of TH-IR neurons (n = 5; P < 0.001) (Fig. 6A–D and G). In contrast, pretreatment of enteric neurons with SB203580 (p38 inhibitor; 10 μm) did not significantly modify the KCl-induced increase in TH expression in enteric neurons (n = 5; P = 0.11) (Fig. 6A, B, E, F and G).
Figure 6. ERK- but not p38-dependent pathways mediate the KCl-induced increase in the proportion of TH-immunoreactive neurons.
Photomicrographs illustrate that in the presence of KCl a proportion of PGP9.5-IR neurons (A) was TH-IR (B). In the presence of KCl and the inhibitor of ERK pathways (PD98059; 50 μm), no PGP9.5-IR neurons (C) were TH-IR (D). In contrast, in the presence of KCl and the inhibitor of p38 pathways (SB203580; 10 μm) a proportion of PGP9.5-IR neurons (E) was still TH-IR (F). Quantification of the results demonstrated that PD98059 (PD), but not SB203580 (SB) significantly reduced the KCl-induced increase in the proportion of TH-IR neurons (G; P < 0.001, n = 5,t test). Scale bar represents 25 μm. Data are presented as the mean ±s.e.m.
Protein kinase C and the cAMP pathway are involved in the KCl-induced increase in the proportion of TH-IR neurons
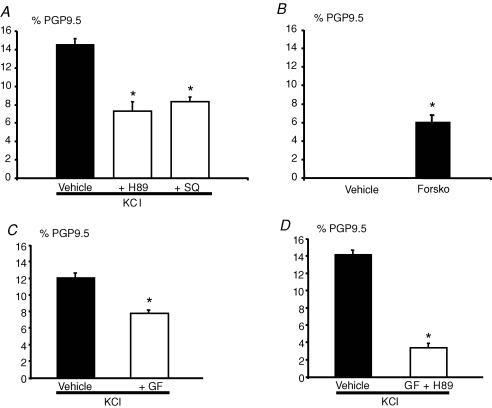
In neuronal cells, Ca2+ influx can activate ERK through several signalling pathways. This can be achieved through activation of the proline rich tyrosine kinase 2 (Pyk2)/Src complex, PKC, PKA and CaM kinase (Derkinderen et al. 1999). The role of these signalling pathways linking Ca2+ influx, ERK and TH expression was studied using a pharmacological approach. First, two inhibitors of the cAMP pathway, SQ22536 (adenylate cyclase inhibitor; 100 μm) and H89 (PKA inhibitor; 2 μm), significantly reduced the KCl-induced increase in the proportion of TH-IR neurons by 43% and 50%, respectively (n = 5; P = 0.008 and P < 0.001, respectively) (Fig. 7A). Treatment with 20 μm forskolin, an activator of adenylyl cyclase induced a significant increase in the number of TH-IR neurons as compared to control (6 ± 1 versus 0 ± 0% of PGP9.5-IR neurons, respectively, n = 5; P = 0.001), further reinforcing the role of the cAMP pathway in the regulation of TH expression (Fig. 7B). Pretreatment of neurons with GF109203X (1 μm), a specific inhibitor of PKC, significantly reduced the KCl-induced increase of TH-IR by 42% (n = 4, P = 0.029) (Fig. 7C). Concomitant addition of both H89 and GF109203X induced a significant additive inhibition of the KCl-induced increase of TH-IR neurons by 74% (Fig. 7D). In contrast, PP2 (an inhibitor of the Src family kinase; 1 μm) did not inhibit KCl effects on TH-IR in enteric neurons (n = 4; data not shown).
Figure 7. Both, cAMP- and PKC-dependent pathways mediate the KCl-induced increase in TH-immunoreactive neurons.
The increase in the proportion of TH-IR neurons induced by KCl was significantly reduced by an inhibitor of adenylate cyclase, SQ22536 (SQ, 100 μm; n = 5; P = 0.008, t test), and by an inhibitor of PKA, H89 (A) (H89 2 μm; n = 5; P≤ 0.001, t test). Forskolin, an activator of adenylate cyclase, induced a significant increase in the proportion of TH-IR neurons as compared to control (B) (Forsko, 20 μm; n = 5; P = 0.001, t test). The increase in the proportion of TH-IR neurons induced by KCl was also significantly reduced by the inhibitor of PKC, GF109203X (C) (GF 1 μm; n = 4; P = 0.029, t test). In the presence of both PKC and PKA inhibitors, the increase in the proportion of TH-IR neurons induced by KCl was further significantly reduced as compared to the condition with the inhibitors alone (D) (n = 5; P≤ 0.001, t test). Data are presented as the mean ±s.e.m.
Discussion
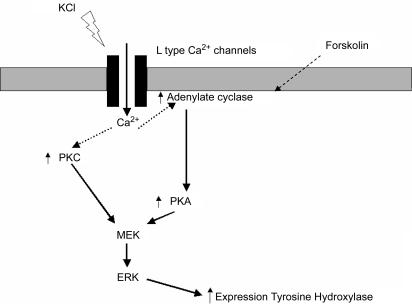
Our study showed that activity-dependent stimuli can modulate TH expression in enteric neurons in part via cholinergic pathways and activation of L-type Ca2+ channels. Furthermore, TH regulation was shown to involve both PKC- and PKA-dependent pathways leading to ERK activation. To the best of our knowledge, this is the first study characterizing the signalling pathways involved in the regulation of a neuromediator in the ENS. In addition, it is also one of the rare studies linking activity-dependent signalling pathways regulating TH expression in primary culture of neurons (Fig. 8).
Figure 8. Signalling pathways involved in the activity-dependent regulation of TH expression in enteric neurons.
Neuronal activation by depolarization results in opening of L-type calcium channels leading to an increase in calcium entry. This increase in intracellular calcium induces an activation of ERKs through an activation of both PKA and PKC, resulting in an increase in TH expression.
Activity-dependent regulation of neuromediators has been well documented in the CNS (West et al. 2001). In particular, depolarization was able to increase the proportion of dopaminergic/TH-IR neurons in primary cultures of mouse olfactory bulb and of rat brain (Brosenitsch et al. 1998; Cigola et al. 1998). Nicotine has also been demonstrated to increase TH expression both in the CNS and in sympathetic ganglia (Otten & Thoenen, 1976; Sun et al. 2003). Consistent with these observations, we have shown that nicotinergic cholinergic pathways can modulate TH expression in the ENS. Although acetylcholine (ACh) is the main fast excitatory neurotransmitter in the ENS, its effect on regulation of gene expression remains largely unknown and needs further study. In our model, we showed that a physiological inhibition of cholinergic activity mimicked by noradrenaline was able to antagonize the KCl-induced increase in the proportion of TH-IR, via activation of α2-receptors. These results are the basis of the observation that in vivo sympathetic denervation increases the TH expression in myenteric and submucosal neurons in mice (Li et al. 2004). Furthermore, the observation that cholinergic activity increases the proportion of TH-IR neurons in the ENS suggests a paradigm for a physiological regulation of TH expression in the ENS. An increase in the activity of the ENS could indeed lead to an up-regulation of dopamine synthesis aimed at reducing this neuronal activity. This hypothesis is reinforced by the observation that dopamine inhibits the evoked release of [3H]ACh from enteric neurons (Kusunoki et al. 1985; Takahashi et al. 1991). A similar homeostatic regulation of neurotransmitters has been shown to occur in the CNS. Indeed, activity has been shown to participate to the homeostatic regulation of transmitter specification (Spitzer et al. 2005). In particular, an increase in neuronal activity down-regulates the expression of excitatory mediators such as ACh while it increases the expression of inhibitory mediators such as GABA or dopamine, through a regulation of TH expression (Spitzer et al. 2005). Modulation of enteric phenotype by sympathetic pathways is probably not restricted to TH as sympathetic denervation has been shown to increase the expression of neuronal nitric oxide synthase in rat jejunal myenteric neurons, an effect mediated by the activation of α2-adrenoreceptors (Nishizaki et al. 2003).
Interestingly, transient TH-IR neurons have been described in the mouse gut during development. These transient TH-IR neurons are the first to exhibit an adult-like phenotype (Gershon et al. 1993; Young et al. 1999, 2002). Thus, it is tempting to speculate that the increase in the number of TH-IR neurons induced by neuronal activity in our experiments could be a correlate of the transient cathecolaminergic phenotype observed during development. However, while TH-IR neurons increase, no change in the proportion of nNOS-IR is reported in this study. Since TH-IR transient neurons have been shown to be the progenitors of nNOS neurons, it is therefore unlikely that TH-IR neurons are the transient TH-IR neurons. Furthermore, KCl also induced a robust increase in the proportion of VIP-IR neurons. As VIP expression has been shown to peak when TH-IR fades during development (Pham et al. 1991), we further believe that the concomitant increase induced by KCl in TH- and VIP-IR neurons is probably not associated with a dedifferentiation.
In our study, activity-dependent regulation of TH expression was fully blocked by an inhibitor of L-type Ca2+ channels and mimicked by an agonist of these channels demonstrating a key role for L-type Ca2+ channels in the regulation of TH expression in the ENS. Such a critical role of L-type Ca2+ channels in the regulation of TH expression has been documented in both mouse olfactory neurons and primary sensory neurons (Brosenitsch et al. 1998; Cigola et al. 1998). Ca2+ channels are present in enteric neurons but their role in ENS physiology has been poorly studied. Isolated myenteric ganglia of newborn rats and neurons from adult rats and guinea pigs express different types of voltage-dependent Ca2+ channels, from which the L- and the N-type seem to be the most important (Kirchgessner & Liu, 1999; Schaufele & Diener, 2005). Our results provide further evidences that L-type Ca2+ channels are not only expressed by enteric neurons but also involved in ENS physiology and neuromediator plasticity.
Using a specific inhibitor of MEK, we showed that ERKs are involved in the regulation of TH expression in enteric neurons. Several studies have provided evidence implicating ERKs as a critical player in synaptic and neuronal plasticity, through their role in the regulation of gene expression (Curtis & Finkbeiner, 1999). Such a critical role of ERK in TH expression has already been documented in neuronal cells treated with uracil nucleotides, short chain fatty acids and fibroblast growth factor (Milosevic et al. 2006; Shah et al. 2006). ERK could thus be a final common pathway to the regulation of TH expression. Depolarization has been shown to increase the expression of TH in primary sensory neurons and PC12 cells (Kilbourne et al. 1992; Brosenitsch & Katz, 2001). Interestingly, in these neuronal cells, the effects of KCl on TH expression were insensitive to specific inhibitors of PKA and PKC (Kilbourne et al. 1992; Brosenitsch & Katz, 2001). This contrasts with our results obtained in primary enteric neurons in which both PKC and PKA are involved in the regulation of TH expression by depolarization. It has been suggested that a PKA-dependent signalling pathway links neuronal Ca2+ influx to ERKs via the small G-protein, Rap1, and the neuronal Raf isoform, B-Raf (Grewal et al. 2000; Baldassa et al. 2003). Depending on the neuronal cell type (PC12 cells or primary cultures of rat hippocampal neurons), depolarization can activate either PKA-dependent or PKA-independent pathways to ERKs. Our results obtained in enteric neurons show that depolarization may be able to activate both PKA-dependent and -independent pathways equally, as already observed in hippocampal neurons (Grewal et al. 2000). They also suggest that both Rap1 and B-Raf could be present in enteric neurons.
Whether activity-dependent regulation of neuromediators also occurs in the ENS is currently unknown. However, in various GI pathologies both in infants and in the adults, plasticity in the neurochemical phenotype of the ENS occurs. These changes have been observed in inflammatory bowel disease such as ulcerative colitis (Neunlist et al. 2003), Crohn's disease (Schneider et al. 2001) or even in atresia (Khen et al. 2004). Consistent with a putative involvement of activity and calcium signalling in these alterations is the observation that inflammatory mediators present in these pathologies such as IL-1β and TNF-α modulate both neuronal excitability and [Ca2+]i (Xia et al. 1999; Kelles et al. 2000; Rehn et al. 2004). Therefore, depending on the inflammatory phenotype (Crohn's disease or ulcerative colitis), the neuronal calcium response could differ and differentially regulates neuromediator gene expression. Besides a role in pathology, activity-dependent control of neuronal phenotype could also be involved in the development of GI functions, in particular motility. Indeed, recent data in mice have shown that the establishment of neural control of motility is paralleled by an increase in the cholinergic phenotype of the ENS (Roberts et al. 2007). One can speculate that activity-dependent signalling due to the muscle distension or mucosal stroking (induced by luminal gut content) could be directly involved in maturation of the ENS neurochemical phenotype.
In conclusion, these results are the first demonstration that the TH phenotype of enteric neurons can be regulated by neuronal activity. They could also form the basis of the study of the pathways and mechanisms involved in the neurochemical plasticity observed both during ENS development and in inflammatory enteric neuropathies.
Acknowledgments
This work was supported by a grant from Agence Nationale de la Recherche (ANR) Nutrisens (to M.N. and P.N.) and the Action Spécifique of the University of Nantes, a grant from Fonds voor Wetenschappelijk Onderzoek (FWO) to P.G. and P.V.B. and a grant from France Parkinson, ADPLA (association des parkinsoniens de Loire Atlantique) and Féderation des groupements de Parkinsoniens (Vendée) to P.D. and M.N.
References
- Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- Anlauf M, Schafer MK, Eiden L, Weihe E. Chemical coding of the human gastrointestinal nervous system: cholinergic, VIPergic, and catecholaminergic phenotypes. J Comp Neurol. 2003;459:90–111. doi: 10.1002/cne.10599. [DOI] [PubMed] [Google Scholar]
- Baldassa S, Zippel R, Sturani E. Depolarization- induced signaling to Ras, Rap1 and MAPKs in cortical neurons. Brain Res Mol Brain Res. 2003;119:111–122. doi: 10.1016/j.molbrainres.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Belai A, Boulos PB, Robson T, Burnstock G. Neurochemical coding in the small intestine of patients with Crohn's disease. Gut. 1997;40:767–774. doi: 10.1136/gut.40.6.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosenitsch TA, Katz DM. Physiological patterns of electrical stimulation can induce neuronal gene expression by activating N-type calcium channels. J Neurosci. 2001;21:2571–2579. doi: 10.1523/JNEUROSCI.21-08-02571.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosenitsch TA, Salgado-Commissariat D, Kunze DL, Katz DM. A role for L-type calcium channels in developmental regulation of transmitter phenotype in primary sensory neurons. J Neurosci. 1998;18:1047–1055. doi: 10.1523/JNEUROSCI.18-03-01047.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chijiwa T, Mishima A, Hagiwara M, Sano M, Hayashi K, Inoue T, Naito K, Toshioka T, Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem. 1990;265:5267–5272. [PubMed] [Google Scholar]
- Cigola E, Volpe BT, Lee JW, Franzen L, Baker H. Tyrosine hydroxylase expression in primary cultures of olfactory bulb: role of L-type calcium channels. J Neurosci. 1998;18:7638–7649. doi: 10.1523/JNEUROSCI.18-19-07638.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuenda A, Rouse J, Doza YN, Meier R, Cohen P, Gallagher TF, Young PR, Lee JC. SB 203580 is a specific inhibitor of a MAP kinase homologue which is stimulated by cellular stresses and interleukin-1. FEBS Lett. 1995;364:229–233. doi: 10.1016/0014-5793(95)00357-f. [DOI] [PubMed] [Google Scholar]
- Curtis J, Finkbeiner S. Sending signals from the synapse to the nucleus: possible roles for CaMK, Ras/ERK, and SAPK pathways in the regulation of synaptic plasticity and neuronal growth. J Neurosci Res. 1999;58:88–95. [PubMed] [Google Scholar]
- Derkinderen P, Enslen H, Girault JA. The ERK/MAP-kinases cascade in the nervous system. Neuroreport. 1999;10:R24–R34. [PubMed] [Google Scholar]
- Du Z, Guo X, Iacovitti L. Regulation of tyrosine hydroxylase gene expression during transdifferentiation of striatal neurons: changes in transcription factors binding the AP-1 site. J Neurosci. 1998;18:8163–8174. doi: 10.1523/JNEUROSCI.18-20-08163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaker EY, Bixler GB, Dunn AJ, Moreshead WV, Mathias JR. Dopamine and norepinephrine in the gastrointestinal tract of mice and the effects of neurotoxins. J Pharmacol Exp Ther. 1988;244:438–442. [PubMed] [Google Scholar]
- Gershon MD, Chalazonitis A, Rothman TP. From neural crest to bowel: development of the enteric nervous system. J Neurobiol. 1993;24:199–214. doi: 10.1002/neu.480240207. [DOI] [PubMed] [Google Scholar]
- Goyal RK, Hirano I. The enteric nervous system. N Engl J Med. 1996;334:1106–1115. doi: 10.1056/NEJM199604253341707. [DOI] [PubMed] [Google Scholar]
- Grewal SS, Horgan AM, York RD, Withers GS, Banker GA, Stork PJ. Neuronal calcium activates a Rap1 and B-Raf signaling pathway via the cyclic adenosine monophosphate-dependent protein kinase. J Biol Chem. 2000;275:3722–3728. doi: 10.1074/jbc.275.5.3722. [DOI] [PubMed] [Google Scholar]
- Hanke JH, Gardner JP, Dow RL, Changelian PS, Brissette WH, Weringer EJ, Pollok BA, Connelly PA. Discovery of a novel, potent, and Src family-selective tyrosine kinase inhibitor. Study of Lck- and FynT-dependent T cell activation. J Biol Chem. 1996;271:695–701. doi: 10.1074/jbc.271.2.695. [DOI] [PubMed] [Google Scholar]
- Haslam RJ, Davidson MM, Desjardins JV. Inhibition of adenylate cyclase by adenosine analogues in preparations of broken and intact human platelets. Evidence for the unidirectional control of platelet function by cyclic AMP. Biochem J. 1978;176:83–95. doi: 10.1042/bj1760083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelles A, Janssens J, Tack J. IL-1β and IL-6 excite neurones and suppress cholinergic neurotransmission in the myenteric plexus of the guinea pig. Neurogastroenterol Motil. 2000;12:531–538. doi: 10.1046/j.1365-2982.2000.00228.x. [DOI] [PubMed] [Google Scholar]
- Khen N, Jaubert F, Sauvat F, Fourcade L, Jan D, Martinovic J, Vekemans M, Landais P, Brousse N, Leborgne M, Nihoul-Fekete C, Cerf-Bensussan N, Sarnacki S. Fetal intestinal obstruction induces alteration of enteric nervous system development in human intestinal atresia. Pediatr Res. 2004;56:975–980. doi: 10.1203/01.PDR.0000145294.11800.71. [DOI] [PubMed] [Google Scholar]
- Kilbourne EJ, Nankova BB, Lewis EJ, McMahon A, Osaka H, Sabban DB, Sabban EL. Regulated expression of the tyrosine hydroxylase gene by membrane depolarization. Identification of the responsive element and possible second messengers. J Biol Chem. 1992;267:7563–7569. [PubMed] [Google Scholar]
- Kirchgessner AL, Liu MT. Differential localization of Ca2+ channel a1 subunits in the enteric nervous system: presence of a1B channel-like immunoreactivity in intrinsic primary afferent neurons. J Comp Neurol. 1999;409:85–104. doi: 10.1002/(sici)1096-9861(19990621)409:1<85::aid-cne7>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Kusunoki M, Taniyama K, Tanaka C. Dopamine regulation of [3H]acetylcholine release from guinea-pig stomach. J Pharmacol Exp Ther. 1985;234:713–719. [PubMed] [Google Scholar]
- Li ZS, Pham TD, Tamir H, Chen JJ, Gershon MD. Enteric dopaminergic neurons: definition, developmental lineage, and effects of extrinsic denervation. J Neurosci. 2004;24:1330–1339. doi: 10.1523/JNEUROSCI.3982-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZS, Schmauss C, Cuenca A, Ratcliffe E, Gershon MD. Physiological modulation of intestinal motility by enteric dopaminergic neurons and the D2 receptor: analysis of dopamine receptor expression, location, development, and function in wild-type and knock-out mice. J Neurosci. 2006;26:2798–2807. doi: 10.1523/JNEUROSCI.4720-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Milosevic J, Brandt A, Roemuss U, Arnold A, Wegner F, Schwarz SC, Storch A, Zimmermann H, Schwarz J. Uracil nucleotides stimulate human neural precursor cell proliferation and dopaminergic differentiation: involvement of MEK/ERK signalling. J Neurochem. 2006;99:913–923. doi: 10.1111/j.1471-4159.2006.04132.x. [DOI] [PubMed] [Google Scholar]
- Neunlist M, Aubert P, Toquet C, Oreshkova T, Barouk J, Lehur PA, Schemann M, Galmiche JP. Changes in chemical coding of myenteric neurones in ulcerative colitis. Gut. 2003;52:84–90. doi: 10.1136/gut.52.1.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizaki K, Nakao K, Ishii H, Yamanaka H, Tokunaga A, Nakagawa K, Yamamura T, Noguchi K. Induction of neuronal nitric oxide synthase by sympathetic denervation is mediated via a2-adrenoceptors in the jejunal myenteric plexus. Brain Res. 2003;965:121–129. doi: 10.1016/s0006-8993(02)04148-3. [DOI] [PubMed] [Google Scholar]
- Otten U, Thoenen H. Mechanisms of tyrosine hydroxylase and dopamine β-hydroxylase induction in organ cultures of rat sympathetic ganglia by potassium depolarization and cholinomimetics. Naunyn Schmiedebergs Arch Pharmacol. 1976;292:153–159. doi: 10.1007/BF00498586. [DOI] [PubMed] [Google Scholar]
- Pham TD, Gershon MD, Rothman TP. Time of origin of neurons in the murine enteric nervous system: sequence in relation to phenotype. J Comp Neurol. 1991;314:789–798. doi: 10.1002/cne.903140411. [DOI] [PubMed] [Google Scholar]
- Rehn M, Hubschle T, Diener M. TNF-α hyperpolarizes membrane potential and potentiates the response to nicotinic receptor stimulation in cultured rat myenteric neurones. Acta Physiol Scand. 2004;181:13–22. doi: 10.1111/j.1365-201X.2004.01269.x. [DOI] [PubMed] [Google Scholar]
- Roberts RR, Murphy JF, Young HM, Bornstein JC. Development of colonic motilty in the neonatal mouse – studies using spatiotemporal maps. Am J Physiol Gastrointest Liver Physiol. 2007;292:G93013–G938. doi: 10.1152/ajpgi.00444.2006. [DOI] [PubMed] [Google Scholar]
- Sann H, Hoppe S, Baldwin L, Grundy D, Schemann M. Presence of putative neurotransmitters in the myenteric plexus of the gastrointestinal tract and in the musculature of the urinary bladder of the ferret. Neurogastroenterol Motil. 1998;10:35–47. doi: 10.1046/j.1365-2982.1998.00083.x. [DOI] [PubMed] [Google Scholar]
- Schaufele N, Diener M. Pharmacological characterisation of voltage-dependent Ca2+ channels in isolated ganglia from the myenteric plexus. Life Sci. 2005;77:2489–2499. doi: 10.1016/j.lfs.2004.12.053. [DOI] [PubMed] [Google Scholar]
- Scheibner J, Trendelenburg AU, Hein L, Starke K, Blandizzi C. α2-Adrenoceptors in the enteric nervous system: a study in α2A-adrenoceptor-deficient mice. Br J Pharmacol. 2002;135:697–704. doi: 10.1038/sj.bjp.0704512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schemann M, Neunlist M. The human enteric nervous system. Neurogastroenterol Motil. 2004;16(Suppl. 1):55–59. doi: 10.1111/j.1743-3150.2004.00476.x. [DOI] [PubMed] [Google Scholar]
- Schemann M, Schaaf C, Mader M. Neurochemical coding of enteric neurons in the guinea pig stomach. J Comp Neurol. 1995;353:161–178. doi: 10.1002/cne.903530202. [DOI] [PubMed] [Google Scholar]
- Schneider J, Jehle EC, Starlinger MJ, Neunlist M, Michel K, Hoppe S, Schemann M. Neurotransmitter coding of enteric neurones in the submucous plexus is changed in non-inflamed rectum of patients with Crohn's disease. Neurogastroenterol Motil. 2001;13:255–264. doi: 10.1046/j.1365-2982.2001.00265.x. [DOI] [PubMed] [Google Scholar]
- Shah P, Nankova BB, Parab S, La Gamma EF. Short chain fatty acids induce TH gene expression via ERK-dependent phosphorylation of CREB protein. Brain Res. 2006;1107:13–23. doi: 10.1016/j.brainres.2006.05.097. [DOI] [PubMed] [Google Scholar]
- Singaram C, Ashraf W, Gaumnitz EA, Torbey C, Sengupta A, Pfeiffer R, Quigley EM. Dopaminergic defect of enteric nervous system in Parkinson's disease patients with chronic constipation. Lancet. 1995;346:861–864. doi: 10.1016/s0140-6736(95)92707-7. [DOI] [PubMed] [Google Scholar]
- Spitzer NC, Borodinsky LN, Root CM. Homeostatic activity-dependent paradigm for neurotransmitter specification. Cell Calcium. 2005;37:417–423. doi: 10.1016/j.ceca.2005.01.021. [DOI] [PubMed] [Google Scholar]
- Sun B, Sterling CR, Tank AW. Chronic nicotine treatment leads to sustained stimulation of tyrosine hydroxylase gene transcription rate in rat adrenal medulla. J Pharmacol Exp Ther. 2003;304:575–588. doi: 10.1124/jpet.102.043596. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Kurosawa S, Wiley JW, Owyang C. Mechanism for the gastrokinetic action of domperidone. In vitro studies in guinea pigs. Gastroenterology. 1991;101:703–710. doi: 10.1016/0016-5085(91)90528-s. [DOI] [PubMed] [Google Scholar]
- Toullec D, Pianetti P, Coste H, Bellevergue P, Grand-Perret T, Ajakane M, Baudet V, Boissin P, Boursier E, Loriolle F, et al. The bisindolylmaleimide GF 109203X is a potent and selective inhibitor of protein kinase C. J Biol Chem. 1991;266:15771–15781. [PubMed] [Google Scholar]
- Walker JK, Gainetdinov RR, Mangel AW, Caron MG, Shetzline MA. Mice lacking the dopamine transporter display altered regulation of distal colonic motility. Am J Physiol Gastrointest Liver Physiol. 2000;279:G311–G318. doi: 10.1152/ajpgi.2000.279.2.G311. [DOI] [PubMed] [Google Scholar]
- West AE, Chen WG, Dalva MB, Dolmetsch RE, Kornhauser JM, Shaywitz AJ, Takasu MA, Tao X, Greenberg ME. Calcium regulation of neuronal gene expression. Proc Natl Acad Sci U S A. 2001;98:11024–11031. doi: 10.1073/pnas.191352298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia Y, Hu HZ, Liu S, Ren J, Zafirov DH, Wood JD. IL-1β and IL-6 excite neurons and suppress nicotinic and noradrenergic neurotransmission in guinea pig enteric nervous system. J Clin Invest. 1999;103:1309–1316. doi: 10.1172/JCI5823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young HM, Ciampoli D, Hsuan J, Canty AJ. Expression of Ret-, p75(NTR)-, Phox2a-, Phox2b-, and tyrosine hydroxylase immunoreactivity by undifferenciated neural crest-derived cells and different classes of enteric neurons in the embryonic mouse gut. Dev Dyn. 1999;216:137–152. doi: 10.1002/(SICI)1097-0177(199910)216:2<137::AID-DVDY5>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Young HM, Jones BR, McKeown SJ. The projections of early enteric neurons are influenced by the direction of neural crest cell migration. J Neurosci. 2002;22:6005–6018. doi: 10.1523/JNEUROSCI.22-14-06005.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang GH, Zhu JX, Xue H, Fan J, Chen X, Tsang LL, Chung YW, Xing Y, Chan HC. Dopamine stimulates Cl− absorption coupled with HCO3− secretion in rat late distal colon. Eur J Pharmacol. 2007;570:188–195. doi: 10.1016/j.ejphar.2007.05.038. [DOI] [PubMed] [Google Scholar]