Abstract
Inwardly rectifying potassium (Kir) channels are gated by the interaction of their cytoplasmic regions with membrane-bound phosphatidylinositol-4,5-bisphosphate (PIP2). In the present study, we examined how PIP2 interaction regulates channel availability and channel openings to various subconductance levels (sublevels) as well as the fully open state in the strong inward rectifier Kir2.1 channel. Various Kir2.1 channel constructs were expressed in Xenopus oocytes and single channel or macroscopic currents were recorded from inside-out patches. The wild-type (WT) channel rarely visited the subconductance levels under control conditions. However, upon reducing Kir2.1 channel interaction with PIP2 by a variety of interventions, including PIP2 antibodies, screening PIP2 with neomycin, or mutating PIP2 binding sites (e.g. K188Q), visitation to the sublevels was markedly increased before channels were converted to an unavailable mode in which they did not open. No channel activity was detected in channels with the double mutation K188A/R189A, a mutant which exhibits extremely weak interaction with PIP2. By linking subunits together in tandem dimers or tetramers containing mixtures of WT and K188A/R189A subunits, we demonstrate that one functional PIP2-interacting WT subunit is sufficient to convert channels from the unavailable to the available mode with a high open probability dominated by the fully open state, with similar kinetics as tetrameric WT channels. Occasional openings to sublevels become progressively less frequent as the number of WT subunits increases. Quantitative analysis reveals that the interaction of PIP2 with WT subunits exerts strong positive cooperativity in both converting the channels from the unavailable to the available mode, and in promoting the fully open state over sublevels. We conclude that the interaction of PIP2 with only one Kir2.1 subunit is sufficient for the channel to become available and to open to its full conductance state. Interaction with additional subunits exerts positive cooperativity at multiple levels to further enhance channel availability and promote the fully open state.
Kir channel activity depends on the interaction of the channel with phosphatidylinosital-4,5-bisphosphate (PIP2) (Hilgemann et al. 2001). Many regulatory signals modulating channel activity are believed to act through the common mechanism of modifying the channel's affinity for PIP2 (see Xie et al. 2007 for review): for example, protein kinase A (PKA) phosphorylation in Kir1.1 channels (Liou et al. 1999; Zeng et al. 2003), polyamines in strong rectifier Kir2.1 channels (Xie et al. 2005), Gβγ in Kir3 channels (Huang et al. 1998; Ho & Murrell-Lagnado, 1999; Zhang et al. 1999), and the sulphonylurea receptor SUR1 in Kir6.2 channels (Song & Ashcroft, 2001).
Single channel recordings revealed that the Kir2.1 channel enters an extra-long closed state when it undergoes rundown due to depleted membrane PIP2 levels (Xiao et al. 2003). In this state, the channel is fully closed and remains in a distinct unavailable mode which can only be recovered by exogenous application of PIP2 or MgATP. Another intriguing feature of Kir channels is the relationship between their PIP2 affinity and the occurrence of subconductance levels (sublevels). In Kir1.1 channels, Leung et al. (2000) found that disruption of the PIP2–channel interaction not only decreased single channel open probability (Po), but also caused the appearance of sublevels. In Kir2.1 channels, Lopes et al. (2002) reported three types of single channels with different amplitudes and/or gating when they coexpressed WT subunits (1: 20) with R218Q, a mutant subunit with reduced PIP2 affinity. They suggested that different WT/mutant stoichiometries resulted in different channel conductances and gating. In addition to disruption of PIP2–channel interaction, sublevels have also been observed in Kir channels during (1) block by cationic ions such as Mg2+ or Ca2+ (Mazzanti et al. 1996; Oishi et al. 1998); (2) partial block of the internal channel pore with methanthiosulphonate (MTS) reagents (Lu et al. 1999a) or Tl+ (Lu et al. 2001b); and (3) conformational changes in the selectivity filter induced by backbone mutations in Kir2.1 (Lu et al. 2001a).
We speculated that the influence of PIP2 on sublevels might provide insights into the molecular mechanisms by which PIP2 regulates Kir2.1 channels. Since most previous studies were performed by expressing monomers to form Kir channels, there is currently limited information on how channel behaviour depends on the interaction of PIP2 with individual subunits in tetrameric channels. Accordingly, in addition to expressing monomers, we examined the effects of disrupting the interaction of PIP2 with Kir2.1 channels formed from tandem dimeric or tetrameric constructs, in which the number of subunits with intact PIP2 binding sites was varied. We addressed the following questions: (1) Since Kir2.1 channels have at least one PIP2 binding site per subunit, how many subunits must interact with PIP2 for the channel to become available to open? (2) When the channel opens, do the sublevels correspond to the number of subunits interacting with PIP2? (3) If not, how many subunits must interact with PIP2 for the channel to enter the fully open state? (4) Is there cooperativity between the interaction of PIP2 with subunits and channel gating?
Using inside-out patches from Xenopus oocytes expressing a variety of Kir2.1 constructs, we show that interaction of a single subunit with PIP2 is sufficient to induce full openings, indicating that the first subunit–PIP2 interaction shifts the channel from an unavailable to an available mode with high open probability (Po). Interaction of PIP2 with additional subunits exerts strong positive cooperativity in promoting the available mode. Once the channel is in the available mode, another level of positive cooperativity between subunits promotes the fully open state and minimizes the occurrence of sublevels, even when only a single subunit is interacting with PIP2. However, interaction of additional subunits with PIP2 further enhances this positive cooperativity, reducing the dwell time in sublevels even further.
Methods
Molecular biology and preparation of oocytes
The Kir2.1 cDNA (Kubo et al. 1993) was generously provided by Dr Lily Y. Jan. ‘Quickchange’ mutagenesis (Stratagene, La Jolla, CA, USA) was used to construct individual mutants such as K188Q and K188A/R189A. In order to construct tandem structure K188A/R189A–WT, the ‘Quickchange’ mutagenic protocol was used to insert an EcoR1 site at the 5′ open reading frame (ORF) of the wild-type and 3′ ORF of the mutant (K188A/R189A) Kir2.1 sequences. Linkage via the EcoR1 sites generated a GGGGEFGGG (single letter amino acid code) sequence between the Kir2.1 repeat sequences. Other tandem dimeric constructs including WT–K188A/R189A, K188A/R189A–K188A/R189A or WT–WT were constructed using the same protocol. To make the tetrameric oligomer: a HindIII site was inserted into the tandem dimers, at the 5′ end (ORF) of Kir 2.1 carrying a 3-EcoRI restriction site, and a HindIII site inserted at the 3′ ORF of a Kir2.1 sequence carrying an EcoR1 site at the 5′ end. Linkage via the HindIII site generated another tandem construct separated by AGGKLGGA, which can be inserted into the tandem construct carrying the EcoRI site in its middle. The final construct therefore resembles 2.1 Eco 2.1 Hind 2.1 Eco 2.1, where 2.1 can either be wild-type or mutant sequences. We therefore can obtain tetrameric channels with any number of mutant and WT subunits. The strategy of constructing dimeric and tetrameric channels has been used in Kir channels extensively (Lu et al. 1999b; Cho et al. 2000; Kono et al. 2000; Zingman et al. 2002; Lin et al. 2003; Matsuda et al. 2003). The cRNAs were synthesized using T7 polymerase (Ambion Inc.)
Oocytes (stage IV–V) were isolated by partial ovariectomy from mature female Xenopus anaesthetized with 0.1% tricaine. All of the frogs were humanely killed at the end of the oocyte collection. The use and care of the animals in these experiments were approved by the Chancellor's Animal Research Committee at UCLA. The oocytes were then defolliculated by treatment for 1 h with 1 mg ml−1 collagenase (TypeII, Life Technologies) in Barth's solution containing (mm): 88 NaCl, 1 KCl, 2.4 NaHCO3, 0.3 Ca(N2O6).4H2O, 0.41 CaCl2, 0.82 MgSO4, 15 Hepes; and 50 mg ml−1 gentamicin and 10 mg ml−1 Baytril; pH 7.6. The day after collagenase treatment, selected oocytes were pressure-injected with ∼50 nl RNA (1–100 ng ml−1). Oocytes were maintained at 18°C in Barth's solution and electrophysiological studies were conducted 1–3 days later. The vitelline membrane was removed immediately before the patch clamp recording.
Electrophysiology and data analysis
Macroscopic currents were recorded from excised inside-out giant patches of RNA-injected oocytes with an Axopatch 200A amplifier (Axon Instruments) at room temperature. Patch electrodes were pulled from thin-wall borosilicate glass (Garner Glass Co., Claremont, CA, USA) and had a tip diameter of 20–30 μm after fire polishing. The patch electrode solution contained (mm): 85 KCl, 1.8 CaCl2, 5 K2HPO4, 5 KH2PO4, pH 7.2 with KOH. The standard bath solution contained (mm): 85 KCl, 5 EDTA, 5 K2HPO4, 5 KH2PO4, pH adjusted to the desired level with KOH. Chemicals were purchased from Sigma (St Louis, MO, USA). Continuous current traces were elicited by voltage ramps from −100 to +100 mV at 0.4 mV ms−1. The currents were measured at −100 mV. The leak current corrections were made in most of the patches by subtracting current after complete rundown. Data were filtered with an 8-pole Bessel filter (Frequency Devices) at 1 kHz and digitized at 2 kHz via a DigiData 1200 interface (Axon Instruments).
Single channel activity was recorded from inside-out patches excised from oocytes injected with WT and mutant channel RNA. Patch electrodes were pulled from thick-wall borosilicate glass (Warner Instrument Corp.) and had a resistance of 5–8 MΩ when filled with the pipette solution. Electrodes were coated near their tips with Sylgard (Dow Corning Co.) or N,N-dimethyltrimethylsilylamine (Fluka) to reduce electrical capacitance. The patch electrode solution contained (mm): 140 KCl, 1.8 CaCl2, 5 K2HPO4, 5 KH2PO4, pH 7.2 with KOH. The standard bath solution contained (mm): 140 KCl, 5 EDTA, 5 K2HPO4, 5 KH2PO4, pH adjusted to the desired level with KOH. The rundown of channel activity was delayed by treating inside-out patches with 2 mm MgATP in the EDTA-free bath solution. Data were filtered at 2–5 kHz and digitized at 5–10 kHz. Data acquisition and analysis were carried out using pCLAMP8 or 9 software (Axon Instruments). The all-point histograms were constructed with a bin width of 0.1 pA from data records of ∼10 s. Dwell-time distributions were constructed and fitted with one or two exponential functions.
All experiments were conducted at room temperature (20–24°C). Data are presented as mean ±s.d. The paired or unpaired Student's t test was conducted with P value < 0.05 being considered as statistically significant.
Simulation of single channel data with kinetic models
Simulation and fitting of single channel data was carried out with the QuB software (The State University of New York at Buffalo). Briefly, pCLAMP data files were firstly exported as .ldt files and then imported into the QuB data window. After generation of the model, the data were idealized by using Idl function and the segmental k-means (SKM) method. The model optimization was carried out by using the Maximum Interval Likelihood rate estimation (MIL) function with setting of the initial values for the rate constants. Simulated single channel events were then generated based on the estimated rate constant values, using the Simulate (SIM) function and compared with the experimental data to establish the validity of the kinetic model.
Abbreviations
Kir channel, inwardly rectifying potassium channel; PIP2, phosphatidylinositol-4,5-bisphosphate; WT, wild-type; M, double mutant K188A/R189A subunit; WT4, channel containing four wild-type monomers or linked tetramer; WT3M1, WT–K188A/R189A–WT–WT tetramer, WT2M2, K188A/R189A–WT dimer or WT–K188A/R189A–K188A/R189A–WT tetramer; WT1M3, K188A/R189A–K188A/R189A–K188A/R189A–WT tetramer; M4, channel containing K188A/R189A monomers or linked tetramer.
Results
Single channel gating of WT Kir2.1 channels under control conditions
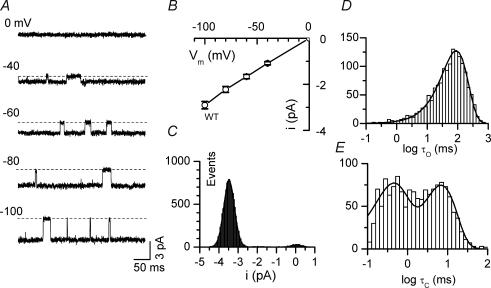
Figure 1A illustrates typical single channel behaviour of WT Kir2.1 channels in recordings from an inside-out patch at various holding potentials. The average unitary current–voltage (i–V) relation from 10 patches is shown in Fig. 1B, with the unitary conductance (g) averaging 29.1 ± 1.6 pS. Single channel transitions between the closed and fully open states at a holding potential of −100 mV were analysed in detail. Channels exhibited a typically high Po of 0.90 ± 0.03 (n = 5). The open-time distribution was well fitted by a single exponential, and the closed-time distribution was well fitted by the sum of two exponential components. These indicate that there are minimally one open state, with open time constant τo, and two closed states, with closed time constants τc1 and τc2 (Fig. 1D and E, Table 1). Under control conditions, sublevels were rarely observed in WT patches, typically showing very brief transitions to 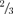 to
to  of the fully open level. Due to the low frequency and short dwell times of the sublevels, however, extra peaks corresponding to sublevels were not apparent in the amplitude histogram (Fig. 1C, histogram).
of the fully open level. Due to the low frequency and short dwell times of the sublevels, however, extra peaks corresponding to sublevels were not apparent in the amplitude histogram (Fig. 1C, histogram).
Figure 1. Single channel gating of WT Kir2.1 channels.
A, representative single channel currents recorded at various holding potentials as indicated. B, single channel current–voltage (i–V) relationship. C, all-point histogram of the current trace recorded at a holding potential of −100 mV. D, open and E, closed time distributions of single channel activity at holding potential of −100 mV. The open time distribution was well fitted by a single exponential with a time constant (τo) of 79.4 ms. The closed time distribution was well fitted by two exponential components with time constants τc1= 7.9 ms and τc2= 0.65 ms, respectively, in this particular patch.
Table 1.
Single channel gating kinetics of Kir 2.1 WT and various channel constructs at −100 mV
| Constructs | Po | τo (ms) | τc1 (ms) | τc2 (ms) |
|---|---|---|---|---|
| WT4 (n = 5) | 0.90 ± 0.01 | 71.5 ± 15.5 | 9.4 ± 2.4 | 0.72 ± 0.12 |
| WT3M1 (n = 6) | 0.91 ± 0.02* | 78.4 ± 12.7* | 10.3 ± 1.6* | 0.57 ± 0.11* |
| WT2M2 (n = 4) | 0.91 ± 0.03* | 67.0 ± 11.5* | 13.3 ± 2.6* | 0.75 ± 0.12* |
| WT1M3 (n = 4) | 0.87 ± 0.03* | 61.2 ± 8.6* | 14.2 ± 2.8* | 0.69 ± 0.12* |
| K188Q (n = 5) | 0.90 ± 0.02* | 82.9 ± 12.4* | 13.4 ± 0.9* | 0.82 ± 0.14* |
Channel constructs are indicated in Abbreviations in Methods. The data were obtained under control condition in the absence of DA12.
P > 0.05 compared to values in WT4 channel.
PIP2 dually regulates channel availability and conductance sublevels
To examine how PIP2 affects single channel properties, we inhibited channel–PIP2 interaction using three interventions: (1) application of neomycin; (2) application of PIP2 antibody; and (3) mutation of putative PIP2 binding residues. Neomycin, a polycation that binds PIP2 (Arbuzova et al. 2000), has been shown to reduce Kir channel activity by screening PIP2 molecules (Schulze et al. 2003; Ribalet et al. 2005; Xie et al. 2005). The effects of neomycin on single channel currents are shown in Fig. 2A. In this patch, two channels opened with high Po under control conditions (Fig. 2Aa). After application of 100 μm neomycin, one of the channels closed immediately. The remaining channel remained functional, but exhibited frequent sublevels, as shown in Fig. 2Ab. The most frequently observed sublevel was  of the fully open level, while other sublevels, such as
of the fully open level, while other sublevels, such as  , were rare. This channel then also closed and did not reopen during continued neomycin application (Fig. 2Ac). K2ATP had no effect on the channel activity after neomycin (Fig. 2Ad), while MgATP rescued channel activity with normal kinetics and unitary amplitude without sublevels (Fig. 2Ae). Recovery of normal channel activity after MgATP but not K2ATP in the continued presence of neomycin (1) excludes the possibility that neomycin directly caused the sublevels by partially blocking the ion-conducting pathway (like Mg); (2) suggests that PIP2 synthesis, not ATP complexation, recovers channel activity and suppresses sublevels. Thus, neomycin induces both sublevels and long-lasting full closure in Kir2.1 channels by disrupting their interaction with PIP2.
, were rare. This channel then also closed and did not reopen during continued neomycin application (Fig. 2Ac). K2ATP had no effect on the channel activity after neomycin (Fig. 2Ad), while MgATP rescued channel activity with normal kinetics and unitary amplitude without sublevels (Fig. 2Ae). Recovery of normal channel activity after MgATP but not K2ATP in the continued presence of neomycin (1) excludes the possibility that neomycin directly caused the sublevels by partially blocking the ion-conducting pathway (like Mg); (2) suggests that PIP2 synthesis, not ATP complexation, recovers channel activity and suppresses sublevels. Thus, neomycin induces both sublevels and long-lasting full closure in Kir2.1 channels by disrupting their interaction with PIP2.
Figure 2. Screening of PIP2 by neomycin or PIP2-antibody inhibits Kir2.1 single channel activity by inducing sublevels and full closure.
A, effect of neomycin (Neo). a, two channels with high Po under control conditions. b, In the presence of 100 μm Neo, one channel closed and the other showed various sublevels. c, both channels then ceased activity. The traces in the presence of Neo were selected before each channel completely ran down. d, K2ATP had no effect on the channel activity after neomycin. e, channel activity with normal kinetics and unitary amplitude was recovered by MgATP, presumably via synthesis of PIP2. B, effect of PIP2-antibody (PIP2-Ab). There were 3 channels under control conditions, and PIP2-Ab caused all three to close abruptly. Three segments in the presence of PIP2-Ab are expanded, showing sublevels (arrows) preceding complete closure of each channel.
It has been previously shown that anti-PIP2 antibodies can bind PIP2 and inhibit macroscopic current in Kir channels (Huang et al. 1997; Zhang et al. 1999). Similar to neomycin, we found that treatment of patches with PIP2 antibody induced sublevels as well as full closure (Fig. 2B). In this patch, there were three channels with high open probability under control conditions. Application of PIP2-Ab caused channels to lose function one by one. Note that multiple sublevels (arrows) were observed before each channel closed completely, especially when only one active channel remained in the patch, as shown in the expanded traces.
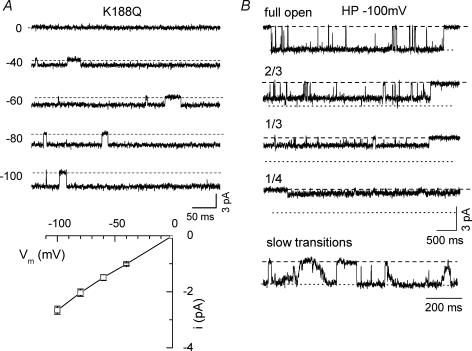
Multiple positively charged residues located in N- and C-termini are critical for the interaction of PIP2 with Kir channels (Fan & Makielski, 1997; Huang et al. 1998; Shyng et al. 2000; Lopes et al. 2002; Schulze et al. 2003), such that neutralization of these putative PIP2 binding residues reduces channel activity. For example, mutant K188Q channels exhibit very small macroscopic currents and low PIP2 affinity as gauged by high sensitivity to neomycin (Schulze et al. 2003; Xie et al. 2005). Consistent with weakened PIP2 affinity, these channels are minimally activated by either MgATP (2 mm) or PIP2 (10 μm), although high activity can be unmasked by adding long polyamines, such as diamine10 (DA10) and diamine12 (DA12) which strengthen the channels' interaction with PIP2 (Schulze et al. 2003; Xie et al. 2005). Although the majority of K188Q channels in the patch were inactive under control conditions, the few spontaneously active channels showed a high open probability with single current amplitude and open–close transition kinetics similar to WT channels (Fig. 3 and Table 1). Unlike WT Kir2.1 channels, however, multiple sublevels, at  and
and  of full amplitude, occurred frequently in 5 out of 7 patches studied. In some of the patches, gradual stepwise transitions between sublevels from the closed to fully open state, or vice versa, were observed (Fig. 3B, bottom panel).
of full amplitude, occurred frequently in 5 out of 7 patches studied. In some of the patches, gradual stepwise transitions between sublevels from the closed to fully open state, or vice versa, were observed (Fig. 3B, bottom panel).
Figure 3. Full openings and multiple sublevels in K188Q, a mutant channel with weak PIP2 binding.
A, single channel current recorded at various holding potentials and single channel i–V relationship. B, full openings and various sublevels with long dwell times recorded from a K188Q mutant single channel. The patch was held at −100 mV. Bottom trace illustrates slow open-to-closed state transitions via continuous sublevel changes shows noted in this channel.
These experiments reveal that reducing channel–PIP2 interaction exerts dual effects on the single channel gating, promoting both sublevels as well as long-lasting full channel closure (unavailable mode). Thus, PIP2 plays a key role in regulating both Kir2.1 channel availability and its ability to open fully.
Regulation of channel availability by PIP2: first level of positive cooperativity between channel subunits
Like K188, R189 has also been shown to regulate PIP2 affinity in Kir2.1 channels (Lopes et al. 2002). We confirmed that the double mutant K188A/R189A traffics normally to the cell membrane by linking the double mutant to green fluorescent protein (GFP) (K188A/R189A–GFP). Cells expressing K188A/R189A–GFP channels showed strong surface membrane labelling, similar to the WT–GFP channels (data not shown). However, in 10 patches tested, no macroscopic or single channel currents were detected from either giant or standard inside-out patches, even in the presence of PIP2 and long diamines (Figs 4A and 5A), suggesting that channel–PIP2 interaction was disrupted sufficiently to trap the channels in an unavailable mode in which channel openings were eliminated altogether.
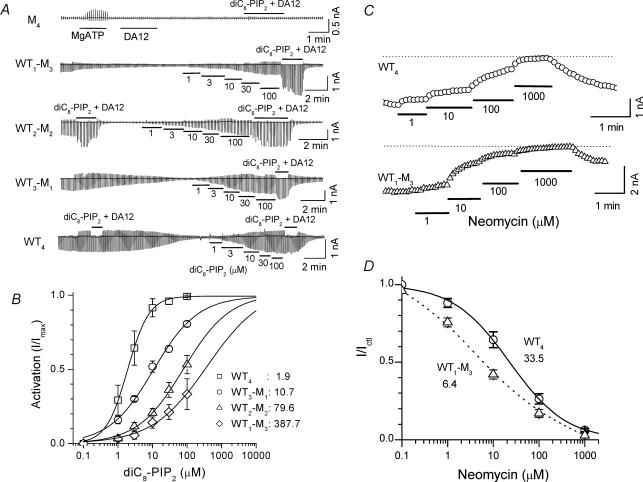
Figure 4. Loss of channel activity in the double mutation K188A/R189A with low PIP2 binding affinity, and progressive rescue by adding back WT subunits in a tandem tetrameric channel to increase PIP2 affinity.
A, continuous macroscopic current traces recorded from inside-out giant patches (electrode diameter ∼20 μm) in channels with the various stoichiometries as indicated. Currents were elicited by voltage ramps from −100 to +100 mV at 0.4 mV ms−1. Short chain PIP2, diC8-PIP2, in various concentrations was applied as indicated by the bars under the traces. DA12 (100 μm) + diC8-PIP2 (100 μm) was used to maximally activate channel activity. The application of MgATP activated an outward current in some patches (e.g. the patch expressing M4), probably due to activation of an endogenous Ca2+- or Mg2+-sensitive Cl− current known to be present in Xenopus oocytes (Weber et al. 1995). B, concentration–activation curves for each channel construct. The activation by diC8-PIP2 was expressed as I/Imax, where Imax is the maximum current activated by diC8-PIP2+ DA12.The mean EC50 values are indicated. C, effects of neomycin in WT4 and WT1M3 constructs. Current at −100 mV was recorded during application of neomycin at various concentrations indicated by the horizontal bars. D, neomycin dose–response curves for WT4 and WT1M3 constructs. The mean EC50 values are indicated.
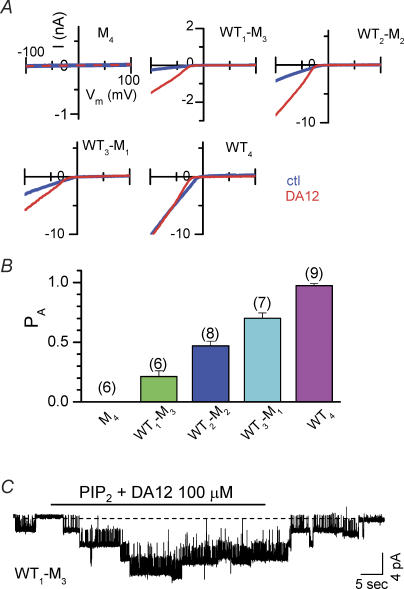
Figure 5. Channel availability measurement in various tandem tetrameric channel constructs.
A, macroscopic current–voltage relations recorded from inside-out giant patches in channels with the various stoichiometries as indicted. Continuous current traces were elicited by voltage ramps from −100 to +100 mV at 0.4 mV ms−1. The patches were pre-treated with 2 mm MgATP (ctl) before application of 100 μm diamine 12 (DA12) to strengthen the channel–PIP2 interaction. B, the observed available probability (PA) values for various channel constructs calculated as Ictl/IDA12, in which Ictl represents control current level at −100 mV, and IDA12 the current level after application of 100 μm DA12 pre-treated with 2 mm MgATP. C, in a patch containing few channels, the application of PIP2 plus DA12 (horizontal bar) caused unavailable channels to open reversibly.
To determine whether channel activity could be rescued by incorporating one or more WT subunits into K188A/R189A channels, we performed studies on tandem dimeric and tandem tetrameric constructs containing mixtures of WT and mutant (M) K188A/R189A subunits. We measured macroscopic currents from giant patches to determine the apparent PIP2 affinity of each construct with short chain PIP2, diC8-PIP2 (Fig. 4). We have demonstrated in our previous study (Xie et al. 2005) that long polyamines, such as DA12, interact with the channel membrane pore region (including negatively charged D172) and stabilize the bundle crossing in its open configuration. This allosteric effect enhances the interaction of the channel cytoplasmic region (including K188, R189) with PIP2 at the cytoplasmic side of the membrane and thus increases PIP2 affinity. Therefore, to activate channels maximally (Imax), we used the combination of 100 μm diC8-PIP2+ 100 μm DA12, since when channel PIP2 binding affinity is decreased, even saturating levels of PIP2 alone cannot fully activate channel activity (Xie et al. 2005) and higher concentrations (200 μm diC8-PIP2+ 100 μm DA12) did not further increase current level. As shown in Fig. 4A and B, the apparent PIP2 affinity strongly depended on the number of PIP2 interacting subunits. WT4 showed highest PIP2 affinity with a mean EC50 value of 1.9 ± 0.3 μm while WT3M1, WT2M2 and WT1M3 had lower PIP2 affinities, with EC50 values of 10.7 ± 1.6, 79.6 ± 7.6 and 387.7 ± 84.2 μm (n = 4 for each group), respectively. These results suggest that the affinity of PIP2 is shifted to a lower level (rightward) when the number of mutant subunit increases. In the presence of 100 μm DA12, all the constructs tended to be fully activated by 100 μm PIP2 and the differences in the affinity for PIP2 among the different constructs were minimized. The Hill coefficient values for the four constructs were 1.21 ± 0.16 (WT4), 0.78 ± 0.08 (WT3M1), 0.75 ± 0.14 (WT2M2) and 0.65 ± 0.15 (WT1M3), respectively.
Consistent with short-chain PIP2 data, we observed that decreasing the number of WT subunits progressively increased the neomycin sensitivity of the channels. Figure 4C and D shows representative data in WT4 and WT1M3 channels. The neomycin concentration causing 50% inhibition of current (IC50) averaged 33.5 ± 5.1 μm in WT4 channels, and 6.4 ± 2.4 μm in WT1M3 channels (P < 0.05).
Figure 5A and B further compares macroscopic current levels under control conditions and after maximal activation. Immediately after patch excision, the patches were treated with 2 mm MgATP for > 1 min to generate high membrane levels of PIP2. A voltage ramp from −100 to +100 mV was applied to measure basal channel activity, and then repeated during exposure to 100 μm DA12 to enhance channel–PIP2 interaction and maximally activate all channels in the patch. Similar to K188A/R189A monomers, the tandem tetrameric construct containing four K188A/R189A subunits (M4 channels) did not show channel activity before or after DA12 treatment, consistent with an extremely low PIP2 affinity. Current levels in channels formed from tandem dimeric or tetrameric constructs containing at least one WT subunit were enhanced by application of DA12 (100 μm). The enhancement of the current depended on the number of WT subunits in the tetrameric channel: it was greatest in channels with a single WT subunit (WT1M3 channels); intermediate in channels with two or three WT subunits, and least in channels with four WT subunits (WT4 channels) (Fig. 5A). The findings indicate that with high ambient basal PIP2 levels in the membrane (i.e. after treatment with 2 mm MgATP), WT4 channels in which all four subunits are capable of interacting with the PIP2 are nearly all active (defined here as the available mode). As the number of subunits with PIP2 interaction sites (WT) decreases, fewer and fewer channels remain in the available mode, unless PIP2 affinity is increased by exposure to long diamines. Nevertheless, channels with only a single WT subunit (WT1M3) can still be active, especially if the PIP2 affinity of this channel is enhanced by the application of long diamines.
The conclusion that PIP2 promotes the available mode was confirmed in the WT1M3 channel as shown in Fig. 5C. Increasing channel–PIP2 interaction by direct exposure to PIP2 (combined with DA12) increased the number of active channels, e.g. from one to four in this specific patch. Please notice this result was obtained in the WT1M3 channel which showed much lower PIP2 affinity, and both PIP2 and DA12 were necessary for the maximal activation. The fast reversibility was mostly probably due to the washout of DA12 causing an immediate reduction in channel–PIP2 interaction, rather than PIP2 depletion per se.
After pretreatment with MgATP (for > 1 min) to synthesize PIP2 in the membrane, DA12 (100 μm) maximally increased channel activities in all channel constructs, since application of PIP2 or diC8-PIP2 in the presence of DA12 did not further increase the currents, suggesting a conversion of all channels into the available mode. Even for channels with a single WT subunit, MgATP pretreatment + DA12 was sufficient to convert all channels in the membrane to the available mode. If this assumption is accurate, then the observed available probability (PA) for various channel constructs can be calculated as a ratio of the basal current to that after maximal activation (Ictl/IDA12) (Fig. 5B and Table 2, 2nd row). For WT1M3 channels, this would correspond to a predicted PA of 0.21 for a single PIP2 interacting subunit. If all four subunits bind PIP2 independently (i.e. neutral cooperativity), then the probability of no subunits binding PIP2 (PNB, equivalent to the channel being in the unavailable mode) should be (0.79)4 for WT4 channels, respectively. Thus, the predicted PA= (1 −PNB) for the WT4 channel should give the value of 0.61, assuming neutral cooperativity. However, this value is much lower than the experimentally observed PA value of 0.97, which was calculated from the Ictl/IDA12 ratio. These observations indicate that the multiple PIP2 interaction sites must exert positive cooperativity to promote the available mode as each additional subunit interacts with PIP2.
Table 2.
Positive cooperativity ratios for PIP2 binding subunits regulating channel available probability PA
| WT1M3 (n = 9) | WT2M2 (n = 7) | WT3M1 (n = 8) | WT4 (n = 6) | |
|---|---|---|---|---|
| Predicted PA for neutral cooperativity | 0.21 | 0.38 | 0.51 | 0.61 |
| Observed PA (Ictl/IDA12) | 0.21 ± 0.05 | 0.47 ± 0.04 | 0.70 ± 0.05 | 0.97 ± 0.02 |
Regulation of sublevels by PIP2: a second level of positive cooperativity
In addition to regulating channel availability, the findings in the first section show that PIP2 also regulates transitions between sublevels. One possibility is that sublevels correspond to the number of subunits that are interacting with PIP2, as has been suggested in voltage-gated Kv2.1 channels (Chapman & VanDongen, 2005). However, this possibility is ruled out for Kir2.1 channels by our observation that in the tetramer WT1M3 with a single WT subunit, the predominant open state, even under basal conditions in the absence of exogenous PIP2 or long diamines, was the fully open state with same single channel conductance as in WT4 channels. Figure 6 and Table 1 show that the typical transitions in all the tandem tetrameric and dimeric channels exhibited WT-like open–close kinetics and with a similar unitary conductance of the fully open state. Figure 6B shows an example in the WT1M3 channel. More frequent sublevels were observed in WT3M1 channel than in WT2M2 or WT4 channels. However,  level was still the most commonly observed sublevel.
level was still the most commonly observed sublevel.
Figure 6. Single channel kinetics in various tandem tetrameric and dimeric constructs with different numbers of mutant K188A/R189A subunits.
A, single channel traces recorded from tandem dimeric WT2M2 channels and B, tandem tetrameric WT1M3 channels at holding potentials as indicated. The data were obtained under control conditions in the absence of DA12. Note sublevels with long dwell times in WT1M3 channels, although the fully opening state remained dominant. All-points histograms for each construct are shown in for WT2M2 (C) and for WT1M3 (D), respectivewly; E, single channel current–voltage relations for the two constructs. F, single channel cord conductances at −100 mV measured in the various constructs. The number of patches in each group are indicated above each bar.
For all the constructs tested, the most frequently sublevel visited was 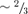 of the fully open state. Transitions to other sublevels such as
of the fully open state. Transitions to other sublevels such as  and
and  of full opening level were rare, precluding detailed quantitative analysis of their kinetics. Therefore, to analyse the kinetics quantitatively, we considered only the
of full opening level were rare, precluding detailed quantitative analysis of their kinetics. Therefore, to analyse the kinetics quantitatively, we considered only the  sublevel and the fully open state.
sublevel and the fully open state.
Figure 6B illustrates that the sublevels were accessible from both the fully open state and closed state. The closed-to-sublevel transitions shared similar kinetics to the closed–full open state transitions. We calculated the sublevel probability (Ps, the fraction of time spent at the sublevel) by measuring the area under the all-point histogram for the sublevel, and taking the ratio of this area (As) to the total area under the all-point histogram (A). (Similar values were obtained from the ratio of the dwell time for the sublevel using the QuB software program.) In WT1M3 channels, sublevels were observed in 6/7 patches with mean Ps= 0.13 ± 0.06; in WT2M2, sublevels occurred in 6/14 patches with Ps= 0.05 ± 0.02; and in WT3M1 and WT4, sublevels had too low a Ps to quantify reliably. Thus, sublevels occurred more frequently as the number of PIP2-interacting subunits decreased.
Markov model quantitatively simulating single channel sublevel kinetics
We constructed a Markovian model (Fig. 7 and Scheme 1) to account for the following observations: (1) interaction of a single Kir2.1 subunit with PIP2 is sufficient to induce full openings shifting the channel from the unavailable to available mode with an intrinsically high Po; (2) once the channel is in the available mode, positive cooperativity between subunits promotes the fully open state and minimizes the occurrence of sublevels; (3) interaction of additional subunits with PIP2 further enhances this positive cooperativity, reducing the dwell time in sublevels even further. We based Fig. 7 on the emerging idea from recent functional and structural studies (see Discussion) that two distinct gates control the behaviour of Kir channels (Proks et al. 2001; So et al. 2001; Phillips & Nichols, 2003). We assume that when the channel is in the available mode, open–closed transitions are mediated by gating of an ‘upper gate’, putatively located at the selectivity filter. Both availability and sublevel behaviour of the channel, on the other hand, are controlled by the interaction of PIP2 with cytoplasmic regions that regulate a ‘lower gate’ (LG), which may relate to conformational changes in the M2 bundle crossing and/or G-loop of the cytoplasmic pore (Xiao et al. 2003). Schematically, the four subunits each contribute a component of the LG, which opens fully when all four components are in the UP position, and closes fully when all four are in the DOWN position. In the absence of PIP2, or in a mutant channel with no PIP2 interaction sites (i.e. K188A/R189A), all subunits remain in the DOWN position such that the channel is unavailable to open. However, interaction of any single subunit with PIP2‘tethers’ that subunit ‘up’ against the membrane, facilitating the UP position with high probability. In addition, when one subunit is tethered by PIP2, strong positive cooperativity between the subunits favour other subunits entering the UP position with slightly lower, but still high, probability even when they are themselves not directly interacting with PIP2 (Fig. 7A). A recent crystal structure study (Nishida et al. 2007), suggesting that PIP2 binding may open the apex of the cytoplasmic pore (G-loop) through a rigid-body movement to an Upper position, fits well with our proposed scheme. We further assume that the sublevels correspond to the number of subunits in the UP position. When one subunit is UP, the LG opens partially ( sublevel), and with two UP, three UP and four UP, the conductance progressively increases from
sublevel), and with two UP, three UP and four UP, the conductance progressively increases from  to
to  (or
(or 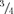 ) to the fully open levels, respectively (Fig. 7B). This model accounts for the observation that tetrameric WT1M3 channels, with only a single PIP2 interacting subunit, exhibit the same fully open state as well as the various sublevels observed as WT4 channels. Because of strong positive cooperativity between subunits, when a single subunit is tethered by PIP2, all four subunits favour the UP position, rarely visiting the
) to the fully open levels, respectively (Fig. 7B). This model accounts for the observation that tetrameric WT1M3 channels, with only a single PIP2 interacting subunit, exhibit the same fully open state as well as the various sublevels observed as WT4 channels. Because of strong positive cooperativity between subunits, when a single subunit is tethered by PIP2, all four subunits favour the UP position, rarely visiting the  substate (3 UP) and almost never visiting the
substate (3 UP) and almost never visiting the  (2 UP) and
(2 UP) and  (1 UP) sublevels.
(1 UP) sublevels.
Figure 7. Putative channel conformational changes corresponding to single channel sublevels – a cartoon image.
A, longitudinal section through the channel showing subunits I and III only. When no subunits are interacting with membrane PIP2 (red circles), the cytoplasmic lower gate (LG) portion of all subunits reside in the DOWN position (a) and the channel is UNAVAILABLE to open. To become AVAILABLE to open, at least one subunit must interact with PIP2 at K188/R189, causing the lower gate (LG) portion of that subunit to move to the UP position, and exerting positive cooperativity on the LG portions of the other subunits to move into the UP position. Once in the AVAILABLE mode, the selectivity filter (SF) in the transmembrane pore controls open–close gatings. The single channel conductance corresponds to the number to LG portions in the UP position, with 1, 2, 3 and 4 UP corresponding to the  or
or  , and fully open levels, respectively, as indicated by the dashed lines through the pore region. Thus, with only one subunit tethered to PIP2, strong coupling allows other subunits to enter the UP position favouring the fully open state. B, schematic representation of a cross-section through the lower gate region, in which each subunits contribution to the lower gate is indicated by a circle, which can be either in the UP (U) or DOWN (D) position. When DOWN, that subunit's component of the lower gate partially occludes the ion-conducting pathway. Thus, with 4 DOWN, the channel is closed, and with 1, 2, 3 or 4 UP, the LG progressively opens corresponding to the
, and fully open levels, respectively, as indicated by the dashed lines through the pore region. Thus, with only one subunit tethered to PIP2, strong coupling allows other subunits to enter the UP position favouring the fully open state. B, schematic representation of a cross-section through the lower gate region, in which each subunits contribution to the lower gate is indicated by a circle, which can be either in the UP (U) or DOWN (D) position. When DOWN, that subunit's component of the lower gate partially occludes the ion-conducting pathway. Thus, with 4 DOWN, the channel is closed, and with 1, 2, 3 or 4 UP, the LG progressively opens corresponding to the  (or
(or  ), and full conductance levels, respectively. Thus, the sublevels correspond to the number of subunits in the UP position.
), and full conductance levels, respectively. Thus, the sublevels correspond to the number of subunits in the UP position.
In the Markov model, we represent a channel in the available mode with one open and two closed states (Sakmann & Trube, 1984) (box in Scheme 1), reflecting the observed ‘upper gate’ gating kinetics with a single open time and two closed times (see Table 1). The values of the rate constants (α, β, δ, γ) were then obtained by fitting to the mean open and closed times for the fully open state. To account for sublevels, we linked the fully open state (O1) to series of partially open states ( ), leading to a closed state (Cn) representing the unavailable mode (Scheme 1). We assumed that the rate constants (α, β, δ) were identical for the sublevel transitions.
), leading to a closed state (Cn) representing the unavailable mode (Scheme 1). We assumed that the rate constants (α, β, δ) were identical for the sublevel transitions.

Since only the  sublevel was observed frequently enough for quantitative analysis, Scheme 1 was further simplified to Scheme 2. Here we assume a high degree of positive cooperativity between the transitions to the fully open state, so that all the sublevels can be condensed into a single Os state, whose open probability Ps quantitatively matches that of the
sublevel was observed frequently enough for quantitative analysis, Scheme 1 was further simplified to Scheme 2. Here we assume a high degree of positive cooperativity between the transitions to the fully open state, so that all the sublevels can be condensed into a single Os state, whose open probability Ps quantitatively matches that of the  state in Scheme 1.
state in Scheme 1.

The transition from Cn to Os, controlling channel availability, was assumed to be PIP2 sensitive, whereas the transitions between Os and O1 were modulated by the number of subunits interacting with PIP2. The main effect on sublevels of decreasing the number of WT subunits in tetrameric channels was attributed to a decrease in the equilibrium coefficient K1 = k1/k−1, giving the mutant channel more opportunity to reside in the Os state. When the single channel data were fitted to Scheme 2 to obtain the rate constants both for gating by the ‘upper gate’ (α, β, δ, γ) and ‘lower gate’ (k1 and k−1) mediating transitions between the sublevel state Os and fully open state O1, we found that for modelling transition of the channel into the available mode, kn and k−n could be set within a wide range as long as kn/k−n > 20 and k−n < k1. Figure 8 shows that with these constraints, simulated single channel traces using QuB software closely matched the experimental recordings from both WT4 and WT1M3 channels. Table 3 lists the average rate constants obtained for the various tetrameric constructs. For the k1 and k−1 values shown in Table 3, the probability of WT1M3 channels residing in the sublevel state is Ps = k−1/(k1+k−1) = 0.11, similar to the observed value of 0.13. For WT2M2 channels, the changes in k1 and k−1 cause Ps to fall to 0.04, similar to the experimental value of 0.05. For either WT3M1 and WT4 channels, k1 and k−1 were chosen to predict very short dwell times (1/k1) and extremely low Ps values [k−1/(k1+k−1)], consistent with experimental finding that sublevels were too infrequent to quantify in these channels.
Figure 8. Recorded and simulated single channel traces using scheme 2.
Representative single channel traces recorded from WT1M3 channels (left) and simulated current traces using Scheme 2 (right). Real and simulated amplitude histograms are shown below. The rate constants obtained from fitting: α= 8.6, β= 86.0, γ= 3.2, δ= 1470, k1= 26.0, k−1= 4.0. B, representative single channel traces recorded from WT4 channels (left) and simulated using Scheme 2 (right). Real and simulated amplitude histograms are shown below. The rate constants obtained from fitting: α= 3.4, β= 67.0, γ= 4.0, δ= 1052, k1= 500, k−1= 3.0. C, direct application of PIP2 reduced sublevel probability. Single channel traces recorded from WT1M3 channel at a holding potential of −100 mV in an inside-out patch under control conditions (left) and in the presence of 10 μm PIP2 (right). All-point histograms for each condition are shown in the lower panels.
Table 3.
Rate constants (1/s) obtained from fitting the experimental data with the proposed schematic model (Scheme 2) with the QuB software
| Constructs | k1 | k−1 | α | β | γ | δ |
|---|---|---|---|---|---|---|
| WT4 | 737.5 ± 37.5 | 2.8 ± 0.6 | 8.4 ± 2.4 | 131.8 ± 43.6 | 11.3 ± 6.3 | 1557 ± 332 |
| WT2M2 | 73.5 ± 15.4 | 3.1 ± 0.5 | 7.3 ± 1.6 | 82.8 ± 13.9 | 9.2 ± 2.0 | 1463 ± 278 |
| WT1M3 | 31.2 ± 4.6 | 3.7 ± 0.5 | 6.9 ± 1.2 | 98.8 ± 13.3 | 7.7 ± 2.1 | 1694 ± 361 |
kn/k−n > 20 andk−n < k1.
In WT1M3 channels, we also found that Ps decreased when the patch was directly exposed to 10 μm PIP2 (Fig. 8C). Thus, although PIP2 and DA12 had no effect on open–close gating kinetics or the unitary conductance of the fully open state, they reduced sublevel probability in WT1M3 channels. These results indicate that sublevel probability is controlled both by number and affinity of PIP2 interaction sites in the channel as well as PIP2 levels in the membrane. The latter factor may account for the variability in Ps between patches for the same channel construct. For instance, in WT1M3, in 1/7 patches did not exhibit sublevels, implicating a high ambient membrane PIP2 level in that patch. Consistent with the experimental data, increasing the concentration of PIP2 by ∼10-fold (overall k1) in the simulation also decreased sublevel visitations.
Sakmann and Trube (Sakmann & Trube, 1984) suggested another model C1↔ C2↔ O that also fitted the kinetic properties of the cardiac inwardly rectifying K channels well. We also tested this model by incorporating it into Scheme 2 and could fit the experimental data equally well. Considering there is no clear structural evidence that two closed states are sequentially connected, we preferred to use the model with two parallel closed states.
Discussion
The major findings of the present study are: (1) PIP2 has dual effects on Kir2.1 channels, modulating both channel availability and the probability of visiting sublevels. Reducing Kir2.1 channel interaction with PIP2 by a variety of interventions, such as PIP2 antibodies, screening PIP2 with neomycin, or mutating known PIP2 binding sites such as K188 and/or R189 promoted both sublevels and long-lasted channel closure into an unavailable mode. Conversely, enhancing channel–PIP2 interaction by direct application of PIP2 and long polyamines increased channel availability and reduced sublevel probability; (2) At least one subunit must interact with PIP2 for the tetrameric channel to exit the unavailable mode and enter the available mode. As the number of PIP2-interacting subunits in tandem tetramers or dimers increased, strong positive cooperativity between these subunits progressively stabilized the channel in the available mode; (3) Once the interaction of a single subunit with PIP2 placed the channel in the available mode, the channel favoured the fully open state rather than sublevels, reflecting a strong inherent second level of positive cooperativity between subunits. Sublevels therefore do not directly correspond to number of channel subunits interacting with PIP2. When additional subunits do interact with PIP2, their effect is to strengthen the inherent positive cooperativity between subunits, decreasing sublevel probability further in favour of the fully open state. In summary, our findings are consistent with a model in which PIP2 binding to a single subunit suffices to induce a transition from an unavailable mode to an available mode. Once in the available mode, strong positive cooperativity between subunits promotes the fully open state with high probability, which is further enhanced when additional subunits interact with PIP2. Thus, PIP2 acts by enhancing positive cooperativity at multiple levels which reinforce Kir2.1 subunit interactions to promote available, fully open channels.
A remaining question is how many PIP2 molecules bind to each individual PIP2-interacting subunit. In the present study, we found that the interaction of a single subunit with PIP2 is sufficient for the Kir2.1 channel to transition readily to the fully open state. One PIP2 molecule per subunit seems a reasonable guess, since the two positively charged residues which we have mutated, K188 and R189, are located together and are likely to contribute to a single PIP2 binding site. However, other PIP2 binding sites in the channel are also likely to modulate sublevel probability (see below).
It is well known that polyamines block outward current through Kir channels and contribute to inward rectification. Therefore, one can wonder if polyamines could also cause discrete subconductance levels. We previously showed that polyamines decreased inward Kir2.1 channel unitary conductance in a continuous manner, probably by a charge screening effect (Xie et al. 2002). However, we did not observe obvious discrete subconductance levels in the presence of polyamines.
Regulation of channel availability by PIP2
Although we cannot exclude the possibility that the K188A/R189A mutation may directly affect the lower gate to generate a non-functional channel in the M4 construct, the results shown in Figs 4 and 5 suggest it is most likely that disrupting channel–PIP2 interactions in one or more subunit causes the channel to progressively enter an unavailable mode, in which the channels still reside in the membrane but do not open. Evidence for a distinct unavailable mode is observed with decreasing membrane PIP2 levels (due to spontaneous degradation, screening or PIP2 antibodies) when the channel suddenly enters a super-long closed state, rather than undergoing a gradual decrease in open time (Xiao et al. 2003) (also see Fig. 2). Under these conditions, normal channel gating with high Po could only be recovered by exogenous application of PIP2 or MgATP (Xiao et al. 2003).
Based on crystal structural and functional studies, two regions have been proposed to control gating in Kir channels, an ‘upper gate’ due to stochastic switching between different conformational states of the selectivity filter, and a ‘lower gate’ involving larger conformational changes in the M2 bundle crossing and/or G-loop of the cytoplasmic pore (Xiao et al. 2003). Recent structural studies (Pegan et al. 2005; Nishida et al. 2007) have suggested that the G-loop is too narrow for K+ ions to permeate in the absence of PIP2 binding, and that the electrostatic interaction between cytoplasmic regions and membrane-bound PIP2 provides the energy to keep the lower gate in the open conformation. Conversely, Yang's group (Xiao et al. 2003) has suggested that although the lower gate region of the pore narrows when PIP2 unbinds from the cytoplasmic domain, the constriction at bundle crossing may not by itself fully close the channel, but is instead coupled to closure of the selectivity filter. Supporting this assumption, Yang's group has demonstrated that backbone mutations in the selectivity filter (Lu et al. 2001a) or partial blockage of the inner pore (Lu et al. 1999a, 2001b) cause sublevels to appear. In addition, there is also a possibility that the slow (C1) transition gating may be controlled by or located at the lower gate, as suggested by Proks et al. in the Kir6.2 channel (Proks et al. 2001; So et al. 2001; Phillips & Nichols, 2003).
Although the precise molecular mechanisms by which PIP2 gates Kir2.1 channels remain to be elucidated, it is well established that there are allosteric links between PIP2 binding and channel gating. PIP2-dependent channel opening has been shown to involve conformational changes in the M2 bundle crossing and G-loop regions, where the ‘lower gate’ may reside. Based on these studies, we favour the hypothesis that the interactions of PIP2 with Kir2.1 primarily regulate a lower gate near the M2 bundle crossing or G-loop (Fig. 7). Recent studies of human Kir2.1 genetic defects causing Andersen's Syndrome (Pegan et al. 2006; Ma et al. 2007) have revealed that several mutations are located in close proximity to the G-loop and may disrupt intra-subunit (R218–T309, R218–V302) or inter-subunit (E303–R312) salt bridges or polar interactions. These interactions are thought to be required to stabilize the G-loop in a functional state permitting ion permeation. Some of these residues are either PIP2 binding sites (e.g. R218) or allosterically affect PIP2 gating (V302, T309). Mechanistically, PIP2 binding to the inner leaflet-facing cytoplasmic portions of the channel may be critical for properly aligning the side chains of the residues for efficient salt bridge formation. Thus, disruption of PIP2–channel interactions may cause channels to become non-functional due to loss of intra- or inter-subunit interactions important for maintenance of tetramer structure. Conversely, facilitation of these intra- or inter-subunit interactions by PIP2 may be the underlying explanation for the positive cooperativity. Our finding that single channel gating kinetics of the 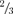 sublevel are similar to those of the fully open conductance level (Figs 3B and 8B) supports the concept that the same gate regulates open–closed transitions within the fully open or sublevel states (i.e. the vertically orientated rate constants in Schemes 1 and 2), independently from a PIP2-sensitive gate which regulates transitions between the fully open state, sublevels and the long-lasting closed state (the horizontally orientated rate constants in Schemes 1 and 2).
sublevel are similar to those of the fully open conductance level (Figs 3B and 8B) supports the concept that the same gate regulates open–closed transitions within the fully open or sublevel states (i.e. the vertically orientated rate constants in Schemes 1 and 2), independently from a PIP2-sensitive gate which regulates transitions between the fully open state, sublevels and the long-lasting closed state (the horizontally orientated rate constants in Schemes 1 and 2).
Regulation of sublevels by PIP2
Leung et al. (2000) showed that disruption of PIP2–channel interaction not only decreased single channel Po, but induced sublevels in the Kir1.1 channels. In Kir2.1 channels, Lopes et al. (2002) identified three types of single channels with different amplitudes and/or gating when they coexpressed WT and the PIP2-insensitive R218Q mutant. They suggested that the different channel conductances and gating behaviours represented different WT/mutant subunit stoichiometries. The present study is the first to show definitively how the number of PIP2-interacting subunits in a Kir channel affects gating and sublevels. A similar approach was previously used in the voltage-gated potassium channel, Kv2.1, which also exhibits sublevels (Chapman & VanDongen, 2005). By constructing tandem dimers that linked two Kv2.1 channel subunits with different activation voltage thresholds, Chapman et al. (1997) reported that heteromeric pore conformations produced sublevels correlating with the voltage gate status of the individual subunits. Considering the general similarity in channel pore structure (Doyle et al. 1998; Jiang et al. 2003; Kuo et al. 2003; Pegan et al. 2005), we originally hypothesized that the Kir2.1 channel might also display stepwise sublevels depending on how many of the channel's four subunits were strongly bound to PIP2. If this were the case, then we would predict that WT1M3 channels, with only a single subunit capable of interacting with PIP2, would exhibit only the  sublevel. However, our finding that WT1M3 channels exhibited all of the sublevels as well as the fully open state excluded this possibility. Thus, the interaction of PIP2 with additional subunits was not required for full channel opening, but rather enhanced the inherent positive cooperativity between subunits to promote the fully open state. This conclusion is supported by the single channel kinetics data listed in Table 1, demonstrating there is no significant difference in Po, to and tc between WT4 and other channel constructs. Thus the different levels of macroscopic current are related to channel availability, i.e. the number of channels available to open in the patches. By adding positive cooperativity to the Markov model, these features were readily simulated with reasonable quantitative accuracy (Fig. 8).
sublevel. However, our finding that WT1M3 channels exhibited all of the sublevels as well as the fully open state excluded this possibility. Thus, the interaction of PIP2 with additional subunits was not required for full channel opening, but rather enhanced the inherent positive cooperativity between subunits to promote the fully open state. This conclusion is supported by the single channel kinetics data listed in Table 1, demonstrating there is no significant difference in Po, to and tc between WT4 and other channel constructs. Thus the different levels of macroscopic current are related to channel availability, i.e. the number of channels available to open in the patches. By adding positive cooperativity to the Markov model, these features were readily simulated with reasonable quantitative accuracy (Fig. 8).
Besides K188 and R189, several other positively charged residues (e.g. R67 in N-terminus; R218 and R312 in C-terminus) have also been demonstrated to affect PIP2 sensitivity in activating Kir channels. However, the K188A/K189A double mutant (M4) experiments suggest that the K188/R189 PIP2 binding site is absolutely required to convert the channel to the available mode; the other PIP2 binding sites are not sufficient, even in the presence of PIP2 and DA12 (Figs 4 and 5). Nevertheless, although they are not essential for the channel to enter the available mode, interaction of these additional PIP2 binding sites appears to further increase the positive cooperativity between the subunits to favour the fully open state when the channel is in the available mode. This would explain why application of exogenous PIP2 further suppressed sublevels in the WT1M3 mutant channels, in which only one subunit contains the critical K188/K189 site (Fig. 8C). This modulatory effect of additional PIP2 binding sites would also explain why Ps varies from patch to patch in each construct.
Acknowledgments
We thank Dr L. Y. Jan for providing the Kir2.1 clone. This work was supported by NIH/NHLBI R37 HL60025 (to J.N.W.), by American Heart Association, National Research Center, Scientist Development Grant (0535143 N to L.-H.X.), and the Kawata and Laubisch Endowments.
References
- Arbuzova A, Martushova K, Hangyas-Mihalyne G, Morris AJ, Ozaki S, Prestwich GD, McLaughlin S. Fluorescently labeled neomycin as a probe of phosphatidylinositol-4,5-bisphosphate in membranes. Biochim Biophys Acta. 2000;1464:35–48. doi: 10.1016/s0005-2736(99)00243-6. [DOI] [PubMed] [Google Scholar]
- Chapman ML, VanDongen AM. K channel subconductance levels result from heteromeric pore conformations. J Gen Physiol. 2005;126:87–103. doi: 10.1085/jgp.200509253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman ML, VanDongen HM, VanDongen AM. Activation-dependent subconductance levels in the drk1 K channel suggest a subunit basis for ion permeation and gating. Biophys J. 1997;72:708–719. doi: 10.1016/s0006-3495(97)78707-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho HC, Tsushima RG, Nguyen TT, Guy HR, Backx PH. Two critical cysteine residues implicated in disulfide bond formation and proper folding of Kir2.1. Biochemistry. 2000;39:4649–4657. doi: 10.1021/bi992469g. [DOI] [PubMed] [Google Scholar]
- Doyle DA, Morais Cabral J, Pfuetzner RA, Kuo A, Gulbis JM, Cohen SL, Chait BT, MacKinnon R. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 1998;280:69–77. doi: 10.1126/science.280.5360.69. [DOI] [PubMed] [Google Scholar]
- Fan Z, Makielski JC. Anionic phospholipids activate ATP-sensitive potassium channels. J Biol Chem. 1997;272:5388–5395. doi: 10.1074/jbc.272.9.5388. [DOI] [PubMed] [Google Scholar]
- Hilgemann DW, Feng S, Nasuhoglu C. The complex and intriguing lives of PIP2 with ion channels and transporters. Sci STKE. 2001 doi: 10.1126/stke.2001.111.re19. RE19. [DOI] [PubMed] [Google Scholar]
- Ho IH, Murrell-Lagnado RD. Molecular determinants for sodium-dependent activation of G protein-gated K+ channels. J Biol Chem. 1999;274:8639–8648. doi: 10.1074/jbc.274.13.8639. [DOI] [PubMed] [Google Scholar]
- Huang CL, Feng S, Hilgemann DW. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gβγ. Nature. 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- Huang CL, Jan YN, Jan LY. Binding of the G protein βγ subunit to multiple regions of G protein-gated inward-rectifying K+ channels. FEBS Lett. 1997;405:291–298. doi: 10.1016/s0014-5793(97)00197-x. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Lee A, Chen J, Ruta V, Cadene M, Chait BT, MacKinnon R. X-ray structure of a voltage-dependent K+ channel. Nature. 2003;423:33–41. doi: 10.1038/nature01580. [DOI] [PubMed] [Google Scholar]
- Kono Y, Horie M, Takano M, Otani H, Xie LH, Akao M, Tsuji K, Sasayama S. The properties of the Kir6.1–6.2 tandem channel co-expressed with SUR2A. Pflugers Arch. 2000;440:692–698. doi: 10.1007/s004240000315. [DOI] [PubMed] [Google Scholar]
- Kubo Y, Baldwin TJ, Jan YN, Jan LY. Primary structure and functional expression of a mouse inward rectifier potassium channel. Nature. 1993;362:127–133. doi: 10.1038/362127a0. [DOI] [PubMed] [Google Scholar]
- Kuo A, Gulbis JM, Antcliff JF, Rahman T, Lowe ED, Zimmer J, Cuthbertson J, Ashcroft FM, Ezaki T, Doyle DA. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science. 2003;300:1922–1926. doi: 10.1126/science.1085028. [DOI] [PubMed] [Google Scholar]
- Leung YM, Zeng WZ, Liou HH, Solaro CR, Huang CL. Phosphatidylinositol 4,5-bisphosphate and intracellular pH regulate the ROMK1 potassium channel via separate but interrelated mechanisms. J Biol Chem. 2000;275:10182–10189. doi: 10.1074/jbc.275.14.10182. [DOI] [PubMed] [Google Scholar]
- Lin YW, Jia T, Weinsoft AM, Shyng SL. Stabilization of the activity of ATP-sensitive potassium channels by ion pairs formed between adjacent Kir6.2 subunits. J Gen Physiol. 2003;122:225–237. doi: 10.1085/jgp.200308822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liou HH, Zhou SS, Huang CL. Regulation of ROMK1 channel by protein kinase A via a phosphatidylinositol 4,5-bisphosphate-dependent mechanism. Proc Natl Acad Sci U S A. 1999;96:5820–5825. doi: 10.1073/pnas.96.10.5820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes CM, Zhang H, Rohacs T, Jin T, Yang J, Logothetis DE. Alterations in conserved Kir channel–PIP2 interactions underlie channelopathies. Neuron. 2002;34:933–944. doi: 10.1016/s0896-6273(02)00725-0. [DOI] [PubMed] [Google Scholar]
- Lu T, Nguyen B, Zhang X, Yang J. Architecture of a K+ channel inner pore revealed by stoichiometric covalent modification. Neuron. 1999a;22:571–580. doi: 10.1016/s0896-6273(00)80711-4. [DOI] [PubMed] [Google Scholar]
- Lu T, Ting AY, Mainland J, Jan LY, Schultz PG, Yang J. Probing ion permeation and gating in a K+ channel with backbone mutations in the selectivity filter. Nat Neurosci. 2001a;4:239–246. doi: 10.1038/85080. [DOI] [PubMed] [Google Scholar]
- Lu T, Wu L, Xiao J, Yang J. Permeant ion-dependent changes in gating of Kir2.1 inward rectifier potassium channels. J Gen Physiol. 2001b;118:509–522. doi: 10.1085/jgp.118.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T, Zhu YG, Yang J. Cytoplasmic amino and carboxyl domains form a wide intracellular vestibule in an inwardly rectifying potassium channel. Proc Natl Acad Sci U S A. 1999b;96:9926–9931. doi: 10.1073/pnas.96.17.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma D, Tang XD, Rogers TB, Welling PA. An Andersen–Tawil syndrome mutation in Kir2.1 (V302M) alters the G-loop cytoplasmic K+ conduction pathway. J Biol Chem. 2007;282:5781–5789. doi: 10.1074/jbc.M608776200. [DOI] [PubMed] [Google Scholar]
- Matsuda H, Oishi K, Omori K. Voltage-dependent gating and block by internal spermine of the murine inwardly rectifying K+ channel, Kir2.1. J Physiol. 2003;548:361–371. doi: 10.1113/jphysiol.2003.038844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzanti M, Assandri R, Ferroni A, DiFrancesco D. Cytoskeletal control of rectification and expression of four substates in cardiac inward rectifier K+ channels. Faseb J. 1996;10:357–361. doi: 10.1096/fasebj.10.2.8641571. [DOI] [PubMed] [Google Scholar]
- Nishida M, Cadene M, Chait BT, Mackinnon R. Crystal structure of a Kir3.1-prokaryotic Kir channel chimera. EMBO J. 2007;26:4005–4015. doi: 10.1038/sj.emboj.7601828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Omori K, Ohyama H, Shingu K, Matsuda H. Neutralization of aspartate residues in the murine inwardly rectifying K+ channel IRK1 affects the substate behaviour in Mg2+ block. J Physiol. 1998;510:675–683. doi: 10.1111/j.1469-7793.1998.675bj.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pegan S, Arrabit C, Slesinger PA, Choe S. Andersen's syndrome mutation effects on the structure and assembly of the cytoplasmic domains of Kir2.1. Biochemistry. 2006;45:8599–8606. doi: 10.1021/bi060653d. [DOI] [PubMed] [Google Scholar]
- Pegan S, Arrabit C, Zhou W, Kwiatkowski W, Collins A, Slesinger PA, Choe S. Cytoplasmic domain structures of Kir2.1 and Kir3.1 show sites for modulating gating and rectification. Nat Neurosci. 2005;8:279–287. doi: 10.1038/nn1411. [DOI] [PubMed] [Google Scholar]
- Phillips LR, Nichols CG. Ligand-induced closure of inward rectifier Kir6.2 channels traps spermine in the pore. J Gen Physiol. 2003;122:795–805. doi: 10.1085/jgp.200308953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proks P, Capener CE, Jones P, Ashcroft FM. Mutations within the P-loop of Kir6.2 modulate the intraburst kinetics of the ATP-sensitive potassium channel. J Gen Physiol. 2001;118:341–353. doi: 10.1085/jgp.118.4.341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribalet B, John SA, Xie LH, Weiss JN. Regulation of the ATP-sensitive K channel Kir6.2 by ATP and PIP2. J Mol Cell Cardiol. 2005;39:71–77. doi: 10.1016/j.yjmcc.2004.11.018. [DOI] [PubMed] [Google Scholar]
- Sakmann B, Trube G. Voltage-dependent inactivation of inward-rectifying single-channel currents in the guinea-pig heart cell membrane. J Physiol. 1984;347:659–683. doi: 10.1113/jphysiol.1984.sp015089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze D, Krauter T, Fritzenschaft H, Soom M, Baukrowitz T. Phosphatidylinositol 4,5-bisphosphate (PIP2) modulation of ATP and pH sensitivity in Kir channels. A tale of an active and a silent PIP2 site in the N terminus. J Biol Chem. 2003;278:10500–10505. doi: 10.1074/jbc.M208413200. [DOI] [PubMed] [Google Scholar]
- Shyng SL, Cukras CA, Harwood J, Nichols CG. Structural determinants of PIP2 regulation of inward rectifier KATP channels. J Gen Physiol. 2000;116:599–608. doi: 10.1085/jgp.116.5.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- So I, Ashmole I, Davies NW, Sutcliffe MJ, Stanfield PR. The K+ channel signature sequence of murine Kir2.1: mutations that affect microscopic gating but not ionic selectivity. J Physiol. 2001;531:37–50. doi: 10.1111/j.1469-7793.2001.0037j.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song DK, Ashcroft FM. ATP modulation of ATP-sensitive potassium channel ATP sensitivity varies with the type of SUR subunit. J Biol Chem. 2001;276:7143–7149. doi: 10.1074/jbc.M009959200. [DOI] [PubMed] [Google Scholar]
- Weber WM, Liebold KM, Reifarth FW, Clauss W. The Ca2+-induced leak current in Xenopus oocytes is indeed mediated through a Cl− channel. J Membr Biol. 1995;148:263–275. doi: 10.1007/BF00235044. [DOI] [PubMed] [Google Scholar]
- Xiao J, Zhen XG, Yang J. Localization of PIP2 activation gate in inward rectifier K+ channels. Nat Neurosci. 2003;6:811–818. doi: 10.1038/nn1090. [DOI] [PubMed] [Google Scholar]
- Xie LH, John SA, Weiss JN. Spermine block of the strong inward rectifier potassium channel Kir 2.1: dual roles of surface charge screening and pore block. J Gen Physiol. 2002;120:53–66. doi: 10.1085/jgp.20028576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie LH, John SA, Ribalet B, Weiss JN. Long polyamines act as cofactors in PIP2 activation of inward rectifier potassium (Kir2.1) channels. J Gen Physiol. 2005;126:541–549. doi: 10.1085/jgp.200509380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie LH, John SA, Ribalet B, Weiss JN. Activation of inwardly rectifying potassium (Kir) channels by phosphatidylinosital-4,5-bisphosphate (PIP2): Interaction with other regulatory ligands. Prog Biophys Mol Biol. 2007;94:320–335. doi: 10.1016/j.pbiomolbio.2006.04.001. [DOI] [PubMed] [Google Scholar]
- Zeng WZ, Li XJ, Hilgemann DW, Huang CL. Protein kinase C inhibits ROMK1 channel activity via a phosphatidylinositol 4,5-bisphosphate-dependent mechanism. J Biol Chem. 2003;278:16852–16856. doi: 10.1074/jbc.M300619200. [DOI] [PubMed] [Google Scholar]
- Zhang H, He C, Yan X, Mirshahi T, Logothetis DE. Activation of inwardly rectifying K+ channels by distinct PtdIns (4,5)P2 interactions. Nat Cell Biol. 1999;1:183–188. doi: 10.1038/11103. [DOI] [PubMed] [Google Scholar]
- Zingman LV, Hodgson DM, Bienengraeber M, Karger AB, Kathmann EC, Alekseev AE, Terzic A. Tandem function of nucleotide binding domains confers competence to sulfonylurea receptor in gating ATP-sensitive K+ channels. J Biol Chem. 2002;277:14206–14210. doi: 10.1074/jbc.M109452200. [DOI] [PubMed] [Google Scholar]