Abstract
Since the airways of control mouse lungs contain few alcian blue/periodic acid–Schiff's (AB/PAS)+ staining ‘goblet’ cells in the absence of an inflammatory stimulus such as allergen sensitization, it was surprising to find that the lungs of mice deficient for the exocytic priming protein Munc13-2 stain prominently with AB/PAS under control conditions. Purinergic agonists (ATP/UTP) stimulated release of accumulated mucins in the Munc13-2-deficient airways, suggesting that the other airway isoform, Munc13-4, supports agonist-regulated secretion. Notably, however, not all of the mucins in Munc13-2-deficient airways were secreted, suggesting a strict Munc13-2 priming requirement for a population of secretory granules. AB/PAS+ staining of Munc13-2-deficient airways was not caused by an inflammatory, metaplastic-like response: bronchial–alveolar lavage leucocyte numbers, Muc5ac and Muc5b mRNA levels, and Clara cell ultrastructure (except for increased secretory granule numbers) were all normal. A Muc5b-specific antibody indicated the presence of this mucin in Clara cells of wildtype (WT) control mice, and increased amounts in Munc13-2-deficient mice. Munc13-2 therefore appears to prime a regulated, baseline secretory pathway, such that Clara cell Muc5b, normally secreted soon after synthesis, accumulates in the gene-deficient animals, making them stain AB/PAS+. The defective priming phenotype is widespread, as goblet cells of several mucosal tissues appear engorged and Clara cells accumulated Clara cell secretory protein (CCSP) in Munc13-2-deficient mice. Additionally, because in the human airways, MUC5AC localizes to the surface epithelium and MUC5B to submucosal glands, the finding that Muc5b is secreted by Clara cells under control conditions may indicate that it is also secreted tonically from human bronchiolar Clara cells.
Oligomeric mucins secreted into the airways of healthy lungs are important for normal mucociliary clearance, and their hypersecretion contributes to pathophysiology of the majority of airways diseases, chronic obstructive pulmonary disease, asthma, cystic fibrosis, primary ciliary dyskinesia, and the morbidity experienced by the patients (see Rose & Voynow, 2006). Secretion of mucins from airway goblet cells appears to be regulated primarily by the agonists ATP and UTP, which activate P2Y2 purinoceptors and phospholipase C to initiate IP3/Ca2+ and diacylglycerol (DAG)/PKC intracellular signalling cascades (Davis, 1997; Kim et al. 2003; von Kugelgen, 2006; Davis & Abdullah, 1997). Of the intracellular events leading to exocytic mucin release (for review, see Davis & Dickey, 2008), PKC and Ca2+ coordinate remodelling of the actin cytoskeleton to allow interaction of mucin secretory granules with apical membrane exocytic docking sites (Trifaro et al. 2002; Ehre et al. 2005). Ca2+ also plays a major role in regulating the final steps of exocytosis in goblet (Rossi et al. 2004, 2007) and other secretory cells (Klenchin & Martin, 2000; Gerber & Sudhof, 2002; Bai & Chapman, 2004; Sudhof, 2004), by interacting with a host of C2-domain-containing proteins, including rabphilin, Munc13 and synaptotagmin (see Cho & Stahelin, 2006). DAG, however, also plays an important role in regulating exocytic proteins by attracting and activating those possessing C1 domains, predominantly Munc13 and diacylglycerol kinase (see Colon-Gonzalez & Kazanietz, 2006). In this study we used Munc13-2-deficient mice to test whether this isoform mediates agonist-regulated mucin secretion in the airways. It was motivated by the findings that high doses of the DAG mimic, phorbol myristic acid, elicit mucin secretion independent of PKC (Abdullah et al. 1997, 2003; Rossi et al. 2004), and that Munc13-2 and -4 are expressed in airways (Koch et al. 2000; Abdullah et al. 2003). The results obtained, however, were unexpected on three significant counts: the hypothesized function of Munc13 in regulated exocytosis, that Clara cells appear to secrete oligomeric mucins under control conditions in the wildtype (WT) mouse airways, and that Muc5b appears to be the major mucin expressed in the superficial epithelium.
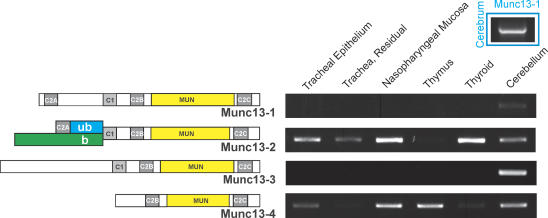
Munc13 is a family of four isoforms (Fig. 1; see Koch et al. 2000; Stevens et al. 2005; Basu et al. 2005), of which, at least in rodents, Munc13-2 is expressed as two splice variants. The brain variant, bMunc13-2, is similar to Munc13-3 in lacking the C2A domain, whereas the variant expressed ‘ubiquitously’, ubMunc13-2, is similar to Munc13-1 in possessing a C2A domain. ubMunc13-2 is expressed in SPOC1 cells, a mucin-secreting cell line derived from rat trachea (Abdullah et al. 2003). Munc13-1, -2 and -3 couple individually with the small GTPases, RIM and Rab3 in a tripartite complex (Betz et al. 2001; Dulubova et al. 2005; Andrews-Zwilling et al. 2006) that is required for exocytic priming of neuronal synaptic vesicles and endocrine secretory granules (Martin, 2002; Varoqueaux et al. 2002). Munc13-4 is a novel isoform lacking both the C2A and the DAG-binding C1 domain of the other Munc13 proteins, and is expressed widely in the body, especially in lung and SPOC1 cells (Koch et al. 2000; Abdullah et al. 2003). Munc13-4 is a Rab27 effector protein known to participate in the regulation of melanosome transport and secretory lysosome secretion (Zhang & Ginsburg, 2003; Neeft et al. 2005; Fukuda, 2005). It is also thought to be broadly active in regulating exocytosis in many endocrine and exocrine secretory cells (Fukuda, 2005; Bossi & Griffiths, 2005). How the isoforms of these two Munc13 subfamilies function coordinately in any individual secretory cell is unknown.
Figure 1. Munc13 domain maps and mRNA expression in mouse tissues.
Left, domain maps representing Munc13. The N-terminal splice variants for Munc13-2 are indicated in green (b, brain) and blue (ub, ubiquitous). Note the lack of an N-terminal C2A domain in some genes, including the brain splice-variant of Munc13-2. MUN, Munc13 homology domain. Right, conventional RT-PCR was used to identify the Munc13 isoforms expressed in tracheal epithelium (luminal extraction), the residual tracheal tissue, and nasopharynx. Thymus, thyroid and cerebellum were used as a source of positive control RNA for all Munc13 isoforms, and cerebrum (inset, upper right), additionally, was used for Munc13-1.
Despite its popularity as a model for allergic mucous metaplasia relevant to asthma and other inflammatory diseases of the lung where mucous metaplasia is prominent, the mouse is unlike larger mammals, including the rat (Kaartinen et al. 1993), in possessing very few identifiable goblet cells in the airway under control conditions. Indeed, a common finding is that alcian blue/periodic acid–Schiff's (AB/PAS)-stained sections of mouse lung exhibit few to no visibly stained goblet cells (e.g. see Cressman et al. 1998), unless metaplasia is induced by allergic challenge, infection, etc. (see Evans et al. 2004; Williams et al. 2006). AB/PAS is a common carbohydrate stain, which in the lung reveals mucin-secreting cells by virtue of mucins being very rich in glycans, ∼90% by weight. The gel-forming mucins are linear, disulphide-linked oligomers with molecular weights in the tens of mega-daltans that function as the molecular scaffolding of mucus (see Thornton & Sheehan, 2004; Rose & Voynow, 2006). The predominant species in the lung are Muc5ac and Muc5b, which in larger mammals are thought to be secreted from goblet cells in the superficial epithelium and from submucosal glands, respectively (Groneberg et al. 2002). In the mouse airways, submucosal glands are restricted to the cranial-most part of the trachea and the primary secretory cell in the superficial epithelium is the Clara cell, which expresses high levels of cytochrome P450 and secretes Clara cell secretory protein (CCSP, which is the same as CC10, uteroglobin), a lipoprotein of poorly understood function (Stripp et al. 2000). Under inflammatory conditions, Clara cells undergo a metaplastic transformation by up-regulating mucin synthesis and becoming AB/PAS+ (Evans et al. 2004; Williams et al. 2006). Hence, the mouse lung presents a non-trivial conundrum in being a commonly used model in biomedical research, one that appears to possess a functional mucociliary clearance system under control conditions (Kurosawa et al. 1995; Cressman et al. 1998; Foster et al. 2001; Grubb et al. 2004), but possesses no obvious source of mucins. Our surprise finding of AB/PAS+ cells during histological examination of the airways from mice deficient for Munc13-2 resolves this conundrum and provides insight into the cellular mechanisms underlying baseline mucin secretion and mucociliary clearance.
Methods
Materials
ATPγS was purchased from Roche Applied Science (Indianapolis, IN, USA); unless noted, all other chemicals were purchased from Sigma Chemical Co. (St Louis, MO, USA). A goat polyclonal antibody to CCSP was purchased from Santa Cruz Biotechnology (S-20). A mouse Muc5b-specific, rabbit polyclonal antibody was raised against a keyhole limit himocyanin-conjugated synthetic peptide with the sequence, CQPQCQWTKWIDVDY, which is repeated 5 times in the molecule in the Cys-rich domains that precede the PTS domains. Pre-immune rabbit serum, tested on agarose Western blots of mouse whole lung extract and in immunohistochemistry (IHC), were uniformly negative.
Mouse care and experimental procedures
Munc13-2+/− C57/B6 mice were kindly provided by Dr Niels Brose, Department of Molecular Neurobiology, Max-Planck-Institute of Experimental Medicine, Germany. All animals were bred and raised at the University of North Carolina, and were allowed food and water ad libitum. Some animals were treated with an ovalbumin tracheal instillation under isoflurane anaesthesia as described below; all animals were ultimately killed with gaseous CO2 prior to tissue harvest by dissection. All experimental procedures using mice were conducted under protocols approved by the University of North Carolina Institutional Animal Use and Care Committee.
Airway mucus metaplasia
Airway mucus metaplasia was induced in mice through ovalbumin (OVA) sensitization and challenge (see Cohn et al. 2002; Evans et al. 2004) using a procedure detailed previously (Ehre et al. 2007). Briefly, OVA was injected i.p. into each mouse on days 0, 7 and 14, and on days 21 and 24, 50 μl of 2.0% OVA in PBS was instilled by aspiration into tracheas of isoflurane-anaesthetized mice. The instillation procedure was completed in < 20 s, well within the ∼1 min period of time it takes the mouse to recover from the isoflurane anaesthesia; recovery from the procedure was 100%. Experimental procedures were performed 3–14 days following the 2nd OVA instillation.
Broncho-alveolar lavage
Broncho-alveolar lavage (BAL) was used to analyse inflammatory cells. Mice were killed with CO2 and the trachea, exposed through a neck incision, was severed and cannulated with a blunt 20G needle. The chest cavity was opened and 3-0 silk suture was used to tie off the left lung lobe, to restrict the BAL to the right lobe. Phosphate-buffered saline (PBS), 17.5 μl (g body weight)−1 and containing Complete Mini Protease Inhibitor protease inhibitor cocktail (1 tablet in 10 ml; Roche, Penzberg, Germany), was slowly injected into the lobe and recovered a total of 3 times, the procedure was repeated once, the two collections were pooled, centrifuged (600 g, 5 min, 4°C), and the pellet resuspended in 100 μl PBS. Total cell number was determined using a haemocytometer, and cell composition by identifying and counting > 200 cells per sample stained with Wright-Giemsa, on air-dried samples prepared by cytospin analysis (StatSpin Cytofuge, Iris International, Westwood, MA, USA).
Naphthalene injury
Naphthalene injury was used to test whether mucins were expressed in Clara cells (Plopper et al. 1992; Van Winkle et al. 1995; Rawlins et al. 2007). Adult mice (8–14 weeks) were injected, between 08:00 and 10:00 h, peritoneally, with 250 mg kg−1 naphthalene dissolved in fresh (< 1 month old) Mazola corn oil; control mice received vehicle only. Two days later, the mice were killed and lung tissue harvested for histological analysis.
Tracheal perfusion, mucin ELISA
Tracheas were harvested from mice killed with CO2, cannulated at the proximal end, and perfused at 50 μl min−1 with warmed, 5% CO2–95% O2-equilibrated DMEM/F12 as detailed previously (Ehre et al. 2007). A pair of preparations were mounted on a custom bracket attached to the arm of a Gilson FC204 microtitre plate fraction collector (Middleton, WI, USA) such that the effluent dripped from the open ends of the tracheas directly into the wells of 96-well, non-binding, polystyrene microtitre storage plates (Corning, no. 3594), with collections timed at 5 min. The entire set-up (pump, solutions, tissues, fraction collector) was housed in a humidified Nuaire DH Autoflow CO2 incubator (Plymouth, MN, USA), factory customized to lie horizontally.
Mucins in the collected fractions were assessed by an ELISA (Ehre et al. 2007), using a mucin ‘subunit antibody’ that recognizes all vertebrate oligomeric mucins (Sheehan et al. 1991). Standard curves were generated from purified SPOC1 mucins (Abdullah et al. 1996) applied to each plate, and the results were expressed as the equivalent nanogram of mucin released per each fraction for each trachea. Apparent differences between sample means in these experiments were subjected to a Student's t test, assuming equal variances, with statistical significance being indicated at P < 0.05.
Histology, immunohistochemistry and confocal microscopy
Tissues fixed in formalin were dehydrated and embedded in paraffin, sections cut at 5 μm were placed on slides, deparaffinized and rehydrated. AB/PAS staining followed standard protocols, using a 5 min incubation in 0.5% periodic acid. Sections for IHC using immunofluorescent probes were first treated for antigen retrieval using HIER solution (SI 700, Dako, Carpinteria, CA, USA) for 30 min, at 98.8°C, before being circled with a liquid repellent pen and placed into a humidified container. The sections were then treated with Image-it FX (Molecular Probes, Eugene, OR, USA) to reduce autofluorescence, and blocked (0.05% saponin, 1% BSA in PBS) for 2 h at room temperature, following which they were incubated with the primary antibody, diluted in blocking solution. They were then incubated overnight at 4°C, then in the appropriate fluorescently conjugated secondary antibody, in blocking solution, for 1 h at room temperature. The sections were washed and coverslips applied with Prolong Gold mounting media (Molecular Probes). For the experiment shown in Fig. 6B, a peroxidase conjugated goat anti-rabbit antibody (1: 200; Vectorlabs, Burlington, VT, USA) and diaminobenzidine (DAB; Vectorlabs) was used. For each experiment, the immunostaining procedure was repeated a minimum of 3 times, and the possibility of non-specific binding was ruled out by staining sections with rabbit or goat IgG.
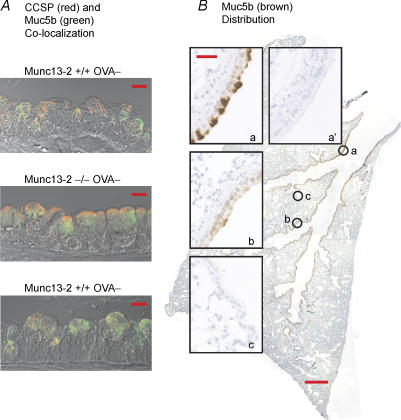
Figure 6. Mucin and CCSP expression in mouse airways.
A, Muc5B and CCSP co-localization. Sections of bronchial epithelium from WT and Munc13-2-deficient mice, without or with OVA sensitization as indicated, were stained for Muc5B (green) and CCSP (red), and examined by confocal microscopy. The images shown, representative of sections from 3 mice each, are overlays of green, red and DIC images, acquired simultaneously. Note that Muc5b staining in the WT control mouse appears sparse with use of the FITC conjugated secondary antibody (scale bars, 10 μm). B, Muc5b distribution in mouse lung. A more sensitive IHC staining technique using a peroxidase conjugated secondary antibody revealed a broad distribution of Muc5b in the airways. The background image shows a tissue section taken through the entire axial bronchus of a 90-day-old wildtype C57BL/6J mouse. Note the dark staining of the airway epithelium deep into the lung. Insets left show that Muc5b is expressed in the axial bronchus (a) and the proximal portion of minor daughter branches (b), but it is absent in the terminal bronchioles (c). a′, demonstration of immunolabel specificity, with normal rabbit serum used in place of anti-Muc5b. The image shown in a′ is taken from the same portion of the axial bronchus in a neighbouring section as shown in a. Scales bars, 250 μm (low magnification) and 25 μm (high magnification).
Immunofluorescence was visualized using a Zeiss 510 Meta (Carl Zeiss International, Oberkochen, Germany) laser scanning confocal microscope. The secondary antibodies were excited with 488 nm argon and 543 nm He–Ne1 lasers. Differential interference microscopy (DIC) was used, simultaneously, to visualize the tissue for general morphology. Photomultiplier gains and offsets for the two fluorescence channels were adjusted at each viewing session to minimize cross-talk. Zeiss 510 imaging software was used for image analysis. The images and protein distributions presented in Results are fully representative of all images examined.
Electron microscopy
Aldehyde-fixed mouse lung tissue was washed in 0.1 m Sorenson's buffer for 1 h at 4°C (×3), post fixed in 1% OsO4 in 0.1 m Sorenson's buffer for 1 h at 4°C, and rinsed in filtered distilled water for 5 min. The tissues were dehydrated in graded ethanol at room temperature for 10 min, immersed in propylene oxide for 20 min (×2), then infiltrated with a 1: 1 mixture of propylene oxide and pure epon resin overnight at room temperature, and then in pure resin for 4 h. Sections 90 nm thick, cut onto copper grids and stained with uranyl acetate and lead citrate, were viewed with a Zeiss EM900 transmission electron microscope.
RT-PCR
Mouse tissues destined for mRNA analysis were cut into small pieces and immersed in RNAlater reagent (Sigma), then stored at −80°C until use. Total RNA was extracted by thawing tissue into RLT buffer (Qiagen, Valencia, CA, USA) and homogenizing with a Fisher PowerGen 125 homogenizer. For selective isolation of RNA from tracheal epithelium, tracheal lumens were exposed directly to RLT buffer for 15 min, then harvested, following which the residual tracheal tissue was extracted, with homogenization, in RLT buffer. Total RNA was purified from extracts using an RNeasy Mini Kit (Qiagen) and quantified using a NanoDrop spectrophotometer (Wilmington, DE, USA).
Conventional RT-PCT was used to identify Munc13 isoforms. SuperScript II (Invitrogen, Carlsbad, CA, USA) was used to reverse transcribe 500 ng total RNA, using oligo(dT) 15-mer primers, following which 50 ng cDNA was amplified by PCR using GoTaq Green Master Mix (Promega, Madison, WI, USA) and forward and reverse primers, respectively, as follows: Munc13-1 (TGCAGGGCTCGGAGCTGG; GCTCACGAGGGGAATGGG); Munc13-2 (CTTCCTACTCCTGTAAGCAGGG; TGTTGACTGGCGCATTGTGG); Munc13-3 (ATCCAGAGTTAAGCAGCACT; GGGCACATGTACTTAGAAGA); and Munc13-4 (AGGGAAGCCCTTCATCCTGT, TCTGTAGACAGCCAAACTCC).
Quantitative, real time PCR (qRT-PCR) was used to analyse mucin and CCSP mRNAs in mouse lung extracts on 20 ng of random-primed, reverse transcribed cDNA using a LightCycler (Roche), and forward and reverse primers, respectively, as follows: Muc5ac (CCATGCAGAGTCCTCAGAACAA, TTACTGGAAAGGCCCAAGCA); Muc5b (CTGGCCTCTGTAACTATGT, CATAGCCACTGCTCTTCT); CCSP (AGCCTCCAACCTCTACCA, AGGGATGCCACATAACCA). For each gene, a standard curve was used to calculate the efficiency of the PCR reaction, and the data were analysed and expressed relative to WT controls using the method of Pfaffl (Pfaffl, 2001).
Collection and analysis of Muc5b glycoprotein
Mucins were solubilized from whole lung tissue using a guanidinium chloride (GuCl) extraction buffer (6 m GuCl, 100 mm Tris-HCl, 5 mm EDTA, pH 8.0, with 1 tablet protease inhibitor per 7 ml working buffer, see BAL section above), either by immersing the left lung lobe in 1 ml of extractant, or by injecting 0.6 ml into the whole lung via the trachea, following which it was immersed in 1 ml of extractant. In both cases, the tissue was chopped into small pieces with scissors, stirred overnight at 4°C, centrifuged (16 000 g, 30 min, 4°C), and the supernatant dialysed overnight at 4°C against 6 m urea buffer (6 m urea, 100 mm Tris-HCl, 5 mm EDTA, pH 8.0) and stored at −80°C until use. Protein concentrations were determined using a BCA assay (Pierce, Rockford, IL, USA). Mucins were analysed by agarose Western blotting, as detailed previously (Kirkham et al. 2002; Holmen et al. 2004). Briefly, mucins in whole lung extract, ≤ 40 μl, were resolved by electrophoresis in 1% agarose gels (80 V, 2 h), and transferred by vacuum blotting (VacuGene XL, GE Heathcare, Piscataway, NJ, USA) to nitrocellulose membranes. Blots were probed with Muc5b antibody and developed with enhanced chemiluminescence (Pierce).
Results
Munc13-4 is expressed in lung (Koch et al. 2000) and with ubMunc13-2 in SPOC1 cells (Abdullah et al. 2003), but it was important to establish a more complete distribution of Munc13 isoforms expressed in mouse airway epithelium because their expression outside the brain is poorly known (e.g. see Augustin et al. 1999a). Figure 1 shows that only Munc13-2 and -4 mRNAs were identified in tracheal epithelium (selective, luminal exposure to the extraction buffer) and nasopharynx, similar to the expression pattern described for SPOC1 cells (Abdullah et al. 2003).
Effects of Munc13-2 deficiency on regulated mucin secretion from control airways
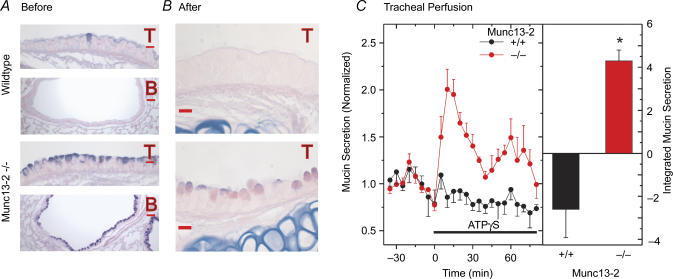
Histological analysis of the airways from WT control mice (Fig. 2A) confirmed the common observation that cells staining with AB/PAS in the healthy epithelium are rare, particularly for C57B/6 mice (see Cressman et al. 1998). In contrast, both the tracheal and bronchial epithelia of Munc13-2-deficient mice contained significant quantities of AB/PAS+ material, presumably mucins (see below). Whether this accumulation of AB/PAS+ material reflects the loss of a Munc13-2-mediated secretory function directly, however, or is the indirect result of a metaplastic-like response in the null mice is not clear from the result. As a first step in answering this question, we tested whether the accumulated mucins in the Munc13-2-deficient mice could be secreted following stimulation by purinergic agonists, which are well known mucin secretagogues in the airways (Davis & Dickey, 2008). Using tracheal isolation and perfusion, and mucin detection protocols established recently (Ehre et al. 2007), tracheas from WT and Munc13-2-deficient mice were challenged with the purinergic agonist ATPγS. As shown in Fig. 2C, perfused tracheas from Munc13-2-deficient mice mounted a healthy secretory response to ATPγS. Analysis of the tracheas following agonist exposure, however, showed that a substantial amount of AB/PAS+ material was retained following the 80 min challenge (Fig. 2B). Agonist-exposed tracheas from the control, WT mice released no detectible mucins above baseline (Fig. 2C), as expected from the general lack of AB/PAS+ material in WT control airways (Fig. 2A). The WT baseline data appear to diminish following agonist exposure, but whether or not this change represents a significant decline is unclear since the baseline secretion rates in these mice are so low.
Figure 2. Mucin stores and secretion from control, non-OVA sensitized Munc13-2-deficient mouse airways.
A and B. AB/PAS-stained lung sections before (A) and after (B) tracheal perfusion and agonist challenge (T, trachea; B, bronchus; scale bars in A = 30 μm, in B = 10 μm) C, tracheal mucin secretion, time course (left) and integrated response (right). Isolated, perfused trachea were challenged with purinergic agonist (ATPγS, 100 μm) during the period indicated by the bar. Perfusates were collected at 5 min intervals and sampled for contained mucins by ELISA. Data (mean ±s.e.m., n = 4) are normalized to the mean baseline period (30 min) preceding the agonist challenge. Integrated response (right). Supra-baseline agonist-stimulated data for WT and Munc13-2-deficient mice were summed, after subtracting the mean baseline, to estimate the integrated mucin secretory response; *P < 0.05.
In summary, mucins accumulate in Munc13-2-deficient mouse airways, possibly reflecting a functional deficiency in a tonically active, baseline mucin secretion pathway. Importantly, given the participation of Munc13-2 in priming the SNARE complex of regulated exocytosis, this pathway, by definition, would be a regulated, Ca2+-dependent secretory pathway, not a constitutive secretory pathway (Miller & Moore, 1991; Arvan & Castle, 1998; Ponnambalam & Baldwin, 2003; Gorr et al. 2005). These results also show that a substantial portion of the accumulated mucins can be released in response to agonist stimulation, presumably under the control of Munc13-4 or other protein(s).
Effects of Munc13-2 deficiency on regulated mucin secretion from metaplastic airways
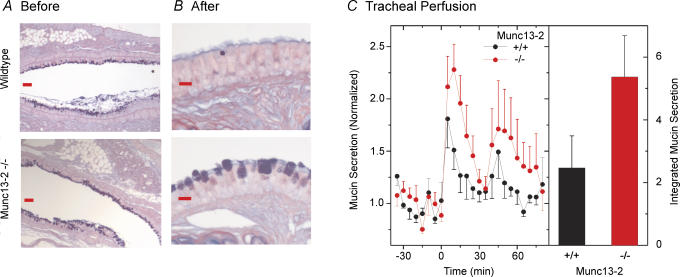
To test whether Munc13-2 participates as a central regulator of the mucin granule exocytic complex during agonist-induced secretion, it was necessary to have AB/PAS+ WT airways as proper controls, which was achieved by inducing allergic mucous metaplasia with OVA treatment of both WT and Munc13-2-deficient mice. Lungs from such mice, sectioned and stained with AB/PAS, indicated that the airways of both WT and null mice were highly metaplastic, staining strongly with AB/PAS (compare Fig. 3A with 2A). As shown in Fig. 3C, tracheas from both the WT and Munc13-2-deficient mice mounted robust mucin secretory responses in response to agonist; however, the peak and integrated responses of the Munc13-2-deficient tracheas appeared, unexpectedly, to be greater than the WT controls. The difference between the two groups of mice, though, was not significant (P/ 0.05). This result indicates, again, that Munc13-2 is apparently not essential for agonist-induced secretion.
Figure 3. Mucin stores and secretion from OVA-sensitized, metaplastic mouse airways.
A and B, AB/PAS-stained lung sections before (A; bronchus) and after (B; trachea) tracheal perfusion and agonist challenge (scale bars in A = 30 μm, in B = 10 μm). C, tracheal mucin secretion, time course (left), integrated response (right). Experiment per Fig. 2C (n = 5).
Interestingly, examination of tracheal epithelia following the 80 min agonist exposure revealed significant residual mucin stores (AB/PAS+ staining) in the Munc13-2-deficient tracheas, whereas the WT control tracheas were completely depleted (Fig. 3B). Since residual mucins were apparent after agonist exposure only in tracheas of Munc13-2-deficient mice, whereas the amounts of mucin in WT and Munc13-2-deficient tracheas of OVA-sensitized mice were nominally similar (Fig. 3, and see oligomeric mucin expression, below), this isoform, again, appears to contribute in some way to the regulation of mucin secretion even though agonist-stimulated secretion can occur in its absence.
Does AB/PAS+ airways in Munc13-2 deficient mice signify mucous metaplasia?
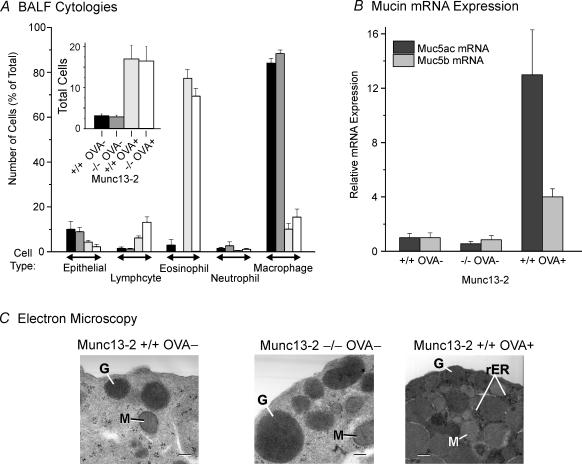
As noted above, the appearance of AB/PAS+ staining in the airways of Munc13-2-deficient mice could be caused by an inflammation-like response leading to mucous metaplasia, such as is known to occur in transgenic mice overexpressing the β subunit of the epithelial Na+ channel (βENaC; Mall et al. 2004). We addressed this possibility in three ways. First, bronchial–alveolar lavage fluids (BALF) were harvested and the cells quantified by cell type. As shown in Fig. 4A, there were no differences in BALF total cells (inset), or in the percentage composition of different cell types between the non-OVA-treated mice, whether they were WT or Munc13-2-deficient mice. OVA treatment, in contrast, caused the appearance of inflammatory cells, predominately eosinophils, to infiltrate the lung. Notably, OVA had the same effects on the BALF cytologies of the WT and Munc13-2-deficient mice. Additionally, examination of haematoxylin and eosin stained sections of non-metaplastic, WT and Munc13-2-deficient mice exhibited no evidence of inflammatory cell infiltrates in the airways (data not shown). Both sets of data suggest the lack of an inflammatory response in the Munc13-2-deficient mice.
Figure 4. Airway epithelium of Munc13-2-deficient mice is not metaplastic.
WT and Munc13-2-deficient mice, without and with OVA treatment as indicated, were assessed for indications of mucous metaplasia. A, BALF inflammatory cell profile. Mouse BAL fluids were assessed for total cells (inset) and for relative cell composition (bar codes in inset). Data presented as the mean +s.e.m. (n = 4–6). Note the effects of OVA treatment, but not of Munc13-2 genetic status. B, mucin gene expression in mouse lung. Whole lung extracts were assessed for relative Muc5AC and Muc5B mRNA expression levels by qRT-PCR. Data are normalized to the WT, control (OVA–) mice, and presented as the mean +s.e.m. (n = 3). C, electron micrographs of bronchial Clara cell apical pole region. G, secretory granule; M, mitochondria; rER, rough ER; scale, 0.5 μm. Micrographs shown are representative of those examined from 3 different mice for each condition.
Second, we tested whether mucin gene expression was up-regulated, as would be expected for an inflammatory or inflammatory-like condition (Evans et al. 2004). Total RNAs, extracted and purified from whole lungs of WT and Munc13-2-deficient mice, and of WT OVA-treated mice, were probed for Muc5ac and Muc5b mRNA levels by qRT-PCR. When expressed against the WT controls, there were no differences in mRNA levels for either mucin in Munc13-2-deficient mice; however, Muc5ac and 5b levels were increased in the OVA-treated WT mice, as expected (Fig. 4B). Indeed, our data are consistent in this regard with the recent findings of Young et al. in that Muc5ac was up regulated in allergen-induced mucous metaplasia by a much larger degree than was Muc5b. Additionally, normalizing the data to 18S rRNA, the levels of Muc5b in WT, control mice are estimated to exceed Muc5ac by 89-fold (data not shown), again in general agreement with published data (Young et al. 2007).
Third, we examined airway Clara cells by electron microscopy. There were greater numbers of secretory granules in the apical pole regions of Munc13-2-deficient, non-metaplastic Clara cells (Fig. 4C); otherwise, there were no notable differences from WT. In contrast, the metaplastic, WT Clara cells in OVA-treated mice showed major changes in appearance, in addition to increased numbers of granules. Chief among these were the presence of dense-core granules, a more electron dense cytoplasm, and the appearance of abundant rough endoplasmic reticulum, a sign of increased secretory protein synthetic activity. That these changes were not observed in non-metaplastic Munc13-2-deficient Clara cells is consistent with a mucin content that is increased by decreased secretory activity, rather than increased synthesis.
Oligomeric mucin expression in mouse lung
If Clara cells in the airways of Munc13-2-deficient mice become AB/PAS+ because oligomeric mucins are retained, rather than secreted, then it should be possible to detect mucins in WT control animals. Additionally, the mucin content of the MUNC13-2-deficient mice should be increased. To test these predictions, we determined the relative amounts of Muc5b glycoprotein in whole lung extracts of WT and Munc13-2-deficient mice by agarose Western blotting. A significant effort was also made to estimate Muc5ac glycoprotein levels, but neither the specific antibodies we made nor any of the commercially available antibodies yielded satisfactory results – both gastric (control) mucins stained poorly, as did airway mucins, even those extracted from lungs of OVA-treated mice in which Muc5ac mRNA levels are up-regulated (Evans et al. 2004). For Muc5b, the lungs were initially extracted by immersion in 6 m GuCl buffer, the samples reduced with DTT, and the relative staining intensities of mucin bands in the blots determined. These data showed consistently that there was more Muc5b in the lungs of Munc13-2-deficient, than WT mice, especially in the non-metaplastic lungs (n = 5 each; data not shown).
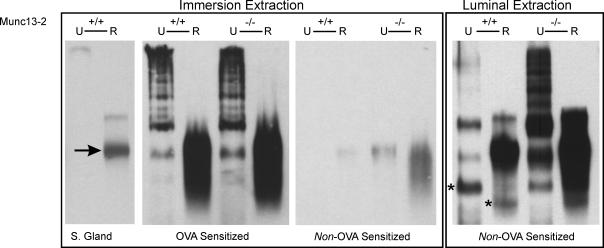
Muc5b agarose Western blots were next prepared from unreduced and DTT-reduced samples, to resolve high molecular weight Muc5b oligomers and monomers, respectively. Material extracted by immersion in GuCl buffer (Fig. 5, ‘Immersion Extraction’) from lungs of OVA-sensitized animals showed clearly that Muc5b in the unreduced (U) samples resolved as a structured ladder of bands with increasing mobility, most likely representing successively smaller mucin oligomers (see Sheehan et al. 2004). When the samples were reduced (R), this material resolved predominately as a single band that had the same relative mobility as the positive control, DTT-reduced material from submucosal glands (Fig. 5, arrow), extracted from the tissue between the cricoid cartilage and second tracheal ring. Hence, this band probably represents mature, i.e. fully glycosylated, Muc5b monomers. The Muc5b staining intensities of the unreduced and reduced samples from the OVA-treated Munc13-2-deficient mouse were more intense than those from WT, but only slightly so.
Figure 5. Muc5b glycoprotein expression in mouse lung.
The presence of Muc5b was assessed in whole lung extracts by agarose Western blotting. The arrow, left, indicates the Muc5b glycosylated monomer band in submucosal gland extract (S. Gland), which served as a positive control. ‘Immersion extraction’ (left-hand blots) is lungs immersed in GuCl buffer. ‘Luminal Extraction’ (right-hand blot) is GuCl buffer injected into airways prior to immersion in GuCl buffer. U, unreduced; R, reduced (DTT). Samples from non-OVA-sensitized and OVA-sensitized animals are indicated at the bottom. The bands at positions in the right-most blot indicated by * are interpreted as the non-glycosylated, Muc5b dimer (unreduced) and monomer (reduced), precursors of the mature mucin. Blots are representative of 3 or more prepared for each condition.
DTT-reduced material extracted by immersion from non-OVA-treated airways also stained positively for monomeric Muc5b, but as expected the staining intensities were uniformly lower than OVA-treated material. In fact, there was little or no apparent oligomeric Muc5b in the samples from both the WT and Munc13-2-deficient mice, and in the WT mice only the reduced sample exhibited any staining at all. In these non-OVA-treated mice, however, there were large differences between reduced samples from WT and Munc13-2-deficient mice, consistent with the notion of accumulated mucins in the deficient animals.
To increase the efficiency of mucin extraction, a modified procedure was used for non-OVA-treated mice. Before immersing the harvested lungs in GuCl buffer, as above, a smaller volume of buffer was injected into the airways through the carina. Hence, the lungs were extracted from the airway epithelium outwards, insuring good solubilization of the mucins in the airway epithelium. As shown in the blot labelled ‘Luminal Extraction’ (Fig. 5), this procedure yielded sufficient Muc5b to visualize mucin oligomeric bands in agarose Western blots, especially for the lungs from Munc13-2-deficient mice. In this case, a well formed oligomeric ladder is apparent, whose bands correlate reasonably well with those for the metaplastic samples in the ‘Immersion Extraction’ blot. Muc5b oligomers in the WT unreduced sample resolved as very faint bands; however, the reduced sample yielded a heavily stained monomeric band indicating the clear presence of Muc5b in lungs of the WT, control mouse. Notably, there was a very large increase in Muc5b in the lungs of Munc13-2-deficient mice, relative to WT. Since there was not a commensurate increase in Muc5b gene expression between these mice, the airways of the Munc13-2-deficient mouse probably accumulate mucins that would be normally secreted under baseline conditions.
Luminal extraction of the mucins produced clear bands of higher mobility in the agarose Western blots (Fig. 5, left, asterisks), that probably represent the non-glycosylated Muc5b dimer (non-reduced) and monomer (reduced) units. Faint bands at these positions can also be identified in the mucins resolved from OVA-treated airways. Also interesting is the apparent increase in Muc5b staining intensity that occurs when the mucins are reduced, particularly in mucins from the WT control mice. Part of the explanation may lie with the material observed in the prominent reduced band, being dispersed over numerous, less mobile bands in the non-reduced material. An additional possibility, however, is that some, or even most, of the five repeats of the antibody target sequence in the non-reduced, oligomeric form of Muc5b (see Experimental procedures) may be hidden from the antibody inside the tertiary structure of the glycoprotein, and exposed following reduction.
Mucin and CCSP expression in Clara cells
Clara cells being the only secretory cell in WT, control mouse airways makes them the likely source of mucins. This notion was tested in two ways. First, Muc5b and CCSP were localized in the airway epithelium by IHC. Figure 6A shows that Muc5b co-localized with CCSP in Clara cells under all conditions, i.e. in WT cells, both without and with OVA treatment, and in Munc13-2-deficient cells. With the FITC conjugated secondary antibody used in these experiments, however, Muc5b did not appear to stain all CCSP-staining cells, especially in the WT control tissue. Importantly, cells that did stain positively for Muc5b were also CCSP positive.
To better assess the distribution of Muc5b glycoprotein expression in WT control mice, we used a more sensitive IHC technique, namely a HRP-conjugated secondary antibody. As Fig. 6B illustrates, this technique showed Muc5b to be distributed widely in the airways, from the axial bronchus, where it is most heavily expressed, down to the small airways. It does not appear, however, to be expressed in the terminal bronchioles (Fig. 6Bc).
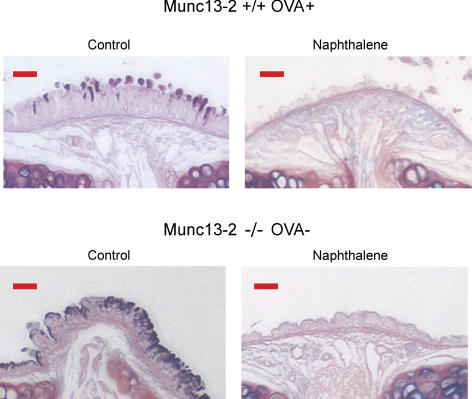
As a second test of mucin expression in Clara cells, naphthalene was used to injure the epithelium, using a protocol developed to direct the injury selectively to Clara cells, which express high levels of P450 (Hong et al. 2001; Rawlins et al. 2007). Trachea and lung sections were stained with AB/PAS 2 days following treatment. As shown in Fig. 7, treating WT OVA sensitized (control) mice and Munc13-2-deficient non-OVA sensitized mice with naphthalene resulted in a loss of AB/PAS+ staining, suggesting that mucins are expressed in Clara cells. The overall reduction in epithelial height seen in the naphthalene-treated tissues reflects the fact that Clara cells are a major cell type in mouse airways. The selective loss of Clara cells apparently causes the ciliated cells to spread out, reducing their overall height.
Figure 7. Naphthalene injury selectively ablates mucin-expressing cells.
WT mice sensitized with OVA (controls), and Munc13-2-deficient non-OVA-treated mice were subjected to naphthalene injury, and the airways examined 2 days later for AB/PAS+ staining. Images shown are representative of 3 mice for each condition. Scale bars, 15 μm.
Munc13-2 involvement in other epithelial secretory processes
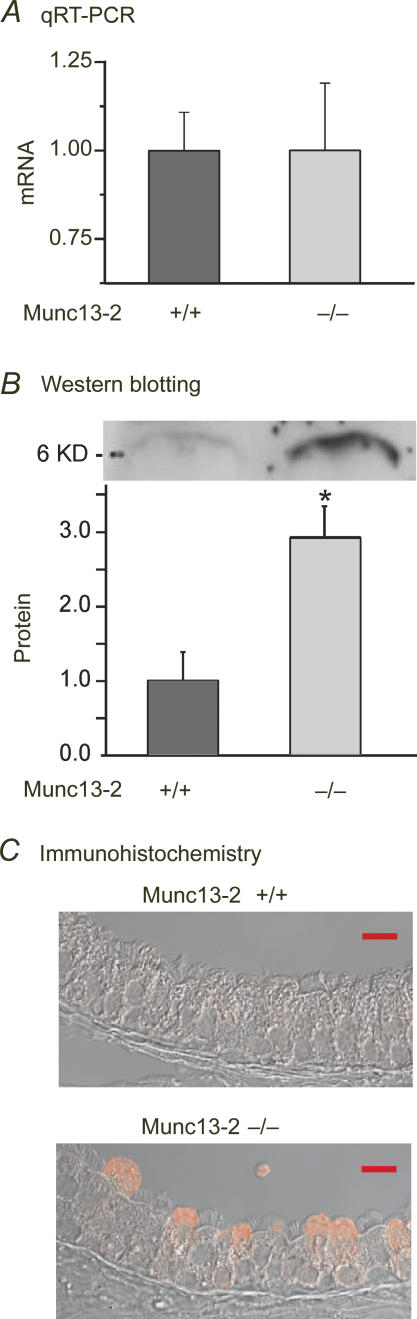
Given the wide distribution of Munc13-2 in secretory cells, it would be surprising were mucins the only secretory cargo affected in the airways of the null animals. It can be appreciated from Fig. 6A, for instance, that CCSP staining appears more intense in the Munc13-2-deficient Clara cells, relative to the WT control. To test whether CCSP secretion might be also affected in the Munc13-2-deficient animals, we used qRT-PCR and Western blotting to determine the relative CCSP mRNA and protein expression levels in lung extracts, and repeated the CCSP IHC without Muc5b co-staining. Figure 8 shows clearly that CCSP mRNA levels are not affected in the Munc13-2-deficient mice, whereas CCSP protein and immunostaining in the Munc13-2-deficient Clara cells was substantially increased, relative to their WT controls. Hence, there also appears to be a CCSP secretory defect in the Munc13-2-deficient mice.
Figure 8. CCSP lipoprotein secretion is affected in Munc13-2-deficient mice.
CCSP mRNA (A; NS, n = 4) and protein (B; n = 3) levels in whole lung extracts were assessed by qRT-PCR and Western blotting, respectively, in non-OVA-sensitized WT and Munc13-2-deficient mice. A sample blot is shown above the graph in B;*P < 0.05, Student's t test. C, bronchial epithelium was assessed for CCSP immunostaining in the tracheas of the same mice. Images shown are representative. Scale bars, 10 μm.
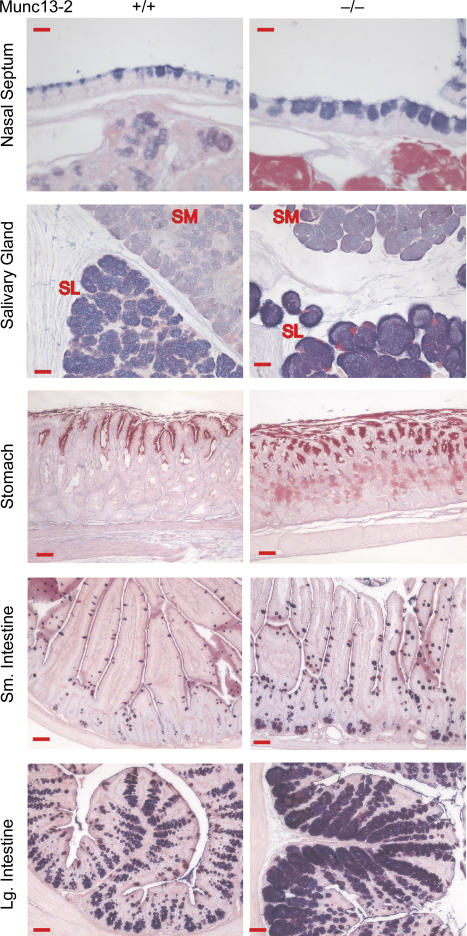
To test whether epithelial secretory tissues other than the airways were affected in the Munc13-2 deficient mice, a series of mucosal organs were sectioned and stained with AB/PAS. Figure 9 shows that AB/PAS+ staining was increased in every tissue examined in the Munc13-2-deficient animals, including nasal septum, salivary gland, stomach, and small and large intestine, dramatically so in some cases. Hence, the apparent defect in baseline secretion observed for the airways of Munc13-2-deficient mice appears to be a widespread abnormality.
Figure 9. Mucin secretory defects in Munc13-2-deficient mice are widespread.
Tissues of several mucin-secreting organs in WT Munc13-2-deficient mice, as indicated, were examined for AB/PAS+ staining. Images shown are representative of 2–3 mice each. For salivary gland: SL, sublingual; SM, submandibular. Nasal septum, scale bars, 10 μm; other scale bars, 50 μm.
Discussion
Munc13 function in the airway epithelium
The archetypal gene encoding exocytic priming protein, Unc-13, was identified in a classic 1975 mutagenic study of C. elegans as producing an severe uncoordinated phenotype, i.e. they were paralysed (Brenner, 1974). A functional problem in neurotransmitter release was suggested in 1991 when the neurons in these worms were discovered to have elevated levels of acethylcholine (Hosono & Kamiya, 1991), which was confirmed in 1999 (Richmond et al. 1999; Augustin et al. 1999b). Cloning and sequencing of the gene revealed that the only regions of Unc-13 with homology to other proteins were its C1 and C2 domains (Maruyama & Brenner, 1991), structural elements subsequently found preserved in its mammalian homolog, Munc13 (Brose et al. 1995). While Munc13-1, -2 and -3 have been studied extensively in the nervous and endocrine system (for review, Brose & Rosenmund, 2002; Martin, 2002), there have been very few studies in other secretory cells. Munc13-4 has received substantial recent attention for its function in the immune system, having had several genetic diseases ascribed to its dysfunction (for review, Fukuda, 2005; Hong, 2005), but few of these studies relate to its molecular physiology.
In hippocampal neurons, all synaptic activity ceases in mice lacking both normally expressed isoforms, Munc13-1 and ubMunc13-2, and nearly full function is restored when either isoform is delivered exogenously to rescue the primary defect (Rosenmund et al. 2002). The priming activities of Munc13, per se, have been associated with the C-terminal half of the molecule, in particular the MUN domain (= MHD1 + MHD2) (Koch et al. 2000; Basu et al. 2005; Stevens et al. 2005), which is consistent with the high sequence similarity in its structure for all the isoforms, including Munc13-4. Although less well conserved and not essential for priming, the half of the molecule lying upstream of the MUN domain appears to be involved in Munc13 localization and recruitment. The C1 domain, for instance, is essential for survival in mice, because it mediates activation by DAG (Rhee et al. 2002), and the C2A domain is essential for the recruitment of Munc13-1 and ubMunc13-2 to the synaptic active zone by RIM1a (Andrews-Zwilling et al. 2006). Munc13-4 lacks both C1 and C2A domains, yet it is a Rab27 effector (Neeft et al. 2005; Fukuda, 2005) through interactions with an unidentified structural element. Given the importance of Munc13 in neurotransmitter and hormone release, the N-terminal structural differences between the airway-expressed ubMunc13-2 and Munc13-4 isoforms, the strong dependence of airway mucin secretion on PLC-coupled P2Y2-R signalling (von Kugelgen, 2006; Davis, 1997; Kim et al. 2003), and the PKC-independent, mucin secretory effects of PMA in airways (Abdullah et al. 1997, 2003; Rossi et al. 2004), we predicted that regulated mucin secretion would suffer severe disruption in the Munc13-2-deficient mouse. Hence, finding that Munc13-2, in fact, is not essential for agonist-induced mucin secretion in metaplastic goblet cells was a major surprise (Figs 2 and 3). Parsimony suggests that Munc13-4 is active in agonist-induced secretion and substitutes for Munc13-2 in the null animals; however, the participation of other, unidentified proteins in the granule priming process cannot be ruled out.
Although not essential for regulated mucin secretion, Munc13-2 deficiency did have two major consequences in the airway epithelium which may provide clues to its function. First, Clara cells in the Munc13-deficient mice were observed to accumulate mucins under control conditions (Figs 2, 5 and 6). The accumulation of mucins did not appear to result from airway inflammation in the Munc13-2-deficient mice, as there was no increase in inflammatory cell numbers in the epithelium or airway lumen, mucin gene expression was not increased, and the mucin synthetic machinery was not up-regulated (Fig. 4). The accumulation of secretory cargo in Munc13-2-deficient mice (Figs 2, 3, 5 and 6) strongly suggests that this isoform regulates baseline exocytic release in these cells. Since there is almost no AB/PAS staining in the WT mouse airways (Fig. 2A) under control conditions, we predict that these mucins are secreted immediately following synthesis, i.e. the stored pool of mucin secretory granules is minimal. Interestingly, this aberrant accumulation affected other secreted proteins and organs other than the airways epithelium: CCSP was also observed to accumulate in Clara cells, in the absence of an up-regulation of its mRNA (Fig. 7), and mucin secretion appeared to be affected in many mucosal organs – in every tissue examined, the mucin-containing cells were engorged in the Munc13-2-deficient animals (Fig. 9). Hence, Munc13-2 control of a regulated, baseline exocytic process may be a general secretory cell phenomenon outside the nervous system, and may require a general rethinking of ‘regulated’versus‘constitutive’ exocytic secretion.
Retention of significant mucin stores following exposure to maximal agonist concentrations was the second consequence of Munc13-2 deficiency; in contrast, mucins were secreted fully from agonist-exposed airways of OVA-treated WT mice (Figs 2 and 3). This observation suggests either that Munc13-2 is substituted incompletely by Munc13-4, or that a population of mucin granules requires Munc13-2 uniquely for exocytic release. One simple possibility requiring attention is that mucin granules are ‘tagged’ with either or both Rab3d (Evans et al. 2004) and Rab27a (Tolmachova et al. 2004), such that they are primed, accordingly, by Munc13-2 or Munc13-4. Deciphering such scenarios, however, will require more cellular level approaches than used in the present study.
Mucins in the mouse airway
Another unanticipated finding of this study on Munc13-2-deficient mice was the accumulation of mucins in Clara cells which serendipitously revealed these cells to be the source of oligomeric mucins in the mouse lung and thereby solved an ancient riddle. Work with allergen-sensitized mice relevant to asthma, has identified important inflammatory mediators underlying allergic mucous metaplasia (e.g. see Cressman et al. 1998; Hershey, 2003; Elias, 2004), but in the great majority of these studies Clara cells were considered to have transformed to ‘goblet cells’. Recent work, however, has shown clearly that these metaplastic ‘goblet cells’ are, in fact, still Clara cells that continue to contain CCSP, but additionally have accumulated mucins (Evans et al. 2004). This work and a recent extension (Young et al. 2007), have shown that Muc5ac and Muc5b mRNAs are, in fact, produced in control mouse airways, with Muc5b dominating by / 40-fold. Both Muc5ac and Muc5b gene expression is up-regulated during allergic mucous metaplasia, Muc5ac dramatically so. Hence, the Clara cell was most probably the cell synthesizing mucins under control conditions, but there was no direct evidence supporting this point of view.
The appearance of AB/PAS staining and the accumulation of Muc5b in Munc13-2-deficient Clara cells under non-inflammatory, non-metaplastic conditions in which neither Muc5ac nor Muc5b mRNAs have changed from control (Figs 2–6) offers the first direct evidence that Clara cells synthesize and secrete oligomeric mucins. The agarose immunoblots probed with a Muc5b-specific antibody show that the mucins under non-reducing conditions are present as an ordered array of oligomers (Fig. 5), as they appear in other mucin-secreting cells (Sheehan et al. 2004). Significantly, our Muc5b antibody also detected mucins in WT control mice, both by agarose immunoblotting (Fig. 5) and in Clara cells by IHC (Fig. 6).
Muc5b
The findings that Muc5b is not only expressed in WT mouse airways, but is the dominant oligomeric mucin mRNA (Evans et al. 2004; Young et al. 2007; and Fig. 4) and is detectible at the glycoprotein level (Figs 5 and 6) was a surprising finding given that Muc5b is thought to be the major mucin expressed in human large airways submucosal glands, but not in superficial epithelium (see Voynow et al. 2006). In the current studies, Muc5b expression in mouse lungs at baseline localized to the same anatomical sites (i.e. proximal airway Clara cells) as seen when mucous metaplasia is induced in mouse lungs following antigen challenge (Evans et al. 2004). This finding supports the concept that multiple, distinct subtypes of CCSP-expressing cells are present in mouse lungs, based upon their tissue localization and progenitor cell origins (Rawlins et al. 2007) and their phenotypic responses to inflammation and injury (Reader et al. 2003; Evans et al. 2004; Homer et al. 2006). The potential significance of these findings is twofold.
First, human bronchioles, small airways < 2 mm diameter, are similar in many respects to mouse airways, including trachea (∼1 mm diameter). Like the mouse airways, most non-ciliated secretory cells in the human bronchiole lack AB/PAS+ staining (e.g. see Jeffery et al. 1992), goblet cells comprise < 1% of the epithelium (Thurlbeck, 1990), and inflammation leads to mucous metaplasia (Thurlbeck, 1990; Hogg, 2004). Given these similarities, our results suggest that MUC5B is probably also expressed in human bronchiolar secretory cells, the ‘Clara cells’ of some investigators (e.g. Boers et al. 1999). Existing evidence from in situ hybridization shows MUC5B gene expression in human bronchiolar epithelium (see Fig. 4 in Reid et al. 1997); however, a cautious interpretation of the result is required by distinctly goblet cell-like distribution of label and the source of tissue, pathology specimens removed during surgeries.
Second, should MUC5B prove to be secreted from human bronchiolar secretory cells, then we speculate that in the normal human lung this mucin functions as the primary mucin in mucociliary clearance: it would transit onto bronchial surfaces from both the small airways, as well as, more proximally, from submucosal glands. If MUC5B/Muc5b indeed functions primarily in mucociliary clearance, then MUC5AC/Muc5ac may subserve some other defensive function in the airways, e.g. cough clearance.
In summary, the Munc13-2-deficient mouse has revealed unexpectedly that this priming protein is not essential for agonist-regulated mucin secretion, but it does appear to regulate a tonically active, baseline secretory pathway in the airways and other mucosal tissues. In the absence of Munc13-2, oligomeric mucins accumulate in airway Clara cells, and, surprisingly, Muc5b proves to comprise a major fraction of the mucins in gene-deficient mice. Muc5b glycoprotein is also expressed in WT Clara cells, though in amounts too small to be visualized with AB/PAS staining.
Acknowledgments
The authors thanks Dr Nils Brose for the gift of the Munc13-2 null mice, and Dr Michael Tuvim for assistance with quantification. Research in the Davis laboratory was supported by grants from the NHLBI (HL063756) and the North American Cystic Fibrosis Foundation; research in the Evans and Dickey laboratories was supported by grants from the NHLBI (HL072984; B.F.D.), North American Cystic Fibrosis Foundation and American Lung Association (RG-22720-N; C.E.), and the MD Anderson Cancer Center (P30CA16672; B.F.D. and C.E.).
References
- Abdullah LH, Bundy JT, Ehre C, Davis CW. Mucin secretion and PKC isoforms in SPOC1 goblet cells: differential activation by purinergic agonist and PMA. Am J Physiol Lung Cell Mol Physiol. 2003;285:L149–L160. doi: 10.1152/ajplung.00359.2002. [DOI] [PubMed] [Google Scholar]
- Abdullah LH, Conway JD, Cohn JA, Davis CW. Protein kinase C and Ca2+ activation of mucin secretion in airway goblet cells. Am J Physiol Lung Cell Mol Physiol. 1997;273:L201–L210. doi: 10.1152/ajplung.1997.273.1.L201. [DOI] [PubMed] [Google Scholar]
- Abdullah LH, Davis SW, Burch L, Yamauchi M, Randell SH, Nettesheim P, Davis CW. P2u purinoceptor regulation of mucin secretion in SPOC1 cells, a goblet cell line from the airways. Biochem J. 1996;316:943–951. doi: 10.1042/bj3160943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Zwilling YS, Kawabe H, Reim K, Varoqueaux F, Brose N. Binding to RAB3A-interacting molecule RIM regulates the presynaptic recruitment of Munc13–1 and ubMunc13–2. J Biol Chem. 2006;281:19720–19731. doi: 10.1074/jbc.M601421200. [DOI] [PubMed] [Google Scholar]
- Arvan P, Castle D. Sorting and storage during secretory granule biogenesis: looking backward and looking forward. Biochem J. 1998;332:593–610. doi: 10.1042/bj3320593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Augustin I, Betz A, Herrmann C, Jo T, Brose N. Differential expression of two novel Munc13 proteins in rat brain. Biochem J. 1999a;337:363–371. [PMC free article] [PubMed] [Google Scholar]
- Augustin I, Rosenmund C, Sudhof TC, Brose N. Munc13-1 is essential for fusion competence of glutamatergic synaptic vesicles. Nature. 1999b;400:457–461. doi: 10.1038/22768. [DOI] [PubMed] [Google Scholar]
- Bai J, Chapman ER. The C2 domains of synaptotagmin – partners in exocytosis. Trends Biochem Sci. 2004;29:143–151. doi: 10.1016/j.tibs.2004.01.008. [DOI] [PubMed] [Google Scholar]
- Basu J, Shen N, Dulubova I, Lu J, Guan R, Guryev O, Grishin NV, Rosenmund C, Rizo J. A minimal domain responsible for Munc13 activity. Nat Struct Mol Biol. 2005;12:1017–1018. doi: 10.1038/nsmb1001. [DOI] [PubMed] [Google Scholar]
- Betz A, Thakur P, Junge HJ, Ashery U, Rhee JS, Scheuss V, Rosenmund C, Rettig J, Brose N. Functional interaction of the active zone proteins Munc13-1 and RIM1 in synaptic vesicle priming. Neuron. 2001;30:183–196. doi: 10.1016/s0896-6273(01)00272-0. [DOI] [PubMed] [Google Scholar]
- Boers JE, Ambergen AW, Thunnissen FB. Number and proliferation of Clara cells in normal human airway epithelium. Am J Respir Crit Care Med. 1999;159:1585–1591. doi: 10.1164/ajrccm.159.5.9806044. [DOI] [PubMed] [Google Scholar]
- Bossi G, Griffiths GM. CTL secretory lysosomes: biogenesis and secretion of a harmful organelle. Semin Immunol. 2005;17:87–94. doi: 10.1016/j.smim.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Brenner S. The genetics of Caenorhabditis elegans. Genetics. 1974;77:71–94. doi: 10.1093/genetics/77.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brose N, Hofmann K, Hata Y, Sudhof TC. Mammalian homologues of Caenorhabditis elegans unc-13 gene define novel family of C2-domain proteins. J Biol Chem. 1995;270:25273–25280. doi: 10.1074/jbc.270.42.25273. [DOI] [PubMed] [Google Scholar]
- Brose N, Rosenmund C. Move over protein kinase C, you've got company: alternative cellular effectors of diacylglycerol and phorbol esters. J Cell Sci. 2002;115:4399–4411. doi: 10.1242/jcs.00122. [DOI] [PubMed] [Google Scholar]
- Cho W, Stahelin RV. Membrane binding and subcellular targeting of C2 domains. Biochim Biophys Acta. 2006;1761:838–849. doi: 10.1016/j.bbalip.2006.06.014. [DOI] [PubMed] [Google Scholar]
- Cohn L, Whittaker L, Niu N, Homer RJ. Cytokine regulation of mucus production in a model of allergic asthma. Novartis Found Symp. 2002;248:201–213. [PubMed] [Google Scholar]
- Colon-Gonzalez F, Kazanietz MG. C1 domains exposed: from diacylglycerol binding to protein–protein interactions. Biochim Biophys Acta. 2006;1761:827–837. doi: 10.1016/j.bbalip.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Cressman VL, Hicks EM, Funkhouser WK, Backlund DC, Koller BH. The relationship of chronic mucin secretion to airway disease in normal and CFTR-deficient mice. Am J Respir Cell Mol Biol. 1998;19:853–866. doi: 10.1165/ajrcmb.19.6.3194. [DOI] [PubMed] [Google Scholar]
- Davis CW. Goblet cells: physiology and pharmacology. In: Rogers DF, Lethem MI, editors. Airway Mucus: Basic Mechanisms and Clinical Perspectives. Basel: Berkhauser; 1997. pp. 150–177. [Google Scholar]
- Davis CW, Abdullah LH. In vitro models for airways mucin secretion. Pulm Pharmacol Ther. 1997;10:145–155. doi: 10.1006/pupt.1997.0088. [DOI] [PubMed] [Google Scholar]
- Davis CW, Dickey BF. Regulated airway goblet cell mucin secretion. Annu Rev Physiol. 2008;70 doi: 10.1146/annurev.physiol.70.113006.100638. in press. [DOI] [PubMed] [Google Scholar]
- Dulubova I, Lou X, Lu J, Huryeva I, Alam A, Schneggenburger R, Sudhof TC, Rizo J. A Munc13/RIM/Rab3 tripartite complex: from priming to plasticity? EMBO J. 2005;24:2839–2850. doi: 10.1038/sj.emboj.7600753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehre C, Rossi AH, Abdullah LH, De Pestel K, Hill S, Olsen JC, Davis CW. Barrier role of actin filaments in regulated mucin secretion from airway goblet cells. Am J Physiol Cell Physiol. 2005;288:C46–C56. doi: 10.1152/ajpcell.00397.2004. [DOI] [PubMed] [Google Scholar]
- Ehre C, Zhu Y, Abdullah LH, Olsen J, Nakayama KI, Nakayama K, Messing RO, Davis CW. nPKCε, a P2Y2-R downstream effector in regulated mucin secretion from airway goblet cells. Am J Physiol Cell Physiol. 2007;293:C1445–C1454. doi: 10.1152/ajpcell.00051.2007. [DOI] [PubMed] [Google Scholar]
- Elias J. The relationship between asthma and COPD. Lessons from transgenic mice. Chest. 2004;126:111S–116S. doi: 10.1378/chest.126.2_suppl_1.111S. [DOI] [PubMed] [Google Scholar]
- Evans CM, Williams OW, Tuvim MJ, Nigam R, Mixides GP, Blackburn MR, DeMayo FJ, Burns AR, Smith C, Reynolds SD, Stripp BR, Dickey BF. Mucin is produced by Clara cells in the proximal airways of antigen-challenged mice. Am J Respir Cell Mol Biol. 2004;31:382–394. doi: 10.1165/rcmb.2004-0060OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster WM, Walters DM, Longphre M, Macri K, Miller LM. Methodology for the measurement of mucociliary function in the mouse by scintigraphy. J Appl Physiol. 2001;90:1111–1117. doi: 10.1152/jappl.2001.90.3.1111. [DOI] [PubMed] [Google Scholar]
- Fukuda M. Versatile role of Rab27 in membrane trafficking: focus on the Rab27 effector families. J Biochem. 2005;137:9–16. doi: 10.1093/jb/mvi002. [DOI] [PubMed] [Google Scholar]
- Gerber SH, Sudhof TC. Molecular determinants of regulated exocytosis. Diabetes. 2002;51(Suppl. 1):S3–S11. doi: 10.2337/diabetes.51.2007.s3. [DOI] [PubMed] [Google Scholar]
- Gorr SU, Venkatesh SG, Darling DS. Parotid secretory granules: crossroads of secretory pathways and protein storage. J Dent Res. 2005;84:500–509. doi: 10.1177/154405910508400604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groneberg DA, Eynott PR, Oates T, Lim S, Wu R, Carlstedt I, Nicholson AG, Chung KF. Expression of MUC5AC and MUC5B mucins in normal and cystic fibrosis lung. Respir Med. 2002;96:81–86. doi: 10.1053/rmed.2001.1221. [DOI] [PubMed] [Google Scholar]
- Grubb BR, Jones JH, Boucher RC. Mucociliary transport determined by in vivo microdialysis in the airways of normal and CF mice. Am J Physiol Lung Cell Mol Physiol. 2004;286:L588–L595. doi: 10.1152/ajplung.00302.2003. [DOI] [PubMed] [Google Scholar]
- Hershey GK. IL-13 receptors and signaling pathways: an evolving web. J Allergy Clin Immunol. 2003;111:677–690. doi: 10.1067/mai.2003.1333. [DOI] [PubMed] [Google Scholar]
- Hogg JC. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet. 2004;364:709–721. doi: 10.1016/S0140-6736(04)16900-6. [DOI] [PubMed] [Google Scholar]
- Holmen JM, Karlsson NG, Abdullah LH, Randell SH, Sheehan JK, Hansson GC, Davis CW. Mucins and their O-Glycans from human bronchial epithelial cell cultures. Am J Physiol Lung Cell Mol Physiol. 2004;287:L824–L834. doi: 10.1152/ajplung.00108.2004. [DOI] [PubMed] [Google Scholar]
- Homer RJ, Zhu Z, Cohn L, Lee CG, White WI, Chen S, Elias JA. Differential expression of chitinases identify subsets of murine airway epithelial cells in allergic inflammation. Am J Physiol Lung Cell Mol Physiol. 2006;291:L502–L511. doi: 10.1152/ajplung.00364.2005. [DOI] [PubMed] [Google Scholar]
- Hong KU, Reynolds SD, Giangreco A, Hurley CM, Stripp BR. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol. 2001;24:671–681. doi: 10.1165/ajrcmb.24.6.4498. [DOI] [PubMed] [Google Scholar]
- Hong W. Cytotoxic T lymphocyte exocytosis: bring on the SNAREs! Trends Cell Biol. 2005;15:644–650. doi: 10.1016/j.tcb.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Hosono R, Kamiya Y. Additional genes which result in an elevation of acetylcholine levels by mutations in Caenorhabditis elegans. Neurosci Lett. 1991;128:243–244. doi: 10.1016/0304-3940(91)90270-4. [DOI] [PubMed] [Google Scholar]
- Jeffery PK, Gaillard D, Moret S. Human airway secretory cells during development and in mature airway epithelium. Eur Respir J. 1992;5:93–104. [PubMed] [Google Scholar]
- Kaartinen L, Nettesheim P, Adler KB, Randell SH. Rat tracheal epithelial cell differentiation in vitro. In Vitro Cell Dev Biol Anim. 1993;29A:481–492. [PubMed] [Google Scholar]
- Kim KC, Hisatsune A, Kim dJ, Miyata T. Pharmacology of airway goblet cell mucin release. J Pharmacol Sci. 2003;92:301–307. doi: 10.1254/jphs.92.301. [DOI] [PubMed] [Google Scholar]
- Kirkham S, Sheehan JK, Knight D, Richardson PS, Thornton DJ. Heterogeneity of airways mucus: variations in the amounts and glycoforms of the major oligomeric mucins MUC5AC and MUC5B. Biochem J. 2002;361:537–546. doi: 10.1042/0264-6021:3610537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenchin VA, Martin TF. Priming in exocytosis: attaining fusion-competence after vesicle docking. Biochimie. 2000;82:399–407. doi: 10.1016/s0300-9084(00)00208-x. [DOI] [PubMed] [Google Scholar]
- Koch H, Hofmann K, Brose N. Definition of Munc13-homology-domains and characterization of a novel ubiquitously expressed Munc13 isoform. Biochem J. 2000;349:247–253. doi: 10.1042/0264-6021:3490247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosawa H, Wang CG, Dandurand RJ, King M, Eidelman DH. Mucociliary function in the mouse measured in explanted lung tissue. J Appl Physiol. 1995;79:41–46. doi: 10.1152/jappl.1995.79.1.41. [DOI] [PubMed] [Google Scholar]
- Mall M, Grubb BR, Harkema JR, O'Neal WK, Boucher RC. Increased airway epithelial Na+ absorption produces cystic fibrosis-like lung disease in mice. Nat Med. 2004;10:487–493. doi: 10.1038/nm1028. [DOI] [PubMed] [Google Scholar]
- Martin TF. Prime movers of synaptic vesicle exocytosis. Neuron. 2002;34:9–12. doi: 10.1016/s0896-6273(02)00651-7. [DOI] [PubMed] [Google Scholar]
- Maruyama IN, Brenner S. A phorbol ester/diacylglycerol-binding protein encoded by the unc-13 gene of Caenorhabditis elegans. Proc Natl Acad Sci U S A. 1991;88:5729–5733. doi: 10.1073/pnas.88.13.5729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SG, Moore HP. Reconstitution of constitutive secretion using semi-intact cells: regulation by GTP but not calcium. J Cell Biol. 1991;112:39–54. doi: 10.1083/jcb.112.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeft M, Wieffer M, de Jong AS, Negroiu G, Metz CH, van Loon A, Griffith J, Krijgsveld J, Wulffraat N, Koch H, Heck AJ, Brose N, van der Kleijmeer M & SP. Munc13-4 is an effector of rab27a and controls secretion of lysosomes in hematopoietic cells. Mol Biol Cell. 2005;16:731–741. doi: 10.1091/mbc.E04-10-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffl MW. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001;29:e45. doi: 10.1093/nar/29.9.e45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plopper CG, Suverkropp C, Morin D, Nishio S, Buckpitt A. Relationship of cytochrome P-450 activity to Clara cell cytotoxicity. I. Histopathologic comparison of the respiratory tract of mice, rats and hamsters after parenteral administration of naphthalene. J Pharmacol Exp Ther. 1992;261:353–363. [PubMed] [Google Scholar]
- Ponnambalam S, Baldwin SA. Constitutive protein secretion from the trans-Golgi network to the plasma membrane. Mol Membr Biol. 2003;20:129–139. doi: 10.1080/0968768031000084172. [DOI] [PubMed] [Google Scholar]
- Rawlins EL, Ostrowski LE, Randell SH, Hogan BL. Lung development and repair: contribution of the ciliated lineage. Proc Natl Acad Sci U S A. 2007;104:410–417. doi: 10.1073/pnas.0610770104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reader JR, Tepper JS, Schelegle ES, Aldrich MC, Putney LF, Pfeiffer JW, Hyde DM. Pathogenesis of mucous cell metaplasia in a murine asthma model. Am J Pathol. 2003;162:2069–2078. doi: 10.1016/S0002-9440(10)64338-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid CJ, Gould S, Harris A. Developmental expression of mucin genes in the human respiratory tract. Am J Respir Cell Mol Biol. 1997;17:592–598. doi: 10.1165/ajrcmb.17.5.2798. [DOI] [PubMed] [Google Scholar]
- Rhee JS, Betz A, Pyott S, Reim K, Varoqueaux F, Augustin I, Hesse D, Sudhof TC, Takahashi M, Rosenmund C, Brose N. β Phorbol ester- and diacylglycerol-induced augmentation of transmitter release is mediated by Munc13s and not by PKCs. Cell. 2002;108:121–133. doi: 10.1016/s0092-8674(01)00635-3. [DOI] [PubMed] [Google Scholar]
- Richmond JE, Davis WS, Jorgensen EM. UNC-13 is required for synaptic vesicle fusion in C. elegans. Nat Neurosci. 1999;2:959–964. doi: 10.1038/14755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose MC, Voynow JA. Respiratory tract mucin genes and mucin glycoproteins in health and disease. Physiol Rev. 2006;86:245–278. doi: 10.1152/physrev.00010.2005. [DOI] [PubMed] [Google Scholar]
- Rosenmund C, Sigler A, Augustin I, Reim K, Brose N, Rhee JS. Differential control of vesicle priming and short-term plasticity by Munc13 isoforms. Neuron. 2002;33:411–424. doi: 10.1016/s0896-6273(02)00568-8. [DOI] [PubMed] [Google Scholar]
- Rossi AH, Salmon WC, Chua M, Davis CW. Calcium signaling in human airway goblet cells following purinergic activation. Am J Physiol Lung Cell Mol Physiol. 2007;292:L92–L98. doi: 10.1152/ajplung.00081.2006. [DOI] [PubMed] [Google Scholar]
- Rossi AH, Sears PR, Davis CW. Ca2+ dependency of ‘Ca2+-independent’ exocytosis in SPOC1 airway goblet cells. J Physiol. 2004;559:555–565. doi: 10.1113/jphysiol.2004.070433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan JK, Boot-Handford RP, Chantler E, Carlstedt I, Thornton DJ. Evidence for shared epitopes within the ‘naked’ protein domains of human mucus glycoproteins. A study performed by using polyclonal antibodies and electron microscopy. Biochem J. 1991;274:293–296. doi: 10.1042/bj2740293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan JK, Kirkham S, Howard M, Woodman P, Kutay S, Brazeau C, Buckley J, Thornton DJ. Identification of molecular intermediates in the assembly pathway of the MUC5AC mucin. J Biol Chem. 2004;279:15698–15705. doi: 10.1074/jbc.M313241200. [DOI] [PubMed] [Google Scholar]
- Stevens DR, Wu ZX, Matti U, Junge HJ, Schirra C, Becherer U, Wojcik SM, Brose N, Rettig J. Identification of the minimal protein domain required for priming activity of Munc13-1. Curr Biol. 2005;15:2243–2248. doi: 10.1016/j.cub.2005.10.055. [DOI] [PubMed] [Google Scholar]
- Stripp BR, Reynolds SD, Plopper CG, Boe IM, Lund J. Pulmonary phenotype of CCSP/UG deficient mice: a consequence of CCSP deficiency or altered Clara cell function? Ann N Y Acad Sci. 2000;923:202–209. doi: 10.1111/j.1749-6632.2000.tb05531.x. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- Thornton DJ, Sheehan JK. From mucins to mucus: toward a more coherent understanding of this essential barrier. Proc Am Thorac Soc. 2004;1:54–61. doi: 10.1513/pats.2306016. [DOI] [PubMed] [Google Scholar]
- Thurlbeck WM. Pathology of chronic airflow obstruction. Chest. 1990;97:6S–10S. [PubMed] [Google Scholar]
- Tolmachova T, Anders R, Stinchcombe J, Bossi G, Griffiths GM, Huxley C, Seabra MC. A general role for Rab27a in secretory cells. Mol Biol Cell. 2004;15:332–344. doi: 10.1091/mbc.E03-07-0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifaro JM, Lejen T, Rose SD, Pene TD, Barkar ND, Seward EP. Pathways that control cortical F-actin dynamics during secretion. Neurochem Res. 2002;27:1371–1385. doi: 10.1023/a:1021627800918. [DOI] [PubMed] [Google Scholar]
- Van Winkle LS, Buckpitt AR, Nishio SJ, Isaac JM, Plopper CG. Cellular response in naphthalene-induced Clara cell injury and bronchiolar epithelial repair in mice. Am J Physiol Lung Cell Mol Physiol. 1995;269:L800–L818. doi: 10.1152/ajplung.1995.269.6.L800. [DOI] [PubMed] [Google Scholar]
- Varoqueaux F, Sigler A, Rhee JS, Brose N, Enk C, Reim K, Rosenmund C. Total arrest of spontaneous and evoked synaptic transmission but normal synaptogenesis in the absence of Munc13-mediated vesicle priming. Proc Natl Acad Sci U S A. 2002;99:9037–9042. doi: 10.1073/pnas.122623799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Kugelgen I. Pharmacological profiles of cloned mammalian P2Y-receptor subtypes. Pharmacol Ther. 2006;110:415–432. doi: 10.1016/j.pharmthera.2005.08.014. [DOI] [PubMed] [Google Scholar]
- Voynow JA, Gendler SJ, Rose MC. Regulation of mucin genes in chronic inflammatory airway diseases. Am J Respir Cell Mol Biol. 2006;34:661–665. doi: 10.1165/rcmb.2006-0035SF. [DOI] [PubMed] [Google Scholar]
- Williams OW, Sharafkhaneh A, Kim V, Dickey BF, Evans CM. Airway mucus: From production to secretion. Am J Respir Cell Mol Biol. 2006;34:527–536. doi: 10.1165/rcmb.2005-0436SF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young HW, Williams OW, Chandra D, Bellinghausen LK, Perez G, Suarez A, Tuvim MJ, Roy MG, Alexander SN, Moghaddam SJ, Adachi R, Blackburn MR, Dickey BF, Evans CM. Central role of Muc5ac expression in mucous metaplasia and its regulation by conserved 5′-elements. Am J Respir Cell Mol Biol. 2007;37:273–290. doi: 10.1165/rcmb.2005-0460OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Ginsburg D. Getting secretory granules ready for prime time. Cell. 2003;115:372–373. doi: 10.1016/s0092-8674(03)00894-8. [DOI] [PubMed] [Google Scholar]