Abstract
Neuronal Kv7 channels (also termed KCNQ channels) are the molecular correlate of the M-current. The Kv7 channels activate at rather negative membrane potentials (≤ 60 mV), thereby ‘fine-tuning’ the resting membrane potential. The Kv7 channels are widely expressed in the brain with the Kv7.2, Kv7.3 and Kv7.5 channels being the most abundant. The Kv7.4 subunit has the most restricted brain regional expression being present in discrete nuclei of brainstem only. Kv7 channels are expressed at different subcellular locations, being on both somatodendritic, axonal and terminal sites. This complex subcellular distribution of Kv7 channels enables them to participate in both pre- and postsynaptic modulation of basal and stimulated excitatory neurotransmission. Activation of neuronal Kv7 channels limits repetitive firing thereby potentially limiting the generation of long bursts, with subsequent inhibition of monoaminergic neurotransmitter release. In this review, we focus on the influence of Kv7 channels on dopaminergic and serotonergic neurotransmission. The data suggest a novel action of Kv7 channel openers which could translate into having therapeutic value in the treatment of disease states characterized by overactivity of dopaminergic (e.g. schizophrenia and drug abuse) and serotonergic neurotransmission (e.g. anxiety).
The KCNQ gene family encodes Kv7 channel subunits that form homo- or heteromeric Kv7 channels (also termed M-channels) which represent the molecular correlate to the M-current (Wang et al. 1998; Jentsch, 2000). Unlike Kv7.1, Kv7.2–5 channels are expressed in the CNS (for a review on brain regional expression, see Dalby-Brown et al. (2006). While the Kv7.2 and Kv7.3 subunits are present in almost all brain regions examined so far (Biervert et al. 1998; Schroeder et al. 1998), the Kv7.4 subunit is expressed only in discrete nuclei of brainstem, including the midbrain (Kharkovets et al. 2000; Dalby-Brown et al. 2006). Although Kv7.2 subunits are able to form homomeric Kv7 channels, heteromerization with the Kv7.3 subunit yields increased M-currents which is largely due to more efficient surface targeting and expression of functional channels (Schwake et al. 2000; Schroeder et al. 2000). The Kv7.4 subunit also coassembles with the Kv7.3, but not the Kv7.2, subunit, which produces larger currents than homomeric Kv7.4 channels per se (Kubisch et al. 1999).
Kv7 channels are voltage-dependent K+ channels; all subtypes start opening at around −60 mV (although the voltage dependency of Kv7 channels differ between heterologous and native cells), thus being functionally active close to the resting membrane potential. In addition, Kv7 channels may be considered as being non-inactivating (‘leaky’) K+ channels at physiologically relevant resting potentials, although a proportion of Kv7 channels may undergo steady-state inactivation (Jensen et al. 2007). These characteristics enable the Kv7 channels to produce the underlying subthreshold M-current, which stabilizes the neuronal resting potential. Consequently, the Kv7 channels are thought to inhibit neuronal excitability and put a ‘brake’ on action potential firing when the neuron is exposed to an excitatory stimulus.
Kv7 channels activate slowly (main τ∼100–300 ms at − 30 mV (Delmas & Brown, 2005; Soldovieri et al. 2007) following excitatory stimuli, the open probability therefore increases during long-lasting depolarization-current pulses or high firing frequencies. Thus, the biophysical nature of the Kv7 channels precludes them from playing a major role in the repolarization phase of fast action potentials, whereas during repetitive or sustained neuronal firing the Kv7 channels provide a strong inhibitory input which causes a reduction in firing frequency in various central and peripheral neurons (Marrion, 1997). The principal Kv7 channel opener, retigabine, is able to accelerate this process by producing a hyperpolarizing shift of the activation curve of the channel by 14–43 mV (depending on the Kv7 channel subtype) at 10 μm (Tatulian et al. 2001). This greatly increases open channel probability at resting membrane potentials (Tatulian & Brown, 2003). In the hippocampus, retigabine inhibits action potential generation (Piccinin et al. 2006; Hu et al. 2007). Conversely, inhibition of Kv7 channel function by application of the Kv7 channel blockers XE991 or linopirdine produces hyperexcitability and triggers burst firing in various hippocampal neurons (Yue & Yaari, 2004; Lawrence et al. 2006). Also, hippocampal neurons have an increased propensity to generate current-induced action potentials in a conditional transgenic mouse model of severe functional Kv7.2 channel deficiency (Peters et al. 2005).
The inhibitory effect of Kv7 channel activity on neuronal spike electrogenesis is likely to be the basis for the effect of Kv7 channel openers in various CNS diseases characterized by neuronal overactivity. Notably, retigabine is effective in preclinical animal models of epilepsy and chronic pain conditions, and is now in clinical development for the treatment of partial complex seizures. Particularly, the inhibitory effect on epileptogenic activity is ascribed to the inhibitory effect of Kv7 channels on hippocampal and cortical glutamatergic neurotransmission (Blackburn-Munro et al. 2005).
It is becoming evident that Kv7 channels also modulate other excitatory neurotransmitter systems in the CNS. In this review, we focus on the current evidence for a close interaction between Kv7 channel function and monoaminergic neurotransmitter activity with special emphasis on dopaminergic and serotonergic neurotransmission. These findings may possibly translate into novel treatment paradigms of disease states characterized by dopaminergic (e.g. schizophrenia, drug abuse) and serotonergic (e.g. anxiety) overactivity.
Kv7 channel-induced modulation of burst firing capacity
Burst firing is an important physiological feature of monoaminergic synaptic neurotransmission, as bursts give rise to a supra-additive release of the monoaminergic neurotransmitters dopamine, noradrenaline and serotonin (Overton & Clark, 1997; Gartside et al. 2000; Harley, 2007). Interestingly, preliminary data from a computational study suggest that elimination of M-currents leads to prolonged burst firing in mesencephalic dopaminergic neurons, but has little effect on tonic firing, during which the membrane potential of these neurons is on average more negative (Bonjean et al. 2007). The basic electrophysiological properties of the Kv7 channel and its influence on repetitive neuronal firing frequency therefore suggest that positive modulation of Kv7 channel activity would lead to changes in neurotransmitter release. Several reports have documented that retigabine-induced Kv7 channel activity inhibits the release of dopamine in vitro and in vivo (Hansen et al. 2006; Martire et al. 2007) and noradrenaline in vitro (Lechner et al. 2003; Martire et al. 2004; Edelbauer et al. 2005). In addition, Kv7 channel modulators also influence the release of other neurotransmitters, including glutamate (Martire et al. 2004), γ-aminobutyric acid (GABA) (Martire et al. 2004), and acetylcholine (Zaczek et al. 1998) (Table 1).
Table 1.
Kv7 channel modulators affect neurotransmitter release in the rat CNS in vitro and in vivo
| Neurotransmitter | Preparation | Compound | Release effect | Reference |
|---|---|---|---|---|
| Dopamine | Rat striatal microdialysates | Retigabine | No effect | Hansen et al. 2006, 2007 |
| Rat striatal synaptosomes | Retigabine | Inhibition | Martire et al. 2007 | |
| Rat striatal synaptosomes | XE991 | Stimulation | ||
| Noradrenaline | Rat hippocampal synaptosomes | Retigabine | Inhibition | Martire et al. 2004 |
| Rat cortical synaptosomes | Retigabine | Inhibition | ||
| Glutamate | Rat hippocampal synaptosomes | Retigabine | Inhibition | Martire et al. 2004 |
| GABA | Rat hippocampal synaptosomes | Retigabine | Inhibition | Martire et al. 2004 |
| Acetylcholine | Rat hippocampal slices | XE991 | Stimulation | Zaczek et al. 1998 |
| Rat hippocampal microdialysates | DMP543 | Stimulation | ||
| Linopirdine | Stimulation |
Note that all in vitro studies have been conducted using depolarization-induced release of tritiated neurotransmitters triggered by high extracellular potassium concentrations (Martire et al. 2004, 2007: 9 mm K+; Zaczek et al. 1998: 25 mm K+).
As described below, the effects of Kv7 channel modulators are likely to occur at both pre- and postsynaptic Kv7 channels (illustrated in the case of a principal dopaminergic neuron, see Fig. 1). Stimulation of Kv7 channel activity may therefore lead to inhibition of neurotransmitter release via different subcellular sites of Kv7 channel action.
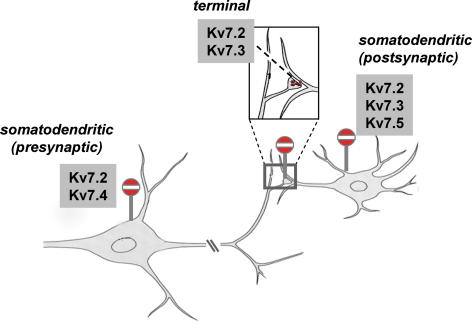
Figure 1. Schematized drawing of the subcellular topography of Kv7 channel expression in a principal mesencephalic dopaminergic neuron.
Kv7.2-Kv7.5 channels are indicated at different principal subcellular positions where Kv7 channel mRNA and/or protein expression has been reported. Note that the Kv7.4 channels are expressed only in mesencephalic dopaminergic neurons at somatodendritic sites, whereas Kv7.2 and Kv7.3 channels are found at several cellular pre- and postsynaptic locations. Modified from Kandal ER, Jessell TM & Schwartz JH (2000), Principles of Neural Science, McGraw-Hill Medical, reproduced with permission of the McGraw-Hill Companies.
Kv7 channel interaction with dopaminergic neurotransmission
Kv7 channel-induced modulation of dopaminergic activity in the striatum
Retigabine exhibits a strong negative modulatory effect on striatal and cortical excitability (see below). This is likely to be the underlying basis for retigabine's pronounced inhibitory effects on both spontaneous and stimulated locomotor activity in rodents (Korsgaard et al. 2005; Hansen et al. 2007) and may also explain the anticataleptic effect of flupirtine, a structural analogue of retigabine (Schmidt et al. 1997).
The site of action could potentially be the basal ganglia, because the striatum is the principal input nucleus to the other nuclei of the basal ganglia, receiving prominent excitatory input from corticostriatal (glutamatergic) and nigrostriatal/mesolimbic (dopaminergic) afferents. Modulation of Kv7 channel function would therefore be expected to influence striatal excitability leading to altered basal ganglia output. In agreement with this idea, retigabine reduces basal striatal excitability, as inferred by reduced c-Fos expression (a marker of postsynaptic neuronal excitation) in both the dorsolateral and ventral part (nucleus accumbens). Most strikingly, retigabine effectively blocks striatal neuronal excitation produced by dopamine D2 receptor antagonists and the psychostimulants cocaine and methylphenidate (Mikkelsen, 2004, 2006, 2007). This generalized inhibitory effect on postsynaptic neuronal activity may be a consequence of attenuated presynaptic dopaminergic neurotransmission, as retigabine administration results in inhibition of basal dopamine synthesis and reduces accumulation of extracellular dopamine following acute blockade of striatal dopamine reuptake in vivo (Hansen et al. 2006, 2007). Also, retigabine exerts an inhibitory effect on depolarization-induced dopamine release from striatal terminals in vitro (Martire et al. 2007).
This principal inhibitory effect of Kv7 channel activation on terminal excitability may also apply to other brain regions, including the hippocampus and cortex (Martire et al. 2004). In line with these observations, retigabine also inhibits cortical neuronal excitation induced by acute systemic administration of the psychotomimetic drug phencyclidine (Hansen et al. 2007).
Thus, activation Kv7 channels leads to a bimodal effect on presynaptic dopaminergic neurotransmission in the striatum, i.e. by reducing both terminal synthesis and release of dopamine. Due to the complexity of striatal gating of excitatory input, it is at present not clear whether the effect of retigabine is a consequence of indirect or direct actions in the striatum. Specifically, current evidence indicates that both extrastriatal and intrastriatal Kv7 channels play an important role in the modulation of basal ganglia tonus.
Extrastriatal Kv7 channels
Kv7.4 channels are prominently expressed in dopaminergic neurons in the substantia nigra pars compacta (SNc) and ventral tegmental area (VTA) (Kharkovets et al. 2000). In both structures, Kv7.4 is expressed somatodendritically (Hansen et al. 2006), see Fig. 2. The Kv7.4 subunit is predominantly localized to the plasma membrane, but Kv7.4 immunoreactivity is also observed in intracellular membranes and processes, in agreement with Kharkovets et al. (2000). Presumably, these Kv7.4 proteins are retained due to incomplete trafficking to the plasma membrane or, alternatively, destined for trafficking to axonal, dendritic, or perisynaptic sites, as suggested for Kv7.2 channels (Cooper et al. 2001).
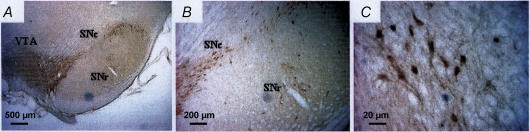
Figure 2. Immunohistochemical staining of Kv7.4 channels in the rat substantia nigra pars compacta (SNc, A–C) and ventral tegmental area (VTA, A).
Note that the substantia nigra pars reticulata (SNr) is practically devoid of Kv7.4 immunoreactivity (A and B, 5x and 20× magnification, respectively). C, Kv7.4 positive neurons in the SNc, showing the presence of somatodendritic Kv7.4 channel protein expression (40× magnification). The Kv7.4 subunit is localized to the plasma membrane and dendrites as well as in intracellular membranes, being in agreement with Kharkovets et al. (2000). A rabbit polyclonal Kv7.4 channel antibody was used (Kharkovets et al. 2000) and immunoreactivity was detected by means of the avidin–biotin method using diaminobenzidine as chromagen and the reaction was amplified using the biotinylated tyramine procedure as previously described (Hansen et al. 2006).
The striatum and cortex do not contain Kv7.4 channel transcripts (Kharkovets et al. 2000; Shen et al. 2005), indicating that Kv7.4 channels are not expressed intrastriatally or in corticostriatal projections. Potentially, this points to an indirect effect of Kv7.4 channel function on striatal dopaminergic activity resulting from inhibition of the somatic excitability of ascending nigrostriatal and mesolimbic dopaminergic projections. In agreement with this view, application of retigabine produces a robust inhibition of dopaminergic neuronal spike activity in the SNc and VTA in vitro and in vivo (see below), thus lending support to the hypothesis of direct influence of Kv.7.4 channel function on mesencephalic dopaminergic neurotransmission.
In addition to Kv7.4, also Kv.7.2, but not Kv7.3 and Kv7.5, subunits are found in the SNc and VTA (Cooper et al. 2001; Hansen et al. 2006; Weber et al. 2006). It is at present unclear whether Kv7.2 channels are localized to dopaminergic or GABAergic subregions of the substantia nigra (Cooper et al. 2001; Hansen et al. 2006; Weber et al. 2006). However, the IC50 value for tetraethylammonium (a fast and reversible K+ channel blocker working in millimolar concentrations) for the blockade of the M-current in dissociated VTA dopaminergic neurons is reported to lie between the IC50 of the blocker on Kv7.2 and Kv7.4 channels, suggesting contribution of K+ currents from both channel isoforms (Koyama & Appel, 2006). Since Kv7.2 and Kv7.4 channels do not assemple to generate functional heteromeric channels in vitro (Kubisch et al. 1999), this might imply the coexistence of functional monomeric Kv7.2 and Kv7.4 channels. This might indicate that both Kv7 channels may impinge on dopaminergic output from the SNc and VTA. However, whereas homomeric Kv7.4 channels yielded significant K+ currents in heterologous expression systems (Kubisch et al. 1999), homomeric Kv7.2 channels gave very low currents and require coexpression of Kv7.3 channels to mediate significant currents in vitro (Wang et al. 1998; Schroeder et al. 1998; Schwake et al. 2000). Consequently, the lack of Kv7.3 channel expression in the SNc and VTA argues for the Kv7.4 channel being the principal Kv7 channel mediating retigabine-induced suppression of mesencephalic dopaminergic activity.
Interestingly, coexpression of the D2 receptor and Kv7 channel combinations in heterologous expression systems yields increased M-currents upon D2 receptor agonism. This effect on D2 receptor stimulation was most pronounced in cells cotransfected with the Kv7.4 channel (Ljungstrom et al. 2003), suggesting a facilitatory coupling of Kv7.4 channel and D2 autoreceptor function. Although this functional interaction remains to be established in native neurons, the localization of Kv7.4 channels in the SNc and VTA corresponds with the somatodendritic expression of dopamine D2 autoreceptors near excitatory-type synapses (Pickel et al. 2002), and this raises the possibility that dendritically released dopamine acting on D2 autoreceptors in the SNc and VTA could increase Kv7.4 channel function.
Retigabine potently inhibits basal dopaminergic neuronal firing in acute SNc and VTA slice preparations and this effect is antagonized by nanomolar concentrations of the Kv7 channel blocker XE991 (Hansen et al. 2006; Koyama & Appel, 2006). In accordance, retigabine completely reverses the excitatory effect of D2 autoreceptor blockade in the SNc in vivo (Hansen et al. 2006). These data suggest that retigabine could prevent striatal dopamine release as a consequence of direct Kv7.4 channel-induced blockade of dopaminergic impulse flow in nigrostriatal and mesolimbic pathways.
An XE991-sensitive, typical M-type current has recently been demonstrated in dissociated VTA dopaminergic neurons (Koyama & Appel, 2006; Koyama et al. 2007). XE991 exhibits very little effect on the membrane potential of mesencephalic dopaminergic neurons and does not affect the spike medium afterhyperpolarization (mAHP) phase; however, XE991 moderately increases the basal excitability of a fraction of both SNc and VTA dopaminergic neurons (Hansen et al. 2006; Koyama & Appel, 2006). Taken together, these studies demonstrate the existence of a retigabine- and XE991-sensitive M-current in mesencephalic DA neurons. The moderate effect of XE991 on the firing of these cells may be due to the fact that it has been tested mostly on tonically firing neurons. As described above, the Kv7 channels are not activated to produce significant M-currents under these circumstances. However, XE991 may have more significant effects when these cells burst (see above).
Intrastriatal Kv7 channels
Kv7.2 channel protein has recently been reported to be present on rat striatal nerve terminals (Martire et al. 2007). Kv7.2 channel expression on striatal nerve terminals colocalizes with tyrosine hydroxylase (the principal rate-limiting enzyme for dopamine synthesis), suggesting expression on dopaminergic nerve terminals, and application of retigabine to striatal synaptosomes preloaded with [3H]dopamine results in an XE991-sensitive reduction of dopamine release (Martire et al. 2007). Although this effect of retigabine needs to be confirmed in in vivo settings, this suggests a direct effect of Kv7 channel modulation in the striatum by controlling terminal excitability of nigrostriatal and mesolimbic dopaminergic neurons.
In the striatum, neuronal fibres (suggestive of axonal projections) express Kv7.2 and Kv7.3 channels (Devaux et al. 2004; Geiger et al. 2006; Weber et al. 2006). It should be noted that dopaminergic fibres are unmyelinated, which rules out the possibility of nodal Kv7 channel expression in mesencephalic dopaminergic projections innervating the striatum. However, although no definitive data have been reported so far as to whether these channels are expressed on myelinated fibres in the striatum, they may potentially be located in striatal initial axon segments and nodes of Ranvier (Devaux et al. 2004).
It is likely that these neurons represent striatal GABAergic output neurons, as Kv7.2, Kv7.3 and Kv7.5 channel transcripts are expressed in medium spiny striatopallidal and striatonigral neurons (Shen et al. 2005). A small proportion of cholinergic interneurons are also reported to be immunoreactive for Kv7.2 protein (Cooper et al. 2001). Based on the finding of Kv7 channel expression in striatal output neurons, Shen et al. (2005) recently proposed a model of intrastriatal Kv7 channel function. Accordingly, excitatory input from intrastriatal cholinergic neurons, via release of acetylcholine inhibits postsynaptic heteromeric Kv7.2/7.3 channel function as a consequence of activation of muscarinic M1 receptors. Conversely, stimulated Kv7 channel activity on medium spiny neurons could lead to reduced gating of excitatory input from cholinergic interneurons. Thus, physiological fluctuations of cholinergic and dopaminergic tone onto striatal Kv7 channel expressing output neurons would allow ‘fine-tuned’ striatal activity for execution of coordinated motor activity (Yamada et al. 2004).
Kv7 channel interaction with serotonergic neurotransmission
The only serotonergic pathways arise in the raphe nuclei, which form a continuous collection of cell groups close to the midline throughout the brainstem. The most rostral nuclei project to an extensive number of forebrain areas, including the amygdala, hippocampus, hypothalamus and virtually all neocortical regions. An important group of serotonergic neurons is contained within the dorsal raphe nucleus (DRN). The DRN contains both serotonergic and non-serotonergic neurons with most of the latter neurons presumably being mainly GABAergic (Day et al. 2004). Serotonergic neurons of the DRN are topographically organized and the subpopulations of neurons are distinguished by cellular morphology, coexpression of other neurotransmitters and neuropeptides, and specific neuronal input and output pathways (Lowry et al. 2005).
Many observations suggest that hyperactivity of DRN neurons is a characteristic feature in stress and anxiety states. Accordingly, exposure to hostile conditions initiates stress responses composed of alterations in behaviour, autonomic function and the release of multiple neurotransmitters and hormones in the DRN. Aversive stimuli producing stress, anxiety and fear increase neuronal activity in subpopulations of serotonergic neurons and increase serotonin levels in the vicinity of DRN neurons (Amat et al. 2005). Behavioural paradigms associated with increased anxiety and conditioned fear increase serotonergic activity arising from the DRN (Maier & Watkins, 2005). Hence, negative modulation of serotonergic activity in the DRN ascending projection pathways may produce anxiolytic responses.
Kv7 channels in the DRN
Interestingly, Kv7.4 channels are expressed in the DRN (Kharkovets et al. 2000). To date, no other Kv7 channels have been identified in the DRN, suggesting the Kv7.4 channel is an attractive and selective target for treating anxiety. The presence of Kv channels in raphe nuclei was initially suggested by Kharkovets et al. (2000), who reported Kv7.4 mRNA and protein expression in both the dorsal and medial raphe nuclei (MRN). We have confirmed and extended these findings using immunohistochemical stainings of the DRN and MRN, showing that Kv7.4 channels are expressed in the DRN at different rostral–caudal levels (Fig. 3) Notably, Kv7.4 immunoreactivity is found specifically on a subpopulation of serotonergic (TH-positive) neurons in the DRN (Fig. 4, upper panels).
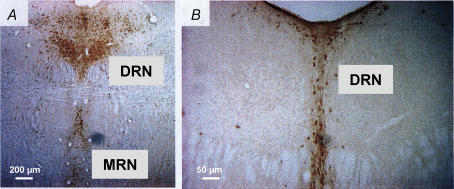
Figure 3. Kv7.4 channel expression in the raphe nuclei.
A, representative immunohistochemical staining of Kv7.4 channels in the rat dorsal (DRN) and median raphe (MNR) nucleus of the rat brainstem (10× magnification, 40 μm section). B, further caudal level of the DRN (20× magnification, 40 μm section). The data indicate that Kv7.4 channels are expressed throughout the DRN at the rostral–caudal level. A rabbit polyclonal Kv7.4 channel antibody (Kharkovets et al. 2000) diluted 1: 5000 and biotinylated donkey anti-rabbit IgG (1: 800; Jackson ImmunoResearch Laboratories, West grove, PA, USA) were used. Immunoreactivity was detected by means of the avidin–biotin amplification method using diaminobenzidine as chromagen and amplified using the biotinylated tyramine procedure as previously described (Hansen et al. 2006).
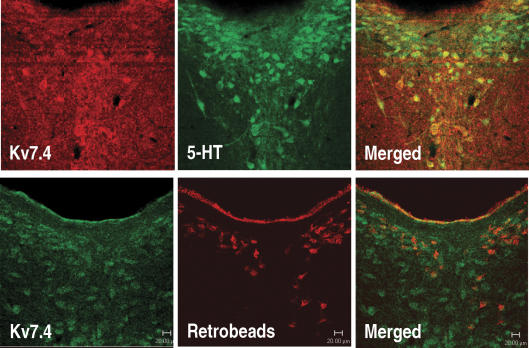
Figure 4. Kv7.4 channels are expressed in serotonergic (5-HT) neurons in the rat DRN.
Upper panels (40× magnification, 40 μm section): Confocal scanning of Kv7.4 and 5-HT immunoreactivity shows an overlap of Kv7.4 channel and 5-HT expression in a subpopulation of neurons in the rat dorsal raphe nucleus (DRN). Lower panels (40× magnification, 40 μm section): Representative retrograde tracing experiment showing that Kv7.4 channels colocalize with rhodamine-coupled latex microsphere retrobeads (Lumaflour, Naples, FL, USA) pressure-injected unilaterally into the rat hippocampus, indicating that Kv7.4 channels are expressed in several DRN neurons projecting to the hippocampus. After 10 days the rat was perfused and 40 μm DRN sections processed for double immunohistochemistry. A rabbit polyclonal Kv7.4 channel (Kharkovets et al. 2000) diluted 1: 5000 and mouse monoclonal 5-HT antibody (1: 5000; DiaSorin, Saluggia, Italy) were used. The secondary antibodies were either Cy3-conjugated sheep anti-mouse IgG (1: 200) or Alexa 633-conjugated donkey anti-mouse/goat IgG (1: 200) mixed with biotinylated donkey anti-rabbit IgG (1: 800) (all from Jackson ImmunoResearch Laboratories, West grove, PA, USA). The immunoreactions were completed using the biotinylated tyramine procedure as previously described together with Fluorescein Avidin D (1: 200; Vector Laboratories, Burlingame, CA, USA) as previously described (Hansen et al. 2006).
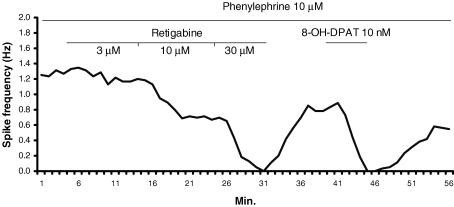
Application of retigabine to acute rat DRN slices produces a potent and robust inhibitory effect on the firing rate of serotonergic DRN neurons (mean IC50= 5.1 μm, Fig. 5). Thus, the potency and efficacy of retigabine-induced inhibition of DRN spike activity is similar to that observed for retigabine on dopaminergic neurons (Hansen et al. 2006). However, not all serotonergic neurons monitored were responsive to retigabine, which correlates well with the Kv7.4 expression being restricted to only a subset of DRN serotonergic neurons. In contrast, the effect of retigabine is not dependent on a specific subregional localization of Kv7 channels in the DRN, because all neurons responded equally regardless of the topographical localization of the recorded cells (not shown). It should be noted, however, that all recordings were made in the medial part of the DRN where most serotonergic neurons are found. This indicates a uniform inhibitory effect of retigabine throughout the DRN, which is consistent with the finding of Kv7.4 expression in the dorsal, ventral and ventrolateral segments (Fig. 3). These results suggest a retigabine-sensitive M-type current mediated by Kv7.4 channels is present in serotonergic DRN neurons. However, experiments with Kv7 channel blockers are needed to confirm this hypothesis, as has been done in the SNc and VTA (Hansen et al. 2006; Koyama & Appel, 2006).
Figure 5. Extracellular recording of a neuron in the anterior part of the rat DRN in vitro.
The method has been previously described (Seutin et al. 1990). Retigabine induces a concentration-dependent inhibition of the firing rate of the cell. Unlike the situation in vivo in which the majority of DRN serotonergic neurons are spontaneously active, most DRN serotonergic neurons are silent in a slice preparation. In the presence of 10 μm phenylephrine, they fire at a rate of 0.5–5 spikes per second and are characterized by long duration (2 ms), often triphasic action potentials. Neuronal spike activity is completely inhibited by nanomolar concentrations of the selective 5-HT1A agonist 8-hydroxy-2-(di-n-propylamine)tetralin (8-OH-DPAT), which strongly suggests that the neuron is serotonergic.
When performing retrograde tracing using retrobeads injected into the rat hippocampus, subsequent immunohistological analysis revealed retrograde transport to several, but not all, Kv7.4 channel-expressing DRN neurons (Fig. 4, lower panels). This invites the possibility that Kv7.4 activity in the DRN may lead to negative modulation of serotonergic function in the hippocampus. Because DRN serotonergic projections innervate major anxiety circuits, including the hippocampus (Lowry et al. 2005), modulated Kv7.4 channel activity could be relevant in the context of anxiety disorders.
Anxiolytic properties of Kv7 channel openers
So far, there are no reports of a specific involvement of Kv7 isoforms in behavioural models of anxiety. However, the anxiolytic potential of broad-spectrum Kv7 channel openers has recently been assessed in both unconditioned and conditioned (shock-based) rodent models of anxiety (Korsgaard et al. 2005). Retigabine showed dose-related anxiolytic efficacy in the mouse zero maze and marble burying test in an XE991-sensitive manner. In comparison, the Kv7 opener BMS-204352 (S-enantiomer) was equally effective in the same models, and also showed efficacy in the rat conditioned emotional response model. Importantly, both compounds in the doses tested had no significant effect on motor activity, indicating no confounding effects by potentially reduced motor performance. Remarkably, in this study the anxiolytic properties of the S-enantiomer were completely antagonized by the R-enantiomer, a Kv7 channel blocker. The S-enantiomer exhibits a slightly higher affinity for the Kv7.4 and Kv7.5 channels as compared to Kv7.2 and Kv7.3 channels (Korsgaard et al. 2005), which could suggest that stimulation of Kv7.4 channel activity in the DRN contributed to the anxiolytic profile of BMS-204352.
It should be noted that retigabine is reported to have inhibitory effects on glutamatergic neurotransmission, i.e. by reducing presynaptic efflux of glutamate (Martire et al. 2004) (see Table 1), which is highly implicated in the precipitation of stress and anxiety behaviour. Thus, it cannot be excluded that anxiolytic activity of Kv7 channel openers requires modulation, direct or indirect, of several neurotransmitter systems in the CNS.
Conclusion
Kv7 channel openers potently and effectively inhibit dopaminergic and serotonergic activity in the CNS. The electrophysiological properties and expression pattern of Kv7 channels suggest a direct inhibitory effect of dopaminergic and serotonergic neurotransmission in the brainstem, presumably resulting from blockade of neuronal impulse flow in the SNc/VTA and DRN, respectively. The specific Kv7 channel isoform operating in these systems is not yet identified, but intriguing data point to a possible key role of the Kv7.4 channel in the control of somatodendritic neuronal excitability in these monoaminergic nuclei. If so, this would indicate a potential target for specific modulation of these neurotransmitter systems with Kv7 channel subtype-selective drugs. Consequently, Kv7.4 channel openers could prove to be attractive pharmacological agents in the treatment of disease states characterized by dopaminergic or serotonergic overactivity, including schizophrenia, drug abuse and anxiety.
Acknowledgments
H.H.H. and J.D.M. were supported by the EU 6th framework programme (grant no. LSHM-CT-2004-503038). V.S. was supported by grant no. 9.4560.03 from the FNRS. (Belgium) and by a grant from the Belgian Science Policy (Inter-University Attraction Poles grant no. P6/31). S.A. was supported by grants from the Lundbeck Foundation, the Danish Medical Research Council, Augustinus Foundation, Simon Fougner Hartmanns Family Foundation, and the Foundation for Research in Neurology.
References
- Amat J, Baratta MV, Paul E, Bland ST, Watkins LR, Maier SF. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat Neurosci. 2005;8:365–371. doi: 10.1038/nn1399. [DOI] [PubMed] [Google Scholar]
- Biervert C, Schroeder BC, Kubisch C, Berkovic SF, Propping P, Jentsch TJ, Steinlein OK. A potassium channel mutation in neonatal human epilepsy. Science. 1998;279:403–406. doi: 10.1126/science.279.5349.403. [DOI] [PubMed] [Google Scholar]
- Blackburn-Munro G, Dalby-Brown W, Mirza NR, Mikkelsen JD, Blackburn-Munro RE. Retigabine: chemical synthesis to clinical application. CNS Drug Rev. 2005;11:1–20. doi: 10.1111/j.1527-3458.2005.tb00033.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonjean M, Waroux O, Dang-Vu T, Phillips C, Lamy C, Scuvee-Moreau J, Sepulchre R, Maquet P, Seutin V. 2007 Abstract Viewer/Itinerary Planner. Washington, DC: Society for Neuroscience; 2007. Effect of the M-current on the excitability of midbrain dopaminergic neurons: a computational study. Program No. 681.6. [Google Scholar]
- Cooper EC, Harrington E, Jan YN, Jan LY. M channel KCNQ2 subunits are localized to key sites for control of neuronal network oscillations and synchronization in mouse brain. J Neurosci. 2001;21:9529–9540. doi: 10.1523/JNEUROSCI.21-24-09529.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalby-Brown W, Hansen HH, Korsgaard MP, Mirza N, Olesen SP. Kv7 channels: function, pharmacology and channel modulators. Curr Top Med Chem. 2006;6:999–1023. doi: 10.2174/156802606777323728. [DOI] [PubMed] [Google Scholar]
- Day HE, Greenwood BN, Hammack SE, Watkins LR, Fleshner M, Maier SF, Campeau S. Differential expression of 5HT-1A, α1b adrenergic, CRF-R1, and CRF-R2 receptor mRNA in serotonergic, γ-aminobutyric acidergic, and catecholaminergic cells of the rat dorsal raphe nucleus. J Comp Neurol. 2004;474:364–378. doi: 10.1002/cne.20138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmas P, Brown DA. Pathways modulating neural KCNQ/M (Kv7) potassium channels. Nat Rev Neurosci. 2005;6:850–862. doi: 10.1038/nrn1785. [DOI] [PubMed] [Google Scholar]
- Devaux JJ, Kleopa KA, Cooper EC, Scherer SS. KCNQ2 is a nodal K+ channel. J Neurosci. 2004;24:1236–1244. doi: 10.1523/JNEUROSCI.4512-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelbauer H, Lechner SG, Mayer M, Scholze T, Boehm S. Presynaptic inhibition of transmitter release from rat sympathetic neurons by bradykinin. J Neurochem. 2005;93:1110–1121. doi: 10.1111/j.1471-4159.2005.03084.x. [DOI] [PubMed] [Google Scholar]
- Gartside SE, Hajos-Korcsok E, Bagdy E, Harsing LG, Jr, Sharp T, Hajos M. Neurochemical and electrophysiological studies on the functional significance of burst firing in serotonergic neurons. Neuroscience. 2000;98:295–300. doi: 10.1016/s0306-4522(00)00060-9. [DOI] [PubMed] [Google Scholar]
- Geiger J, Weber YG, Landwehrmeyer B, Sommer C, Lerche H. Immunohistochemical analysis of KCNQ3 potassium channels in mouse brain. Neurosci Lett. 2006;400:101–104. doi: 10.1016/j.neulet.2006.02.017. [DOI] [PubMed] [Google Scholar]
- Hansen HH, Andreasen JT, Weikop P, Mirza N, Scheel-Kruger J, Mikkelsen JD. The neuronal KCNQ channel opener retigabine inhibits locomotor activity and reduces forebrain excitatory responses to the psychostimulants cocaine, methylphenidate and phencyclidine. Eur J Pharmacol. 2007;570:77–88. doi: 10.1016/j.ejphar.2007.05.029. [DOI] [PubMed] [Google Scholar]
- Hansen HH, Ebbesen C, Mathiesen C, Weikop P, Rønn LC, Waroux O, Scuvee-Moreau J, Seutin V, Mikkelsen JD. The KCNQ channel opener retigabine inhibits the activity of mesencephalic dopaminergic systems of the rat. J Pharmacol Exp Ther. 2006;318:1006–1019. doi: 10.1124/jpet.106.106757. [DOI] [PubMed] [Google Scholar]
- Harley CW. Norepinephrine and the dentate gyrus. Prog Brain Res. 2007;163:299–318. doi: 10.1016/S0079-6123(07)63018-0. [DOI] [PubMed] [Google Scholar]
- Hu H, Vervaeke K, Storm JF. M-channels (Kv7/KCNQ channels) that regulate synaptic integration, excitability, and spike pattern of CA1 pyramidal cells are located in the perisomatic region. J Neurosci. 2007;27:1853–1867. doi: 10.1523/JNEUROSCI.4463-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen HS, Grunnet M, Olesen SP. Inactivation as a new regulatory mechanism for neuronal Kv7 channels. Biophys J. 2007;92:2747–2756. doi: 10.1529/biophysj.106.101287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jentsch TJ. Neuronal KCNQ potassium channels: physiology and role in disease. Nat Rev Neurosci. 2000;1:21–30. doi: 10.1038/35036198. [DOI] [PubMed] [Google Scholar]
- Kharkovets T, Hardelin JP, Safieddine S, Schweizer M, El-Amraoui A, Petit C, Jentsch TJ. KCNQ4, a K+ channel mutated in a form of dominant deafness, is expressed in the inner ear and the central auditory pathway. Proc Natl Acad Sci U S A. 2000;97:4333–4338. doi: 10.1073/pnas.97.8.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korsgaard MP, Hartz BP, Brown WD, Ahring PK, Strobaek D, Mirza NR. Anxiolytic effects of maxipost (BMS-204352) and retigabine via activation of neuronal Kv7 channels. J Pharmacol Exp Ther. 2005;314:282–292. doi: 10.1124/jpet.105.083923. [DOI] [PubMed] [Google Scholar]
- Koyama S, Appel SB. Characterization of M-current in ventral tegmental area dopamine neurons. J Neurophysiol. 2006;96:535–543. doi: 10.1152/jn.00574.2005. [DOI] [PubMed] [Google Scholar]
- Koyama S, Brodie MS, Appel SB. Ethanol inhibition of m-current and ethanol-induced direct excitation of ventral tegmental area dopamine neurons. J Neurophysiol. 2007;97:1977–1985. doi: 10.1152/jn.00270.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubisch C, Schroeder BC, Friedrich T, Lutjohann B, El Amraoui A, Marlin S, Petit C, Jentsch TJ. KCNQ4, a novel potassium channel expressed in sensory outer hair cells, is mutated in dominant deafness. Cell. 1999;96:437–446. doi: 10.1016/s0092-8674(00)80556-5. [DOI] [PubMed] [Google Scholar]
- Lawrence JJ, Saraga F, Churchill JF, Statland JM, Travis KE, Skinner FK, McBain CJ. Somatodendritic Kv7/KCNQ/M channels control interspike interval in hippocampal interneurons. J Neurosci. 2006;26:12325–12338. doi: 10.1523/JNEUROSCI.3521-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lechner SG, Mayer M, Boehm S. Activation of M1 muscarinic receptors triggers transmitter release from rat sympathetic neurons through an inhibition of M-type K+ channels. J Physiol. 2003;553:789–802. doi: 10.1113/jphysiol.2003.052449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungstrom T, Grunnet M, Jensen BS, Olesen SP. Functional coupling between heterologously expressed dopamine D2 receptors and KCNQ channels. Pflugers Arch. 2003;446:684–694. doi: 10.1007/s00424-003-1111-2. [DOI] [PubMed] [Google Scholar]
- Lowry CA, Johnson PL, Hay-Schmidt A, Mikkelsen J, Shekhar A. Modulation of anxiety circuits by serotonergic systems. Stress. 2005;8:233–246. doi: 10.1080/10253890500492787. [DOI] [PubMed] [Google Scholar]
- Maier SF, Watkins LR. Stressor controllability and learned helplessness: the roles of the dorsal raphe nucleus, serotonin, and corticotropin-releasing factor. Neurosci Biobehav Rev. 2005;29:829–841. doi: 10.1016/j.neubiorev.2005.03.021. [DOI] [PubMed] [Google Scholar]
- Marrion NV. Control of M-current. Annu Rev Physiol. 1997;59:483–504. doi: 10.1146/annurev.physiol.59.1.483. [DOI] [PubMed] [Google Scholar]
- Martire M, Castaldo P, D'Amico M, Preziosi P, Annunziato L, Taglialatela M. M channels containing KCNQ2 subunits modulate norepinephrine, aspartate, and GABA release from hippocampal nerve terminals. J Neurosci. 2004;24:592–597. doi: 10.1523/JNEUROSCI.3143-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martire M, D'Amico M, Panza E, Miceli F, Viggiano D, Lavergata F, Iannotti FA, Barrese V, Preziosi P, Annunziato L, Taglialatela M. Involvement of KCNQ2 subunits in [3H]dopamine release triggered by depolarization and pre-synaptic muscarinic receptor activation from rat striatal synaptosomes. J Neurochem. 2007;102:179–193. doi: 10.1111/j.1471-4159.2007.04562.x. [DOI] [PubMed] [Google Scholar]
- Mikkelsen JD. The KCNQ channel activator retigabine blocks haloperidol-induced c-Fos expression in the striatum of the rat. Neurosci Lett. 2004;362:240–243. doi: 10.1016/j.neulet.2004.03.025. [DOI] [PubMed] [Google Scholar]
- Overton PG, Clark D. Burst firing in midbrain dopaminergic neurons. Brain Res Brain Res Rev. 1997;25:312–334. doi: 10.1016/s0165-0173(97)00039-8. [DOI] [PubMed] [Google Scholar]
- Peters HC, Hu H, Pongs O, Storm JF, Isbrandt D. Conditional transgenic suppression of M channels in mouse brain reveals functions in neuronal excitability, resonance and behavior. Nat Neurosci. 2005;8:51–60. doi: 10.1038/nn1375. [DOI] [PubMed] [Google Scholar]
- Piccinin S, Randall AD, Brown JT. KCNQ/Kv7 channel regulation of hippocampal gamma-frequency firing in the absence of synaptic transmission. J Neurophysiol. 2006;95:3105–3112. doi: 10.1152/jn.01083.2005. [DOI] [PubMed] [Google Scholar]
- Pickel VM, Chan J, Nirenberg MJ. Region-specific targeting of dopamine D2-receptors and somatodendritic vesicular monoamine transporter 2 (VMAT2) within ventral tegmental area subdivisions. Synapse. 2002;45:113–124. doi: 10.1002/syn.10092. [DOI] [PubMed] [Google Scholar]
- Schmidt WJ, Schuster G, Wacker E, Pergande G. Antiparkinsonian and other motor effects of flupirtine alone and in combination with dopaminergic drugs. Eur J Pharmacol. 1997;327:1–9. doi: 10.1016/s0014-2999(97)89671-9. [DOI] [PubMed] [Google Scholar]
- Schroeder BC, Hechenberger M, Weinreich F, Kubisch C, Jentsch TJ. KCNQ5, a novel potassium channel broadly expressed in brain, mediates M-type currents. J Biol Chem. 2000;275:24089–24095. doi: 10.1074/jbc.M003245200. [DOI] [PubMed] [Google Scholar]
- Schroeder BC, Kubisch C, Stein V, Jentsch TJ. Moderate loss of function of cyclic-AMP-modulated KCNQ2/KCNQ3 K+ channels causes epilepsy. Nature. 1998;396:687–690. doi: 10.1038/25367. [DOI] [PubMed] [Google Scholar]
- Schwake M, Pusch M, Kharkovets T, Jentsch TJ. Surface expression and single channel properties of KCNQ2/KCNQ3, M-type K+ channels involved in epilepsy. J Biol Chem. 2000;275:13343–13348. doi: 10.1074/jbc.275.18.13343. [DOI] [PubMed] [Google Scholar]
- Seutin V, Scuvee-Moreau J, Giesbers I, Massotte L, Dresse A. Effect of BHT 920 on monoaminergic neurons of the rat brain: an electrophysiological in vivo and in vitro study. Naunyn Schmiedebergs Arch Pharmacol. 1990;342:502–507. doi: 10.1007/BF00169036. [DOI] [PubMed] [Google Scholar]
- Shen W, Hamilton SE, Nathanson NM, Surmeier DJ. Cholinergic suppression of KCNQ channel currents enhances excitability of striatal medium spiny neurons. J Neurosci. 2005;25:7449–7458. doi: 10.1523/JNEUROSCI.1381-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soldovieri MV, Cilio MR, Miceli F, Bellini G, Miraglia del Giudice E, Castaldo P, Hernandez CC, Shapiro MS, Pascotto A, Annunziato L, Taglialatela M. Atypical gating of M-type potassium channels conferred by mutations in uncharged residues in the S4 region of KCNQ2 causing benign familial neonatal convulsions. J Neurosci. 2007;27:4919–4928. doi: 10.1523/JNEUROSCI.0580-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatulian L, Brown DA. Effect of the KCNQ potassium channel opener retigabine on single KCNQ2/3 channels expressed in CHO cells. J Physiol. 2003;549:57–63. doi: 10.1113/jphysiol.2003.039842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatulian L, Delmas P, Abogadie FC, Brown DA. Activation of expressed KCNQ potassium currents and native neuronal M-type potassium currents by the anti-convulsant drug retigabine. J Neurosci. 2001;21:5535–5545. doi: 10.1523/JNEUROSCI.21-15-05535.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HS, Pan Z, Shi W, Brown BS, Wymore RS, Cohen IS, Dixon JE, McKinnon D. KCNQ2 and KCNQ3 potassium channel subunits: molecular correlates of the M-channel. Science. 1998;282:1890–1893. doi: 10.1126/science.282.5395.1890. [DOI] [PubMed] [Google Scholar]
- Weber YG, Geiger J, Kampchen K, Landwehrmeyer B, Sommer C, Lerche H. Immunohistochemical analysis of KCNQ2 potassium channels in adult and developing mouse brain. Brain Res. 2006;1077:1–6. doi: 10.1016/j.brainres.2006.01.023. [DOI] [PubMed] [Google Scholar]
- Yamada H, Matsumoto N, Kimura M. Tonically active neurons in the primate caudate nucleus and putamen differentially encode instructed motivational outcomes of action. J Neurosci. 2004;24:3500–3510. doi: 10.1523/JNEUROSCI.0068-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue C, Yaari Y. KCNQ/M channels control spike afterdepolarization and burst generation in hippocampal neurons. J Neurosci. 2004;24:4614–4624. doi: 10.1523/JNEUROSCI.0765-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaczek R, Chorvat RJ, Saye JA, Pierdomenico ME, Maciag CM, Logue AR, Fisher BN, Rominger DH, Earl RA. Two new potent neurotransmitter release enhancers, 10,10-bis(4-pyridinylmethyl)-9(10H)-anthracenone and 10,10-bis(2-fluoro-4-pyridinylmethyl)-9(10H)-anthracenone: comparison to linopirdine. J Pharmacol Exp Ther. 1998;285:724–730. [PubMed] [Google Scholar]