Abstract
Apoptosis is an essential physiological process in embryonic development. In the developing eye of vertebrates, three periods of developmental apoptosis can be distinguished: early, intermediate and later. Within the apoptosis pathway, caspases play a crucial role. It has also been shown that HSP110 may have a potential role in apoptosis. The aim of this research was to study the expression of HSP110, caspase-3 and -9 in physiological, retinoic- or irradiation-induced apoptosis during early eye development. Seven pregnant C57Bl/6J mice received 80 mg kg−1 of all-trans retinoic acid mixed with sesame oil. Seven pregnant NMRI mice received 2 Gy irradiation at the same gestational day. Control mice of both strains (seven mice of each) were not submitted to any treatment. Embryos were harvested at 3, 6, 12 and 24 h after exposition, fixed, dehydrated and embedded. Coronal sections (5 µm) were made. Slide staining occurred alternatively using anti-caspase-3, anti-caspase-9 and anti-HSP110 immunohistochemistry. HSP110 and caspase-3 expression presented similar topographic and chronological patterns, whereas expression of HSP110 was more precocious in retinoic acid-treated embryos. After retinoic exposure, caspase-3- and HSP110-positive cells were increased in the region of the optic vesicle. By contrast, after irradiation, caspase-3- and HSP110-positive cells were noticeably increased in the optic vesicle, peri-optical mesoderm but less in lens placode. HSP110 was expressed before caspase-3. By contrast, caspase-9 was expressed by a very small number of cells in the optic vesicle either under physiological or under teratogenic conditions. Thus, it seems that activation of caspase-9 is dispensable in early eye developmental apoptosis.
Keywords: apoptosis, caspases, embryos, eye, HSP 110, irradiation, retinoic acid
Introduction
Apoptosis is an active form of cell death characterized by a series of distinct morphological and biochemical alterations. It plays an essential part in development (Meier et al. 2000; Zakeri & Lockshin, 2002). In the developing eye of vertebrates, and especially in the developing retina, three periods of developmental apoptosis can be distinguished (Callerino et al. 2000). Early developmental apoptosis occurs within the optic vesicle and lens placode of mice from embryonic day (E)9 to E11 (Frade et al. 1997; Laemle et al. 1999; Callerino et al. 2000; Bozanic & Saraga-Barbic, 2003). Intermediate apoptosis, which occurs during neurogenesis within the retina when differentiation of specific neurons of the retina (retinal ganglion cells, amacrine cells, bipolar cells, Müller's cells, horizontal cells and photo-sensitive cells) takes place, corresponds to E13–E15 of mouse embryogenesis (Linden et al. 2005; Sanders et al. 2005). Late developmental apoptosis occurs during synaptogenesis and involves the connection of retinal ganglion cells with the tectum/thalamus and the connection of different retinal neurons. It occurs between E17 and the first two postnatal weeks of mouse development. Cells commit to apoptosis after they differentiate. Thus, retinal ganglion cells are the first to differentiate and to be eliminated by cell death and this is followed by apoptosis of amacrine cells, bipolar cells and Müller's cells. Apoptosis of rod cells, which occurs last in the postnatal period, is discrete and sporadic (Bahr, 2000; Kawabata et al. 2003; Zeiss et al. 2004).
Although the role of apoptosis in the developing eye has been established, the underlying biochemical mechanisms are not yet completely understood. We studied the expression of three proteins associated with the process of apoptosis, namely caspase-3, caspase-9 and HSP110.
It is known that the initiation and execution of apoptosis involves a cascade of biochemical reactions that culminate in the activation of a family of proteases known as caspases (Chang & Yang, 2000; Blatt & Glick, 2001).
At present, at least 14 caspases have been identified in mammals, some of which are known to play a critical role in apoptosis. Caspases are found in normal cells as inactive proenzymes and are activated in cells undergoing apoptosis (Chang & Yang, 2000).
Two distinct caspase activation pathways are known: an extrinsic pathway, which is induced by the ligation of the death receptor, a member of the tumour necrosis factor receptor family called TNFr (e.g. Fas/CD95), and its ligand tumour necrosis factor TNF (e.g. FasL/CD95L), and the intrinsic pathway, or mitochondrial apoptotic pathway, which is initiated by numerous stimuli (e.g. irradiation and oxidative stress). Both apoptotic pathways converge on the activation of executioner caspases, mainly caspase-3 (Scaffidi et al. 1998; Varfolomeev et al. 1998; Wang, 2001). By contrast, heat shock proteins (HSPs) are expressed transitorily as a response to cellular stresses, be they environmental or biochemical (high temperature, heavy metals, hypoxia, anoxia, toxins, etc.). In cellular stress conditions, HSPs prevent aggregation of damaged, misfolded or unfolded proteins and thus ensure cell survival (Alexandrov, 1994; Schirmer et al. 1996; Sreedhar & Csermely, 2004). Some HSPs have, however, been shown to play a role in cell death (Vanmuylder et al. 1997; Evrard et al. 1999, 2000; Abdelwahid et al. 2001). Vanmuylder et al. (1997) have demonstrated that HSP110 is expressed in apoptotic chondrocytes in growth-plate cartilage of young rats. The same HSP110 has been shown to be expressed in neural crest cells undergoing physiological or retinoic-induced apoptosis in mouse embryos (Evrard et al. 1999, 2000). These results suggest that HSP110 is strongly associated with developmental apoptosis, but the mechanism involved has not yet been elucidated.
The aim of the present investigation was to study the expression of HSP110, caspase-3 and caspase-9 in physiological, retinoic acid (RA)- or irradiation-induced apoptosis during early eye development. This will further our understanding of the mechanism of caspase activation during physiologically and teratogenically induced apoptosis in eye development. We also discuss the correlation of HSP110 expression with active-form caspase-3 expression.
Materials and methods
Embryos and teratogenic models
Mature and nulliparous female mice were mated overnight with a male and the day on which vaginal plugs were found was considered day 0 of gestation (E0). On day 9 of gestation, a group of seven pregnant NMRI mice was irradiated (2 Gy) using a linear accelerator (Clinac 2100C, Varian Medical Systems) with an energy of 6 MV. Seven other pregnant NMRI mice were kept as controls and did not receive any irradiation. The irradiation protocol has been previously validated (Glineur et al. 1998).
Another group of seven pregnant E9 C57Bl/6J mice received per os 80 mg kg−1 of all-trans RA in sesame oil. A group of seven C57Bl/6J pregnant mice were kept as controls and did not receive any treatment. Previous experimentations have validated this protocol (Mulder et al. 2000). The C57Bl/6J mouse strain was chosen because it shows sensitivity to RA teratogenicity (Sulik et al. 1987; Louryan et al. 1990; Glineur et al. 1999; Mulder et al. 2000). NMRI mice show less sensibility to RA but exhibit the same kind of malformations. Furthermore, the cell death pattern appears to be very similar in both strains (Louryan et al. 1990).
Pregnant females were killed by cervical dislocation on day 9 (E9), or day 9 plus 3, 6, 12 or 24 h. Embryo staging was performed by determination of crown–rump length and counting of somites (Theiler, 1987) to ensure that embryos were at the same stage of development. Embryos were fixed for 2–3 h in Serra's fixative medium. After dehydratation in alcohol and paraffin embedding according to standard procedures, 5-µm coronal sections were placed on slides and stored until further processing. For each case, serial alternative sections were performed.
Caspase-3 immunohistochemistry
Tissue sections mounted on slides were deparaffinized and rehydrated through graded alcohol and water. To permeabilize the cellular membranes, slides were placed in citrate buffer and irradiated in a microwave at 650 W for 2 min. After progressive cooling in citrate and washing in phosphate-buffered saline (PBS), endogenous peroxidase was blocked by incubation in methanol containing 0.3% hydrogen peroxide. After washing in PBS containing 0.1% Triton X-100® to permeabilize further the cytoplasmic membrane, the slides were incubated in normal goat serum (NGS; IHC Select Chemicon, Temecula, CA, USA) for 120 min to block non-specific binding sites. NGS was removed and slides were incubated overnight in a humidified chamber with rabbit-polyclonal anti-caspase-3 (BD Biosciences Pharmingen, San José, CA, USA), diluted 1 : 500 in PBS. This antibody is recognized as specific to the active form of caspase-3. The slides were washed in PBS and incubated with goat anti-rabbit IgG (IHC Select Chemicon) for 30 min. After washing with PBS, slides were incubated avidin–biotin–peroxidase complex (ABC; IHC Select Chemicon) for 30 min. After PBS washing, the slides were incubated with peroxidase substrate solution of diaminobenzidine (DAB; Vector Industries, Burlingame, CA, USA) for 4 min. Finally, the slides were rinsed in tap water, dehydrated through a graded alcohol series, mounted in DPX and examined under light microscopy.
Caspase-9 and HSP110 immunohistochemistry
We used the same method to identify HSP110- or caspase-9-positive cells. For caspase-9, we used a rabbit-polyclonal anti-caspase, specific to caspase-9 active form (Santa Cruz Biotechnology), diluted 1 : 50 in PBS.
For HSP110, we used a rabbit-polyclonal anti-HSP104 (Affinity Bioreagens Inc.), diluted 1 : 250 in PBS. This antibody is directed against the yeast Saccharomyces cerevisiae HSP104, but specifically recognized mammmalian (human and mouse) HSP100 and HSP105. These two mammalian proteins belong to the HSP110 family (Vanmuylder et al. 1997; Evrard et al. 1999, 2000).
Quantification HSP110-, caspase-3- and caspase-9-positive cells
Quantification of HSP110-, caspase-3- and caspase-9-positive cells was performed in the right optic vesicle area of (E9+3, E9+6, E9+12, E9+24) mouse embryos, both in control embryos and after irradiation or RA administration according to a protocol used in a previous study (Evrard et al. 2000). Control E9 NMRI or C57Bl/6J embryos were used. For each stage, three embryos were chosen randomly and a total of 54 embryos were used. The same section levels were used for expression of the three proteins to ensure that the quantification was performed for similar eye regions of the embryos. Data were collected from three consecutive sections from each embryo. For data acquisition, each slide was digitized by a CCD camera (Higekami, Japan) and a frame-grabber (DATA TRANSLATION 2871).
The digitalized image was sent to a TV monitor for visual control. One square of labelled cells per section was chosen and remained the same for the entire process. For each protein we determined the cell labelling level to be considered as positive. Positive cells only were then automatically counted. For each case, mean and standard error were calculated.
Abbreviations used in figures
des, desquamation cells; di, diencephalon; inf, infundibulum; lp, lens placode; mes, mesectoderm; os, optic stalk; Rp, Rathke's pouch; vent, ventricle.
Results
Normal early eye development
HSP110 and caspase-3 were expressed mainly by cells in the optic vesicle. A small number of cells in the lens placode and peri-optical mesectoderm showed expression of caspase-3 and HSP110 (Figs 1A,C and 2A,C). As caspase-3 is activated only in cells undergoing apoptosis, the expression of the active form of this protein is evidence of apoptosis (Umpierre et al. 2001). Furthermore, even in the optic vesicle, HSP110 and caspase-3 expression showed a particular spatial pattern. These two proteins were expressed by cells within the central area of the optic vesicle and stalk, ventral area of the optic vesicle and stalk, and cells of the optic vesicle close to the lens placode. By contrast, caspase-9 was expressed by a very small number of cells in the optic vesicle (Figs 4 and 6). Cells expressing caspase-3 and HSP110 were more numerous in the optic vesicle of NMRI embryos than of C57Bl/6J embryos. The spatial pattern of caspase-3-positive cells and HSP110-positive cells was, however, the same within embryos from the two strains (Figs 1A,C and 2A,C).
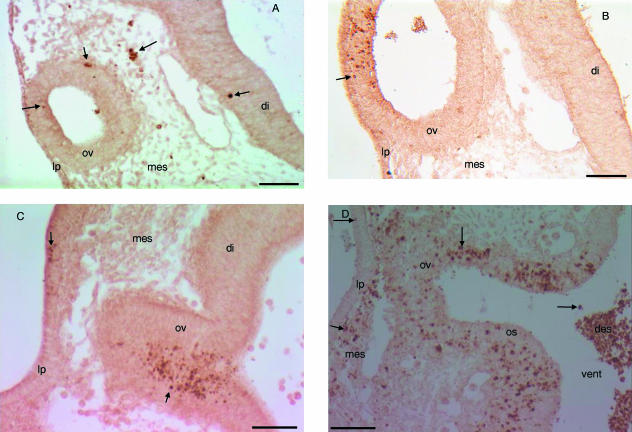
Fig. 1.
Transverse sections through the optic vesicle of control, RA-treated or irradiated mouse embryos. Immunohistochemical staining with anti-caspase-3. (A) E9+12 C57Bl/6J control embryo. Caspase-3-positive cells (arrow) are seen in the optic vesicle, peri-optical mesectoderm and diencephalon. (B) E9+12, C57Bl/6J RA-treated embryo. We observe an increase of caspase-3-positive cells (arrow) in the optic vesicle. (C) E9+6 NMRI control embryo. Numerous caspase-3-positive cells (arrow) are seen in the central and ventral portion of the optic vesicle. Some caspase-3-positive cells are also seen in the lens placode. (D) E9+3 NMRI irradiated embryo. Caspase-3-positive cells (arrow) are increased in the optic vesicle, optic stalk and peri-optical mesectoderm. Fewer caspase-3-positive cells are seen within the lens placode.
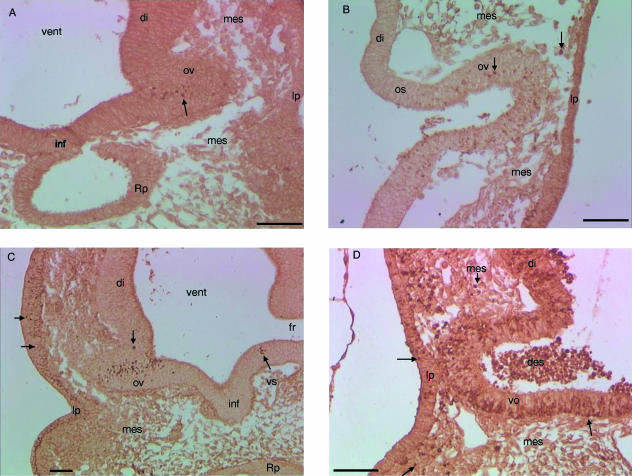
Fig. 2.
Transverse sections trough the optic vesicle of control, RA-treated or irradiated mice embryo. HSP110-positive cells are shown. (A) E9+6, C57Bl/6J control embryo. Cells expressing HSP110 (arrow) are seen in the central and ventral area of the optic vesicle. Some HSP110-positive cells are observed in the lens placode. (B) E9+6, C57Bl/6J embryo exposed to RA. Cells expressing HSP110 (arrow) are increased within the optic vesicle and optic stalk. (C) E9+3, NMRI control embryo. Cells expressing HSP110 (arrow) are seen in the optic vesicle and in the ectoderm cranial to the lens placode. (D) E9+3, NMRI irradiated embryo. Cells expressing HSP110 (arrow) are increased in the diencephalon, optic vesicle, optic stalk and peri-optical mesectoderm. Fewer HSP110-positive cells are seen in the lens placode and only a few cells of the ectoderm expressed HSP110.
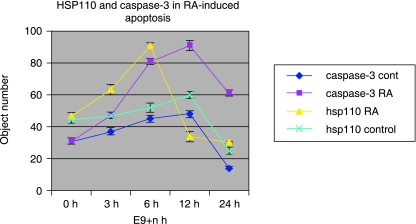
Fig. 4.
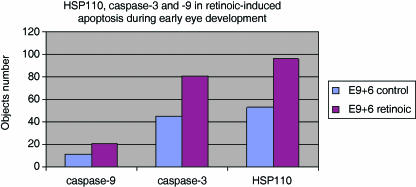
Evolution kinematics of HSP110 and caspase-3 expression in control B57Bl/6J and after embryonic exposure to RA. Cells expressing either caspase-3 or HSP110 are increased after RA treatment. Cells of the developing eye expressing HSP110 were at maximum after 6 h of RA exposure whereas caspase-3 expression was maximal after 12 h of exposure.
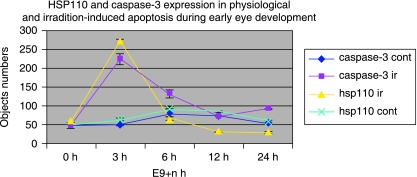
Fig. 6.
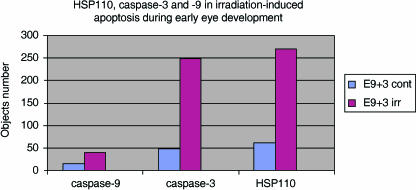
Evolution kinematics of HSP110 and caspase-3 expression in control NMRI embryos and after irradiation. Maximum HSP110-positive cells or caspase-3-positive cells are observed after only 3 h of embryonic irradiation. Bar: 100 µm.
Fig. 3.
HSP110, caspase-3 and caspase-9 expression in control E9/6 B57Bl/6J embryo and after embryonic exposure to RA. In contrast to HSP110 and caspase-3, caspase-9 is expressed by fewer cells undergoing physiological or RA-induced apoptosis in the developing eye.
Teratogenic eye development
After embryonic exposure to all-trans RA, the spatial pattern of cells expressing caspase-3 and HSP110 was the same as in normal eye development. Expression of both caspase-3 and HSP110 was increased. Numerous cells in the area of the optic vesicle, particularly near the lens placode, expressed caspase-3 and HSP110 (Figs 1B and 2B). HSP110-positive cells were more numerous than caspase-3-positive cells (Fig. 5). As in physiological apoptosis, only a few cells expressed caspase-9 in RA-induced apoptosis (Fig. 4).
Fig. 5.
Evolution of HSP110, caspase-3 and caspase-9 expression in control E9+3 NMRI embryos and after embryonic irradiation. Caspase-9 is expressed by few cells undergoing physiological or irradiation-induced apoptosis in the developing eye.
The kinetics of caspase-3 and HSP110 expression showed that expression of HSP110 was maximal after 6 h of RA treatment whereas caspase-3 expression was maximal after 12 h of RA administration. Therefore, HSP110 was expressed before caspase-3 activation (Fig. 5).
After embryonic irradiation, caspase-9 was still expressed by relatively few cells of the optic vesicle or lens placode (Fig. 6). By contrast, caspase-3 and HSP110 were highly expressed after embryonic irradiation. Cells expressing caspase-3 and HSP110 were found in all embryonic eye tissues. Numerous caspase-3-positive cells and HSP110-positive cells were observed within the optic vesicle, optic vesicle stalk and peri-optic mesectoderm. Fewer cells of the lens placode expressed caspase-3 and HSP110, and only a few cells of the ectoderm were caspase-3-positive or HSP110-positive (Figs 1D and 2D).
Maximum expression of caspase-3 and HSP110 occurred after 3 h of irradiation (Fig. 5). Numerous cells of the optic vesicle desquamated and were seen in the retinal space or in the third vesicle of the developing diencephalon. Nearly all these cells were expressing caspase-3 and HSP110 (Figs 1D and 2D). In RA-induced apotosis, HSP110 expression occurs more precociously than caspase-3 staining (Fig. 4), but in physiological apoptosis and irradiation, both phenomena occur simultaneously (Figs 4 and 5).
Discussion
During early eye development, caspase-3 was expressed by numerous cells within the optic vesicle but fewer cells within the lens placode. This observation confirms earlier studies on apoptosis in the developing eye (Laemle et al. 1999; Callerino et al. 2000; Bozanic & Saraga-Barbic, 2003). Unlike caspase-3, caspase-9 was expressed only by a few cells in the developing eye. As caspase-9 is the main initiator caspase, which activates the executioner caspase-3 (Blatt & Glick, 2001; Chang & Yang, 2000), it is likely that caspase-3 is activated by other caspases during physiological apoptosis of the developing eye. Consequently, the mitochondrial pathway may not play an essential role in normal apoptosis during early eye development. Zeiss et al. (2004) showed that caspase-3, but not caspase-9, is essential for normal eye development. Indeed, caspase-3−/– embryos show ocular anomalies such as protrusion of the neuro-retina due to excess of retinal ganglion cells, and cataracts located directly on the optic axis, their opacity being caused by an accumulation of epithelioid cells at the pole of the lens. By contrast, caspase-9−/– embryos do not show any ocular defects even if these embryos show the same severe defects of the central nervous system as caspase-3−/– embryos. These central nervous system anomalies are due to the decrease of developmental apoptosis and are characterized by excessive numbers of cells (Kuida et al. 1996, 1998; Zeiss et al. 2004).
During early eye development, apoptosis is triggered by the endogenous nerve growth factor (NGF) acting through its p57 receptor, which belongs to the TNF receptor family (Frade et al. 1996). The p57 protein is expressed before the trkA, a tyrosine kinase receptor known to bind to NGF and to mediate its anti-apoptotic function. It has been demonstrated that anti-NGF or anti-p57 antibodies substantially reduce the number of apoptotic cells in the developing retina (Frade & Barde, 1999). Moreover, treatment of embryos with exogenous brain-derived neurotropic factor (BDNF), a natural antagonist of NGF, significantly reduces apoptosis in the developing neural retina (Frade et al. 1997). In addition, it has been demonstrated that binding of NGF to p57 leads to the activation of the apoptotic machinery similar to that described following TNF or Fas receptor activation (Frade et al. 1996; Frade & Barde, 1999). However, the initiator caspase of this apoptotic pathway needs further studies, as caspase-8 activation has not yet been reported in this model.
After embryonic exposure to RA, caspase-9 expression was still observed in just a few cells of the developing eye. By contrast, caspase-3 expression was increased. This demonstrates clearly that the developing eye is sensitive to the teratogenic action of RA, as has been previously demonstrated. Indeed, previous studies showed that a deficit in endogenous RA induces eye defects but also that excessive exogenous RA causes abnormal eye development (Dickman et al. 1997; Holson et al. 1997; Umpierre et al. 2001). RA plays an essential role in the establishment of the ventro-dorsal axis of the early developing retina (Marsh-Armstrong et al. 1994). This axis is necessary for accurate topographic targeted projection within the visual system. Embryonic exposition to RA induces the posterior extension of homeobox genes, which are markers of the ventral developing retina (e.g. pax), whereas the dorsal marker genes (e.g. msh) are repressed (MacCaffery et al. 1992; Marsh-Armstrong et al. 1994; Hyatt et al. 1996; Wagner et al. 2000).
Caspase-3 expression was increased after embryonic RA exposure, which enlarges the areas of apoptosis in the developing eye. As regards the physiological apoptosis, the RA-induced apoptosis is not mediated through caspase-9 activation, the classical initiator caspase in the mitochondrial apoptosis pathway. However, caspase-9 activation has been shown in RA-induced apoptosis within neural crest cells (Gashegu et al. 2006). The caspase activation cascade is probably dependent on the type of cells undergoing cell death, as reported by others (Kuida et al. 1996, 1998; Haken et al. 1998; Varfolomeev et al. 1998; Singh et al. 2001).
After embryonic irradiation, numerous cells of the optic vesicle, optic stalk and peri-optic mesectoderm were undergoing apoptosis and expressed caspase-3. This could explain the eye abnormalities observed after mouse embryo irradiation (Glineur et al. 1998). Fewer cells of the lens placode also expressed caspase-3. The pattern of irradiation-induced apoptosis is different from physiological and RA-induced apoptosis in the developing eye. In addition, caspase-9 expression was not increased in irradiation-induced apoptosis during early eye development. This finding is surprising as it is well established that irradiation induces DNA lesions and p53-dependent apoptosis, which is a mitochondrial pathway apoptosis (Vanmuylder et al. 2004; Gashegu et al. 2005). Many neural cells also express caspase-9 after irradiation (Gashegu et al. 2006). Mitochondrial involvement in irradiation-induced apoptosis is clear. It is suggested that, in accordance with recent studies (Zhivotovsky & Orrenius, 2005; Vandna et al. 2006), caspase-9 may not be the only initiator caspase activated in mitochondrial pathway apoptosis. Depending on the cell type, other initiator caspases might be activated in irradiation-induced apoptosis. A good putative candidate may be caspase-2. Numerous studies have recently linked caspase-2 activation with p53-dependent apoptosis. Caspase-2 activation is considered as an alternative of caspase-9 activation in the mitochondrial apoptotic pathway (Susin et al. 1999; Zheng et al. 2000). It has even been shown that caspase-2 acts upstream of mitochondria events (Guo et al. 2002; Berube et al. 2005; Seth et al. 2005; Gogvadze et al. 2006). Moreover, Kojima et al. (1998) have shown caspase-2 expression in the developing rat retina. Thus, apoptosis may be understood as a dynamic and flexible process (Haken et al. 1998; Zheng et al. 2000; Singh et al. 2001).
We also showed that the pattern of HSP110 expression was similar to the pattern of caspase-3 expression in our three experimental models of apoptosis. Hence, HSP110 may be involved in the apoptosis machinery. Our findings are supported by previous studies. Indeed, Evrard et al. (1999), 2000 demonstrated that HSP110 is expressed by TUNEL-positive cells in RA-induced or physiological apoptosis during early craniofacial development.
HSP110 was expressed before caspase-3 activation. Thus, HSP110 may play a role in caspase activation. Other studies have suggested the putative role of HSP110 in activation of caspases. Yamagishi et al. (2002) showed that the over-expression of HSP105α, a member of the HSP110 family, by E9 embryonic cells induces apoptosis. The over-expression of HSP105α increases reactive oxidative species (ROS), enhances the release of cytochrome c from mitochondria and subsequently triggers caspase activation (Yamagishi et al. 2002). However, we did not observe any caspase-9 activation. However, it has been suggested that ROS can also trigger the activation of caspase-2 (Vandna et al. 2006).
Conclusion and perspectives
In early eye development, a window of physiological apoptosis is observed that corresponds to the onset of development of the retina and lens progenitor. This apoptosis depends on caspase-3 activation, but not on the classical mitochondrial apoptosis pathway. This suggests that further studies are needed to identify the initiator. HSP110 expression is similar to caspase-3 expression. The combination of our results with previous findings suggests that HSP110 is involved in apoptosis during embryonic development and can be a good marker of apoptosis. It would thus be of interest to study the apoptotic pathways in neuro-degenerative diseases and oxidative stress associated with ocular diseases.
References
- Abdelwahid E, Eriksson M, Pelliniemi LJ, Jokinen E. Heat shock proteins, HSP25 and HSP70, and apoptosis in developping endocardial cushion of mouse heart. Histochem Cell Biol. 2001;115:95–104. doi: 10.1007/s004180000227. [DOI] [PubMed] [Google Scholar]
- Alexandrov V. Functional aspects of cell response to heat shock. Int Rev Cytol. 1994;148:171–227. doi: 10.1016/s0074-7696(08)62408-0. [DOI] [PubMed] [Google Scholar]
- Bahr M. Live or let die – retinal ganglion cell death and survival during development and in the lesioned adult CNS. Trends Neurosci. 2000;23:483–490. doi: 10.1016/s0166-2236(00)01637-4. [DOI] [PubMed] [Google Scholar]
- Berube C, Boucher LM, Ma W, et al. Apoptosis caused by p53-induced protein with death domain (PIDD) depends on the death adapter RAIDD. Proc Natl Acad Sci USA. 2005;102:14314–14319. doi: 10.1073/pnas.0506475102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatt BN, Glick DG. Signaling pathways and effectors mechanisms pre-programmed cell death. Bioorg Med Chem. 2001;9:1371–1384. doi: 10.1016/s0968-0896(01)00041-4. [DOI] [PubMed] [Google Scholar]
- Bozanic D, Saraga-Barbic M. Role of apoptosis and mitosis during human eye development. Eur J Cell Biol. 2003;82:421–429. doi: 10.1078/0171-9335-00328. [DOI] [PubMed] [Google Scholar]
- Callerino A, Bahr M, Isenman S. Apoptosis in the developing visual system. Cell Tissue Res. 2000;301:53–69. doi: 10.1007/s004410000178. [DOI] [PubMed] [Google Scholar]
- Chang HY, Yang X. Proteases for cell suicide: functions and regulation of caspase. Microbiol Molec Biol Rev. 2000;64:821–846. doi: 10.1128/mmbr.64.4.821-846.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickman ED, Thaller C, Smith SM. Temporally-regulated retinoic acid depletion produces specific neural crest, ocular and nervous system defects. Development. 1997;124:3111–3121. doi: 10.1242/dev.124.16.3111. [DOI] [PubMed] [Google Scholar]
- Evrard L, Vanmuylder N, Glineur R, Louryan S. Cytochemical identification of HSP110 during early mouse facial development. J Craniofac Genet Dev Biol. 1999;19:24–32. [PubMed] [Google Scholar]
- Evrard L, Vanmuylder N, Dourov N, et al. Correlation of HSP110 expression with all-trans retinoic acid-induced apoptosis. J Craniofac Genet Dev Biol. 2000;20:183–192. [PubMed] [Google Scholar]
- Frade JM, Rodriguez-Tébar A, Barde YA. Induction of cell death by endogenous nerve growth factor through its p57 receptor. Nature. 1996;383:166–168. doi: 10.1038/383166a0. [DOI] [PubMed] [Google Scholar]
- Frade JM, Bovolenta P, Martinez-Morales JR, Arribas A, Barbas JA, Rodriguez-Tébar A. Control of early cell death by BDNF in the retina. Development. 1997;124:3313–3320. doi: 10.1242/dev.124.17.3313. [DOI] [PubMed] [Google Scholar]
- Frade JM, Barde YA. Genetic evidence of cell death mediated by nerve growth factor and neurotrophin receptor p57 in developing retina and spinal cord. Development. 1999;126:683–690. doi: 10.1242/dev.126.4.683. [DOI] [PubMed] [Google Scholar]
- Gashegu J, Vanmuylder N, Kassengera Z, et al. L’expression de la caspase 3 et la protéine p53 au cours de l’apoptose physiologique et induite par 3 agents tératogènes au cours du développement crânio-facial précoce de l’embryon de souris. Morphologie. 2005;89:82–89. doi: 10.1016/s1286-0115(05)83243-6. [DOI] [PubMed] [Google Scholar]
- Gashegu J, Vanmuylder N, Philippson C, Choa-Duterre M, Rooze M, Louryan S. Correlation of HSP110 expression with caspase-3 and -9 during apoptosis induced by in vivo embryonic exposition to retinoic acid or irradiation in early mouse cranio-facial development. Orthod Craniofacial Res. 2006;9:84–92. doi: 10.1111/j.1601-6343.2006.00361.x. [DOI] [PubMed] [Google Scholar]
- Glineur R, Louryan S, Philippson C, Evrard L, de Vos L. Effects of irradiation on facial development in mouse embryos. Eur J Morph. 1998;36:245–252. doi: 10.1076/ejom.36.4.245.5816. [DOI] [PubMed] [Google Scholar]
- Glineur R, Louryan S, Lemaître A, Evrard L, Rooze M, De Vos L. Mouse craniofacial abnormalities induced by retinoic acid methyl-triazene. Surg Radiol Anat. 1999;21:41–47. doi: 10.1007/BF01635051. [DOI] [PubMed] [Google Scholar]
- Gogvadze V, Orrenius S, Zhivotovsky B. Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim Biophys Acta. 2006;1757:639–647. doi: 10.1016/j.bbabio.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Guo Y, Srinivasula SM, Druilhe A, Fernandes-Alnemri T, Alnemri ES. Caspase-2 induces apoptosis by releasing proapoptotic proteins from mitochondria. J Biol Chem. 2002;277:13430–13437. doi: 10.1074/jbc.M108029200. [DOI] [PubMed] [Google Scholar]
- Haken R, Haken A, Duncan SG, et al. Differential requirement for caspase 9 in apoptotic pathways in vivo. Cell. 1998;95:339–352. doi: 10.1016/s0092-8674(00)81477-4. [DOI] [PubMed] [Google Scholar]
- Holson RR, Gazzara RA, Ferguson SA, Ali SF, Laborde JB, Adams J. Gestational retinoic acid exposure: a sensitive periode for effects on neonatal mortality and cerebellar development. Neurotox Teratol. 1997;19:335–346. doi: 10.1016/s0892-0362(97)00039-1. [DOI] [PubMed] [Google Scholar]
- Hyatt GA, Schmitt EA, Marsh-Armstrong N, MacCaffery P, Dräger UC, Dowling JE. Retinoic acid establishes ventral retinal characteristics. Development. 1996;122:195–204. doi: 10.1242/dev.122.1.195. [DOI] [PubMed] [Google Scholar]
- Kawabata K, Maeda S, Takanaga A, Ito H, Tanaka K, Hayakawa T, Seki M. Apoptosis and retinal projections in dorsal lateral geniculate nucleus after monocular deprivation during later phase of critical period in rat. Anat Sci Int. 2003;78:104–110. doi: 10.1046/j.0022-7722.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- Kojima M, Asahi M, Kikuchi H, Hashimoto N, Noda M, Hoshimaru M. Expression of Nedd2/ICH-1 (caspase-2) in developing rat retina. Neurosci Res. 1998;31:211–217. doi: 10.1016/s0168-0102(98)00039-x. [DOI] [PubMed] [Google Scholar]
- Kuida K, Zheng ST, Na S, et al. Decreased apoptosis in the brain and premature lethality in CPP32-deficient mice. Nature. 1996;384:368–375. doi: 10.1038/384368a0. [DOI] [PubMed] [Google Scholar]
- Kuida K, Hydar TF, Kuan CY, et al. Reduced apoptosis and cytochrome c-mediated caspase activation in mice lacking caspase 9. Cell. 1998;94:325–337. doi: 10.1016/s0092-8674(00)81476-2. [DOI] [PubMed] [Google Scholar]
- Laemle LK, Puszkarczuk M, Feinberg RN. Apoptosis in early ocular morphogenesis in mouse. Dev Brain Res. 1999;112:129–133. doi: 10.1016/s0165-3806(98)00153-9. [DOI] [PubMed] [Google Scholar]
- Linden R, Martins RA, Silveira MS. Control of programmed cell death by neurotransmitters and neuropeptides in developing mammalian retina. Prog Retin Eye Res. 2005;24:457–491. doi: 10.1016/j.preteyeres.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Louryan S, Glineur R, Tainmont S, Vandam P. Tératogénicité de l’acide 13-cis rétinoïque sur les ébauches mandibulo-otiques de l’embryon de souris: approche histologique et histochimique. Bull GIRSO. 1990;33:147–153. [PubMed] [Google Scholar]
- MacCaffery P, Lee MO, Wagner MA, Sladek NE, Dräger UC. Asymmetrical retinoic acid synthesis in dorsoventral axis of retina. Development. 1992;115:371–382. doi: 10.1242/dev.115.2.371. [DOI] [PubMed] [Google Scholar]
- Marsh-Armstrong N, MacCaffery P, Gilbert W, Dowling JE, Dräger UC. Retinoic acid is necessary for development of ventral retina in zebrafish. Proc Natl Acad Sci USA. 1994;91:7286–7290. doi: 10.1073/pnas.91.15.7286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier P, Finch A, Evan G. Apoptosis in development. Nature. 2000;407:796–801. doi: 10.1038/35037734. [DOI] [PubMed] [Google Scholar]
- Mulder BG, Maneley N, Grant J, et al. Effects of excess vitamin A on development of cranial neural crest-derived structures: a neonatal and embryologic study. Teratology. 2000;61:297–304. doi: 10.1002/1096-9926(200010)62:4<214::AID-TERA7>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- Sanders EJ, Parker E, Aramburo C, Harvey S. Retinal growth hormone is an anti-apoptotic factor in embryonic retinal ganglion cell differentiation. Exp Eye Res. 2005;81:551–560. doi: 10.1016/j.exer.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Scaffidi C, Fulda S, Srinivasan A, et al. Two CD95 (APO1/Fas) signaling pathways. EMBO J. 1998;17:1675–1687. doi: 10.1093/emboj/17.6.1675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schirmer EC, Glover JR, Singer MA, Lindquist S. HSP100/Clp proteins: a common mechanism explains diverse functions. Trends Biochem Sci. 1996;21:289–296. [PubMed] [Google Scholar]
- Seth R, Yang C, Kaushal V, Shah SV, Kaushal GP. p53-dependant caspase-2 activation in mitochondrial release of apoptosis-inducing factor and its role in tubular epithelial cell injury. J Biol Chem. 2005;280:31230–31239. doi: 10.1074/jbc.M503305200. [DOI] [PubMed] [Google Scholar]
- Singh M, Savitz SI, Hoque R, et al. Cell-specific caspase expression by different neuronal phenotypes in transient retinal ischemia. J Neuroch. 2001;77:466–475. doi: 10.1046/j.1471-4159.2001.00258.x. [DOI] [PubMed] [Google Scholar]
- Sreedhar SA, Csermely P. Heat shock proteins in regulation of apoptosis: new strategies in tumor therapy, a comprehensive review. Pharmacol Therap. 2004;101:227–257. doi: 10.1016/j.pharmthera.2003.11.004. [DOI] [PubMed] [Google Scholar]
- Sulik KK, Johnston MC, Smiley JS, Speight SH, Jarvis EB. Mandibulofacial dysostosis (Treacher–Collins syndrome): a new proposal for its pathogenesis. Am J Med Genet. 1987;27:359–372. doi: 10.1002/ajmg.1320270214. [DOI] [PubMed] [Google Scholar]
- Susin SA, Lorenzo HK, Zamzami N, et al. Mitochondrial release of caspase-2 and -9 during apoptotic process. J Exp Med. 1999;189:381–393. doi: 10.1084/jem.189.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiler K. The House MouseAtlas of Embryonic Development. Berlin: Springer; 1987. [Google Scholar]
- Umpierre CC, Little SA, Mirkes PE. Co-localisation of active caspase-3 and DNA fragmentation (TUNEL) in normal and hyperthermia-induced abdominal mouse development. Teratology. 2001;63:134–143. doi: 10.1002/tera.1024. [DOI] [PubMed] [Google Scholar]
- Vandna P, Anmol C, Tayashree CJ, Sudheer KP, Padma S. ROS-triggered caspase-2 activation and feedback amplification loop in β-carotene-induced apoptosis. Free Radic Biol Med. 2006;41:431–442. doi: 10.1016/j.freeradbiomed.2006.03.009. [DOI] [PubMed] [Google Scholar]
- Vanmuylder N, Evrard L, Dourov N. Strong expression of heat shock proteins in growth plate cartilage, an immunohistochemical study of HSP28, HSP70, HSP110. Anat Embryol. 1997;195:359–362. doi: 10.1007/s004290050056. [DOI] [PubMed] [Google Scholar]
- Vanmuylder N, Evrard L, Glineur R, et al. Aspects histologiques et ultrastructuraux de la mort cellulaire programme et induite par deux agents tératogènes dans les arcs viscéraux de l’embryon de souris. Rev Méd Brux. 2004;25:14–20. [PubMed] [Google Scholar]
- Varfolomeev EE, Schuchmann M, Luria V, et al. Targeted disruption of the mouse caspase 8 gene ablates cell death induction by TNF receptors, Fas/Apo1, and DR3 and lethal prenatally. Immunity. 1998;9:267–276. doi: 10.1016/s1074-7613(00)80609-3. [DOI] [PubMed] [Google Scholar]
- Wagner E, MacCaffery P, Dräger UC. Retinoic acid in the formation of the dorsoventral retina and its central connections. Dev Biol. 2000;222:460–470. doi: 10.1006/dbio.2000.9719. [DOI] [PubMed] [Google Scholar]
- Wang X. The expanding role of mitochondria in apoptosis. Genes Dev. 2001;15:2922–2933. [PubMed] [Google Scholar]
- Yamagishi N, Saito Y, Ishahara K, Hatayama T. Enhancement of oxidative stress-induced apoptosis by hsp105α in mouse embryonal F9 cells. Eur J Biochem. 2002;269:4143–4151. doi: 10.1046/j.1432-1033.2002.03109.x. [DOI] [PubMed] [Google Scholar]
- Zakeri Z, Lockshin RA. Cell death in development. J Immunol Meth. 2002;265:3–20. doi: 10.1016/s0022-1759(02)00067-4. [DOI] [PubMed] [Google Scholar]
- Zeiss JC, Neal J, Johnson EA. Caspase-3 in postnatal retinal development and degeneration. Invest Ophthalmol Vis Sci. 2004;45:964–970. doi: 10.1167/iovs.03-0439. [DOI] [PubMed] [Google Scholar]
- Zheng TS, Hunot S, Kuida K, Momoi T, Srinivasan A, Nicholson DW. Deficiency in caspase-9 or caspase-3 induces compensatory caspase activation. Nat Med. 2000;6:1241–1247. doi: 10.1038/81343. [DOI] [PubMed] [Google Scholar]
- Zhivotovsky B, Orrenius S. Caspase-2 function in response to DNA damage. Biochem Biophys Res Commun. 2005;331:859–867. doi: 10.1016/j.bbrc.2005.03.191. [DOI] [PubMed] [Google Scholar]