Abstract
Changes in glomerular volume (Vglom) play an important role in the initiation and progression of various glomerulopathies. Estimation of Vglom in the normal kidney provides baseline values for studies of glomerular hypertrophy in disease. The traditional model-based method of Weibel and Gomez is widely applied to estimate Vglom in clinical biopsy specimens. Assumptions of glomerular size distribution and shape required by this method are potential sources of bias that have not been verified. We evaluated the applicability of the glomerular size distribution coefficient in estimating Vglom in human kidneys. Vglom of 720 non-sclerotic glomeruli in histologically normal kidneys of 24 males (20–69 years) was estimated by the unbiased disector/Cavalieri approach. Accurate glomerular diameters were calculated from Cavalieri estimates of Vglom assuming glomerular sphericity. The coefficients of variation (CV) of glomerular diameters were compared with the corresponding values of the size distribution coefficient predicted by the Weibel and Gomez method. Mean (SD) glomerular diameter was 201 (28) mm (range 110–276 mm). The CV of glomerular diameter within each kidney ranged from 4.9 to 14.6%. Corresponding glomerular size distribution coefficients predicted by the formula of Weibel and Gomez ranged from 1.00 to just 1.03. The value of the size distribution coefficient required by the Weibel and Gomez technique when estimating Vglom in normal human kidneys is remarkably constant. This is despite large variations in Vglom. Future studies should examine the extent of bias introduced by the glomerular shape assumptions of this method.
Keywords: glomerular diameters, glomerular profiles, human kidney, stereology
Introduction
Accurate estimation of glomerular volume (Vglom) has become increasingly important because Vglom varies significantly in normal human kidneys (Samuel et al. 2005), and because alterations in Vglom may play a significant pathophysiological role in the development or progression of a range of nephropathies (Bilous et al. 1989; Keller et al. 2003). Glomerular enlargement precedes glomerulosclerosis in idiopathic focal segmental glomerulosclerosis (FSGS), diabetic nephropathy, obesity-related glomerulopathy, HIV nephropathy, reflux nephropathy, pre-eclampsia, sickle cell nephropathy and transplant glomerulopathy (El-Khatib et al. 1987; Fogo et al. 1990; Pardo et al. 1991; Bathena, 1993; Nochy et al. 1994; Bertram et al. 1998; Praga et al. 2001). Furthermore, changes in Vglom that occur with different degrees of glomerulosclerosis in conditions characterized by FSGS may be of prognostic significance (Bertram et al. 1998; Fogo et al. 1990). Vglom can be reduced in ischaemic renal injury and may be associated with long-term passive smoking (Moran et al. 1992; Dundar et al. 2004).
Unbiased design-based stereological methods are currently the preferred approach for estimation of Vglom (Bertram, 1995; Madsen, 1999). However, the model-based stereological method of Weibel and Gomez (Weibel, 1980), which originally attempted to estimate glomerular number based on the relationship between volume and mean cross-section area – which depend on glomerular shape and a size distribution factor, is still widely applied to measure Vglom (Bertram et al. 1998). This method requires only a single random section through each glomerulus and is therefore well suited to clinical biopsy specimens with a limited number of glomerular profiles. The Weibel and Gomez method is also more time efficient over the ‘gold standard’ unbiased Cavalieri principle (Gundersen & Jensen, 1987; Bertram, 1995).
The Cavalieri method requires exhaustive sectioning of the glomerulus in large kidney tissue samples and requires sizing glomerular profiles in consecutive serial sections of complete glomeruli (Samuel et al. 2005). Unlike the Cavalieri principle, the method of Weibel and Gomez (Weibel, 1980) requires knowledge or assumptions about the shape and size of glomeruli. To the extent that these assumptions differ from true values, estimates of Vglom will be biased. According to the Weibel and Gomez formula, Vglom = glomerular profile area1.5 × β/K, where β is a shape coefficient of 1.38 for a sphere and K is a size distribution coefficient. Values for K vary between 1 and 1.05 for glomerular size distributions with standard deviations of less than 20% of the mean within a single specimen of kidney tissue. It is well known that glomeruli vary in size and shape within the same kidney (Samuel et al. 2005), and therefore any bias introduced in the estimation of Vglom using the method of Weibel and Gomez needs to be determined.
The aim of this investigation was to assess the applicability of the Weibel and Gomez glomerular size distribution coefficient K, when estimating Vglom within normal human kidneys.
Materials and methods
The study population consisted of 24 North American males (12 African Americans and 12 Caucasians) aged 20–69 years who had died suddenly of non-renal causes (Samuel et al. 2005). Post-mortem kidney specimens were collected at the University of Mississippi Medical Center (Jackson, MS). Ethical approval for the use of autopsy tissue for clinical research was obtained by informed consent from the first of kin and approved by the Internal Review Board of the University of Mississippi Medical Center. Exclusion criteria included a history of kidney disease, significant size asymmetry between the right and left kidney, and histologically proven glomerular or tubulointerstitial disease. Details of tissue processing and estimates of individual glomerular volumes have previously been reported (Samuel et al. 2005). Briefly, one mid-sagittal half of each perfusion-fixed right kidney was selected randomly for analysis. A block of tissue was cut from the mid-hilar region of the kidney, embedded in glycolmethacrylate (Technovit 7100, Heraeus Kulzer Gmbh, Germany) and serially sectioned at 10 µm. Glycolmethacrylate was preferred to paraffin as the embedding medium because the dimensional changes associated with tissue processing for glycolmethacrylate embedding are significantly smaller than those associated with paraffin embedding (Bertram, 1995). Glomeruli were sampled with equal probability regardless of their shape or size using disectors (Sterio, 1984; Bertram, 1995). Thirty non-sclerotic glomeruli were sampled per kidney. Each glomerulus was exhaustively sectioned at a known section thickness, t (10 µm). The glomerular profile tuft area of every second section was measured by the point counting method of Henning (Henning & Meyer-Arendt, 1963) using an orthogonal grid system with points 1 cm apart at a final magnification of ×320. On average 12 sections were measured from each glomerulus.
The area of each glomerular profile (Aglom) was determined using Aglom = ∑ P × a(p), where ∑ P was the number of points hitting the glomerular profile and a(p) was the area associated with each test point on the grid. Glomerular tuft volume was estimated using the Cavalieri principle: Vglom = ∑ Aglom × 2(t). The radius (r) and diameter of each of the 30 sampled glomeruli per kidney were calculated from Vglom = 4/3 × πr3, assuming glomerular sphericity. The mean diameter (d) of the 30 glomeruli and the standard deviation (SD) of the diameter were used to calculate the coefficient of variation (CV) of glomerular diameter per kidney, CV = SD/d. The calculated values for CV were compared with the corresponding values for K predicted by the Weibel and Gomez method (Weibel, 1979).
Results
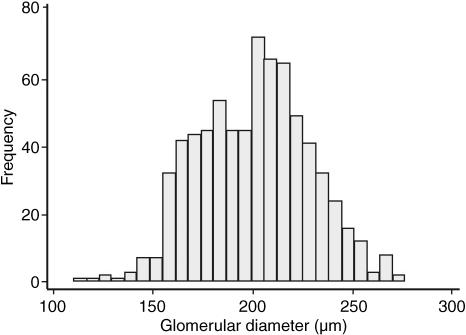
The diameters of each of 30 non-sclerotic glomeruli per kidney were calculated from previous Cavalieri estimates of Vglom in 24 male subjects (Samuel et al. 2005). The frequency distribution of the diameters of a total of 720 glomeruli is shown in Fig. 1. Glomeruli were of varying diameters with a 2.5-fold range. The diameter of the largest glomerulus was 276 µm and the smallest 110 µm. Mean (± SD) glomerular diameter was 201 ± 28 µm.
Fig. 1.
Distribution of diameters of 720 non-sclerotic glomeruli in 24 males aged 20–69 years. The normal distribution was confirmed using the Shapiro Wilk normality test.
The CV of glomerular diameter within each kidney was less than 15% with a three-fold range from 4.9 to 14.6% (Table 1). However, the corresponding glomerular size distribution coefficients (K) predicted by the formula of Weibel and Gomez (Weibel, 1980) were quite constant, ranging from 1.00 to 1.03 (Table 1).
Table 1.
Glomerular diameters, coefficients of variation (CV) of glomerular diameters derived from Cavalieri estimates of glomerular volume, and the Weibel and Gomez glomerular size distribution constant (K) in kidneys of 24 males aged 20–69 years
| Subject | Age (years) | Race | No. of glomeruli analysed | Minimum glomerular diameter (µm) | Maximum glomerular diameter (µm) | Mean (SD) glomerular diameter (µm) | CV of glomerular diameter CV (%) | Size distribution coefficient (K)* |
|---|---|---|---|---|---|---|---|---|
| 1 | 20 | African American | 30 | 153 | 201 | 172 (11) | 6.6 | 1.00 |
| 2 | 21 | African American | 30 | 138 | 205 | 176 (17) | 9.6 | 1.00 |
| 3 | 21 | Caucasian | 30 | 195 | 250 | 219 (13) | 5.7 | 1.00 |
| 4 | 22 | African American | 30 | 188 | 231 | 207 (10) | 4.9 | 1.00 |
| 5 | 22 | Caucasian | 30 | 149 | 205 | 178 (13) | 7 | 1.00 |
| 6 | 22 | Caucasian | 30 | 155 | 202 | 176 (12) | 6.6 | 1.00 |
| 7 | 25 | African American | 30 | 160 | 252 | 208 (22) | 10.7 | 1.01 |
| 8 | 28 | African American | 30 | 127 | 248 | 209 (31) | 14.6 | 1.03 |
| 9 | 29 | African American | 30 | 184 | 268 | 221 (22) | 9.8 | 1.01 |
| 10 | 29 | Caucasian | 30 | 136 | 193 | 163 (10) | 6.1 | 1.00 |
| 11 | 30 | Caucasian | 30 | 132 | 219 | 191 (19) | 9.9 | 1.01 |
| 12 | 30 | Caucasian | 30 | 149 | 276 | 221 (26) | 11.8 | 1.02 |
| 13 | 51 | African American | 30 | 163 | 237 | 218 (14) | 6.5 | 1.00 |
| 14 | 51 | African American | 30 | 184 | 268 | 214 (16) | 7.6 | 1.00 |
| 15 | 51 | African American | 30 | 165 | 249 | 224 (22) | 10 | 1.01 |
| 16 | 56 | African American | 30 | 175 | 275 | 237 (28) | 12 | 1.02 |
| 17 | 61 | African American | 30 | 158 | 265 | 229 (21) | 9.4 | 1.00 |
| 18 | 63 | Caucasian | 30 | 159 | 220 | 181 (14) | 7.6 | 1.00 |
| 19 | 65 | African American | 30 | 156 | 224 | 186 (18) | 9.8 | 1.01 |
| 20 | 65 | Caucasian | 30 | 163 | 244 | 202 (17) | 8.2 | 1.00 |
| 21 | 67 | Caucasian | 30 | 127 | 237 | 204 (23) | 11.2 | 1.01 |
| 22 | 67 | Caucasian | 30 | 110 | 209 | 177 (23) | 13 | 1.02 |
| 23 | 68 | Caucasian | 30 | 159 | 227 | 193 (18) | 9.6 | 1.00 |
| 24 | 69 | Caucasian | 30 | 142 | 246 | 213 (19) | 9 | 1.00 |
| Mean (SD) | 43 (19.3) | 201 (28) | 9.1 |
Values of K for a given value of CV were obtained from the estimates of Weibel and Gomez.
Discussion
The development of unbiased design-based stereological techniques over the past 20 years has revolutionized the quantification of three-dimensional cell, tissue and organ structure (Nyengaard, 1999; Gundersen et al. 1988a,b; Bertram et al. 1992; Bertram, 1995). Unfortunately, these methods are not suitable for estimation of glomerular dimensions in limited clinical core biopsy specimens. However, the unbiased techniques provide a mechanism whereby traditional model-based and potentially biased stereological methods that are more practical in clinical application, such as the method of Weibel and Gomez (Weibel, 1980), can be validated. In the present study, we utilized the gold standard disector/Cavalieri combination, to assess the applicability of the Weibel and Gomez method to estimate Vglom in normal human kidneys.
‘True’ glomerular diameters were derived using estimates of Vglom obtained with the unbiased Cavalieri method in glomeruli sampled with the disector method and embedded in glycolmethacrylate. This approach overcame several potential sources of bias and error associated with alternative methods for estimating glomerular diameter. First, all glomeruli had the same chance of being included in the sample, because of the use of the disector sampling approach. This overcame the problem of preferentially sampling large glomeruli. Second, there was no need to estimate diameter in maximal glomerular profiles. And finally, tissue shrinkage and deformations were minimized with the use of glycolmethacrylate as the embedding medium.
In a previous study from our laboratory (Bertram et al. 1998) the method of Weibel and Gomez was found to overestimate mean Vglom of 17 initial transplant biopsy specimens by 23% compared with Cavalieri estimates based on 238 glomeruli. Paraffin sections were analysed in this earlier study. It should be noted that the use of glycolmethacrylate as the embedding medium in the present study of a larger sample of 720 glomeruli very likely contributed to the good agreement between the CV of glomerular diameters derived from the Cavalieri principle and the Weibel and Gomez estimates of glomerular size distribution. Tissue embedded in glycolmethacrylate undergoes far less shrinkage and distortion than tissue processed for embedding in paraffin (Miller & Meyer, 1990).
Another critical parameter to consider in studies of this kind is section thickness. Macleod et al. (2000) demonstrated that when using the Cavalieri principle, section intervals greater than 20 µm could result in a significant increase in the variance of the estimate of Vglom in biopsies of diabetics. This is particularly relevant as various disease processes can distort both glomerular size and shape, thereby creating a different distribution of glomerular size. Glomeruli deviate from the shape of a sphere and can be ellipsoidal even in the normal kidney (Abrams et al. 1963).
We have recently addressed the other important consideration of how many glomerular profiles must be measured to obtain reliable estimates of mean glomerular areas in human renal biopsies and found that estimates based on random sampling of five or more glomerular profiles per biopsy reliably estimated the ‘true’ population mean of a group of at least 30 biopsies (Hoy et al. 2006). The remaining challenge is to determine the values of the glomerular shape coefficient (β) of the Weibel and Gomez approach to estimation of Vglom in normal and diseased human kidneys.
In conclusion, the present findings indicate the remarkable stability of the values of the size distribution coefficient (K) when estimating glomerular volume in normal human kidneys using the method of Weibel and Gomez (Weibel, 1980).
Acknowledgments
We would like to thank emeritus Professor Ewald Weibel for his constructive comments regarding this manuscript.
References
- Abrams RL, Lipkin LE, Hennigar GR. A quantitative estimation of variation among human renal glomeruli. Lab Invest. 1963;12:69–76. [PubMed] [Google Scholar]
- Bathena DB. Glomerular size and the association of focal glomerulosclerosis in long-surviving human renal allografts. J Am Soc Nephrol. 1993;4:1316–1326. doi: 10.1681/ASN.V461316. [DOI] [PubMed] [Google Scholar]
- Bertram JF, Soosaipillai MC, Ricardo SD, Ryan GB. Total numbers of glomeruli and individual cell types in the normal rat kidney. Cell Tissue Res. 1992;270:37–45. doi: 10.1007/BF00381877. [DOI] [PubMed] [Google Scholar]
- Bertram JF. Analyzing renal glomeruli with the new stereology. Int Rev Cytol. 1995;161:111–172. doi: 10.1016/s0074-7696(08)62497-3. [DOI] [PubMed] [Google Scholar]
- Bertram JF, Young RJ, Seymour AE, Kincaid-Smith P, Hoy W. Glomerulomegaly in Australian Aborigines. Nephrology. 1998;4:S46–S53. [Google Scholar]
- Bilous RW, Mauer SM, Sutherland SER, Steffes MW. Mean glomerular volume and rate of development of diabetic nephropathy. Diabetes. 1989;38:1142–1147. doi: 10.2337/diab.38.9.1142. [DOI] [PubMed] [Google Scholar]
- Dundar M, Kocak I, Culhaci N. Effects of long-term passive smoking on the diameter of glomeruli in rats: histopathological evaluation. Nephrology. 2004;9:53–57. doi: 10.1111/j.1440-1797.2003.00231.x. [DOI] [PubMed] [Google Scholar]
- El-Khatib MT, Becker GJ, Kincaid-Smith PS. Morphological aspects of reflux nephropathy. Kidney Int. 1987;32:261–266. doi: 10.1038/ki.1987.201. [DOI] [PubMed] [Google Scholar]
- Fogo A, Hawkins EP, Berry PL, et al. Glomerular hypertrophy in minimal change disease predicts subsequent progression to focal glomerular sclerosis. Kidney Int. 1990;38:115–123. doi: 10.1038/ki.1990.175. [DOI] [PubMed] [Google Scholar]
- Gundersen HJG, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Gundersen HJG, Bagger P, Bendtsen TF, et al. The new stereological tools: disector, fractionator, nucleator and point sampled intercepts and their use in pathological research and diagnosis. Acta Pathol Microbiol Immunol Scand. 1988a;96:857–881. doi: 10.1111/j.1699-0463.1988.tb00954.x. [DOI] [PubMed] [Google Scholar]
- Gundersen HJG, Bendtsen TF, Evans SM, et al. Some new, simple and efficient stereological methods and their use in pathological research and diagnosis. Acta Pathol Microbiol Immunol Scand. 1988b;96:379–394. doi: 10.1111/j.1699-0463.1988.tb05320.x. [DOI] [PubMed] [Google Scholar]
- Henning A, Meyer-Arendt JR. Microscopic volume determination and probability. Lab Invest. 1963;12:460–464. [PubMed] [Google Scholar]
- Hoy WE, Samuel T, Hughson MD, Nicol JL, Bertram JF. How many glomerular profiles must be measured to obtain reliable estimates of mean glomerular areas in human renal biopsies. J Am Soc Nephrol. 2006;17:556–563. doi: 10.1681/ASN.2005070772. [DOI] [PubMed] [Google Scholar]
- Keller G, Zimmer G, Mall G, Ritz E, Amann K. Nephron number in patients with primary hypertension. N Engl J Med. 2003;348:101–108. doi: 10.1056/NEJMoa020549. [DOI] [PubMed] [Google Scholar]
- MacLeod JM, White KE, Tate H, Bilous RW. Measurement of glomerular volume in needle biopsy specimens. Nephrol Dial Transplant. 2000;15:239–243. doi: 10.1093/ndt/15.2.239. [DOI] [PubMed] [Google Scholar]
- Madsen KM. The art of counting. J Am Soc Nephrol. 1999;10:1121–1125. doi: 10.1681/ASN.V1051124. [DOI] [PubMed] [Google Scholar]
- Miller PL, Meyer TW. Effects of tissue preparation on glomerular volume and capillary structure in the rat. Lab Invest. 1990;63:862–886. [PubMed] [Google Scholar]
- Moran K, Mulhall J, Kelly D, et al. Morphological changes and alterations in regional intrarenal blood flow induced by graded ishaemia. J Urol. 1992;148:463–466. doi: 10.1016/s0022-5347(17)36629-6. [DOI] [PubMed] [Google Scholar]
- Nochy D, Heudes D, Glotz D, et al. Pre-eclampsia associated focal segmental glomerulosclerosis and glomerular hypertrophy; a morphometric analysis. Clin Nephrol. 1994;42:9–17. [PubMed] [Google Scholar]
- Nyengaard JR. Stereologic methods and their application to kidney research. J Am Soc Nephrol. 1999;10:1100–1123. doi: 10.1681/ASN.V1051100. [DOI] [PubMed] [Google Scholar]
- Pardo V, Howard C, Bell M, Longone AC, Hernandez I, Strauss J. Hypertophic glomeruli in AIDS: possible relationship to HIV-associated glomerulosclerosis. Lab Invest. 1991;64:98. (abstract) [Google Scholar]
- Praga M, Hernandez E, Morales E. Clinical features and long-term outcome of obesity-associated focal segmental glomerulosclerosis. Nephrol Dial Transplant. 2001;16:1790–1798. doi: 10.1093/ndt/16.9.1790. [DOI] [PubMed] [Google Scholar]
- Samuel T, Hoy WE, Douglas-Denton R, Hughson MD, Bertram JF. Determinants of glomerular volume in different cortical zones of the human kidney. J Am Soc Nephrol. 2005;16:3102–3109. doi: 10.1681/ASN.2005010123. [DOI] [PubMed] [Google Scholar]
- Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc. 1984;134:127–136. doi: 10.1111/j.1365-2818.1984.tb02501.x. [DOI] [PubMed] [Google Scholar]
- Weibel ER. Elementary introduction to stereological principles. In: Weibel ER, editor. Stereological Methods, Practical Methods for Biological Morphometry. Vol. 1. London: Academic Press; 1979. pp. 44–45. [Google Scholar]
- Weibel ER. Numerical density: shape and size of particles. In: Weibel ER, editor. Stereological Methods, Vol. 2 Theoretical Foundations. London: Academic Press; 1980. pp. 149–152. [Google Scholar]