Abstract
The distribution of perineuronal nets and the potassium channel subunit Kv3.1b was studied in the subdivisions of the cochlear nucleus, the medial nucleus of the trapezoid body, the medial and lateral superior olivary nuclei, the lateral lemniscal nucleus and the inferior colliculus of the rhesus monkey. Additional sections were used for receptor autoradiography to visualize the patterns of GABAA and GABAB receptor distribution. The Kv3.1b protein and perineuronal nets [visualized as Wisteria floribunda agglutinin (WFA) binding] were revealed, showing corresponding region-specific patterns of distribution. There was a gradient of labelled perineuronal nets which corresponded to that seen for the intensity of Kv3.1b expression. In the cochlear nucleus intensely and faintly stained perineuronal nets were intermingled, whereas in the medial nucleus of the trapezoid body the pattern changed to intensely stained perineuronal nets in the medial part and weakly labelled nets in its lateral part. In the inferior colliculus, intensely labelled perineuronal nets were arranged in clusters and faintly labelled nets were arranged in sheets. Using receptor autoradiography, GABAB receptor expression in the anterior ventral cochlear nucleus was revealed. The medial part of the medial nucleus of the trapezoid body showed a high number of GABAA binding sites whereas the lateral part exhibited more binding sites for GABAB. In the inferior colliculus, we found moderate GABAB receptor expression. In conclusion, intensely WFA-labelled structures are those known to be functionally involved in high-frequency processing.
Keywords: GABA receptors, lectin, potassium channel
Introduction
The auditory brainstem of most mammals consists of two components, one devoted to higher-frequency hearing and the other to lower frequencies (Heffner & Masterton, 1990). The distribution patterns of higher- and lower-frequency subnuclei or subsets of neurons may provide evidence for certain phylogenetic traits in primates (Bazwinsky et al. 2003, 2005). Previous comparative studies have indicated that a well-developed medial nucleus of the trapezoid body (MNTB) seems to be associated with auditory high-frequency processing, whereas a lack of MNTB in humans seems to be related to the processing of lower frequencies (Richter et al. 1983; Bazwinsky et al. 2003). In the rat, the MNTB is characterized by the presence of perineuronal nets and the potassium channel subunit Kv3.1b (Härtig et al. 2001). Macica et al. (2003) provided evidence that a loss of the Kv3.1b gene in mice results in the failure of the neurons to respond to high-frequency stimulation. Song et al. (2005) used computational modelling to show that high levels of Kv3.1b currents enable neurons to follow high-frequency stimuli. The authors stated that their ‘results indicate that the intrinsic electrical properties of auditory neurons are rapidly modified to adjust to the ambient acoustic environment’. Härtig et al. (1999) and Brückner et al. (2006) revealed that perineuronal nets contribute to electrophysiological properties of different cell domains. This suggests that the expression of perineuronal nets and Kv3.1b-containing potassium channels may be characteristic markers for auditory neuronal circuitries involved in high-frequency processing within the auditory pathways. In order to address this hypothesis, we studied the distribution of perineuronal nets and Kv3.1b expression in the subdivisions of the cochlear nucleus (CN), in the superior olivary complex (SOC), consisting of the lateral and medial olivary nuclei (MSO, LSO), and in the MNTB, in the lateral lemniscal nucleus (LLN) and in the inferior colliculus (IC) of the rhesus monkey. In addition, we provide first results for a characteristic, region-specific presence of GABAA and GABAB receptors as revealed by in vitro autoradiography.
Materials and methods
Tissue processing and sectioning
All procedures were approved by the local animal welfare legislation and were in accordance with the European Communities Council Directive of 24 November 1986 (86/609/EEC).
Six brains of adult female Macaca fascicularis (3 years old) were obtained from Covance, Münster, Germany. The brainstems were fixed in a mixture of 4% paraformaldehyde and 15% saturated picric acid in sodium phosphate buffer, pH 7.4 (Zamboni's fixative) at 4 °C under constant stirring for at least 1 week prior to further processing. The specimens were dissected and sectioned after clearing the arachnoid and blood vessels from the surface. Each specimen was cut into blocks of 1 cm thickness in the frontal plane. The blocks were cryoprotected by immersion in 0.1 m phosphate-buffered saline (PBS, pH 7.4) containing 15% sucrose for 1 day, followed by 30% sucrose in PBS at 4 °C for a further day. Frozen sections (50 µm) were cut on a cryotome. Wisteria floribunda agglutinin (WFA) histochemistry was used to reveal perineuronal nets and immunohistochemistry for Kv3.1b protein was performed on serially cut sections as detailed below. Certain sections were double-stained with the lectin and antibody directed toward Kv3.1b to reveal a correlation of those markers.
WFA histochemistry
Sections were treated free-floating with 0.6% H2O2 in PBS for 20 min. After two rinses with PBS followed by two rinses with 0.05 m Tris-HCl + 0.9% NaCl (TBS) they were incubated with biotinylated WFA (b-WFA, Sigma L-1766, Munich, Germany) at a concentration of 10 µg b-WFA mL−1 TBS containing 2% bovine serum albumin (TBS-BSA) overnight at 4 °C. Sections were rinsed four times (15 min each) with TBS and further incubated for 1 h in Extravidin-peroxidase (Sigma-Immunochemicals). They were rinsed twice in TBS followed by two rinses in TB (pH 7.6). The visualization of the lectin-binding sites was performed with diaminobenzidine (DAB/H2O2) as detailed by Hilbig et al. (2001). After several rinses with TB the sections were mounted onto glass slides, air-dried and coverslipped with Entellan (Merck, Heidelberg, Germany). For fluorescence microscopy the sections were incubated in steptavidin-Cy3 (20 µg mL−1; Dianova, Hamburg, Germany), rinsed, and double stained immunohistochemically or dried and coverslipped.
Immunohistochemistry
Sections were incubated free-floating as described for the WFA labelling. After blocking with normal horse serum, the primary antibody against Kv3.1b (diluted 1 : 1000; DAKO, Cologne, Germany) was used. For visualization the Vectastain ABC-kit was used. Fluorescence immunoreactivities were visualized by means of Cy3-conjugated goat anti-mouse antibody (Fab-fragment, diluted 1 : 150; Dianova).
Control sections were treated similarly, but using a non-specific mouse IgG1 (DAKO) instead of primary antibodies. Sections were studied by light or fluorescence microscopy with an ‘Axiophot’ photomicroscope (Zeiss, Jena, Germany) equipped with epifluorescence. The confocal laser scanning microscope TCS 4D (Leica, Germany) was used for visualization of cellular details in the fluorescence sections.
Receptor autoradiography
Additional unfixed brainstems from six Macaca fascicularis were frozen in isopentane (Merck) cooled over dry-ice. Frozen sections of 20 µm were cut and mounted onto glass slides which were then processed for either Nissl staining or in vitro receptor autoradiography according to established protocols for GABAA using [3H]muscimol (6 nm; PerkinElmer, Rodgau, Germany) in 50 mm Tris-citrate buffer (pH 7.0) for 40 min at 4 °C. γ-Aminobutric acid (10 µm) was used as competitor as outlined in detail by Zilles et al. (2002). GABAB-receptor binding was visualized by [3H]CGP 54626 (1.5 nm; Tocris-Cookson, Bristol, UK) binding in Tris-HCl buffer (pH 7.2) plus 2.5 mm CaCl2 for 60 min at 4 °C in the presence of or without CGP 55845 (100 µm) as a competitor and processed and evaluated exactly as described previously (Zilles et al. 2002).
Results
We found a strong and consistent co-localization of the Kv3.1b protein and WFA binding sites in the CN, the SOC, the LLN and the IC of the rhesus monkey brainstem. Additionally, differences in WFA staining intensity partially reflected regional differences in the distribution patterns of GABAA and/or GABAB receptors.
CN
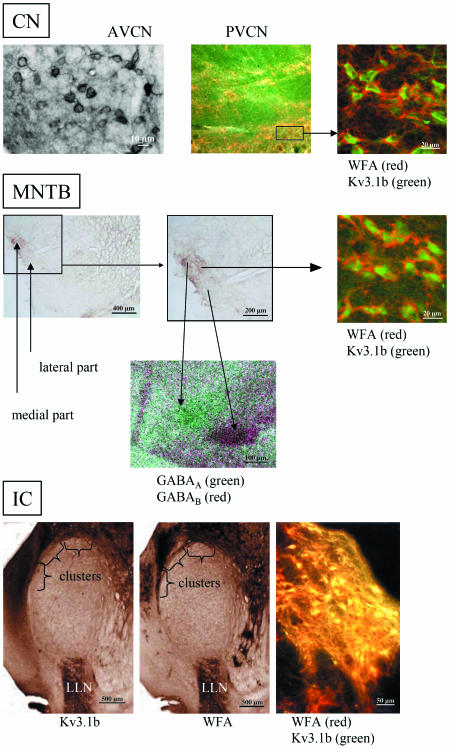
The distribution pattern of the WFA binding sites in the CN was inhomogeneous. In the caudal part of the CN, WFA-positive perineuronal nets were homogeneously distributed. Between the anterior ventral CN (AVCN) and the posterior ventral CN (PVCN) the homogeneous distribution patterns of the perineuronal nets changed. In the PVCN, we recognized shells and clusters, as shown in Fig. 1 (PVCN). In the AVCN and the dorsal CN, the nearly homogeneous distribution pattern persisted. Nevertheless, the staining intensity altered (Fig. 1, AVCN). Cells (most of them multipolar cells) with dark perineuronal nets were intermingled with cells surrounded by more faintly stained perineuronal nets. Nevertheless, no compartmentalization was found. The so-called octopus cells and multipolar cells of the posterior ventral CN were surrounded by diffuse perineuronal nets, as shown in Fig. 2, and expressed the Kv3.1b protein (see Fig. 1 for co-localization). Both somatodendritic and axonal labelling of the Kv3.1b protein was seen. In some instances the lack of contrast in the staining between somata and their surrounding fibres made it difficult to decide whether the somatic staining was due to the presence of protein in somatic membrane or whether it was the result of the presence of protein in terminals surrounding the cell body as with the perineuronal nets. The octopus cells and a subset of multipolar cells were heavily WFA-stained. Not only were the soma surrounded by perineuronal nets (Fig. 2), but dendrites were labelled in a manner similar to that described for the gerbil by Cant & Benson (2005).
Fig. 1.
Comparison of the distribution patterns of perineuronal nets as revealed by WFA binding sites and Kv3.1b protein in the relay stations of the auditory brainstem of the rhesus monkey. The distribution patterns change from non-compartmentalization in the anterior ventral cochlear nucleus (AVCN) with intermingled intensely and weakly stained WFA to clusters (see window) in the posterior ventral cochlear nucleus (PVCN), via subnucleation into a medial part with intensely stained WFA and a lateral part of the MNTB (additionally revealed by GABAA and GABAB receptors) to clusters in the external nucleus of the inferior colliculus (IC); LLN, lateral lemniscal nucleus. The heavily stained WFA binding was revealed by confocal laser scanning microscopy as thick, red perineuronal nets and is co-localized with green labelled Kv3.1b protein at the soma of the auditory neuron. In the overview, yellow colour indicates co-localization of Kv3.1b with dark WFA binding sites in a cluster.
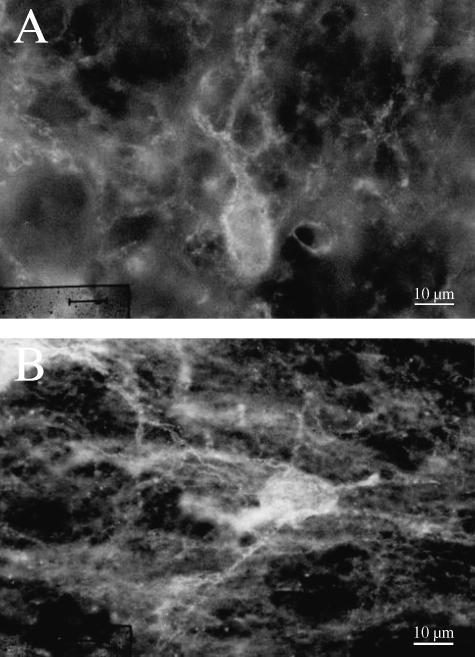
Fig. 2.
Fluorescence photomicrographs of an octopus cell (A) and a multipolar cell (B) surrounded by WFA-stained perineuronal nets.
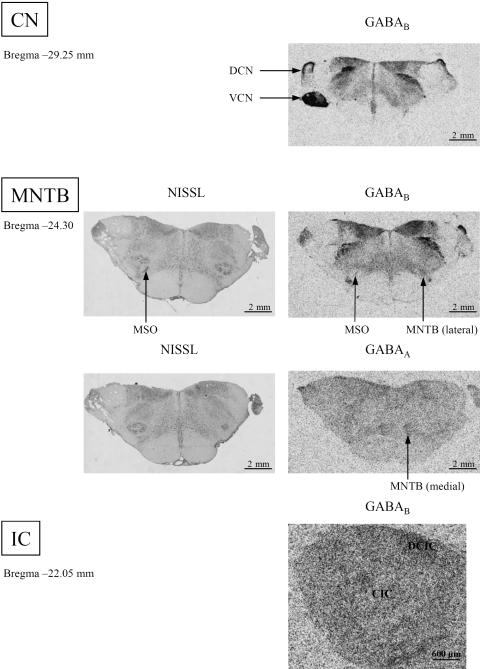
In the whole CN of the rhesus monkey, there were no detectable GABAA binding sites. Conversely, high GABAB receptor densities were revealed in the VCN and in the superficial granular cell layer of the dorsal CN (Fig. 3, DCN, VCN).
Fig. 3.
Comparison of the distribution patterns of GABAA and GABAB receptors in the relay stations of the auditory brainstem of the rhesus monkey. At the level of the dorsal cochlear nucleus (DCN) the superficial granular cell layer is labelled; VCN, ventral cochlear nucleus. For a better orientation, at the level of the SOC Nissl stainings are provided. The MSO is the needle-shaped structure indicated by arrows; MNTB, medial nucleus of the trapezoid body. For the CN and the IC, GABAA receptor densities were below detection levels. GABAB receptors were found especially in the DCIC (dorsal cortex of the IC) whereas the CIC (central nucleus of the IC) was weakly labelled.
SOC
In the SOC, the medial part of the MNTB and the medial peduncle of the LSO (not shown) were revealed by co-localized WFA binding sites and Kv3.1b protein immunoreactivity. The cells surrounded by WFA seemed to belong to the class of principal globular cells of the MNTB, as described previously by Elezgarai et al. (2003). It is, however, noteworthy that the intensity of the WFA binding sites varied, thereby clearly demarcating a medial (strongly labelled) and a lateral (weakly labelled) part of the MNTB. The neurons of the MSO were not surrounded by perineuronal nets and did not express the Kv 3.1b protein.
In contrast to the CN, the medial part of the MNTB was clearly visualized by its GABAA receptor content, whereas the lateral part of the MNTB contained higher GABAB binding site densities (Figs 1 and 3). In particular for the localization of the weak GABAA receptor staining it was useful to compare the images with Nissl staining (Fig. 3). Therefore, the medial (GABAA) and the lateral part of the MNTB (GABAB) could be localized and matched using false-colour features (Fig. 1, MNTB). The needle-shaped MSO could be clearly visualized by GABAB receptors (Fig. 3).
IC
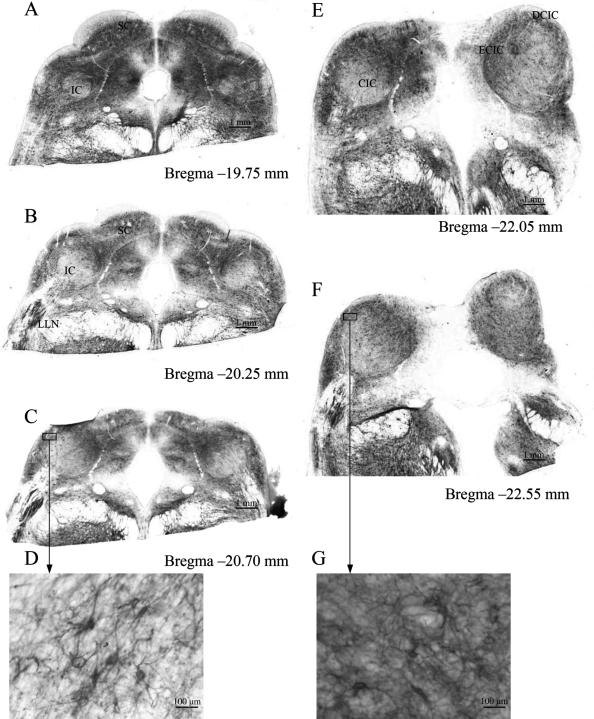
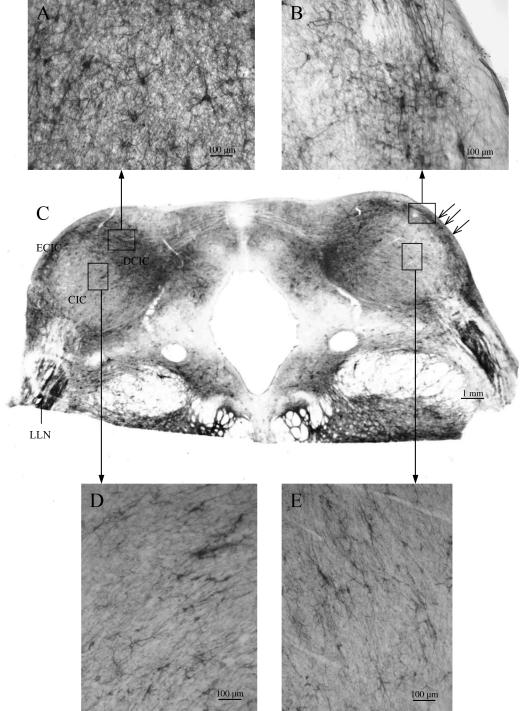
In the LLN and in the IC, a strong co-localization of both WFA binding sites and Kv3.1b protein existed (Fig. 1, IC). In the medial part and the external nuclei (external cortex, ECIC, and dorsal cortex, DCIC) of the IC, large multipolar Kv3.1b protein-containing neurons were surrounded by intensely stained perineuronal nets (Figs 4 and 5), scattered in clusters similar to that described by Caicedo et al. (1996) for the guinea-pig and by Chernock et al. (2004) for the rat. Overviews of WFA binding sites in the IC revealed changes in staining intensity at the different anterior–posterior levels (Fig. 4). Comparing caudal and rostral parts of the rhesus IC, the intensity of the WFA binding sites in the neuropil decreased rostrally (Fig. 4D,G) whereas the soma sizes of the neurons arranged in clusters increased (Fig. 5A,B). Different staining intensities at the same level of the IC resulted in the demarcation of several clusters and layers. The clusters were formed by large neurons, which showed strong WFA binding and a strong staining of the neuropil, which was orientated in parallel to the surface of the IC (Fig. 5B). In the central nucleus of the rhesus IC (CIC), smaller neurons were surrounded by faintly stained perineuronal nets and weakly stained neuropil. The layers consisted of medium-sized neurons with oval or circular somata that were aligned parallel to the surface of the rostral IC (Fig. 5D,E).
Fig. 4.
Overviews of WFA binding sites in the IC of the rhesus monkey revealing changes in the distribution patterns at different levels: the intensity of the WFA binding sites in the neuropil decreased rostrally (D). DCIC, dorsal cortex of the IC; LLN, lateral lemniscal nucleus; ECIC, external cortex of the IC; CIC, central nucleus of the IC; SC, superior colliculus.
Fig. 5.
Distribution patterns of WFA binding sites in the IC of the rhesus monkey at Bregma −21.75 mm. DCIC, dorsal cortex of the IC; LLN, lateral lemniscal nucleus; ECIC, external cortex of the IC; CIC, central nucleus of the IC; open arrows indicate clusters of very large neurons with intensely stained WFA binding sites (B); windows in the CIC indicate examples of sheets of small neurons surrounded by faintly labelled perineuronal nets (D,E).
GABAA receptor densities were below detection level in both the LLN and the IC. The distribution of the GABAB receptor binding sites shows a nearly homogeneous pattern with some weak indications of clusters in the external nucleus and a dark stripe in the medial part of the IC. This stripe was found exclusively in the rostral IC.
Taken together, we found (1) a strong co-localization of the expression of the Kv3.1b protein and WFA binding sites showing the presence of perineuronal nets in the nuclei of the auditory brainstem of the rhesus monkey. (2) There was a gradient in the staining of perineuronal nets. (3) Whereas in the CN the distribution pattern of intensely and faintly stained perineuronal nets was intermingled, the pattern changed in the MNTB to intensely stained perineuronal nets in the medial part and weakly stained nets in the lateral part and in the IC the arrangement of cells surrounded by dark perineuronal nets showed the formation of clusters in the external nucleus and faintly stained sheets in the internal nucleus.
In addition, the observed staining patterns of WFA and Kv3.1b were also demarcated by their differential presence of GABAA and GABAB receptors, especially in the SOC, where the intensely labelled perineuronal nets corresponded to heavy Kv3.1b immunoreactivity and GABAA receptor binding.
Discussion
Functional implications of the clear differences between the rhesus monkey and human SOC have been discussed previously (Bazwinsky et al. 2005). Here we focus on the question of whether it will be possible to find comparable association patterns between WFA and Kv3.1b in primates with those known from rodents.
Within the auditory pathways of the rhesus brainstem, our current data showed distinct patterns of perineuronal nets, Kv3.1b protein, and for GABAA and GABAB receptor distributions. We found a co-localization of WFA binding and Kv3.1b protein within distinct functional compartments, indicating that compartments involved in high-frequency processing were composed of neurons that expressed binding sites for both WFA and Kv3.1b potassium channels in the monkey brainstem. The auditory system of the rhesus monkey uses generally comparable hearing frequencies to those of humans in the range of about 200–20 000 Hz (Fay, 1988; Jackson et al. 1999; Heffner, 2004). Nevertheless, monkeys are more sensitive to higher frequencies than are humans (Stebbins, 1973). Thus, it is possible to compare the findings with those human regions known to be involved in the auditory processing of specific ranges of frequencies. Trussell (2002) pointed to the fact that the relay nuclei of the auditory brainstem contain some of the largest nerve terminals in the mammalian brain. The relatively few neurons compared with other sensory systems require numerous and remarkably diverse mechanisms for the modulation of neurotransmitter release and the fine-tuning of their synaptic transmission patterns (Macica et al. 2003). Elezgarai et al. (2003) investigated the postnatal development of the expression of the Kv3.1b protein in the rat MNTB and stated that expression of the Kv3.1b protein in MNTB neurons coincided with the functional maturation of the calyx of Held–principal globular neuron synapse. The presence of that voltage-dependent potassium channel in this system is crucial for the high-frequency synaptic transmission of auditory signals (Li et al. 2001; Kaczmarek et al. 2005). The Kv3.1b protein was predominantly localized in the principal cells of the MNTB. A functional heterogeneity and diverse sensitivity to neuromodulators was proposed (Koch & Grothe, 2003; Koch et al. 2004). This hypothesis is supported by our findings regarding the differential presence of GABA binding sites in the MNTB. Moreover, the high expression of Kv3.1b protein found in the giant terminals of the synapses of Held in the MNTB pointed to a targeted clustering at GABAergic synapses similar to findings reported for the localization of Kv4.2 channels in the hippocampus (Jinno et al. 2005). Developmental changes in distribution of GABA-receptor subunits have been reported for the gerbil LSO by Korada & Schwartz (1999), indicating that not the endogenous transmitters but specifically their receptors are indicators for a functional maturation within the auditory pathway. By contrast, an age-related decrease of GABAA receptor subunits in the rat IC was reported by Gutiérrez et al. (1994). Milbrandt et al. (1996) found the highest number of GABAA binding sites in the dorsal nucleus of the IC of young rats (2 months of age), which lost about half of the binding sites up to the age of 23 months.
In the CN, many inhibitory nerve terminals contain both GABA and glycine (Lim et al. 2000). For the rat CN heavily labelled glycine receptors were observed accompanied by ‘weak diffuse immunolabeling of GABAA receptors’ (Lim et al. 2000). Taking into account that the rhesus monkeys of the investigations reported here were ‘aged’, those observations could explain our low GABAA receptor densities. Nevertheless, here we were able to show for the first time that differences in the distribution of ligand binding to GABAA and GABAB receptors may be useful to elucidate morphologically the functional organizations of the MNTB in addition to that known for perineuronal nets and Kv3.1b protein (WFA binding sites, rat: Brückner et al. 1993; Härtig et al. 2001; neurocan, dog: Atoji et al. 1997; chondroitin sulfate proteoglycans, rat: Friauf, 2000). Interestingly, the distribution patterns for the dog differed from those for gerbil or rat (although neurocan is a chondroitin sulfate proteoglycan). Atoji et al. (1997) did not find any immunoreactivity in the MNTB or the LLN. In the dog, only a small number of cells in the AVCN was surrounded by neurocan-positive perineuronal nets with weak staining intensity. In the IC very weakly stained neurons were seen in the central nucleus tending to be located at the periphery of the nucleus.
Li et al. (2001) reported that ‘the intensity of the Kv3.1b immunoreactivity varied across the tonotopic map in the medial nucleus of the trapezoid body with neurons responding best to high-frequency tones most intensely labeled’.
For the IC, it is known that the dorsal and the lateral parts of the central nucleus are considered to be involved in lower-frequency hearing (Aitkin & Schuck, 1985). High-frequency characteristics were reported for the ventral part of the central nucleus of the IC (Aitkin et al. 1975, 1994; Maffi & Aitkin, 1987) and in laminar structures of the central nucleus (Schreiner & Langner, 1997). A topographic representation of auditory space in the external nucleus of the IC of the guinea-pig (Binns et al. 1992) correlates with a periodic network of neurochemical modules in the external nuclei of the IC of the rat (Chernock et al. 2004). All these findings point to a similarity in the organization of the neuronal circuits involved in the auditory high-frequency processing in rodents and rhesus monkeys, therefore suggesting that WFA-positive perineuronal nets associated with the high-frequency circuits of hearing in monkey may represent a general feature which may be useful for its comparative characterization on a morphological basis. The described modular patterns for the rat IC (Bertolotto et al. 1996; Chernock et al. 2004) were revealed in the rhesus monkey IC by WFA binding sites and Kv3.1b protein expression. Interestingly, in 1999 we described a comparable modular pattern in the SC of humans (Hilbig et al. 1999).
In the central nucleus of the IC of the rat, GABAA receptors mediate postsynaptic responses which could follow impulses over the whole frequency range but show depression at low rates and facilitation at higher rates (Wu et al. 2004). For the MSO and the lateral part of the MNTB, which were weakly stained by WFA histochemistry and which express small amounts of Kv3.1b protein accompanied by GABAB receptors, involvement in low-frequency hearing is well known. Other intensely labelled structures of the auditory brainstem, such as the external nucleus of the IC or the LLN, only showed very low densities of GABAergic receptors, and form part of the auditory motor control, which is controlled by reflexes.
It remains to be elucidated why the distribution patterns of the neurons surrounded by perineuronal nets in the auditory system changed from mixed populations in CN via subnuclear parcellation in SOC to a clustered pattern in the IC.
In conclusion, our results could contribute to the discussion of the models of sound localization during the evolution of mammals which was forced by Grothe and colleagues (Grothe & Neuweiler, 2000; Grothe, 2000; McAlpine & Grothe, 2003).
Acknowledgments
We thank Dr N. Palomero-Gallagher, Institute for Medicine, Research Ctr. Jülich, for helpful comments and corrections.
References
- Aitkin LM, Webster WR, Veale JL, Crosby DC. Inferior colliculus. I. Comparison of response properties of neurons in central, pericentral, and external nuclei of adult cat. J Neurophysiol. 1975;38:1196–1207. doi: 10.1152/jn.1975.38.5.1196. [DOI] [PubMed] [Google Scholar]
- Aitkin L, Schuck D. Low frequency neurons in the lateral central nucleus of the cat inferior colliculus receive their input predominantly from the medial superior olive. Hear Res. 1985;17:87–93. doi: 10.1016/0378-5955(85)90134-0. [DOI] [PubMed] [Google Scholar]
- Aitkin L, Tran L, Syka J. The responses of neurons in subdivisions of the inferior colliculus of cats to tonal, noise and vocal stimuli. Exp Brain Res. 1994;98:53–64. doi: 10.1007/BF00229109. [DOI] [PubMed] [Google Scholar]
- Atoji Y, Yamamoto Y, Suzuki Y, Matsui F, Oohira A. Immunohistochemical localization of neurocan in the lower auditory nuclei of the dog. Hear Res. 1997;110:200–208. doi: 10.1016/s0378-5955(97)00079-8. [DOI] [PubMed] [Google Scholar]
- Bazwinsky I, Hilbig H, Bidmon HJ, Rübsamen R. Characterization of the human superior olivary complex by calcium binding proteins and neurofilament H (SMI-32) J Comp Neurol. 2003;456:292–303. doi: 10.1002/cne.10526. [DOI] [PubMed] [Google Scholar]
- Bazwinsky I, Bidmon HJ, Zilles K, Hilbig H. Characterization of the rhresus monkey superior olivary complex by calcium binding proteins and synaptophysin. J Anat. 2005;204:745–761. doi: 10.1111/j.1469-7580.2005.00491.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertolotto A, Manzardo E, Guglielmone R. Immunohistochemical mapping of perineuronal nets containing chondroitin unsulfate proteoglycan in the rat central nervous system. Cell Tissue Res. 1996;283:283–295. doi: 10.1007/s004410050538. [DOI] [PubMed] [Google Scholar]
- Binns KE, Grant S, Withington DJ, Keating MJ. A topographic representation of auditory space in the external nucleus of the inferior colliculus of the guinea pig. Brain Res. 1992;589:321–242. doi: 10.1016/0006-8993(92)91282-j. [DOI] [PubMed] [Google Scholar]
- Brückner G, Brauer K, Härtig W, et al. Perineuronal nets provide a polyanionic, glia-associated form of microenvironment around certain neurons in many parts of the rat brain. Glia. 1993;8:183–200. doi: 10.1002/glia.440080306. [DOI] [PubMed] [Google Scholar]
- Brückner G, Szeöke S, Pavlica S, Grosche J, Kacza J. Axon initial segment ensheathed by extracellular matrix in perineuronal nets. Neuroscience. 2006;138:365–375. doi: 10.1016/j.neuroscience.2005.11.068. [DOI] [PubMed] [Google Scholar]
- Caicedo A, d’Aldin C, Puel JL, Eybalin M. Distribution of calcium binding protein immunoreactivities in the guinea pig auditory brainstem. Anat Embryol. 1996;194:465–487. doi: 10.1007/BF00185994. [DOI] [PubMed] [Google Scholar]
- Cant NB, Benson CG. Wisteria floribunda lectin is associated with specific cell types in the ventral cochlear nucleus of the gerbil, Meriones unguiculatus. Hear Res. 2005;216–217:64–72. doi: 10.1016/j.heares.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Chernock ML, Larue DT, Winer JA. A periodic network of neurochemical modules in the inferior colliculus. Hear Res. 2004;188:12–20. doi: 10.1016/S0378-5955(03)00340-X. [DOI] [PubMed] [Google Scholar]
- Elezgarai I, Diez J, Puente N, et al. Subcellular localization of the voltage-dependent potassium channel Kv3.1b in the postnatal and adult rat medial nucleus of the trapezoid body. Neuroscience. 2003;118:889–898. doi: 10.1016/s0306-4522(03)00068-x. [DOI] [PubMed] [Google Scholar]
- Fay RR. Hearing in Vertebrates – a Psychophysics Databook. Worchester, MA: Heffernan Press; 1988. [Google Scholar]
- Friauf E. Development of chondroitin sulphate proteoglycans in the central auditory system of rats correlates with acquisition of mature properties. Audiol Neurotol. 2000;5:251–262. doi: 10.1159/000013889. [DOI] [PubMed] [Google Scholar]
- Grothe B. The evolution of temporal processing in the medial superior olive, an auditory brainstem structure. Prog Neurobiol. 2000;61:581–610. doi: 10.1016/s0301-0082(99)00068-4. [DOI] [PubMed] [Google Scholar]
- Grothe B, Neuweiler G. The function of the medial superior olive in small mammals: temporal receptive fields in auditory anaysis. J Comp Physiol. 2000;186:413–423. doi: 10.1007/s003590050441. [DOI] [PubMed] [Google Scholar]
- Gutiérrez A, Khan ZU, Morris SJ, De Blas AL. Age-related decrease of GABAA receptor subunits and glutamic acid decarboxylase in the rat inferior colliculus. J Neurosci. 1994;14:7469–7477. doi: 10.1523/JNEUROSCI.14-12-07469.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Härtig W, Derouiche A, Welt K, et al. Cortical neurons immunoreactive for the potassium channel Kv3.1b subunit are predominantly surrounded by perineuronal nets presumed as a buffering system for cations. Brain Res. 1999;842:15–29. doi: 10.1016/s0006-8993(99)01784-9. [DOI] [PubMed] [Google Scholar]
- Härtig W, Singer A, Grosche J, Brauer K, Ottersen OP, Brückner G. Perineuronal nets in the rat medial nucleus of the trapezoid body surround neurons immunoreactive for various amino acids, calcium-binding proteins and potassium channel subunit Kv3.1b. Brain Res. 2001;899:123–133. doi: 10.1016/s0006-8993(01)02211-9. [DOI] [PubMed] [Google Scholar]
- Heffner RS, Masterton RB. Sound localization in mammals: brain-stem mechanisms. In: Berkley MA, Stebbins WC, editors. Comparative Perception. I. New York: John Wiley; 1990. pp. 285–314. [Google Scholar]
- Heffner RS. Primate hearing from a mammalian perspective. Anat Rec. 2004;281A:1111–1122. doi: 10.1002/ar.a.20117. [DOI] [PubMed] [Google Scholar]
- Hilbig H, Bidmon HJ, Zilles K, Busecke K. Neuronal and glial structures of the superficial layers of the human superior colliculus. Anat Embryol. 1999;200:103–115. doi: 10.1007/s004290050264. [DOI] [PubMed] [Google Scholar]
- Hilbig H, Bidmon HJ, Blohm U, Zilles K. Wisteria floribunda agglutinin labeling patterns in the human cortex: a tool for revealing areal borders and subdivisions in parallel with immunocytochemistry. Anat Embryol. 2001;203:45–52. doi: 10.1007/s004290000135. [DOI] [PubMed] [Google Scholar]
- Jackson LL, Heffner RS, Heffner HE. Free-field audiogram of the Japanese macaque (Macaca fuscata) J Acoust Soc Am. 1999;106:3017–3023. doi: 10.1121/1.428121. [DOI] [PubMed] [Google Scholar]
- Jinno S, Jeromin A, Kosaka T. Postsynaptic and extrasynaptic localization of Kv4.2 channels in the mouse hippocampal region, with special reference to targeted clustering at gabaergic synapses. Neuroscience. 2005;134:483–494. doi: 10.1016/j.neuroscience.2005.04.065. [DOI] [PubMed] [Google Scholar]
- Kaczmarek LK, Bhattacharjee A, Desai R, et al. Regulation of the timing of MNTB neurons by short-term and long-term modulation of potassium channels. Hear Res. 2005;206:133–145. doi: 10.1016/j.heares.2004.11.023. [DOI] [PubMed] [Google Scholar]
- Koch U, Grothe B. Hyperpolarization-activated current (Ih) in the inferior colliculus: distribution and contribution to temporal processing. J Neurophysiol. 2003;90:3679–3688. doi: 10.1152/jn.00375.2003. [DOI] [PubMed] [Google Scholar]
- Koch U, Braun M, Kapfer C, Grothe B. Distribution of HCN1 and HCN2 in rat auditory brainstem nuclei. J Neurosci. 2004;20:79–91. doi: 10.1111/j.0953-816X.2004.03456.x. [DOI] [PubMed] [Google Scholar]
- Korada S, Schwartz IR. Development of GABA, glycine, and their receptors in the auditory brainstem of gerbil: a light and electron microscopic study. J Comp Neurol. 1999;409:664–681. doi: 10.1002/(sici)1096-9861(19990712)409:4<664::aid-cne10>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Li W, Kaczmarek LK, Perney TM. Localization of two high-threshold potassium channel subunits in the rat central auditory system. J Comp Neurol. 2001;437:196–218. doi: 10.1002/cne.1279. [DOI] [PubMed] [Google Scholar]
- Lim R, Alvarez FJ, Walmsley B. GABA mediates presynaptic inhibition at glycinergic synapses in a rat auditory brainstem nucleus. J Physiol. 2000;525:447–459. doi: 10.1111/j.1469-7793.2000.t01-1-00447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macica CM, von Hehn CAA, Wang LY, et al. Modulation of the Kv3.1b potassium channel isoform adjusts the fidelity of the firing pattern of auditory neurons. J Neurosci. 2003;23:1133–1153. doi: 10.1523/JNEUROSCI.23-04-01133.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maffi CL, Aitkin LM. Differential neural projections to regions of the inferior colliculus of the cat responsive to high frequency sounds. Hear Res. 1987;26:211–219. doi: 10.1016/0378-5955(87)90113-4. [DOI] [PubMed] [Google Scholar]
- McAlpine D, Grothe B. Sound localization and delay lines- do mammals fit the model? Trends Neurosci. 2003;26:347–350. doi: 10.1016/S0166-2236(03)00140-1. [DOI] [PubMed] [Google Scholar]
- Milbrandt JC, Albin RL, Turgeon SM, Caspary DM. GABAA receptor binding in the aging rat inferior colliculus. Neuroscience. 1996;73:449–458. doi: 10.1016/0306-4522(96)00050-4. [DOI] [PubMed] [Google Scholar]
- Richter EA, Norris BE, Fullerton BC, Levine RA, Kiang NYS. Is there a medial nucleus of the trapezoid body in humans. Am J Anat. 1983;68:157–166. doi: 10.1002/aja.1001680205. [DOI] [PubMed] [Google Scholar]
- Schreiner CE, Langner G. Laminar fine structure of frequency organization in auditory midbrain. Nature. 1997;388:383–386. doi: 10.1038/41106. [DOI] [PubMed] [Google Scholar]
- Song P, Yang Y, Barnes-Davies M, et al. Acoustic environment determines phosphorylation state of the Kv3.1b potassium channel in auditory neurons. Nat Neurosci. 2005;8:1335–1342. doi: 10.1038/nn1533. [DOI] [PubMed] [Google Scholar]
- Stebbins WC. Hearing of Old World monkeys. (Cercopithecinae) Am J Phys Anthropol. 1973;38:357–364. doi: 10.1002/ajpa.1330380233. [DOI] [PubMed] [Google Scholar]
- Trussell LO. Modulation of transmitter rease at giant synapses of the auditory system. Curr Opin Neurosci. 2002;12:400–404. doi: 10.1016/s0959-4388(02)00335-5. [DOI] [PubMed] [Google Scholar]
- Wu SH, Ma CL, Kelly JB. Contribution of AMPA, NMDA, and GABA(A) receptors to temporal pattern of postsynaptic responses in the inferior colliculus of the rat. J Neurosci. 2004;24:4625–4634. doi: 10.1523/JNEUROSCI.0318-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilles K, Palomero-Gallagher N, Grefkes C, et al. Architectonics of the human cerebral cortex and transmitter receptor fingerprints: reconciling functional neuroanatomy and neurochemistry. Eur Neuropsychopharmacol. 2002;12:587–599. doi: 10.1016/s0924-977x(02)00108-6. [DOI] [PubMed] [Google Scholar]