Abstract
Thioredoxin-related protein of 14 kDa, TRP14, has previously been identified only in humans. Here we report the identification and expression of an amphioxus TRP14 gene, named AmphiTRP14, the first such data in a non-mammalian organism. AmphiTRP14 consists of a 372-bp open reading frame coding for a 123-amino-acid protein with a calculated molecular weight of 14 kDa. It shares 56% identity with human TRP14 and possesses a highly conserved motif CPDC. Sequence comparison suggests the evolutionary appearance of the four-exon-three-intron organization of TRP14 genes after the split of protostome/deuterostome, which is highly conserved since then. AmphiTRP14 has been successfully expressed in Escherichia coli and purified. The recombinant protein exhibited features characteristic of human TRP14, including a reductase activity towards insulin. Both in situ hybridization histochemistry and immunohistochemistry revealed that AmphiTRP14 was expressed in a tissue-specific manner, with the most abundant expression in the hepatic caecum, ovary and hind-gut. This suggests that AmphiTRP14 plays a fundamental but tissue-specific role, or alternatively reflects differences in the tissue susceptibility to oxidative damage.
Keywords: amphioxus, Branchiostoma, evolution, expression, thioredoxin-related protein
Introduction
Thioredoxin (Trx), identified first in Escherichia coli as an electron donor for ribonucleotide reductase (Laurent et al. 1964), is a 12-kDa redox protein which is present in virtually every living species from prokaryotes to eukaryotes, including humans (Powis & Montfort, 2001). It functions as a protein-disulfide reductase (Arnér & Holmgren, 2000; Carvalho et al. 2006) participating in many physiological processes including the regulation of transcription factor DNA-binding activity, antioxidant defence, modulation of apoptosis, immune response and morphogenesis (for reviews see Arnér & Holmgren, 2000; Das, 2004; Carvalho et al. 2006). Trx is also correlated with a number of pathophysiological conditions such as cancer, Alzheimer's and Parkinson's diseases (Hirota et al. 2002; Powis et al. 2000; Arnér & Holmgren, 2006). The redox activity of Trx resides in a highly conserved active site, Cys-Gly-Pro-Cys (CGPC), where the two Cys residues undergo a reversible oxidation, converting their dithiol group to a disulfide bond and transferring the reducing equivalents to a disulfide substrate (Powis & Montfort, 2001). The oxidized inactive forms are reduced by the selenoprotein thioredoxin reductase (TrR), which uses the reducing power of NADPH (Powis & Montfort, 2001).
Primary structures of many Trx are known. They vary in length from 105 to 110 amino acids, exhibit 27–69% sequence identity to that of E. coli (Eklund et al. 1991), and share a common globular structure consisting of a central core of β-sheets surrounded by α-helixes with the active site situated in a protrusion of the protein surface (Jeng et al. 1994; Martin, 1995). Proteins containing the Trx-like active site have also been identified in various species and classified as part of the Trx superfamily (Matsuo et al. 2002; Nakamura, 2005; Carvalho et al. 2006). Among them, thioredoxin-related protein of 14 kDa, TRP14, a widely expressed cytosolic protein with a modified active site sequence Cys-Pro-Asp-Cys (CPDC), has been found to act as disulfide reductase like Trx1 (Jeong et al. 2004a), and to regulate TNF-α-induced signalling pathways in a different manner from Trx1 (Jeong et al. 2004b). However, little information is available regarding TRP14 in non-mammalian organisms although some hypothetical proteins with a CXXC motif have been documented in several species such as cow (GenBank GeneID: 404159), mouse (GenBank GeneID: 52700), rat (GenBank GeneID: 287474), sea urchin (GenBank GeneID: 582604), fruit-fly (GenBank GeneID: 43938) and nematode (GenBank GeneID: 175400). The purpose of this study was thus to identify TRP14 cDNA from amphioxus Branchiostoma belcheri, a basal chordate bridging the gap between invertebrates and vertebrates (Stokes & Holland, 1998; Holland et al. 2004) and to examine its expression and biochemical properties.
Materials and methods
Cloning and sequence analysis of cDNA
The gut cDNA library of adult amphioxus was constructed with SMART cDNA Library Construction Kit (CLONTECH, Palo Alto, CA, USA) according to the method described by Liu et al. (2002). In a large-scale sequencing of the gut cDNA library with ABI PRISM 377XL DNA sequencer, more than 5000 clones were analysed for coding probability with the DNATools program (Rehm, 2001). Comparison against the GenBank protein database was performed using the BLAST network server at the National Center for Biotechnology Information (Altschul et al. 1997). Multiple protein sequences were aligned using the MegAlign program by using the CLUSTAL W method in DNASTAR software package (Burland, 2000). The phylogenetic tree was constructed by the neighbour-joining method within the package PHYLIP 3.6c software package (Felsenstein, 1993) using 1000 bootstrap replicates.
Northern blotting
Total RNA was extracted with Trizol (Gibco) from the adult amphioxus. An aliquot of 5 µg RNA was electrophoresed and blotted onto nylon membrane (Osmonics Inc.). The blots were hybridized at high stringency with digoxigenin (Dig)-labelled amphioxus TRP14 riboprobes of about 775 bp (1 µg mL−1 in DIG Easy Hyb) for 16 h at 58 °C and washed twice in 2× SSC with 0.1% sodium dodecylsulfate (SDS) at room temperature for 5 min each and twice in 0.1× SSC with 0.1% SDS at 65 °C for 20 min each. They were incubated in the blocking solution (pH 7.5) containing 100 mm maleic acid, 150 mm NaCl and 1% blocking reagent (Roche) for 2 h, and then in the blocking solution with anti-Dig-alkaline phosphatase (AP)-conjugated antibody (Roche) diluted 1 : 10 000 for 1 h at room temperature. After washing with 100 mm maleic acid buffer (pH 7.5) containing 150 mm NaCl and 0.3% Tween-20 and with 100 mm Tris-HCl buffer (pH 9.5) containing 100 mm NaCl, the hybridized bands were visualized by BM-Purple (Roche).
In situ hybridization histochemistry
Sexually mature B. belcheri was cut into 3–4 pieces and fixed in freshly prepared 4% paraformaldehyde in 100 mm phosphate-buffered saline (PBS; pH 7.4) at 4 °C for 8 h. The samples were dehydrated in an ethanol gradient, embedded in paraffin and sectioned at 7 µm. The sections were mounted on poly-l-lysine-coated slides, dried at 42 °C for 36 h, and de-paraffinized in xylene for 20 min (two changes for 10 min each) followed by immersion in absolute ethanol for 10 min (two changes for 5 min each). They were re-hydrated, and finally equilibrated in double-distilled water containing 0.1% DEPC. In situ hybridization histochemistry was carried out as described by Xue et al. (2006).
Expression and purification of recombinant protein
The complete coding region of the amphioxus TRP14 gene was amplified by polymerase chain reaction (PCR) with the upstream primer 5′-CGCGGATCCATGGTTGTCTCTGAAAAG-3′ (BamHI site underlined) and the downstream primer 5′-ATAAGAATGCGGCCGCTAGGGTTTCCTCCATACT-3′ (NotI site underlined). The reaction was carried out under the following conditions: initial denaturation at 94 °C for 5 min followed by 30 cycles of denaturation at 94 °C for 30 s, annealing for 30 s at 53 °C, and extension at 72 °C for 1 min. The PCR product was digested with BamHI and NotI and subcloned into the pET-28a expression vector (Novagen) previously cut with the same restriction enzymes. The identity of the insert was verified by sequencing, and the plasmid was designated pET28a-AmphiTRP14.
Cells of Escherichia coli BL21 were transformed with the plasmid pET28a-AmphiTRP14, and cultured overnight in LB broth containing kanamycin (30 µg mL−1). The culture was diluted 1 : 100 with LB broth and subjected to further incubation at 37 °C for 3 h. The expression of AmphiTRP14 was induced by addition of isopropyl β-d-thiogalactoside (IPTG) to the culture at a final concentration of 1.0 mm. After incubation at 37 °C for 4 h, bacterial cells were harvested by centrifugation, re-suspended in 50 mm PBS (pH 8.0) containing 0.3 m NaCl and 10 mm imidazole, and sonicated on ice. Cell debris was removed by centrifugation at 15 000 g for 10 min, and the supernatant was loaded onto a Ni-NTA resin column (Novagen). The column was washed with 50 mm PBS (pH 8.0) containing 20 mm imidazole and with 50 mm PBS (pH 8.0) containing 40 mm imidazole, respectively, and then eluted with 50 mm PBS (pH 8.0) containing 250 mm imidazole. The purity of eluted samples was analysed by 12% SDS-polyacrylamide gel electrophoresis (PAGE) as described by Laemmli (1970), and stained with Coomassie Brilliant Blue R-250.
Protein concentrations were determined by the method of Bradford using bovine serum albumin as a standard (Bradford, 1976).
Fluorescence spectroscopy analysis
The fluorescence spectrum of amphioxus TRP14 was measured with a SpectraMax M5 (Molecular Devices Corp., USA) by excitation at 280 nm (Holmgren, 1972). The emission spectrum was recorded from 300 to 400 nm and the band-width of emission was 5 nm. All measurements were made at 25 °C in a final volume of 4 mL reaction mixture containing 3.96 mL of 50 mm Tris-HCl (pH 7.4) with 1 mm EDTA and 40 µL of 100 µm amphioxus TRP14. Complete reduction of amphioxus TRP14 was achieved by addition of 1 mm dithiothreitol (DTT) to the reaction mixture. Control blanks were processed similarly except that an equal volume of 50 mm PBS (pH 8.0) with 250 mm imidazole replaced the amphioxus TRP14 solution. The fluorescence intensity of control blanks was subtracted from the sample spectra (Windle et al. 2000).
Reductase activity assay
Reductase activity was assayed at 25 °C by monitoring the precipitation of insulin β-chain that followed the reduction of the disulfide bond (Holmgren, 1979). Each reaction was performed at least three times. The assay mixture contained 100 mm PBS (pH 7.0), 1 mm EDTA (pH 8.0), 340 µm insulin and either 5 or 10 µm recombinant amphioxus TRP14 protein in a final volume of 1.5 mL. The reaction was initiated by addition of 100 mm DTT into the reaction mixture to a final concentration of 0.5 mm DTT. Absorbance was monitored at 650 nm every 30 s. In all experiments, the non-enzymatic reduction of insulin by DTT solution in the absence of amphioxus TRP14 was recorded as a control.
Preparation of polyclonal antibody
The purified recombinant amphioxus TRP14 protein was used for raising antibody in two rabbits. Approximately 400 µg of the purified AmphiTRP14 was emulsified with Freund's complete adjuvant and injected subcutaneously at multiple sites of the rabbits. Two booster injections of 100 µg antigen mixed with Freund's incomplete adjuvant were administered subcutaneously at intervals of 2 weeks. Eight days after the final booster, the blood was collected from the rabbits by carotid puncture and serum was prepared. The antisera were aliquoted and stored at −70 °C. Antibody titre in the sera was determined by dot blot assay.
Western blotting
Two amphioxus were homogenized in 1 mL of 50 mm Tris-HCl (pH 7.2) with 50 mm NaCl on ice and centrifuged at 15 000 g for 20 min at 4 °C. The supernatant was pooled and run on 12% SDS-PAGE. The cell lysates of IPTG-induced E. coli BL21, control cell lysates and purified protein were also run on the same gel. The gel was washed for 15 min in 20 mm PBS (pH 7.4) containing 0.1% Tween-20, and proteins on the gel were blotted on nitrocellulose membrane (Hybond, Amersham Pharmacia). The membrane was incubated in 20 mm PBS (pH 7.4) containing 3% defatted milk powder at 30 °C for 1.5 h, and then in the rabbit antisera diluted 1 : 500 with the same buffer for 2 h at 30 °C. After washing in 20 mm PBS (pH 7.4), the membrane was incubated in the peroxidase-conjugated goat anti-rabbit antibody diluted 1 : 2000 at 30 °C for 2 h. Bands were visualized using 4-dimethylaminobenzene (DAB) and 0.03% H2O2.
Immunohistochemistry
Amphioxus was cut into four pieces and fixed in freshly prepared 4% paraformaldehyde (w/v) in 100 mm PBS (pH 7.4) at 4 °C for 12 h. After dehydration, the samples were embedded in paraffin and sectioned at 7 µm. The sections were mounted on slides and dried at 42 °C for 36 h, and the immunohistochemical assay was performed as described by Liang et al. (2006).
Results and discussion
Sequence and phylogeny of amphioxus TRP14
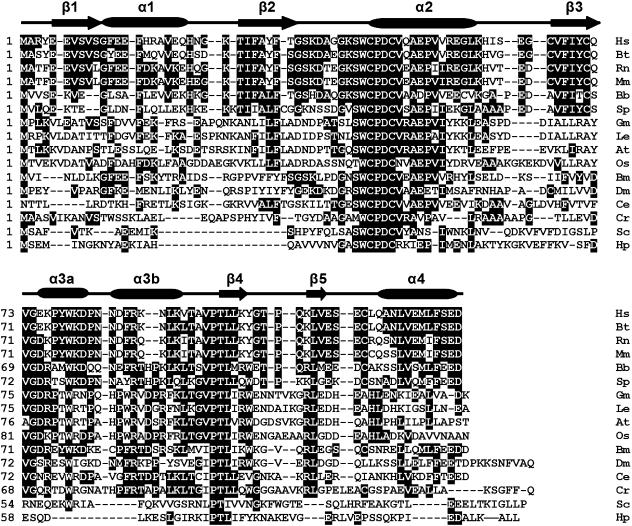
The cDNA (GenBank accession number: EF065518) obtained from the gut cDNA library of amphioxus B. belcheri was 775 bp long and its longest open-reading frame (ORF) coded for a protein of 123 amino acids with a predicted molecular mass of approximately 14 kDa and an isoelectric point of 4.8 (EditSeq 5.01, DNASTAR Inc). The 5′-untranslated region (UTR) is 57 bp long and the 3′-UTR is 346 bp long with two polyadenylation signals (AATAAA) and a polyadenylation tail. The start codon (ATG) was assigned on the basis that there was no ATG in the 5′-UTR 57 nt and the start codon was flanked by a purine at positions of both −3 and +4, matching the Kozak consensus sequence (Kozak, 1987). The BLASTp searching at NCBI showed that the protein encoded by the cDNA shared 56% (66/117) identity to human TRP14, and had a Trx-like motif Cys-Pro-Asp-Cys (CPDC). Moreover, prediction by the SWISS-MODEL Program (http://swissmodel.expasy.org/workspace/) revealed the presence of five β-sheets and five α-helixes (Fig. 1), characteristic of human TRP14 (Woo et al. 2004). The cDNA therefore encodes an amphioxus TRP14 gene and is designated AmphiTRP14.
Fig. 1.
Alignment of the amino acid sequences of AmphiTRP14 with other TRP14 proteins including human TRP14 and putative TRP14 proteins using the MegAlign program (DNASTAR) by the CLUSTAL W method. Shaded (with solid black) residues are the amino acids that match the consensus. Gaps introduced into sequences to optimize alignment are represented by a dash. The secondary structures of human TRP14 protein (PDB: 1WOU) are marked at the peak of the corresponding sequences. β-strands are shown as arrows and α-helices are shown as cylinder. The conserved active motif is marked by closed inverted triangles. The sequences used are: Hs (Homo sapiens, BC006405), Bt (Bos taurus, AW917424), Rn (Rattus norvegicus, AW917424), Mm (Mus musculus, NM_026559), Bb (Branchiostoma belcheri, EF065518), Sp (Strongylocentrotus purpuratus, XP_787642), Gm (Glycine max, AW734061), Le (Lycopersicon esculentum, AW223476), At (Arabidopsis thaliana, BAB09186), Os (Oryza sativa, BE040654), Bm (Bombyx mori, AU003992), Dm (Drosophila melanogaster, CAB58075), Sc (Saccharomyces cerevisiae, NP_013468), Ce (Caenorhabditis elegans, AV193251), Cr (Chlamydomonas reinhardtii, BE056551), Hp (Helicobacter pylori, B64702).
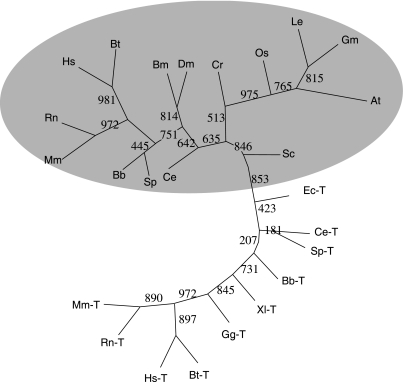
Using the deduced AmphiTRP14 as template, a homology search in the GenBank database revealed the presence of a number of putative TRP14 sequences from a variety of organisms including animals, plants and bacteria. The phylogenetic tree constructed by the neighbour-joining method using the sequences of TRP14 proteins, putative TRP14 proteins and representative Trx proteins showed that both amphioxus TRP14 and human TRP14 as well as putative TRP14 proteins were clustered together, separate from Trx proteins (Fig. 2). An alignment of TRP14 proteins and putative TRP14 proteins demonstrated that they all had the same active site motif CPDC (Fig. 1). These imply that TRP14 is a highly conserved ubiquitous protein with the active site CPDC.
Fig. 2.
Unrooted phylogenetic tree constructed by the neighbour-joining method using TRP14 proteins, putative TRP14 proteins and representative Trx proteins. Bootstrap value based on 1000 replicates is indicated at each branch point. Sequences of TRP14 proteins are included in Fig. 1. ‘-T’ stands for thioredoxin protein. The sequences used are: Hs-T (Homo sapiens, NP_003320), Bt-T (Bos taurus, O97680), Rn-T (Rattus norvegicus, P11232), Mm-T (Mus musculus, P10639), Bb-T (Branchiostoma belcheri, AAK72483), Gg-T (Gallus gallus, P08629), Xl-T (Xenopus laevis, AAH72884), Sc-T (Saccharomyces cerevisiae, O14463), Ec-T (Escherichia coli, AAA24534), Ce-T (Caenorhabditis elegans, Q09433).
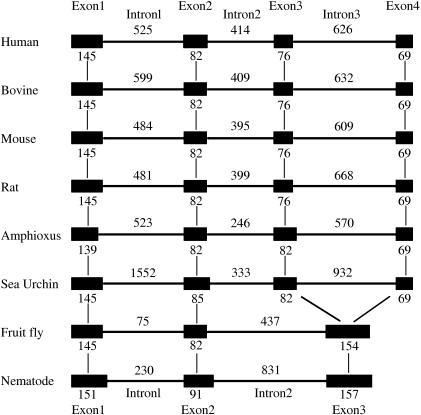
A search of the recently completed draft assembly and automated annotation of the B. floridae genome was also carried out. It revealed the presence of a Florida amphioxus TRP14 cDNA and its genomic DNA sequence (fgenesh2_pg.scaffold_364000050; scaffold_364; http://shake.jgi-psf.org/cgi-bin/dispTranscript?db=Brafl1&id=100641&useCoords=1). Sequence comparison demonstrated that AmphiTRP14 shared 94% identity to the deduced protein encoded by the Florida amphioxus gene at the amino acid level. Analysis of the genomic structure exhibited that the Florida amphioxus TRP14 gene consisted of four exons and three introns (Fig. 3). The four exons of 139, 82, 82 and 69 bp, respectively, were interspaced by the three introns of 523, 246 and 570 bp, which all begin with GT and end with an AG dinucleotide (Fig. 3), sequences thought necessary for correct RNA splicing of various other eukaryotic genes (Breathnach et al. 1978). It is notable that TRP14 genes in deuterostomes such as the human TRP14 gene and bovine, mouse, rat and sea urchin putative TRP14 genes all encompassed four exons and three introns, whereas TRP14 genes in protostomes like fruit-fly and nematode putative TRP14 genes comprised three exons and two introns. Moreover, the first two exons in all the species mentioned above encode the respective homologue protein domains, while exons 3 and 4 in deuterostomes are combined in a single third exon in protostome putative TRP14 genes (Fig. 3). This indicates the evolutionary emergence of the four-exon-three-intron organization of TRP14 genes after the split of protostome/deuterostome, which is highly conserved since then.
Fig. 3.
Diagram of the genomic structures of TRP14 genes from human, mouse, rat, bovine, amphioxus, sea urchin, fruit fly and nematode. Solid boxes represent the region coding for the structural sequences of the protein. Thin lines indicate the sequences of introns. The number of nucleotides above the thin lines represents the size of introns, and the number below the solid boxes the size of exons. The genomic sequences correspond to: human (Homo sapiens, GenBank GeneID: 84817), bovine (Bos taurus, GenBank GeneID: 404159), mouse (Mus musculus, GenBank GeneID: 52700), rat (Rattus norvegicus, GenBank GeneID: 287474), sea urchin (Strongylocentrotus purpuratus, GenBank GeneID: 582604), fruit fly (Drosophila melanogaster, GenBank GeneID: 43938), nematode (Caenorhabditis elegans, GenBank GeneID: 175400).
Biochemical properties of recombinant AmphiTRP14
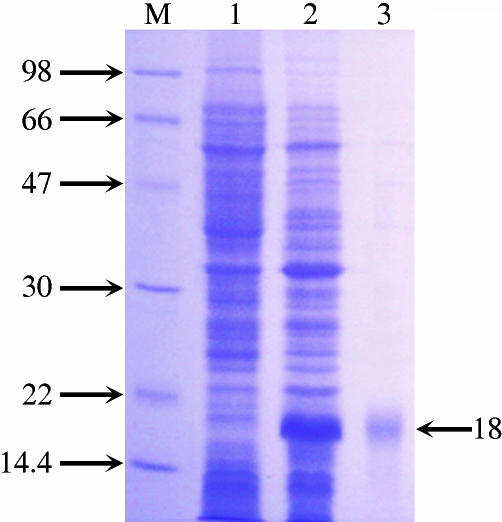
An expression vector including the entire ORF of AmphiTRP14 and a 5′ additional tag of pET28a was constructed and successfully transformed into E. coli, and this resulted in the original N-terminal Met in the recombinant protein being replaced by Met-Gly-Ser-Ser-(His)6-Ser-Ser-Gly-Leu-Val-Pro-Arg-Gly-Ser-His-Met-Ala-Ser-Met-Thr-Gly-Gly-Gln-Gln-Met-Gly-Arg-Gly-Ser-Met, which increases the size of the recombinant protein by an approximately additional 4 kDa. The recombinant protein was purified by affinity chromatography on an Ni-NTA resin column, and the purified AmphiTRP14 with the His6 tag produced a single band of ∼18 kDa on SDS-PAGE gel after Coomassie blue staining, coinciding with its theoretical size (Fig. 4).
Fig. 4.
SDS-PAGE of AmphiTRP14. Lane M, marker; Lane 1, extracts from E. coli BL21 containing pET28a/AmphiTRP14 before induction; Lane 2, extracts from E. coli BL21 containing pET28a/AmphiTRP14 after IPTG-induction; Lane 3, recombinant AmphiTRP14 purified on Ni-NTA resin column. The arrow indicates the location and size of recombinant AmphiTRP14.
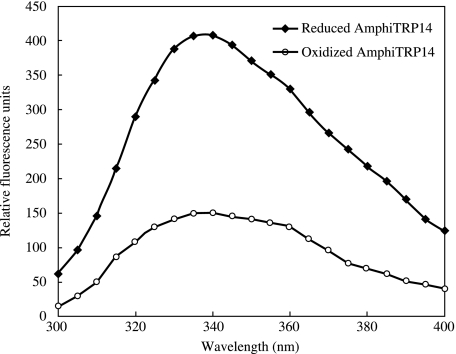
The human TRP14 has been shown to produce a 2.6-fold increase in fluorescence intensity upon reduction of the active site Cys residues. This is attributable to the quenching effect of the active site disulfide bond on the fluorescence of adjacent tryptophan (W) residues. AmphiTRP14, which contains W40, W75 and W99, also yielded a 2.7-fold increase in tryptophan fluorencence when reduced (Fig. 5), suggesting changes in the microenvironment around the Trp (W) residues juxtaposing the CXXC active site. The similar phenomenon has also been observed in horse crab TRP16 (Wang et al. 2007).
Fig. 5.
Fluorescence emission spectra of oxidized and reduced AmphiTRP14. The assay was repeated three times and a repesentative assay is shown. The excitation spectrum was 280 nm and the emission spectrum was recorded from 300 to 400 nm. The fluorescence intensity of control blanks was subtracted from the sample spectra.
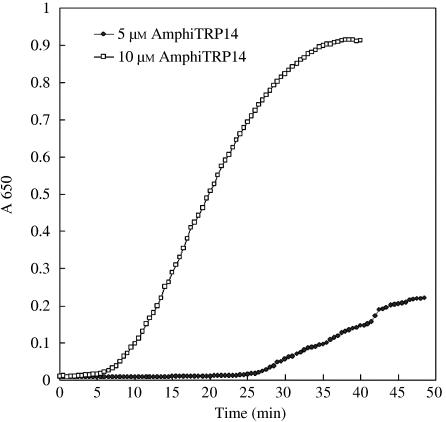
The reductase activity of AmphiTRP14 was assayed by its capacity to reduce the disulphide bonds of insulin, which contains one intramolecular and two intermolecular disulfides. It was found that AmphiTRP14 exhibited a concentration-dependent disulfide reductase activity toward insulin (Fig. 6), which is comparable with that of human TRP14. This indicates that like human TRP14, AmphiTRP14 is not only a disulfide reductase but functional as well.
Fig. 6.
Reduction assay of AmphiTRP14. AmphiTRP14 at 5 µm and 10 µm was assayed for its ability to reduce insulin β-chain in the presence of DTT. Each assay was repeated at least three times and one representative assay is shown. The absorbance of control blanks has been subtracted.
Antibody specificity
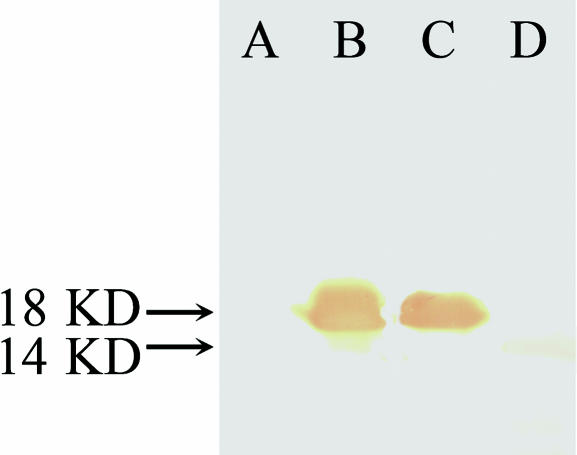
Rabbit antiserum against the purified recombinant AmphiTRP14 with a titre of 1 : 1000 was obtained. Western blotting analysis exhibited that the rabbit antiserum reacted with the supernatant of the cell lysate of IPTG-induced E. coli BL21 with expression vector, forming a band of approximately 18 kDa, but it was not reactive to the supernatant of the lysate of the same E. coli cells before induction by IPTG. The antiserum also reacted with the amphioxus homogenates, forming a single band of approximately 14 kDa (Fig. 7), corresponding to the molecular mass predicted by AmphiTRP14 cDNA. These show that the rabbit antiserum prepared has a conspicuous antigen-specific reactivity.
Fig. 7.
Western blotting. Lane A, extracts from E. coli BL21 containing pET28a/AmphiTRP14 before induction; Lane B, extracts from IPTG-induced E. coli BL21 containing pET28a/AmphiTRP14; Lane C, recombinant AmphiTRP14 protein purified on Ni-NTA resin column; Lane D, amphioxus tissue homogenates. The arrows indicate the location and size of the recombinant protein and the native protein in amphioxus, respectively.
Tissue-specific expression of AmphiTRP14
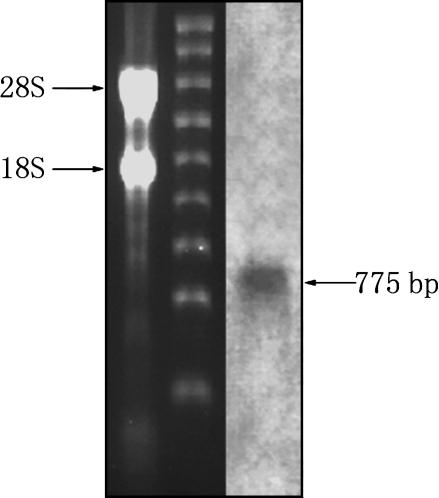
Northern blotting was conducted to assess the presence and size of AmphiTRP14 transcript. As shown in Fig. 8, an approximately 770-bp band of AmphiTRP14 transcript was detected. In situ hybridization histochemistry revealed that AmphiTRP14 transcript was abundant in the hepatic caecum and ovary, and at a lower level present in the hind-gut, endostyle, epipharyngeal groove, gill and testis, while it was absent in the neural tube, notochord and muscle (Fig. 9). It is clear that the TRP14 gene is expressed in amphioxus in a tissue-specific manner. This was further corroborated by immunohistochemical staining using the rabbit antiserum against the purified recombinant AmphiTRP14, which showed that AmphiTRP14 was predominantly localized in the hepatic caecum, ovary and hind-gut, and weakly in the testis and gill (Fig. 10). These are in contrast to the widespread expression pattern of human TRP14, where the human TRP14 gene was detected in all tissues examined (Jeong et al. 2004a), and suggests that AmphiTRP14 may play a fundamental but tissue-specific role in food digestion for example, or alternatively reflect differences in the tissue susceptibility to oxidative damage (Doi et al. 2004).
Fig. 8.
Northern blotting. A total of 5 µg RNA was analysed in 1.2% agarose formaldehyde-denaturing gel. The two main bands are 28S and 18S RNA. The blot was hybridized with Dig-labelled AmphiTRP14 RNA probe. The arrow indicates the molecular size equivalent to ∼775 bp.
Fig. 9.
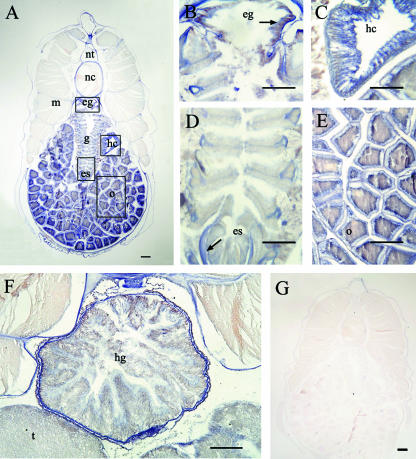
Localization of AmphiTRP14 transcripts in different tissues of adult amphioxus. A is the low magnification view of tissues of female amphioxus. AmphiTRP14 transcripts were observed in the epipharyngeal groove, endostyle, hepatic caecum, gill and the ovary. B, C, D and E are enlargements of the boxes in A. F shows the positive signals in gut and testis. G is the control of a female amphioxus. Arrows in the figure indicate the positive signals. es, endostyle; eg, epipharyngeal groove; g, gill; hc, hepatic caecum; hg, hind-gut; m, muscle; nc, notochord; nt, neural tube; o, ovary; t, testis. Bars represent 100 µm in B–F and 200 µm in A and G.
Fig. 10.
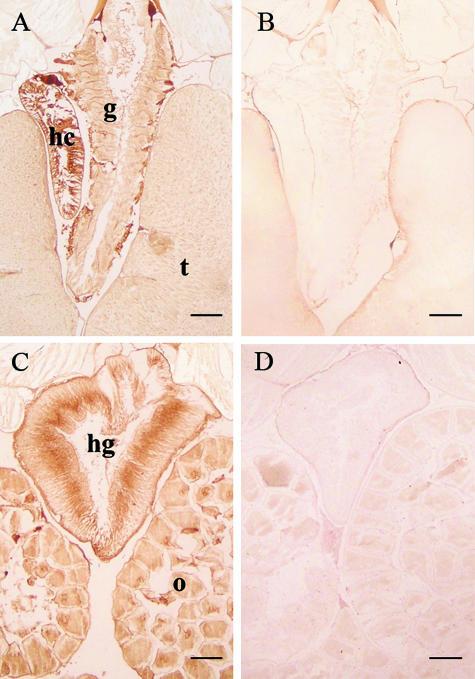
Localization of AmphiTRP14 by immnohistochemistry. (A,C) Micrographs show the strong presence of AmphiTRP14 in the hepatic caecum, hind-gut and ovary in amphioxus. Weak signals are also detected in the gill and testis. B and D are the control sections. hc, hepatic caecum; hg, hind-gut; g, gill; o, ovary; t, testis. Bars, 100 µm.
Acknowledgments
This work was supported by the Ministry of Science and Technology (MOST) of China 2006CB101805 and by the National Science Foundation of China (NSFC; 30470203).
References
- Altschul SF, Madden TL, Schaffer AA, et al. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnér ESJ, Holmgren A. Physiological functions of thioredoxin and thioredoxin reductase. Eur J Biochem. 2000;267:6102–6109. doi: 10.1046/j.1432-1327.2000.01701.x. [DOI] [PubMed] [Google Scholar]
- Arnér ESJ, Holmgren A. The thioredoxin system in cancer. Semin Cancer Biol. 2006;16:420–426. doi: 10.1016/j.semcancer.2006.10.009. [DOI] [PubMed] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Breathnach R, Benoist C, O’Hare K, Cannon F, Chambon P. Ovalbumin gene: evidence for a leader sequence in mRNA and DNA sequences at the exon-intron boundaries. Proc Natl Acad Sci USA. 1978;75:4853–4857. doi: 10.1073/pnas.75.10.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burland TG. DNASTAR's Lasergene sequence analysis software. Meth Mol Biol. 2000;132:71–91. doi: 10.1385/1-59259-192-2:71. [DOI] [PubMed] [Google Scholar]
- Carvalho AP, Fernandes PA, Ramos MJ. Similarities and differences in the thioredoxin superfamily. Prog Biophys Mol Biol. 2006;91:229–248. doi: 10.1016/j.pbiomolbio.2005.06.012. [DOI] [PubMed] [Google Scholar]
- Das KC. Thioredoxin system in premature and newborn biology. Antioxid Redox Signal. 2004;6:405–412. doi: 10.1089/152308604771978480. [DOI] [PubMed] [Google Scholar]
- Doi AM, Pham RT, Hughes EM, Barber DS, Gallagher EP. Molecular cloning and characterization of a glutathione S-transferase from largemouth bass (Micropterus salmoides) liver that is involved in the detoxification of 4-hydroxynonenal. Biochem Pharmacol. 2004;67:2129–2139. doi: 10.1016/j.bcp.2004.01.024. [DOI] [PubMed] [Google Scholar]
- Eklund H, Gleason FK, Holmgren A. Structural and functional relations among thioredoxins of different species. Protein. 1991;11:13–28. doi: 10.1002/prot.340110103. [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Phylip (Phylogeny Inference Package) Seattle: Department of Genetics, University of Washington; 1993. [Google Scholar]
- Hirota K, Nakamura H, Masutani H, Yodoi J. Thioredoxin superfamily and thioredoxin inducing agents. Ann NY Acad Sci. 2002;957:189–199. doi: 10.1111/j.1749-6632.2002.tb02916.x. [DOI] [PubMed] [Google Scholar]
- Holland LZ, Laudet V, Schubert M. The chordate amphioxus: an emerging model organism for developmental biology. Cell Mol Life Sci. 2004;61:2290–2308. doi: 10.1007/s00018-004-4075-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren A. Tryptophan fluorescence study of conformational transitions of the oxidized and reduced form of thioredoxin. J Biol Chem. 1972;247:1992–1998. [PubMed] [Google Scholar]
- Holmgren A. Thioredoxin catalyzes the reduction of insulin disulfides by dithiothreitol and dihydrolipoamide. J Biol Chem. 1979;254:9627–9632. [PubMed] [Google Scholar]
- Jeng MF, Campbell AP, Begley T, et al. High-resolution solution structures of oxidized and reduced Escherichia coli thioredoxin. Structure. 1994;2:853–868. doi: 10.1016/s0969-2126(94)00086-7. [DOI] [PubMed] [Google Scholar]
- Jeong WJ, Yoon HW, Lee SR, Rhee SG. Identification and characterization of TRP14, a thioredoxin-related protein of 14 kDa. New insights into the specificity of thioredoxin function. J Biol Chem. 2004a;279:3142–3150. doi: 10.1074/jbc.M307932200. [DOI] [PubMed] [Google Scholar]
- Jeong WJ, Chang TS, Boja ES, Fales HM, Rhee SG. Roles of TRP14, a thioredoxin-related protein in tumor necrosis factor-alpha signaling pathways. J Biol Chem. 2004b;279:3151–3159. doi: 10.1074/jbc.M307959200. [DOI] [PubMed] [Google Scholar]
- Kozak M. An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987;15:8125–8148. doi: 10.1093/nar/15.20.8125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laurent TC, Moore EC, Reichard P. Enzymatic synthesis of deoxyribonucleotides. Iv. Isolation and characterization of thioredoxin, the hydrogen donor from Escherichia coli B. J Biol Chem. 1964;239:3436–3444. [PubMed] [Google Scholar]
- Liang Y, Zhang S, Lun L, Han L. Presence and localization of antithrombin and its regulation after acute lipopolysaccharide exposure in amphioxus, with implications for the origin of vertebrate liver. Cell Tissue Res. 2006;323:537–541. doi: 10.1007/s00441-005-0088-x. [DOI] [PubMed] [Google Scholar]
- Liu Z, Zhang S, Yuan J, Sawant MS, Wei J, Xu A. Molecular cloning and phylogenetic analysis of AmphiUbf80, a new member of Ubiquitin family from the amphioxus Branchiostoma belcheri tsingtauense. Curr Sci. 2002;83:50–53. [Google Scholar]
- Martin JL. Thioredoxin – a fold for all reasons. Structure. 1995;3:245–250. doi: 10.1016/s0969-2126(01)00154-x. [DOI] [PubMed] [Google Scholar]
- Matsuo Y, Hirota K, Nakamura H, Yodoi J. Redox regulation by thioredoxin and its related molecules. Drug News Perspect. 2002;15:575–580. doi: 10.1358/dnp.2002.15.9.840062. [DOI] [PubMed] [Google Scholar]
- Nakamura H. Thioredoxin and its related molecules: update 2005. Antioxidants Redox Signaling. 2005;7:823–828. doi: 10.1089/ars.2005.7.823. [DOI] [PubMed] [Google Scholar]
- Powis G, Mustacich D, Coon A. The role of the redox protein thiuoredoxin in cell growth and cancer. Free Rad Biol Med. 2000;29:312–322. doi: 10.1016/s0891-5849(00)00313-0. [DOI] [PubMed] [Google Scholar]
- Powis G, Montfort WR. Properties and biological activities of thioredoxins. Annu Rev Pharmacol Toxicol. 2001;41:261–295. doi: 10.1146/annurev.pharmtox.41.1.261. [DOI] [PubMed] [Google Scholar]
- Rehm BH. Bioinformatic tools for DNA/protein sequence analysis, functional assignment of genes and protein classification. Appl Microbiol Biotechnol. 2001;57:579–592. doi: 10.1007/s00253-001-0844-0. [DOI] [PubMed] [Google Scholar]
- Stokes MD, Holland ND. The lancelet: also known as ‘amphioxus’, this curious creature has returned to the limelight as a player in the phylogenetic history of the vertebrates. Am Sci. 1998;86:552–560. [Google Scholar]
- Wang XW, Liou YC, Ho B, Ding JL. An evolutionarily conserved 16 kDa thioredoxin-related protein is an antioxidant which regulates the NF-κB signaling pathway. Free Radical Biol Med. 2007;42:247–259. doi: 10.1016/j.freeradbiomed.2006.10.040. [DOI] [PubMed] [Google Scholar]
- Windle HJ, Fox A, Ni ED, Kelleher D. The thioredoxin system of Helicobacter pylori. J Biol Chem. 2000;275:5081–5089. doi: 10.1074/jbc.275.7.5081. [DOI] [PubMed] [Google Scholar]
- Woo JR, Kim SJ, Jeong W, et al. Structural basis of cellular redox regulation by human TRP14. J Biol Chem. 2004;279:48120–48125. doi: 10.1074/jbc.M407079200. [DOI] [PubMed] [Google Scholar]
- Xue JY, Zhang SC, Liu NG, Liu ZH. Verification, characterization and tissue-specific expression of UreG, a urease accessory protein gene, from the amphioxus Branchiostoma belcheri. Acta Biochim Biophys Sin. 2006;38:549–555. doi: 10.1111/j.1745-7270.2006.00197.x. [DOI] [PubMed] [Google Scholar]