Abstract
Voltage-gated Ca2+ channels activated by action potentials evoke Ca2+ entry into presynaptic terminals thus briefly distorting the resting Ca2+ concentration. When this happens, a number of processes are initiated to re-establish the Ca2+ equilibrium. During the post-spike period, the increased Ca2+ concentration could enhance the presynaptic Ca2+ signalling. Some of the mechanisms contributing to presynaptic Ca2+ dynamics involve endogenous Ca2+ buffers, Ca2+ stores, mitochondria, the sodium–calcium exchanger, extraterminal Ca2+ depletion and presynaptic receptors. Additionally, subthreshold presynaptic depolarization has been proposed to have an effect on release of neurotransmitters through a mechanism involving changes in resting Ca2+. Direct evidence for the role of any of these participants in shaping the presynaptic Ca2+ dynamics comes from direct recordings of giant presynaptic terminals and from fluorescent Ca2+ imaging of axonal boutons. Here, some of this evidence is presented and discussed.
Keywords: analogue coding, calcium buffers, calcium depletion, calcium dynamics, calcium stores, mitochondria, presynaptic receptors
Introduction
Neurotransmitter release occurs via an exocytotic mechanism triggered by rises of the Ca2+ concentration inside the synaptic terminals (Sudhof, 2004). When neurons send chemical information to other neurons they do it through a fast chain of events (several milliseconds) that generally starts by the generation of currents at dendritic inputs (Fatt, 1957; Williams & Stuart, 2003). During their trip throughout the neuron these currents are integrated owing to the biophysical properties of the plasmatic membrane, and modulate excitability (Yuste & Tank, 1996; Gulledge et al. 2005). If the currents are excitatory and reach the initial segment of the axon, where there is a high density of Na+ channels (Inda et al. 2006), a massive depolarization can occur, producing an action potential (Fuortes et al. 1957; Colbert & Johnston, 1996). If no failures occur during the propagation of the action potential, the axonal terminals will be briefly but intensely depolarized, boosting the intraterminal Ca2+ concentration (Koester & Sakmann, 2000), mainly through the activation of voltage-dependent Ca2+ channels, triggering the exocytotic machinery (Schneggenburger & Neher, 2005).
The increase of the intraterminal Ca2+ concentration disrupts the resting condition and triggers a number of mechanisms to re-establish the Ca2+ equilibrium: (1) endogenous Ca2+ buffers will trap some of the excess of Ca2+, (2) Ca2+ pumps from three different sources (plasmatic membrane, endoplasmic reticulum and mitochondria) will use the energy of ATP to extrude Ca2+ from the cytoplasm, and (3) the Na+/Ca2+ exchanger will generally use the electrochemical gradient of Na+ ions to pump Ca2+ out of the presynaptic site. During these processes, the residual increase in Ca2+ concentration is likely to facilitate neurotransmitter release (Kamiya & Zucker, 1994).
Most plastic phenomena in synaptic transmission are related to the dynamics of presynaptic Ca2+ (if not directly explained by them). Although presynaptic Ca2+ dynamics has been intensely studied for many years, recent breakthroughs have come from electrophysiological recordings from single giant terminals (Geiger & Jonas, 2000) and from Ca2+ imaging in individual presynaptic boutons (Koester & Sakmann, 2000). Here, some well-documented mechanisms affecting presynaptic Ca2+ signalling, together with other contributing factors, are discussed.
Residual Ca2+ hypothesis and endogenous Ca2+ buffers
Paired pulse facilitation (PPF) is a short-term plasticity phenomenon occurring when the postsynaptic response to an action potential (AP) is larger than the response to a previous AP, the two stimuli being separated by less than a few hundred milliseconds. Katz & Miledi (1968) proposed that facilitation was caused by the slow decay of the Ca2+ transient following an AP so that if a subsequent AP occurs shortly thereafter, the residual Ca2+ adds to the new Ca2+ elevation, increasing the probability of release (Fig. 1A). This model was called the ‘(residual) Ca2+ hypothesis’ and was proposed for the neuromuscular junction.
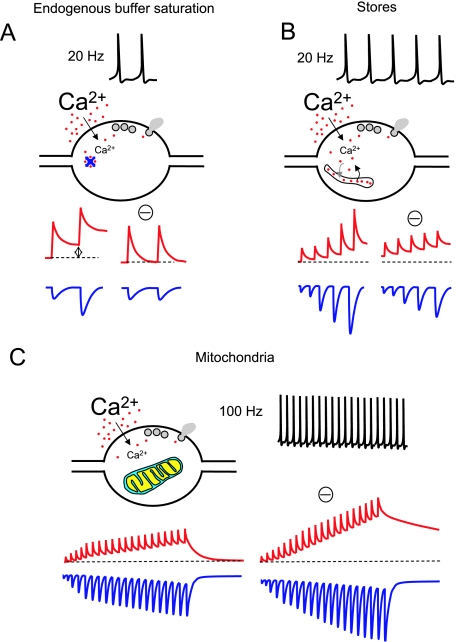
Fig. 1.
Mechanisms of control of presynaptic Ca2+ dynamics (I). For all the panels in this figure and Fig. 2 a general idealistic illustration of each of the mechanisms described in the text is shown. The stimulating protocols at which these mechanisms occur are depicted on top of each panel. Red traces represent free Ca2+ concentration. Blue traces represent postsynaptic responses. The hypothetical blockade of each mechanism is indicated with a minus symbol (‘–’). All panels represent general concepts and are not intended to show quantitative data. For an accurate idea of a realistic view of free Ca2+ dynamics and postsynaptic responses see Fig. 3. Only non-depressing synapses with a low release probability are considered. (A) The role of endogenous buffers in paired pulse facilitation (PPF) at a synaptic terminal. Two APs at 20 Hz produce two consecutive increases in Ca2+ concentration inside the terminal. The endogenous presynaptic Ca2+ buffers rapidly trap Ca2+ ions and can be partially saturated after the first AP at relatively high frequencies. Therefore, the residual Ca2+ when the second AP occurs is higher (left red trace), and this enhances transmitter release (left blue trace). The effect on free Ca2+ and EPSCs of a hypothetic blockade of buffer saturation is also illustrated. (B) Upon repetitive stimulation at 20 Hz, Ca2+ release from presynaptic Ca2+ stores can occur, contributing to the global Ca2+ signal. Through this mechanism neurotransmitter release is facilitated. The blockade of Ca2+ stores should reduce facilitation (only partially due to the presence of mechanism A) and increase resting Ca2+. (C) Intense (100 Hz) stimulation recruits presynaptic mitochondria and contributes to buffer the excess of Ca2+. In the absence of mitochondria the presynaptic Ca2+ concentration would reach higher levels during such a repetitive high-frequency stimulation, increasing the release rate.
Experimental evidence has been reported extending this hypothesis to central synapses (Regehr et al. 1994; for a review see Burnashev & Rozov, 2005). Direct measurements of Ca2+-dependent fluorescent transients in individual axonal boutons show that intraterminal Ca2+ dynamics after an AP is altered for several hundred seconds (Koester & Sakmann, 2000). The kinetics of free Ca2+ transients (Ca2+ available to trigger release) has been assessed by two-photon imaging in single hippocampal mossy fibre terminals, suggesting that high-frequency trains of AP elevate residual Ca2+ to about 0.2–1.5 µm during a train of five APs (Fig. 3; Scott & Rusakov, 2006). Normally, such Ca2+ rises do not to produce important (if any) release at central synapses but would be enough to induce facilitation when they are added to the local peak Ca2+ concentration (Bollmann et al. 2000; Schneggenburger & Neher, 2000).
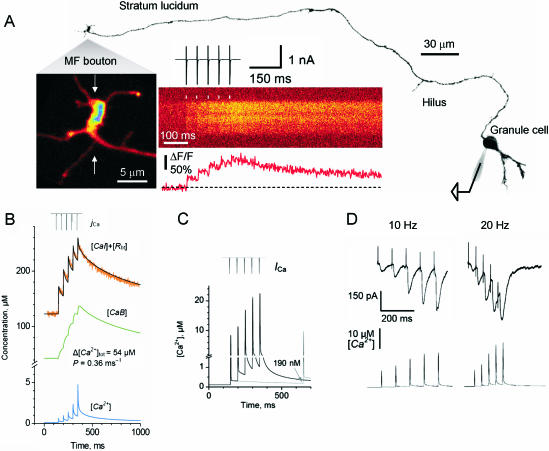
Fig. 3.
Ca2+ dynamics in mossy fibre giant terminals at the stratum lucidum. (A) Giant terminal (with visible filopodia) emerging from the mossy fibre of a granule cell loaded with alexa-594 and fluo-4 through a patch pipette. The morphology of the cell and the main axon is shown (collateral branches were out of focus). The cell body was briefly depolarized evoking action currents that propagated along the axon producing Ca2+-dependent fluorescence transients at the giant terminals, almost 1 mm distant. These transients were recorded by line-scanning two-photon microscopy. The average of ten fluorescent transients is shown. (B) Average response for 18 giant boutons (orange trace) in similar conditions to A. Several parameters involved in Ca2+ dynamics could be assumed (AP-dependent Ca2+ influx, calbindin concentration, dye concentration, and Kd of the dye; Scott & Rusakov, 2006). An accurate fitting of the experimental data was obtained, also rendering the kinetics of the endogenous buffer bound to Ca2+ (green trace) and the free Ca2+ concentration changes during trains of AP (blue trace). In order to constrain the unknown parameters (total Ca2+ entry and removal rate, Δ[Ca]tot and P, respectively), the experiments were repeated with fluo-4 (50 µm) and fluo-5 (200 µm). Similar values were obtained in the three conditions for these unknowns (not shown). (C) The model allowed us to extrapolate the dynamics of free Ca2+ concentration in the absence of exogenous buffers. It was also possible to simulate the free Ca2+ dynamics during different frequencies of AP. (D) Typical EPSCs recorded in CA3 pyramidal cells at 20 Hz and 50 Hz. Below, the simulations of free Ca2+ at the corresponding frequencies (modified from Scott & Rusakov, 2006).
Endogenous Ca2+ buffers are widely expressed in many neurons throughout the nervous system. These buffers are proteins such as parvalbumin, calretinin and calbindin with Ca2+ binding domains with different affinity for Ca2+. Their presence and concentration in synaptic terminals can explain certain properties of neurotransmission, especially PPF. One of the proposed mechanisms responsible for the slow residual Ca2+ decay after an AP is the local saturation of the fast endogenous Ca2+ buffers in the terminal during a train of APs (Klingauf & Neher, 1997). Recently, this mechanism has been shown to act at cortical calbindin-containing terminals (Blatow et al. 2003) as well as at giant calyx of Held synapses (Felmy et al. 2003).
Exogenous buffers have been widely used as a tool to investigate PPF. EGTA is a Ca2+ buffer which is too slow to compete with the fast Ca2+ sensor of the release machinery but fast enough to buffer residual Ca2+ during the interval between a pair of APs. Low concentrations of EGTA can abolish PPF (Blatow et al. 2003). Interestingly, terminals that contain parvalbumin, a slow endogenous buffer, do not show the strong facilitation occurring in parvalbumin knock-out mice (Caillard et al. 2000). The effect of slow buffers in PPF suggests that one of the requisites for a terminal to be facilitating is a long distance between the AP-dependent Ca2+ source and the Ca2+ sensor of the release machinery.
Unlike facilitation, short-term depression is generally explained by depletion of the ready releasable pool of vesicles in synaptic terminals. Therefore, the mechanism for depression does not seem to involve presynaptic Ca2+ dynamics. Consequently, for a terminal to be facilitating (in the context of the residual Ca2+ hypothesis) its release probability p should normally be low. At many synapses therefore repetitive activation results in an interplay between facilitation and depression of transmission.
Ca2+ stores
Ca2+-induced Ca2+ release (CICR) mediated by ryanodine receptors (RyRs) from the sarcoplasmic reticulum has been well described in cardiac, skeletal and smooth muscles. In the brain, RyRs are localized in the endoplasmic reticulum (ER) at postsynaptic sites and glia (for a review see Verkhratsky, 2005). There is no general consensus on whether RyRs are located in presynaptic terminals in the brain, nor is it clear whether RyRs participate in the dynamics of intraterminal Ca2+.
Although not universally, electron microscopy provides evidence for the presence of ER in presynaptic terminals (McGraw et al. 1980; Hartter et al. 1987; Lysakowski et al. 1999).
Carter et al. (2002) loaded bunches of hippocampal axons with membrane-permeable Ca2+-sensitive dyes and directly measured (global) Ca2+-dependent fluorescence transients (CaFT) induced by pairs of pulses. They reported no effect of thapsigargin and ryanodine (both drugs altering the Ca2+ store dynamics) in four different types of excitatory synaptic terminals from the hippocampus and cerebellum, including the mossy fibre large terminals in contact with CA3 pyramidal cells in the hippocampus.
However, Ca2+ imaging of individual terminals (rather than wide-field imaging of the bulk of fibres) in conditions of repetitive stimuli has revealed that these drugs could reduce the AP-evoked Ca2+ signal (Fig. 1B; Llano et al. 2000; Emptage et al. 2001; Liang et al. 2002; Scott & Rusakov, 2006). In addition, Ca2+ stores regulate the resting levels of Ca2+ at presynaptic terminals, thus playing a dual role, one more dependent and one less dependent on cell activity (Scott & Rusakov, 2006).
The evidence proposing a role for presynaptic CICR from stores in Ca2+ dynamics and therefore synaptic transmission is based on the effects of bath-applied ryanodine and thapsigargin (for a review see Bouchard et al. 2003). Because Ca2+ stores are present in dendrites and soma, the disruption of the Ca2+ dynamics in these compartments could affect the Ca2+ homeostasis in synaptic terminals simply because of diffusion of Ca2+ from one compartment to another. Recording at synaptic terminals located 1 mm or more far from the soma makes this problem less likely (Scott & Rusakov, 2006). However, such diffusional artefacts cannot be completely ruled out. A clear-cut demonstration of the functional role of presynaptic Ca2+ stores would require Ca2+ imaging inside the presynaptic stores.
Mitochondria
Blaustein et al. (1978) showed that a fraction of the Ca45 uptake in patched synaptosomes was associated with the intraterminal mitochondria. Whether mitochondria are important for transmitter release in the central nervous system has been a matter of intense studies during the last two decades. In a detailed electron microscopy study Shepherd & Harris (1998) showed that mitochondria are present only in < 50% of typical central presynaptic boutons from hippocampal CA1 axons, and a similar conclusion has been found by imaging mitochondria with fluorescent dyes captured by the organelle, in combination with imaging of FM dye destaining as an assay of neurotransmitter release (Waters & Smith, 2003). This evidence seems to raise the question if mitochondria are essential participants in the presynaptic function.
Direct imaging of mitochondrial and cytoplasmic Ca2+ in whole cell recordings of central giant terminals (calyx of Held) and simultaneous postsynaptic recordings have elegantly demonstrated a role of mitochondrial Ca2+ sequestration in synaptic transmission in the brain (Billups & Forsythe, 2002; see also Fig. 1C). This phenomenon has also been reported in other systems (Tang & Zucker, 1997; David et al. 1998; David & Barrett, 2000), although not universally (Kobayashi & Tachibana, 1995; Zenisek & Matthews, 2000). Mitochondrial interaction with presynaptic Ca2+ dynamics seems to affect synaptic plasticity (Tang & Zucker, 1997; Levy et al. 2003), for instance, by maintaining transmission accelerating recovery from synaptic depression after periods of moderate activity (Billups & Forsythe, 2002).
Apart from their role in Ca2+ clearance during repetitive activity, mitochondria have also been proposed to act as Ca2+ reservoirs that are mobilized by the Na+ influx during high-frequency and long-lasting stimulation in excitable cells. The increase of intracellular Na+ concentration activates the mitochondrial Na+/Ca2+ exchanger and induces Ca2+ efflux from the organelle (Rizzuto, 2003; Yang et al. 2003).
By directly patching mitochondria inside synaptic terminals of the giant squid axon, Jonas et al. (1999) detected large conductances (several nS) occurring with an occasional frequency that increased up to 60-fold during trains of AP several seconds long at high frequency, and continued to increase after the stimulation had stopped, to recover after tens of seconds. Interestingly, when mitochondria become overloaded with Ca2+, they can undergo the so-called mitochondrial permeability transition. This includes formation of a non-selective pore that allows solutes of 1500 Da or smaller to pass through the inner mitochondrial membrane with a resultant rupture of the outer mitochondrial membrane caused by osmotic swelling. If occurring in axonal mitochondria, such mechanisms would affect presynaptic Ca2+ dynamics. Interestingly, synaptic mitochondria seem to be more susceptible than somatic or dendritic mitochondria of suffering mitochondrial permeability transition (Brown et al. 2006).
Na+/Ca2+ exchanger
There is evidence supporting a functional role for the presynaptic Na+/Ca2+ exchanger (NCX) in cultured hippocampal neurons (Reuter & Porzig, 1995; Doi et al. 2002), in cerebellar granule cells (Doi et al. 2002; Regehr, 1997), in neurohypophysis (Lee et al. 2002) and in the calyx of Held (Kim et al. 2005).
The NCX has a high capacity for Ca2+ transport, but a low affinity for Ca2+ (Allen et al. 1989). It exchanges three Na+ ions for one Ca2+ ion and, in the case of the Na+/Ca2+K+ exchanger (NCKX), four Na+ ions for one Ca2+ and one K+ ion. This electrogenic mechanism, depending on the membrane potential and the intracellular and extracellular concentrations of Na+ and Ca2+ (and K+), can either remove Ca2+ from the cell or transport Ca2+ from the extracellular space into the cytoplasm. Actually, there is evidence showing that presynaptic residual Ca2+ after a period of high-frequency activity is caused by Ca2+ influx through a reverse mode Na+/Ca2+ exchange (Zhong et al. 2001).
Study of the role of NCX in central synapses has been difficult owing to the lack of specific pharmacology. Using Na+- and Ca2+-sensitive fluorescent indicators it has been possible to record spike-evoked Na+ and Ca2+ presynaptic transients in granule cell parallel fibres in brain slices from rat cerebellum. A model of the kinetics of the NCX and the Ca-ATPase at presynaptic terminals fitted well with the recorded Na+- and Ca2+-dependent fluorescence transients. Immediately following stimulation, the NCX removed Ca2+ from the terminal more rapidly than does the Ca-ATPase. However, eventually, the large Na+ influx drives the exchanger into steady state, being the Ca-ATPase the only one to extrude Ca2+. This disrupts the equilibrium of the NCX, which acts opposite to the Ca-ATPase, and thus Ca2+ and Na+ slowly return to resting levels (Regehr, 1997).
At the Calix of Held, Kim et al. (2005) compared the presynaptic Ca2+ removal after a 50-ms voltage depolarization in the presence of normal extracellular Na+ concentration or the same concentration of Li+. They also studied the effect of substituting internal K+ by tetraethylammonium (TEA). Their results suggest that, in response to small Ca2+ transients (2 µm), Ca2+ loads are cleared from the calyx of Held primarily by NCKX (42%) and NCX (26%). Additionally, they conclude that in these conditions plasmatic membrane Ca2+ ATPase participates with 23% of Ca2+ clearance, and that mitochondria participate when the Ca2+ load is larger or prolonged.
New pharmacological selective tools are needed to characterize further the role of presynaptic NCX with different patterns of stimulation.
Ca2+ depletion in the synaptic cleft
A potentially important mechanism of regulating presynaptic (and postsynaptic) Ca2+ dynamics is depletion of the extracellular concentration of Ca2+ in the synaptic cleft due to Ca2+ flux from the synaptic cleft into the pre- and postsynaptic sites (Fig. 2A). This would reduce the driving force for Ca2+ and, during high-frequency activity, would diminish subsequent Ca2+ influx.
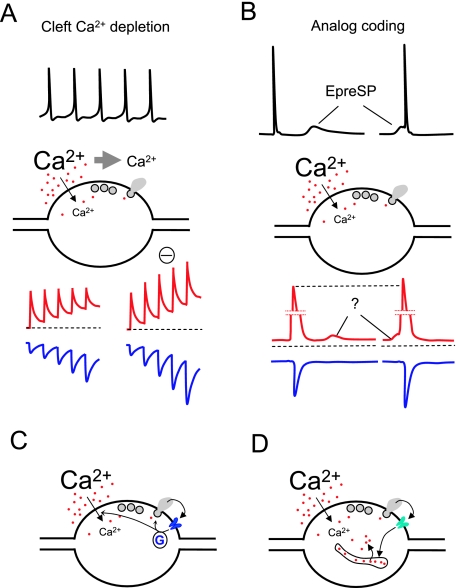
Fig. 2.
Mechanisms of control of presynaptic Ca2+ dynamics (II). See also legend to Fig. 1. (A) High-frequency stimulation transiently decreases the extracellular concentration of Ca2+ in the synaptic cleft due to Ca2+ ions passing into the cells during the electrical activity. The reduction of the electrochemical gradient for Ca2+ produces a decrease of presynaptic Ca2+ entry during subsequent AP and a reduction of neurotransmitter release. A hypothetical blockade of Ca2+ depletion restablishes the amplitude of consecutive Ca2+ transients and enhances facilitation. (B) Subthreshold voltage changes in the dendrites or soma can passively travel relatively long distances along the axons and regulate neurotransmitter release when they immediately precede an AP. A suggested mechanism for this phenomenon would be that small changes in resting Ca2+ produced by such subthreshold voltage signals would add to the Ca2+ transient elicited by an AP, resulting in an enhancement of transmitter release. (C) Presynaptic metabotropic receptors can modulate Ca2+ entry and or neurotransmitter release via G-proteins. (D) Ionotropic receptors can regulate neurotransmitter release interacting with intracellular stores.
The concentration of Ca2+ in the extracellular volume of the brain decreases during repetitive activity in both physiological and pathophysiological conditions (Nicholson et al. 1978; Krnjevic et al. 1980; Heinemann et al. 1986).
Changes of Ca2+ concentration in the synaptic cleft have been theoretically predicted (Smith, 1992; Vassilev et al. 1997; Egelman & Montague, 1998; Rusakov et al. 1998). Indeed, several lines of experimental evidence propose that such reductions in presynaptic Ca2+ influx may occur due to activity-dependent depletion of Ca2+ in the synaptic cleft of calyceal synapses (Borst & Sakmann, 1999; Stanley, 2000; Rabl & Thoreson, 2002). Additionally, in more common types of central synapses depletion of extracellular Ca2+ was optically measured in response to brief trains of APs. This phenomenon seems to be due to postsynaptic NMDA receptor activation and modulates presynaptic release (Rusakov & Fine, 2003).
A role for the glial sheath covering synapses has been proposed to enhance Ca2+ depletion in the synaptic cleft in a computational model (Rusakov, 2001). Finally, it has recently been shown that depletion of extracellular Ca2+ can regulate neuronal Ca2+/CaM-dependent protein kinase via a depletion of intracellular stores (Cohen & Fields, 2006). Whether enzyme activities in synaptic terminals can be modulated during brain activity by extracellular Ca2+ depletion requires further investigation.
Presynaptic receptors
Presynaptic receptors (metabotropic as well as ionotropic) affecting Ca2+ dynamics at synaptic terminals have been investigated by using intracellular Ca2+ chelants and, more recently, by directly imaging presynaptic boutons. Presynaptic group II and III metabotropic glutamate receptors modulate excitatory transmission by decreasing AP-dependent presynaptic Ca2+ transients at area CA1 synapses in the hippocampus (Faas et al. 2002). They could act on both cytoplasmic and membrane-bound effectors by differential activation of either diffusible Gα proteins or the membrane-associated Gβγ subunits, respectively, or both (Fig. 2C). Potentially, these actions of mGluRs could affect both the release cascade directly (mainly through Gα signalling) or by down-modulating Ca2+ channels (mainly through Gβγ signalling).
Presynaptic ionotropic receptors (Fig. 2D), like kainate receptors (KARs), also affect presynaptic Ca2+ signalling (Kullmann, 2001). Ca2+ imaging of the bulk of mossy fibres in hippocampal area CA3 suggests that activation of KARs reduces presynaptic Ca2+ transients (Kamiya et al. 2002). Consistent with this finding, electrophysiological recordings suggest a KAR-dependent triggering of Ca2+ release from internal stores at mossy fibre synapses (Lauri et al. 2003). Besides the extensively studied KARs, other types of ionotropic receptors have also been implicated in presynaptic modulation of transmission at central synapses. In the cerebellum, presynaptic NMDA receptors enhance GABA release at inhibitory synapses onto Purkinje cells (Duguid & Smart, 2004) and also contribute to long-term depression at parallel fibre–Purkinje cell synapses (Casado et al. 2002). A role for presynaptic NMDA receptors in synaptic depression has also been described in neocortical pyramidal cells (Sjostrom et al. 2003). Regulation of neurotransmitter release by presynaptic GABAA receptors, a well-established mechanism of inhibition of presynaptic activity in the spinal cord, has been found to occur in the calyx of Held (Turecek & Trussell, 2001) and in developing cerebellar interneurons (Pouzat & Marty, 1999). In individual axonal boutons from hippocampal mossy fibres, GABAA receptors reduce presynaptic AP-induced Ca2+ entry and elevate resting Ca2+ (Ruiz et al. 2003). Depending on the intracellular concentration of Cl−, the activation of these receptors could either depolarize or hyperpolarize the terminals allowing, in principle, a bi-directional control of the release probability.
Analogue coding
Neurotransmitter release at synaptic terminals located at relatively short distances can occur or be modulated by subthreshold changes of the somatic or dendritic membrane potential. Such a graded transmission (in contrast to transmission due to APs) occurs in small invertebrates (Juusola et al. 1996) and it is possible in part because of the specific expression of presynaptic L-type Ca2+ channels, which activate relatively rapidly and do not (or almost do not) inactivate.
Recently, a graded (also termed analogue) modulation of synaptic transmission has been described for neurons in the mammalian brain (Alle & Geiger, 2006; Shu et al. 2006). Subthreshold voltage changes caused by synaptic activity in dendrites can be passively transmitted along the axon and reach far distances due to the long length constant of the axon (~430 µm). These voltage signals, when overlapping with an AP, enhance transmitter release (Fig. 2B). Because intracellular EGTA decreases this facilitation, it has been suggested that analogue coding could be related to presynaptic Ca2+ changes. Nevertheless, the reduction of facilitation in the presence of intracellular EGTA could also be explained by a combined voltage and Ca2+ dependence of the release machinery. At mossy fibre terminals in area CA3, Ca2+ channels do not seem to activate at voltages more negative than –60 mV (Bischofberger et al. 2002). This appears to be inconsistent with an effect of subthreshold dendritic activity on Ca2+ channels from axonal terminals located 500–1000 µm from the soma, assuming a Vm of approximately –80 mV at granule cell soma and terminals and a length constant of 430 µm for the mossy fibre (Alle & Geiger, 2006). At the calyx of Held, subthreshold depolarization of the terminals produces Ca2+ entry through P/Q-type Ca2+ channels, thus enhancing neurotransmitter release (Awatramani et al. 2005). Direct evidence for subthreshold resting Ca2+ changes in axonal varicosities has been reported for the hippocampal mossy fibre up to 200 µm from the soma. However, these changes in resting Ca2+ were detected when the soma underwent long (tens of seconds) periods of subthreshold depolarization (Ruiz et al. 2003). Additionally, somatic effects on AP-dependent Ca2+ signal have been reported for this axon, occurring with a length constant of ~175 µm (Scott & Rusakov, 2006). To determine whether Ca2+ signalling could underlie the mechanism of analogue electronic control in these conditions, it would be advantageous to monitor the dendrosomatic subthreshold effects on presynaptic Ca2+ dynamics – mediated by Ca2+ channels, Ca2+ stores or other mechanisms – directly in individual remote terminals.
Concluding remarks
A number of mechanisms, which often interact with one another, take part in controlling presynaptic Ca2+ dynamics in different conditions. An ideal research objective would be to build a mechanistic model describing the integrated effect of these mechanisms in the intraterminal Ca2+ concentration for specific synapses. Novel imaging techniques will surely provide interesting data on local presynaptic Ca2+, and, ultimately, on the integration of the kinetics of Ca2+ microdomains with exocytotic events. In the next few years our knowledge of the dynamics of presynaptic Ca2+ will reach the submicrometre resolution for several types of synapses. However, many questions will remain. How could the signature of presynaptic Ca2+ dynamics of certain synapses affect neural circuits? Can these circuits alter their pattern of activity by changes in presynaptic Ca2+ dynamics of some (how many?) of their individual synapses? Would these network changes occur during development and during leaning?
Acknowledgments
Thanks to Dmitri Rusakov for his careful reading of the manuscript and his valuable comments.
References
- Alle H, Geiger JR. Combined analog and action potential coding in hippocampal mossy fibers. Science. 2006;311:1290–1293. doi: 10.1126/science.1119055. [DOI] [PubMed] [Google Scholar]
- Allen TJA, Noble D, Reuter H. Sodium–Calcium Exchange. Oxford: Oxford University Press; 1989. [Google Scholar]
- Awatramani GB, Price GD, Trussell LO. Modulation of transmitter release by presynaptic resting potential and background calcium levels. Neuron. 2005;48:109–121. doi: 10.1016/j.neuron.2005.08.038. [DOI] [PubMed] [Google Scholar]
- Billups B, Forsythe ID. Presynaptic mitochondrial calcium sequestration influences transmission at mammalian central synapses. J Neurosci. 2002;22:5840–5847. doi: 10.1523/JNEUROSCI.22-14-05840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischofberger J, Geiger JR, Jonas P. Timing and efficacy of Ca2+ channel activation in hippocampal mossy fiber boutons. J Neurosci. 2002;22:10593–10602. doi: 10.1523/JNEUROSCI.22-24-10593.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blatow M, Caputi A, Burnashev N, Monyer H, Rozov A. Ca2+ buffer saturation underlies paired pulse facilitation in calbindin-D28k-containing terminals. Neuron. 2003;38:79–88. doi: 10.1016/s0896-6273(03)00196-x. [DOI] [PubMed] [Google Scholar]
- Blaustein MP, Ratzlaff RW, Schweitzer ES. Calcium buffering in presynaptic nerve terminals. II. Kinetic properties of the nonmitochondrial Ca sequestration mechanism. J Gen Physiol. 1978;72:43–66. doi: 10.1085/jgp.72.1.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollmann JH, Sakmann B, Gerard J, Borst G. Calcium sensitivity of glutamate release in a calyx-type terminal. Science. 2000;289:953–957. doi: 10.1126/science.289.5481.953. [DOI] [PubMed] [Google Scholar]
- Borst JG, Sakmann B. Depletion of calcium in the synaptic cleft of a calyx-type synapse in the rat brainstem. J Physiol. 1999;521:123–133. doi: 10.1111/j.1469-7793.1999.00123.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouchard R, Pattarini R, Geiger JD. Presence and functional significance of presynaptic ryanodine receptors. Prog Neurobiol. 2003;69:391–418. doi: 10.1016/s0301-0082(03)00053-4. [DOI] [PubMed] [Google Scholar]
- Brown MR, Sullivan PG, Geddes JW. Synaptic mitochondria are more susceptible to Ca2+ overload than nonsynaptic mitochondria. J Biol Chem. 2006;281:11658–11668. doi: 10.1074/jbc.M510303200. [DOI] [PubMed] [Google Scholar]
- Burnashev N, Rozov A. Presynaptic Ca2+ dynamics, Ca2+ buffers and synaptic efficacy. Cell Calcium. 2005;37:489–495. doi: 10.1016/j.ceca.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Caillard O, Moreno H, Schwaller B, Llano I, Celio MR, Marty A. Role of the calcium-binding protein parvalbumin in short-term synaptic plasticity. Proc Natl Acad Sci USA. 2000;97:13372–13377. doi: 10.1073/pnas.230362997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AG, Vogt KE, Foster KA, Regehr WG. Assessing the role of calcium-induced calcium release in short-term presynaptic plasticity at excitatory central synapses. J Neurosci. 2002;22:21–28. doi: 10.1523/JNEUROSCI.22-01-00021.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casado M, Isope P, Ascher P. Involvement of presynaptic N-methyl-D-aspartate receptors in cerebellar long-term depression. Neuron. 2002;33:123–130. doi: 10.1016/s0896-6273(01)00568-2. [DOI] [PubMed] [Google Scholar]
- Cohen JE, Fields RD. CaMKII inactivation by extracellular Ca(2+) depletion in dorsal root ganglion neurons. Cell Calcium. 2006;39:445–454. doi: 10.1016/j.ceca.2006.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colbert CM, Johnston D. Axonal action-potential initiation and Na+ channel densities in the soma and axon initial segment of subicular pyramidal neurons. J Neurosci. 1996;16:6676–6686. doi: 10.1523/JNEUROSCI.16-21-06676.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G, Barrett JN, Barrett EF. Evidence that mitochondria buffer physiological Ca2+ loads in lizard motor nerve terminals. J Physiol. 1998;509:59–65. doi: 10.1111/j.1469-7793.1998.059bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David G, Barrett EF. Stimulation-evoked increases in cytosolic [Ca(2+)] in mouse motor nerve terminals are limited by mitochondrial uptake and are temperature-dependent. J Neurosci. 2000;20:7290–7296. doi: 10.1523/JNEUROSCI.20-19-07290.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi A, Kakazu Y, Akaike N. Na+/Ca2+ exchanger in GABAergic presynaptic boutons of rat central neurons. J Neurophysiol. 2002;87:1694–1702. doi: 10.1152/jn.00400.2001. [DOI] [PubMed] [Google Scholar]
- Duguid IC, Smart TG. Retrograde activation of presynaptic NMDA receptors enhances GABA release at cerebellar interneuron-Purkinje cell synapses. Nat Neurosci. 2004;7:525–533. doi: 10.1038/nn1227. [DOI] [PubMed] [Google Scholar]
- Egelman DM, Montague PR. Computational properties of peri-dendritic calcium fluctuations. J Neurosci. 1998;18:8580–8589. doi: 10.1523/JNEUROSCI.18-21-08580.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emptage NJ, Reid CA, Fine A. Calcium stores in hippocampal synaptic boutons mediate short-term plasticity, store-operated Ca2+ entry, and spontaneous transmitter release. Neuron. 2001;29:197–208. doi: 10.1016/s0896-6273(01)00190-8. [DOI] [PubMed] [Google Scholar]
- Faas GC, Adwanikar H, Gereau RW, Saggau P. Modulation of presynaptic calcium transients by metabotropic glutamate receptor activation: a differential role in acute depression of synaptic transmission and long-term depression. J Neurosci. 2002;22:6885–6890. doi: 10.1523/JNEUROSCI.22-16-06885.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatt P. Sequence of events in synaptic activation of a motoneurone. J Neurophysiol. 1957;20:61–80. doi: 10.1152/jn.1957.20.1.61. [DOI] [PubMed] [Google Scholar]
- Felmy F, Neher E, Schneggenburger R. Probing the intracellular calcium sensitivity of transmitter release during synaptic facilitation. Neuron. 2003;37:801–811. doi: 10.1016/s0896-6273(03)00085-0. [DOI] [PubMed] [Google Scholar]
- Fuortes MGF, Frank K, Becker MC. Steps in the production of motoneuron spikes. J Gen Physiol. 1957;40:735–752. doi: 10.1085/jgp.40.5.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger JRP, Jonas P. Dynamic control of presynaptic Ca2+ inflow by fast-inactivating K+ channels in hippocampal mossy fiber boutons. Neuron. 2000;28:927–939. doi: 10.1016/s0896-6273(00)00164-1. [DOI] [PubMed] [Google Scholar]
- Gulledge AT, Kampa BM, Stuart GJ. Synaptic integration in dendritic trees. J Neurobiol. 2005;64:75–90. doi: 10.1002/neu.20144. [DOI] [PubMed] [Google Scholar]
- Hartter DE, Burton PR, Laveri LA. Distribution and calcium-sequestering ability of smooth endoplasmic reticulum in olfactory axon terminals of frog brain. Neuroscience. 1987;23:371–386. doi: 10.1016/0306-4522(87)90297-1. [DOI] [PubMed] [Google Scholar]
- Heinemann U, Konnerth A, Pumain R, Wadman WJ. Extracellular calcium and potassium concentration changes in chronic epileptic brain tissue. Adv Neurol. 1986;44:641–661. [PubMed] [Google Scholar]
- Inda MC, DeFelipe J, Munoz A. Voltage-gated ion channels in the axon initial segment of human cortical pyramidal cells and their relationship with chandelier cells. Proc Natl Acad Sci USA. 2006;103:2920–2925. doi: 10.1073/pnas.0511197103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas EA, Buchanan J, Kaczmarek LK. Prolonged activation of mitochondrial conductances during synaptic transmission. Science. 1999;286:1347–1350. doi: 10.1126/science.286.5443.1347. [DOI] [PubMed] [Google Scholar]
- Juusola M, French AS, Uusitalo RO, Weckstrom M. Information processing by graded-potential transmission through tonically active synapses. Trends Neurosci. 1996;19:292–297. doi: 10.1016/S0166-2236(96)10028-X. [DOI] [PubMed] [Google Scholar]
- Kamiya H, Zucker RS. Residual Ca2+ and short-term synaptic plasticity. Nature. 1994;371:603–606. doi: 10.1038/371603a0. [DOI] [PubMed] [Google Scholar]
- Kamiya H, Ozawa S, Manabe T. Kainate receptor-dependent short-term plasticity of presynaptic Ca2+ influx at the hippocampal mossy fiber synapses. J Neurosci. 2002;22:9237–9243. doi: 10.1523/JNEUROSCI.22-21-09237.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz B, Miledi R. Role of calcium in neuromuscular facilitation. J Physiol. 1968;195:481–492. doi: 10.1113/jphysiol.1968.sp008469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MH, Korogod N, Schneggenburger R, Ho WK, Lee SH. Interplay between Na+/Ca2+ exchangers and mitochondria in Ca2+ clearance at the calyx of Held. J Neurosci. 2005;25:6057–6065. doi: 10.1523/JNEUROSCI.0454-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingauf J, Neher E. Modeling buffered Ca2+ diffusion near the membrane: Implications for secretion in neuroendocrine cells. Biophys J. 1997;72:674–690. doi: 10.1016/s0006-3495(97)78704-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi K, Tachibana M. Ca2+ regulation in the presynaptic terminals of goldfish retinal bipolar cells. J Physiol. 1995;483:79–94. doi: 10.1113/jphysiol.1995.sp020569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koester HJ, Sakmann B. Calcium dynamics associated with action potentials in single nerve terminals of pyramidal cells in layer 2/3 of the young rat neocortex. J Physiol. 2000;529:625–646. doi: 10.1111/j.1469-7793.2000.00625.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krnjevic K, Morris ME, Reiffenstein RJ. Changes in extracellular Ca2+ and K+ activity accompanying hippocampal discharges. Can J Physiol Pharmacol. 1980;58:579–582. doi: 10.1139/y80-097. [DOI] [PubMed] [Google Scholar]
- Kullmann DM. Presynaptic kainate receptors in the hippocampus: slowly emerging from obscurity. Neuron. 2001;32:561–564. doi: 10.1016/s0896-6273(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Lauri SE, Bortolotto ZA, Nistico R, et al. A role for Ca2+ stores in kainate receptor-dependent synaptic facilitation and LTP at mossy fiber synapses in the hippocampus. Neuron. 2003;39:327–341. doi: 10.1016/s0896-6273(03)00369-6. [DOI] [PubMed] [Google Scholar]
- Lee SH, Kim MH, Park KH, Earm YE, Ho WK. K+-dependent Na+/Ca2+ exchange is a major Ca2+ clearance mechanism in axon terminals of rat neurohypophysis. J Neurosci. 2002;22:6891–6899. doi: 10.1523/JNEUROSCI.22-16-06891.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy M, Faas GC, Saggau P, Craigen WJ, Sweatt JD. Mitochondrial regulation of synaptic plasticity in the hippocampus. J Biol Chem. 2003;278:17727–17734. doi: 10.1074/jbc.M212878200. [DOI] [PubMed] [Google Scholar]
- Liang Y, Yuan LL, Johnston D, Gray R. Calcium signaling at single mossy fiber presynaptic terminals in the rat hippocampus. J Neurophysiol. 2002;87:1132–1137. doi: 10.1152/jn.00661.2001. [DOI] [PubMed] [Google Scholar]
- Llano I, Gonzalez J, Caputo C, et al. Presynaptic calcium stores underlie large-amplitude miniature IPSCs and spontaneous calcium transients. Nat Neurosci. 2000;3:1256–1265. doi: 10.1038/81781. [DOI] [PubMed] [Google Scholar]
- Lysakowski A, Figueras H, Price SD, Peng YY. Dense-cored vesicles, smooth endoplasmic reticulum, and mitochondria are closely associated with non-specialized parts of plasma membrane of nerve terminals: implications for exocytosis and calcium buffering by intraterminal organelles. J Comp Neurol. 1999;403:378–390. doi: 10.1002/(sici)1096-9861(19990118)403:3<378::aid-cne7>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- McGraw CF, Somlyo AV, Blaustein MP. Localization of calcium in presynaptic nerve terminals. An ultrastructural and electron microprobe analysis. J Cell Biol. 1980;85:228–241. doi: 10.1083/jcb.85.2.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson C, ten Bruggencate G, Stockle H, Steinberg R. Calcium and potassium changes in extracellular microenvironment of cat cerebellar cortex. J Neurophysiol. 1978;41:1026–1039. doi: 10.1152/jn.1978.41.4.1026. [DOI] [PubMed] [Google Scholar]
- Pouzat C, Marty A. Somatic recording of GABAergic autoreceptor current in cerebellar stellate and basket cells. J Neurosci. 1999;19:1675–1690. doi: 10.1523/JNEUROSCI.19-05-01675.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabl K, Thoreson WB. Calcium-dependent inactivation and depletion of synaptic cleft calcium ions combine to regulate rod calcium currents under physiological conditions. Eur J Neurosci. 2002;16:2070–2077. doi: 10.1046/j.1460-9568.2002.02277.x. [DOI] [PubMed] [Google Scholar]
- Regehr WG. Interplay between sodium and calcium dynamics in granule cell presynaptic terminals. Biophys J. 1997;73:2476–2488. doi: 10.1016/S0006-3495(97)78276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regehr WG, Delaney KR, Tank DW. The role of presynaptic calcium in short-term enhancement at the hippocampal messy fiber synapse. J Neurosci. 1994;14:523–537. doi: 10.1523/JNEUROSCI.14-02-00523.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuter H, Porzig H. Localization and functional-significance of the Na+/Ca2+ exchanger in presynaptic boutons of hippocampal cells in culture. Neuron. 1995;15:1077–1084. doi: 10.1016/0896-6273(95)90096-9. [DOI] [PubMed] [Google Scholar]
- Rizzuto R. Calcium mobilization from mitochondria in synaptic transmitter release. J Cell Biol. 2003;163:441–443. doi: 10.1083/jcb.200309111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz A, Fabian-Fine R, Scott R, Walker MC, Rusakov DA, Kullmann DM. GABAA receptors at hippocampal mossy fibers. Neuron. 2003;39:961–973. doi: 10.1016/s0896-6273(03)00559-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusakov DA, Harrison E, Stewart MG. Synapses in hippocampus occupy only 1–2% of cell membranes and are spaced less than half-micron apart: a quantitative ultrastructural analysis with discussion of physiological implications. Neuropharmacology. 1998;37:513–521. doi: 10.1016/s0028-3908(98)00023-9. [DOI] [PubMed] [Google Scholar]
- Rusakov DA. The role of perisynaptic glial sheaths in glutamate spillover and extracellular Ca(2+) depletion. Biophys J. 2001;81:1947–1959. doi: 10.1016/S0006-3495(01)75846-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rusakov DA, Fine A. Extracellular Ca2+ depletion contributes to fast activity-dependent modulation of synaptic transmission in the brain. Neuron. 2003;37:287–297. doi: 10.1016/s0896-6273(03)00025-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneggenburger R, Neher E. Intracellular calcium dependence of transmitter release rates at a fast central synapse. Nature. 2000;406:889–893. doi: 10.1038/35022702. [DOI] [PubMed] [Google Scholar]
- Schneggenburger R, Neher E. Presynaptic calcium and control of vesicle fusion. Current Opinion Neurobiol. 2005;15:266–274. doi: 10.1016/j.conb.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Scott R, Rusakov DA. Main determinants of presynaptic Ca2+ dynamics at individual mossy fiber-CA3 pyramidal cell synapses. J Neurosci. 2006;26:7071–7081. doi: 10.1523/JNEUROSCI.0946-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd GM, Harris KM. Three-dimensional structure and composition of CA3→CA1 axons in rat hippocampal slices: implications for presynaptic connectivity and compartmentalization. J Neurosci. 1998;18:8300–8310. doi: 10.1523/JNEUROSCI.18-20-08300.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu Y, Hasenstaub A, Duque A, Yu Y, McCormick DA. Modulation of intracortical synaptic potentials by presynaptic somatic membrane potential. Nature. 2006;441:761–765. doi: 10.1038/nature04720. [DOI] [PubMed] [Google Scholar]
- Sjostrom PJ, Turrigiano GG, Nelson SB. Neocortical LTD via coincident activation of presynaptic NMDA and cannabinoid receptors. Neuron. 2003;39:641–654. doi: 10.1016/s0896-6273(03)00476-8. [DOI] [PubMed] [Google Scholar]
- Smith SJ. Do astrocytes process neural information? Prog Brain Res. 1992;94:119–136. doi: 10.1016/s0079-6123(08)61744-6. [DOI] [PubMed] [Google Scholar]
- Stanley EF. Presynaptic calcium channels and the depletion of synaptic cleft calcium ions. J Neurophysiol. 2000;83:477–482. doi: 10.1152/jn.2000.83.1.477. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. The synaptic vesicle cycle. Annu Rev Neurosci. 2004;27:509–547. doi: 10.1146/annurev.neuro.26.041002.131412. [DOI] [PubMed] [Google Scholar]
- Tang Y, Zucker RS. Mitochondrial involvement in post-tetanic potentiation of synaptic transmission. Neuron. 1997;18:483–491. doi: 10.1016/s0896-6273(00)81248-9. [DOI] [PubMed] [Google Scholar]
- Turecek R, Trussell LO. Presynaptic glycine receptors enhance transmitter release at a mammalian central synapse. Nature. 2001;411:587–590. doi: 10.1038/35079084. [DOI] [PubMed] [Google Scholar]
- Vassilev PM, Mitchel J, Vassilev M, Kanazirska M, Brown EM. Assessment of frequency-dependent alterations in the level of extracellular Ca2+ in the synaptic cleft. Biophys J. 1997;72:2103–2116. doi: 10.1016/S0006-3495(97)78853-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verkhratsky A. Physiology and pathophysiology of the calcium store in the endoplasmic reticulum of neurons. Physiol Rev. 2005;85:201–279. doi: 10.1152/physrev.00004.2004. [DOI] [PubMed] [Google Scholar]
- Waters J, Smith SJ. Mitochondria and release at hippocampal synapses. Pflugers Arch. 2003;447:363–370. doi: 10.1007/s00424-003-1182-0. [DOI] [PubMed] [Google Scholar]
- Williams SR, Stuart GJ. Role of dendritic synapse location in the control of action potential output. Trends Neurosci. 2003;26:147–154. doi: 10.1016/S0166-2236(03)00035-3. [DOI] [PubMed] [Google Scholar]
- Yang F, He XP, Russell J, Lu B. Ca2+ influx-independent synaptic potentiation mediated by mitochondrial Na(+)-Ca2+ exchanger and protein kinase C. J Cell Biol. 2003;163:511–523. doi: 10.1083/jcb.200307027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuste R, Tank DW. Dendritic integration in mammalian neurons, a century after Cajal. Neuron. 1996;16:701–716. doi: 10.1016/s0896-6273(00)80091-4. [DOI] [PubMed] [Google Scholar]
- Zenisek D, Matthews G. The role of mitochondria in presynaptic calcium handling at a ribbon synapse. Neuron. 2000;25:229–237. doi: 10.1016/s0896-6273(00)80885-5. [DOI] [PubMed] [Google Scholar]
- Zhong N, Beaumont V, Zucker RS. Roles for mitochondrial and reverse mode Na+/Ca2+ exchange and the plasmalemma Ca2+ ATPase in post-tetanic potentiation at crayfish neuromuscular junctions. J Neurosci. 2001;21:9598–9607. doi: 10.1523/JNEUROSCI.21-24-09598.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]