Abstract
The testicular capsule was studied histologically, morphometrically, ultrastructurally and immunohistochemically in the Japanese quail, domestic fowl, turkey and duck (all members of the Galloanserae). The testicular capsule was, relative to mammals, thin, being 81.5 ± 13.7 µm in the quail, 91.7 ± 6.2 µm in the domestic fowl, 104.5 ± 29.8 µm in the turkey and 91.8 ± 18.9 µm in the duck. The orchido-epididymal border (hilus) of the capsule was much thicker than elsewhere in all birds (from 233.7 ± 50.7 µm in the duck to 550.0 ± 147.3 µm thick in the turkey). The testicular capsule, other than the tunica serosa and tunica vasculosa, comprised, in the main, smooth muscle-like or myoid cells running mainly in one direction, and disposed in one main mass. Peritubular tissue was similarly composed of smooth muscle-like cells disposed in several layers. Actin and desmin intermediate filaments were immunolocalized in the inner cellular layers of the capsule in the quail, domestic fowl and duck, but uniformly in the turkey. Vimentin intermediate filament immunoreaction in the capsule was moderately and uniformly positive in the testicular capsule of only the quail. Actin and desmin, but not vimentin (except very faintly in the turkey) or cytokeratin, were immunolocalized in the peritubular tissue of all birds. The results therefore establish, or complement, some previous observations that these birds have contractile cells in their testicular capsule and peritubular tissue, whose function probably includes the transport of testicular fluid into the excurrent duct system.
Keywords: birds, immunohistochemistry, peritubular tissue, structure, testicular capsule, thickness
Introduction
The testis of most vertebrates is enclosed in a testicular tissue capsule through which blood vessels and nerves enter and leave the substance of the organ. Histologically, it is composed of three layers of tissue, namely the outermost layer, the tunica serosa, which is derived from the peritoneum, the middle and by far the thickest layer, the tunica albuginea, and the innermost and often poorly differentiated layer, the tunica vasculosa. In those animals studied to date, the tunica albuginea forms the bulk of the testicular capsule, and is composed of collagen, elastic fibres and abundant fibroblasts (Davis et al. 1970; Hodges, 1974). In mammals, testicular septa branch from the inner part of the capsule, and conduct blood vessels and nerves into and out of the testicular substance (Davis et al. 1970). However, testicular septa are thought to be absent in birds (Lake, 1971, 1981; Hodges, 1974).
The function of the testicular capsule has also been a subject of much speculation and conjecture. Davis et al. (1970), Holstein & Weiss (1967) and Hargrove et al. (1977) have demonstrated, in some mammals, the presence of smooth muscle cells in the testicular capsule, the contraction of which may assist in transporting immobile testicular spermatozoa into the excurrent duct system. The testicular capsule has been found to respond, by contraction, to various chemical and electrical stimulations (Davis et al. 1970; Banks et al. 2006).
Peritubular tissue (tunica or lamina propria) that invests the seminiferous tubules is present in all mammalian species (Maekawa et al. 1996) and in several birds (Rothwell & Tingari, 1973; Aire, 1997). Although its organization seems to vary by species, its widespread occurrence among various species of animals indicates this tissue is an important component of the testis (Maekawa et al. 1996). The peritubular tissue comprises smooth muscle-like or myoid cells in various mammalian species (see Virtanen et al. 1986; Maekawa et al. 1996) and birds (Rothwell & Tingari, 1973; Aire, 1997).
The testicular capsule and peritubular tissue in men and some other mammals have received considerable attention recently (see Maekawa et al. 1996; Arenas et al. 1997; Banks et al. 2006) but very little is known about these important tissues in birds. Morphometric data on the testicular capsule are lacking, and therefore comparisons between animals are difficult to make with regard to thickness of the entire capsule or parts thereof. It is generally thought that birds, relative to mammals, have very thin testicular capsules, but there are no data to substantiate these assertions.
Are avian testicular capsules and peritubular tissue capable of contracting and facilitating the movement of testicular fluid into the excurrent duct system? There is a paucity of information on the testicular capsule and peritubular tissue of birds. Initial studies have indicated that there might be smooth muscle cells in the testicular capsule and peritubular tissue of the Japanese quail and domestic fowl (Van Nassauw et al. 1993; Maretta & Marettova, 2004). The histology and ultrastructure of the testicular capsule and comparative measurements of its thickness in the domestic fowl, turkey and Japanese quail (all domestic members of the Galliformes) and duck (Anseriformes) are reported in this paper. In addition, the presence or absence of actin, desmin, vimentin and cytokeratin intermediate filaments (IFs), detected by immunohistochemical methods, in the testicular capsule and peritubular tissue of these birds is reported.
Materials and methods
Testicular capsule and testicular parenchyma were obtained from sexually mature and active male turkeys (n = 5), domestic fowls (n = 5), Japanese quails (n = 5) and ducks (n = 6). Testicular capsule, with attached testis parenchyma, was taken from both poles, lateral and medial surfaces of the testis, and from the ‘hilus’ or orchido-epididymal border of the testis. For light microscopy and immunohistochemistry, tissue samples from three birds were fixed, by immersion, in Bouin's fluid. For electron microscopy, the entire bird was intravascularly perfused with 3% glutaraldehyde buffered in sodium cacodylate, at pH 7.4, using the left ventricle in the quail, and descending aorta in the other bigger birds. Fixation of tissues was for 12–24 h in Bouin's fluid, and up to 72 h in glutaraldehyde.
For light microscopy, tissue blocks were subsequently processed conventionally for paraffin embedment, and 5-µm-thick sections were cut and stained with haematoxylin and eosin (H&E). For electron microscopy, the tissue blocks were processed conventionally for plastic embedment, 1-µm-thick sections were stained with toluidine blue, and ultrathin sections were cut and stained in the usual manner for viewing in the electron microscope.
For morphometry, Bouin's fluid-fixed tissue samples were used for all measurements because they presented fewer preparation artefacts, and were better and more consistently fixed. The thickness of the testicular capsule was measured at ×20 or ×40 objective, using a calibrated eye-piece micrometer. At least ten measurements were taken from each free segment of the testis, namely caudal and cranial poles, and lateral and medial surfaces, of each bird. The thickness of the testicular capsule on the dorsomedial border (hilus or orchido-epididymal interface) was similarly measured specifically in all four species of birds. Analysis of variance (anova) was used to compare the testicular segments (free surfaces), excluding the hilus, of each species of bird. The thickness of the hilus of each species of bird was compared with the pooled free surface values of the testis using Student t-test. The hilus of the four species of bird as well as the pooled free surface values were statistically tested using anova. The level of significance was P < 0.05.
For immunohistochemistry, Bouin's fluid-fixed testicular tissue (containing both the capsule and the parenchyma) was processed routinely and embedded in paraffin wax. Sections 5 µm thick were cut and mounted on slides precoated with polylysine, deparaffinized and rehydrated. Immunostaining of slides for smooth muscle actin, cytokeratin, desmin and vimentin was performed as recommended by DakoCytomation (Denmark), the supplier of the LSAB+ Kit (HRP) used in this study. Briefly, the rehydrated tissue section was microwaved at 750 W for two cycles of 7 min each in citrate buffer (pH 6). Thereafter, the slide was allowed to cool for 20 min and then rinsed in phosphate-buffered saline (PBS) containing bovine serum albumen (pH 7.6) for 5 min. Endogenous peroxidase activity was blocked using hydrogen peroxide (3% in distilled water) for 5 min. Smooth muscle actin (DakoCytomation, Denmark, Code: M085101), cytokeratin (M082101), desmin (A0611; polyclonal) and vimentin (M072501) immunodetection was carried out, at room temperature for 30 min, using respective mouse primary antibodies at dilutions of 1 : 50, 1 : 100, 1 : 300 and 1 : 100, respectively. After rinsing in PBS, each slide was incubated with ready-to-use biotinylated anti-rabbit, anti-mouse and anti-goat immunoglobin for 15 min in a humidified chamber at room temperature. Each slide was again washed in PBS, followed by incubation in streptavidin peroxidase for another 15 min. Reactivity was visualized, after rinsing in PBS, by applying substrate-chromogen solution (either LSAB® +3,3′-diaminobenzidine or VECTOR® NovaRED™) for 20 s. Sections were counter-stained with haematoxylin. Control slides were treated identically, except that the primary antibody was replaced by bovine serum albumin.
Results and observations
Morphometry
The free portions of the testicular capsule varied slightly in their thickness. In the domestic fowl, for example, the caudal pole was 88.3 ± 8.0 µm, the cranial pole was 79.6 ± 13.3 µm, and both the lateral and medial surfaces were 74.1 ± 6.6 µm thick. The differences between sites were not statistically significant (P > 0.5), and therefore all data were pooled. The testicular capsule was remarkably thicker (between 2.5- and 6.3-fold) along the dorso-medial border of the testis, which is also the hilus that lies adjacent to the medial surface of the epididymal (the orchido-epididymal) border or interface, than elsewhere (Table 1). There were no significant differences in the width of this portion of the capsule between the duck and quail or between the domestic fowl and turkey, but there were significant differences (P < 0.01) between the former and latter pairs of birds in this regard.
Table 1.
Thickness (mean ± SEM, µm) of the various parts of the testicular capsule in birds
| Part of capsule | Quail | Domestic fowl | Turkey | Duck |
|---|---|---|---|---|
| (1) Free parts | 91.7 ± 6.2*** | 81.5 ± 13.7*** | 105 ± 29.8*** | 91.8 ± 18.9*** |
| (2) Hilus | 255.4 ± 71.8b | 515 ± 88.2a | 550 ± 147.3a | 233.7 ± 50.7b |
P < 0.001 (free parts vs. hilus; within species). Means with different superscript letters are significantly different (P < 0.001).
Histology and ultrastructure
Testicular capsule
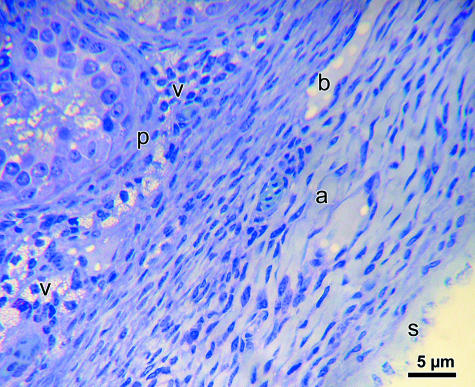
The testicular capsule in all four species of birds was similar in composition, both histologically and ultrastructurally. Three layers of tissue, namely tunica serosa, tunica albuginea and tunica vasculosa, were identified (Fig. 1). The tunica serosa was the outermost layer and corresponded to the mesothelium derived from the peritoneal lining of the structures within the abdominal cavity of the bird. Histologically and ultrastructurally, this tissue layer comprised flattened or squamous cells, which were only occasionally cuboidal, and displayed a few stubby microvilli on their free surfaces. The nucleus was highly elongated, slightly irregular in outline, heterochromatic and occupied most of the cytoplasm, which was highly dense. Only a few organelles were discernible in the cytoplasm. The mesothelial cells lay on a basal lamina, which was separated from the middle layer, the tunica albuginea. This layer is, by far, the thickest portion of the testicular capsule.
Fig. 1.
Photomicrographs of the testicular capsule (tunica serosa, s; albuginea, a; and vasculosa, v) and peritubular tissue (p) of the domestic fowl. b, blood vessel. The cellular elements are orientated longitudinally in the tunica albuginea.
In all four species of birds, the bulk of the tunica albuginea was almost exclusively populated by circularly arranged spindle-shaped, overlapping cells whose fine features were typical of smooth muscle cells (Fig. 2). The nuclei were regularly elongated, euchromatic and displayed an eccentric nucleolus. The organelles, such as mitochondria and short strands of rough endoplasmic reticulum (RER), were few and located mainly at both poles of the nucleus, from which they extended variably into the central part of the cytoplasm (inset to Fig. 2). The cytoplasm contained abundant filaments that radiated obliquely, from the nucleus or central zone occupied by organelles toward the sarcolemma. Some cells that were uncommonly scattered between the smooth muscle cells contained elongated heterochromatic nuclei, which were surrounded by a small rim of cytoplasm and displayed very poorly developed cytoplasmic filaments. These were probably fibroblasts. Cytoplasmic densities either occurred within the cytoplasm or were attached to the sarcolemma. In all birds examined, bundles of collagen were only infrequently seen between the smooth muscle cells, which appeared compactly packed. Large blood vessels, mostly veins, ran circularly through the tunica albuginea. The tunica vasculosa comprised loose connective tissue, fibroblasts and blood vessels. There were no clearly defined branches of tissue leaving the tunica albuginea to enter the testicular parenchyma as septa.
Fig. 2.
Testicular capsule of the duck. Smooth muscle cells, predominant over collagen in the capsule, contain abundant microfilaments (F), cytoplasmic densities (arrow) and mitochondria (M) in the organelle space of the cell. Inset: arrowheads, pinocytic vesicles; M, mitochondria and R, rough endoplasmic reticulum in the organelle space; F, microfilaments; N, nucleus of the smooth muscle cell.
Peritubular tissue
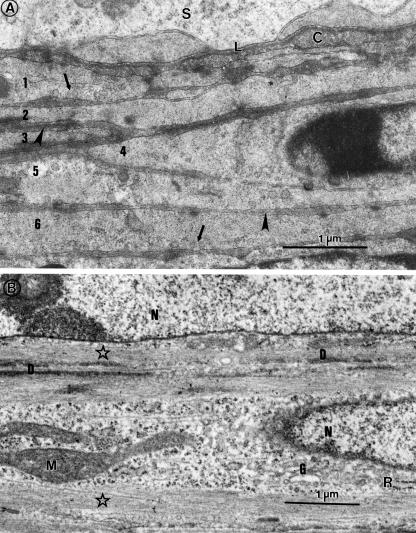
The peritubular tissue was composed of several overlapping layers of cells (Fig. 3) whose composition revealed a moderate abundance of filaments attaching to cytoplasmic densities that lay within the cytoplasm or were adherent to the plasmalemma, which bore numerous micropinocytotic vesicles (Fig. 3a). Organelle content included an elongated, generally euchromatic nucleus, a number of mitochondria, free and rosettes of ribosomes, and a few, short profiles of RER, all contained in a central, filament-free zone of the cytoplasm, extending for variable lengths from either pole of the nucleus into the rest of the cytoplasm (Fig. 3b).
Fig. 3.
Peritubular tissue of the quail (A), displaying several (1–6) layers of myoid cells, separated by basal lamina-like material (arrowheads); arrows, pinocytic vesicles; C, collagen fibres; L, basal lamina; S, seminiferous epithelium. (B) Higher power view of myoid cells. Stars, microfilaments; D, cytoplasmic densities; G, Golgi complex; M, mitochondrion; N, nucleus; R, rough endoplasmic reticulum.
Immunohistochemistry
Testicular capsule
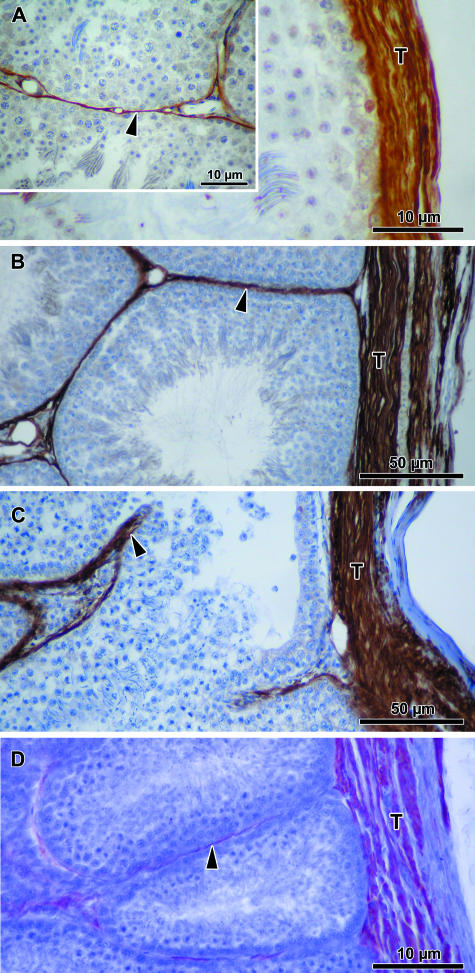
Actin IFs were moderately immunolocalized in bundles of fibres in the inner part of the testicular capsule of the quail and domestic fowl (Fig. 4; Table 2). However, in the turkey and duck, thick bundles of fibres occupying most of the tunica albuginea of the capsule were strongly immunopositive for actin.
Fig. 4.
Testicular capsule (T) and peritubular tissue (arrowhead) show varying levels of immunoreactivity for actin in the quail (A), domestic fowl (B), turkey (C) and duck (D).
Table 2.
Scores of intensity (maximum of 5+) of reaction to various intermediate filaments (IF) in the capsule of birds
| IF type | Quail | Domestic fowl | Turkey | Duck |
|---|---|---|---|---|
| ACTIN | +++ (inner zone only) | ++++ (outer zone only) | ++++ (outer zone only) | ++++ (uniform) |
| DESMIN | +++ (inner zone) | 11/2+ to ++ (inner zone) | +++ to ++++ (uniform) | +++ (inner zone) |
| VIMENTIN | +++ | –ve | + (scattered in the inner zone) | –ve |
| CYTOKERATIN | –ve | –ve | –ve | –ve |
There was a moderately to strongly immunopositive reaction for desmin in the inner half of the tunica albuginea in the duck and domestic fowl, while in the turkey a moderate to strong immunoreaction to desmin was localized throughout the capsule (Fig. 5; Table 2).
Fig. 5.
Testicular capsule (T) and peritubular tissue (arrowhead) show varying levels of immunopositivity for desmin in the quail (A), domestic fowl (B), turkey (C) and duck (D).
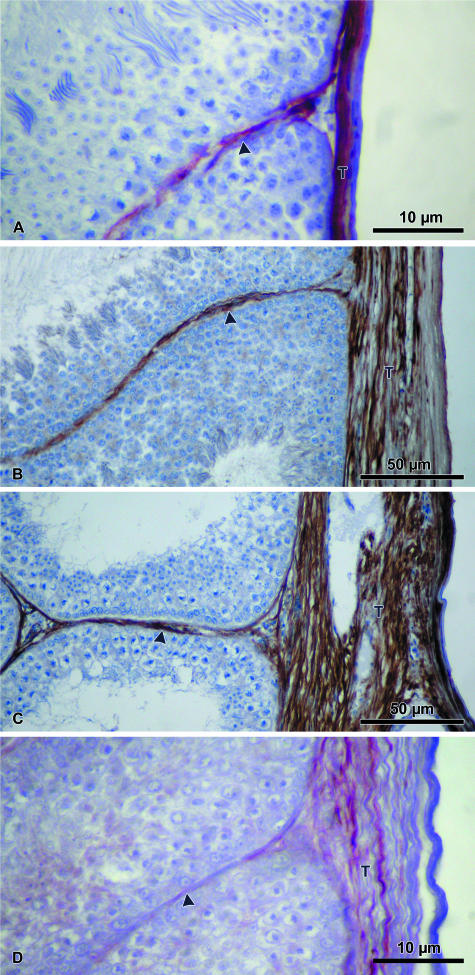
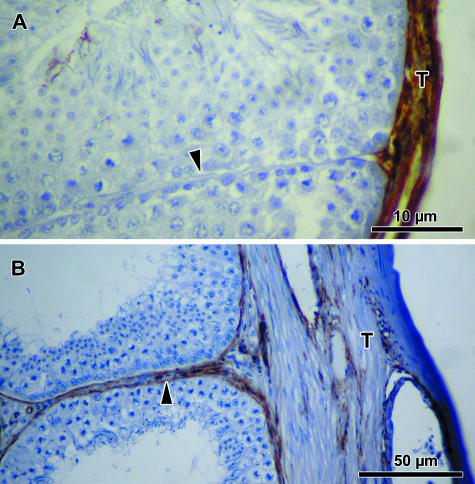
Vimentin immunoreactivity was moderately demonstrated in the testicular capsule of the quail, and sparsely in the turkey, but not in the other two bird species (Fig. 6; Table 2). Cytokeratin immunoreaction was absent in all four species of birds.
Fig. 6.
The testicular capsule (T) of both the quail (A) and the turkey (B) show varying vimentin immunopositivity. The peritubular tissue (arrowhead) is immunonegative for vimentin in the quail (A), but moderately immunopositive in the turkey (B).
Peritubular tissue
In the quail, domestic fowl and turkey, moderate to strong actin immunoreaction was demonstrated in the peritubular tissue of the testis, but in the duck, this reaction was weakly expressed in a very thin sleeve of tissue around the seminiferous tubule (Fig. 4; Table 3). The tunica adventitia of blood vessels immunoreacted moderately to strongly, but the tunica intima was immunonegative in all birds.
Table 3.
Scores of intensity (maximum of 5+) of reaction to various intermediate filaments (IF) in the peritubular tissue of birds
| IF type | Quail | Domestic fowl | Turkey | Duck |
|---|---|---|---|---|
| ACTIN | +++(thin sleeve) | ++++ | +++ | ++ |
| DESMIN | + to ++(thin layer) | ++(thin layer) | ++ to +++ | + to 11/2+ |
| VIMENTIN | –ve | –ve | + | –ve |
| CYTOKERATIN | –ve | –ve | –ve | –ve |
Immunoexpression of desmin was weak to moderate in most of the peritubular tissue layer in the turkey and duck, but in only a thin sleeve of the cell layer in the quail and domestic fowl (Fig. 5; Table 3). Desmin immunoreaction in the tunica adventitia of blood vessels was moderate to strong, but negative in the tunica intima in all birds.
Only the turkey displayed faint to weak immunoreaction to vimentin in the peritubular tissue. Vimentin immunoreaction in the tunica intima of blood vessels was moderate, but negative in the tunica adventitia (Fig. 6; Table 3).
The peritubular tissue as well as blood vessels in all birds was negatively immunoreactive to cytokeratin.
Discussion
As in mammals (Davis et al. 1970), the avian testicular capsule displays an outer tunica serosa, a thick, middle tunica albuginea and a poorly differentiated innermost layer of tunica vasculosa. The avian capsule is generally regarded as being very thin (Lake, 1971; Hodges, 1974). Our thickness data for all birds, including the domestic fowl (81.5 ± 13.7 µm), are higher than has been reported for the domestic fowl (30–60 µm) by Hodges (1974), but much lower (by over five-fold) than in young and older men (Arenas et al. 1997). The reasons for this discrepancy in testicular capsule thickness in the domestic fowl between the present study and that of Hodges (1974) is not known, but could be due to fixatives and fixation methods used, as well as age of the subjects and possibly but not probably reproductive activity. Arenas et al. (1997) have shown that although testicular capsule thickness increases with age, its total volume does not change. Relative to control birds, in the Japanese quail the thickness of the testicular capsule increased remarkably in organs whose seminiferous tubules were considerably atrophic due to carbendazim treatment (unpublished observations).
There are variations in the thickness of the testicular capsule from one surface of the testis to another and between both poles. These differences, except at the testicular hilus, are not significant, but suffice it to say that the tunic on both the medial and the lateral surfaces is thinner than that of the cranial and caudal poles, the caudal pole of the capsule being generally thicker than that of the cranial pole. The capsule in the hilus or orchido-epididymal interface is, however, unique in being much thicker (between two- and six-fold) than on other surfaces of the capsule. Variations in the thickness of the capsule, even on the same surface, are attributed to the presence of segments of the rete testis (usually on the medial and lateral as well as peri-epididymal surfaces) and/or blood vessels, which are usually large veins and abound in the capsule on both the medial and the lateral surfaces of the testis. This is reflected in the large standard errors of the means. The thickness of the capsule on the orchido-epididymal surface is a reflection of the number of rete testis lacunae, blood vessels and/or the amount of adipose tissue that are present in this segment of the capsule. Both the domestic fowl and the turkey display a large network of rete testis lacunae in this region of the testicular capsule.
Histology and ultrastructure
Testicular capsule
All birds studied here displayed similar morphological characteristics of the testicular capsule. Only one, broad, distinct layer of smooth muscle cells interspersed with sparse collagen tissue was observed. A few profiles of cell groups appeared longitudinally sectioned, while others appeared transversely or obliquely sectioned, histologically, in the orchido-epididymal zone of the testicular capsule, especially in the duck. The rabbit has two layers of smooth muscles, running perpendicularly to each other (Holstein, 1967) but other mammals, as in birds reported in this study, have only a single, distinct layer of smooth muscle cells (Leeson & Forman, 1981).
The tunica albuginea of the birds examined in this study, unlike in the human, rabbit, rat and mouse (Banks et al. 2006), was composed mainly by cells that exhibited typical smooth muscle cell features. These features include, but are not limited to, an abundance of thin filaments as well as cytoplasmic densities occupying most of the cytoplasm. A major difference between these birds and the mammals referred to above is the relatively small volume proportion of collagen tissue in the tunica albuginea of the birds. It is thus clear that the testicular capsule in the Galloanserae, although much thinner than that of mammals, contains a highly contractile amount of tissue, which probably contributes significantly in transporting testicular fluid from the testis into its excurrent ducts.
Peritubular tissue
Peritubular tissue in birds, namely domestic fowl, guinea fowl, Japanese quail and duck, has been studied and found to contain myoid cells (Rothwell & Tingari, 1973; van Nassauw et al. 1993; Aire, 1997; this study). Unlike in rodents, which have only one layer of smooth muscle (myoid) cells in the peritubular tissue (Maekawa et al. 1996), the birds studied thus far display multiple layers of myoid cells (Rothwell & Tingari, 1973; van Nassauw et al. 1993; Aire, 1997; this study) as has been described for larger mammals (Virtanen et al. 1986; Maekawa et al. 1996). The functional significance of this species variation in the number of myoid cell layers is also not clearly understood. It is noteworthy, however, that the cells in the peritubular tissue in our birds and those of van Nassauw et al. (1993) were of one type, i.e. myoid or smooth muscle cells, unlike the observations made by Rothwell & Tingari (1973) that both fibroblast-like and myoid cells were present in two different layers (inner and outer, respectively) in the peritubular tissue of the domestic fowl.
Immunohistochemistry
Smooth muscle actin and desmin IFs are specific for contractile cells (van Nassauw et al. 1993) while vimentin IFs are abundant in fibroblasts (Maekawa et al. 1996). In mammals, both myoid and smooth muscle contractile cells are found in the tunica albuginea of the testis (Holstein & Weiss, 1967; Holstein, 1967; Davis & Langford, 1970; Hargrove et al. 1977). Contractile cells have only recently been described in the tunica albuginea of birds, in the Japanese quail (van Nassauw et al. 1993) and domestic fowl (Maretta & Marettova, 2004). Both actin and desmin IFs were immunolocalized in the testicular capsule in both bird species. The present report shows that actin and desmin IFs are present in the tunica albuginea of the quail, domestic fowl, duck and turkey, and vimentin IFs moderately and uniquely in the testicular capsule of the quail. There are variations in the immunolocalization of IF types in the tunica albuginea between species of birds. Neither van Nassauw et al. (1993) in the quail nor Maretta & Marettova (2004) in the domestic fowl indicated whether there were differential or focal variations in IF immunolocalizations in the testicular capsule. Contractile activity, as evidenced by IF concentration, in the testicular capsule is therefore expected to be greater in the inner than in the outer part of the capsule of the quail and duck, where they appear to be more concentrated. Conversely, contractility of the entire tunica albuginea would be expected to be uniform in the quail because although both actin and desmin are immunoexpressed in the inner zone of the tunica albuginea, vimentin is present throughout the tissue layer. Similarly, the uniform immunoexpression of actin throughout the tunica albuginea in the duck suggests that the entire tissue layer is contractile in this bird. It has been suggested that species-specific differences in the degree of differentiation of fibroblasts to smooth muscle cells in the myoid cell population might occur (Bustos-Obregon & Courot, 1974; Wrobel et al. 1979, 1988; Leeson & Forman, 1981).
The present observations also indicate that contractile cells in different parts of the testicular capsule may contain more than one type of IF (e.g. both actin and desmin are expressed in the inner half of the capsule in the quail). According to Georgatos (1993), certain cells possess more than one IF system but the different networks do not overlap. In humans, fibroblast-like cells in the tunica albuginea were immunoreactive to α-smooth muscle actin and vimentin but not desmin, while the myoid cells immunoexpressed all three types of IFs (Arenas et al. 1997). The amount and distribution of smooth muscle-like cells also vary from one mammalian species to another (Arenas et al. 1997; Banks et al. 2006). The differential immunoexpression of actin, desmin and vimentin in the tunica albuginea of the birds studied here tends to support this observation. Banks et al. (2006) have also observed that the differences seen in the IF content of smooth muscle-like cells in either the outer or the inner zones of the tunica albuginea may be due to the fact that a smooth muscle cell is not a single entity but a heterogeneous cell that differentially expresses both contractile and synthetic activities at opposite ends of the spectrum.
Our results also show that the myoid cells of the peritubular tissue in all birds examined exhibited actin and desmin immunoreaction moderately or strongly. This confirms the presence of IFs in these cells in all birds examined thus far (van Nassauw et al. 1993; Maretta & Marettova, 2004; this study) and that more than one type of these filaments can occur in the same cell, as explained by Banks et al. (2006). Actin and desmin IFs are abundant in myoid cells (Maekawa et al. 1991) and have also been immunolocalized in peritubular myoid cells of the testis in the monkey (Schlatt et al. 1993).
The results presented in this paper on the testicular capsule thickness, histology, ultrastructure and immunohistochemistry of IFs are those of normal, adult, sexually active birds. It was not possible to evaluate the testicular capsule and peritubular boundary tissues in either prepubertal or ageing birds, or in degenerate testes of birds. Aire (1997) has observed profound changes in the basal lamina, and possible transformational changes in the peritubular layer of myoid cells in seminiferous tubules of physiologically regressed testes. Changes have not been observed in the volume of interstitial connective tissue and number of peritubular cells in the testes of ageing men (Johnson, 1986), but changes in immunoexpression of IFs in peritubular myoid cells have been reported in only severely degenerated human seminiferous tubules (Davidoff et al. 1990; Martin et al. 1992; Santamaria et al. 1992; Arenas et al. 1997). A study of the possible effect of development of puberty, ageing and seminiferous tubular degeneration on the thickness, structure, and immunohistochemistry of IFs in myoid/smooth muscle cells in the testicular capsule and peritubular tissue of birds is advocated.
In conclusion, the avian testicular capsule in members of the Galloanserae studied is much thinner than, but similar histologically to, that of mammals. Actin, desmin and vimentin IFs are variably immunolocalized in this tissue of the birds. Vimentin, an abundant IF in fibroblasts, is immunolocalized moderately only in the quail. Both actin and desmin IFs are also present in the peritubular myoid cells of birds. Avian testes, compared with those of mammals, produce large quantities of both fluid and spermatozoa (Aire, 1979, 1980; Aire, 2007a,b; Clulow & Jones, 1988). It is therefore not inconceivable that both the peritubular tissue and testicular capsule are capable of, and, indeed, by their contractile activities function in, propelling the enormous amount of testicular fluid produced in the seminiferous tubules into the excurrent ducts of the testis.
Acknowledgments
We are grateful for a University of Pretoria research grant that aided this work. P.C.O. is a recipient of the University of Pretoria Post-Doctoral Fellowship. Ms Alexandra Blomqvist kindly provided the plastic embedded tissue from which the light micrographs of the testicular capsule of the domestic fowl were taken.
References
- Aire TA. The epididymal region of the Japanese quail (Coturnix coturnix japonica) Acta Anat. 1979;103:305–312. [PubMed] [Google Scholar]
- Aire TA. The ductuli efferentes of birds. J Anat. 1980;130:707–723. [PMC free article] [PubMed] [Google Scholar]
- Aire TA. The structure of the interstitial tissue of the active and resting avian testis. Onderstepoort J Vet Res. 1997;64:291–299. [PubMed] [Google Scholar]
- Aire TA. Anatomy of the testis and male reproductive tract. In: Jamieson BGM, editor. Reproductive Biology and Phylogeny of Birds. 6A. New Hampshire, USA; Plymouth, UK: Science Publishers, Inc; 2007a. pp. 37–113. [Google Scholar]
- Aire TA. Spermatogenesis and testicular cycles. In: Jamieson BGM, editor. Reproductive Biology and Phylogeny of Birds. 6A. New Hampshire, USA; Plymouth, UK: Science Publishers, Inc; 2007b. pp. 279–347. [Google Scholar]
- Arenas MI, Betencourt FR, Fraile B, Panaigua R. Immunocytochemical and quantitative study of the tunica albuginea testis in young and ageing men. Histochem Cell Biol. 1997;107:469–477. doi: 10.1007/s004180050134. [DOI] [PubMed] [Google Scholar]
- Banks FCL, Knight GE, Calvert RC, et al. Smooth muscle and purinergic contraction of the human, rabbit, rat, and mouse testicular capsule. Biol Reprod. 2006;74:473–480. doi: 10.1095/biolreprod.105.044602. [DOI] [PubMed] [Google Scholar]
- Bustos-Obregon E, Courot M. Ultrastructure of the lamina propria in the ovine seminiferous tubule. Development and some endocrine considerations. Cell Tissue Res. 1974;150:481–492. doi: 10.1007/BF00225971. [DOI] [PubMed] [Google Scholar]
- Clulow J, Jones RC. Studies of fluid and spermatozoal transport in the extratesticular ducts of the Japanese quail. J Anat. 1988;157:1–11. [PMC free article] [PubMed] [Google Scholar]
- Davidoff MS, Breuker H, Holstein AF, Seidel K. Cellular architecture of the lamina propria of human seminiferous tubules. Cell Tissue Res. 1990;262:253–261. doi: 10.1007/BF00309880. [DOI] [PubMed] [Google Scholar]
- Davis JR, Langford GA. Pharmacological studies of the testicular capsule in relation to sperm transport. In: Rosenberg E, Paulsen CA, editors. The Human Testis. New York: Plenum; 1970. pp. 495–514. [Google Scholar]
- Davis JR, Langford GA, Kirby PJ. The testicular capsule. In: Johnson AD, Gomes R, Vandemark NL, editors. The Testis. Development, Anatomy and Physiology. London: Academic Press; 1970. pp. 281–337. [Google Scholar]
- Georgatos SD. Dynamics of intermediate filaments. Recent progress and unanswered questions. FEBS Lett. 1993;318:101–107. doi: 10.1016/0014-5793(93)80001-b. [DOI] [PubMed] [Google Scholar]
- Hargrove JL, MacIndoe JH, Ellis LC. Testicular contractile cells and sperm transport. Fertil Steril. 1977;28:146–1157. [PubMed] [Google Scholar]
- Hodges RD. The Histology of the Fowl. London: Academic Press; 1974. [Google Scholar]
- Holstein AF. Smooth musculature of the testicular tunica albuginea and its influence on the transport of spermatozoa into the epididymis. Verb Anat Ges. 1967;62:103–108. [PubMed] [Google Scholar]
- Holstein AF, Weiss C. Ûber die Wirkung der glatten Muskulatur in der Tunica albuginea in Hoden des Kaninchens; Messungen des intersitiellen Druckes. Z Gesamte Experimentelle Med Einschliesslich Experimentelle Chirurgie. 1967;142:334–337. [PubMed] [Google Scholar]
- Johnson L. Spermatogenesis and ageing in the human. J Androl. 1986;7:331–335. doi: 10.1002/j.1939-4640.1986.tb00943.x. [DOI] [PubMed] [Google Scholar]
- Lake PE. The male in reproduction. In: Bell DJ, Freeman BM, editors. Physiology and Biochemistry of the Domestic Fowl. Vol. 3. New York: Academic Press; 1971. pp. 1411–1447. [Google Scholar]
- Lake PE. Male genital organs. In: King AS, McLelland J, editors. Form and Functions in Birds. Vol. 2. London: Academic Press; 1981. pp. 1–61. [Google Scholar]
- Leeson CR, Forman DE. Postnatal development and differentiation of contractile cells within the rabbit testis. J Anat. 1981;132:491–511. [PMC free article] [PubMed] [Google Scholar]
- Maekawa M, Nagano T, Kamimura T, Ishikawa H, Dezawa M. Distribution of actin-filament bundles in myoid cells, Sertoli cells, and tunica albuginea of rat and mouse testes. Cell Tissue Res. 1991;266:295–300. doi: 10.1007/BF00318185. [DOI] [PubMed] [Google Scholar]
- Maekawa M, Kamimura K, Nagano T. Peritubular myoid cells in the testis: their structure and function. Arch Histol Cytol. 1996;59:1–13. doi: 10.1679/aohc.59.1. [DOI] [PubMed] [Google Scholar]
- Maretta M, Marettova E. Immunohistochemical demonstration of myoid cells in the testis and its excurrent ducts in the domestic fowl. Br Poultry Sci. 2004;45:585–589. doi: 10.1080/00071660400006313. [DOI] [PubMed] [Google Scholar]
- Martin R, Santamaria L, Nistal M, Fraile B, Paniagua R. The peritubular myofibroblasts in the testes from normal men and men with Klinefelter's syndrome. A quantitative, ultrastructural, and immunohistochemical study. J Pathol. 1992;168:59–66. doi: 10.1002/path.1711680111. [DOI] [PubMed] [Google Scholar]
- van Nassauw L, Harrison F, Callebaut M. Smooth muscle cells in the peritubular tissue of the quail testis. Eur J Morph. 1993;31:60–64. [PubMed] [Google Scholar]
- Rothwell B, Tingari MD. The ultrastructure of the boundary tissue of the seminiferous tubule in the testis of the domestic fowl (Gallus domesticus) J Anat. 1973;114:321–328. [PMC free article] [PubMed] [Google Scholar]
- Santamaria L, Martin R, Nistal M, Paniagua R. The peritubular myoid cells in the testes from men with varicocele: an ultrastructural, immunohistochemical and quantitative study. Histopathology. 1992;21:423–443. doi: 10.1111/j.1365-2559.1992.tb00426.x. [DOI] [PubMed] [Google Scholar]
- Schlatt S, Weinbauer GF, Arslan M, Nieschlag E. Appearance of α-smooth muscle actin in peritubular cells of monkey testes is induced by androgens, modulated by follicle-stimulating hormone, and maintained after hormonal withdrawal. J Androl. 1993;14:340–350. [PubMed] [Google Scholar]
- Virtanen I, Kalljoki M, Närvänen O, Paranko LE, Miettinen M, Lehto V-P. Peritubular myoid cells of human and rat testis are smooth muscle cells that contain desmin-type intermediate filaments. Anat Rec. 1986;215:10–20. doi: 10.1002/ar.1092150103. [DOI] [PubMed] [Google Scholar]
- Wrobel K-H, Dostal S, Schimmer M. Postnatal development of the tubular lamina propria and the intertubular tissue in the bovine testis. Cell Tissue Res. 1988;252:639–653. doi: 10.1007/BF00216652. [DOI] [PubMed] [Google Scholar]
- Wrobel K-H, Mademann R, Sinowatz F. The lamina propria of the bovine seminiferous tubule. Cell Tissue Res. 1979;202:357–377. doi: 10.1007/BF00220431. [DOI] [PubMed] [Google Scholar]