Abstract
The present study describes the biological meaning of the asymmetrical shape in avian reproduction using quail. During the incubation of eggs, water was gradually lost and the air chamber which appeared in between the inner and outer shell membranes at the blunt end expanded, so that the angle made by the long egg-axis and the horizontal line increased, presumably because the centre of gravity of the egg contents moved toward the sharp end. The increase in angle occurred in both fertile and infertile eggs, suggesting that this phenomenon occurs irrespective of fertility and is due to the asymmetrical shape. The increase in the volume of the air chamber resulted in an increase in the area of the inner shell membrane at the chamber to satisfy the amount of gas exchange needed by the developing embryo for better hatching. We isolated a 300-kDa protein from the inner shell membrane. It was produced by cells in the luminal epithelium of the oviductal isthmus and was found in the cortex of the fibres of shell membranes and a lining surrounding the air chamber. The lining comprised a medial layer between the inner and outer shell membranes in uterine eggs. The asymmetrical ellipsoid produces the air chamber at the blunt end of the avian egg during its sojourn in the oviductal isthmus, to maintain the blunt end up after oviposition and to raise that end during incubation in a dry environment, leading to high hatchability.
Keywords: air chamber, fertile egg, hatchability, quail, shell membrane
Introduction
Most birds lay their eggs in a nest made on land or in a tree, almost always in a dry environment. Their embryos develop completely within an eggshell, a relatively self-enclosed organism; therefore, the egg must provide all requirements for the development of the embryo, adapting its reproductive strategy to do so. Avian eggs supply the embryo with all the nutrients it needs except oxygen. Furthermore, they have particular features ensuring the normal development of embryos. The limiting membrane, a component of shell membranes, is a smooth layer of homogeneous, dense material that thins out during incubation (Yoshizaki & Saito, 2002). The thinning was thought to be one particular element of the avian reproductive strategy: the limiting membrane may be involved in preventing the early embryo from drying out, and thins out to satisfy the requirement for gas exchange (Kutchai & Steen, 1971) and for the mobilization of calcium from the eggshell (Johnston & Comar, 1955; Simkiss, 1961; Ono & Wakasugi, 1984) later in development. It has also been suggested that the turning of eggs during incubation allows the width of the limiting membrane to be sufficiently and equally reduced over the entire surface (Yoshizaki & Saito, 2002).
The avian eggshell is an asymmetrical prolate ellipsoid; a cross-section is a circle and a longitudinal section is an asymmetrical ellipse (Burley & Vadehra, 1989). The circlular shape of the cross section might enable the egg to turn as mentioned above. But the biological significance of the asymmetrical ellipse of the longitudinal section is not clear. In almost all avian species, the egg is placed at a certain angle with its sharp end down owing to such asymmetry. In the artificial incubation of eggs, this angle has been considered important for satisfactory hatchability: horizontal orientation of the egg-axis leads to low hatchability. In the modern poultry industry, eggs are generally incubated with the blunt end up in order to obtain optimum hatchability. Positions during incubation affect the development of avian embryos and thus hatchability (Cain & Abbott, 1971; Van Schalkwyk et al. 2000; Deeming, 2002). Here we show the biological significance of the asymmetry of eggs to the success of avian hatching.
Materials and methods
Japanese quails (Coturnix japonica) were housed individually in cages located in a poultry house, which was illuminated for 16 h each day. A layer's diet and water were given ad libitum. The quails were killed by cervical dislocation; this protocol was approved by an Institutional Animal Care and Use Committee (Document No. 04111). The muscle tissue of the oviductal isthmus was removed and the mucosa was divided into anterior and posterior portions. Extracts of the mucosa were obtained by homogenizing in Ringer's solution and filtrating through a 0.22-µm nylon mesh.
Fertilized quail eggs collected at about 10:00 h each day were incubated at 39 °C and at 60% humidity, with automatic turning every 2 h. In some experiments, egg turning was performed manually twice daily. Hatchability was measured on the 18th day. Data were analysed by a one-way anova.
To measure angles, the eggs were placed on a horizontal platform and photographed with a digital camera. The angle was measured on the digital image with ACD See Image Management Software (ACD systems, Victoria, Canada). To measure the volume of the air chamber, the blunt end half of the eggshell with shell membranes was removed from eggs and grape-seed oil was poured carefully into the chamber with a calibrated syringe through one of two holes made in the eggshell. After its volume had been measured, the air chamber was isolated from the shell proper by cutting along its edges; the shell membranes with the shell were cut, expanded under glass, and then analysed via Photoshop Software (Adobe systems, Inc., San Jose, CA, USA).
To isolate a medial-layer substance from fertile eggs, incubated for 5 days, the inner shell membranes at air chambers were isolated, rinsed with distilled water, degassed and then incubated in 8 m guanidine hydrochloride (Nacalai tesque, Tokyo, Japan) at 37 °C overnight. The extract was dialtsed in distilled water, and after centrifugation at 1500 g for 10 min, the precipitate was stored at –30 °C prior to use. To raise an antibody, the extract from about 250 inner shell membranes was suspended in 2 mL of phosphate-buffered saline with the ultrasonic disruptor, emulsified with 2 mL of Freund's complete adjuvant (Difco, Detroit, MI, USA), and injected subscapularly into a rabbit.
For light microscopy, specimens were fixed in Bouin's solution and embedded in paraffin. Sections were stained with haematoxylin and eosin. Some sections were treated with the antiserum for 1 h at 37 °C, after which they were washed with phosphate-buffered saline and treated with fluorescein isothiocyanate-conjugated goat antiserum against rabbit IgG (E-Y Laboratories, San Mateo, CA, USA) for 1 h at 37 °C.
For transmission electron microscopy, specimens were fixed with 2.5% (v/v) glutaraldehyde in 0.1 m cacodylate buffer (pH 7.4), cut into pieces and kept overnight at 4 °C in the same solution. They were then rinsed with the buffer and post-fixed with 1% (w/v) OsO4 in the same buffer for 3 h at 4 °C. After fixation they were dehydrated in acetone and embedded in epoxy resin. Ultrathin sections were stained with uranyl acetate and lead citrate and observed with a model H-8000 electron microscope (Hitachi, Tokyo, Japan). For scanning electron microscopy, specimens were dehydrated in acetone, dried in a critical point apparatus (HCP-2; Hitachi), coated with a layer of platinum in an E102 ion sputter (Hitachi) and examined with a model S-4300 electron microscope (Hitachi). For immunoelectron microscopy, the glutaraldehyde-fixed specimens were embedded in Lowicryl K4M resin (Polysciences, Warrington, PA, USA). Thin sections were treated first with the antiserum for 2 h, and then with a gold-conjugated goat antiserum against rabbit IgG (E-Y Laboratories) for 1 h. Samples were next stained with uranyl acetate.
The extracts of the inner shell membrane and the isthmus mucosa were separated through 6% sodium dodecylsulphate (SDS)-polyacrylamide gel electrophoresis (PAGE) (Laemmli, 1970) under reducing conditions. The gels were stained with Coomassie Brilliant Blue. For immunoblot analyses, samples in the gels were electroblotted onto a polyvinyliden difluoride membrane (Atto, Tokyo, Japan) and the membrane was treated with the antiserum against the extracts of the inner shell membrane for 1 h at 37 °C. After a wash with PBS, the membrane was treated with an alkaline phosphatase-conjugated anti-rabbit IgG antibody (Promega, Madison, WI, USA) for 2 h. The colour reaction was carried out with the stabilized substrate for alkaline phosphatase (Promega).
Results
Hatching rate of embryos at various egg positions
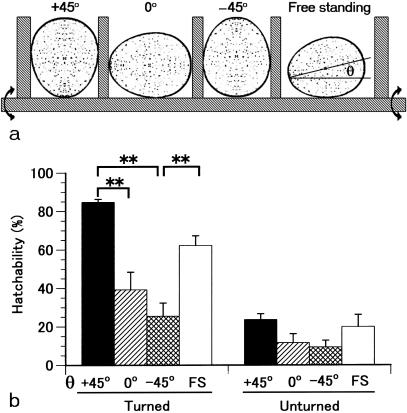
A comparison of the effect of an egg's position on hatchability is shown in Fig. 1. With the standard method of artificial incubation in which eggs are placed with the blunt end up, tilted (θ +45°) and turned, hatchability was 84.8% of fertile eggs, significantly higher than when the eggs were placed with the side up (θ 0°) or with the sharp end up (θ –45°). The 62.3% hatchability obtained for the free-standing eggs did not differ significantly from the rate obtained at θ +45° or θ 0°, but was significantly different from that at θ –45°. The hatching rate decreased significantly for unturned eggs. However, again hatchability was correlated with the angle of the egg's position. Thus, maintaining the blunt end upwards is a key to improving hatchability in avian reproduction.
Fig. 1.
Hatchability at various egg positions. (a) Schematic illustration of the positions of eggs during incubation. The positions +45°, 0°, –45° and free standing (FS) mean that eggs were placed with the blunt end up, the side up, the sharp end up and no control, respectively. Incubation was done by tilting an egg-stand 45° and turning it 90° every 2 h. θ, the angle made by the long egg-axis line and the horizontal line with the sharp end down. (b) Hatchability was dependent on the egg's position. A single experiment was conducted with eight plots of around 30 eggs each, depending on the egg-angle and egg-turning. Values are means ± SEM of five experiments. **Significance differences (P < 0.01).
Changes of the egg-axis angle and the air-chamber volume
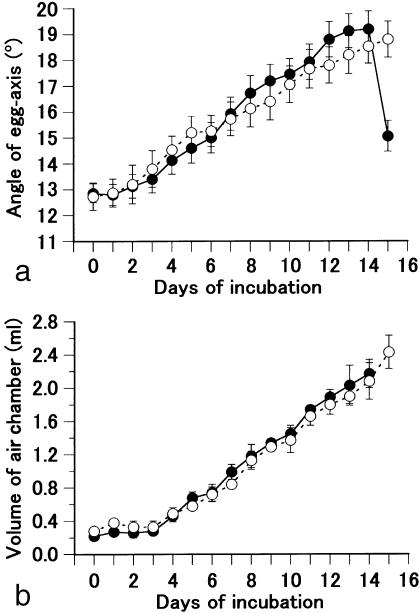
During the process of hatching, the angle of the egg-axis in free-standing eggs increased gradually from about 13° at day 0 of incubation to 19° at day 14 (Figs 2a and 3a,b). It then dropped to 15° at day 15, probably because the embryo inside the shell had stretched its head into an air chamber located at the blunt end of the egg. The embryo then hatches in the next 2 days by breaking the shell with a pipping action. In infertile eggs, the angle increased as in fertile eggs (Fig. 2a). There was no difference in angle between fertile and infertile eggs until day 14. However, in infertile eggs, the increase continued beyond day 15, and reached a maximum of 44.0 ± 1.4° (means ± SEM; n = 9) at day 70 (Fig. 3c,d).
Fig. 2.
Changes in the egg-axis angle and air-chamber volume. (a) The change in egg-axis angle during incubation of fertile (closed circle) and infertile (open circle) eggs with automatic egg-turning. Values are means ± SEM (n = 14). (b) The change in air-chamber volume during incubation. Values are means ± SEM (n = 5).
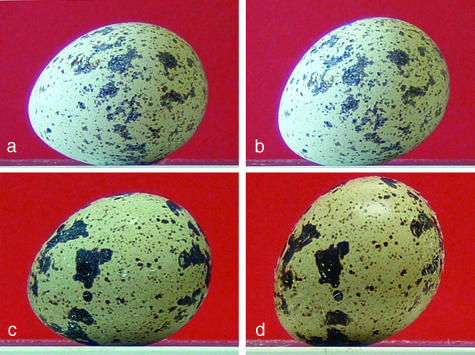
Fig. 3.
Photographs of a fertile eggs at day 0 (a: θ = +13°) and day 14 (b: θ =+27°) and of an infertile egg at day 0 (c: θ = +13°) and day 70 (d: θ = +44°).
We next measured the volume of the air chamber. The volume increased steadily from 0.22 mL at day 0 to 2.16 mL at day 14 in fertile eggs (Fig. 2b). No significant difference in volume was observed between fertile and infertile eggs (Fig. 2b). The contents of infertile eggs at day 70 were completely dried out and had accumulated at the sharp end. The volume of the air chamber and the angle of the egg axis in day-50 infertile eggs were 5.15 ± 0.25 mL and 37.2 ± 1.4° (means ± SEM; n = 10), respectively.
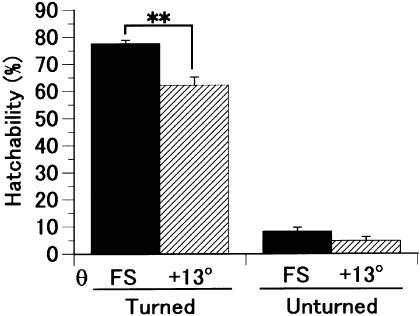
To verify the significance of the change in egg-axis angle during the hatching process, hatchability was compared between eggs in the free-standing position and those fixed at 13°. The hatchability of free-standing eggs was significantly higher than that of eggs fixed at 13° if the eggs were turned, but not otherwise (Fig. 4). This means that the naturally occurring increase in angle, although small, improves hatchability.
Fig. 4.
The hatchability of the free standing (FS) eggs compared with that of the eggs positioned at θ = +13° (**, P < 0.01). A single experiment was conducted with four plots of around 25 eggs each, depending on the egg-angle and egg-turning. Values are means ± SEM of 5 experiments. The eggs positioned at θ = +13° were encased individually in a paper frame and tilted. Egg turning was performed manually every 12 h.
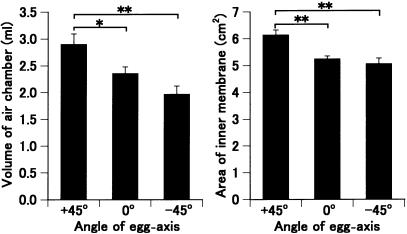
Next, an experiment was performed to find the parameters that determine hatchability. Eggs whose egg-axis angle was fixed at θ +45°, 0° or –45° were incubated with turning for 14 days, and the volume of the air chamber and area of the inner shell membrane in the chamber were measured. Values were highest for the eggs kept at θ +45° and lowest for those incubated at θ –45° (Fig. 5). Significant differences were observed between the eggs at θ +45° and those at θ 0° or –45° for both parameters. The increase in egg-axis angle during incubation was 9.9 ± 0.9, 8.6 ± 0.7 and 7.0 ± 0.5° (means ± SEM; n = 17) in eggs positioned at θ +45°, 0° and – 45°, respectively.
Fig. 5.
Parameters for hatchability. The eggs fixed at θ +45°, 0° and –45° were incubated with turning for 14 days, and the volume of their air chamber (a) and the area of their inner shell membrane in the chamber (b) were measured. Asterisks indicate significant differences (**P < 0.01, *P < 0.05). Values are means ± SEM (n = 17).
Morphology of shell membrane and oviductal isthmus
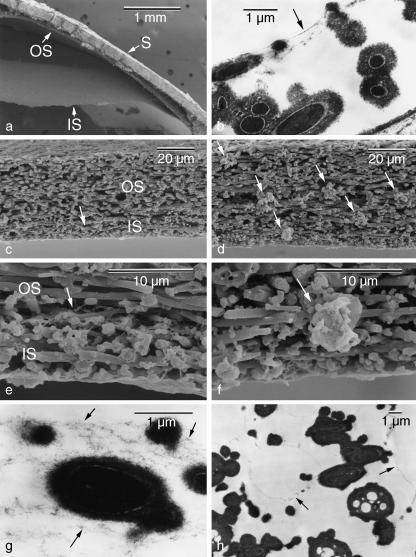
The last experiment was performed to find out why the air chamber is formed only at the blunt end of eggs. The shell membranes are made up of roughly parallel intertwining fibres arranged in alternate layers parallel to the egg surface and are divided into two parts, inner and outer, of different thickness. The air chamber at the blunt end of oviposited eggs is a space formed between the inner and outer shell membranes (Fig. 6a) and apparently demarcated by several membranous layers (Fig. 6b). Observation of the shell membranes of uterine eggs with a scanning electron microscope revealed that the air chamber was not yet formed at the blunt end but there was a membranous structure, called the medial layer, between the inner and outer shell membranes (Fig. 6c,e). The medial layer consisted of several layers of thin sheets as observed under the transmission electron microscope (Fig. 6g). Away from the blunt end, a distortion was observed in the disposition of shell membranes; some fibres were orientated randomly and aggregated (Fig. 6d,f). Such aggregates distributed in and between the inner and outer shell membranes. Distinct layers of the medial layer were not present but minute chamber-like structures were observed between the fibres in this region (Fig. 6h).
Fig. 6.
Electron micrographs showing the structural difference of the shell membrane between the air chamber and other regions. (a) An air chamber appeared with the splitting of the inner (IS) and outer shell membranes (OS) in an oviposited egg. S, shell. (b) Micrograph of inner shell membrane showing that the chamber is demarcated by several thin membranous layers (arrow). (c–h) In uterine eggs, a medial layer (arrows) was present between the inner and outer shell membranes at the blunt end (c,e) and consisted of several layers of thin sheets (g). In other regions, a distortion was observed in the disposition of shell membranes, some fibres of which orientated randomly and aggregated (arrows in d,f), and minute chamber-like structures (arrows) were present in the space between the fibres (h).
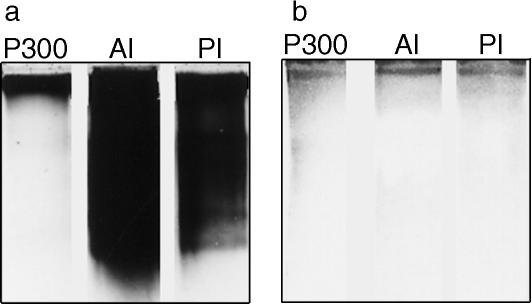
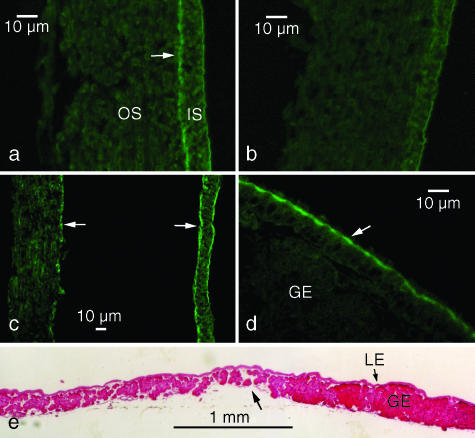
We isolated 300-kDa proteins from inner shell membranes (P300; Fig. 7a). An antiserum produced against P300 specifically recognized the same band in extracts of mucosae from the anterior and posterior isthmus (Fig. 7b). With immunofluorescence microscopy, the antiserum stained the inner shell membrane moderately and the outer shell membrane weakly both at the blunt end (Fig. 8a) and in other regions (Fig. 8b) of uterine eggs. In the case of the former, however, strong staining occurred in the medial layer (Fig. 8a). In oviposited eggs, the blunt end medial layer was divided into halves, and both were heavily stained (Fig. 8c). Immunoelectron microscopic observations showed that P300 distributed in the medial layer demarcating the air chamber and in the cortex surrounding the core of fibres (Fig. 9).
Fig. 7.
SDS-PAGE (6% gels) profiles of the substance isolated from inner shell membranes (P300; 10-µg protein) and the extracts (500 µg) from the epithelial mucosae of the anterior (AI) and posterior (PI) isthmus. Stained with Coomassie Brilliant Blue (a) or an antiserum against P300 (b).
Fig. 8.
Histochemistry of shell membranes and the oviductal isthmus. (a–d) The antiserum stained a medial layer (arrow in a) between the inner (IS) and outer shell membrane (OS) at the blunt end in a uterine egg. This layer was not present in any other region of the shell membrane (b). In an oviposited egg, the medial layer was divided into two (arrows in c). The antiserum stained an apical region of the luminal epithelium of the isthmus (arrow in d). Immunofluorescent staining. (e) A sagittal section of the luminal epithelium (LE) showed that the glandular epithelium (GE) is poorly developed at the boundary (arrow) between the anterior (to the left) and posterior (to the right) of the isthmus. Haematoxylin-eosin staining.
Fig. 9.
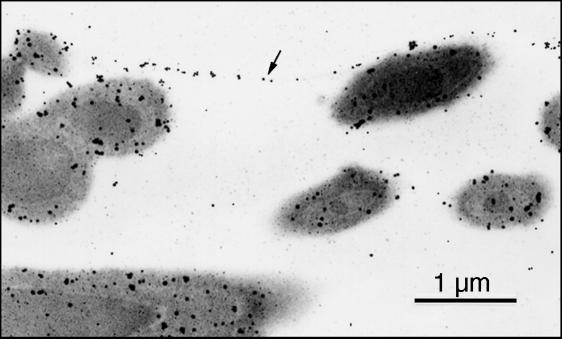
Immunoelectron micrograph showing the localization of P300 in the medial layer (arrow) demarcating an air chamber and in the cortex of fibres. Immunogold labelling.
An apical region of the luminal epithelium at the isthmus was also stained by the antiserum (Fig. 8d), indicating that the substance of both the medial layer and the cortex of fibres is produced by the luminal epithelium. This staining occurred along the entire length of the isthmus from an anterior portion that produces the inner shell membrane to a posterior portion that produces the outer shell membrane. The epithelium of the isthmus consisted of two parts, the luminal epithelium and the glandular epithelium. Cross sections showed that the development of the glandular epithelium varied depending on location. The glands were the sparsest and smallest at the boundary between the anterior and posterior portions of the isthmus (Fig. 8e).
Discussion
In almost all avian species, the egg is placed in the nest at an angle with its sharp end down owing to its asymmetrical shape. In the artificial incubation of eggs, this angle has been considered to be important for satisfactory hatchability; hatchability greatly decreased if the eggs were incubated in an inverted position (Cain & Abbott, 1971). By incubating eggs with different angles, at θ = 45° (the blunt end up), +13°~19° (the free standing), 0° (the side up) and –45° (the sharp end up), the present study showed that hatchability decreased in this same order, suggesting that the position of the blunt end up has biological meaning in avian reproduction.
The major finding of the present study is that avian eggs themselves change their angle during incubation: from 13° at day 0 of incubation to 19° at day 14 in fertile eggs. Interestingly, the increase in angle parallels an increase in the volume of the air chamber and there are no differences between fertile and infertile eggs in terms of these changes. In infertile eggs incubated for 70 days, the angle reached 44° and the egg contents had completely dried out and accumulated at the sharp end. These results indicate that the increase in the volume of the air chamber might be caused by the evaporation of water and the increase in the egg-axis angle might occur through movement of the centre of gravity toward the sharp end. About 10–11% of the water in an embryo is lost during the incubation period (Tullett & Deeming, 1987), and the average constant water loss throughout incubation is a valid approximation in 475 species of birds (Rahn & Ar, 1974). Thus, the change to the egg-axis angle is a natural phenomenon that occurs in the dry environment inhabited by avian eggs, the shape of which is asymmetrical.
The shell membranes consist of roughly parallel intertwining fibres which are arranged in alternate layers parallel to the egg surface (Burley & Vadehra, 1989). The fibres consist of a core surrounded by a cortex (Bellairs & Boyde, 1969). They contain type I, V and X collagens (Wong et al. 1984; Arias et al. 1992). Recently, type X collagen was detected in the core of the fibres and in the glandular epithelium of the isthmus (Fernandez et al. 2001; Wang et al. 2002). A P300 protein isolated in the present study was located in the cortex of the fibres and a lining surrounding the air chamber, and in the luminal epithelium of the isthmus.
The air chamber is a space formed between the inner and outer shell membranes and first appears at the moment of oviposition presumably as a result of a reduction in the volume of the egg contents induced by a sharp drop in temperature. Why then is the air chamber formed only at the blunt end of the eggshell? We found here the third element of the shell membrane, a medial layer, between the inner and outer shell membranes at the blunt end of the shell of uterine eggs. After oviposition, the medial layer split and produced the lining surrounding the air chamber. However, in regions other than the blunt end, no such layer was observed but minute air-chamber-like structures were present. Interestingly, the fibres of shell membranes there were distorted in their arrangement; some fibres were orientated randomly and aggregated.
The disturbance to the arrangement of the shell membrane in regions other than at the blunt end may be caused by the instability of a stratified shear flow. The asymmetrical egg-shape arises as a configuration of a layer of egg-white which is deposited in the posterior-most region of the oviductal magnum and coated thereafter by the peri-albumen layer and the shell membranes produced around it at the magnum–isthmus junction (Sultana et al. 2003) and the isthmus (Mao et al. 2006), respectively. The egg penetrates into and passes through the isthmus in a screwing motion with its sharp end first. When the matrices of the inner shell membrane, the medial layer, and the outer shell membrane are given sequentially upon the side of an egg, a stratified shear flow might be produced at the diffuse interface between these layers. The actual movement of an egg is not smooth but often interrupted at the turning point in the twisted oviduct. Then, the shear flow might become unstable and take the form of growing waves (Thorpe, 1971), resulting in a disruption of the arrangement in parallel layers of shell membranes and in the fragmentation of the medial layer. At the blunt end of the egg, this shear flow is cancelled out and the three matrices are layered neatly. Thus, the air chamber is formed only at the blunt end.
At the start of the study, we expected there to be three different segments in the isthmus that produce the substances of the inner shell membrane, the medial layer, and the outer shell membrane, in this order. However, the cell types which comprise the luminal epithelium and the glandular epithelium of the isthmus were mostly the same over the entire length (Hoffer, 1971; Draper et al. 1972). The only difference was in the size of the gland, observed in the cross-section of the tissue. The gland was sparsest and smallest at the junction between the anterior and posterior portions of the isthmus. Then, the amount of product of the luminal epithelium relative to that of the glandular epithelium might be greatest at the boundary. By immunoelectron microscopy, it was shown that a component of the medial layer and the cortex of fibres, P300, is produced by the cells in the luminal epithelium. Thus, the medial layer consists of the same substances as those of the cortex of fibers and might be produced by the accumulation of an excess of cortex substances.
Gas exchange is essential to embryonic development. It has been shown that there is a gradual reduction in gas conductance through the shell and shell membrane and in pore density in the shell from the blunt end of the egg toward the sharp end (Rokitka & Rahn, 1987). Gas exchange of the avian embryo takes place by diffusion through the chorioallantoic membrane and the shell membrane before pulmonary respiration starts. The air chamber at the blunt end enables a more effective gas exchange (Rokitka & Rahn, 1987; Seymour & Visschedijk, 1988) and the increase in its volume during incubation is vital in sustaining the metabolism of a growing embryo. But why was there a difference in the volume of the air chamber in eggs that were incubated at different angles? The answer might reside in the structure of the shell membranes. The shell membranes consist of a meshwork of fibers. The meshwork acts as a cushion at the bottom of the egg to protect its contents, and as a hanger in the upper portion to protect against the crush of the egg's own weight. If the egg is inverted, the air chamber, now at the bottom, will be made narrower by the hanging interior. The decrease in the volume of the air chamber negatively affects gas exchange, embryonic development and hatchability.
In conclusion, the asymmetrical ellipsoid produces an air chamber at the blunt end of the avian egg during its sojourn in the oviductal isthmus, to maintain the blunt end up after oviposition and to raise that end during incubation in a dry environment, leading to high hatchability. Our data indicate that the Aves might have evolved an asymmetrical egg-shape as a strategy to invade inland areas and reproduce there.
Acknowledgments
We wish to thank S. Ito for supplying the quail eggs.
References
- Arias JL, Carrino DA, Fernandez MS, Rodriguez JP, Dennis JE, Caplan AI. Partial biochemical and immunochemical characterization of avian eggshell extracellular matrices. Arch Biochem Biophys. 1992;298:293–302. doi: 10.1016/0003-9861(92)90126-h. [DOI] [PubMed] [Google Scholar]
- Bellairs R, Boyde A. Scanning electron microscopy of the shell membranes of the hen's egg. Z Zellforsch. 1969;96:237–249. doi: 10.1007/BF00338771. [DOI] [PubMed] [Google Scholar]
- Burley RW, Vadehra DV. The Avian Egg: Chemistry and Biology. New York, NY: John Wiley & Sons; 1989. [Google Scholar]
- Cain JR, Abbott UK. Incubation of avian eggs in an inverted position. Poult Sci. 1971;50:1223–1226. [Google Scholar]
- Deeming DC. Avian Incubation. Oxford: Oxford University Press; 2002. [Google Scholar]
- Draper MH, Davidson MF, Wyburn GM, Johnston HS. The fine structure of the fibrous membrane forming region of the isthmus of the oviduct of Gallus domesticus. Quart J Exper Physiol. 1972;57:297–309. doi: 10.1113/expphysiol.1972.sp002163. [DOI] [PubMed] [Google Scholar]
- Fernandez MS, Moya A, Lopez L, Arias JL. Secretion pattern, ultrastructural localization and function of extracellular matrix molecules involved in eggshell formation. Matrix Biol. 2001;19:793–803. doi: 10.1016/s0945-053x(00)00128-1. [DOI] [PubMed] [Google Scholar]
- Hoffer AP. The ultrastructure and cytochemistry of the shell membrane-secreting region of the Japanese quail oviduct. Am J Anat. 1971;131:253–288. doi: 10.1002/aja.1001310302. [DOI] [PubMed] [Google Scholar]
- Johnston PM, Comar CL. Distribution and contribution of calcium from the albumen, yolk and shell to the developing chick embryo. Am J Physiol. 1955;183:365–370. doi: 10.1152/ajplegacy.1955.183.3.365. [DOI] [PubMed] [Google Scholar]
- Kutchai H, Steen JB. Permeability of the shell and shell membranes of hens’ eggs during development. Respir Physiol. 1971;11:265–278. doi: 10.1016/0034-5687(71)90001-6. [DOI] [PubMed] [Google Scholar]
- Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mao KM, Sultana F, Howlider MAR, Iwasawa A, Yoshizaki N. The magnum–isthmus junction of the fowl oviduct participates in the formation of the avian-type shell membrane. Zool Sci. 2006;23:41–47. doi: 10.2108/zsj.23.41. [DOI] [PubMed] [Google Scholar]
- Ono T, Wakasugi N. Mineral content of quail embryos cultured in mineral-rich and mineral-free conditions. Poult Sci. 1984;63:159–166. doi: 10.3382/ps.0630159. [DOI] [PubMed] [Google Scholar]
- Rahn H, Ar A. The avian egg: incubation time and water loss. Condor. 1974;76:147–152. [Google Scholar]
- Rokitka MA, Rahn H. Regional differences in shell conductance and pore density of avian eggs. Respir Physiol. 1987;68:371–376. doi: 10.1016/s0034-5687(87)80021-x. [DOI] [PubMed] [Google Scholar]
- Seymour RS, Visschedijk AHJ. Effects of variation in total and regional shell conductance on air cell gas tensions and regional gas exchange in chicken eggs. J Comp Physiol B. 1988;158:229–236. [Google Scholar]
- Simkiss K. Calcium metabolism and avian reproduction. Biol Rev. 1961;36:321–367. [Google Scholar]
- Sultana F, Yokoe A, Ito Y, Mao KM, Yoshizaki N. The peri-albumen layer: a novel structure in the envelopes of an avian egg. J Anat. 2003;203:115–122. doi: 10.1046/j.1469-7580.2003.00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorpe SA. Experiments on the instability of stratified shear flows: miscible fluids. J Fluid Mech. 1971;46:299–319. [Google Scholar]
- Tullett SG, Deeming DC. Failure to turn eggs during incubation: effects on embryo weight, development of the chorioallantois and absorption of albumen. Br Poult Sci. 1987;28:239–243. doi: 10.1080/00071668708416958. [DOI] [PubMed] [Google Scholar]
- Van Schalkwyk SJ, Cloete SW, Brown CR, Brand Z. Hatching success of ostrich eggs in relation to setting, turning and angle of rotation. Br Poult Sci. 2000;41:46–52. doi: 10.1080/00071660086394. [DOI] [PubMed] [Google Scholar]
- Wang X, Ford BC, Praul CA, Leach RM. Collagen X expression in oviduct tissue during the different stages of the egg laying cycle. Poult Sci. 2002;81:805–808. doi: 10.1093/ps/81.6.805. [DOI] [PubMed] [Google Scholar]
- Wong M, Hendrix MJC, von der Mark K, Little C, Stern R. Collagen in the eggshell membranes of the hen. Dev Biol. 1984;104:28–36. doi: 10.1016/0012-1606(84)90033-2. [DOI] [PubMed] [Google Scholar]
- Yoshizaki N, Saito H. Changes in shell membranes during development of quail embryos. Poult Sci. 2002;81:246–251. doi: 10.1093/ps/81.2.246. [DOI] [PubMed] [Google Scholar]