Abstract
The endogenous pain modulatory system is a complex network of brain areas that control nociceptive transmission at the spinal cord by inhibitory and facilitatory actions. The balance between these actions ensures effective modulation of acute pain, while during chronic pain the pronociceptive effects appear to prevail. The mechanisms underlying this imbalance were studied as to the role of two medullary components of the pain modulatory system: the dorsal reticular nucleus and the caudal ventrolateral medulla, which function primarily as pronociceptive and antinociceptive centres, respectively. Both areas are connected with the spinal dorsal horn by closed reciprocal loops. In the spino-dorsal reticular nucleus loop, the ascending branch is strongly inhibited by spinal GABAergic neurons, which may act as a buffering system of the dorsal reticular nucleus-centred amplifying effect. In the spino-caudal ventrolateral medulla loop, the ascending branch is under potent excitation of substance P (SP) released from primary afferents, which is likely to trigger the intense descending inhibition detected in acute pain. During chronic pain, the activity in the lateral reticular formation of the caudal ventrolateral medulla changes, so that the action of the caudal ventrolateral medulla upon SP-responsive spinal neurons shifts from inhibitory to excitatory. The mechanisms of this modulatory shift are unknown but probably relate to the decresed expression of µ-opioid, δ-opioid and GABAB receptors. Normalizing receptor expression in the caudal ventrolateral medulla or controlling noci-evoked activity at the dorsal reticular nucleus or caudal ventrolateral medulla by interfering with neurotransmitter release is now possible by the use of gene therapy, an approach that stands out as a unique tool to manipulate the supraspinal endogenous pain control system.
Keywords: caudal ventrolateral medulla, chronic pain, dorsal reticular nucleus, receptor systems.
Introduction
Nociceptive transmission from the spinal dorsal horn is modulated from several brain areas, which are collectively known as the supraspinal endogenous pain control system. The existence of a system that modulates pain transmission at the spinal dorsal horn was already postulated by the gate control theory (Melzack & Wall, 1965). It is currently accepted that the endogenous pain control system sets up the level of excitability of spinal nociceptive second-order neurons. Departing from initial observations that electrical stimulation of the periaqueductal grey (PAG) inhibits behavioural responses in acute pain tests (Mayer et al. 1971) and nociceptive activation of spinal dorsal horn neurons (Lieberskind et al. 1973), it is now established that stimulation-produced analgesia can be triggered from several supraspinal areas, most of which are located in the brainstem.
The endogenous pain control system is organized in a very complex way (cf. initial and recent reviews in Basbaum & Fields, 1984; Millan, 2002). Descending modulation is exerted by three main neurochemical systems – noradrenergic, serotonergic and opioidergic – which interact in an intricate manner (reviewed by Jones, 1992). Besides the extensively explored anti-nociceptive effects, the system also triggers descending pain facilitation (pronociception). Pain facilitating components are mainly located at the medulla oblongata. The rostroventromedial medulla (RVM), which encompasses the nucleus raphe magnus and adjacent reticular formation, is known for its biphasic (i.e, pronociceptive and antinociceptive effects). The dorsal reticular nucleus (DRt) exerts unique pronociceptive effects revealed by the enhancement of nociceptive responses of spinal dorsal horn neurons and behavioural responses to pain upon local stimulation (reviewed by Lima & Almeida, 2002). The biological significance of pain facilitation is a matter of dispute (Ossipov & Porreca, 2006).
Chronic pain appears to be related to imbalance of descending modulation, with pro-nociceptive actions prevailing over anti-nociceptive effects (reviewed by Ren & Dubner, 2002; Dubner & Ren, 2004). The mechanisms underlying this modulatory shift are starting to be unravelled (Danzinger et al. 2001) and probably account for the establishment and perpetuation of chronic pain (Ren & Dubner, 2002; Dubner & Ren, 2004). Further complexity is added to the endogenous pain modulatory system by its role in integrating nociception with other body functions (e.g. cardiovascular and motor responses). One of the most representative examples is provided by the involvement of the caudal ventrolateral medulla (cVLM) in the integration of blood pressure and pain responses (Maixner, 1991; Lima et al. 2002). Such integrative processes are only possible because of the network properties of the circuits that compose the endogenous pain modulatory system and play a fundamental role in homeostasis (reviewed by Mason, 2006).
Direct targeting of the supraspinal pain control system through local electrical stimulation (Simpson, 1994) has been used as a therapeutic approach mainly in intractable pain (Bittar et al. 2005). Two main limitations impair, however, a generalized use of electrical stimulation for pain control: (1) it interferes with other physiological functions of the pain modulatory centres leading to deleterious side-effects, and (2) it excludes specific manipulation of pain facilitatory centres, which are known to play a major role in chronic pain maintenance. Based on the special role of the DRt and cVLM in pain modulation, the next two sections will present anatomo-functional data indicating that both areas can be more effectively manipulated by targeting specifically neuronal groups misfunctionning during chronic pain.
The dorsal reticular nucleus plays a unique pronociceptive role
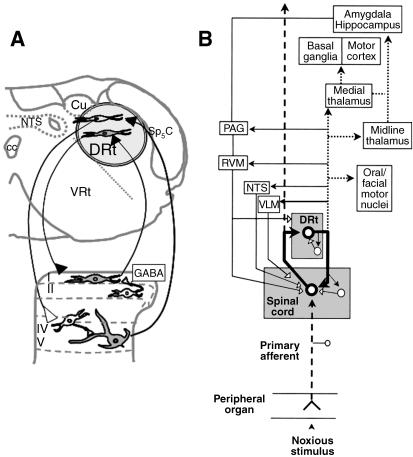
The DRt is situated in the reticular formation medial to the spinal trigeminal nucleus, pars caudalis (Sp5C), lateral to the nucleus tractus solitarius, ventral to the cuneate nucleus (Cu) and dorsal to the ventral reticular nucleus (VRt; Fig. 1A). DRt neurons respond exclusively or mainly to noxious stimulation at the periphery (Villanueva et al. 1988). DRt stimulation induces hyperalgesia in acute pain (Almeida et al. 1996), whereas lesioning the nucleus induces analgesia both in acute (Almeida et al. 1996) and in sustained (Almeida et al. 1999) pain. These pronociceptive actions are mediated by spinal cord neurons, as demonstrated by the increase in post-discharge after DRt stimulation (Dugast et al. 2003) and the decrease in spinal Fos expression after DRt lesioning (Almeida et al. 1999). The descending pronociceptive effects of the DRt appear to be conveyed by direct descending projections to the spinal dorsal horn (Tavares & Lima, 1994). DRt fibres establish putatively excitatory synaptic contacts upon lamina I neurons that project back to the DRt (Fig. 1A; Almeida et al. 1993). Excitatory synaptic contacts also occur between DRt-projecting spinal cells and spinally projecting DRt neurons (Almeida et al. 2000). A similar facilitatory effect upon the deep dorsal horn (Dugast et al. 2003) was proposed to be mediated by dorsal horn inhibitory interneurons (Fig. 1A).
Fig. 1.
Pain facilitating circuits centred in the DRt. (A) Location of the DRt in a coronal section of the medulla oblongata obtained at 5.3 mm caudal to the interaural line (Paxinos & Watson, 1998) depicting the spino-DRt-spinal loops. The buffering role of GABAergic spinal neurons in counteracting the amplifying effect of the loop is included. (B) Interactions of the DRt, not only with the spinal cord, but also with other brain structures, namely the components of the pain control system (PAG, RVM, NTS and cVLM). The bold thick lines represent the circuit involved in signal amplification; dotted lines represent the circuit mediating aversive pain reactions; thin solid lines represent the circuit involved in counteracting signal amplification; dashed lines represent the signal transmission pathway. Filled neurons/arrows: excitatory actions; empty neurons/arrows: inhibitory actions. Adapted from Lima & Almeida (2002). Abbreviations: cc, central canal; Cu, cuneate nucleus; cVLM, caudal ventrolateral medulla; DRt, dorsal reticular nucleus; NTS, nucleus tractus solitarius; PAG, periaqueductal grey; RVM, rostroventromedial medulla; Sp5C, spinal trigeminal nucleus, pars caudalis; VRt, ventral reticular nucleus.
Mechanisms buffering the amplifying effect of the reverberative spino-DRt-spinal loops probably include GABAergic inhibition at the spinal level. The expression of GABAB receptors in DRt-projecting spinal neurons is higher than in other spinofugal pathways (Castro et al. 2006b). The projection of amplified signals from the DRt to supraspinal pain inhibitory centres is also likely to play a role in the buffering process (Fig. 1B; Lima and Almeida, 2002). However, the pattern of DRt projections to the brain is widespread, targeting areas involved in motivational-affective aspects of pain along with pain-associated motor behaviours (Fig. 1B; Leite-Almeida et al. 2006). Direct targeting of DRt neurons involved in spino-DRt-spinal amplifying loops would therefore be an important strategy for pain control.
The caudal ventrolateral medulla strongly inhibits acute pain and is a nociceptive integration centre
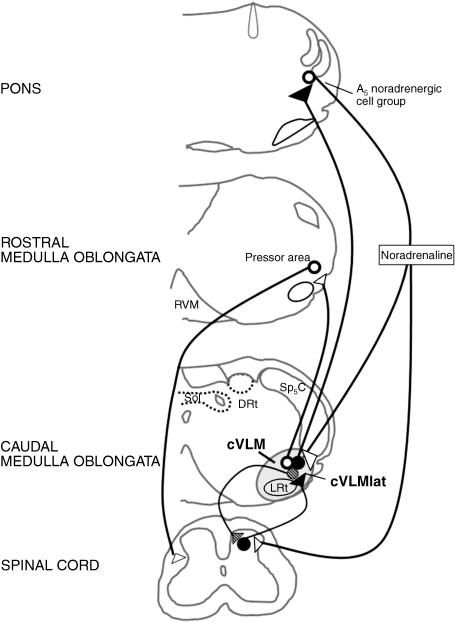
The cVLM is one of the main inhibitory components of the endogenous pain modulatory system (reviewed by Tavares & Lima, 2002). Stimulation of the cVLM induces analgesia in acute pain and inhibits nociceptive responses of spinal cord neurons (Gebhart & Ossipov, 1986), some of which participate in the spinothalamic tract (Janss & Gebhart, 1988). The reticular formation known as cVLMlat (Fig. 2), located in the lateralmost part of the cVLM between the Sp5C and the lateral reticular nucleus (LRt), appears to be the cVLM region responsible for pain modulation. The analgesia induced upon cVLMlat stimulation is more intense and long-lasting than from the other cVLM regions (Gebhart & Ossipov, 1986). Furthermore, projections from the cVLMlat, but not from the LRt, specifically target spinal laminae involved in pain transmission, namely laminae I, IV–V and X (Tavares et al. 1998). The cVLMlat also targets the dorsal horn indirectly through other components of the pain modulatory system. A disynaptic pathway relaying in the pontine A5noradrenergic cell group was described which conveys the α2-adrenoreceptor-mediated analgesia triggered from the cVLM (Tavares et al. 1996). Spinally projecting noradrenergic efferents from the pontine A5noradrenergiccell group receive excitatory afferents from fibres originating in the cVLMlat (Fig. 2; Tavares et al. 1996). An A5-cVLMlat negative feedback control loop was revealed based on the demonstration that most A5 noradrenergic neurons that participate in the cVLMlat-A5-spinal pathway send collaterals back to the cVLMlat (Fig. 2; Tavares et al. 1997a). The cVLMlat also projects to other components of the supraspinal endogenous pain modulatory system, namely the RVM (Tavares et al. 1996), suggesting that it may activate several components of the supraspinal pain modulatory system. Arrival of nociceptive input at the cVLMlat is a probable trigger of descending modulation. Spinal neurons projecting to the cVLM are activated by noxious stimuli of various natures (Tavares et al. 1993), probably in response to substance P release by primary afferents, as suggested by the large amount of spinal-cVLM neurons that express NK1 receptors (Todd, 2002; Castro et al. 2006b).
Fig. 2.
Diagram of circuits centred in the cVLMlat and participating in pain modulation (continuous lines) and cardiovascular control (punctated lines). The arrival of nociceptive input to the cVLMlat (the reticular formation lying between the Sp5C and the LRt) triggers pain control by activation of the A5 noradrenergic cell group, which induces noradrenaline-mediated inhibition of pain transmission at the spinal dorsal horn, along with feedback inhibition of the cVLMlat. A direct effect of the cVLMlat in pain descending control by closed spino-cVLMlat loops was also included. Blood pressure rises activate cVLM neurons by afferents from the nucleus tractus solitarius (NTS), which will inhibit the pressor area located at the rostroventrolateral medulla. For simplification of the diagram the connections of the LRt relevant for motor control (see text) were not represented. Filled circles/arrows: excitatory actions; empty circles/arrows: inhibitory actions. Abbreviations: cc, central canal; cVLM, caudal ventrolateral medulla; DRt, dorsal reticular nucleus; LRt, lateral reticular nucleus; NTS, nucleus tractus solitarius; RVM, rostroventromedial medulla; Sp5C, spinal trigeminal nucleus, pars caudalis.
The extensive anatomo-functional characterization of circuits centred in the cVLM indicates that this region is particularly suited to integrate cardiovascular and motor responses to the noxious event, which can be related to a putative role of that medullary area in maintenance of homeostasis (Mason, 2006). The cVLM exerts vasodepressive effects (Day & Ro, 1983; Murugaian et al. 1989) from areas that include the cVLMlat (Tavares et al. 1997b), through GABA-mediated inhibition of the vasopressor centre located at the rostroventrolateral medulla (Fig. 2; Agarwal & Calaresu, 1991). cVLMlat lesioning prevents the hypertension-related inhibition of noxious-evoked Fos expression in spinal neurons in several animal models of hypertension (Randich & Robertson, 1994; Tavares et al. 1997b; Morato et al. 2006). The cVLM is also likely to instigate motor reactions to noxious events, through nociceptive activation of the LRt, an area with a well-established involvement in motor control (Alstermark et al. 1981; Cledenin et al. 1974).
The multiple anatomical studies carried so far indicate that different cVLM neurons participate in the anti-nociceptive, cardiovascular and motor control (Fig. 2). It is therefore possible to take advantage of the strong analgesic efficacy of the cVLM by elucidating more direct and specific ways of manipulating cVLM nociceptive neurons than the current approaches that interfere with the integrative role of this medullary area.
Chronic pain affects the endogenous supraspinal pain modulatory system
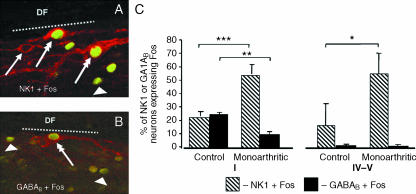
The pain modulatory system has been mainly studied in acute pain tests but it is reasonable to assume that the nociceptive barrage from the spinal cord during chronic pain deeply affects the system (Ren & Dubner, 2002; Vanegas & Schaible, 2004). During chronic pain, increases in nociceptive activation of spinal neurons bearing NK1 receptors are detected, but the nociceptive activation of GABAB-expressing spinal neurons decreases (Fig. 3; Castro et al. 2005). Thus, chronic pain is likely to interfere with the activity of the DRt and cVLM given that both areas are targeted by spinal neurons expressing those receptors (Castro et al. 2006b). As regards the DRt, the decrease in nociceptive activation of GABAB-expressing neurons during chronic inflammatory pain, together with the strong participation of these neurons in the ascending branch of the spino-DRt-spinal loop (Fig. 1; Castro et al. 2006b), indicates that the amplification process carried out by that loop is depressed by chronic pain. These GABA-mediated changes in the spinal inhibitory control are in agreement with the increase in GABA levels during chronic inflammatory pain (Castro-Lopes et al. 1992). Neuronal activity in the supraspinal pain modulatory system appears, however, to be generally increased during chronic pain (Porro et al. 1991; Neto et al. 1999; reviewed by Porreca et al. 2002). This might be related to the specific supraspinal effects of chronic pain in the decreased expression of receptors involved in pain inhibition, as demonstrated for µ-opioid and GABAB receptors in the DRt and cVLM during chronic inflammatory pain (Pinto et al. 2003, 2006b; Castro-Lopes et al. 2006). These changes appear to be functionally relevant as ongoing studies show that the effects of opioid agonists administered in the DRt differ between acute and chronic pain conditions (Pinto et al. 2006b).
Fig. 3.
Effect of chronic pain in the nociceptive activation of spinal neurons bearing NK1 or GABAB receptors. (A,B) Confocal images of neurons double-immunostained for NK1 receptors and Fos protein (double arrows in A) or GABAB receptors and Fos protein (double arrow in A,B). The photomicrographs also depict cells labelled only for NK1 (arrow in A) or for the Fos protein (arrowheads) which was induced in response to noxious mechanical stimulation of the skin close to a chronically inflammed joint. The dotted lines mark the border between lamina I and the dorsal funiculus (DF). (C) Percentages of NK1- or GABAB-immunoreactive neurons in spinal laminae I or IV–V that express Fos in non-inflamed (control) or inflamed (monoarthritic) animals. Nociceptive activation of NK1-expressing neurons increases significantly in lamina I (P < 0.001) and IV–V (P < 0.05) whereas that of GABAB-expressing neurons decreases in lamina I (P < 0.01). Adapted from Castro et al. (2005).
The precise effect of chronic pain in the functioning of spino-medullary loops centred in the DRt and cVLMlat needs to be ascertained. Regarding the DRt, the increase in spinal GABAergic buffering mechanisms discussed above probably results in a decreased recruitment of supraspinal pain-inhibitory components targeted by the DRt (Fig. 1B). In the cVLM, a shift from anti-nociceptive to pronociceptive in the descending modulatory action upon spinal NK1-positive neurons takes place (Castro et al. 2006a). This nicely matches structural data showing that the descending branch of the cVLM-spinal loop includes both inhibitory and facilitatory components (Tavares et al. 1998) and recent electrophysiological data showing that the cVLM contains cells associated with pain inhibition (OFF cells) along with neurons associated with pain facilitation (ON cells) (Pinto-Ribeiro et al. 2006). The decrease in both µ-opioid and GABAB receptor expression in the cVLM (Pinto et al. 2003, 2006b; Castro-Lopes et al. 2006) may account for the overtake of descending facilitation during chronic pain.
Based on the network organization of the pain modulatory system, changes induced by chronic pain in one component will affect the activity of the others (Pinto et al. 2006a). In the case of the DRt-cVLM reciprocal connection (Almeida et al. 2002; Cobos et al. 2003; Leite-Almeida et al. 2006), a positive correlation of nociceptive activation between the two areas occurs during chronic pain (Pinto et al. 2006a). In general, the data indicate that the effects of chronic pain on descending pain modulation from the DRt and cVLM are very complex and that specific targeting of system components for chronic pain control cannot be achieved with the currently available methods.
Gene therapy: an eclectic way to manipulate the supraspinal endogenous pain modulatory system
Gene therapy allows local expression of missing receptors (Xu et al. 2003) and to direct gene expression to specific neuronal populations (reviewed by Cronin et al. 2005). Both issues should be taken into account in the design of strategies to manipulate the supraspinal pain modulatory system, considering the data reviewed in the sections above. Encouraging results were provided by gene therapy studies directed to anti-nociceptive components of the supraspinal pain modulatory system, with inhibition of acute pain (Kang et al. 1998; Jasmin et al. 2003). In chronic pain, gene therapy has been directed only to the spinal level (Glorioso et al. 2003; Pohl et al. 2003). Replication-defective forms of Herpes Simplex Virus type 1 (HSV-1) have been the vectors of choice due to specific affinities of this vector to the nervous system, namely its affinity to neuronal cells and ability to be retrogradely transported (Frampton et al. 2005). HSV-1 vectors codying for opioid peptides were peripherally applied and retrogradely transported to the dorsal root ganglion and produced a significant release of the transgene product at the spinal dorsal horn, as well as sustained analgesia (Braz et al. 2001). Other viral vectors have been used in gene therapy of pain, each presenting specific features that should be considered, namely the levels of immunogenicity, toxicity, transduction efficiency and capability of transgene insertion (Cope & Lariviere, 2006). Besides HSV-1, the most promising viral vectors are the adenoviruses, owing to their high transduction efficiency of both dividing and non-dividing cells (Finegold et al. 1999; Milligan et al. 2005). However, adenoviral expression is transient and the virus induces inflammatory responses, leading to the appearance of adeno-associated viruses (AAV), which have been used in chronic inflammatory pain models (Gu et al. 2005). Recent studies used lentiviral vectors to overexpress neurotrophic factors at the spinal cord in a sustained manner, which opened new perspectives in the use of these delivering systems in gene therapy for chronic pain (Pezet et al. 2006). Non-viral vectors, in which naked plasmid DNA is introduced in liposomes, have the advantage over viral vectors of decreasing the immunological responses. Although the efficiency of non-viral vectors needs to be improved, promising results were obtained in bladder pain (Chuang et al. 2003).
Based on the affinity of HSV-1 to the neuron and the appearance of improved HSV-based constructs, like amplicons (Jerusalinsky & Epstein, 2006), this will still be a viral of choice in gene therapy for chronic pain. In order to use HSV-1-based gene therapy to target the endogenous pain modulatory system, it is important (1) to distinguish neurons involved in nociceptive processing from neurons participating in other brain functions, and (2) to ascertain how HSV-1 constructs will migrate retrogradely upon local delivery. The first requirement has been addressed for the cVLM, in which vasodepressor, motor control and anti-nociceptive neurons have been distinguished on the basis of the anatomy and neurochemical organization of the respective circuits, as described in earlier sections of this review. The study of the migration dynamics of an HSV-1 vector containing the lacZ reporter gene, under the control of the human cytomegalovirus promoter (hCMV), from the DRt and cVLM has revealed viral expression in several of their afferents. However, transgene product release was also observed in axonal terminal arborizations in few central areas other than the DRt and cVLM through axonal collaterals, which was more pronounced in the case of the cVLM (Martins et al. 2004). These data indicated that the desired amplification of the HSV-1-based gene therapy effects, which would be achieved by targeting multiple DRt and cVLM afferent areas, can only be obtained without widespread collateral effects by directing viral expression to relevant components of the pain modulatory system. A detailed neurochemical characterization of brain neurons transduced upon HSV-1 injected in the DRt or cVLM showed that the noradrenergic components of the endogenous pain modulatory system are strongly targeted without significant release of transgene product in axonal terminations (Martins et al. 2005).
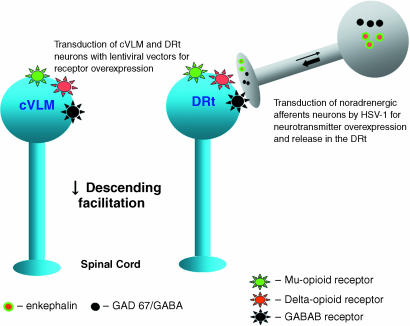
Based on all the results summarized above, a major outcome for chronic pain control would be to decrease the pronociceptive effects of the DRt and cVLM. Two complementary gene therapy strategies (Fig. 4) could be used: local transduction of DRt and cVLM nociceptive neurons to overexpress the receptors depressed during chronic pain (µ-opioid, δ-opioid and GABAB), and transduction of DRt and cVLM noradrenergic afferent neurons. For the first strategy, lentiviral vectors appear to be especially suited owing to its transduction being restricted to local neurons. The second strategy should rely on the administration in the DRt and cVLM of HSV-1 constructs in which the noradrenaline synthesizing promoter – tyrosine hydroxylase (TH) promoter – is used to restrict the expression of various transgenes, such as the enkephalin precursor (preproenkephalin) or the GABA-synthesizing enzyme (GAD 67) to noradrenergic neurons. This would be even more relevant inasmuch as the TH promoter was shown to allow a very long transgene expression (Wang et al. 2004). Although it is important to discard effects in functions besides pain control by detailed studies of the transduced circuits and animal behaviour, it is likely that this gene therapy strategy will produce the specific and sustained effects necessary for chronic pain control. Studies of other components of the supraspinal endogenous pain modulatory system, similar to those summarized in the present review for the DRt and cVLM, may allow to expand the applications of gene therapy to other pain control areas.
Fig. 4.
Gene therapy strategies to decrease descending facilitation from the DRt and cVLM. Overexpression of depressed receptors (µ-opioid, δ-opioid and GABAB receptors) in neurons of both pain control areas can be induced by lentiviral vectors containing transgenes for each receptor. A synaptically mediated increase in the release of opioids and GABA in the DRt can be achieved by HSV-1 constructs retrogradely transported (thin arrow) to the noradrenergic DRt afferents, using a TH promoter to control the expression of preproenkephalin or the GABA synthesizing enzyme GAD 67. The transgene product is then anterogradely released in the DRt (thick arrow).
Concluding remarks
Manipulating the endogenous pain control system for pain control purposes prompts the development of methods that interfere in a specific and sustained manner with neurons involved in nociceptive control. A detailed anatomical and functional characterization of the system is required as regards the characteristics of pain modulatory units and their interplay with other body functions. Gene therapy may represent an important strategy for pain control from supraspinal levels as very specific viral vectors can be constructed based on the specific characteristics of the components of the endogenous pain control system to be targeted. These studies may provide the basis for translational application of gene therapy to pain control areas that are more easily accessed surgically than the DRt and cVLM. As animal studies have not demonstrated adverse effects of gene therapy, and the improvement of transgene-delivering systems is a fast-growing field, it is possible that the application of gene therapy to the endogenous supraspinal pain modulatory system may be used in the near future to control refractory and severe pain.
Acknowledgments
Supported by Grant 15/04 of Fundação Bial (Portugal) and FCT grant PTDC/SAU-OSM/64643/2006
References
- Agarwal SK, Calaresu FR. Monosynaptic connection from caudal to rostroventrolateral medulla in the baroreceptor reflex pathway. Brain Res. 1991;555:70–74. doi: 10.1016/0006-8993(91)90861-o. [DOI] [PubMed] [Google Scholar]
- Almeida A, Tavares I, Lima D, Coimbra A. Descending projections from the medullary dorsal reticular nucleus make synaptic contacts with spinal cord lamina I cells projecting to that nucleus: an electron microscopic tracer study in the rat. Neuroscience. 1993;55:1093–1106. doi: 10.1016/0306-4522(93)90323-8. [DOI] [PubMed] [Google Scholar]
- Almeida A, Tjolsen A, Lima D, Coimbra A, Hole H. The medullary dorsal reticular nucleus facilitates acute nociception in the rat. Brain Res Bull. 1996;39:7–15. doi: 10.1016/0361-9230(95)02027-6. [DOI] [PubMed] [Google Scholar]
- Almeida A, Storkson R, Hole H, Lima D, Tjolsen A. The medullary dorsal reticular nucleus facilitates pain behaviour induced by formalin in the rat. Eur J Neurosci. 1999;11:110–122. doi: 10.1046/j.1460-9568.1999.00411.x. [DOI] [PubMed] [Google Scholar]
- Almeida A, Tavares I, Lima D. Reciprocal connections between the medullary dorsal reticular nucleus and the spinal dorsal h orn in the rat. Eur J Pain. 2000;4:373–387. doi: 10.1053/eujp.2000.0193. [DOI] [PubMed] [Google Scholar]
- Almeida A, Cobos A, Tavares I, Lima D. Brain afferents to the medullary dorsal reticular nucleus: a retrograde and anterograde tracing study in the rat. Eur J Neurosci. 2002;16:81–95. doi: 10.1046/j.1460-9568.2002.02058.x. [DOI] [PubMed] [Google Scholar]
- Alstermark B, Lundberg A, Norsell U, Sybierska E. Integration in descending motor pathways controlling the forelimb in the cat: differential behavioural defects after spinal cord lesions interrupting defined pathways from higher motor centers to motoneurones. Exp Brain Res. 1981;42:299–318. doi: 10.1007/BF00237496. [DOI] [PubMed] [Google Scholar]
- Basbaum AI, Fields HL. Endogenous pain control systems: brainstem spinal pathways and endorphin circuitry. Ann Rev Neurosci. 1984;7:309–338. doi: 10.1146/annurev.ne.07.030184.001521. [DOI] [PubMed] [Google Scholar]
- Bittar RG, Kar-Purkayastha I, Owen SL, et al. Deep brain stimulation for pain relief: a meta-analysis. J Clin Neurosci. 2005;12:515–519. doi: 10.1016/j.jocn.2004.10.005. [DOI] [PubMed] [Google Scholar]
- Braz J, Beaufour C, Coutaux A, et al. Therapeutic efficacy in experimental polyarthritis of viral-driven enkephalin overproduction in sensory neurons. J Neurosci. 2001;21:7881–7888. doi: 10.1523/JNEUROSCI.21-20-07881.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro AR, Pinto M, Lima D, Tavares I. Imbalance between the expression of NK1 and GABAB receptors in nociceptive spinal neurons during secondary hyperalgesia: a c-fos study in the monoarthritic rat. Neuroscience. 2005;132:905–916. doi: 10.1016/j.neuroscience.2005.01.048. [DOI] [PubMed] [Google Scholar]
- Castro AR, Lima D, Tavares I. Program No. 248.13/R11 2006 Neuroscience Meeting Planner. Atlanta, GA: Society for Neuroscience; 2006. Descending modulation of secondary hyperalgesia in the monoarthritic rat: role of antinociceptive and pronociceptive medullary centres in the differential nociceptive activation of NK1- and GABAB-expressing spinal cord neurons. [Google Scholar]
- Castro AR, Morgado C, Lima D, Tavares I. Differential expression of NK1 and GABAB receptors in spinal neurons projecting to antinociceptive or pronociceptive medullary centers. Brain Res Bull. 2006b;69:266–275. doi: 10.1016/j.brainresbull.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Castro-Lopes JM, Tavares I, Tölle TR, Coito A, Coimbra A. Increase in GABAergic cells and GABA levels in the spinal cord in unilateral inflammation of the hindlimb. Eur J Neurosci. 1992;4:296–301. doi: 10.1111/j.1460-9568.1992.tb00877.x. [DOI] [PubMed] [Google Scholar]
- Castro-Lopes JM, Carvalhosa R, Ferreira-Gomes J, Reguenga C, Neto F. Expression of delta opioid receptor (DOR) mRNA is regulated in thalamic and brainstem nuclei of monoarthritic rats. FENS Abstract. 2006;3:A004.4. [Google Scholar]
- Chuang YC, Chou AK, Wu PC, et al. Gene therapy for bladder pain with gene gun particle encoding pro-opiomelacortin cDNA. J Urol. 2003;170:2044–2048. doi: 10.1097/01.ju.0000092945.76827.47. [DOI] [PubMed] [Google Scholar]
- Cledenin M, Ekerot CF, Oscarsson O, Rosén I. The lateral reticular nucleus in the cat. II. Organization of component activated from bilateral central flexor reflex tract (bVFRT) Exp Brain Res. 1974;21:487–500. doi: 10.1007/BF00237167. [DOI] [PubMed] [Google Scholar]
- Cobos A, Lima D, Almeida A, Tavares I. Brain afferents to the caudal ventrolateral medulla: a retrograde and anterograde tracing study in the rat. Neuroscience. 2003;120:485–498. doi: 10.1016/s0306-4522(03)00209-4. [DOI] [PubMed] [Google Scholar]
- Cope DK, Lariviere WR. Gene therapy and chronic pain. TheScientificWorldJOURNAL. 2006;6:1066–1074. doi: 10.1100/tsw.2006.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cronin J, Zhang XY, Reiser J. Altering the tropism of lentiviral vectors through pseudotyping. Curr Gene Ther. 2005;5:387–398. doi: 10.2174/1566523054546224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danzinger N, Weill-Fugazza J, Le Bars D, Bouhassira D. Stage-dependent changes in the modulation of spinal nociceptive neuronal activity during the course of inflammation. Eur J Neurosci. 2001;13:230–240. doi: 10.1046/j.0953-816x.2000.01375.x. [DOI] [PubMed] [Google Scholar]
- Day TA, Ro A. Depressor area within the caudal ventrolateral medulla of the rat does not correspond to the A1 catecholaminergic cell group. Brain Res. 1983;279:299–302. doi: 10.1016/0006-8993(83)90197-x. [DOI] [PubMed] [Google Scholar]
- Dubner R, Ren K. Brainstem modulation of pain after inflammation. Prog Brain Res Manage. 2004;31:107–120. [Google Scholar]
- Dugast C, Almeida A, Lima D. The medullary dorsal reticular nucleus enhances the responsiveness of spinal nociceptive neurons to peripheral stimulation in the rat. Eur J Neurosci. 2003;18:580–588. doi: 10.1046/j.1460-9568.2003.02782.x. [DOI] [PubMed] [Google Scholar]
- Finegold AA, Mannes AJ, Iadarola MJ. A paracrine paradigm for in vivo gene therapy in the central nervous system: treatment of chronic pain. Hum Gene Ther. 1999;10:1251–1257. doi: 10.1089/10430349950018238. [DOI] [PubMed] [Google Scholar]
- Frampton AR, Jr, Goins WF, Nakano K, Burton EA, Glorioso JC. HSV trafficking and development of gene therapy vectors with applications in the nervous system. Gene Ther. 2005;12:891–901. doi: 10.1038/sj.gt.3302545. [DOI] [PubMed] [Google Scholar]
- Gebhart JF, Ossipov MH. Characterization of inhibition of the spinal nociceptive tail-flick reflex effect in the rat from the medullary lateral reticular nucleus. J Neurosci. 1986;6:701–713. doi: 10.1523/JNEUROSCI.06-03-00701.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glorioso JC, Mata M, Fink DJ. Gene therapy for chronic pain. Curr Gene Ther. 2003;3:223–238. [PubMed] [Google Scholar]
- Gu Y, Xu Y, Li GW, Huang LY. Remote nerve injection of mu opioid receptor adeno-associated viral vector increases antinociception of intrathecal morphine. J Pain. 2005;6:447–454. doi: 10.1016/j.jpain.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Janss AJ, Gebhart JF. Spinal monoaminergic receptors mediate the antinociception produced by glutamate in the medullary lateral reticular nucleus. J Neurosci. 1988;7:2862–2873. doi: 10.1523/JNEUROSCI.07-09-02862.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasmin L, Rabkin SD, Granato A, Boudah A, Ohara PT. Analgesia and hyperalgesia from GABA-mediated modulation of the cerebral cortex. Nature. 2003;424:316–320. doi: 10.1038/nature01808. [DOI] [PubMed] [Google Scholar]
- Jerusalinsky D, Epstein AL. Amplicon vectors as outsnading tools to study and modify cognitive functions. Curr Gene Ther. 2006;6:351–360. doi: 10.2174/156652306777592027. [DOI] [PubMed] [Google Scholar]
- Jones SL. Descending control of nociception. In: Gilderberg P, editor. The Initial Processing of Pain and its Descending Control: Spinal and Trigeminal Systems. Basel, Switzerland: Karger; 1992. pp. 203–295. [Google Scholar]
- Kang W, Wilson MA, Bender MA, Glorioso JC, Wilson SP. Herpes virus-mediated preproenkephalin gene transfer to the amygdale is antinociceptive. Brain Res. 1998;792:133–135. doi: 10.1016/s0006-8993(98)00194-2. [DOI] [PubMed] [Google Scholar]
- Leite-Almeida H, Valle-Fernandes A, Almeida A. Brain projections from the medullary dorsal reticular nucleus: an anterograde and retrograde tracing study in the rat. Neuroscience. 2006;140:577–595. doi: 10.1016/j.neuroscience.2006.02.022. [DOI] [PubMed] [Google Scholar]
- Lieberskind JC, Guilbaud G, Besson JM, Oliveras JL. Analgesia from electrical stimulation of the periaqueductal grey matter in the cat: behavioural observations and inhibitory effects on spinal cord interneurons. Brain Res. 1973;50:441–446. doi: 10.1016/0006-8993(73)90748-8. [DOI] [PubMed] [Google Scholar]
- Lima D, Albino-Teixeira A, Tavares I. The caudal medullary ventrolateral reticular formation in nociceptive-cardiovascular integration. An experimental study in the rat. Exp Physiol. 2002;87:267–274. doi: 10.1113/eph8702354. [DOI] [PubMed] [Google Scholar]
- Lima D, Almeida A. The medullary dorsal reticular nucleus as a pronociceptive centre of the pain control system. Prog Neurobiol. 2002;66:81–108. doi: 10.1016/s0301-0082(01)00025-9. [DOI] [PubMed] [Google Scholar]
- Maixner W. Interactions between cardiovascular and pain modulatory systems: physiological and pathophysiological implications. J Cardiovasc Electrophysiol. 1991;2:S3–S12. [Google Scholar]
- Martins I, Pinto M, Wilson SP, Lima D, Tavares I. Dynamics of a recombinant HSV-1 vector migration from the medulla oblongata: implications for gene therapy of pain. FENS Abstract. 2004;2:A192.20. [Google Scholar]
- Martins I, Pinto M, Pereira S, Wilson SP, Lima D, Tavares I. Gene therapy for chronic pain control: perspectives of HSV-1 mediated gene transfer to supraspinal centres. Mol Ther. 2005;11:S63. [Google Scholar]
- Mason P. Descending pain modulation as a component of homeostasis. In: Cervero F, Jensen TS, editors. Handbook of Clinical Neurology, 81 (3rd series) pain. London: Elsevier; 2006. pp. 211–220. [DOI] [PubMed] [Google Scholar]
- Mayer DJ, Woolfe TL, Akil H, Carder B, Liebeskind JC. Analgesia from electrical stimulation in the brainstem of the rat. Science. 1971;174:1351–1354. doi: 10.1126/science.174.4016.1351. [DOI] [PubMed] [Google Scholar]
- Melzack R, Wall PD. Pain mechanisms: a new theory. Science. 1965;150:971–979. doi: 10.1126/science.150.3699.971. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Morato M, Pinho D, Sousa T, Tavares I, Albino-Teixeira A. The inhibition of nociceptive responses of spinal cord neurones during hypertension involves the spinal GABAergic system and a pain modulatory center located at the caudal ventrolateral medulla. J Neurosci Res. 2006;83:647–655. doi: 10.1002/jnr.20770. [DOI] [PubMed] [Google Scholar]
- Milligan ED, Langer SJ, Sloane EM, et al. Controlling pathological pain by adenovirally driven spinal production of the anti-inflammatory cytokine, interleukin-10. Eur J Neurosci. 2005;21:2136–2148. doi: 10.1111/j.1460-9568.2005.04057.x. [DOI] [PubMed] [Google Scholar]
- Murugaian J, Sundaram K, Krieger A, Sapru H. Electrolytic lesions of the depresor area of the ventrolateral medulla abolish depresor responses to the aortic nerve stimulation. Brain Res. 1989;499:371–377. doi: 10.1016/0006-8993(89)90787-7. [DOI] [PubMed] [Google Scholar]
- Neto FL, Schadrack A, Ableitner A, et al. Supraspinal metabolic activity changes in the rat during adjuvant monoarthritis. Neuroscience. 1999;94:607–621. doi: 10.1016/s0306-4522(99)00185-2. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Porreca F. Descending excitatory systems. In: Cervero F, Jensen TS, editors. Handbook of Clinical Neurology, 81 (3rd series) Pain. pp. 193–210. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 4. San Diego: Academic Press; 1998. [Google Scholar]
- Pezet S, Krzyzanowska A, Wong L-F, et al. Reversal of neuroschemical changes and pain-related behaviour in a model of neuropathic pain using modified lentiviral vectors expressing GDNF. Mol Ther. 2006;13:1101–1109. doi: 10.1016/j.ymthe.2005.11.026. [DOI] [PubMed] [Google Scholar]
- Pinto M, Lima D, Castro-Lopes JM, Tavares I. Noxious-evoked c-fos expression in brainstem neurons immunoreactive for GABAB, m-opioid and NK-1 receptors. Eur J Neurosci. 2003;17:1393–1402. doi: 10.1046/j.1460-9568.2003.02586.x. [DOI] [PubMed] [Google Scholar]
- Pinto M, Lima D, Tavares I. Correlation of noxious evoked c-fos expression in areas of the somatosensory system during chronic pain: involvement of spino-medullary and intra-medullary connections. Neurosci Lett. 2006a;409:100–105. doi: 10.1016/j.neulet.2006.08.031. [DOI] [PubMed] [Google Scholar]
- Pinto M, Tschudy F, Sousa M, Wilson SP, Lima D, Tavares I. Program No. 249.21/S24 2006 Neuroscience Meeting Planner. Atlanta, GA: Society for Neuroscience; 2006b. Opioid-mediated control of pain modulation from the medullary dorsal reticular nucleus: a gene therapy and pharmacological study in the monoarthritic rat. [Google Scholar]
- Pinto-Ribeiro F, Ansah O, Almeida A, Pertovaara A. Descending pain modulatory influence from the hypothalamic paraventricular nucleus through the caudal ventrolateral medulla in arthritic and control rats. FENS Abstract. 2006;3:A004.13. [Google Scholar]
- Pohl M, Braz J. Gene therapy of pain: emergig strategies and future directions. Eur J Pharmacol. 2001;429:39–48. doi: 10.1016/s0014-2999(01)01304-8. [DOI] [PubMed] [Google Scholar]
- Pohl M, Meunier A, Hamon M, Braz J. Gene therapy of chronic pain. Curr Gene Ther. 2003;3:223–238. doi: 10.2174/1566523034578348. [DOI] [PubMed] [Google Scholar]
- Porreca F, Ossipov M, Gebhart GF. Chronic pain and medullary descending facilitation. TINS. 2002;25:319–325. doi: 10.1016/s0166-2236(02)02157-4. [DOI] [PubMed] [Google Scholar]
- Porro CA, Cavazzuti M, Galetti A, Sassatelli L. Functional activity mapping of the rat brainstem during formalin-induced noxious stimulation. Neuroscience. 1991;41:667–680. doi: 10.1016/0306-4522(91)90358-u. [DOI] [PubMed] [Google Scholar]
- Randich A, Robertson JD. Spinal nociceptive transmission in the spontaneously hypertensive and Wistar-Kyoto normotensive rat. Pain. 1994;58:169–183. doi: 10.1016/0304-3959(94)90197-X. [DOI] [PubMed] [Google Scholar]
- Ren K, Dubner R. Descending modulation in persistent pain: an uptake. Pain. 2002;100:1–6. doi: 10.1016/s0304-3959(02)00368-8. [DOI] [PubMed] [Google Scholar]
- Simpson BA. Spinal cord and brain stimulation. In: Wall PD, Melzak R, editors. Textbook of Pain. 4. Edinburgh: Churchill Livingstone; 1999. pp. 1353–1381. [Google Scholar]
- Tavares I, Lima D, Coimbra A. Neurons in the superficial dorsal horn of the rat spinal cord projecting to the medullary reticular formation express c-fos after noxious stimulation of the skin. Brain Res. 1993;623:278–286. doi: 10.1016/0006-8993(93)91438-x. [DOI] [PubMed] [Google Scholar]
- Tavares I, Lima D. Descending projections from the caudal medulla oblongata to the superficial or deep dorsal horn of the rat spinal cord. Exp Brain Res. 1994;99:455–463. doi: 10.1007/BF00228982. [DOI] [PubMed] [Google Scholar]
- Tavares I, Lima D, Coimbra A. The ventrolateral medulla of the rat is connected with the spinal cord dorsal horn by an indirect descending pathway relayed in the A5 noradrenergic cell group. J Comp Neurol. 1996;374:84–95. doi: 10.1002/(SICI)1096-9861(19961007)374:1<84::AID-CNE6>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Tavares I, Lima D, Coimbra A. The pontine A5 noradrenergic cells which project to the spinal cord dorsal horn are reciprocally connected with the caudal ventrolateral medulla in the rat. Eur J Neurosci. 1997a;9:2452–2461. doi: 10.1111/j.1460-9568.1997.tb01662.x. [DOI] [PubMed] [Google Scholar]
- Tavares I, Almeida A, Albino-Teixeira A, Lima D. Lesions of the caudal ventrolateral medulla block the hypertension-induced inhibition of noxious-evoked c-fos expression in the rat spinal cord. Eur J Pain. 1997b;1:149–116. doi: 10.1016/s1090-3801(97)90073-2. [DOI] [PubMed] [Google Scholar]
- Tavares I, Almeida A, Esteves F, Lima D, Coimbra A. The caudal ventrolateral medullary reticular formation is reciprocally connected with the spinal cord. Soc Neurosci Abstract. 1998;24:1132. [Google Scholar]
- Tavares I, Lima D. The caudal ventrolateral medulla as an inhibitory modulator of pain transmission in the spinal cord. J Pain. 2002;3:337–346. doi: 10.1054/jpai.2002.127775. [DOI] [PubMed] [Google Scholar]
- Todd AJ. Anatomy of primary afferents and projection neurones in the rat spinal dorsal horn with particular emphasis on substance P and the neurokinin 1 receptor. Exp Physiol. 2002;87:245–249. doi: 10.1113/eph8702351. [DOI] [PubMed] [Google Scholar]
- Vanegas H, Schaible HG. Descending control of persistent pain: inhibitory or facilitatory. Brain Res Rev. 2004;46:295–309. doi: 10.1016/j.brainresrev.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Villanueva L, Bouhassira D, Bing Z, Le Bars D. Convergence of heterotopic nociceptive information onto subnucleus reticularis dorsalis neurons in the rat medulla. J Neurophysiol. 1988;60:980–1009. doi: 10.1152/jn.1988.60.3.980. [DOI] [PubMed] [Google Scholar]
- Wang X, Kong L, Zhang GR, Sun M, Geller AI. A preproenkephalin-neurofilament chimeric promoter in a helper virus-free herpes simplex virus vector enhances long-term expression in the rat striatum. Neurobiol Dis. 2004;16:596–603. doi: 10.1016/j.nbd.2004.04.016. [DOI] [PubMed] [Google Scholar]
- Xu Y, Gu G-Y, Xu P, Wu G-W, Li L-Y, Huang M. Adeno-associated viral transfer of opioid receptor gene to primary sensory neurons: a strategy to increase opioid antinociception. Proc Natl Acad Sci USA. 2003;100:6204–6209. doi: 10.1073/pnas.0930324100. [DOI] [PMC free article] [PubMed] [Google Scholar]