Abstract
Since the very first detailed description of the different types of cortical interneurons by Cajal, the tremendous variation in the morphology, physiology and neurochemical properties of these cells has become apparent. However, it still remains unclear whether all types of interneurons are present in all cortical areas and species. Here we have focused on tyrosine hydroxylase (TH)-immunoreactive cortical interneurons, which although only present in certain species, are particularly abundant in the human neocortex. We argue that this type of interneuron is more widespread in the human neocortex than in any other species examined so far and that, therefore, it is probably involved in a larger variety of cortical circuits. In addition, notable regional variation can be seen in relation to these interneurons. These differences further emphasize the variability in the design of microcircuits between cortical areas and species, and they probably reflect an evolutionary adaptation of cortical circuits to particular functions.
Keywords: catecholamines, cortical specializations, evolution, interneurons.
Introduction
It is thought that during evolution, the human cerebral cortex has become more differentiated and has increased in size. However, there is still no answer to the fundamental and longstanding question of what is so special about the human brain and how does it differ from that of other species?
During the first two-thirds of the nineteenth century, there was a lack of appropriate techniques to analyse the organization of the brain. Given the apparent similarities in the morphology of neurons throughout the cerebral cortex, it was accepted at that time that the functional differences between species were mainly based on quantitative differences in the number of nerve cells in the brain. In 1873, Golgi introduced the method of the reazione nera (black reaction; Golgi, 1873) and for the first time neurons could be fully visualized in a histological preparation. Furthermore, only a small portion of the neurons were stained, permitting individual neurons to be examined with the utmost morphological detail. Thus, it became possible to characterize and classify neurons, and to study their possible connections (DeFelipe, 2002a).
Golgi was the first to suggest that, in general, there were two morphologically and physiologically different types of neurons: motor (type I) and sensory (type II). Motor neurons had long axons that generated collaterals although the main axon left the grey matter (projection neurons). These cells would have a motor function as their axons were considered to be in contact with the motor roots. Sensory neurons had short axons that branched near the parent cell but that did not leave the grey matter (intrinsic neurons). These neurons were considered to be sensory because their axonal branches were linked with afferent fibres.
Thanks to this new method, Cajal embarked on the detailed study of the cerebral cortex. Indeed, he was the first to achieve a comprehensive analysis of the microanatomy of the cerebral cortex using the Golgi method, giving rise to the first diagrams of cortical circuits (DeFelipe, 2002a,b). According to Cajal (1892), it was not physiologically possible to maintain the distinction between the two cell types proposed by Golgi (for instance, there were a large number of cells with long axons in the retina). Thus, Cajal designated Golgi's two types as cells with a long axon and cells with a short axon, avoiding any consideration of their possible physiological roles (Cajal, 1891, 1892). Since then, the term short-axon cell has commonly been considered to be synonymous with interneuron. This term includes short axon cells that are excitatory (glutamatergic) and are typically located in the middle layers (spiny interneurons) and short-axon cells with smooth dendrites or dendrites that have only sparse spines (smooth interneurons). These latter cells are GABAergic and are found in all layers. They represent the vast majority of short-axon cells and 15–30% of the total population of neurons (DeFelipe, 2002a). For simplicity, here we will refer to smooth interneurons as inteneurons.
Cajal was not persuaded by the common opinion of many researchers that the quantitative differences between different cortical regions and species were solely responsible for the functional characteristics they displayed. He felt that with the new and powerful method of Golgi to visualize neurons, he could try to elucidate what is special about human cortical circuits:
‘… the generally accepted idea that the differences between the brain of [non-human] mammals (cat, dog, monkey, etc.) and that of man are only quantitative, seemed to me unlikely and even a little offensive to human dignity [ … ] language, the capability of abstraction, the ability to create concepts and finally, the art of inventing ingenious instruments [ … ] do [these facets] not seem to indicate (even admitting fundamental structural correspondences with the animals) the existence of original resources, of something qualitatively new which justifies the psychological nobility of Homo sapiens? Microscope at the ready, I then launched with my usual ardor to conquer the supposed anatomical characteristic of the king of Creation, to reveal these enigmatic, strictly human neurons upon which our zoological superiority is founded.’ (Cajal, 1917)
For a number of years Cajal was immersed in the detailed examination of the cerebral cortex of small mammals (rat, rabbit, cat, among others) and that of the human. Having described many morphological types of neurons (DeFelipe & Jones, 1988), he reached the following conclusion:
‘My investigations showed that the functional excellence of the human brain is intimately linked to the prodigious abundance and unwonted wealth of forms of the so-called neurons with short axon.’ (Cajal, 1897)
In spite of the influence of Cajal's studies, most researchers, including his disciple Lorente de Nó, maintained the idea that the same types of neurons could be found in the mouse and humans (Lorente de Nó, 1922) and, therefore, the conclusion of Cajal was not further verified (DeFelipe et al. 2007).
With the introduction and exploitation of immunocytochemical techniques by a number of authors in the 1970s and 1980s, it became clear that most interneurons expressed the inhibitory neurotransmiter GABA or its synthesizing enzyme GAD. Thus, it became possible to obtain an accurate picture of the proportion of these interneurons. Using these approaches, it was found that GABAergic cells represent no more than 15% of the total neuron population in all cortical areas and in layers II–VI of the rat. By contrast, they constitute 20% of the population in the visual cortex of the macaque monkey and up to 25% in other cortical areas. Indeed, they can even reach up to 34–44% of the population in supragranular layers of certain areas of the macaque and human (reviewed in DeFelipe et al. 2007). However, in spite of the many comparative studies performed since the studies of Cajal, it is somewhat surprising that we have not yet obtained a clear answer to the question of whether there is a higher proportion of all types of interneurons in primates or simply an increase in the number of certain neuronal types, and/or the addition of new cell types. At present it is known that interneurons are characterized by their particular synaptic connectivity, physiological properties and the expression of a variety of neurotransmitters, neuroactive peptides and other molecules [reviewed in DeFelipe (1993), Kawaguchi & Kubota (1997), Somogyi et al. (1998), Gupta et al. (2000), Markram et al. (2004) and DeFelipe et al. (2006)]. There are a number of studies that support the theory of morphological or neurochemical interneuron diversity across evolution (e.g. Lewis & Lund, 1990; Glezer et al. 1993; del Río & DeFelipe, 1997; Hof et al. 2000; Preuss & Coleman, 2002; see also Benavides-Piccione & DeFelipe, 2003 Ballesteros-Yáñez et al. 2005). These authors hold that distinct cell types may only be found in particular cortical areas and species. For example, double bouquet cells, which are thought to constitute a key element of the minicolumnar organization of the primate cortex (DeFelipe et al. 1990; del Río & DeFelipe, 1997; Peters & Sethares, 1997; reviewed in DeFelipe, 1997, 2002a; Jones, 2000), are highly modified or less abundant and even absent in the neocortex of other species (e.g. Ballesteros et al. 2005; DeFelipe et al. 2007). Conversely, other studies emphasize the similarities of cortical circuits, supporting the idea of basic uniformity within the cortex. In this case, the differences in function among different cortical areas and/or species are believed to be due to differences in the inputs they receive rather than to intrinsic differences in cortical processing. Accordingly, the same basic organization of neuronal cell types would be valid throughout the neocortex (reviewed in Douglas & Martin, 2004).
Therefore, it is necessary to investigate further the different types of interneurons present in different species and cortical areas in order to determine which are the common types and which are specific to a given cortical area or species. In the present study, we have focused on a type of cortical neuron that is immunoreactive (-ir) for tyrosine hydroxylase (TH). While these interneurons are only present in certain species they are particularly widespread in the human neocortex. Additionally, we have examined the distribution and the density of TH-ir neurons in various cytoarchitectonic areas of the human cerebral cortex that are involved in different functions.
General aspects of the catecholaminergic system
Catecholamines are a group of molecules that appear to act as neurotransmitters or neuromodulators in a variety of processes, including higher cognitive functions and pathologies such as Alzheimer's disease and schizophrenia (Palmer, 1996; Goldman-Rakic, 1998; Lidow et al. 1998; Benes et al. 2000; Lewis & Lieberman, 2000; Akil et al. 2003). These molecules are synthesized from l-tyrosine using the rate-limiting catecholamine-synthesizing enzyme TH. The catecholaminergic system arises mainly from the major catecholaminergic cell groups in the upper brainstem (sustancia nigra, ventral tegmental area and locus coeruleus). Moreover, it constitutes one of the main subcortical non-thalamic afferent systems to the neocortex, whose fibres are widely distributed throughout the nervous system (Hökfelt et al. 1976, 1977; Lindvall et al. 1983; reviewed in Smeets & Gonzalez, 2000). In the neocortex, the axons from these neurons give rise to a plexus that displays different laminar and regional distributions depending on the species and the cortical area. In general, cortical layers I–II of primates are the most densely innervated, followed by layer V–VI (Fig. 1), whereas in rodents the densest innervation is found in layers V–VI (reviewed in Berger et al. 1991; Berger & Gaspar, 1994). Catecholaminergic axons are particularly prominent in the motor and anterior cingulate areas of humans, in the motor cortex of the macaque with a relatively high density in the cingulate cortex, and in the cingulate, frontal and entorhinal cortex of the rat, in which the motor cortex is poorly innervated (Berger et al. 1985, 1988, 1991; Lewis et al. 1987; Gaspar et al. 1989). Furthermore, certain species also present an intrinsic cortical catecholaminergic system constituted by the presence of TH-ir cortical neurons. This system results in an additional source of TH-ir fibres in the cerebral cortex of these species.
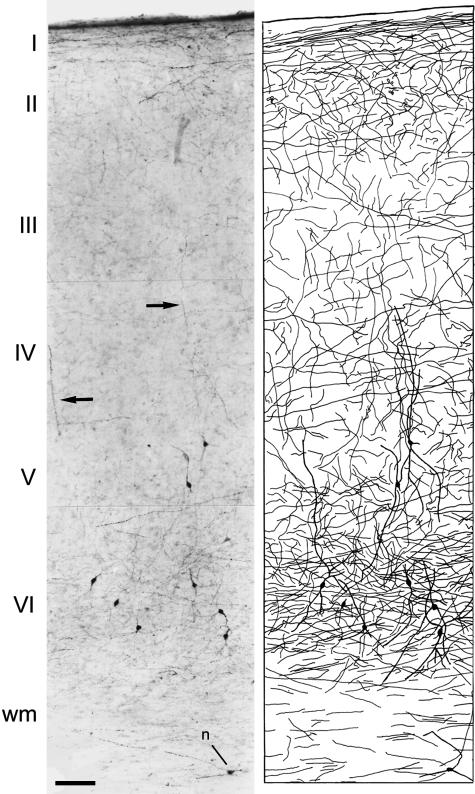
Fig. 1.
Left: low-power photomicrograph from a biopsy of the human temporal cortex (Brodmann's area 20) showing TH immunostaining throughout layer I to the white matter. Note the labelling of neurons in layers V–VI. Arrows indicate vertically orientated fibres. n, a TH-ir neuron in the white matter. Right: Camera lucida drawing of the section on the left showing the laminar distribution of TH-ir neurons and fibres. Scale bar = 120 µm. Taken from Benavides-Piccione & DeFelipe (2003).
Laminar distribution and morphology of TH-ir neurons
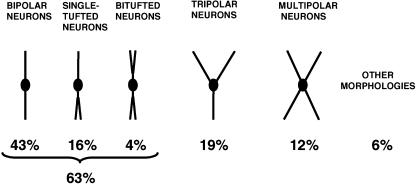
Cortical TH-ir neurons have been described in a number of species and they have consistently been described as smooth, mainly bipolar interneurons. For example, in the human cortex, 63% of the TH-ir population are bipolar and bitufted, with vertically orientated dendrites. The remaining TH-ir neurons are either tripolar (19%) or multipolar (12%: Benavides-Piccione & DeFelipe, 2003; Fig. 2). In addition, some TH-ir neurons have been identified as neurons with ascending axons (Martinotti cells). Conversely, the typical bundles of long, tight, vertically orientated axonal collaterals of double bouquet cells are not stained for TH, nor are the short, vertically orientated rows of boutons in chandelier cell axon terminals and the perisomatic terminals originating from basket cells (DeFelipe, 2002a; Benavides-Piccione & DeFelipe, 2003). Thus, the populations of TH-ir neurons may include Martinotti cells but they do not contain double bouquet cells, chandelier cells or basket cells.
Fig. 2.
Schematic drawing showing the main morphological types of TH-ir neurons in layers V–VI of the human temporal cortex. Taken from Benavides-Piccione & DeFelipe (2003).
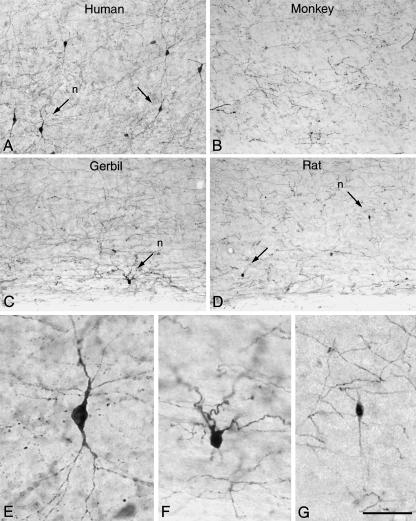
The laminar distribution of TH-ir neurons is markedly different across species (Fig. 3). For example, in the neocortex of certain cetaceans TH-ir neurons are mainly confined to layer I (Hof et al. 1995), whereas in the rat they are found in all cortical layers, even though they are relatively more abundant in layers II–III (Kosaka et al. 1987a). TH-positive cells are found scattered in the deeper cortical layers of the hamster (Vincent, 1988) and they are rarely observed in the gerbil and the rabbit (DeFelipe et al. 2007; our unpublished observations). In the macaque monkey, TH-ir neurons are only occasionally found. Indeed, while they exist in deeper layers of the prefrontal or entorhinal cortex and in the superficial layers of the piriform cortex, they have not been observed in other cortical regions such as the temporal cortex (Köhler et al. 1983; Lewis et al. 1988; Dubach, 1994; DeFelipe et al. 2007, unpublished results). In the human neocortex, TH-ir neurons are present in all cortical regions analysed to date, including primary and secondary sensory and motor areas, temporal and frontal association areas, and paralimbic regions. Moreover, they have predominantly been found in layers V–VI of the cortex and in the underlying white matter (Gaspar et al. 1987; Hornung et al. 1989; Kuljis et al. 1989; Lewis, 1992; Benavides-Piccione & DeFelipe, 2003). The lack of expression of TH-ir neurons in certain cortical regions/species may reflect the absence of a particular type of interneuron. Alternatively, it may indicate that although the same cell type exists in these regions it does not express TH.
Fig. 3.
Low-power photomicrographs of TH immunostaining in the human temporal cortex (Brodmann's area 20) (A), macaque temporal cortex (area TE) (B), gerbil somatosensory cortex (C), and rat somatosensory cortex (D), extending from the lower part of layer V to the white matter. Some TH-ir neurons are indicated by arrows. E–G, high-power magnification of TH-ir neurons indicated (n) in A, C, and D, respectively. Scale bar = 160 µm for A–D and 40 µm for E–G. Taken from DeFelipe et al. (2006).
Neurochemical profiles
There have been relatively few neurochemical studies of TH-ir neurons. However, it has been shown that the majority of TH-ir neurons in the rat cortex co-express GABA or GAD, whereas in the human neocortex only 50% contain GABA (Kosaka et al. 1987b; Trottier et al. 1989). This observation is striking because it is thought that most interneurons are GABAergic and thus inhibitory (Houser et al. 1984). Furthermore, it has been shown that 26% of TH neurons in the human temporal cortex co-express the nitric oxide synthase (nNOS; Benavides-Piccione & DeFelipe, 2003). Nitrergic neurons have been chemically defined as a subpopulation of GABAergic cells that contain the peptides somatostatin and neuropeptide Y. Furthermore, these cells frequently contain the calcium binding protein calbindin, but not parvalbumin or calretinin. Given that they do not appear to co-express other peptides (Gonzalez-Albo et al. 2001), it seems likely that the population of TH-nNOS-ir neurons also express GABA, and they possibly contain calbindin, somatostatin and neuropeptide Y, but not other calcium-binding proteins or peptides. In addition, the population of TH-ir neurons that do not contain nNOS probably also includes those TH-ir neurons that lack GABA. In conclusion, chemical subtypes of TH-ir neurons may be recognized based on their GABA and nNOS content: TH-nNOS-GABA-positive, TH-GABA-positive/nNOS-negative and TH-positive/GABA-nNOS-negative.
The catecholaminergic end-product of human cortical TH-ir neurons is still unknown. Previous immunocytochemical studies have shown that these neurons appear to synthesize only DOPA. Indeed, they do not express the aromatic amino acid decarboxylase and dopamine-β-hydroxylase enzymes necessary for the synthesis of dopamine and noradrenaline, respectively (Gaspar et al. 1987; Lewis, 1992; Ikemoto et al. 1999). However, it cannot be excluded that the levels of one or both of these enzymes in these cells may be below the sensitivity of the currently available immunocytochemical techniques. Alternatively, although TH is present it might be inactive in cortical neurons. Hence, further studies will be necessary to elucidate the neurochemical characteristics of intrinsic TH-ir neurons.
Heterogeneity of TH-ir human cortical neurons
Inter-areal variations
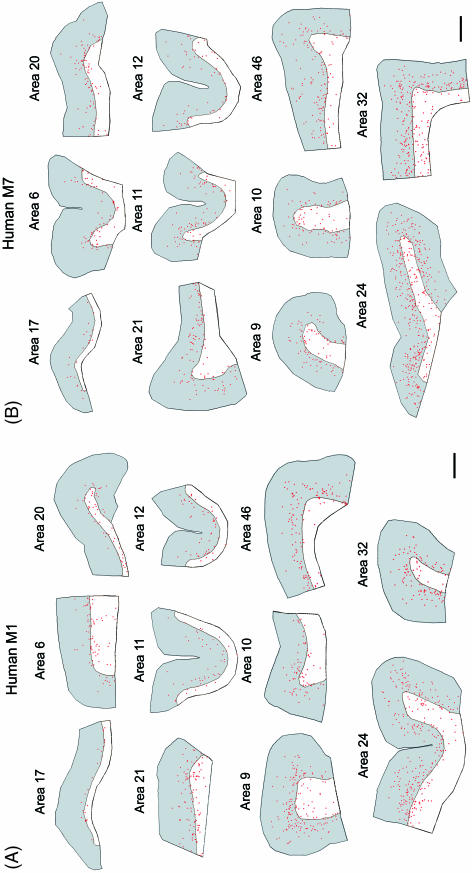
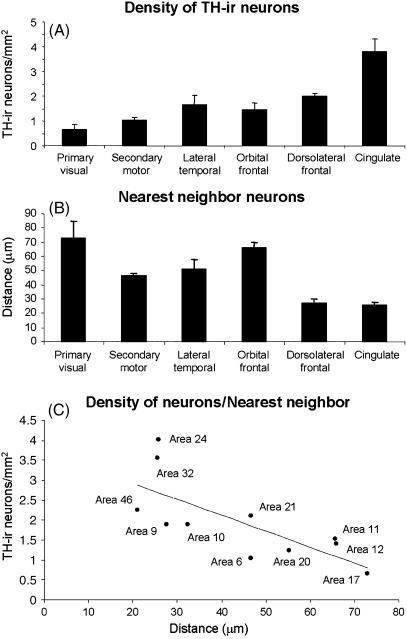
We present here the estimated density and distribution of TH-ir cortical neurons in different areas of the neocortex (Table 1, Figs 4 and 5) from two normal human males (see Materials and Methods for details). According to Brodman's cytoarchitectonic subdivisions (Garey, 1994), we have analysed the primary visual area 17, secondary motor area 6, associative lateral temporal areas 20 and 21, orbital frontal areas 11 and 12, dorsolateral frontal areas 9, 10 and 46, and anterior limbic cingulate areas 24 and 32. As shown in Fig. 4, TH-ir neurons presented similar laminar distribution in the different cortical areas of the human neocortex, concentrating mainly in the deep layers of the cortex and the underlying white matter. However, in some areas (9, 46, 24 and 32) TH-ir neurons were found more frequently in the superficial layers than in the rest of the cortical areas examined. The density of TH-ir neurons in the primary sensory area was the lowest, followed by the secondary motor area and then the temporal, orbital and dorsolateral frontal areas. The highest density of TH-ir neurons was observed in the limbic cingulate cortex (Table 1 and Fig. 5A). These results confirm and extend previous findings on the regional distribution of TH-ir neurons in the human neocortex (Gaspar et al. 1987; Hornung et al. 1989; Lewis et al. 1992). Furthermore, the lowest and highest density of TH-ir neurons found in area 17 and 24, respectively, coincides with the relatively low and high density of TH-ir fibres found in these regions (Fig. 6; see also Gaspar et al. 1987, 1989; Berger & Gaspar, 1994). Nevertheless, TH-ir fibres and neurons do not necessarily correlate given that in other regions such as the motor cortex, a very rich plexus of TH-ir fibres is associated with a relatively low number of TH-ir neurons (Fig. 6; see also Gaspar et al. 1987, 1989; Berger & Gaspar, 1994). Hence, the regional distributions in the cortical and subcortical catecholaminergic system are not necessarily correlated in the human neocortex. The high density of TH-ir neurons and fibres in the anterior cingulate cortex suggests that this limbic region is a primary target for both cortical and subcortical catecholaminergic systems.
Table 1.
Density of TH-ir neurons in different areas of the human neocortex. TH-ir cell density (no. of cells mm−2) was estimated in layers I–VI in different cortical areas of primary visual, secondary motor, associative lateral temporal, associative orbital frontal, associative dorsolateral frontal and limbic cingulate cortex of two individuals (M1 and M7)
| Primary visual | Secondary motor | Associative temporal | Associative orbital frontal | Associative dorsolateral frontal | Limbic cingulate | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Case | area 17 | area 6 | area 20 | area 21 | area 11 | area 12 | area 9 | area 10 | area 46 | area 24 |
| M1 | 0.437 | 1.131 | 0.592 | 2.145 | 0.937 | 1.669 | 1.957 | 1.898 | 2.052 | 3.045 |
| M7 | 0.862 | 0.945 | 1.859 | 2.046 | 2.100 | 1.117 | 1.793 | 1.920 | 2.436 | 5.034 |
| Mean ± SEM | 0.650 ± 0.212 | 1.038 ± 0.093 | 1.225 ± 0.633 | 2.096 ± 0.05 | 1.519 ± 0.587 | 1.393 ± 0.276 | 1.875 ± 0.082 | 1.909 ± 0.011 | 2.24 ± 0.192 | 4.0397 ±0.995 |
Fig. 4.
Camera lucida drawings showing the laminar distribution along layers I–VI (in grey) and underlying white matter (in white) of TH-ir neurons (red dots) in different cortical regions of the human M1 (A) and M7 (B). These regions correspond to the following Brodman's cytoarchitectonic subdivisions: primary visual area 17, secondary motor area 6, associative lateral temporal areas 20 and 21, associative orbital frontal areas 11 and 12, associative dorsolateral frontal areas 9, 10 and 46, and anterior limbic cingulate areas 24 and 32. Note the relative scarcity of TH-ir neurons in layers V–VI of area 17 and the high cell density in cingulate areas compared with other areas. Scale bar = 2 mm.
Fig. 5.
Plots showing the density of TH-ir neurons (A), nearest TH-ir cell neighbour analysis (B) and correlation analysis of these variables (C) from the primary visual cortex area 17, secondary motor area 6, associative lateral temporal cortex (areas 20 and 21), associative orbital prefrontal cortex (area 11 and 12), dorsolateral frontal cortex (area 9, 10 and 46) and anterior limbic cingulate cortex (areas 24 and 32). Data are expressed as the mean ± SEM. Statistical analysis showed that the differences in the TH-ir neuron density were significant (Kruskal–Wallis test H = 14.63, d.f. = 5, P < 0.05) between primary visual and limbic cingulate areas (post-hoc analyses, Dunn's multiple comparisons test, P < 0.05). Regarding TH-ir cell neighbour analysis, the distance between the closest pair of neurons in both dorsolateral frontal and cingulate cortex was significantly smaller than that in the orbital cortex (Kruskal–Wallis test H = 17.30, d.f. = 5, P < 0.005; post-hoc analyses, Dunn's multiple comparisons test, P < 0.05).
Fig. 6.
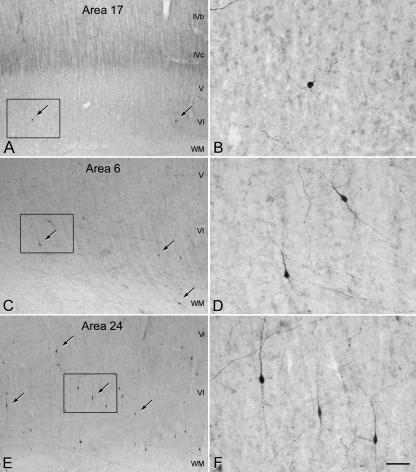
Photomicrographs showing TH-ir neurons (some indicated by arrows) and fibres in the deeper layers of areas 17 (A), 6 (C) and 24 (E) of the human neocortex. B, D and F are higher magnifications of the boxed areas in A, C and E, respectively. Note the differences in the number of TH-ir neurons between these three cortical areas. Scale bar = 190 µm in A, C and E, and 50 µm in B, D and F.
We also studied the distribution of the TH-ir neuron population by analysing the closest neighbour distance (defined as the distance between the closest pair of neurons). We found that the cells in dorsolateral frontal and cingulate cortex are closer to each other than in any other cortical area analysed (Fig. 5B). Furthermore, these neurons seem to occupy the space within a given region of the cortex uniformly, as there was a negative correlation (correlation coefficient = –0.73) between the minimum distance among TH-ir neurons and the estimated cell density in each cortical region (Fig. 5C). Hence, the less dense the cell packing, the further away the cells are from one another.
Functional implications
Catecholaminergic transmission in the cortex has been shown mainly to modulate excitatory inputs to pyramidal neurons, although GABAergic neurons are also innervated by these terminals (Cepeda et al. 1992; Sesack et al. 1995a; Williams & Goldman-Rakic, 1995; Jedema & Moghddam, 1996; Gao et al. 2001; Tseng & O'Donnell, 2004). In fact, it has been shown that the dendrites of pyramidal cells are the main targets for catecholaminergic axon terminals (Goldman-Rakic et al. 1989; Smiley et al. 1992; Smiley & Goldman-Rakic, 1993; Cowan et al. 1994; Sesack et al. 1995b, 2003; Carr & Sesack, 1996; Krimer et al. 1997; Carr et al. 1999; Erickson et al. 2000; Benavides-Piccione & DeFelipe, 2003). In particular, it has been estimated in the human temporal cortex that only 6% and 5% of the TH-ir axonal boutons are in contact with the soma of NeuN-ir neurons in layers II and VI, respectively (Benavides-Piccione & DeFelipe, 2003). Through intracellular injections of Lucifer Yellow combined with immunostaining for TH, the distribution of TH-ir axons on pyramidal cells has been demonstrated in different layers of the macaque (Krimer et al. 1997) and human cortex (Benavides-Piccione et al. 2005; Fig. 7). These studies showed that the density of catecholaminergic inputs to the dendrites of pyramidal cells varies from layer to layer. Nevertheless, in both species the highest numbers of appositions corresponded to the layers with the highest density of TH-ir fibres. This is important when considering that pyramidal cells represent the most common type of cortical neuron, and that the different circuits in which they participate depend on the layer in which they are located (Jones, 1984; White, 1989; Felleman & Van Essen, 1991; Morrison et al. 1998). Furthermore, the distribution of these inputs was structured in a rather regular pattern throughout the apical and basal dendritic arbors of pyramidal neurons in all cortical layers. This would suggest that, in principle, catecholaminergic inputs may influence many afferent systems involving pyramidal neurons, thereby modifying the activity of a large variety of circuits.
Fig. 7.
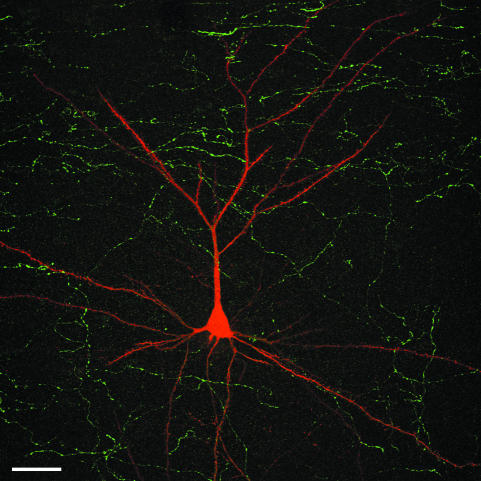
Confocal microscopy stack of 64 serial images showing an intracellularly injected pyramidal neuron (red) with Lucifer Yellow (LY) in the human temporal cortex, as well as the TH-ir fibres (green) in order to illustrate the relative location of TH-ir axons in the neuropil with respect to the pyramidal cell. The section was double-stained using a rabbit and mouse antiserum against LY and TH, respectively. The antibodies were visualized with biotinylated horse anti-mouse IgG and a mixture of Alexa fluor 594-conjugated goat anti-rabbit and streptavidin-conjugated Alexa fluor 488. Unpublished material from Benavides-Piccione et al. (2005). Scale bar = 40 µm.
As a result, it is hard to believe that there is any specificity among the inputs other than at the laminar level. Nevertheless, variability has been observed in the innervation of individual pyramidal neurons in the human temporal cortex, such that some pyramidal neurons are preferentially innervated (Benavides-Piccione et al. 2005). This feature appears not to occur in the monkey as a relatively low variance in the innervation of individual pyramidal neurons in the prefrontal cortex has been reported (Krimer et al. 1997). One possible source of the differential targeting in the human neocortex could involve the axons of TH-ir intrinsic neurons throughout the cortex. Although these neurons are preferentially found in deep layers of the cortex, superficial layers of the cortex could also be the target of these cortical neurons, as some of them have been identified as Martinotti cells or neurons that have an ascending axon in addition to local axonal plexus (Benavides-Piccione & DeFelipe, 2003).
Furthermore, the more widespread distribution and higher density of TH-ir neurons in the human cortex when compared with other species (including monkeys) is clearly indicative of differences in cortical circuits between species. Particularly, the intrinsic catecholaminergic system in the human neocortex is probably involved in a larger variety of cortical circuits than that in any other species examined so far. In addition, TH-ir neurons display a particular distribution within different neocortical regions. As TH-ir neurons are not yet well defined neurochemically it is difficult to ascertain the functional significance of these variations. Nevertheless, the remarkable differences between different areas of the human neocortex further highlight the regional specialization of cortical circuits.
Finally, the variations in the density of TH-ir neurons are in line with other studies performed in our laboratory on the same human cortical tissue from which significant differences in the density of other types of interneurons were identified, including chandelier and double bouquet cells (Ballesteros et al. 2005; Inda et al. 2006; DeFelipe et al. 2007). Interestingly, regional variations in the density of chandelier terminals were similar to those of TH-ir neurons, whereas there was no such correlation for double bouquet cell axons. For example, there is a high density of double bouquet cell axons in the visual cortex, where the density of chandelier terminals is low and there are few TH-ir neurons. Therefore, differences in the density, distribution and connectivity of various types of interneurons in different cortical areas emphasize that cortical regions possess particular neuronal circuits. It seems likely that this would be related to the functional characteristics of each cortical region.
Coming back to Cajal's idea about the structure of the cerebral cortex, it is amazing that 100 years ago he reached a similar conclusion using the rudimentary techniques available at that time:
‘The specific activities of each cortical region depend as much on the quality of the sensory excitation received, as upon the peculiar structure of the gray substance. Such structural particularities probably represent a secondary phenomenon, an adaptation to function, [and this] adaptation would progressively lead to the perfection of the function itself.’ (Cajal, 1904)
Methods
Tissue preparation
Human tissue (kindly supplied by Dr R. Alcaraz, Forensic Pathology Service, Basque Institute of Legal Medicine, Bilbao, Spain) was obtained at autopsy (2–3 h post-mortem) from two normal males, M1 and M7 (aged 23 and 49 years, respectively), who died in traffic accidents. Their brains were immersed in cold 4% paraformaldehyde in 0.1 m phosphate buffer, pH 7.4 (PB) and sectioned into 1.5-cm-thick coronal slices. Small blocks of cortex were then transferred to a second solution of 4% paraformaldehyde in PB for 24 h at 4 °C. The tissue blocks were then cryoprotected in 25% sucrose in PB and stored at –20 °C in a solution of glycerol, ethylene glycol and PB. The neocortex used was from the following Brodmann's cytoarchitectonic subdivitions (Garey, 1994): the primary visual area 17, secondary motor area 6, associative lateral temporal areas 20 and 21, orbital frontal areas 11 and 12, dorsolateral frontal areas 9, 10 and 46, and anterior limbic cingulate areas 24 and 32 of each individual (M1 and M7). Vibratome sections (100 µm) of the tissue from the selected cytoarchitectonic areas were obtained and pretreated with a solution of ethanol and hydrogen peroxide in PB to remove endogenous peroxidase activity.
Immunocytochemistry
Sections were pre-incubated with 3% normal horse serum in PB with Triton X-100 (0.25%) for 2 h at room temperature. The sections were then incubated for 24 h at 4 °C in the same solution containing mouse anti-tyrosine hydroxylase (1 : 1000; Diasorin, Stillwater, MN, USA). The sections were then washed in PB and incubated for 1 h at room temperature in a biotinylated horse anti-mouse antibody diluted 1 : 200 in PB (Vector, Burlingame, CA, USA). Thereafter, the sections were processed using the Vectastain ABC immunoperoxidase kit (Vector) and the antibody distribution was detected histochemically with 0.05% 3,3′-diaminobenzidine tetrahydrochloride (DAB; Sigma) and 0.01% hydrogen peroxide. Finally, the sections were mounted, dehydrated, cleared with xylene and coverslipped.
Quantitative analyses
The Neurolucida package (MicroBrightField Europe, Magdeburg, Germany) was used to trace the cortical surface and white matter contour of each cortical region analysed. Surface areas of the selected cortical regions ranged from 22.034 to 97.075 mm2 (mean area ± SD = 43.728 ± 18.438 mm2). TH-ir neurons observed within these surface areas were marked and the density of these neurons was determined as the number of TH-ir neurons divided by the surface area (including layers I–VI) of each cytoarchitectonic area. Statistical comparisons between cortical regions were carried out with the aid of the GraphPad statistical package (Prism, San Diego, CA, USA).
References
- Akil M, Kolachana BS, Rothmond DA, Hyde TM, Weinberger DR, Kleinman JE. Catechol-O-methyltransferase genotype and dopamine regulation in the human brain. J Neurosci. 2003;23:2008–2013. doi: 10.1523/JNEUROSCI.23-06-02008.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros-Yáñez I, Muñoz A, Contreras J, Gonzalez J, Rodriguez-Veiga E, DeFelipe J. The double bouquet cell in the human cerebral cortex and a comparison with other mammals. J Comp Neurol. 2005;486:344–360. doi: 10.1002/cne.20533. [DOI] [PubMed] [Google Scholar]
- Benavides-Piccione R, DeFelipe J. Different populations of tyrosine-hydroxylase-immunoreactive neurons defined by differential expression of nitric oxide synthase in the human temporal cortex. Cereb Cortex. 2003;13:297–307. doi: 10.1093/cercor/13.3.297. [DOI] [PubMed] [Google Scholar]
- Benavides-Piccione R, Arellano J, DeFelipe J. Catecholamine innervation of pyramidal neurons in the human temporal cortex. Cereb Cortex. 2005;15:1584–1591. doi: 10.1093/cercor/bhi036. [DOI] [PubMed] [Google Scholar]
- Benes FM, Taylor JB, Cunningham MC. Convergence and plasticity of monoaminergic systems in the medial prefrontal cortex during the postnatal period: implications for the development of psychopathology. Cereb Cortex. 2000;10:1014–1027. doi: 10.1093/cercor/10.10.1014. [DOI] [PubMed] [Google Scholar]
- Berger B, Verney C, Alvarez C, Vigny A, Helle KB. New dopaminergic terminal fields in the motor, visual (area 18b) and retrosplenial cortex in the young and adult rat. Immunocytochemical and catecholamine histochemical analices. Neuroscience. 1985;15:983–998. doi: 10.1016/0306-4522(85)90248-9. [DOI] [PubMed] [Google Scholar]
- Berger B, Trottier S, Verney C, Gaspar P, Alvarez C. Regional and laminar distribution of the dopamine and serotonin innervation in macaque cerebral cortex. J Comp Neurol. 1988;273:99–119. doi: 10.1002/cne.902730109. A radioautographic study. [DOI] [PubMed] [Google Scholar]
- Berger B, Gaspar P, Verney C. Dopaminergic innervation of the cerebral cortex: unexpected differences between rodents and primates. Trends Neurosci. 1991;14:21–27. doi: 10.1016/0166-2236(91)90179-x. [DOI] [PubMed] [Google Scholar]
- Berger B, Gaspar P. Comparative anatomy of the catecholaminergic innervation of rat and primate cerebral cortex. In: Smeets WJAJ, Reiner A, editors. Philogeny and Development of Catecholamine System in the CNS of Vertebrates. Cambridge: Cambridge University Press; 1994. pp. 293–324. [Google Scholar]
- Cajal SR. Sur la structure de l’ecorce cerebrale de quelques mammifercs. La Cellule. 1891;7:125–176. [Google Scholar]
- Cajal SR. El nuevo concepto de la histología de los centros nerviosos. Rev Ciencias Méd. 1892;18:457–476. [Google Scholar]
- Cajal SR. Las células de cilindro-eje corto de la capa molecular de1 cerebro. Rev Trim Micrográf. 1897;2:105–127. [Google Scholar]
- Cajal SR. Textura del Sistema Nervioso del Hombre y de Los Vertebrados. Vol. 2. Madrid: Moya; 1904. [Google Scholar]
- Cajal SR. Recuerdos de Mi Vida. Vol. 2. Madrid: Moya; 1917. Historia de Mi Labor Científica. [Google Scholar]
- Carr DB, Sesack SR. Hippocampal afferents to the rat prefrontal cortex: synaptic targets and relation to dopamine terminals. J Comp Neurol. 1996;369:1–15. doi: 10.1002/(SICI)1096-9861(19960520)369:1<1::AID-CNE1>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Carr DB, O'Donnell P, Card JP, Sesack SR. Dopamine terminals in the rat prefrontal cortex synapse on pyramidal cells that project to the nucleus accumbens. J Neurosci. 1999;19:11049–11060. doi: 10.1523/JNEUROSCI.19-24-11049.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cepeda C, Radisavljevic Z, Peacock W, Levine MS, Buchwald NA. Differential modulation by dopamine of responses evoked by excitatory amino acids in human cortex. Synapse. 1992;11:330–341. doi: 10.1002/syn.890110408. [DOI] [PubMed] [Google Scholar]
- Cowan RL, Sesack SR, Van Bockstaele EJ, Branchereau P, Chain J, Pickel VM. Analysis of synaptic inputs and targets of physiologically characterized neurons in rat frontal cortex: combined in vivo intracellular recording and immunolabeling. Synapse. 1994;17:101–114. doi: 10.1002/syn.890170206. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Hendry SH, Hashikawa T, Molinari M, Jones EG. A microcolumnar structure of monkey cerebral cortex revealed by immunocytochemical studies of double bouquet cell axons. Neuroscience. 1990;37:655–673. doi: 10.1016/0306-4522(90)90097-n. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Jones EG. New York: Oxford University Press; 1988. Cajal on the Cerebral Cortex. [Google Scholar]
- DeFelipe J. Neocortical neuronal diversity: Chemical heterogeneity revealed by colocalization studies of classic neurotransmitters, neuropeptides, calcium-binding proteins, and cell surface molecules. Cereb Cortex. 1993;3:273–289. doi: 10.1093/cercor/3.4.273. [DOI] [PubMed] [Google Scholar]
- DeFelipe J. Types of neurons, synaptic connections and chemical characteristics of cells immunoreactive for calbindin-D28K, parvalbumin and calretinin in the neocortex. J Chem Neuroanat. 1997;14:1–19. doi: 10.1016/s0891-0618(97)10013-8. [DOI] [PubMed] [Google Scholar]
- DeFelipe J. Cortical interneurons: from Cajal to 2001. Prog Brain Res. 2002a;136:215–238. doi: 10.1016/s0079-6123(02)36019-9. [DOI] [PubMed] [Google Scholar]
- DeFelipe J. Sesquicentenary of the birthday of Santiago Ramon y Cajal, the father of modern neuroscience. Trends Neurosci. 2002b;25:481–484. doi: 10.1016/s0166-2236(02)02214-2. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Alonso-Nanclares L, Blatow M, Caputi A, Monyer H. Anatomical and molecular heterogeneity of cortical GABAergic interneurons. In: Grillner S, editor. Microcircuits: the Interface Between Neurons and Global Brain Function. Dahlem Workshop Report 93. Cambridge, MA: MIT Press; 2006. pp. 295–325. [Google Scholar]
- DeFelipe J, Alonso-Nanclares L, Arellano J, Ballesteros-Yáñez I, Benavides-Piccione R, Muñoz A. Specializations of cortical microestructure of humans. In: Kaas JH, editor. The Evolution of Primate Nervous Systems. Vol. 5. Oxford: Elsevier; 2007. pp. 167–190. From: Evolution of the Nervous System. [Google Scholar]
- Del Río MJ, DeFelipe J. Colocalization of parvalbumin and calbindin d-28k in neurons including chandelier cells of the huma temporal neocortex. J Chem Neuroanat. 1997;12:165–173. doi: 10.1016/s0891-0618(96)00191-3. [DOI] [PubMed] [Google Scholar]
- Douglas RJ, Martin KA. Neuronal circuits of the neocortex. Annu Rev Neurosci. 2004;27:419–451. doi: 10.1146/annurev.neuro.27.070203.144152. [DOI] [PubMed] [Google Scholar]
- Dubach M. Telencephalic dopamine cells in monkeys, humans and rats. In: Smeets WJAJ, Reiner A, editors. Philogeny and Development of Catecholamine System in the CNS of Vertebrates. Cambridge: Cambridge University Press; 1994. pp. 273–292. [Google Scholar]
- Erickson SL, Sesack SR, Lewis DA. Dopamine innervation of monkey entorhinal cortex: postsynaptic targets of tyrosine hydroxylase-immunoreactive terminals. Synapse. 2000;36:47–56. doi: 10.1002/(SICI)1098-2396(200004)36:1<47::AID-SYN5>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Gao WJ, Krimer LS, Goldman-Rakic PS. Presynaptic regulation of recurrent excitation by D1 receptors in prefrontal circuits. Proc Natl Acad Sci USA. 2001;98:295–300. doi: 10.1073/pnas.011524298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garey LJ. Brodmann's Localisation in the Cerebral Cortex. London: Smith-Gordon; 1994. [Google Scholar]
- Gaspar P, Berger B, Febvret A, Vigny A, Krieger-Poulet M, Borri-Voltattorni C. Tyrosine hydroxylase-immunoreactive neurons in the human cerebral cortex: a novel catecholaminergic group? Neurosci Lett. 1987;80:257–262. doi: 10.1016/0304-3940(87)90464-2. [DOI] [PubMed] [Google Scholar]
- Gaspar P, Berger B, Febvret A, Vigny A, Henry JP. Catecholamine innervation of the human cerebral cortex as revealed by comparative immunohistochemistry of tyrosine hydroxylase and dopamine-beta-hydroxylase. J Comp Neurol. 1989;279:249–271. doi: 10.1002/cne.902790208. [DOI] [PubMed] [Google Scholar]
- Glezer II, Hof PR, Leranth C, Morgane PJ. Calcium-binding protein-containing neuronal populations in mammalian visual cortex: a comparative study in whales, insectivores, bats, rodents, and primates. Cerebral Cortex. 1993;3:249–272. doi: 10.1093/cercor/3.3.249. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. The cortical dopamine system: role in memory and cognition. Adv Pharmacol. 1998;42:707–711. doi: 10.1016/s1054-3589(08)60846-7. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS, Leranth C, Williams SM, Mons N, Geffard M. Dopamine synaptic complex with pyramidal neurons in primate cerebral cortex. Proc Natl Acad Sci USA. 1989;86:9015–9019. doi: 10.1073/pnas.86.22.9015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golgi C. Sulla stmttura della sostanza grigia de1 cervello (Comunicazione preventiva) Gazz Med Ital Lombardia. 1873;33:244–246. Reimpress in: Opera Omnia, Vol. I. Istologia Normale, pp. 91–98. Ulrico Hoepli, Milano. [Google Scholar]
- Gonzalez-Albo MC, Gomez-Utrero E, Sanchez A, Sola RG, DeFelipe J. Changes in the colocalization of glutamate ionotropic receptor subunits in the human epileptic temporal lobe cortex. Exp Brain Res. 2001;138:398–402. doi: 10.1007/s002210100720. [DOI] [PubMed] [Google Scholar]
- Gupta A, Wang Y, Markram H. Organizing principles for a diversity of GABAergic interneurones and synapses in the neocortex. Science. 2000;287:273–278. doi: 10.1126/science.287.5451.273. [DOI] [PubMed] [Google Scholar]
- Hof PR, Glezer II, Revishchin AV, Bouras C, Charnay Y, Morgane PJ. Distribution of dopaminergic fibers and neurons in visual and auditory cortices of the harbor porpoise and pilot whale. Brain Res Bull. 1995;36:275–284. doi: 10.1016/0361-9230(94)00202-c. [DOI] [PubMed] [Google Scholar]
- Hof PR, Glezer II, Nimchinsky EA, Erwin JM. Neurochemical and cellular specializations in the mammalian neocortex reflect phylogenetic relationships: evidence from primates, cetaceans, and artiodactyls. Brain, Behav Evol. 2000;55:300–310. doi: 10.1159/000006665. [DOI] [PubMed] [Google Scholar]
- Hokfelt T, Johansson O, Fuxe K, Goldstein M, Park D. Immunohistochemical studies on the localization and distribution of monoamine neuron systems in the rat brain. I. Tyrosine hydroxylase in the mesand diencephalon. Med Biol. 1976;54:427–453. [PubMed] [Google Scholar]
- Hokfelt T, Johansson O, Fuxe K, Goldstein M, Park D. Immunohistochemical studies on the localization and distribution of monoamine neuron systems in the rat brain II. Tyrosine hydroxylase in the telencephalon. Med Biol. 1977;55:21–40. [PubMed] [Google Scholar]
- Hornung JP, Tork I, De Tribolet N. Morphology of tyrosine hydroxylase-immunoreactive neurons in the human cerebral cortex. Exp Brain Res. 1989;76:12–20. doi: 10.1007/BF00253618. [DOI] [PubMed] [Google Scholar]
- Houser CR, Vaughn JE, Hendry SHC, Jones EG, Peters A. GABA neurons in the cerebral cortex. In: Jones EG, Peters A, editors. Cerebral Cortex, Functional Properties of Cortical Cells. Vol. 2. New York: Plenum Press; 1984. pp. 63–89. [Google Scholar]
- Ikemoto K, Kitahama K, Nishimura A, et al. Tyrosine hydroxylase and aromatic 1-amino acid decarboxylase do not coexist in neurons in the human anterior cingulate cortex. Neurosci Lett. 1999;269:37–40. doi: 10.1016/s0304-3940(99)00409-7. [DOI] [PubMed] [Google Scholar]
- Inda MC, DeFelipe J, Muñoz A. The distribution of chandelier cell axon terminals that express the GABA plasma membrane transporter GAT-1 in the human neocortex. Cereb Cortex. 2006 doi: 10.1093/cercor/bhl114. in press. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Jedema HP, Moghddam B. Characterization of excitatory amino acid modulation of dopamine release in the prefrontal cortex of conscious rats. J Neurochem. 1996;66:1448–1453. doi: 10.1046/j.1471-4159.1996.66041448.x. [DOI] [PubMed] [Google Scholar]
- Jones EG. Laminar distributions of cortical efferent cells. In: Peters A, Jones EG, editors. Cerebral Cortex: Cellular Components of the Cerebral Cortex. Vol. 1. New York: Plenum Press; 1984. pp. 521–552. [Google Scholar]
- Jones EG. Microcolumns in the cerebral cortex. Proc Natl Acad Sci USA. 2000;97:5019–5021. doi: 10.1073/pnas.97.10.5019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Köhler C, Everitt BJ, Pearson J, Goldstein M. Immunohistochemical evidence for a new group of catecholamine-containing neurons in the basal forebrain of the monkey. Neurosci Lett. 1983;37:161–166. doi: 10.1016/0304-3940(83)90147-7. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Hama K, Nagatsu I. Tyrosine hydroxylase-immunoreactive intrinsic neurons in the rat cerebral cortex. Exp Brain Res. 1987a;68:393–405. doi: 10.1007/BF00248804. [DOI] [PubMed] [Google Scholar]
- Kosaka T, Kosaka K, Hataguchi Y, et al. Catecholaminergic neurons containing GABA-like and/or glutamic acid decarboxylase-like immunoreactivities in various brain regions of the rat. Exp Brain Res. 1987b;66:191–210. doi: 10.1007/BF00236215. [DOI] [PubMed] [Google Scholar]
- Krimer LS, Jakab RL, Goldman-Rakic PS. Quantitative three-dimensional analysis of the catecholaminergic innervation of identified neurons in the macaque prefrontal cortex. J Neurosci. 1997;17:7450–7461. doi: 10.1523/JNEUROSCI.17-19-07450.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuljis RO, Martin-Vasallo P, Peress NS. Lewy bodies in tyrosine hydroxylase-synthesizing neurons of the human cerebral cortex. Neurosci Lett. 1989;106:49–54. doi: 10.1016/0304-3940(89)90200-0. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Campbell MJ, Foote SL, Goldstein M, Morrison JH. The distribution of tyrosine hydroxylase immunoreactive fibers in primate neocortex is widespread but regionally specific. J Neurosci. 1987;7:279–290. doi: 10.1523/JNEUROSCI.07-01-00279.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis DA, Foote SL, Goldstein M, Morrison JH. The dopaminergic innervation of monkey prefrontal cortex: a tyrosine hydroxylase immunohistochemical study. Brain Res. 1988;449:225–243. doi: 10.1016/0006-8993(88)91040-2. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Lund JS. Heterogeneity of chandelier neurons in monkey neocortex: corticotropinreleasing factor- and parvalbumin-immunoreactive populations. J Comp Neurol. 1990;293:599–615. doi: 10.1002/cne.902930406. [DOI] [PubMed] [Google Scholar]
- Lewis DA. The catecholaminergic innervation of primate prefrontal cortex. J Neural Transm Suppl. 1992;36:179–200. doi: 10.1007/978-3-7091-9211-5_9. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Lieberman JA. Catching up on schizophrenia: natural history and neurobiology. Neuron. 2000;28:325–334. doi: 10.1016/s0896-6273(00)00111-2. [DOI] [PubMed] [Google Scholar]
- Lidow MS, Williams GV, Goldman-Rakic PS. The cerebral cortex: a case for a common site of action of antipsychotics. Trends Pharmacol Sci. 1998;19:136–140. doi: 10.1016/s0165-6147(98)01186-9. [DOI] [PubMed] [Google Scholar]
- Lindvall O, Bjorklund A, Skagerberg G. Dopamine-containing neurons in the spinal cord: anatomy and some functional aspects. Ann Neurol. 1983;14:255–260. doi: 10.1002/ana.410140302. [DOI] [PubMed] [Google Scholar]
- Lorente de No′ R. La corteza cerebral del rato’n (Primera contribución – La corteza acústica) Somatosens Mot Res. 1922;9:3–36. Trab Labo Invest Biol (University Madrid)20, 41–78. Translated in Fairén A, Regidor J, Kruger L (1992) The cerebral cortex of the mouse (A first contribution – The acoustic cortex) [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Morrison JH, Hof PR, Huntley GW. Neurochemical organization of the primate visual cortex. In: Bloom FE, Björklund A, Hökfelt T, editors. Handbook of Chemical Neuroanatomy, Vol. 14: the Primate Nervous System, Part II. Amsterdam: Elsevier; 1998. pp. 299–433. [Google Scholar]
- Palmer AM. Neurochemical studies of Alzheimer's disease. Neurodegeneration. 1996;5:381–391. doi: 10.1006/neur.1996.0051. [DOI] [PubMed] [Google Scholar]
- Peters A, Sethares C. The organization of double bouquet cells in monkey striate cortex. J Neurocytol. 1997;26:779–797. doi: 10.1023/a:1018518515982. [DOI] [PubMed] [Google Scholar]
- Preuss TM, Coleman GQ. Human-specific organization of primary visual cortex: alternating compartments of dense Cat 301 and calbindin immunoreactivity in layer 4A. Cereb Cortex. 2002;12:671–691. doi: 10.1093/cercor/12.7.671. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Snyder CL, Lewis DA. Axon terminals immunolabeled for dopamine or tyrosine hydroxylase synapse on GABA-immunoreactive dendrites in rat and monkey cortex. J Comp Neurol. 1995a;363:264–280. doi: 10.1002/cne.903630208. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Bressler CN, Lewis DA. Ultrastructural associations between dopamine terminals and local circuit neurons in the monkey prefrontal cortex: a study of calretinin-immunoreactive cells. Neurosci Lett. 1995b;200:9–12. doi: 10.1016/0304-3940(95)12076-g. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Carr DB, Omelchenko N, Pinto A. Anatomical substrates for glutamate–dopamine interactions: evidence for specificity of connections and extrasynaptic actions. Ann NY Acad Sci. 2003;1003:36–52. doi: 10.1196/annals.1300.066. [DOI] [PubMed] [Google Scholar]
- Smeets WJ, Gonzalez A. Catecholamine systems in the brain of vertebrates: new perspectives through a comparative approach. Brain Res Rev. 2000;33:308–379. doi: 10.1016/s0165-0173(00)00034-5. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Williams SM, Szigeti K, Goldman-Rakic PS. Light and electron microscopic characterization of dopamine-immunoreactive axons in human cerebral cortex. J Comp Neurol. 1992;321:325–335. doi: 10.1002/cne.903210302. [DOI] [PubMed] [Google Scholar]
- Smiley JF, Goldman-Rakic PS. Heterogeneous targets of dopamine synapses in monkey prefrontal cortex demonstrated by serial section electron microscopy: a laminar analysis using the silver-enhanced diaminobenzidine sulfide (SEDS) immunolabeling technique. Cereb Cortex. 1993;3:223–238. doi: 10.1093/cercor/3.3.223. [DOI] [PubMed] [Google Scholar]
- Somogyi P, Tamás G, Lujan R, Buhl EH. Salient features of synaptic organisation in the cerebral cortex. Brain Res Rev. 1998;26:113–135. doi: 10.1016/s0165-0173(97)00061-1. [DOI] [PubMed] [Google Scholar]
- Trottier S, Geffard M, Evrard B. Co-localization of tyrosine hydroxylase and GABA immunoreactivities in human cortical neurons. Neurosci Lett. 1989;106:76–82. doi: 10.1016/0304-3940(89)90205-x. [DOI] [PubMed] [Google Scholar]
- Tseng KY, O'Donnell P. Dopamine–glutamate interactions controlling prefrontal cortical pyramidal cell excitability involve multiple signaling mechanisms. J Neurosci. 2004;24:5131–5139. doi: 10.1523/JNEUROSCI.1021-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vincent SR. Distributions of tyrosine hydroxylase-, dopamine-beta-hydroxylase-, and phenylethanolamine-N-methyltransferase-immunoreactive neurons in the brain of the hamster (Mesocricetus auratus) J Comp Neurol. 1988;268:584–599. doi: 10.1002/cne.902680408. [DOI] [PubMed] [Google Scholar]
- White EL. Cortical Circuits. Synaptic Organization of the Cerebral Cortex. Structure, Function and Theory. Boston: Birkhäuser; 1989. [Google Scholar]
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]