Abstract
The mammalian neocortex consists of six layers. By contrast, the reptilian and avian cortices have only three, which are believed to be equivalent to layers I, V and VI of mammals. In mammals, the majority of cortical cell proliferation occurs in the ventricular and subventricular zones, but there are a small number of scattered individual divisions throughout the cortex. Neurogenesis in the cortical subventricular zone is believed to contribute to the supragranular layers. To estimate the proportions of different forms of divisions in reptiles and birds, we examined the site of proliferation in embryonic turtle (stages 18–25) and chick (embryonic days 8–15) brains using phospho-histone H3 (a G2 and M phase marker) immunohistochemistry. In turtle, only few scattered abventricular H3-immunoreactive cells were found outside the ventricular zone; the majority of the H3-immunoreactive cells were located in the ventricular zone throughout the entire turtle brain. Ventricular zone cell proliferation peaks at stages 18 and 20, before an increase of abventricular proliferation at stages 23 and 25. In turtle cortex, however, abventricular proliferation at any given stage never exceeded 17.5 ± 2.47% of the total division and the mitotic profiles did not align parallel to the ventricular zone. Phospho-histone H3 immunoreactivity in embryonic chick brains suggests the lack of subventricular zone in the dorsal cortex, but the presence of subventricular zone in the ventral telencephalon. We were able to demonstrate that the avian subventricular zone is present in both pallial and subpallial regions of the ventral telencephalon during embryonic development, and we characterize the spatial and temporal organization of the subventricular zone. Comparative studies suggest that the subventricular zone was involved in the laminar expansion of the cortex to six layers in mammals from the three-layered cortex found in reptiles and birds. Within mammals, the number of neurons in a cortical column appears to be largely constant; nevertheless, there are considerable differences between the germinal zones in mammalian species. It is yet to be determined whether these elaborations of the subventricular zone may have contributed to cell diversity, tangential expansion or gyrus formation of the neocortex and whether it might have been the major driving force behind the evolution of the six-layered neocortex in mammals.
Keywords: abventricular division, cell cycle, neurogenesis, radial and tangential neuronal migration, subventricular zone, ventricular zone.
Elaboration of the telencephalon in amniote evolution
In the evolution of reptiles, birds and mammals, major brain regions have largely been conserved (Puelles et al. 2000). However, the telencephalon has undergone a great elaboration since the divergence of these lineages (Striedter, 1997, 2005). Particularly noteworthy are the radial expansion of the neocortex to a six-layered structure in mammals, and the tangential expansion of the cortex within several mammalian lineages (Molnár et al. 2006a). In avian evolution, telencephalic structures such as the hyperpallium, mesopallium and nidopallium have also undergone significant elaborations (Medina & Reiner, 2000; Timmermans et al. 2000; Iwaniuk & Hurd, 2005). Evolution must have acted on developmental mechanisms in the telencephalon in order to produce the observed diversity of adult forms.
The mammalian cortex contains drastically more neurons, and a greater diversity of neural subtypes (Ramón y Cajal, 1909). In contrast to the six-layered mammalian cortex, reptiles possess a three-layered cortex with similarity to layers I, V and VI of mammals (Goffinet, 1983; Goffinet et al. 1986; Reiner, 1991). The isocortical homologue of birds has a pseudo-layered structure, which is considerably different to the mammalian organization (Medina & Reiner, 2000). The laminar expansion of the cortex in the common ancestor of all mammals was accompanied by increased neural diversity. Similar to turtles, birds have a pseudo-layered structure, the hyperpallium (wulst) in which apparent layers are actually generated from adjacent portions of the ventricular zone (VZ) (Medina & Reiner, 2000). Ebner (1976) proposed that the reptile dorsal cortex lacked neuronal subtypes found in the upper layers of the mammalian neocortex. Indeed, based on morphology, connectivity and neurotransmitters, neither birds nor reptiles have homologues to the upper layer pyramidal neurons (Reiner, 1991; Butler & Hodos, 1996). Therefore, a question arises: Where did the extra cortical cells come from in the mammalian brain and what are the major changes in cortical neurogenesis in mammals? There are numerous theories on the increase of mammalian cortical neurons (Northcutt & Kaas, 1995; Butler & Hodos, 2005). Most of these theories suggest that there are accessory sites of neurogenesis for the mammalian cortex. In the absence of ancestral fossils, comparative studies can reveal the developmental mechanisms that change most considerably in mammals and other vertebrates.
Karten argued that the mammalian neocortex evolved from a rearrangement of elements already present in other vertebrates. He demonstrated that equivalent circuits are present in the visual and auditory pathways in avian and mammalian telencephali (Karten, 1969, 1997). While these components are arranged into cortical layers in mammals (thalamic recipient layers IV, cortico-cortical projection neurons in II–III and efferent projection neurons in V–VI), they are mostly situated subcortically in birds (Karten, 1991). Karten postulated that a considerable population of mammalian neurons are generated outside the cortex and migrate into the cortex during development (Karten, 1997). This theory predicts relocation of corresponding cell groups in ancestral species at the reptilian–mammalian transformation.
Initial reports that the mammalian subpallium, a region outside the cortical neuroepithelium, contributes tangentially migrating neurons to the mammalian cortex appeared to support Karten's theory (de Carlos et al. 1996; Anderson et al. 1997; Karten, 1997; Tamamaki et al. 1997). However, several expectations from Karten's theory were not fulfilled by the tangentially migrating neurons. It is now well established that the tangentially migrating neurons are purely inhibitory (GABAergic) and do not contain any excitatory pyramidal neurons. Additionally, tangential migration is not unique to mammalian brains. Recent comparative analysis of tangentially migrating neurons in birds and reptiles revealed that, just as in mammals, most GABAergic interneurons originate in the ventral telencephalon (Cobos et al. 2001; Métin et al. 2006). In slice cultures prepared from embryonic chick and turtle brains, GABAergic cells follow similar tangential routes in both subpallium and pallium, and show similar branched leading processes (Tuorto et al. 2003; Métin et al. 2006). These studies also demonstrated that the origin of the migrating cells in mammals does not coincide with the domain that is considered homologous to the dorsal ventricular ridge (DVR). In avian and reptilian brains, GABAergic interneurons also arise from a Dlx domain and migrate tangentially to the dorsal cortex (Cobos et al. 2001; Tuorto et al. 2003; Métin et al. 2006).
An alternative theory suggests that the generation of extra cortical neurons for the expanding sheet of cortical neuroepithelium and elaboration of the granular and supragranular cortical layers in mammals occurred de novo in mammals and required an accessory site of proliferation within the cortical subventricular zone (SVZ) and also the appearance of an intermediate progenitor population (Martínez-Cerdeno et al. 2006; Molnár et al. 2006a). This paper reviews the possible role of the SVZ in the expansion of the neocortex and the generation of neural complexity, using original comparative data from amphibian, turtle, chick, mouse, rat, macaque and human (Striedter & Keefer, 2000; Smart et al. 2002; Carney, 2005; Wullimann et al. 2005; Bystron et al. 2006; Martínez-Cerdeno et al. 2006). This paper also uses our own original data to discuss the absence of SVZ in embryonic turtle cortex and the presence of an SVZ in the chick DVR during embryonic neurogenesis.
Neurogenesis in the SVZ could increase the number of neurons produced during development
The majority of neurons in the adult brain are produced during embryonic development (Au & Fishell, 2006). In the telencephalon of reptiles, birds and mammals, embryonic neurogenesis occurs at several sites. In all three groups, neurogenesis occurs in the VZ which lines the lateral ventricle and in abventricular cells which are located away from the VZ (Haubensak et al. 2004). However, in mammals, but not in other vertebrates, cortical neurons are also generated in the dorsal cortex by a discrete mitotic compartment called the SVZ, which is located radially above the VZ (Privat, 1975; Sturrock & Smart, 1980; Bayer & Altman, 1991).
The progenitor cells of the mammalian SVZ and of abventricular regions differ from progenitors in the VZ. In the VZ, neuroepithelial cells give rise to early born neurons and to radial glia (Götz & Huttner, 2005; see Gal et al. 2006 for evidence of an additional subclass of progenitor in the VZ). The nuclei of radial glia remain in the VZ and radial glia divide asymmetrically to produce another radial glia and either a mature neuron or an intermediate progenitor cell (IPC). IPCs are found in both the SVZ and in abventricular regions (Haubensak et al. 2004). In contrast to radial glia, IPCs divide symmetrically to produce two neurons, or occasionally, two daughter IPCs (Noctor et al. 2004). Thus, the two-step pattern of neurogenesis in which radial glia produce IPCs increases the overall rate of neurogenesis relative to the one-step pattern in which radial glia produce single neurons directly by asymmetric divisions.
The laminar expansion from a three- to a six-layered cortex that occurred in the common ancestor of all mammals, and the tangential expansion of the cortex that has occurred within several mammalian orders, each required increased neural production during embryonic neurogenesis. The two-step pattern of neurogenesis that occurs in the SVZ represents a mechanism that could amplify the number of neurons produced by increasing the rate of neurogenesis and by prolonging the period of neurogenesis (Martínez-Cerdeno et al. 2006). A radial glia that produces an IPC produces at least two neurons, doubling the output of a radial glia that directly produces a single neuron by asymmetric division. Meanwhile, the production of an IPC by asymmetric division still maintains the progenitor pool of radial glia, allowing for an extended period of neurogenesis (Hevner, 2006).
Neurogenesis in the SVZ could increase neural complexity
In addition to increasing the rate of neurogenesis, progenitor cells in the mammalian SVZ also produce neurons with a distinct laminar fate. Originally, the SVZ was thought to produce only glia (Privat, 1975; Sturrock & Smart, 1980), but indirect evidence led Tarabykin et al. (2001) to propose a model in which SVZ progenitors give rise to pyramidal neurons in upper cortical layers (layers II, III and IV) while VZ progenitors give rise to pyramidal neurons in deep cortical layers (layers V and VI). Tarabykin et al. (2001) linked SVZ progenitors to upper layer neurons because the expression of the gene Svet1 was restricted to both sets of cells, and reduced expression of Svet1 in the SVZ corresponded to the absence of upper layer pyramidal neurons in Pax6 mutant mice. Since the proposal of Tarabykin and colleagues, time-lapse photography of GFP-labelled progenitor cells in cultured brain slices (Noctor et al. 2004) and direct in vivo labelling of SVZ cells in a transgenic mouse (Wu et al. 2005) have confirmed the model that SVZ cells generate upper layer pyramidal neurons. Thus, cells of the SVZ produce subtypes of neurons with distinct properties.
The spatial separation of the SVZ from the VZ could provide a mechanism for increasing neural complexity. During embryonic neurogenesis, the SVZ provides an additional compartment for responses to signalling molecules and localized transcription factor expression that could contribute to the generation of diverse neural subtypes (Guillemot et al. 2006; Hevner et al. 2006; Molnár et al. 2006a). Indeed, localized expression of several transcription factors has been observed in the SVZ that could influence the fate of progenitors including Svet1 (Tarabykin et al. 2001), Cux1 and Cux2 (Nieto et al. 2004), Tbr2 and NeuroD (Hevner et al. 2006). Additionally, thalamic afferents have been shown to affect cell cycle duration in progenitor cells (Dehay et al. 2001), and the SVZ could provide an additional compartment by which thalamic inputs influence cell cycle and thereby affect cell fate (Calegari et al. 2005; Lukaszewicz et al. 2005). Thus, the SVZ could contribute to neural complexity by providing an additional compartment for expression of subsets of transcription factors and responses to thalamic inputs and environmental signals.
The SVZ and expansion of the neocortex within mammals
The same functions of the SVZ that supported the laminar expansion of the cortex to six layers in the common ancestor of all mammals could also have supported expansion of the neocortex within mammalian lineages. Specifically, the SVZ may contribute to increased neural production and to increased neural complexity. Increased neural production could potentially be reflected in the adult neocortex in two ways: an increased number of neurons within a single cortical column, or an increased number of neurons along the tangential sheet. Similarly, increased neural complexity could be reflected in the adult neocortex by the presence of unique neural subtypes. Comparative studies of neurogenesis can test the hypothesis of whether elaboration of the SVZ is correlated with changes in neural number and neural complexity within mammalian species.
Comparative studies in mammals demonstrate that the SVZ has been elaborated across mammalian species. For example, in the developing primate cortex, the SVZ further contains an additional compartment, the outer SVZ (OSVZ), which has been shown to produce the majority of supragranular layer neurons (Smart et al. 2002; Lukaszewicz et al. 2005). Could these elaborations of the SVZ correlate with a radial expansion or a tangential expansion of the number of neurons in the cortex?
Conserved neuron number in the cortical column
In mammals, cortical neurons are organized in minicolumns orientated perpendicular to the cortical surface, in cylinders 30–50 µm in diameter (Mountcastle, 1998). The radial unit hypothesis (Rakic, 1988) postulates that the size of cortical areas is determined by the number of ontogenetic units of neuroepithelial founder cells. These radial units sequentially generate neurons for the various cortical layers. Elaboration of the SVZ in mammalian evolution might be expected to have contributed to a radial expansion of cortical columns. The role of the SVZ in the laminar expansion of the cortex indicates that further elaboration of the SVZ would increase the number of supragranular layer neurons produced. Indeed, the additional SVZ compartment in primates, the OSVZ, produces the majority of cells in supragranular layers of macaques (Lukaszewicz et al. 2005), and supragranular layers in macaque are correspondingly much larger than in rodents, which have a smaller, less elaborate SVZ. However, despite such changes in cortical thickness and relative proportion of layers across species, the number of neurons in a cortical column appears to be largely constant in different species (Rockel et al. 1980). This constant number of cells within a cortical column would indicate that further elaboration of the SVZ in mammals has not contributed to a radial expansion of cell number in the neocortex. Instead, the elaboration of the supragranular layers occurred at the expense of the infragranular layers, resulting in a constant cell number.
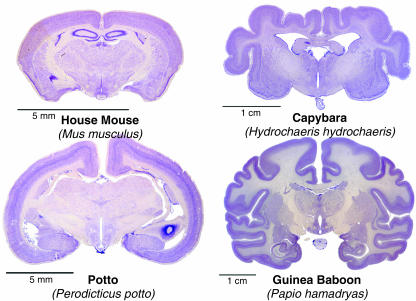
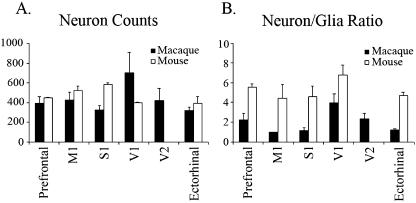
Rockel et al. (1980) counted the number of neuronal cell bodies in a narrow radial strip (30 µm wide) through the depth of the neocortex in different functional areas (motor, somatic sensory, area 17, frontal, parietal and temporal) in mouse, rat, cat, monkey and human. The same absolute number of neurons (110 per radial strip) was found in all areas of all species with only one exception: the binocular part of area 17 of primates, which had approximately 2.5 times more neurons. The numbers and proportions of GABA-immunoreactive neurons in different areas of mammalian cerebral cortices are also relatively constant (Hendry et al. 1987). Mammalian brains exhibit considerable variations in shape, size and weight (Fig. 1). The conserved cortical cell number dogma is so surprising that we decided to re-count the number of neurons. With the discovery of several neuronal-specific markers after Rockel's report, we initially performed NeuN, β-tubulin III ‘Tuj-1’ or MAP-2 immunohistochemistry to distinguish neurons from glia. However, even using a cocktail of all these antibodies, it is not possible to label all neurons. Instead, we counted on Nissl-stained sections of a wider range of mammals. Section samples from monotremes (platypus and echidna), mouse, macaque (Old World monkey), aotus (New World monkey), dog, dolphin and whale are currently being counted and compared in collaboration with Patrick Hof (Mount Sinai Medical School, NY, USA). As some neuronal cell bodies, notably the Betz cells in the primate primary motor cortex, can reach 100 µm in diameter (Nolte, 2002), we increased the width of the radial strips to 100 µm. Our preliminary data on mouse and macaque suggest that the variation of cell count in different functional regions is greater than previously reported, but the cortical cell number dogma appears to be conserved (Fig. 2).
Fig. 1.
The shape and size of mammalian brains are different in spite of the basic uniformity of the six-layered mammalian neocortex. Examples for lissencephalic (left column) and gyrencephalic (right column) brains from rodents (upper row) and from primates (lower row). Images were taken from the University of Wisconsin-Madison Brain Collection (http://www.brainmuseum.org/).
Fig. 2.
Average (± SEM) (A) neuronal cell count, and (B) neuron/glia ratio in mouse and macaque. Data were obtained from three radial strips in each functional region (except M1 of macaque, n = 2). The variation of cell count in different functional regions is greater than previously reported, and one-way anova analysis indicates that there are significant differences between regions in the neuronal cell count in mouse (P < 0.005).
The increased number of neurons in primate area 17 may correlate with the increased OSVZ of that region. As the boundary between areas 17 and 18 is distinct, one would expect a similar transition in the germinal zone. Nevertheless, Smart et al. (2002) describes no such sharp transition and suggests such radial expansion would only account for a fraction of the overall expansion in the primate occipital lobe. How gradients of embryonic gene expression patterns, proliferation and migration produce the sharp 17/18 boundary is a highly exciting area of current research (Lukaszewicz et al. 2006).
Interestingly, while the number of neurons may not change across species, the ratio of neurons to glia does appear to change both across the area 17/18 boundary and across species. Previous reports find a significant decrease in the neuron/glia ratio between area 17 (1.5) and 18 (1.0) in human (Leuba & Garey, 1989), and we observe a change from 4.0 to 2.4 in macaque. The reduction in the neuron/glia ratio may favour the hypothesis that this ratio is linked to function. Comparative studies demonstrate that the neuron/glia ratio decreases along the phylogenetic scale from mouse to primates and humans (Friede, 1954). This increase in relative glial number from lower mammals to primates may reflect an increased neuropil complexity. The same could be true for different cortical regions with different specializations in the same species: secondary visual area 18 is involved in higher order processes of visual analysis than primary visual area 17. It was proposed that increased neuronal activity in an enriched environment was linked, in the rat, with an increase in glia (Szeligo & Leblond, 1977).
The SVZ and the tangential expansion of the neocortex
In contrast to the relatively constant features of mammalian radial columns, a more noticeable change in the evolution of the neocortex is the tangential expansion of the cortical sheet associated with the transformation of lissencephalic cortex typical of rodents, to the gyrencephalic cortex typical of primates. The total surface area of the cerebral cortex has increased exponentially from lesser shrew (0.8 cm2), rat (6 cm2) and cat (83 cm2), to human (2500 cm2), bottlenose dolphin (3745 cm2) and African elephant (6300 cm2) (Peters & Jones, 1984; Ridgway, 1988; Nieuwenhuys et al. 1998). This tangential expansion of the cortex may be linked to elaboration of the SVZ.
Within mammals, the relative size of the SVZ and the duration of SVZ proliferation correlate with the tangential expansion of the cortex. Martínez-Cerdeno et al. (2006) compared progenitor cells in rats which have a smooth lissencephalic cortex and ferrets which have a folded gyrencephalic cortex. In ferrets, the proportion of total cell divisions that occur in the SVZ and in abventricular cells is higher than in rats. The number of cell divisions in the SVZ also remains at peak levels for a longer duration in ferrets than in rats. These data suggest that the increased proportion and duration of SVZ divisions could relate to the tangential expansion of the ferret cortex. Meanwhile, in macaques, the SVZ has expanded to include the OSVZ, which becomes the major proliferative component of the SVZ during development, and the primate cortex is disproportionately expanded compared with in other studied mammals (Smart et al. 2002). Thus, comparative studies support a role for the SVZ in the tangential expansion of the cortex. It is currently not known whether a larger SVZ is related to sulcus and gyrus formation. Whether the large SVZ is correlated with gyrencephalic mammalian brains should be further investigated in rodents with gyrencephalic and in primates with lissencephalic brains (see examples in Fig. 1). The SVZ in human lissencephalies also deserves further study.
The SVZ and neural complexity within the mammal cortex
Tangential expansion of the cortex has also been associated with unique neural subtypes. Thus, elaboration of the SVZ may relate to both tangential expansion and increased neural diversity in mammals. Over a century ago, Ramón y Cajal proposed that unique types of neurons were present in the massively expanded human neocortex (Ramón y Cajal, 1909; DeFelipe & Jones, 1988). Indeed, an interneuron, the double bouquet cell, has been identified that is numerous in primates, sparse in carnivores and absent in rodents (Yañez et al. 2005). Similarly, a projection neuron, the von Economo neuron, has been identified that is unique to great apes, and most numerous in humans (Nimchinsky et al. 1999).
In addition to these examples of unique neurons in the primate neocortex, the pattern of GABAergic neurogenesis has also changed in the primate cortex. Around 70–85% of cortical neurons are excitatory glutamatergic pyramidal neurons, while the rest are inhibitory GABAergic interneurons. These basic proportions also seem to be constant in all mammals (Hendry et al. 1987; DeFelipe, 2002). However, in human, gene expression evidence suggests that a substantial fraction (65%) of cortical interneurons might be generated by the pallium (Letinic & Rakic, 2001) whereas in rodents this estimate is only 5% (Letinic et al. 2002). It is possible that the increased local generation of GABAergic neurons could also be linked to elaboration of the cortical germinal zones in primates.
The SVZ and the appearance of a six-layered cortex in mammals
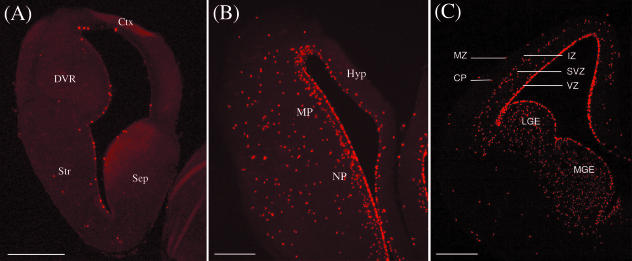
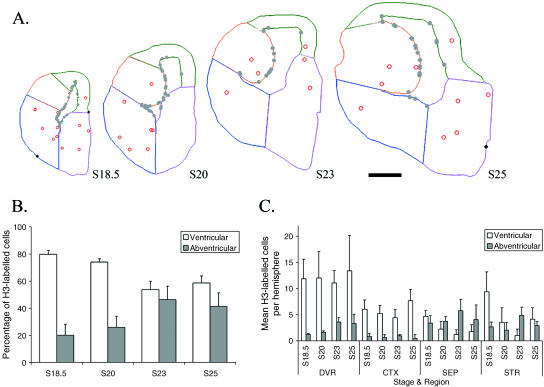
Turtles have a three-layer cortex, with neurons corresponding to layers I, V and VI (Reiner, 1991). To examine whether turtles have SVZ we used an antibody against phospho-histone H3 (H3) to label mitotic cells in G2 and M phase of cell proliferation in turtles staged between 18 and 25. Figures 3(A) and 4(A) show that in both the pallium and the subpallium of turtles, cell divisions occur along the VZ and in abventricular cells, but never in an organized SVZ. Across the entire telencephalon, VZ mitosis peaks in earlier stages (S18 and S20) before shifting to an increasingly abventricular site of proliferation (Fig. 4B) (Molnár et al. 2006b).
Fig. 3.
Coronal sections through the telencephali of (A) stage 25 turtle, (B) E8 chick and (C) E15 rat were immunolabelled for the mitotic marker phospho-histone H3. H3 reveals cells in a specific phase of their cell cycle corresponding to G2 and M. Scale bars = 400 µm in (A,B), 100 µm in (C). Abbreviations: (A) Ctx = cortex; DVR = dorsal ventricular ridge; Str = striatum; Sep = septum. (B) Hyp = hyperpallium; MP = mesopallium; NP = nidopallium. (C) MZ = marginal zone, CP = cortical plate, IZ = intermediate zone, SVZ = subventricular zone, VZ = ventricular zone, LGE = lateral ganglionic eminence, MGE = medial ganglionic eminence.
Fig. 4.
Lack of SVZ is revealed in turtle telencephalon with H3 immunohistochemistry. (A) The distribution of the mitotic figures is plotted on Neurolucida image of a single coronal section of stage 18.5, 20, 23 and 25 turtle brains. Grey dots represent ventricular divisions, open circles represent abventricular divisions and black diamonds represent pial cell division. Different sectors are outlined on the coronal sections: orange = DVR, green = cortex, magenta = septum, blue = striatum. Scale bar = 500 µm. (B,C) Distribution of cell divisions occurring in the VZ or outside the VZ during cortical development. Abventricular division becomes increasingly more prominent in later stages (B). However, the proportion of abventricular division in the dorsal cortex at any given stage remains low (C). Error bars = standard deviation. Abbreviations: DVR = dorsal ventricular ridge, CTX = cerebral cortex, SEP = septum, STR = striatum.
The shift to increased abventricular division occurred in ventral regions, but was not mirrored in the dorsal cortex. Previously, Martínez-Cerdeno et al. (2006) showed that in earlier stages (13–21), only around 15% of total cell divisions in the turtle dorsal cortex come from abventricular sites. Similarly, we found that the proportion of abventricular proliferation in the turtle dorsal cortex at any given stage remains small and constant (never exceeding 17.5 ± 2.47% of the total division, Fig. 4C). Thus, the absence of the SVZ and the low level of abventricular cell divisions in the turtle dorsal cortex are consistent with the hypothesis that IPCs and the SVZ contributed to the radial expansion of the cortex to six layers in mammals. Of note, there may exist a rudimentary SVZ in the lateral part of the dorsal cortex based on Nissl-stained sections (Martínez-Cerdeno et al. 2006). However, our H3 expression study does not support the idea of residual SVZ in turtle, and evidence for expression of SVZ-specific genes is currently lacking.
The subcortical SVZ in mammals and birds
An SVZ has been identified in subcortical regions of the mammalian and avian telencephalon (Bhide, 1996; Striedter & Keefer, 2000). The organization of the avian telencephalon differs from that of mammals. The hyperpallium is believed to be the homologue to the dorsal cortex of reptiles and neocortex of mammals (Medina & Reiner, 2000). The mesopallium and the nidopallium form the DVR. The DVR structures were classically considered to be part of a hypertrophied striatum in birds, but are now understood to be pallial (Puelles, 2001), and may be a site of higher cognitive function in birds (Timmermans et al. 2000; Güntürkün, 2005; Jarvis et al. 2005). The basal ganglia are composed of the striatum and the pallidum and are highly conserved between birds and mammals (Puelles, 2001).
In mammals, an SVZ was identified in the lateral and medial ganglionic eminences (LGE, MGE) of the ventral telencephalon (Bhide, 1996). The subpallial SVZ of mammals differs from the pallial SVZ in several respects. First, the subpallial SVZ appears before the pallial SVZ during development, at embryonic day (E)12 compared with E15 in rodents. Second, the subpallial SVZ appears to produce tangentially migrating interneurons (Anderson et al. 1997, 2001). Additionally, in contrast to the pallial SVZ, the subpallial SVZ of mammals does not form a discrete band running parallel to the VZ (see Fig. 3C). Instead, mitotic cells are abundant at similar levels throughout the bulged ganglionic eminences (Bhide, 1996; Carney, 2005). Thus, in mammals, the organization and fate of mitotic cells in the SVZ differ in the pallial SVZ and the subpallial SVZ.
Although reptiles and birds lack an SVZ in the dorsal cortex and hyperpallium, respectively, an SVZ has been reported in ventral regions of the telencephalon in birds. Striedter & Keefer (2000) describe a 100–200-µm-thick band of labelled cells superficial to the VZ in the ventral telencephalon of E6 chick. The observed SVZ appeared to be prominent in subpallial regions, leading to the suggestion that the SVZ is confined to the chick subpallium, like the subpallial SVZ in mammals (Szele et al. 2002).
However, basic characteristics remain unknown about the avian SVZ. The compartment has only been characterized in chick, and the spatial and temporal distribution of mitotic cells has not been systematically quantified as in the mouse pallial SVZ (Takahashi et al. 1995) and mouse subpallial SVZ (Bhide, 1996). Additionally, the labelling method used in chick (30 min of incubation with bromodeoxyuridine, BrdU) does not reveal the precise location of dividing cells (Striedter & Keefer, 2000). Finally, the fate of avian embryonic SVZ cells remains unknown and gene expression has not been studied in the avian SVZ.
Thus, our laboratory has begun to investigate the avian SVZ in four major regions of the telencephalon: the hyperpallium, the mesopallium and the nidopallium which form the pallium, and the basal ganglia which form the subpallium.
Spatial and temporal organization of the avian SVZ
We used H3 to label mitotic cells during late phases of embryonic neurogenesis and also gliogenesis in chick and for comparison in turtle. Figures 3(A) and 4(A) show that in both the pallium and the subpallium of turtles, cell divisions occur along the VZ and in abventricular cells, but never in an organized SVZ. This is in contrast to chick, where H3 labelling revealed a clear SVZ at E8 and E10 during late phases of neurogenesis. At E8 and E10, the SVZ appeared to be restricted from the hyperpallium, but to span the DVR and basal ganglia. Labelling of a neighbouring section with the pallial/subpallial marker Tbr1 confirmed that the chick SVZ is present in both pallial and subpallial regions at E8 and E10 (data not shown). The SVZ could no longer be observed at E12 and E15, stages that are believed to be predominantly gliogenic in chick (Tsai et al. 1981).
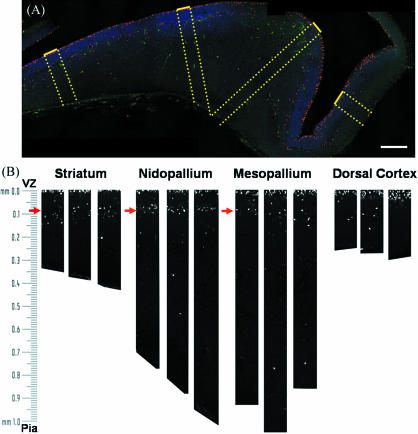
To characterize systematically the size of the SVZ and distance of the SVZ from the ventricle, the frequency of mitotic cells was plotted against distance from the ventricle. Figure 5(A) shows an example of an E8 coronal section reconstructed from four confocal images. For each telencephalon region, three 100-µm-wide bands were selected that extended perpendicular from the VZ to the pia (Fig. 5b). Within the bands, cells were counted in bins with a vertical height of 23 µm, and counts were averaged for each region. This methodology allowed us to characterize the size and organization of the SVZ at each developmental stage.
Fig. 5.
Reconstructions of the H3-immunoreactive nuclei in an E8 chick telencephalon. (A) Confocal microscopic reconstruction of a coronal section of an E8 chick brain. Phospho-histone H3-labelled cells (red) were counted in 100-µm bands running perpendicular from the VZ (dotted yellow lines). Nuclei are labelled with bisbenzimide (blue) and are most dense at the VZ. The outline of the section can be observed in the green channel. Bar, 200 µm. (B) Phospho-histone H3-positive nuclei were counted in three 100-µm bands from each region. Note that H3-labelled cells (white) cluster in the VZ (within 20 µm of the ventricle) and in the SVZ starting around 90 µm from the ventricle (red arrows).
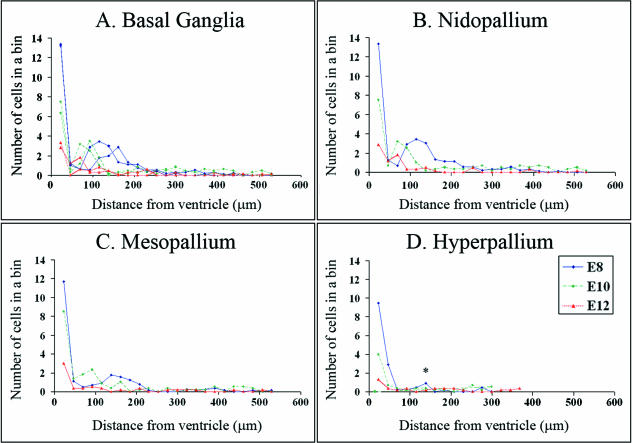
Figure 6 demonstrates that the frequency of H3-labelled cells peaks within 23 µm from the ventricle in all regions of the telencephalon. This peak corresponds to cell division in the VZ. A second peak was clearly observed at E8 and E10 in the basal ganglia, nidopallium and mesopallium. This second peak corresponds to the SVZ and suggests that the SVZ is also present in the DVR regions. At E8 in all three regions, the frequency of mitotic SVZ cells was greatest in the bins that begin at 115 and 138 µm from the ventricle, and the SVZ stretched across 5–6 bins. At E10 in all three regions, the SVZ peaked closer to the ventricle (69–92 µm), and the SVZ appeared narrower, stretching across 3–4 bins. At E8, E10 and E12, the broad region spanned by the SVZ (69–207 µm) accounted for roughly half of the mitotic cells in all three regions.
Fig. 6.
Frequency histograms representing the average number of cells in a region 23 µm by 100 µm in an E8 chick telencephalon (see Fig. 5). A peak corresponding to the SVZ was visible at E8 (blue lines) and E10 (green lines) in the (A) basal ganglia, (B) nidopallium and (C) mesopallium, and the SVZ appeared to become narrower and closer to the ventricle at E10. At E8, a small peak corresponds to the abventricular cells observed in the lateral portion of the hyperpallium (asterisk in D). At E12 (red line), the overall level of cell division was reduced, and peaks corresponding to the SVZ were absent except for a small peak in the nidopallium (B).
While frequency histograms did not identify an SVZ in the chick hyperpallium, a small number of abventricular divisions were observed in the lateral portion at E8 at the same distance from the ventricle as the SVZ in ventral regions. These cells correspond to a small peak in Fig. 6(D) at the same distance from the ventricle as the ventral SVZ. The peak spanned only 1–2 bins and density of cells was significantly lower than in the ventral SVZ of all three regions (anova, P < 0.0001, n = 3; LSD post-hoc test, P < 0.01 for all regions, n = 3). Additional samples and earlier timepoints will be necessary to evaluate whether this thin band of cells corresponds to a rudimentary SVZ in chick. Currently we are characterizing additional E8 samples and samples from earlier phases of neurogenesis to examine this region further.
We have also begun investigating the fate of chick SVZ cells using electroporation of GFP followed by a slice culture preparation. By labelling radial glia with GFP, it is possible to observe patterns of migration, cell division and differentiation. Noctor et al. (2004) used a similar strategy to determine the fate and migration pattern of SVZ cells in mouse.
Transcription factor expression in the pallial and subpallial SVZ
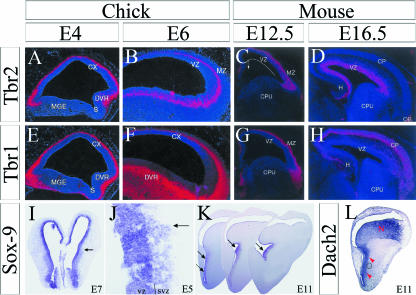
In mammals, several transcription factors uniquely label the pallial SVZ during cortical neurogenesis, including Cux1, Cux2, Svet1, Tbr2 and NeuroD (reviewed by Guillemot et al. 2006; Hevner et al. 2006). The expression of these transcription factors in chick has not yet been studied in relation to the chick SVZ, but previous work intimates that Tbr2 may be expressed in the SVZ of the chick DVR. Prior to the description of the avian SVZ, Bulfone et al. (1999) conducted an in situ hybridization study for Tbr1 and Tbr2 in chick and mouse. They comment that while Tbr1 labelling is restricted to the periphery in chick (Fig. 7E,F), Tbr2 labelling reaches the proliferative zone in the DVR at E4.5 and E6 during neurogenesis (Fig. 7A,B). Similarly, in mouse, they observe that Tbr2 is expressed along and superficial to the pallial VZ at E12.5 and more heavily at E16.5 (Fig. 7C,D) while Tbr1 is again further to the periphery (Fig. 7G,H), and both are excluded from the subpallium. In the context of the present study demonstrating the presence of the SVZ in pallial and subpallial regions, the results of Bulfone et al. suggest that Tbr2 could be expressed in the pallial SVZ of both mammals and birds.
Fig. 7.
Molecular markers may distinguish the pallial and subpallial SVZ in chick. In situ hybridization for Tbr2 in chick demonstrates that Tbr2 expression reaches the proliferative zone of the DVR at E4.5 and E6 (white arrows in C,D), and Tbr2 is excluded from the subpallium (A). In mice, Tbr2 expression reaches the cortical proliferative zone at E12.5 and E16.5 (white arrows in C,D). Tbr1 expression is confined to the periphery and restricted from the subpallium in chick (E,F) and mouse (G,H). Sox-9 is expressed all along the chick ventricular zone, but is only expressed in the subpallial SVZ at E7 (I), E5 (J) and E11 (K). Dach2 is expressed in the subpallial SVZ at E11, but also appears to express along the pallial SVZ described in this paper. Panels A–H from Bulfone et al. (1999); panels I–L from Szele et al. (2002).
While Tbr2 may be expressed in the pallial SVZ, another transcription factor, Sox-9, is expressed in the subpallial, but not the pallial, SVZ. Using in situ hybridization, Szele et al. (2002) show that while Sox-9 is expressed across the entire VZ, Sox-9 is also expressed in the subpallial SVZ from E5 to E11 (Fig. 7I–K). Restriction to subpallial regions was confirmed by staining adjacent sections with the subpallial marker Dlx, and the pallial VZ marker Pax6. The authors also demonstrate that the gene Dach2 is expressed in the chick subpallial SVZ at E11 (Fig. 7L). Based on the previous characterization of the SVZ in ventral regions (Striedter & Keefer, 2000), and on the patterns observed, the authors assume the chick SVZ is restricted to the subpallium. However, Dach2 expression also coincides with the pallial SVZ observed in this study (Fig. 7L).
Thus, in chick, the subpallial and the pallial SVZ may likely be distinguished by the expression of different transcription factors. Tbr2 mRNA appears to be in the DVR, in the region where this study describes a pallial SVZ. Meanwhile, Sox-9 mRNA is confined to the subpallial SVZ (in addition to the entire VZ) from E5 through to E11, and Dach2 mRNA may be found in both the subpallial and the pallial SVZ.
Comparing sites of proliferation in chick with reptiles and mammals
The sites of proliferation in the chick telencephalon can be compared to recent descriptions of sites of proliferation in reptiles and mammals at early and late phases of neurogenesis (Martínez-Cerdeno et al. 2006). Patterns of proliferation in the chick hyperpallium and the turtle dorsal cortex are similar. Like turtles, chicks also appear to lack an SVZ in the dorsal cortex/hyperpallium. Interestingly, in both species, signs of a rudimentary SVZ were observed in the lateral portion of the dorsal cortex/hyperpallium, but these do not extend into the entire structure. At E8 in chick, the contribution of abventricular cells to total proliferation was about 18%, which was similar to the roughly 15% contribution observed in turtle during late stages of neurogenesis.
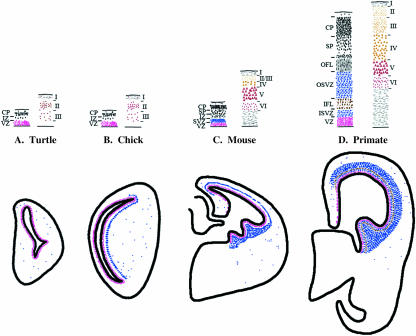
Patterns of proliferation in the chick hyperpallium and mouse neocortex are different (Fig. 8). In mouse, the pallial SVZ is found in the cortex, while in chick, the pallial SVZ is found in the DVR, but not the hyperpallium. The difference in SVZ location may reflect the contribution of abventricular cells to total proliferation. At stages of neurogenesis in rat and ferret comparable with E8 in chick, 20–30% of cortical cell divisions occur away from the VZ during the peak of neurogenesis, compared with a slightly lower percentage (18%) observed in E8 chick (Martínez-Cerdeno et al. 2006; this study). No SVZ was observed in reptile and amphibian telencephalon (Wullimann et al. 2005; Martínez-Cerdeno et al. 2006; Molnár et al. 2006b). Thus, the present data are consistent with the argument that the appearance and elaboration of the SVZ in mammals contributed to the laminar expansion of the neocortex.
Fig. 8.
Comparisons of the sites of cell division during embryonic neurogenesis in turtle, chick, mouse and monkey and overview of the layering patterns in the dorsal cortex. In turtle (A), cell division occurs at the VZ (red) and in abventricular cells (blue), but an organized SVZ is absent. In chick (B), an organized SVZ is absent from the dorsal cortex (hyperpallium), but is present in ventral regions. In mouse (C) and primate (D), an SVZ is present in the dorsal cortex and in the ganglionic eminences. The upper panel demonstrates a correlation between the size of the SVZ and the increase in supragranular layer complexity in the adult cortex. In turtle and chick, the absence of an SVZ (A, B: left column) corresponds to an absence of supragranular layers in the adult (right column). In primate, the SVZ is much larger than in mouse (C, D: left column) and the supragranular layers are larger and more complex (right column). Adapted from Smart et al. (2002), Bhide (1996) and Molnár et al. (2006a).
The pallial SVZ in the chick DVR shares similarities with the pallial SVZ in mouse (Fig. 8). In addition to being present in the pallial field, the pallial SVZ in chick also contributes roughly 50% of the total cell division in the DVR at E8. In addition, the pallial SVZ in chick may express Tbr2 as in mouse. Still, the fate of pallial SVZ cells in chick has not yet been characterized, and the pallial SVZ also shares similarities with the subpallial SVZ. The chick SVZ can be observed continuously across the ventral portion of the telencephalon, and the chick subpallial SVZ also makes a roughly 50% contribution to cell generation. Additionally, the density of the SVZ does not appear to differ across ventral regions. In mouse, the subpallial SVZ appears several days prior to the pallial SVZ, but both can be observed simultaneously at E14 (Carney, 2005). As we process chick samples from earlier timepoints, it will be interesting to see whether the subpallial SVZ also appears prior to the pallial SVZ in chick.
The subpallial SVZ in chick also shares characteristics with the subpallial SVZ in mouse. At E12, the mouse SVZ contributes 30–60% of total cell divisions. Additionally, the subpallial SVZ in mouse produces tangentially migrating GABAergic interneurons, which are also produced in the chick subpallium (Anderson et al. 1997; Cobos et al. 2001). Because of the high level of conservation between the basal ganglia in birds and mammals (Puelles, 2001), one can speculate that the subpallial SVZ may have a common function in birds and mammals.
What molecular factors regulate the formation of the SVZ and are they conserved in birds and mammals?
The restriction of the SVZ from the hyperpallium in chick is intriguing because chicks produce an SVZ along the rest of the ventricle. Future experiments can use electroporation to explore innate and environmental influences on the formation of the SVZ. By electroporating a GFP label into the hyperpallium, and then transplanting these cells to the DVR in slice culture, we can test whether environmental signals in the DVR are sufficient to induce hyperpallium progenitors to arrest for an additional cell division in the SVZ. If progenitors from the hyperpallium do arrest for an extra cell division when transplanted to the DVR, we can test whether common environmental signals are used in mammals by transplanting labelled hyperpallium progenitors to a mammalian cortical slice. Alternatively, the fate of dorsal progenitors may already be restricted, in which case transplanted cells would simply undergo asymmetric divisions producing single neurons.
We can also use the chick to investigate whether transcription factors uniquely expressed in the mammalian SVZ are sufficient to induce the formation of an SVZ in the chick hyperpallium. Candidate transcription factors to test include Tbr2, NeuroD and Svet1 (Tarabykin et al. 2001; Hevner et al. 2006). Tbr2 is particularly attractive because in mammals, Tbr2 is expressed in cells as they leave the VZ and the expression precedes NeuroD expression. Additionally, Tbr2 may be expressed along the chick proliferative zone in the DVR during neurogenesis, but not in the hyperpallium (Bulfone et al. 1999).
Could the pallial SVZ in birds be associated with the evolutionary elaboration of the avian telencephalon?
The presence of a pallial SVZ in the DVR of chick is also intriguing because of the role the pallial SVZ may have played in the evolutionary expansion of the mammalian neocortex. The telencephalon has also been elaborated in avian evolution. Comparative neuroanatomy studies across a range of adult bird species show that, as in mammals, the size of certain telencephalic regions correlates with ecological and cognitive specializations (Iwaniuk & Hurd, 2005). For example, the relative size of a region of the DVR was demonstrated to correlate significantly with feeding innovation rates in birds as categorized by a meta-analysis of reported innovations (Timmermans et al. 2000). If the chick pallial SVZ is neurogenic, then future comparative developmental studies in birds can explore whether elaboration of the avian pallial SVZ corresponds to changes in neural number and neural complexity, as is predicted for the pallial SVZ in mammals.
Conclusion
The mammalian neocortex is the product of evolution of its basic developmental programme. Determining where the extra cortical neurons to the mammalian isocortex came from is a challenge. Comparative embryology allows us to study the alterations from the basic developmental patterns which might have brought about these changes. The SVZ is believed to produce the supragranular neurons, which are absent from the dorsal cortex of sauropsids. Pallial SVZ in the mammalian cortex and a two-step pattern of neurogenesis may function to increase the rate and duration of neurogenesis and the generation of neural complexity. The appearance of the cortical SVZ correlates with expansion of neocortex to six layers and an increasingly complex mammalian cortex. Additional neural subtypes are also observed in the tangentially expanded cortex, and more interneurons are generated locally, intimating a possible role for the SVZ. Within mammals, the tangential expansion of the cortex in several lineages correlates with increased size of the SVZ. However, the numbers of neurons within a unit column of the cortex appear to be surprisingly constant. Thus, elaboration of the SVZ within mammals appears to have primarily contributed to tangential but not radial expansion. Our study suggests that there is no SVZ in the reptilian telencephalon, but there is SVZ in chick in several regions, but not in dorsal cortex. Additional comparative work is needed to relate the chick pallial SVZ and potential similarities with mammalian SVZ. Study of SVZ could be important in the understanding of sulcus and gyrus formation and also in the understanding of human pathologies (lissencephalies). In addition to further comparative work in different species, cell biological studies on transcription factors and cell fate will bring us closer to the evolutionary mechanisms that culminated in the production of the mammalian neocortex.
Acknowledgments
This work was supported by MRC (G300200).
References
- Anderson SA, Qiu M, Bulfone A, et al. Mutations of the homeobox genes Dlx-1 and Dlx-2 disrupt the striatal subventricular zone and differentiation of late born striatal neurons. Neuron. 1997;19:27–37. doi: 10.1016/s0896-6273(00)80345-1. [DOI] [PubMed] [Google Scholar]
- Anderson SA, Marín O, Horn C, Jennings K, Rubenstein JL. Distinct cortical migrations from the medial and lateral ganglionic eminences. Development. 2001;28:353–363. doi: 10.1242/dev.128.3.353. [DOI] [PubMed] [Google Scholar]
- Au E, Fishell G. Adult cortical neurogenesis: nuanced, negligible or nonexistent? Nat Neurosci. 2006;9:1086–1088. doi: 10.1038/nn0906-1086. [DOI] [PubMed] [Google Scholar]
- Bayer SA, Altman J. Development of the endopiriform nucleus and the claustrum in the rat brain. Neuroscience. 1991;45:391–412. doi: 10.1016/0306-4522(91)90236-h. [DOI] [PubMed] [Google Scholar]
- Bhide PG. Cell cycle kinetics in the embryonic mouse corpus striatum. J Comp Neurol. 1996;374:506–522. doi: 10.1002/(SICI)1096-9861(19961028)374:4<506::AID-CNE3>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Bulfone A, Martínez S, Marigo V, et al. Expression pattern of the Tbr2 (Eomesodermin) gene during mouse and chick brain development. Mech Dev. 1999;84:133–138. doi: 10.1016/s0925-4773(99)00053-2. [DOI] [PubMed] [Google Scholar]
- Butler AB, Hodos W. Comparative Vertebrate Neuroanatomy: Evolution and Adaptation. New York: Wiley-Liss; 1996. [Google Scholar]
- Butler AB, Hodos W. Comparative Vertebrate Neuroanatomy. 2. New York: Wiley and Sons; 2005. [Google Scholar]
- Bystron I, Rakic P, Molnár Z, Blakemore C. The first neurons of the human cerebral cortex. Nature Neurosci. 2006;9:880–886. doi: 10.1038/nn1726. [DOI] [PubMed] [Google Scholar]
- Calegari F, Haubensak W, Haffner C, Huttner WB. Selective lengthening of the cell cycle in the neurogenic subpopulation of neural progenitor cells during mouse brain development. J Neurosci. 2005;25:6533–6538. doi: 10.1523/JNEUROSCI.0778-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Carlos JA, Lopez-Mascaraque L, Valverde F. Dynamics of cell migration from the lateral ganglionic eminence in the rat. J Neurosci. 1996;16:6146–6156. doi: 10.1523/JNEUROSCI.16-19-06146.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carney RSE. Thalamocortical development and cell proliferation in fetal primate and rodent cortex. University of Oxford; 2005. D. Phil thesis. [Google Scholar]
- Cobos I, Puelles L, Martínez S. The avian telencephalic subpallium originates inhibitory neurons that invade tangentially the pallium (dorsal ventricular ridge and cortical areas) Dev Biol. 2001;239:30–45. doi: 10.1006/dbio.2001.0422. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Jones EG. Cajal on the Cerebral Cortex. New York: Oxford University Press; 1988. [Google Scholar]
- DeFelipe J. Cortical interneurons: from Cajal to 2001. Prog Brain Res. 2002;136:215–238. doi: 10.1016/s0079-6123(02)36019-9. [DOI] [PubMed] [Google Scholar]
- Dehay C, Savatier P, Cortay V, Kennedy H. Cell-cycle kinetics of neocortical precursors are influenced by embryonic thalamic axons. J Neurosci. 2001;21:201–214. doi: 10.1523/JNEUROSCI.21-01-00201.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebner FF. The forebrain of reptiles and mammals. In: Masterton RB, Bitterman ME, Campbell CBG, Hotton N, editors. Evolution of Brain and Behavior in Vertebrates. New York: John Wiley; 1976. pp. 147–167. [Google Scholar]
- Friede R. Quantitative share of the glia in development of the cortex. Acta Anat (Basel) 1954;20:290–296. [PubMed] [Google Scholar]
- Gal JS, Morozov YM, Ayoub AE, Chatterjee M, Rakic P, Haydar TF. Molecular and morphological heterogeneity of neural precursors in the mouse neocortical proliferative zones. J Neurosci. 2006;26:1045–1056. doi: 10.1523/JNEUROSCI.4499-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goffinet AM. The embryonic development of the cortical plate in reptiles: a comparative study in Emys orbicularis and Lacerta agilis. J Comp Neurol. 1983;215:437–452. doi: 10.1002/cne.902150408. [DOI] [PubMed] [Google Scholar]
- Goffinet AM, Daumerie C, Langerwerf B, Pieau C. Neurogenesis in reptilian cortical structures: 3H-thymidine autoradiographic analysis. J Comp Neurol. 1986;243:106–116. doi: 10.1002/cne.902430109. [DOI] [PubMed] [Google Scholar]
- Götz M, Huttner WB. The cell biology of neurogenesis. Nat Rev Mol Cell Biol. 2005;6:777–788. doi: 10.1038/nrm1739. [DOI] [PubMed] [Google Scholar]
- Guillemot F, Molnár Z, Tarabykin V, Stoykova A. Molecular mechanisms of cortical differentiation. Eur J Neurosci. 2006;23:857–868. doi: 10.1111/j.1460-9568.2006.04626.x. [DOI] [PubMed] [Google Scholar]
- Güntürkün O. The avian ‘prefrontal cortex’ and cognition. Curr Opin Neurobiol. 2005;5:686–693. doi: 10.1016/j.conb.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Haubensak W, Attardo A, Denk W, Huttner WB. Neurons arise in the basal neuroepithelium of the early mammalian telencephalon: a major site of neurogenesis. Proc Natl Acad Sci USA. 2004;101:3196–3201. doi: 10.1073/pnas.0308600100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendry SH, Schwark HD, Jones EG, Yan J. Numbers and proportions of GABA-immunoreactive neurons in different areas of monkey cerebral cortex. J Neurosci. 1987;7:1503–1519. doi: 10.1523/JNEUROSCI.07-05-01503.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hevner RF. From radial glia to pyramidal-projection neuron: transcription factor cascades in cerebral cortex development. Mol Neurobiol. 2006;33:33–50. doi: 10.1385/MN:33:1:033. [DOI] [PubMed] [Google Scholar]
- Hevner RF, Hodge RD, Daza RA, Englund C. Transcription factors in glutamatergic neurogenesis: conserved programs in neocortex, cerebellum, and adult hippocampus. Neurosci Res. 2006;55:223–233. doi: 10.1016/j.neures.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Iwaniuk AN, Hurd PL. The evolution of cerebrotypes in birds. Brain Behav Evol. 2005;65:215–230. doi: 10.1159/000084313. [DOI] [PubMed] [Google Scholar]
- Jarvis ED, Güntürkün O, Bruce L, et al. Avian brains and a new understanding of vertebrate brain evolution. Nat Rev Neurosci. 2005;6:151–159. doi: 10.1038/nrn1606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karten HJ. The organization of the avian telencephalon and some speculations on the phylogeny of the amniote telencephalon. In: Noback CR, Petras JM, editors. Ann NY Acad Sci. Vol. 167. 1969. pp. 146–179. [Google Scholar]
- Karten HJ. Homology and evolutionary origins of the ‘neocortex’. Brain Behav Evol. 1991;38:264–272. doi: 10.1159/000114393. [DOI] [PubMed] [Google Scholar]
- Karten HJ. Evolutionary developmental biology meets the brain: the origins of mammalian cortex. Proc Natl Acad Sci USA. 1997;94:2800–2804. doi: 10.1073/pnas.94.7.2800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letinic K, Rakic P. Telencephalic origin of human thalamic GABAergic neurons. Nat Neurosci. 2001;4:931–936. doi: 10.1038/nn0901-931. [DOI] [PubMed] [Google Scholar]
- Letinic K, Zoncu R, Rakic P. Origin of GABAergic neurons in the human neocortex. Nature. 2002;417:645–649. doi: 10.1038/nature00779. [DOI] [PubMed] [Google Scholar]
- Leuba G, Garey LJ. Comparison of neuronal and glial numerical density in primary and secondary visual cortex of man. Exp Brain Res. 1989;77:31–38. doi: 10.1007/BF00250564. [DOI] [PubMed] [Google Scholar]
- Lukaszewicz A, Savatier P, Cortay V, et al. G1 phase regulation, area-specific cell cycle control, and cytoarchitectonics in the primate cortex. Neuron. 2005;47:353–364. doi: 10.1016/j.neuron.2005.06.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukaszewicz A, Cortay V, Giroud P, et al. The concerted modulation of proliferation and migration contributes to the specification of the cytoarchitecture and dimensions of cortical areas. Cereb Cortex. 2006;16(Suppl. 1):i26–34. doi: 10.1093/cercor/bhk011. [DOI] [PubMed] [Google Scholar]
- Martínez-Cerdeno V, Noctor SC, Kriegstein AR. The role of intermediate progenitor cells in the evolutionary expansion of the cerebral cortex. Cereb Cortex. 2006;16(Suppl. 1):i152–161. doi: 10.1093/cercor/bhk017. [DOI] [PubMed] [Google Scholar]
- Medina L, Reiner A. Do birds possess homologues of mammalian primary visual, somatosensory and motor cortices? Trends Neurosci. 2000;23:1–12. doi: 10.1016/s0166-2236(99)01486-1. [DOI] [PubMed] [Google Scholar]
- Métin C, Alvarez C, Modoux D, Vitalis T, Pieau C, Molnár Z. Conserved pattern of tangential neuronal migration during forebrain development: analysis in reptiles. Development. 2006. in press. [DOI] [PubMed]
- Molnár Z, Metin C, Stoykova A, et al. Comparative aspects of cerebral cortical development. Eur J Neurosci. 2006a;23:921–934. doi: 10.1111/j.1460-9568.2006.04611.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnár Z, Tavare A, Cheung AFP. The origin of neocortex: Lessons from comparative embryology. Evolution of Nervous Systems. In: Kaas J, Kubritzer L, editors. The Evolution of Nervous Systems in Mammals, Volume IV. Vol. 3. London: Elsevier; 2006b. pp. 13–26. [Google Scholar]
- Mountcastle V. Perceptual Neuroscience: the Cerebral Cortex. Cambridge, MA: Harvard University Press; 1998. [Google Scholar]
- Nieto M, Monuki ES, Tang H, et al. Expression of Cux-1 and Cux-2 in the subventricular zone and upper layers II–IV of the cerebral cortex. J Comp Neurol. 2004;479:168–180. doi: 10.1002/cne.20322. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuys R, ten Donkelaar HJ, Nicholson C. The Central Nervous System of Vertebrates. Vol. 3. Berlin: Springer; 1998. [Google Scholar]
- Nimchinsky EA, Gilissen E, Allman JM, Perl DP, Erwin JM, Hof PR. A neuronal morphologic type unique to humans and great apes. Proc Natl Acad Sci USA. 1999;96:5268–5273. doi: 10.1073/pnas.96.9.5268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noctor SC, Martínez-Cerdeno V, Ivic L, Kriegstein AR. Cortical neurons arise in symmetric and asymmetric division zones and migrate through specific phases. Nat Neurosci. 2004;7:136–144. doi: 10.1038/nn1172. [DOI] [PubMed] [Google Scholar]
- Nolte J. The Human Brain. 5. St Louis, MO: Mosby; 2002. [Google Scholar]
- Northcutt RG, Kaas JH. The emergence and evolution of mammalian neocortex. Trends Neurosci. 1995;18:373–379. doi: 10.1016/0166-2236(95)93932-n. [DOI] [PubMed] [Google Scholar]
- Peters A, Jones EG. Classification of cortical neurons. In: Peters A, Jones EG, editors. Cerebral Cortex. Vol. 1. New York: Plenum Press; 1984. pp. 107–121. [Google Scholar]
- Privat A. Postnatal gliogenesis in the mammalian brain. Int Rev Cytol. 1975;40:281–323. doi: 10.1016/s0074-7696(08)60955-9. [DOI] [PubMed] [Google Scholar]
- Puelles L, Kuwana E, Puelles E, et al. Pallial and subpallial derivatives in the embryonic chick and mouse telencephalon, traced by the expression of the genes Dlx-2, Emx-1, Nkx-2.1, Pax-6, and Tbr-1. J Comp Neurol. 2000;424:409–438. doi: 10.1002/1096-9861(20000828)424:3<409::aid-cne3>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Puelles L. Thoughts on the development, structure and evolution of the mammalian and avian telencephalic pallium. Phil Trans R Soc Lond B Biol Sci. 2001;356:1583–1598. doi: 10.1098/rstb.2001.0973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakic P. Specification of cerebral cortical areas. Science. 1988;241:170–176. doi: 10.1126/science.3291116. [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal SR. Histologie du Système Nerveux de l’Homme et des Vertébrés, 2 Volumes. (translated by L Azoulay), repr. Instituto Ramón y Cajal de C.S.I.C., Madrid, 1952–1955.
- Reiner A. A comparison of neurotransmitter-specific and neuropeptide-specific neuronal cell types present in the dorsal cortex in turtles with those present in the isocortex in mammals: implications for the evolution of isocortex. Brain Behav Evol. 1991;38:53–91. doi: 10.1159/000114379. [DOI] [PubMed] [Google Scholar]
- Ridgway SH. The cetacean central nervous system. Compar Neurosci Neurobiol. 1988;1:20–25. [Google Scholar]
- Rockel AJ, Hiorns RW, Powell TP. The basic uniformity in structure of the neocortex. Brain. 1980;103:221–244. doi: 10.1093/brain/103.2.221. [DOI] [PubMed] [Google Scholar]
- Smart IH, Dehay C, Giroud P, Berland M, Kennedy H. Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey. Cereb Cortex. 2002;12:37–53. doi: 10.1093/cercor/12.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striedter GF. The telencephalon of tetrapods in evolution. Brain Behav Evol. 1997;49:179–213. doi: 10.1159/000112991. [DOI] [PubMed] [Google Scholar]
- Striedter GF, Keefer BP. Cell migration and aggregation in the developing telencephalon: pulse-labeling chick embryos with bromodeoxyuridine. J Neurosci. 2000;20:8021–8030. doi: 10.1523/JNEUROSCI.20-21-08021.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striedter GF. Principles of Brain Evolution. Sunderland, MA: Sinauer Associates; 2005. [Google Scholar]
- Sturrock RR, Smart IH. A morphological study of the mouse subependymal layer from embryonic life to old age. J Anat. 1980;130:391–415. [PMC free article] [PubMed] [Google Scholar]
- Szele FG, Chin HK, Rowlson MA, Cepko CL. Sox-9 and cDachsund-2 expression in the developing chick telencephalon. Mech Dev. 2002;112:179–182. doi: 10.1016/s0925-4773(01)00641-4. [DOI] [PubMed] [Google Scholar]
- Szeligo F, Leblond CP. Response of the three main types of glial cells of cortex and corpus callosum in rats handled during suckling or exposed to enriched, control and impoverished environments following weaning. J Comp Neurol. 1977;172:247–263. doi: 10.1002/cne.901720205. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Nowakowski RS, Caviness VS., Jr Early ontogeny of the secondary proliferative population of the embryonic murine cerebral wall. J Neurosci. 1995;15:6058–6068. doi: 10.1523/JNEUROSCI.15-09-06058.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamaki N, Fujimori KE, Takauji R. Origin and route of tangentially migrating neurons in the developing neocortical intermediate zone. J Neurosci. 1997;17:8313–8323. doi: 10.1523/JNEUROSCI.17-21-08313.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarabykin V, Stoykova A, Usman N, Gruss P. Cortical upper layer neurons derive from the subventricular zone as indicated by Svet1 gene expression. Development. 2001;28:1983–1993. doi: 10.1242/dev.128.11.1983. [DOI] [PubMed] [Google Scholar]
- Timmermans S, Lefebvre L, Boire D, Basu P. Relative size of the hyperstriatum ventrale is the best predictor of feeding innovation rate in birds. Brain Behav Evol. 2000;56:196–203. doi: 10.1159/000047204. [DOI] [PubMed] [Google Scholar]
- Tsai HM, Garber BB, Larramendi LM. 3H-thymidine autoradiographic analysis of telencephalic histogenesis in the chick embryo. I. Neuronal birthdates of telencephalic compartments in situ. J Comp Neurol. 1981;198:275–292. doi: 10.1002/cne.901980207. [DOI] [PubMed] [Google Scholar]
- Tuorto F, Alifragis P, Failla V, Parnavelas JG, Gulisano M. Tangential migration of cells from the basal to the dorsal telencephalic regions in the chick. Eur J Neurosci. 2003;18:3388–3393. doi: 10.1111/j.1460-9568.2003.03059.x. [DOI] [PubMed] [Google Scholar]
- Wu SX, Goebbels S, Nakamura K, et al. Pyramidal neurons of upper cortical layers generated by NEX-positive progenitor cells in the subventricular zone. Proc Natl Acad Sci USA. 2005;102:17172–17177. doi: 10.1073/pnas.0508560102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wullimann MF, Rink E, Vernier P, Schlosser G. Secondary neurogenesis in the brain of the African clawed frog, Xenopus laevis, as revealed by PCNA, Delta-1, Neurogenin-related-1, and NeuroD expression. J Comp Neurol. 2005;489:387–402. doi: 10.1002/cne.20634. [DOI] [PubMed] [Google Scholar]
- Yañez IB, Muñoz A, Contreras J, González J, Rodríguez-Veiga E, DeFelipe J. Double bouquet cell in the human cerebral cortex and a comparison with other mammals. J Comp Neurol. 2005;486:344–360. doi: 10.1002/cne.20533. [DOI] [PubMed] [Google Scholar]