Abstract
Interneurons are an integral part of cortical neuronal circuits. During the past decade, numerous studies have shown that these cells, unlike their pyramidal counterparts that are derived from the neuroepithelium along the lumen of the lateral ventricles, are generated in the ganglionic eminences in the subpallium. They use tangential migratory paths to reach the cortex, guided by intrinsic and extrinsic cues. Evidence is now emerging which suggests that the family of Slit proteins, acting through Robo receptors, play a role not only in axon guidance in the developing forebrain, but also as guiding signals in the migration of cortical interneurons. Here we describe the patterns of expression of Slit and Robo at different stages of forebrain development and review the evidence in support of their role in cortical interneuron migration. Slit–Robo signal transduction mechanisms are also important during normal development in a number of systems in the body and in disease states, making them potential therapeutic targets for the treatment of neurological disorders and certain types of cancer.
Keywords: developing cortex, interneurons, neuronal migration, Robo, Slit.
Introduction
Santiago Ramón y Cajal, widely acknowledged as the greatest neurohistologist, investigated brain structures in a wide variety of species (Ramón y Cajal, 1909, 1911). Cajal's first contributions were to the studies of the anatomical organization of the vertebrate retina and cerebellum, and these early observations provided the basis for all his subsequent studies. He discovered and described, always with a penchant for interpreting anatomy in functional terms, an enormous variety of cells in terms of their shapes and sizes, and of the morphology and branching patterns of their processes. In the late 1880s, Cajal launched himself into the study of the cerebral cortex, and devoted much of his time and energy in the subsequent three decades to its structural and functional organization (Ramón y Cajal, 1891, 1894, 1911). This embryonic and neonatal material was crucial in Cajal's success with the Golgi method, in comparison with the inconsistent results of other workers who used adult tissues, including Camillo Golgi himself (see DeFelipe & Jones, 1988). This material was also used by Cajal to make forays into the field of development, allowing him to describe the different histogenetic phases of the cortex of the human fetus and other lower mammals. In these studies, he confirmed the observations of Wilhelm His and other investigators of the late 19th century, and added considerable detail on the morphological and cytological differentiation of the cortical cell types (Ramón y Cajal, 1960; DeFelipe & Jones, 1988). Cajal dealt quite extensively with the differentiation of the neurons with long axons (projection neurons; pyramidal cells), but had less to convey about the cells with short axons (non-pyramidal cells; interneurons) because of the extreme rarity with which these cells were impregnated in his newborn and fetal material (mostly mouse cortex). Based on recent discoveries on the origin and migration of interneurons (see below), it seems likely that many of these cells either had not yet appeared in the cortex at the time of impregnation or had not differentiated sufficiently to reveal their characteristic features.
The exquisite observations of mammalian embryonic tissue by Wilhelm His and other old masters (including Cajal) in the late 19th century prompted the suggestion that cortical neurons are generated near the cerebral ventricle and migrate to their final destinations in the overlying cortex. The crucial finding was that mitotic figures were situated along the lumen of the cerebral ventricles and were virtually absent from the developing cortex forming below the pial surface. This basic concept withstood the test of time and remained, for the most part, valid. However, nearly 10 years ago, reports appeared in the literature suggesting that neurons arising in the ganglionic eminence (GE), the primordium of the basal ganglia in the subpallium, migrate to the developing pallium (De Carlos et al. 1996; Tamamaki et al. 1997). Tracing studies in rodents have since confirmed these observations and demonstrated that these migrating cells are GABA-containing interneurons destined not only for the neocortex, but also for the hippocampus and olfactory bulb (Anderson et al. 1997; Lavdas et al. 1999; Wichterle et al. 1999, 2001; Pleasure et al. 2000). These studies identified different streams of interneurons that migrate around the corticostriatal notch and follow tangentially orientated paths to enter the cortex. An early cohort [embryonic day (E)12 in mouse; E14 in rat], originating in the medial ganglionic eminence (MGE), enters mainly the preplate. A second and more prominent cohort, composed also of MGE cells, migrates mainly through the intermediate zone (IZ) slightly later in development (E13–15 mouse; E15–17 rat). At the late stages of corticogenesis, cells originating mostly from the lateral ganglionic eminence (LGE), but also from the MGE, appear in the lower IZ and subventricular zone (SVZ). More recent studies have shown that the caudal ganglionic eminence (CGE) also contributes to the group of tangentially migrating interneurons to the cortex and hippocampus (Nery et al. 2002). The existence of distinct sources of cortical interneurons has raised the question of whether distinct interneuron subtypes are derived from each of the identified sources in the subpallium. Cortical interneurons show remarkable diversity of subtypes identified by morphological, physiological and molecular properties (Fairén et al. 1984; Cauli et al. 1997; Kawaguchi & Kubota, 1997; Markram et al. 2004). Anderson and colleagues have recently studied the origins and specification of distinct subgroups of cortical interneurons. These investigators used in vitro transplantation assays and analysis of Nkx2.1 mutants and found that somatostatin- and parvalbumin-expressing interneurons derive for the most part from the MGE, while calretinin-containing cells originate predominantly in the CGE (Xu et al. 2004; Wonders & Anderson, 2006). More recently, using in utero fate mapping studies, coupled with electrophysiological and immunological analysis, Butt et al. (2005) have elegantly demonstrated that at E13.5 medial and caudal GEs give rise to inteneuron populations with distinct electrophysiological properties. Interestingly, analysis of the CGE neurons at E15.5 appear to show a different class of regular-spiking inteneurons from those generated at E13.5, suggesting that different spatial and temporal origins of interneurons within the developing telencephalic eminences give rise to mature neurons with predicted physiological properties.
Cortical interneurons generated in the subpallium use intrinsic and extrinsic cues along their tortuous journey to the cortex, and disperse in all cortical layers where they assemble into functional circuits with a precise balance of synaptic excitation and inhibition (for a review, see Flames & Marín, 2005). It has been suggested that disruption in this balance results in neuropathological conditions such as epilepsy and Parkinson's disease (Ribak et al. 1982; Sloviter, 1987; De Lanerolle et al. 2003; Cobos et al. 2005; Magloczky & Freund, 2005; Kumar & Buckmaster, 2006; Mallet et al. 2006). Recent work has also suggested a role for interneurons in the neuropathology and development of Alzheimer's disease (Koliatsos et al. 2006). Thus, understanding the molecular mechanisms that control interneuron migration and the roles these cells play in cortical function is of significant clinical relevance and therapeutic importance.
Santiago Ramón y Cajal (1892) was also the first to propose the idea that chemo-attractive molecules secreted by target cells regulate developmental events such as axon guidance and migration. However, it took nearly 100 years before experimental evidence confirmed that diffusible factors secreted by floor-plate cells in the developing spinal cord regulate commissural axonal outgrowth (Tessier-Lavigne et al. 1988). The molecules were later identified as netrin-1 and netrin-2 (Kennedy et al. 1994). Subsequently, a plethora of chemotropic molecules have been shown to play roles in axon guidance and cell migration. These include three pairs of ligands and their cognate receptors: the Semaphorins (Semas) and their receptors, the neuropilins (Npns); Ephrins and their receptors (Eph); and Slits and their receptors Robo (roundabout in Drosophila). Much of the work pertaining to the role of these ligands and their receptors during cortical neuron migration has been reviewed by Marín & Rubenstein (2003). Here, we review recent findings regarding the role(s) of Slit/Robo interactions in forebrain development with emphasis on cortical interneuron migration.
The family of Slit proteins are large secreted axon guidance molecules, which are evolutionarily conserved (Rothberg et al. 1990). Slit was first identified as a factor involved in the development of midline glia and was subsequently found to be a midline axon repellent (Battye et al. 1999; Kidd et al. 1999). In Drosophila, Slit has been shown to prevent ipsilateral projecting fibres from crossing the midline, and contralaterally from recrossing via repulsive axon guidance activity (Rajagopalan et al. 2000; Simpson et al. 2000a). The repulsion is mediated by members of the Roundabout (Robo) receptor family, which are expressed in commissural axons (Kidd et al. 1998a). In Drosophila, robo and slit mutants exhibit severe midline crossing defects (Kidd et al. 1999; Simpson et al. 2000b).
Robo is a novel member of the immunoglobulin (Ig) super family of cell adhesion molecules (CAMs), which are conserved throughout evolution from Drosophila to humans (Sundaresan et al. 1998a,b). Robo molecules contain five Ig domains (two only in Robo4), three type III fibronectin motifs, a transmembrane segment, and a cytoplasmic tail containing four conserved signalling motifs which are thought to interact with downstream signalling molecules (Bashaw et al. 2000). To date, four family members have been identified in vertebrates: Robo1, Robo2, Robo3 (also known as Rig1) and Robo4 (also known as magic roundabout) (Kidd et al. 1998a,b; Yuan et al. 1999a; Huminiecki et al. 2002). Robo1 and Robo2 are expressed in many tissues and organs during development and in adult life, but show strongest expression in the developing nervous system (Holmes et al. 1998). Robo3 expression seems limited to the developing CNS, while Robo4 is specifically found in endothelial cells. All four Robos have been shown to bind to Slit proteins (Park et al. 2003; Liu et al. 2004; Cammuri et al. 2005; Mambetisaeva et al. 2005). For Robo1, at least, this binding activity has been delineated to reside within Ig domains 1 and 2 (Liu et al. 2004), which are also the most highly conserved parts, highlighting the importance of these domains in Robo function. Recently, these two Ig domains of Robo have been shown to interact with the leucine-rich regions in Slit proteins (Howitt et al. 2004).
Like other CAMs, human Robo1, Robo2 and Robo3 have recently been shown to mediate homophilic adhesion, functioning as both a ligand on one cell and a receptor on another, as well as to function as heterophilic ligands (Hivert et al. 2002; Liu et al. 2004; Cammuri et al. 2005). The significance of such homophilic and heterophilic interactions in terms of Robo function is unknown at present, but for other CAM family members such as NCAM, L1 and the netrin receptor DCC, these interactions have been shown to be important in promoting neurite outgrowth (reviewed in Walsh & Doherty, 1996).
Expression of Slit/Robo in the developing cerebral cortex
The relevance of Robo and Slit expression has been extensively highlighted in axon guidance systems in rodents (reviewed in Brose & Tessier-Lavigne, 2000), specifically in the development of major forebrain axonal tracts and commissures (Bagri et al. 2002; Andrews et al. 2006), in the visual system (Plump et al. 2002; Thompson et al. 2006) and in the spinal cord (Shu et al. 2003; Long et al. 2004; Sabatier et al. 2004; Mambetisaeva et al. 2005). Here, we review Slit and Robo expression patterns in the context of interneuron migration in the developing rodent forebrain. Interneurons follow distinct migratory routes from their origins within the germinal zone of the GE to the developing cortex (see above), and the position and extent of these routes are developmentally regulated. We therefore describe Robo and Slit expression patterns during early (E13), mid (E15) and late (E17) phases of tangential migration.
The expression of slit/robo genes has been investigated predominantly by in situ hybridization, and these studies have shown that robo (robo1 and robo2) and slit (slit1, slit2, slit3) genes are dynamically expressed in complementary patterns during cortical development (Yuan et al. 1999a; Bagri et al. 2002; Marillat et al. 2002; Whitford et al. 2002). The dynamic expression of Robo proteins was subsequently confirmed in the developing mouse CNS using a pan Robo (Robo1 and Robo2) (Sundaresan et al. 2004), and here using previously described Robo1- and Robo2-specific antibodies (see Fig. 1; Long et al. 2004; Andrews et al. 2006). Lack of specific Slit antibodies has prevented the visualization of Slit protein gradients. Whilst the full extent of diffusible Slit chemorepulsive activity remains to be elucidated, transgenic mice that express marker proteins in slit1, slit2 and slit3 loci have made it possible to visualize slit-expressing cells, thus confirming results from previous in situ hybridization studies (Plump et al. 2002; Bagri et al. 2002; Yuan et al. 2003).
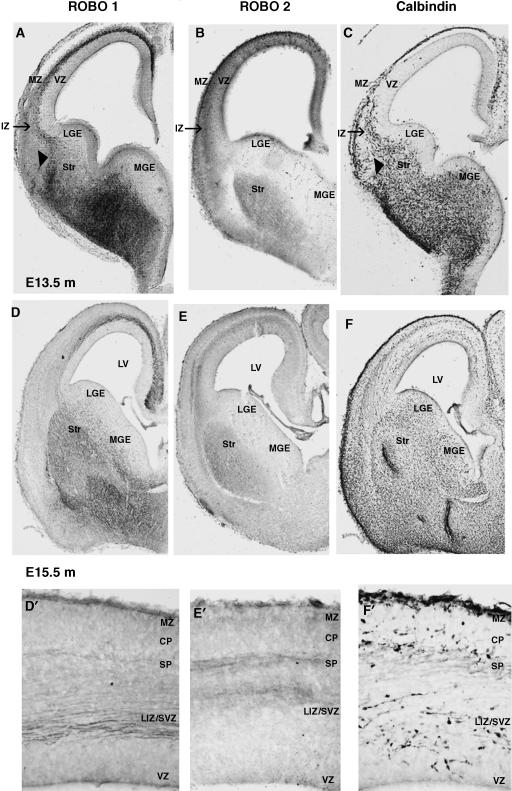
Fig. 1.
Expression patterns of Robo proteins during cortical development. Images illustrate the immunohistochemical localization of Robo1, Robo2 and Calbindin proteins in coronal sections through the developing mouse forebrain at E13.5 (A–C) and E15.5 (D–F). Enlarged portions of the dorsal cortex at E15.5 are shown for Robo1 (D′), Robo 2 (E′) and Calbindin (F′) localization. MZ, marginal zone; IZ, intermediate zone; VZ, ventricular zone; LGE, lateral ganglionic eminence; MGE, medial ganglionic eminence; Str, striatum; LV, lateral ventricle; CP, cortical plate; SP, subplate; LIZ/SVZ, lower intermediate zone/subventricular zone.
During the early phase of cortical development (E13), slit1 is strongly expressed throughout the VZ and SVZ of the GE, as well as at the ventral midline and in basal regions of the forebrain (Yuan et al. 1999b; Bagri et al. 2002; Marillat et al. 2002; Whitford et al. 2002). This is a time when early born interneurons migrate away from the germinal MGE and follow a route superficial to the differentiating striatum (Figs 1C and 2) on their way to the cortex.
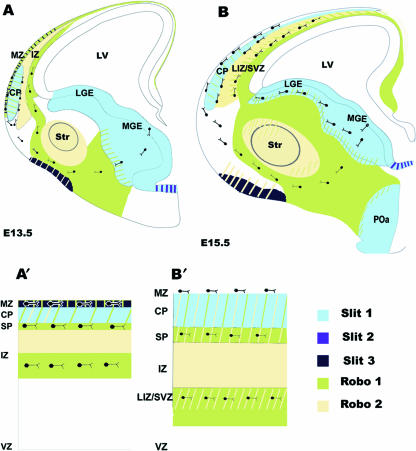
Fig. 2.
Schematic representation of Robo and Slit localization in the developing forebrain. Schematic drawings, based on in situ and immunohistochemical studies, illustrate robo and slit expression patterns during early (E13.5) (A) and mid (E15.5) (B) phases of cortical interneuron migration. Hatched patterns indicate regions of overlap in expression. Enlarged portions of the dorsal cortex at E13.5 (A′) and E15.5 (B′) show the localization of robo and slit and the regions of overlapping expression in greater detail. MZ, marginal zone; IZ, intermediate zone; VZ, ventricular zone; LGE, lateral ganglionic eminence; MGE, medial ganglionic eminence; Str, striatum; LV, lateral ventricle; CP, cortical plate; SP, subplate; LIZ/SVZ, lower intermediate zone/subventricular zone; POa, preoptic area.
Experimental evidence suggests that Slit secreted from the ventricular zone of the lateral ganglionic eminence (LGE) repels cortical interneurons from the subventricular zone of LGE explants and inhibits tangential migration when added locally at the corticostriatal boundary of brain slices (Zhu et al. 1999), suggesting that Slit plays a role in directing inteneurons towards the cortex. This slit expression is maintained within the germinal region of the GE throughout the period of interneuron migration (E13–E17) (Fig. 2) consistent with the notion that slit has a chemorepulsive role in propelling these neurons away from the GE.
Robo1 and Robo2 show distinct but complementary expression patterns in the subpallium at E13, and they are also complementary to slit expression. Robo1 expression corresponds to regions through which early born interneurons migrate (Figs 1A and 2A), and overlaps with the expression of the calcium binding protein calbindin, a marker of GABA-containing cortical interneurons during embryonic development (Fig. 1A,C). Robo2 expression is primarily restricted to the differentiating striatum (Fig. 1B). The striatum remains an exclusion zone throughout the period of generation and migration of cortical interneurons, and has been suggested to have a role in streaming these cells to their appropriate tangential routes (Marín et al. 2001, 2003). Whilst the presence of slits has not been conclusively demonstrated in the striatum to date, the expression patterns of Robo1 and calbindin are consistent with a Robo1-mediated response to repulsive activity within the striatum (arrowheads in Fig. 1A,C) at E13. Thus, Robo1 (but not Robo2) and slit expression patterns are consistent with a role for Robo/Slit in directing early born interneurons along the superficial route within the subpallium.
In the developing cortex, early born interneurons migrate predominantly at the level of the marginal zone (MZ) and IZ, and to a lesser extent within the subplate (SP) (Fig. 1C). Robo1 is distinctly expressed in the IZ and MZ where it overlaps extensively with calbindin expression (Fig. 1A,C). Robo2 is also expressed in the IZ, but is more superficial to Robo1. Thus, it appears that Robo1 (but not Robo2) is expressed in regions that correspond to the tangential migratory routes of early born interneurons, and complements Slit expression in the developing cortical plate (CP) (slit1) and MZ (slit3) (Fig. 2A′). The complementary expression patterns of Robo1 and Slits are therefore consistent with them having a role in directing interneurons within the subpallium as well as in the cortex during the early phases of tangential migration.
During mid phases of tangential interneuron migration, strong staining for both Robo1 and Robo2 receptors is present in the subpallium (Fig. 1D,E, respectively). Both Robo receptors are distinctly expressed, but overlap within the mantle regions of the differentiating striatum and, to a lesser extent, in the differentiating pallidum (Fig. 2B). Punctate Robo1 staining is clearly visible in the SVZ of the GE, and it expands dorsally to the corticostriatal boundary and overlaps with calbindin expression (Fig. 1D,F). This corresponds to the stream of migrating interneurons deep in the differentiating striatum and complements slit expression, which is maintained within the germinal epithelium of the GE and at the ventral midline (Bagri et al. 2002; Marillat et al. 2002; Fig. 2B).
Once later born (E15.5) interneurons arrive at the corticostriatal boundary, they migrate predominately along the lower IZ/SVZ as well as along the SP and MZ (Figs 1F,F′ and 2). Strong Robo1 expression is seen in the lower IZ/SVZ, but considerably weaker expression is noted at the level of the SP, where Robo2 is distinctly present (Fig. 1D′,E′). Both Robos overlap to a small degree within the lower IZ, with diffuse Robo2 expression extending superficially throughout the IZ (Fig. 1D′,E′). Discrepancies between in situ and immunohistochemical studies have been reported for the lower IZ/SVZ. Thus, while Robo1 protein has clearly been localized in this zone (Fig. 1D′), Robo1 mRNA has not been reported in either the rat or the mouse at corresponding developmental stages (Yuan et al. 1999b; Marillat et al. 2002). This could be explained by the presence of Robo1 protein in axonal tracts that traverse the lower IZ/SVZ. Whilst the immunohistochemical labelling for Robo1 appears fibrous, staining of a punctate nature is clearly interspersed within it. The coexpression of calbindin and Robo1 within this zone at E15 further confirms that Robo-expressing interneurons are present within this stream (Andrews et al. 2006). Double labelling experiments using TAG-1, a marker of corticofugal axons, and Robo1 could further clarify this point. A close association between interneurons and TAG-1-expressing axonal tracts has been reported, suggesting that interneurons use this system as scaffold for their migration into the neocortex (Denaxa et al. 2001); however, this idea is not universally accepted (Wichterle et al. 2001). If these corticofugal fibres are Robo1 positive, it is possible that this migration function is facilitated by homophilic Robo interactions, which has been demonstrated previously for Robo1 in axon guidance mechanisms (Hivert et al. 2002).
Robo1 and Robo2 are mostly down-regulated in the differentiating basal ganglia by E17 (data not shown). However, a population of post-mitotic neurons of the caudate/putamen, nucleus accumbens and lateral globus pallidus continue to express robo1 and robo2 mRNA (Marillat et al. 2002). Late born cortical interneurons continue to migrate through the SVZ of the GE and deep in the striatum, and follow a tangential route within the lower IZ/SVZ in the cortex. robo1 is robustly expressed in the SVZ/IZ of the cortex at this time, while robo2 is also present within this region, but at a relatively low level. robo and slit1 are therefore still expressed in regions through which late born interneurons migrate within the striatum. robo1, robo2 as well as slit1 and slit2 continue to be expressed at low levels within the developing CP at E17.
The complementary expression patterns of Slit and Robo are therefore consistent with these molecules having a role in directing interneurons within the subpallium as well as along their tangential routes within the cortex during the early and mid phases of their migration. Slit and Robo continue to be expressed dynamically in layer-specific patterns during early postnatal periods, a time of extensive dendritic growth and afferent axonal branching in which Slit has been suggested to play a role (Marillat et al. 2002; Whitford et al. 2002). Slit and Robo expression is further maintained within the adult cortex and in neurons of the basal ganglia, suggesting that this signalling pathway is important for cellular events in the adult forebrain.
The role of Slit/Robo in cortical development
The expression of Robo proteins in major axonal tracts and cortical interneurons has prompted speculation that Slit/Robo molecules are involved in the development of major axonal pathways and in neuronal migration within the cerebral cortex. Previous in vitro experiments have demonstrated that cortical, thalamocortical and callosal axons are repelled by Slits (Shu & Richards, 2001; Whitford et al. 2002), raising the possibility that these molecules may play important roles in corticofugal, thalamocortical and corpus callosum development. These results have been confirmed in subsequent in vivo experiments using both gene mutations and antisense knockdown of the protein (Bagri et al. 2002; Plump et al. 2002; Shu et al. 2003). Furthermore, these authors have proposed that Slit proteins are involved in maintaining the position of axonal tracts, in preventing axonal extension towards and across the midline, and in channelling axons into particular regions.
Interestingly, we have recently found significant differences between the phenotype of Robo1 mutant mice and that of Slit1 and Slit1/2 double knockout mice (Fig. 3; Andrews et al. 2006). The largest differences were noted in the formation of the thalamocortical and corticothalamic pathways between the two groups of mutants. In particular, in Robo1 knockouts, thalamocortical and corticothalamic axons reach their targets earlier than in control mice, and no ectopic commissures are present in the diencephalon as observed in the Slit1/2 double mutants. Also, when Slit2 is removed, axons tend to defasciculate (Bagri et al. 2002; Plump et al. 2002; Shu et al. 2003) whereas when Robo1 is absent, the axons form tight bundles. These results suggest that Robo1 may be involved in maintaining a crucial distance between the axons, keeping them in distinct tracts either through their use of Robo/Slit signal transduction mechanisms or through Robo1 homophilic/heterophilic interactions.
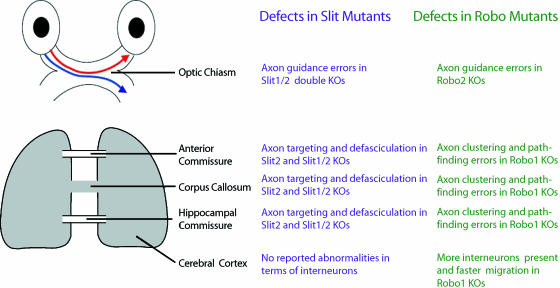
Fig. 3.
Different developmental abnormalities observed in Slit and Robo null mice. Different phenotypical manifestations have been reported between Slit and Robo1 null mice in several systems. In both types of mutants an ectopic chiasm is observed at the optic chiasm, but only in slit mutants do we observe a dorsally projecting axon into the controlateral optic tract (Plump et al. 2002). In cortical commissures and at the corpus callosum Slit mutants demonstrate defects in axon targeting and defasciculation (Bagri et al. 2002; Whitford et al. 2002; Shu et al. 2003), whereas Robo mutants show axon pathfinding errors and axon clustering (Andrews et al. 2006).
Evidence is emerging to indicate that Robo proteins also play a role in cortical interneuron migration. Previously, it was suggested that the migration of interneurons from the MGE is mediated by the repulsive activity of Slit1 present in the VZ of the subpallium (Figs 2 and 4; Marillat et al. 2002). Slit1 has been shown to repel GABAergic cells derived from the LGE in vitro (Zhu et al. 1999). Another diffusible guidance protein, netrin1 (Ntr1), has also been implicated in the repulsion of striatal SVZ cells towards the developing striatum (Hamasaki et al. 2001). Moreover, our own data would suggest a more significant role for Slit/Robo molecules in cortical interneuron migration than previously suggested, as we have shown that they strongly express Robo1 both in vivo and in dissociated cell cultures (Andrews et al. 2006).
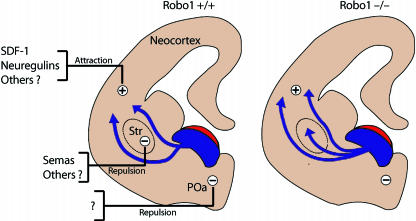
Fig. 4.
Mechanism regulating migration of interneurons from the subpalium to the cerebral cortex in Robo1 wild-type (+/+) and Robo1 null (–/–) mice. Schematic drawings of coronal sections through the mouse telencephalon illustrate how in Robo1 wild-type (+/+) mice cortical inteneurons, generated in the subventricular zone of the medial ganglionic eminence (shown in blue), are expelled from this region by the repulsive action of Slit (shown in red). Unidentified repulsive activity (minus sign) present in the preoptic area (POa) prevents the migration of these neurons ventrally. Expression of Semaphorins in the developing striatum (Str) prevents cortical interneurons that express neuropilin and Robo from entering this structure in mice. Attractive factors (plus sign) including SDF-1 and neuregulins guide interneurons towards the cortex. However, in Robo1–/– mice, interneurons invade the striatal region ignoring the repulsive effects of Semaphorins and other factors, and enter the cortex earlier and in greater numbers than in wild-type littermates.
Analysis of Slit knockout mice has shown that tangential migration from the GE to the cortex was normal both in double (Slit1/Slit2) and in triple (Slit1/Slit2/Ntr1) mutant animals, suggesting that these molecules are not required for the migration of interneurons from the subpallium to the pallium (Marín et al. 2003). However, they do appear to be required for the migration of a subset of subcortical (NPY-expressing) and cholinergic neurons (Marín et al. 2003). These results seem surprising given the high levels of Robo1 expression in GABAergic neurons. It is also difficult to reconcile these data with our finding that Robo1-null mice show a significant increase (approximately 30%) in the number of GABAergic (calbindin positive) neurons entering the neocortex compared with their wild-type littermates throughout the period of corticogenesis (Andrews et al. 2006). These phenotypical differences may be accounted for by: Slit-independent mechanisms; subtle differences in the Robo–Slit signalling pathways; or differences in interneuron proliferation between Slit and Robo1 knockouts. Our recent observations provide evidence for the last of these, as BrdU pulse experiments in dissociated GE cell cultures, similar to those performed by Cavanagh et al. (1997), revealed that calbindin-positive interneurons in Robo1 mutant cultures incorporate more BrdU than cultures from wild-type littermates, indicating that these neurons show an increase in cell proliferation rate (W.D.A., unpublished observations). Such changes in proliferation rates have not been reported for Slit knockout mice.
We have also found that the distribution of cortical interneurons is altered within the cortex of Robo1 knockouts, and that these neurons show altered process configuration (neurite branching and length) compared with wild-type littermates (W.D.A., unpublished observations). Comparable changes have not been reported for Slit knockout mice. However, Slit has previously been shown to have an effect on process length and branching in neuronal populations (Wang et al. 1999; Sang et al. 2002), suggesting an important role for Slit/Robo interactions in the morphology of cortical interneurons.
While these recent observations support an important role for Robo in cortical interneuron migration, other chemotropic molecules have also been implicated. These include the Semas and their receptors, the Npns. Interneurons destined for the cortex have been shown to express both Npn1 and Npn2, which enable them to respond to chemorepulsion exerted by class 3 Semas in the striatal mantle (Marín et al. 2001), thus creating an exclusion zone in this region. This exclusion zone enables migrating interneurons to be channelled into adjacent paths, leading to the formation of the migratory routes into the cortex (Fig. 4). Loss of Npn1 or Npn2 function results not only in an increased number of interneurons invading the striatum, but also affects the number and distribution of interneurons in the cortex as a consequence of their failure to respond to Semas secreted by cells in the CP (Marín et al. 2001; Tamamaki et al. 2003). However, we have recently found that Robo1 is also required for interneurons to avoid the striatum, as loss of Robo1 function leads to an increased number of cells entering this region (Fig. 4; Andrews et al. 2006). Interestingly, such a phenotype was not observed in Slit1/Slit2 double mutant mice (Marín et al. 2003), suggesting that it may be a Slit-independent event or that an as yet unidentified member of the Slit family of molecules is involved. Taken together, these observations suggest that both Sema–Npn and Slit–Robo signalling pathways are required to steer interneurons around the striatum and into their correct positions in the neocortex. Such a dual role for these two families of molecules has recently been proposed in the development of primary sensory projections in the olfactory system (Cloutier et al. 2004).
Role of Slit–Robo interactions in other systems
Mammalian slit/robo homologues have been shown to play vital roles in a number of developmental processes within the nervous system other than cortical development. These include: the formation of the olfactory tract (Nguyen-Ba-Charvet et al. 1999, 2002), the development of the optic chiasm and optic tract (Plump et al. 2002), midline axon crossing (Long et al. 2004; Marillat et al. 2004; Sabatier et al. 2004), and motor axon path finding in the hindbrain (Hammond et al. 2005).
Roles for Slit–Robo proteins have also been proposed in developmental processes outside the brain, including the formation of several organs. The Dutt/Robo1 gene has been shown to be critical for lung maturation and lack of Robo1 results in the development of bronchial hyperplasia in transgenic mice (Xian et al. 2001; W.D.A., unpublished data). In the kidney, aberrant Slit2-mediated Robo2 signalling restricts kidney induction, resulting in the development of supernumerary ureteric buds which fail to connect to the bladder (Grieshammer et al. 2004), and more recently defective Robo2 signalling has been implicated in the pathogenesis of congenital abnormalities of the kidney and urinary tract including vesicoureteral reflux (Weining et al. 2007). Slit2–Robo2-mediated signalling has recently been shown to play a dual role during assembly of the heart tube in Drospohila, by regulating both cell positioning and adhesive interactions between migrating cardiac precursor cells (Santiago-Martinez et al. 2006). Interestingly, Slit3-deficient mice show defects in multiple organ systems including congenital diaphragmatic hernia, kidney agenesis and cardiac defects (Liu et al. 2003).
Robo1 expression has also been reported on endothelial cells, with a role proposed for Slit2–Robo1 signalling in promoting tumour angiogenesis (Wang et al. 2003). These authors also demonstrated that tumour xenografts overexpressing Slit2 showed increased tumour angiogenesis and accelerated tumour growth, whereas xenografts expressing soluble Robo1, or the addition of blocking antibodies, showed reduced tumour growth and microvessel density. These results demonstrate a critical role for Slit–Robo signalling in mediating tumour angiogenesis. A recent report has also suggested a possible role for Robo1/Robo4 in angiogenesis in colorectal cancer (Grone et al. 2006).
Tumour suppressor gene activity of Slit2 and Robo1 has previously been proposed in lung and breast cancer (Sundaresan et al. 1998a), and two recent studies have shown that both genes are frequently inactivated in lung adenocarcinomas and lymphoma by methylation of the gene promoters (Dallol et al. 2002; Xian et al. 2004). Moreover, Xian et al. (2004) have demonstrated that Dutt1/Robo1 transgenic mice show a higher incidence of lymphomas and carcimomas than wild-type littermates, suggesting that Dutt1/Robo1 acts as a tumour suppressor gene. Several other reports have also demonstrated roles for Slit–Robo signalling in various cancer processes (Latil et al. 2003; Zarubin et al. 2005; Narayan et al. 2006). More recently, medulloblastomas and gliomas, which are the most common brain tumours in children and adults, respectively, have been shown to express Robo1 and Slit2, and that the addition of soluble Slit2 reduces tumour invasion rates in a variety of in vitro models (Werbowetski-Ogilvie et al. 2006). Thus, Slit–Robo signalling pathways appear to play an important role in tumour development in many different types of carcinomas and lymphomas.
Conclusions
We have reviewed evidence for strong expression of Slit and Robo molecules in the developing forebrain and for the role of Slit/Robo interactions in the formation of major axonal tracts and interneuron migration. Slit–Robo genes have also been implicated in the development of other areas of the nervous system, the vasculature and in the formation of a number of organs. Understanding the functional role of these pleiotropic molecules in cortical development and in disease processes, such as angiogenesis and tumourogenesis, may result in the development of treatments of vascular disorders and different forms of cancer, and in the establishment of potential therapeutic targets in the treatment of some neurological disorders.
Acknowledgments
The work presented here was supported by the Wellcome Trust (grant no. 074549). We would like to thank Sonja Rakić and Clare Faux for their helpful comments in the preparation of this manuscript, and Professor Fujio Murakami, Osaka University, Japan, for generously providing the Robo1- and Robo2-specific antibodies.
References
- Anderson SA, Eisenstat DD, Shi L, Rubenstein JL. Interneuron migration from basal forebrain to neocortex: dependence on Dlx genes. Science. 1997;278:474–476. doi: 10.1126/science.278.5337.474. [DOI] [PubMed] [Google Scholar]
- Andrews W, Liapi A, Plachez A. Robo1 regulates the development of major axon tracts and interneuron migration in the forebrain. Development. 2006;133:2243–2252. doi: 10.1242/dev.02379. [DOI] [PubMed] [Google Scholar]
- Bagri A, Marín O, Plump AS. Slit prevents midline crossing and determines the dorsoventral position of major axonal pathways in the mammalian forebrain. Neuron. 2002;33:233–248. doi: 10.1016/s0896-6273(02)00561-5. [DOI] [PubMed] [Google Scholar]
- Bashaw GJ, Kidd T, Murray D, Pawson T, Goodman CS. Repulsion axon guidance: Abelson and Enabled play opposing roles downstream of the roundabout receptor. Cell. 2000;101:703–715. doi: 10.1016/s0092-8674(00)80883-1. [DOI] [PubMed] [Google Scholar]
- Battye R, Stevens A, Jacobs JR. Axon repulsion from the midline of the Drosophila CNS requires slit function. Development. 1999;126:2475–2481. doi: 10.1242/dev.126.11.2475. [DOI] [PubMed] [Google Scholar]
- Brose K, Tessier-Lavigne M. Slit proteins: key regulators of axon guidance, axonal branching, and cell migration. Curr Opin Neurobiol. 2000;10:95–102. doi: 10.1016/s0959-4388(99)00066-5. [DOI] [PubMed] [Google Scholar]
- Butt SJ, Fuccillo M, Nery S. The temporal and spatial origins of cortical interneurons predict their physiological subtype. Neuron. 2005;48:591–604. doi: 10.1016/j.neuron.2005.09.034. [DOI] [PubMed] [Google Scholar]
- Cammuri L, Mambetisaeva E, Davies D, Parnavelas J, Sundaresan V, Andrews W. Evidence for the existence of two Robo3 isoforms with divergent biochemical properties. Mol Cell Neurosci. 2005;30:485–493. doi: 10.1016/j.mcn.2005.07.014. [DOI] [PubMed] [Google Scholar]
- Cauli B, Audinat E, Lambolez B. Molecular and physiological diversity of cortical nonpyramidal cells. J Neurosci. 1997;17:3894–3906. doi: 10.1523/JNEUROSCI.17-10-03894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanagh JF, Mione MC, Pappas IS, Parnavelas JG. Basic fibroblast growth factors prolongs the proliferation of rat cortical progenitor cells in vitro without altering their cell cycle parameters. Cereb Cortex. 1997;4:293–302. doi: 10.1093/cercor/7.4.293. [DOI] [PubMed] [Google Scholar]
- Cloutier JF, Sahay A, Chang EC, Tessier-Lavigne M, Dulac C, Kolodkin AL. Differential requirements for semaphorin 3F and Slit-1 in axonal targeting, fasciculation, and segregation of olfactory sensory neuron projections. J Neurosci. 2004;24:9087–9096. doi: 10.1523/JNEUROSCI.2786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobos I, Calcagnotto ME, Vilaythong AJ. Mice lacking Dlx1 show subtype-specific loss of interneurons, reduced inhibition and epilepsy. Nature Neurosci. 2005;8:1059–1068. doi: 10.1038/nn1499. [DOI] [PubMed] [Google Scholar]
- Dallol A, Fernandes Da Silva N, Viacava P. Slit2, a human homologue of the Drosophila Slit2 gene, has tumour suppressor activity and is frequently inactivated in lung and breast cancers. Cancer Res. 2002;62:5874–5880. [PubMed] [Google Scholar]
- De Carlos JA, Lopez-Mascaraque L, Valverde F. Dynamics of cell migration from the lateral ganglionic eminence in the rat. J Neurosci. 1996;16:6146–6156. doi: 10.1523/JNEUROSCI.16-19-06146.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Lanerolle NC, Kim JH, Williamson A. A retrospective analysis of hippocampal pathology in human temporal lobe epilepsy: evidence for distinctive patient subcategories. Epilepsia. 2003;44:677–687. doi: 10.1046/j.1528-1157.2003.32701.x. [DOI] [PubMed] [Google Scholar]
- DeFelipe J, Jones EG. Cajal on the Cerebral Cortex. An Annotated Translation of the Complete Writings. Oxford: Oxford University Press; 1988. [Google Scholar]
- Denaxa M, Chan CH, Schachner M, Parnavelas JG, Karagogeos D. The adhesion molecule TAG-1 mediates the migration of cortical interneurons from the ganglionic eminence along the corticofugal fiber system. Development. 2001;128:4635–4644. doi: 10.1242/dev.128.22.4635. [DOI] [PubMed] [Google Scholar]
- Fairén A, DeFelipe J, Regodor J. Nonpyramidal Neurons. In: Peters A, Jones EG, editors. Cerebral Cortex. Vol. 1. London: Plenum Press; 1984. pp. 201–254. [Google Scholar]
- Flames N, Marín O. Developmental mechanisms underlying the generation of cortical interneuron diversity. Neuron. 2005;46:377–381. doi: 10.1016/j.neuron.2005.04.020. [DOI] [PubMed] [Google Scholar]
- Grieshammer U, Ma L, Plump AS, Wang F, Tessier-Lavigne M, Martin GR. Slit2-mediated Robo2 signalling restricts kidney induction to a single site. Dev Cell. 2004;6:709–717. doi: 10.1016/s1534-5807(04)00108-x. [DOI] [PubMed] [Google Scholar]
- Grone J, Doebler O, Loddenkemper C, Hotz B, Buhr HJ, Bhargava S. Robo1/Robo4: differential expression of angiogenic markers in colorectal cancer. Oncol Report. 2006;15:1437–1443. [PubMed] [Google Scholar]
- Hamasaki T, Goto S, Nishikawa S, Ushio Y. A role of netrin-1 in the formation of the subcortical structure striatum: repulsive action on the late-born striatal neurons. J Neurosci. 2001;21:4271–4280. doi: 10.1523/JNEUROSCI.21-12-04272.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond R, Vivancos V, Naeem A. Slit-mediated repulsion is a key regulator of motor axon path finding in the hind brain. Development. 2005;132:4483–4495. doi: 10.1242/dev.02038. [DOI] [PubMed] [Google Scholar]
- Hivert B, Liu Z, Chuang CY, Doherty P, Sundaresan V. Robo1 and Robo2 are homophilic binding molecules that promote axonal growth. Mol Cell Neurosci. 2002;21:534–545. doi: 10.1006/mcne.2002.1193. [DOI] [PubMed] [Google Scholar]
- Holmes GP, Negus K, Burridge L. Distinct but overlapping expression patterns of two vertebrate slit homologs implies functional roles in CNS development and organogenesis. Mech Dev. 1998;79:57–72. doi: 10.1016/s0925-4773(98)00174-9. [DOI] [PubMed] [Google Scholar]
- Howitt JA, Clout NJ, Hohenester E. Binding site for Robo receptors revealed by dissection of the leucine-rich repeat region of Slit. EMBO J. 2004;23:4406–4412. doi: 10.1038/sj.emboj.7600446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huminiecki L, Gorn M, Suchting S, Poulsom R, Bicknell R. Magic roundabout is a new member of the roundabout receptor family that is endothelial specific and expressed at sites of active angiogenesis. Genomics. 2002;79:547–552. doi: 10.1006/geno.2002.6745. [DOI] [PubMed] [Google Scholar]
- Kawaguchi Y, Kubota Y. GABAergic cell subtypes and their synaptic connections in rat frontal cortex. Cereb Cortex. 1997;7:476–486. doi: 10.1093/cercor/7.6.476. [DOI] [PubMed] [Google Scholar]
- Kennedy TE, Serafini T, de la Torre JR, Tessier-Lavigne M. Netrins are diffusible chemotropic factors for commissural axons of the embryonic spinal cord. Cell. 1994;78:425–435. doi: 10.1016/0092-8674(94)90421-9. [DOI] [PubMed] [Google Scholar]
- Kidd T, Brose K, Mitchell KJ. Roundabout controls axon crossing of the CNS midline and defines a novel subfamily of evolutionary conserved guidance receptors. Cell. 1998a;92:205–215. doi: 10.1016/s0092-8674(00)80915-0. [DOI] [PubMed] [Google Scholar]
- Kidd T, Russell C, Goodman CS, Tear G. Dosage-sensitive and complementary functions of roundabout and commissureless control axon crossing of the CNS midline. Neuron. 1998b;20:25–33. doi: 10.1016/s0896-6273(00)80431-6. [DOI] [PubMed] [Google Scholar]
- Kidd T, Bland KS, Goodman CS. Slit is the midline repellent for the robo receptor in Drosophila. Cell. 1999;96:785–794. doi: 10.1016/s0092-8674(00)80589-9. [DOI] [PubMed] [Google Scholar]
- Koliatsos VE, Kecojevic A, Troncoso JC, Gastard MC, Bennett DA, Schneider JA. Early involvement of small inhibitory cortical interneurons in Alzheimer's disease. Acta Neuropathol. 2006;112:147–162. doi: 10.1007/s00401-006-0068-6. [DOI] [PubMed] [Google Scholar]
- Kumar SS, Buckmaster PS. Hyperexcitability, interneurons, and loss of GABAergic synapses in entorhinal cortex in a model of temporal lobe epilepsy. J Neurosci. 2006;26:4613–4623. doi: 10.1523/JNEUROSCI.0064-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Latil A, Chene L, Cochant-Priollet B. Quantification of expression of netrins, slits and their receptors in human prostate tumours. Int J Cancer. 2003;103:306–315. doi: 10.1002/ijc.10821. [DOI] [PubMed] [Google Scholar]
- Lavdas AA, Grigoriou M, Pachnis V, Parnavelas JG. The medial ganglionic eminence gives rise to a population of early neurons in the developing cerebral cortex. J Neurosci. 1999;19:7881–7888. doi: 10.1523/JNEUROSCI.19-18-07881.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Zhang L, Wang D. Congenital diaphragmatic hernia, kidney agenesis and cardiac defects associated with Slit3-deficiency in mice. Mech Dev. 2003;120:1059–1070. doi: 10.1016/s0925-4773(03)00161-8. [DOI] [PubMed] [Google Scholar]
- Liu Z, Patel K, Schmidt H, Andrews W, Pini A, Sundaresan V. Extracellular Ig domains 1 and 2 are important for ligand (Slit) binding. Mol Cell Neurosci. 2004;26:232–240. doi: 10.1016/j.mcn.2004.01.002. [DOI] [PubMed] [Google Scholar]
- Long H, Sabatier C, Ma L. Conserved roles for Slit and Robo proteins in midline commissural axon guidance. Neuron. 2004;42:213–223. doi: 10.1016/s0896-6273(04)00179-5. [DOI] [PubMed] [Google Scholar]
- Magloczky Z, Freund TF. Impaired and repaired inhibitory circuits in the epileptic human hippocampus. Trends Neurosci. 2005;28:334–340. doi: 10.1016/j.tins.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Mallet N, Ballion B, Le Moine C, Gonon F. Cortical inputs and GABA interneurons imbalance projection neurons in the striatum of Parkinsonian rats. J Neurosci. 2006;26:3875–3884. doi: 10.1523/JNEUROSCI.4439-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mambetisaeva ET, Andrews WD, Cammuri L, Annan A, Sundaresan V. Robo family of proteins exhibit differential expression in murine spinal cord and Robo–Slit interaction is required for midline crossing in vertebrate spinal cord. Dev Dyn. 2005;233:41–51. doi: 10.1002/dvdy.20324. [DOI] [PubMed] [Google Scholar]
- Marillat V, Cases O, Nguyen-Ba-Charvet KT. Spatiotemporal expression patterns of slit and robo genes in the rat brain. J Comp Neurol. 2002;442:130–155. doi: 10.1002/cne.10068. [DOI] [PubMed] [Google Scholar]
- Marillat V, Sabatier C, Failli V. The Slit receptor Rig-1/Robo3 controls midline crossing by hindbrain precerebellar neurons and axons. Neuron. 2004;43:69–79. doi: 10.1016/j.neuron.2004.06.018. [DOI] [PubMed] [Google Scholar]
- Marín O, Yaron A, Bagri A, Tessier-Lavigne M, Rubenstein JL. Sorting of striatal and cortical interneurons regulated by semaphorin–neuropilin interactions. Science. 2001;293:872–875. doi: 10.1126/science.1061891. [DOI] [PubMed] [Google Scholar]
- Marín O, Plump AS, Flames N, Sánchez-Camacho C, Tessier-Lavigne M, Rubenstein JLR. Directional guidance of interneuron migration to the cerebral cortex relies on subcortical Slit1/2-independent repulsion and cortical attraction. Development. 2003;130:1889–1901. doi: 10.1242/dev.00417. [DOI] [PubMed] [Google Scholar]
- Marín O, Rubenstein JL. Cell migration in the forebrain. Annu Rev Neurosci. 2003;26:441–483. doi: 10.1146/annurev.neuro.26.041002.131058. [DOI] [PubMed] [Google Scholar]
- Markram H, Toledo-Rodriguez M, Wang Y, Gupta A, Silberberg G, Wu C. Interneurons of the neocortical inhibitory system. Nat Rev Neurosci. 2004;5:793–807. doi: 10.1038/nrn1519. [DOI] [PubMed] [Google Scholar]
- Narayan G, Goparaju C, Arias-Pulido H. Promoter hypermethylation-mediated inactivation of multiple Slit-Robo pathway genes in cervical cancer progression. Mol Cancer. 2006;15:5–16. doi: 10.1186/1476-4598-5-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nery S, Fishell G, Corbin JG. The caudal ganglionic eminence is a source of distinct cortical and subcortical cell populations. Nature Neurosci. 2002;5:1279–1287. doi: 10.1038/nn971. [DOI] [PubMed] [Google Scholar]
- Nguyen-Ba-Charvet KT, Brose K, Marillat V. Slit2-mediated chemorepulsion and collapse of developing forebrain axons. Neuron. 1999;22:463–473. doi: 10.1016/s0896-6273(00)80702-3. [DOI] [PubMed] [Google Scholar]
- Nguyen-Ba-Charvet KT, Plump AS, Tessier-Lavigne M, Sotelo C, Chedotal A. Slit1 and slit2 proteins control the development of the lateral olfactory tract. J Neurosci. 2002;20:4962–4974. doi: 10.1523/JNEUROSCI.22-13-05473.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KW, Morrison CM, Sorensen LK. Robo4 is a vascular specific receptor that inhibits endothelial migration. Dev Biol. 2003;261:251–267. doi: 10.1016/s0012-1606(03)00258-6. [DOI] [PubMed] [Google Scholar]
- Pleasure SJ, Anderson S, Hevner R. Cell migration from the ganglionic eminences is required for the development of hippocampal GABAergic interneurons. Neuron. 2000;28:727–740. doi: 10.1016/s0896-6273(00)00149-5. [DOI] [PubMed] [Google Scholar]
- Plump AS, Erskine L, Sabatier C. Slit1 and Slit2 cooperate to prevent premature midline crossing of retinal axons in the mouse visual system. Neuron. 2002;33:219–232. doi: 10.1016/s0896-6273(01)00586-4. [DOI] [PubMed] [Google Scholar]
- Rajagopalan S, Vivancos V, Nicolas E, Dickson BJ. Selecting a longitudinal pathway: Robo receptors specifiy the lateral positions of axons in the Drosophila CNS. Cell. 2000;103:1033–1045. doi: 10.1016/s0092-8674(00)00207-5. [DOI] [PubMed] [Google Scholar]
- Ramón y Cajal S. Sur la structure de l'écorce cérébrale de quelques mammifères. La Cellule. 1891;7:125–176. [Google Scholar]
- Ramón y Cajal S. La Rétinedes vertebras. La Cellule. 1892;9:119–258. [Google Scholar]
- Ramón y Cajal S. The Croonian lecture: La fine structure des centres nerveux. Proc R Soc Lond. 1894;55:444–467. [Google Scholar]
- Ramón y Cajal S. Histologie du Système Nerveux de l’Homme et des Vertébrés. Vol. 1. Paris: Maloine; 1909. [Google Scholar]
- Ramón y Cajal S. Histologie du Système Nerveux de l'Homme et des Vertébrés. Vol. 2. Paris: Maloine; 1911. [Google Scholar]
- Ramón y Cajal S. Studies on Vertebrate Neurogenesis. Springfield, IL: Thomas CC; 1960. pp. 325–335. [Google Scholar]
- Ribak CE, Bradburne RM, Harris AB. A preferential loss of GABAergic, symmetric synapses in epileptic foci: a quantitative ultrastructural analysis of monkey neocortex. J Neurosci. 1982;2:1725–1735. doi: 10.1523/JNEUROSCI.02-12-01725.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg JM, Jacobs JR, Goodman CS, Artavanis-Tsakonas S. Slit: an extracellular protein necessary for development of midline glia and commissural axon pathways contains both EGF and LRR domains. Genes Dev. 1990;4:2169–2187. doi: 10.1101/gad.4.12a.2169. [DOI] [PubMed] [Google Scholar]
- Sabatier C, Plump A, Ma L. The divergent Robo family protein Rig-1/Robo3 is a negative regulator of slit responsiveness required for midline crossing of commissural axons. Cell. 2004;117:157–169. doi: 10.1016/s0092-8674(04)00303-4. [DOI] [PubMed] [Google Scholar]
- Sang Q, Wu J, Rao Y, Hsueh YP, Tan SS. Slit promotes branching and elongation of neurites of interneurons but not projection neurons from the developing telencephalon. Mol Cell Neurosci. 2002;21:250–265. doi: 10.1006/mcne.2002.1156. [DOI] [PubMed] [Google Scholar]
- Santiago-Martinez E, Soplop NH, Kramer SG. Lateral positioning at the dorsal midline: Slit and Roundabout receptors guide Drosophila heart cell migration. Proc Natl Acad Sci USA. 2006;103:12441–12446. doi: 10.1073/pnas.0605284103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu T, Richards LJ. Cortical axon guidance by the glia wedge during the development of the corpus callosum. J Neurosci. 2001;21:2749–2758. doi: 10.1523/JNEUROSCI.21-08-02749.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shu T, Sundaresan V, McCarthy MM, Richards LJ. Slit2 guides both precrossing and postcrossing callosal axons at the midline in vivo. J Neurosci. 2003;23:8176–8184. doi: 10.1523/JNEUROSCI.23-22-08176.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson JH, Bland KS, Fetter RD, Goodman CS. Short range and long range guidance by Slit and its Robo receptors: a combinatorial code of Robo receptors control lateral position. Cell. 2000a;103:1019–1032. doi: 10.1016/s0092-8674(00)00206-3. [DOI] [PubMed] [Google Scholar]
- Simpson JH, Kidd T, Bland KS, Goodman CS. Short range and long range guidance by Slit and its Robo receptors: Robo and Robo2 play distinct roles in midline guidance. Neuron. 2000b;28:753–766. doi: 10.1016/s0896-6273(00)00151-3. [DOI] [PubMed] [Google Scholar]
- Sloviter RS. Decreased hippocampal inhibition and a selective loss of interneurons in experimental epilepsy. Science. 1987;235:73–76. doi: 10.1126/science.2879352. [DOI] [PubMed] [Google Scholar]
- Sundaresan V, Chung G, Heppel-Parton A. Homozygous deletions at 3p12 in breast and lung cancer. Oncogene. 1998a;17:1723–1729. doi: 10.1038/sj.onc.1202103. [DOI] [PubMed] [Google Scholar]
- Sundaresan V, Roberts I, Bateman A. The DUTT1 gene, a novel NCAM family member is expressed in developing murine neural tissue and has an unusual broad pattern of expression. Mol Cell Neurosci. 1998b;11:29–35. doi: 10.1006/mcne.1998.0672. [DOI] [PubMed] [Google Scholar]
- Sundaresan V, Mambetisaeva E, Andrews W. Dynamic expression patterns of Robo (Robo1 and Robo2) in the developing central nervous system. J Comp Neurol. 2004;468:467–481. doi: 10.1002/cne.10984. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Fujimori KE, Takauji R. Origin and route of tangentially migrating neurons in the developing neocortical intermediate zone. J Neurosci. 1997;17:8313–8323. doi: 10.1523/JNEUROSCI.17-21-08313.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamamaki N, Fujimori KE, Kaneko T, Takauji R. Evidence that Sema3A and 3F regulate the migration of GABAergic neurons in the developing neocortex. J Comp Neurol. 2003;455:238–248. doi: 10.1002/cne.10476. [DOI] [PubMed] [Google Scholar]
- Tessier-Lavigne M, Placzek M, Lumsden AG, Dodd J, Jessell TM. Chemotropic guidance of developing axons in the mammalian central nervous system. Nature. 1988;336:775–778. doi: 10.1038/336775a0. [DOI] [PubMed] [Google Scholar]
- Thompson H, Camand O, Barker D, Erskine L. Slit proteins regulated distinct aspects of retinal ganglion cell axon guidance within the dorsal and ventral retina. J Neurosci. 2006;26:8082–8091. doi: 10.1523/JNEUROSCI.1342-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh FS, Doherty P. Cell adhesion molecules and neuronal regeneration. Curr Opin Cell Biol. 1996;8:707–713. doi: 10.1016/s0955-0674(96)80113-x. [DOI] [PubMed] [Google Scholar]
- Wang KH, Brose K, Arnott D. Biochemical purification of a mammalian slit protein as a positive regulator of sensory axon elongation and branching. Cell. 1999;96:771–784. doi: 10.1016/s0092-8674(00)80588-7. [DOI] [PubMed] [Google Scholar]
- Wang B, Xiao Y, Ding BB. Induction of tumour angiogenesis by Slit-Robo signalling and inhibition of cancer growth by blocking Robo activity. Cancer Cell. 2003;4:19–29. doi: 10.1016/s1535-6108(03)00164-8. [DOI] [PubMed] [Google Scholar]
- Weining L, van Eerde AM, Fan X, Quintero-Rivera F, Kulkarni S, Ferguson H, Kim HG, Fan Y, Xi Q, Li QG, Sanlaville D, Andrews W, Sundaresan V, Bi W, Yan J, Giltay JC, Wijmenga C, de Jong TP, Feather SA, Woolf AS, Rao Y, Lupski JR, Eccles MR, Quade BJ, Gusella JF, Morton CC, Maas RL. Disruption of ROBO2 is associated with urinary tract anomalies and confers risk of vesicoureteral reflux. Am J Human Genet. 2007;80:616–632. doi: 10.1086/512735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werbowetski-Ogilvie TE, Seyed Sadr M, Jabado N. Inhibition of medulloblastoma cell invasion by Slit. Oncogene. 2006;24:1–10. doi: 10.1038/sj.onc.1209524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitford KL, Marillat V, Stein E. Regulation of cortical dendritic development by Slit–Robo interactions. Neuron. 2002;33:47–61. doi: 10.1016/s0896-6273(01)00566-9. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Garcia-Verdugo JM, Herrera DG, Alvarez-Buylla A. Young neurons from medial ganglionic eminence disperse in adult and embryonic brain. Nat Neurosci. 1999;2:461–466. doi: 10.1038/8131. [DOI] [PubMed] [Google Scholar]
- Wichterle H, Turnbull DH, Nery S, Fishell G, Alvarez-Buylla A. In utero fate mapping reveals distinct migratory pathways and fates of neurons born in the mammalian basal forebrain. Development. 2001;128:3759–3771. doi: 10.1242/dev.128.19.3759. [DOI] [PubMed] [Google Scholar]
- Wonders CP, Anderson SA. The origin and specification of cortical interneurons. Nature Rev Neurosci. 2006;7:687–696. doi: 10.1038/nrn1954. [DOI] [PubMed] [Google Scholar]
- Xian J, Clark KJ, Fordham R, Pannell R, Rabbitts TH, Rabbitts PH. Inadequate lung development and bronchial hyperplasia in mice with a targeted deletion in the Dutt/Robo1 gene. Proc Natl Acad Sci USA. 2001;98:15062–15066. doi: 10.1073/pnas.251407098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian J, Aitchison A, Bobrow L. Targeted disruption of the 3p12 gene, Dutt1/Robo1, predisposes mice to lung adenocarcinomas and lymphomas with methylation of the gene promoter. Cancer Res. 2004;64:6432–6437. doi: 10.1158/0008-5472.CAN-04-2561. [DOI] [PubMed] [Google Scholar]
- Xu Q, Cobos I, De La Cruz E, Rubenstein JL, Anderson SA. Origins of cortical interneuron subtypes. J Neurosci. 2004;24:2612–2622. doi: 10.1523/JNEUROSCI.5667-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan SS, Cox LA, Dasika GK, Lee EY. Cloning and functional studies of a novel gene aberrantly expressed in RB-deficient embryos. Dev Biol. 1999a;207:62–75. doi: 10.1006/dbio.1998.9141. [DOI] [PubMed] [Google Scholar]
- Yuan W, Zhou L, Chen JH, Wu JY, Rao Y, Ornitz DM. The mouse SLIT family: secreted ligands for ROBO expressed in patterns that suggest a role in morphogenesis and axon guidance. Dev Biol. 1999b;21:290–306. doi: 10.1006/dbio.1999.9371. [DOI] [PubMed] [Google Scholar]
- Yuan W, Rao Y, Babiuk RP, Greer JJ, Wu JY, Ornitz DM. A genetic model for a central (septum transversum) congenital diaphragmatic hernia in mice lacking Slit3. Proc Natl Acad Sci USA. 2003;100:5217–5222. doi: 10.1073/pnas.0730709100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarubin T, Jing Q, New L, Han J. Identification of eight genes that are potentially involved in tamoxifen sensitivity in breast cancer cells. Cell Res. 2005;15:439–446. doi: 10.1038/sj.cr.7290312. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Li H, Zhou L, Wu JY, Rao Y. Cellular and molecular guidance of GABAergic neuronal migration from an extracortical origin to the neocortex. Neuron. 1999;23:473–485. doi: 10.1016/s0896-6273(00)80801-6. [DOI] [PubMed] [Google Scholar]