Abstract
Rat and chick studies show that the earliest motor rootlet axon bundles emerge from all levels of the neural tube between radial glial end feet which comprise the presumptive glia limitans. The loose arrangement of the end feet at the time of emergence facilitates this passage. The points of emergence are regularly spaced in relation to the long axis of the neural tube and are not defined by any cell contact with its surface. Each rootlet carries a covering of basal lamina from the neural tube surface, which forms a sleeve around it. It is only after bundles of ventral rootlet axons have emerged that cells associate with them, forming clusters on the rootlet surface at a distance peripheral to the CNS surface of both species. A tight collar of glial end feet develops around the axon bundle at the neural tube surface shortly after initial emergence. These arrangements are in sharp contrast to those seen in the sensory rootlets, where clusters of boundary cap cells prefigure the sensory entry zones at the attachments of the prospective dorsal spinal and cranial sensory rootlets. Boundary cap cells resemble cluster cells and a neural crest origin seems the most likely for them. The study clearly demonstrates that no features resembling boundary caps are found in relation to the developing motor exit points.
Keywords: axon bundle outgrowth, cell density, cell distribution, glia limitans, transitional zone
Introduction
Most cranial and spinal nerves are attached to the neuraxis by series of rootlets. For both motor and sensory rootlets, the Transitional Zone (TZ) (see Table 1 for explanation of abbreviations) is the interface between central and peripheral nervous tissues (Fraher, 1992, 2002; Golding et al. 1997). The sensory entry zones (SEZs) represent the TZs of the sensory rootlets at which the axon bundles grow into the developing neural tube. The SEZs of spinal nerves are termed dorsal rootlet entry zones (DREZs). Bundles of cranial and spinal motor axons emerge from the neuraxis through the modified glia limitans at motor exit points (MEPs) (Fraher, 1992; O'Brien et al. 1998, 2001). At the time of earliest rootlet outgrowth, the end feet of radial glia form the presumptive glia limitans (Elmquist et al. 1994; Lazzari et al. 1997; Lazzari & Franceschini, 2004). Previous studies have rigorously defined spinal MEP development and maturation from the stage following motor axon bundle emergence and ventral rootlet formation, up to the stage of CNS-PNS transitional node formation (Fraher, 1992; O'Brien et al. 1998, 2001). They highlighted the role of glial elements in axon segregation at the cord surface, TZ glial barrier formation, and development of the intramedullary bundles (IMBs) of motor axons, as they cross the developing ventral white matter. However, the earliest stages, namely initial motor axon bundle outgrowth and associated changes in the glia limitans, remain unclear.
Table 1.
Abbreviations
| BC | Boundary Cap |
| BM | Branchiomotor |
| DREZ | Dorsal root entry zone |
| E | Embryonic day |
| IMB | Intramedullary bundle |
| MEP | Motor exit point |
| SEZ | Sensory entry zone |
| SM | Somatomotor |
| TZ | CNS-PNS transitional zone |
Accordingly, this study investigates the earliest developmental stages of MEP and SEZ TZs at cranial and spinal neural tube levels, and compares these in rat and chick at corresponding stages and levels. The stages investigated include those preceding motor rootlet emergence, as well as dorsal rootlet axon bundle ingrowth into the CNS. Somatic motor (SM) and branchiomotor (BM) axon bundle outgrowth is studied. The former emerge from the neural tube ventrally or ventrolaterally as motor rootlets, except for those of the trochlear nerve which emerge dorsally. BM axon bundles emerge further laterally than SM bundles, through fewer, larger MEPs. For the cranial nerves, both motor and sensory components of the BM trigeminal, facial and vago-accessory TZs were examined, as were the purely SM oculomotor, trochlear, abducens and hypoglossal nerves. At spinal levels, MEP and SEZ development at cervical and sacral levels were studied.
Specific features examined included: the presumptive glia limitans before motor rootlet axon bundle emergence; the appearance of the earliest rootlets at emergence; the association of blood vessels with the surface of the neural tube at the presumptive MEP loci, as well as with the rootlets and their TZs.
The study examines in particular the degree and timing of association of cells with the rootlets and the tissues overlying the glia limitans, in relation to axon growth through the glia limitans. Associated cells include leptomeningeal precursors, as well as the cell clusters related to early motor and sensory rootlets (Fraher & Rossiter, 1990) and boundary cap (BC) cells (Niederländer & Lumsden, 1996; Golding & Cohen, 1997; Vermeren et al. 2003). Previous studies have shown that BC cells, derived from the neural crest, prefigure SEZ loci, before sensory axons have grown into the CNS (Golding & Cohen, 1997; Golding et al. 1997; Vermeren et al. 2003). These authors and others (Niederländer & Lumsden, 1996) suggest that BCs are also present at the presumptive loci of MEPs, before motor axons emerge from the cord in the chick and mouse. However, other studies (Fraher & Rossiter, 1983a) indicate that cell association with motor axon bundles takes place only after their exit from the CNS and then at some distance peripheral to the TZ (Fraher, 2002).
Materials and methods
Sprague Dawley rat and Gallus gallus chick embryos from E11 to E13 and E2 to E4 (developmentally corresponding stages), respectively, were used. Developmental ages were known accurately. The levels of the neuraxis chosen in both species included a number of developing cranial and spinal nerve attachment loci with (i) purely somatomotor and (ii) motor and sensory branchial components. They included the abducens, trochlear, oculomotor, trigeminal, vago-accessory, hypoglossal, cervical and sacral nerve levels of the rat neural tube, and the cervical, trigeminal and abducens nerve levels in the chick. A minimum of five embryos was used for all studies at each level and age in both species.
For the rat material, the dams were killed by lethal injection under deep anaesthesia and the embryos were immediately delivered thereafter by caesarean section directly into the following primary fixative: 2% glutaraldehyde, 2.5% paraformaldehyde and 0.05% glucose in 0.10 M phosphate buffer. For the chick, the embryos were dissected away from the vitelline membrane and placed in the following primary fixative: 2% glutaraldehyde, 2.5% paraformaldehyde and 0.05 M glucose in 0.165 M phosphate buffer. For both species exposure to primary fixative was for a total of 2 h. Specimens were then washed in buffer, postfixed with osmium tetroxide, dehydrated in graded alcohols and epoxypropane, and embedded in Araldite.
In both species, transverse, serial semithin (0.5 µm) sections of the neural tube were made at each level studied. The individual cranial nerves and their loci were identified from the unique transverse sectional appearance of the appropriate part of the brainstem. For each area of interest, serial sections extending over 80 to 100 µm were taken. Semi-thin sections were stained with toluidine blue and photographed on an Olympus Provis microscope with an Olympus DP50 digital camera (Olympus, UK) attached.
The ultrastructure of the MEPs and SEZs, both presumptive and developing, was examined at cervical levels of the rat neural tube from E11 to E13. Alternating sequences of thin (90 nm) and semi-thin (0.5 µm) sections were made transverse to the cord and also tangential to its surface. Thin sections were stained with uranyl acetate and lead citrate and examined in a JEOL 2000FX (Jeol, UK) electron microscope. The following features were examined both morphologically and morphometrically: the fine details of the CNS surface, the morphology of the earliest emergent rootlets, as well as of any associated cells. The morphometric study determined the characteristics of the various types of cell (leptomeningeal precursor, boundary cap and cluster cells) which are associated with the early TZs and rootlets. In both species, a central objective of the study was to define comprehensively, before and after rootlet emergence, the precise loci of presumptive TZs, their features and those of the associated tissues. These included the glia limitans and the overlying and underlying areas as well as the appearance, density and location of associated cells and rootlets. To assess the distribution of peripheral cells at the MEPs and SEZs, the numbers of cells per mm3 in defined volumes adjacent to the MEPs and SEZs were examined. These were determined as follows (Fig. 1):
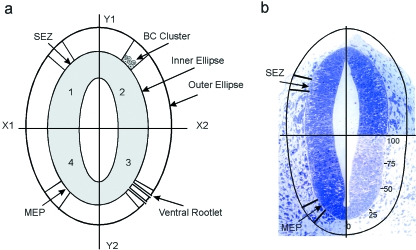
Fig. 1.
Method of determining cell density in the regions of the presumptive MEPs and SEZs. (For details see Text). (a) Diagrammatic representation of transversely sectioned neural tube (shaded), with a pale lumen and bounded by the presumptive glia limitans (inner ellipse). (b) Transverse section of a rat E11 neural tube prior to rootlet emergence. The outer ellipse is shown, 80 µm above the neural tube surface.
The relative positions of the MEPs and SEZs were first ascertained (Fig. 1a). To do this their loci were identified on the perimeter of the transversely sectioned neural tube (the inner ellipse), just after the first appearance of the motor and sensory rootlets, with the aid of a Camera Lucida (Olympus BX40 F). The neural tube profile was divided into quadrants (Fig. 1a). The Y-axis bisected it, while the X-axis divided it transversely into ventral and dorsal halves. The perimeter of the neural tube in each quadrant was divided into 100 units, beginning at the midline in each case (Fig. 1b). Using this scale the exact locations on the neural tube perimeter of the MEPs and the SEZs were determined, as appropriate, for each cranial and spinal nerve examined. A line was then drawn parallel to the neural tube surface, 80 µm superficial to it. This created an outer ellipse.
Radii were extended from the neural tube surface to the outer ellipse from the margins of the presumptive TZs (Fig. 1a). This yielded two areas on each side: one within which the ventral rootlets emerge from the CNS through the MEP and travel away from it, and another within which sensory rootlets approach and enter the CNS through the SEZ.
These measurements were then scaled appropriately and applied to earlier profiles of the developing neural tube which lacked rootlets, to reveal the exact loci of the presumptive MEPs and SEZs before ventral rootlet emergence, or dorsal rootlet entry have taken place (Fig. 1b). Defining the areas in this way enabled rigorous determination of cellular distributions and calculation of cell densities in the tissues immediately overlying the presumptive MEPs and SEZs.
Estimates were made of the numbers, numerical densities and volume fractions of cells in the defined areas. Cells were classified into two types, one consisting of those which were free within the developing leptomeningeal space, the other of those which contacted either rootlets (motor or sensory) or the glia limitans at the presumptive TZ loci on the CNS surface. The former were termed cluster cells, the latter boundary cap (BC) cells. The cytological features of BC and cluster cells were compared. Cell shape was estimated as a form factor. The Cavalieri Principle (Gundersen, 1977; Mayhew, 1992) was used to estimate cell and nuclear volumes.
Every feature measured or counted was initially calculated as a value for each specimen. Individual specimen values were pooled within groups. Means and standard errors were then calculated for each group. Some of the steps outlined above depend on the assumption that the cells in question have on average one nucleus. This was acceptable, since during the course of the studies no cell with more than one nucleus was encountered.
Findings
Transitional Zones
Light microscopy
MEP and SEZ development.
The locations of the presumptive TZs at the neural tube surface were determined (Fig. 1b). On average, at the cervical and sacral levels the MEP extended from the 20% to the 35% point of the perimeter of the ventral quadrant. The SEZ extended between the 18% and 31% points of the dorsal quadrant.
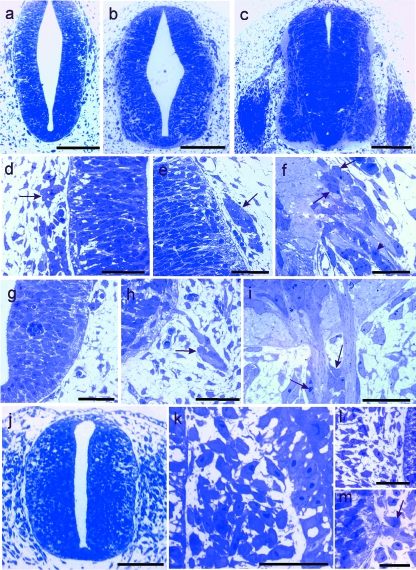
Figure 2shows the earliest stages of rat (a–i) and chick (j–m) spinal MEP and SEZ development, as well as cell relationships with the neural tube and rootlet surfaces before and after rootlet outgrowth. Cluster cells were related to both ventral and dorsal rootlets (Fig. 2f,h,i). BC cells were found only in relation to the presumptive SEZ neural tube surface.
Fig. 2.
(a–i) Rat spinal nerves. Light micrographs of transverse sections of rat neural tube at cervical level. (a–c): overviews; (d–i): enlargements of (a–c). (a, d, g) E11, showing the loci of presumptive SEZ (d) and MEP (g); note the presence of cell clusters overlying the SEZ (d, arrow) and the absence of such cells in (g); (b, e, h) E12, showing (e) cell clusters in relation to the presumptive SEZ; and (h) an early ventral rootlet, to the surface of which cells (arrow) are related at some distance into the periphery; (c, f, i) E13, showing (f) boundary cap cells (arrows) and an early cell cluster (arrowhead) associated with a dorsal rootlet, and (i) bare proximal segments of ventral rootlets, distal to which cell clusters (arrows) are forming. (j–m) Chick spinal nerves. (j–l) Light micrographs of transverse sections of chick cervical neural tube at E2; (j): overview; (k, l): enlargements of SEZ and MEP loci, respectively, in (j); (k) accumulations of neural crest-derived cells are present between the neural tube (left) and the somite (right) in the region of the presumptive SEZ; (l) no MEPs have yet developed; (m) At E3 ventral rootlets have developed and cells are becoming apposed to them at some distance into the periphery (arrow). Stain: toluidine blue. Scale bars, a–c: 200 µm; d–i: 50 µm; j: 200 µm; k–m: 25 µm.
At all ages and all levels, accumulations of neural crest-derived cells flanked the dorsal neural tube in rat (Fig. 2a,b,d,e) and chick (Fig. 2j,k). Some of these were associated with the presumptive SEZ glia limitans and soon after their first appearance, came to overlie it closely, prior to dorsal rootlet axon entry into the neural tube (Fig. 2d,e,k). However, presumptive BC cells frequently could not be distinguished from the mass of cells comprising the primordia of the related sensory ganglia, spinal or cranial (Fig. 2k). In the rat, at E13, BC cells were intimately related to the neural tube surface (Fig. 2f).
No cells were apposed to the loci of any MEPs before motoneuron axon outgrowth (Fig. 2a,g). The first cell-ventral rootlet contact took place well after rootlet outgrowth and was at some distance peripheral to the MEP (Fig. 2h,i,m). Accordingly, the proximal rootlet segment was bare. Soon after the first contact, these cells began to form clusters (Fig. 2i).
Ventral rootlet axon bundles had emerged from the neural tube before dorsal rootlet axon bundles entered it. Cell clusters appeared at ventral rootlet surfaces at around the same time as BCs at presumptive SEZs. Cell clusters also formed in relation to both rat and chick dorsal rootlets, at E13 and E4, respectively (Fig. 2f). In both cases, this was subsequent to rootlet axon ingrowth. These clusters closely resembled those related to ventral rootlets (Fig. 2i).
Figure 3shows the earliest stages of development of the oculomotor (a–c) and trochlear (d,e) as well as the trigeminal (f–l) cranial nerves. As with the spinal nerves, no cells prefigured the loci of the presumptive motor roots of either kind, in either rat or chick. Nor were they evident in relation to the MEP of the dorsally emerging trochlear nerve (Fig. 3d,e). They were also completely absent from presumptive hypoglossal and abducens rootlets (not shown). In all cases, as with the spinal nerves, the proximal lengths of the rootlets were bare (Fig. 3b,c,g). In the developing trigeminal nerve, the clump of ganglioblasts related to the developing sensory root was prominent (Fig. 3h). At E4 in the chick, axon bundles ran centrally from it, to pierce the glia limitans at the SEZ (Fig. 3j,k). Close to this, on its medial side, the motor root was clearly evident and its MEP was distinct from the SEZ (Fig. 3h–j). The motor root joined the medial aspect of the ganglion primordium at some distance peripheral to the MEP (Fig. 3h,j). At E4 in the chick, cell clusters had developed in relation to the trigeminal motor rootlet and had come to lie close to the CNS surface (Fig. 3l).
Fig. 3.
(a–g) Light micrographs of transverse sections through rat brainstem. (a–c) At oculomotor level, rootlets are absent at E11 (a), present at E12 (b), but without cellular contact close to the neural tube (c); (d, e) at superior medullary velum level, no evidence of the trochlear nerve is present at E11 (d); by E13 (e) the axon bundle has grown out; it lacks cellular contact and is closely related to overlying blood vessels (asterisks). (f, g) Light micrographs of the developing rat brainstem at trigeminal nerve level; (f) At E11 neither an MEP nor an SEZ is present; (g) at E12 a motor rootlet (arrow) is present and lacks any cellular contact. (h–l) Light micrographs of chick brainstem at trigeminal nerve level. (h) At E3 both the motor root (arrow) and the sensory root, together with associated ganglioblasts (asterisk), are present and distinct from one another; (i) Enlargement of motor root in (h) showing the constituent fibres accumulating on the external aspect of the basal plate (arrows) and the absence of cellular contacts on the most proximal length of rootlet; (j–l) By E4 the SEZ (j, asterisk) is clearly distinct from the MEP (arrow); large numbers of cells are stacked between axon bundles immediately distal to the CNS-PNS interface (k), while at the MEP (l) cell clusters (arrows) have become closely associated with the axon bundles near the brainstem surface. Stain: toluidine blue. Scale bars, a: 200 µm; b, d–g: 50 µm; c, l: 20 µm; h, j: 100 µm; i, k: 25 µm.
The length of the bare motor rootlet from the CNS surface to the first peripheral cell association decreased by more than 50% in both cervical ventral rootlets and in the trigeminal root. The initial length tended to be less in the chick than in the rat. The extent of the MEP was small relative to that of the SEZ. In the trigeminal nerve, it extended between the 40% and 45% points (see Fig. 1) of the perimeter of the ventral neural tube quadrant. The SEZ lay close to it, extending between the 55% and 70% points in the same quadrant. Also, the present study indicates that at all levels the tissues adjacent to the presumptive MEP were substantially more vascularised than those at the SEZ.
Morphometry
At all levels and in both species the differences noted in cell association with the loci of motor and sensory rootlet attachments were reflected in the quantitative measurements (Tables 2, 3) at equivalent stages in rat and chick.
Table 2.
Mean numbers of peripheral cells (1000s/mm3) overlying TZs
(a) Rat
| Cervical | Sacral | Trigeminal | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Hypoglossal | Oculomotor | Trochlear | |||||||
| Rat | MEP | SEZ | MEP | SEZ | MEP | SEZ | MEP | MEP | MEP |
| E11 | 13 | 20 | – | – | 10 | 91 | 7 | 7 | |
| E12 | 62 | 94 | 31 | 90 | 20 | 117 | 9 | 18 | 9 |
| E13 | 74 | 151 | 66 | 100 | 24 | 10 | |||
| (b) Chick | |||||||||
| Cervical | Trigeminal | ||||||||
| Trochlear | |||||||||
| Chick | MEP | SEZ | MEP | SEZ | MEP | ||||
| E2 | 50 | 78 | |||||||
| E3 | 77 | 274 | 37 | 421 | 47 | ||||
| E4 | 97 | 387 | 279 | 416 | 147 | ||||
Table 3.
Proportion of all cells in areas associated with MEPs and SEZs which make contact with rootlet tissue
(a) Rat
| Cervical | Sacral | Trigeminal | |||||||
|---|---|---|---|---|---|---|---|---|---|
| XII | III | IV | |||||||
| Rat | MEP | SEZ | MEP | SEZ | MEP | SEZ | MEP | MEP | MEP |
| E11 | 0 | 0.67 | 0 | 0.02 | 0 | ||||
| E12 | 0.20 | 0.82 | 0.05 | 0.68 | 0.12 | 0.87 | 0 | 0.10 | 0 |
| E13 | 0.59 | 0.74 | 0.30 | 0.81 | 0.31 | 0.94 | 0.11 | 0.19 | |
| (b) Chick | |||||||||
| Cervical | Trigeminal | Trochlear | |||||||
| Chick | MEP | SEZ | MEP | SEZ | MEP | ||||
| E2 | 0 | 0.56 | 0 | 0.78 | |||||
| E3 | 0 | 0.68 | 0.08 | 0.95 | 0 | ||||
| E4 | 0.18 | 0.96 | 0.58 | 0.99 | 0.33 | ||||
All SEMs were 0.04 or less.
No cells were noted in apposition to the glia limitans at any presumptive MEP, prior to axon bundle outgrowth. The extent to which cells were associated with the rat MEPs and SEZs, presumptive and developing, was estimated as cell numbers per mm3 in the defined tissue space overlying the TZ (Fig. 1). A consistent pattern was found in the rat (Table 2a). In all nerves with sensory and motor roots, cell density in the tissue space overlying the former significantly (P < 0.05) exceeded that related to the latter. The extent of the difference varied: In spinal nerves it ranged from around 50% to 300%. In the trigeminal nerve it was more marked: at E11 it was nine-fold, at E12 six-fold. In those purely motor cranial nerves studied the cell densities were consistently lower than those for the trigeminal or the spinal nerves studied at corresponding ages. Findings in the chick were broadly similar (Table 2b): cell densities were greater at spinal SEZs than at MEPs, by factors of around 50% on first appearance and thereafter of around 400%. A similar disparity was present in relation to the trigeminal nerve: the densities at the SEZ were greater, by a factor of around 12 at E3 and by 50% at E4. In all cases of rat and chick, spinal and cranial, cell densities overlying presumptive SEZs were greater than those overlying presumptive MEPs.
The proportions of cells lying within the defined tissue space, which contacted the motor rootlets increased over time (Table 3). The proportion of all cells which contacted the cervical and cranial motor rootlets in both species showed a clear tendency to increase with development (Table 3). It was in all cases higher at SEZs than at MEPs at corresponding ages. The proportions of cells lacking any contact decreased reciprocally, which is consistent with cells in the latter category becoming associated with the rootlet and forming cluster cell precursors. Cells made contact with SEZs and dorsal rootlets from their first appearance. At E13, the category included both BC and cluster cells.
Ultrastructure
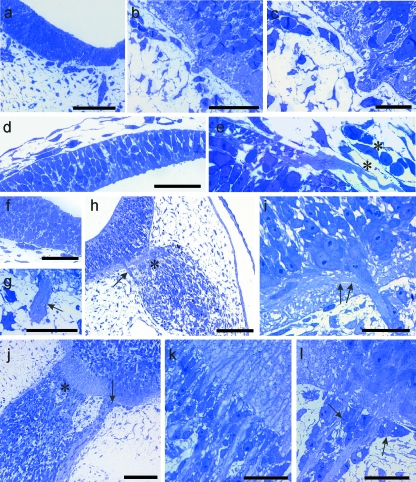
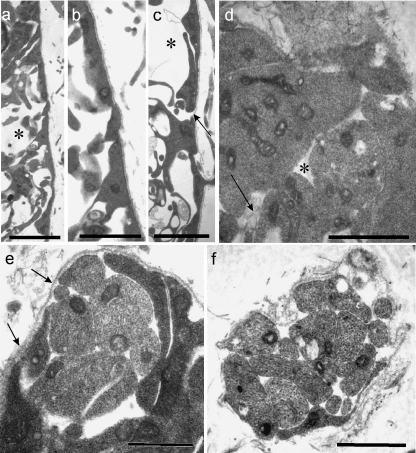
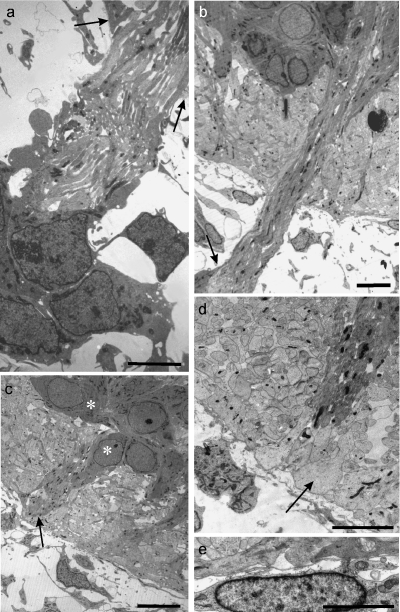
At E11 and E12 the radial glial end feet of rat cervical MEPs varied considerably in appearance (Fig. 4a–c). Some were attenuated, others were thicker, commonly with a triangular profile. Prior to rootlet outgrowth, they formed an incomplete covering over all parts of the neural tube, with numerous gaps between them (Fig. 4c,d). By contrast, the basal lamina of the neural tube surface was well defined and complete at E11 (Fig. 4c). Intercellular spaces were prominent in the ventral white matter at E12, enabling passage for axons, including those of ventral motoneurons (Fig. 4a,c). Flocculent material was prominent within these spaces, especially at E11 (Fig. 4d). The earliest ventral rootlets appeared at E12 and consisted of bundles of a few axons, most of which were naked (Fig. 4e,f). At E12, at the earliest stage of rootlet outgrowth, the basal lamina was continued as a sleeve around the axon bundle (Fig. 4f) as far distally as the developing pia mater, where it was open-ended. By E13 the basal lamina had extended further distally and covered the presumptive cluster cells apposed to the rootlet. Processes extended centrally from these cells along the rootlet bundle towards the cord surface (Fig. 5a,b). Soon after rootlet emergence, the glia limitans processes formed a well-defined, thick glial collar around the axon bundle at the level of the neural tube surface (Fig. 5c,d).
Fig. 4.
Electron micrographs of E11 rat neural tube at presumptive MEP loci. (a–c) Transverse sections of the neural tube show that the end-feet of glial processes comprise the presumptive glia limitans and form a tenuous or incomplete layer (c, arrow), which is covered by a continuous basal lamina (b, c); the underlying presumptive white matter contains prominent spaces (a, c asterisks), some of which contain flocculent material. (d) Tangential section through the glia limitans at E11; the glial end-feet contain prominent mitochondria; gaps (asterisk) are evident between them, containing in places folds of material resembling basal lamina (arrow). (e, f) Tangential sections parallel to the neural tube surface, showing: (e) a transversely sectioned early rootlet consisting of a few axons emerging through the glia limitans, surrounded by dark, loosely arranged glial end-feet, and also the continuity of the basal lamina covering the neural tube and rootlet (arrows); (f) a transversely sectioned early rootlet distal to the neural tube surface, consisting mostly of bare axons, with an extensive covering of basal lamina and an attenuated glial process apposed to part of its surface (below). Scale bars, a: 5 µm; b: 2.5 µm; c: 2 µm; d: 1.5 µm; e, f: 1 µm.
Fig. 5.
(a–d) Electron micrographs of transversely sectioned rat neural tubes at E13, showing features of established, developing ventral motoneuron axon bundles at central and peripheral levels. (a) A cell cluster (below) is prominent in relation to a motor rootlet, distal to its proximal bare length; cytoplasmic processes extend centrally from the cluster cells and cover the distal length of the rootlet (arrows: plane of neural tube surface). (b) An axon bundle emerges intact from within the basal plate, traverses the presumptive ventral white matter and passes through the glia limitans to become a ventral rootlet. A cluster cell (below, left) sends a process (arrow) centrally along the rootlet surface. (c, d) Developing motoneuron perikarya (c, asterisks) lie at the surface of the basal plate; glial processes form prominent collars (arrows) around the obliquely sectioned motoneuron axon bundle at the cord surface; the processes of leptomeningeal cells were commonly attenuated and lay parallel to the neural tube surface (d, bottom right). (e) The elongated perikaryon of a developing leptomeningeal cell (below) closely overlies the presumptive glia limitans (above). Scale bars, a, d: 5 µm; b: 7.5 µm; c: 10 µm; e: 2.5 µm.
Presumptive leptomeningeal cells.
Leptomeningeal precursor cells were commonly flattened and bipolar, with elongated nuclei and long axes orientated parallel to the glia limitans surface (Fig. 5b,e). They were evenly distributed around the lateral aspects of the perimeter of the neural tube, with no apparent specificity for the MEP or SEZ (Fig. 2b). They differed markedly in appearance from the more rounded and compact cluster and BC cells. Their slender processes (Fig. 5d), like their elongated perikarya (Fig. 5b,e), tended to lie approximately parallel to the underlying neural tube surface.
Intramedullary bundle formation.
Within the neural tube, at E12, the axons forming the earliest rootlets converged towards the presumptive MEP on the surface of the basal plate, deep to the matrix of glial processes forming the presumptive ventral white matter (Fig. 3i). From the focus of convergence the axons turned sharply to run towards the glia limitans, forming an intramedullary bundle (IMB), accompanied by radial glial processes from the earliest stages. Some of these processes continued out into the rootlets for a short distance.
By E13, the numbers of rootlet axons had considerably increased. The bundles emerged from deep within the developing ventral horn (Fig. 5b). The glial processes now formed a thick, tight collar around the MEP (Fig. 5c,d). The glial end feet had rounded profiles and granular, electron-lucent cytoplasm.
SEZ formation.
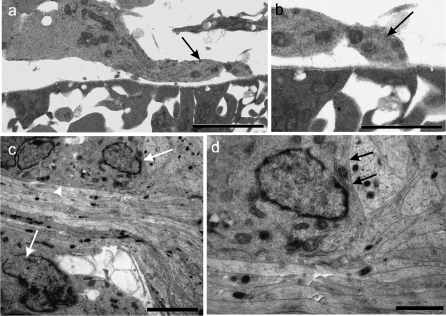
In the rat dorsal neural tube rootlets were not present at E12. Probable BC cell precursors were becoming associated with the, still complete, basal lamina at the presumptive SEZ (Fig. 6a,b). By E13 rounded or polygonal cells were present (Figs 2f, 6c). They were closely apposed to dorsal rootlet axons, to one another and to subjacent CNS tissues, without intervening basal lamina (Fig. 6c,d). More distally, the BC cells tended to be elongated parallel to the long axis of the rootlet (Fig. 2f). After dorsal rootlet formation, the naked axons streamed into the neural tube and diverged from one another, once through its surface (Fig. 6c). BC cell processes extended centrally in relation to the axon bundles, to approximately as far as the plane of the surrounding CNS surface (Fig. 6c).
Fig. 6.
(a–d) Electron micrographs of transversely sectioned neural tubes, showing the locus of the early developing rat SEZ at E12 (a, b) and E13 (c, d). (b) and (d) are enlargements of parts of (a) and (c), respectively. (a, b) At E12, a process from a possible presumptive BC cell overlies the basal lamina and approaches the presumptive SEZ basal lamina (b), which is continuous over the neural tube surface. (c, d) At E13, closely packed axons of dorsal rootlet bundles enter the neural tube and radiate deep to the glial surface; BC cells at the TZ (c, arrows) are apposed to dorsal rootlet axons (c, arrowhead), to the rootlet surface distal to the TZ and to CNS elements (d, arrows). Scale bars, a: 5 µm; b: 2 µm; c: 4.5 µm; d: 1.5 µm.
Ventral motoneuron development.
The neuroepithelial cells of the basal plate were largely darkly staining and included motor neuron precursors (Fig. 5c). At E12 these lay close to the early MEPs (Figs 2i, 3i, 5c). Their somata displayed large nuclei, many containing a prominent nucleolus. One large axonal process was evident, which traversed the ventral white matter and emerged from the neural tube as a component of the ventral rootlet. At E13, no dendrites were seen.
Cell form, size and distribution.
Measurement of cell shape showed the leptomeningeal precursor cells to have the most elongated profiles of all types examined (Table 4). This was evident in their low form factor values. BC cells at the SEZ were more circular than the cluster cells at the MEP. The most rounded types were the motor neuron precursor perikarya. The individual shape factors were discrete from one another and very few intermediate values were found, emphasising the early establishment of differences between the cell types.
Table 4.
Stereological data on cells related to neural tube surface and TZs
| Cell type | Volume (µm3 ± SEM) | Form factor (± SEM) |
|---|---|---|
| BC cell | 153.4 (2.6) | 0.50 (0.02) |
| Cluster cell | 157.7 (2.5) | 0.38 (0.02) |
| Leptomeningeal cell (dorsal)* | 48.8 (0.6) | 0.27 (0.01) |
| Leptomeningeal cell (ventral)* | 48.2 (1.25) | 0.27 (0.01) |
| Neuroepithelial cell (dorsal)* | 139.6 (2.1) | 0.61 (0.01) |
| Neuroepithelial cell (ventral)* | 313.1 (5.6) | 0.74 (0.02) |
The terms dorsal and ventral refer to the position of the cell dorsal or ventral to the horizontal plane dividing the neural tube into equal dorsal and ventral halves.
Leptomeningeal cells had the smallest cell volume of those examined; the motor neuron precursors were the largest. BC and cluster cells showed no significant differences from each other in volume (Table 4).
Discussion
Bundles of motor axons emerge from the neural tube independently
The study shows that the earliest motor rootlets emerge through the glia limitans as small bundles of naked axons; no cells or cell clusters are associated with the neural tube surface before or at the stage of initial axon outgrowth. The use of rigorous longitudinal and transverse serial sectioning demonstrates this unequivocally. This is true for the presumptive MEPs at all cranial and spinal levels of rat and chick studied, including oculomotor, trochlear, trigeminal, abducens and hypoglossal, as well as cervical and sacral. Electron microscopy shows that the emerging axon bundles are surrounded by a complete sheath of basal lamina and are accompanied for a short distance by a few slender glial processes. The presumptive MEPs contrast sharply with the SEZs, the loci of which are prefigured by prominent clumps of BC cells which are commonly continuous with the mass of sensory ganglion precursors. The absence of peripheral cells at the dorsally located presumptive trochlear nerve MEP further indicates that the association of cells with the presumptive TZ is related to axon type and not location.
The distinction of MEP from SEZ loci is clear in the case of spinal nerve roots, where these are widely separated. However, for some cranial nerves, such as the trigeminal and facial, where the two are close together, the difference may escape notice without close inspection and accurate location of the locus of the presumptive MEP (and of the SEZ). This may account for claims in some studies (Niederländer & Lumsden, 1996; Golding et al.1997; Vermeren et al. 2003) that BC cells are present at presumptive MEPs and that motor axon bundles emerge from the neural tube even after neural crest (the most likely source of cluster cells) ablation. From ablation studies, it has been contended that neither neural crest nor BC cells are required for the guidance of motor axons to exit points within the ventral spinal cord, or for their growth into the periphery (Vermeren et al. 2003). The present study shows that this is true under normal developmental circumstances and so does not need recourse to ablation studies to demonstrate it. It is readily demonstrable when the position, form and cellular associations of presumptive MEPs are assessed rigorously using simple morphometry. The findings strongly indicate that it is the neural tube that plays the key role in motor axon bundle outgrowth, supporting the conjecture of Niederländer & Lumsden (1996).
This study differs in approach from previous ones (Niederländer & Lumsden, 1996; Vermeren et al. 2003) in several respects. It rigorously identifies the positions of the SEZ and MEP (before and after rootlet outgrowth) as specific loci on the perimeter of the neural tube surface, and also defines an area of tissue overlying each TZ locus. This enables examination of the glia limitans, the underlying neural tube tissue and elements in the developing leptomeninges overlying the precise locations of the presumptive MEPs and SEZs, before and during earliest rootlet formation. The use of extensive series of serial semithin and ultrathin sections enabled individual rootlets and their relationships, as well as associated cells, to be observed with precision, and at all necessary levels of magnification. It demonstrates unequivocally that the mode of ventral rootlet axon outgrowth is radically different from that of dorsal/sensory rootlet formation. Furthermore, the fact that leptomeningeal precursor cells, and any other cell types that might be intermingled with these, were evenly distributed around the CNS surface with no apparent specificity to the MEP and SEZ, rules out the possibility that any type of cell is preferentially located close to the locus of the MEP before axon outgrowth.
Cluster cells and BC cells
Cluster cell precursors become associated with motor rootlets only after their axons have emerged from the neural tube, and at some distance peripheral to the MEP. They develop their characteristic cluster-forming morphology only after they have become associated with the rootlets. By contrast, BC cells become intimately associated with the glia limitans at about the same time, but before axon bundles traverse it. The relationships of cluster cells and BC cells to the glia limitans are in this respect opposites of one another.
However, cluster cells and BC cells have many features in common. They resemble one another in appearance, as do the clusters formed by them. Both are intimately related to axon bundles traversing the CNS-PNS interface, but at different stages of axon growth and at slightly but crucially different levels along the axon bundles. Some at least of both types segregate axons and appear to develop into Schwann cells (Carlstedt, 1981; Fraher & Rossiter, 1983a,b; Fraher, unpublished observations). However, BC cells have the additional, qualitatively different functions of influencing axon growth through the neural tube surface, and of preventing motoneuron perikarya from emigrating out of the neural tube by translation along their own axons (Vermeren et al. 2003). The two cell classes share a number of other features. Studies using BC cell markers to examine their migration pattern, origin and location have noted these at both the MEPs and SEZs (Wilkinson et al. 1989; Ernfors et al. 1992; Ghislain et al. 2002; Vitalis et al. 2003; Knabe et al. 2005; Okafuji & Tanaka, 2005). It is important to note that in all of these studies both sensory and motor rootlets were already present. Nevertheless, the fact that cells at both the SEZ (BC cells) and the MEP (cluster cells) share the same immunological markers, indicates that the two cell types are closely related or are at least from the same lineage (i.e. the neural crest). Such markers include Krox 20 (Wilkinson et al. 1989), LINGO-1 (Okafuji & Tanaka, 2005), erythropoietin receptor (Knabe et al. 2005), monoamine oxidase B (Vitalis et al. 2003) and neurocan.
Motor rootlet cluster cells could be of neural crest origin, and may arrive at the rootlets using the ventral stream of neural crest migration. In the course of this, they may intermingle with leptomeningeal precursors (O'Rahilly & Müller, 1986). The nearby mass of neural crest cells, including sensory ganglioblasts, is well placed as a possible source of cluster cells for the motor roots, for example, in the trigeminal nerve. The fact that cluster cells segregate and subsequently myelinate the growing motor axon bundles (Fraher & Rossiter, 1983b) is further evidence in support of their neural crest origin. Their morphological similarity to BC cells, which are neural crest derivatives (Altman & Bayer, 1984; Vermeren et al. 2003) also favours this possibility. The neural tube itself has been implicated as a possible source of rootlet Schwann cells (Lunn et al. 1987). The extensive series of examples examined in the present study provided no evidence for this possibility.
Basal lamina and glia limitans
Basal lamina is impenetrable to neural crest cells (Martins-Green & Erickson, 1987). At first, it forms a complete covering over the early neural tube. This is subsequently maintained, except at specific loci where the dorsal rootlets enter (DREZs) and the motor rootlets emerge from it (MEPs). Basal lamina is absent when axons traverse the DREZ and also between the adjacent CNS surface and the associated BC cells (Golding & Cohen, 1997). The basal lamina also comes to be interrupted at the MEPs, but in the absence of any cluster cells or any similar cellular elements in its vicinity. The presence of gaps between the glial end-feet facilitates axonal emergence into the periphery. This appears to be a general feature, not specific to the MEP; the glia limitans is also incomplete in developing rat forebrain up to E16 (Balslev et al. 1997). The earliest rootlets formed consist of only a few axons. The relationship of basal lamina to the rootlet axon bundles accords with the possibility that the latter actively grow out of the neural tube through the gaps between the glial end-feet, and as they do so carry evaginations of the basal lamina over them. They seem to break through the basal lamina at the level of the pial surface. Whether the leading ends (growth cones) of the emergent axons express properties to degrade it remains unknown. Distal to this, they are soon ensheathed by processes growing centrally along the rootlet surface from presumptive cluster cells apposed to the rootlet more distally.
The BC cells at the DREZ could possess the ability to degrade the surface basal lamina. Valinsky & Le Douarin (1985) showed that neural crest cells in the cephalic region of the avian embryo produce high levels of proteolytic enzymes that can actively digest basal lamina. These include serine proteases and particularly plasminogen activator. It may be that BC cells maintain this capability and degrade the basal lamina overlying the incipient DREZ upon contact. The findings of the present study are consistent with this possibility (see Fig. 6): basal lamina is present initially at the presumptive DREZ locus; it is contacted by a developing BC cell precursor and comes to be absent shortly afterwards, when BC cells are apposed to those of the neural tube.
Ventral rootlet axon bundle formation
At the earliest stage of ventral rootlet formation, motoneuron axons converge along the interface between the basal plate and the presumptive ventral white matter towards one site subjacent to the presumptive MEP. From here, the bundle so formed turns sharply ventrally, to become an IMB, traverses the short distance to the glia limitans, and emerges through it as a ventral rootlet. A few axons are seen crossing the deeper part of the white matter obliquely to join the IMBs along their courses (at E13). Thus, the focus for axon convergence to form IMBs is predominantly at the surface of the developing basal plate, rather than at the deep surface of the glia limitans at the presumptive MEP.
Overall, it is clear that motor axon trajectories show different patterns at spinal and cranial levels. The mechanisms involved in the establishment of these paths remain unclear, but several molecules (Sema 3a, Netrin-1), genes (homeobox genes) and cells (BC cells) have been implicated. This study shows that cells acting at the neural tube surface are unlikely to be involved. The specific point of ventral root emergence may be established by cues expressed by somatic mesoderm, although Tosney & Landmesser (1985) showed that chick motor axons emerged from the spinal cord in the absence of somite tissue. The characteristic segmental pattern of ventral root emergence is influenced by the repellent properties of the caudal sclerotome (Oakley & Tosney, 1993), exerted through molecules such as ephrin and semaphorin (Eickholt et al. 1999; Raper, 2000). Nevertheless, removal of such molecules does not disrupt segmental motor axon outgrowth. It is clear that the emergence of motor rootlets through specific loci (MEPs) of the neural tube requires a highly orchestrated sequence of events, many of which remain to be elucidated.
Shortly after emergence, the circumscribed axon bundles have become much larger. In the neural tube, they emerge from within the mass of developing ventral motoneuron perikarya. Their deepest parts appear to have been overgrown by the mass of developing basal plate cells.
At all levels in both species the length of motor rootlet lacking any cell contact decreased significantly with age. This decrease may result from the migration of cluster cells towards the CNS surface or through the expansion of the neural tube engulfing the proximal segments of the motor rootlet axon bundles. The bare rootlet segments were longer in purely motor nerves than in nerves which have both motor and sensory rootlets in relatively close proximity, such as the rat trigeminal. The shorter bare length in the latter may be due to the presence nearby of abundant potential sources of cluster cells, namely the masses of neural crest-derived cells in the loci of the associated sensory ganglion, which are well positioned to migrate the short distance to the ventral rootlets after these have emerged.
MEP-associated blood vessels
Recently, an intimate relationship between the development of blood vessels and nerves has been identified (Carmeliet, 2003). The present findings suggest that at all levels the tissues adjacent to the presumptive MEP, and into which the emerging motoneuron axon bundles grow, are well vascularised. This is true also for the area overlying the dorsally emerging trochlear nerve. Whether or not the presence of the vessels has an influence on axon outgrowth remains unclear. Nevertheless, ventral rootlets and blood vessels are associated in another way: at the time of initial ventral rootlet outgrowth, the neural tube is itself avascular. No blood vessels were found growing into or out of it with the developing rootlets at the MEP or the SEZ at E12. These blood vessels appear at later stages of MEP TZ development (Kaar & Fraher, 1987). Therefore, at the MEP the growing motor axon bundles may serve as guides for growth of blood vessels into the neural tube. The opposite is not the case.
Concluding remarks
To investigate the earliest stages of motor and sensory CNS-PNS transitional zone formation, it is necessary first to determine precisely their presumptive loci. When this is done, it is evident that the two TZs begin development in very different ways. Motor axon bundles grow out of the neural tube as naked bundles; the loci of outgrowth are not prefigured by any cells, nor are any cells in close attendance on the outgrowing axon bundles. Cells become associated with these only after the motor rootlets have been formed, and then at a distance peripheral to the neural tube surface. By contrast, BC cells are associated with the neural tube surface at the presumptive sensory root entry zones before axons have arrived there from the sensory ganglia. The cells which associate with the motor rootlets form clusters and come to bear a close resemblance to the BC cells. It is likely that the neural tube itself plays a major part in determining the behaviour of motor axons as these accumulate into bundles and grow towards and through the developing glia limitans to form the peripheral nerve rootlets.
Acknowledgments
The work reported here was funded through the Higher Education Authority PRTLI3 programme.
References
- Altman J, Bayer SA. The Development of the Rat Spinal Cord. Berlin: Springer Verlag; 1984. [DOI] [PubMed] [Google Scholar]
- Balslev Y, Saunders NR, Møllgård K. Ontogenetic development of diffusional restriction to protein at the pial surface of the rat brain: an electron microscopical study. J Neurocytol. 1997;26:133–148. doi: 10.1023/a:1018527928760. [DOI] [PubMed] [Google Scholar]
- Carlstedt T. An electron-microscopical study of the developing transitional region in feline S1 dorsal rootlets. J Neurol Sci. pp. 357–372. [DOI] [PubMed]
- Carmeliet P. Blood vessels and nerves: common signals, pathways and diseases. Nature Rev Genetic. 2003;4:710–720. doi: 10.1038/nrg1158. [DOI] [PubMed] [Google Scholar]
- Eickholt BJ, Mackenzie SL, Graham A, Walsh FS, Doherty P. Evidence for collapsin-1 functioning in the control of neural crest migration in both trunk and hindbrain regions. Development. 1999;126:2181–2189. doi: 10.1242/dev.126.10.2181. [DOI] [PubMed] [Google Scholar]
- Elmquist JK, Swanson JJ, Sakaguchi DS, Ross LR, Jacobson CD. Developmental distribution of GFAP and vimentin in the Brazilian opossum brain. J Comp Neurol. 1994;344:283–296. doi: 10.1002/cne.903440209. [DOI] [PubMed] [Google Scholar]
- Ernfors P, Merlio JP, Persson H. Cells expressing mRNA for neurotrophins and their receptors during embryonic rat development. Eur J Neurosci. 1992;4:1140–1158. doi: 10.1111/j.1460-9568.1992.tb00141.x. [DOI] [PubMed] [Google Scholar]
- Fraher JP. The CNS-PNS transitional zone in the rat. Morphometric studies at cranial and spinal levels. Prog Neurobiol. 1992;38:261–316. doi: 10.1016/0301-0082(92)90022-7. [DOI] [PubMed] [Google Scholar]
- Fraher JP. Axons and glial interfaces: ultrastructural studies. J Anat. 2002;200:415–430. doi: 10.1046/j.1469-7580.2002.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraher JP, Rossiter JP. Cell clusters on developing rat ventral roots. Prenatal development. J Anat. 1983a;136:111–128. [PMC free article] [PubMed] [Google Scholar]
- Fraher JP, Rossiter JP. Cell clusters on rat ventral roots. II. Postnatal development. J Anat. 1983b;137:555–571. [PMC free article] [PubMed] [Google Scholar]
- Fraher JP, Rossiter JP. Intermingling of central and peripheral nervous tissues in rat dorsolateral vagal rootlet transitional zones. J Neurocytol. 1990;19:385–407. doi: 10.1007/BF01188406. [DOI] [PubMed] [Google Scholar]
- Ghislain J, Desmarquet-Trin-Dinh C, Jaegle M, Meijer D, Charnay P, Frain M. Characterisation of cis-acting sequences reveals a biphasic, axon-dependent regulation of Krox20 during Schwann cell development. Development. 2002;129:155–166. doi: 10.1242/dev.129.1.155. [DOI] [PubMed] [Google Scholar]
- Golding J, Shewan D, Cohen J. Maturation of the mammalian dorsal root entry zone-from entry to no entry. Trends Neurosci. 1997;20:303–308. doi: 10.1016/s0166-2236(96)01044-2. [DOI] [PubMed] [Google Scholar]
- Golding JP, Cohen J. Border controls at the mammalian spinal cord: late-surviving neural crest boundary cap cells at dorsal root entry sites may regulate sensory afferent ingrowth and entry zone morphogenesis. Molec Cell Neurosci. 1997;9:381–396. doi: 10.1006/mcne.1997.0647. [DOI] [PubMed] [Google Scholar]
- Gundersen HJG. Notes on the estimation of the numerical density of arbitary profiles: the edge effect. J Microscopy. 1977;111:219–223. [Google Scholar]
- Kaar GF, Fraher JP. The vascularisation of the central-peripheral transitional zone of rat lumbar ventral rootlets: a morphological and morphometric study. J Anat. 1987;150:145–154. [PMC free article] [PubMed] [Google Scholar]
- Knabe W, Siren AL, Ehrenreich H, Kuhn HJ. Expression patterns of erythropoietin and its receptor in the developing spinal cord and dorsal root ganglia. Anat Embryol. 2005;210:209–219. doi: 10.1007/s00429-005-0019-3. [DOI] [PubMed] [Google Scholar]
- Lazzari M, Franceschini V. Glial fibrillary acidic protein and vimentin immunoreactivity of astroglial cells in the central nervous system of the African lungfish, Protopterus annectens (Dipnoi: Lepidosirenidae) J Morphol. 2004;262:741–749. doi: 10.1002/jmor.10274. [DOI] [PubMed] [Google Scholar]
- Lazzari M, Franceschini V, Ciani F. Glial fibrillary acidic protein and vimentin in radial glia of Ambystoma mexicanum and Triturus carnifex: an immunocytochemical study. J Brain Res. 1997;38:187–194. [PubMed] [Google Scholar]
- Lunn ER, Scourfield J, Keynes RJ, Stern CD. The neural tube origin of ventral root sheath cells in the chick embryo. Development. 1987;101:247–254. doi: 10.1242/dev.101.2.247. [DOI] [PubMed] [Google Scholar]
- Martins-Green M, Erickson CA. Basal lamina is not a barrier to neural crest cell emigration: documentation by TEM and by immunofluorescent and immunogold labelling. Development. 1987;101:517–533. doi: 10.1242/dev.101.3.517. [DOI] [PubMed] [Google Scholar]
- Mayhew TM. A review of recent advances in stereology for quantifying neural structure. J Neurocytol. 1992;21:313–328. doi: 10.1007/BF01191700. [DOI] [PubMed] [Google Scholar]
- Niederländer C, Lumsden A. Late emigrating neural crest cells migrate specifically to the exit points of cranial branchiomotor nerves. Development. 1996;122:2367–2374. doi: 10.1242/dev.122.8.2367. [DOI] [PubMed] [Google Scholar]
- O'Brien D, Dockery P, McDermott K, Fraher JP. The ventral motoneuron axon bundle in the CNS – a cordone system? J Neurocytol. 1998;27:247–258. doi: 10.1023/a:1006932931160. [DOI] [PubMed] [Google Scholar]
- O'Brien D, Dockery P, McDermott K, Fraher JP. Early development of rat ventral root transitional zone: an immunohistochemical and morphometric study. J Neurocytol. 2001;30:11–20. doi: 10.1023/a:1011961106703. [DOI] [PubMed] [Google Scholar]
- O'Rahilly R, Müller F. The meninges in human development. J Neuropathol Exper Neurol. 1986;45:588–608. [PubMed] [Google Scholar]
- Oakley RA, Tosney KW. Contact-mediated mechanisms of motor axon segmentation. J Neurosci. 1993;13:3773–3792. doi: 10.1523/JNEUROSCI.13-09-03773.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okafuji T, Tanaka H. Expression pattern of LINGO-1 in the developing nervous system of the chick embryo. Gene Expr Patterns. 2005;6:57–62. doi: 10.1016/j.modgep.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Raper JA. Semaphorins and their receptors in vertebrates and invertebrates. Curr Opin Neurobiol. 2000;10:88–94. doi: 10.1016/s0959-4388(99)00057-4. [DOI] [PubMed] [Google Scholar]
- Tosney KW, Landmesser LT. Development of the major pathways for neurite outgrowth in the chick hindlimb. Devel Biol. 1985;109:193–214. doi: 10.1016/0012-1606(85)90360-4. [DOI] [PubMed] [Google Scholar]
- Valinsky JE, Le Dourain NM. Production of plasminogen activator by migrating cephalic neural crest cells. EMBO J. 1985;4:1403–1046. doi: 10.1002/j.1460-2075.1985.tb03793.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vermeren M, Maro GS, Bron R, et al. Integrity of developing spinal motor columns is regulated by neural crest derivatives at motor exit points. Neuron. 2003;37:403–415. doi: 10.1016/s0896-6273(02)01188-1. [DOI] [PubMed] [Google Scholar]
- Vitalis T, Alvarez C, Chen K, Shih JC, Gaspar P, Cases O. Developmental expression pattern of monoamine oxidases in sensory organs and neural crest derivatives. J Comp Neurol. 2003;464:392–403. doi: 10.1002/cne.10804. [DOI] [PubMed] [Google Scholar]
- Wilkinson DG, Bhatt S, Chavrier P, Bravo R, Charnay P. Segment-specific expression of a zinc-finger gene in the developing nervous system of the mouse. Nature. 1989;337:461–464. doi: 10.1038/337461a0. [DOI] [PubMed] [Google Scholar]