Abstract
Lewy-type pathology is a characteristic of a number of neurodegenerative disorders, including Parkinson's disease and dementia with Lewy bodies. Thus far, the definitive diagnosis of these dementias can only be confirmed at post-mortem. However, it is known that the loss of smell (anosmia) is an early symptom in patients who develop dementia, and the use of the smell test has been proposed as an early diagnostic procedure. The aim of this study was to understand further the extent of Lewy pathology in the olfactory system of patients with neurodegenerative disorders. Post-mortem tissue from 250 subjects was obtained from the OPTIMA brain bank. Five areas of the olfactory pathway were examined by immunolabelling for alpha-synuclein – a major component of Lewy pathology: the olfactory tract/bulb (n = 79), the anterior olfactory nucleus in the lateral olfactory gyrus (n = 193), the region of olfactory projection to the orbito-frontal cortex (n = 225), the hippocampus (n = 236) and the amygdala (n = 201). Results show that Lewy pathology affects different parts of the olfactory pathways differentially, suggesting a specific pattern of development of pathology. Clinical Parkinson's disease is most likely to be identified if the orbito-frontal cortex is affected, while the diagnosis is less likely if the pathology is restricted to the olfactory bulb or tract. These results suggest that pathology in the olfactory bulb and tract occurs prior to clinical signs of Parkinson's disease. Furthermore, the results presented here provide further evidence supporting the possible value of a smell test to aid the clinical diagnosis of neurodegenerative diseases.
Keywords: alpha-synuclein, Alzheimer's disease, dementia with Lewy dodies, Lewy pathology, Parkinson's disease
Introduction
Lewy pathology is a feature of many neurodegenerative diseases including Parkinson's disease (PD) and dementia with Lewy bodies (DLB). The classical Lewy body was first described by Friedrich Heinrich Lewy in 1912 as a cytoplasmic inclusion in PD patients (Lewy, 1912; Perry et al. 1996). In post-mortem tissue the identification of Lewy bodies is easiest using immunolabelling for alpha-synuclein. As yet, the normal function of alpha-synuclein has still to be elucidated. However, it is known that alpha-synuclein is abnormally deposited in the brains of those suffering from neurodegenerative diseases and is the main constituent of the Lewy bodies (Spillantini et al. 1997).
More recently, Lewy pathology has been shown to be more widespread than previously thought and estimates place DLB as the third most common form of dementia in the elderly after Alzheimer's and cerebrovascular diseases (Stevens et al. 2002; Rahkonen et al. 2003). In addition to Lewy bodies, alpha-synuclein immunolabelling also detects Lewy neurites, representing deposition of alpha-synuclein in neurites (Arima et al. 1998; Braak & Braak, 1999; Crowther et al. 2000). Collectively, neurodegenerative disorders that present with neuronal alpha-synuclein inclusions are termed synucleinopathies (or synucleopathies). Therefore, further to PD and DLB, synucleopathies are also considered to encompass amyotrophic lateral sclerosis (ALS) (Mezey et al. 1998) and multiple system atrophy (Arima et al. 1998; Tu et al. 1998; Wakabayashi et al. 1998; Gai et al. 2000; Fujiwara et al. 2002).
Lewy pathology does not affect the entire brain uniformly. It has been shown that the development of Lewy pathology follows a well-defined spatio-temporal pattern, allowing the development of a six-point staging system (Braak et al. 2003, 2004; Del Tredici et al. 2002), similar to the neurofibrillary tangle staging for Alzheimer's disease (AD). Such pathological staging in AD led to a relatively accurate prediction of the length of time required for the development of pathology characteristic to each phase of the disease and thus can be used to estimate the time of onset of pathology (Ohm et al. 1995).
The olfactory system can be split into two olfactory pathways, termed here the cortical and limbic pathways. The cortical pathway consists of fibres from the olfactory tract which terminate in the primary olfactory cortex, situated inferior to the nucleus accumbens in the lateral olfactory gyrus (also known as the pyriform cortex when taken together with the uncus). From the primary olfactory cortex, neurons project to the orbitofrontal cortex (OF). Although the pathways through which olfactory projections pass to the frontal cortex are not fully elucidated in humans, it is generally accepted that the majority of projections to the OF are direct projections from the primary olfactory cortex. However, some fibres do pass via the thalamus before reaching the OF (Price, 1990; Nolte, 1993).
The limbic olfactory pathway consists of the indirect bulbar input into the hippocampus and direct input to the nucleus of the lateral olfactory tract of the amygdala. In the rostral portion of the amygdala the nucleus of the lateral olfactory tract is situated ventral to the pyriform cortex and the anterior cortical nucleus, and dorsolateral to the periamygdaloid cortex. In caudal regions of the amygdala the olfactory nucleus is more lateral. Here the olfactory nucleus is a region ventral to the medial nucleus but dorsomedial to the magnocellular division of the accessory basal nucleus [see Purves et al. (2004) for olfactory pathways].
These regions are all affected by Lewy pathology. Indeed, pathology in the olfactory bulb is present before clinical symptoms occur or the pathology affects the substantia nigra (SN) (Del Tredici et al. 2002; Braak et al. 2003, 2004). However, Lewy pathology in olfactory structures is always associated with Lewy pathology in the brainstem (Del Tredici et al. 2002). This early appearance of Lewy pathology in the olfactory system has led to the suggestion that a simple smell test may aid the early diagnosis of neurodegenerative diseases. Previous work has shown that anosmia is more likely in subjects diagnosed with DLB as compared with either AD or control subjects (Daniel & Hawkes, 1992; Hawkes et al. 1999; McShane et al. 2001). Similarly, this association between anosmia and Lewy pathology has been confirmed by other studies (Doty et al. 1988, 1989; Olichney et al. 2005). Olichney and colleagues showed that although anosmia is sometimes present in AD, it is very common in DLB and may increase the sensitivity of diagnosis of diseases with Lewy pathology in patients with dementia.
Despite these reports, a systematic study of Lewy pathology in all major regions of the olfactory system has yet to be completed (Daniel & Hawkes, 1992; Tsuboi et al. 2003; Uchikado et al. 2006). Here we map the distribution of Lewy pathology in the olfactory bulb, the anterior olfactory nucleus near to the insertion of the olfactory tract into the primary olfactory cortex, the OF, the amygdala and the hippocampus in subjects with dementia.
Materials and methods
Tissue bank
All tissues were acquired from the Oxford-based OPTIMA brain bank. The collection of brain tissue was done according to the requirements of the local ethics committee, with full informed consent of participants and relatives. Brains were fixed in 4% formalin for at least 4 weeks. We collected olfactory regions of interest from 250 brains. Of the 250 subjects, post-mortem (PM) time was available for 207. Of these, the average PM time was 45.7 h (standard deviation from the mean: 26.3 h, standard error 1.8 h). Unfortunately, not all regions of the olfactory system were available in all subjects. However, where available, the olfactory bulb and tract were taken intact and sectioned in the horizontal plane. The hippocampus (at the level of the lateral geniculate body), amygdala, anterior olfactory nucleus and the orbito-frontal cortex were taken in the coronal plane. Within the amygdala analysis was restricted to the olfactory nucleus. We dissected the olfactory tract/bulb (n = 79), the anterior olfactory nucleus (AON) at the point of insertion of the olfactory tract into the lateral olfactory gyrus (n = 193), the region of olfactory projection to the orbito-frontal cortex (n = 225), the hippocampus (n = 236) and the amygdala (n = 201). Tissue blocks were embedded in paraffin and 10-µm sections were cut and mounted on silane-coated microscope slides (Sigma and VWR International Limited, respectively). The low number of olfactory bulbs/tract (n = 79) available to the study is due to the difficulty in removing and retaining this piece of tissue intact.
Immunohistochemistry
Sections were de-waxed in histoclear (Raymond Lamb, Eastbourne, UK) and re-hydrated through successive ethanol baths (100, 100, 70%) and water. The antibody used for the detection of alpha-synuclein recognizes an epitope hidden by the misfolding of the protein, and thus a pretreatment with formic acid (FA; Fisher Scientific, Loughborough, UK) for antigen retrieval (5 min, 90% FA) was required. The FA pretreatment was followed by a thorough wash in water. Sections were then incubated in 3% H2O2 for 10 min at room temperature (RT) to reduce endogenous peroxidase activity. Following further washes, sections were incubated in fetal calf serum (FCS, 1 : 20) in phosphate-buffered saline (PBS) + 0.1% Triton X-100 (PBST) (VWR International Limited, Dorset, UK) for 10 min at RT to reduce non-specific binding of antibodies. Tissues were then incubated in rabbit anti-alpha-synuclein primary antibody (1 : 200, Sigma, Dorset, UK) for 1 h at RT. Sections were then washed with PBST before incubation in a species-specific anti-IgG conjugated to biotin (Vector Laboratories, Cambridgeshire, UK) at 1 : 200 in 0.1 m PBST for 30 min at RT. Following further washes and incubation in an avidin–biotin peroxidase complex using the Vectastain© Elite ABC kit (Vector Laboratories), tissue was washed again before being reacted in 0.06% diaminobenzidine (DAB; Sigma) containing H2O2 (1.5 µL H2O2 per 5 mL DAB solution). To gain a better contrast, three drops of nickel solution (Vector DAB peroxidase substrate kit, Vector Laboratories) were added to the DAB/H2O2 solution to create a silver/black colour reaction. The colour reaction was terminated by washing the sections in PBST. For toluidine blue counter staining, slides were dipped in 1% toluidine blue (Sigma) for 30 s before dehydrating through alcohol and mounting in Histomount (Raymond Lamb). For Luxol fast blue counterstaining sections were taken to 95% ethanol then left in Luxol fast blue (0.1% solution in 95% ethanol; Sigma) overnight at 37 °C. Sections were then washed through repeated cycles of 5 min in 95% ethanol, 5 min in dH2O and 10 s in 0.005% lithium carbonate (Sigma) and 30 s in 70% ethanol until myelin stain was clear and crisp. Photomicrographs were taken using a Leica DME microscope and attached to a digital camera using XLI capture software (XL Imaging Ltd and Microscopeservices, UK). Sections of all cases were studied blind to their disease category. The PM time did not affect the intensity of immunolabelling.
Examination of Lewy pathology and statistical analysis
We analysed all sections to determine the presence or absence of Lewy bodies and/or Lewy neurites. The presence of one or more Lewy body or Lewy neurite was sufficient for a positive score. For the olfactory bulb/tract and the hippocampus, the entire region was examined. In the amygdala, analysis was restricted to the olfactory nucleus. In the anterior olfactory nucleus, the cortical areas immediate to the insertion of the olfactory tract were examined. In the OF examination was restricted to the specific cortex of the projection from the primary olfactory region.
To determine the power of association between Lewy pathology in different brain regions as well as the association between Lewy pathology and the diagnosis of PD we used odds-ratio (OR) calculations. Statistical significance was based on Pearson's chi-squared and P values.
Results
In total, tissue was available from 250 subjects. Table 1 gives a breakdown of the patient group by diagnosis made at PM examination. Samples used in this study are summarized in Table 2.
Table 1.
Distribution of pathological diagnosis of subjects within the study
| Diagnosis | Number in study |
|---|---|
| AD | 89 |
| AD/OD | 7 |
| AD/PD | 32 |
| AD/VD | 50 |
| PD | 6 |
| PD/VD | 4 |
| VD | 22 |
| CONTROL | 19 |
| Others | 21 |
| Total | 250 |
AD, Alzheimer's disease; OD, other diseases; PD, Parkinson's disease; VD, vascular dementia.
Table 2.
Numbers of subject samples available for each region, broken down into: total number, number with Lewy pathology present/absent, and percentage affected. Not all regions were available for all subjects
| Present | Absent | % Affected | ||
|---|---|---|---|---|
| Total subjects in study | 250 | 94 | 156 | 37.6 |
| Hippocampus | 236 | 52 | 284 | 22 |
| Amygdala | 201 | 64 | 137 | 31.8 |
| Anterior olfactory nucleus | 193 | 57 | 136 | 29.5 |
| Orbitofrontal | 225 | 31 | 194 | 13.7 |
| Olfactory bulb | 79 | 29 | 57 | 36.7 |
Lewy pathology can be present in all regions of the olfactory system
Alpha-synuclein immunolabelling allows the identification of both Lewy bodies (Fig. 1A) and Lewy neurites (Fig. 1B). The Lewy body is an intracellular cytoplasmic inclusion which typically presents as a spherical component within neuronal cell bodies. Lewy neurites are present in axons and, like Lewy bodies, consist of aberrant deposition of proteins, of which alpha-synuclein is a major component (Fig. 1B). Alpha-synuclein deposition in Lewy neurites can stretch for many hundreds of micrometres (Fig. 1B) and are sometimes present in regions even where Lewy bodies are absent. It is therefore a combination of these two types of pathology that allows the observer to define if a subject presents with Lewy pathology and provides a more accurate assessment of pathology. Furthermore, an interesting aspect of the Lewy pathology seen in many subjects is the appearance of Lewy neurites within Alzheimer-type plaques (Fig. 1C).
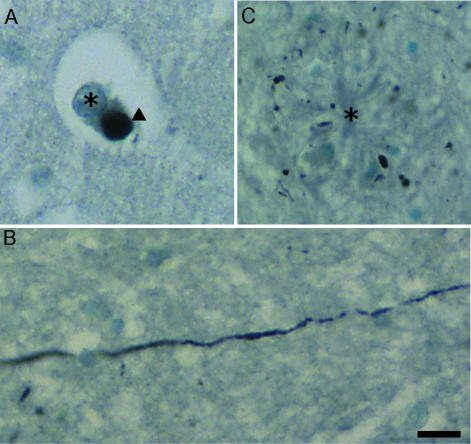
Fig. 1.
Lewy pathology. (A) High-magnification photomicrograph of a Lewy body (arrowhead) in a pyramidal neuron of the OF of a subject diagnosed with neocortical stage AD. (B) Lewy neurites in an amyloid plaque. Toluidine blue counterstaining allows the visualization of Alzheimer-type plaques (centre, labelled with asterisk), into which Lewy neurites have developed. The majority of Lewy neurites are observed in cross-section (transverse), appearing as dots rather than lines of axonal labelling. (C) A Lewy neurite in horizontal section. It is notable that Lewy neurites can extend for long distances. The neurite visible in C is from a region of the AON and is approximately 220 µm in length. Scale bar = 25 µm.
In the olfactory bulb and tract (OBT), Lewy bodies are present in the neurons of the anterior olfactory nucleus pars bulbaris (AONpb). Lewy neurites were observed throughout the olfactory tract and did not appear to be specific to any particular region or area. Within the AON, Lewy bodies were situated in the deep layers of the cortex (V, internal pyramidal; VI, multiform layer) and were interspersed with Lewy neurites. The OF was the least affected region of the olfactory system examined (only 13.3% of subjects exhibiting pathology). In those subjects where the OF was affected, Lewy bodies were present throughout the deep cortical layers, in neurons of the external pyramidal layer, internal granule layer and internal pyramidal layer. Lewy neurites were very rarely seen in the OF; however, when present, they were situated within the same neuronal layers. In the hippocampus the most susceptible region for the formation of Lewy bodies and Lewy neurites was the CAII. Here Lewy bodies were present in the pyramidal neurons and were interspersed with Lewy neurites. Additional to Lewy pathology the CAII region showed misshapen neuronal cell bodies heavily stained with toluidine blue, reminiscent of the ‘dark neurons’ (Liposits et al. 1997) identified as dying neurons. In the amygdala Lewy bodies were also present in individual neurons. Unlike other regions studied the olfactory area of the amygdala contained clusters of neurons in which both Lewy bodies and Lewy neurites were present.
Presence of Lewy pathology in patient groups
In total, 37.6% of all subjects included in the study showed evidence of Lewy pathology in at least one brain region (Table 2). Also, 36.7% of the OBT specimens had Lewy pathology, 29.5% of the AON specimens, 13.7% of the OF cortex specimens, 22% of the hippocampi (Hi) and 31.8% of the amygdalas (Am). Two of the control subjects (as diagnosed at PM) showed signs of Lewy pathology in the amygdala.
Lewy pathology progresses along the olfactory pathways
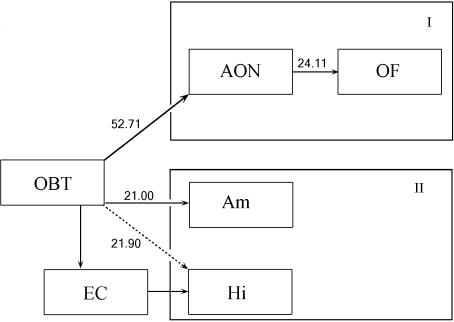
To assess the possible progression of Lewy pathology through the olfactory pathways we analysed the relative risk of each region along the pathway being affected if there was pathology in the OBT (Figs 2 and 3). In 72% of cases where Lewy pathology was present in the OBT, Lewy pathology was also present in the AON (OR: 52.71, Pearson's χ2 = 34.539, P = 0.0000; Fig. 3F). However, only 37% of patients with AON pathology showed pathology in the OF as well (OR: 24.11, Pearson's χ2 = 40.735, P = 0.0000; Fig. 3E). Similarly, there was a 54% chance that subjects with Lewy pathology in the OBT also had pathology in the amygdala (OR: 21, Pearson's χ2 = 19.433, P = 0.0000; Fig. 3G). This likelihood dropped to 45% for the hippocampus (OR: 21.90, Pearson's χ2 = 24.944, P = 0.0000).
Fig. 2.
Lewy pathology progresses along olfactory pathways. The odds of Lewy pathology in each region if the lower-order region in the pathway is affected. OBT = olfactory bulb and tract, AON = anterior olfactory nucleus, OF = orbitofrontal cortex, Am = amygdala, Hi = hippocampus.
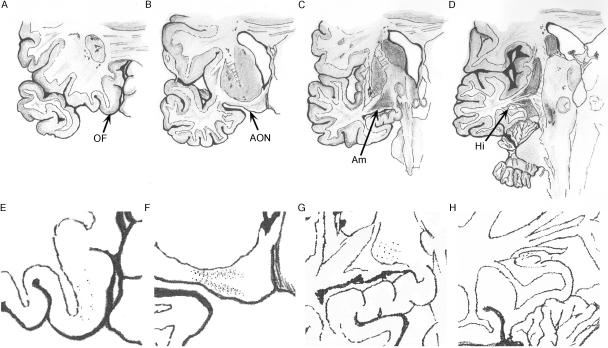
Fig. 3.
Progression of Lewy pathology along different parts of the olfactory system. (A–D) Orbitofrontal cortex (A), anterior olfactory nucleus near the insertion of the olfactory tract (B), amygdala (C) and hippocampus (D). (E–H) If the olfactory bulb and tract are affected by Lewy pathology the likelihood of similar pathology is highest in the anterior olfactory nucleus (E) followed by the amygdala and hippocampus (F and G, respectively), with the orbitofrontal cortex (H) least affected. AON = anterior olfactory nucleus, OF = orbitofrontal cortex, Am = amygdala, EC = entorhinal cortex, Hi = hippocampus.
Relationship between Lewy pathology and PD – Braak staging and diagnosis
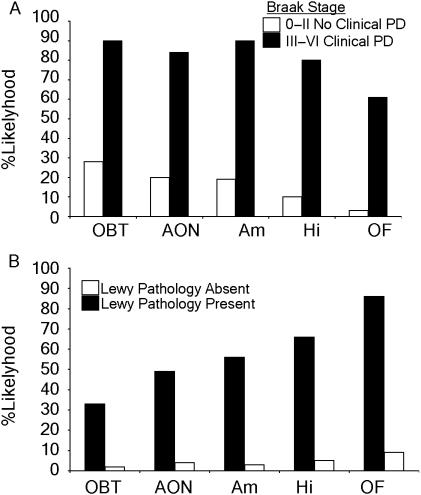
Almost one-third of the subjects without clinical parkinsonism had olfactory bulb Lewy pathology (28%) qualifying for Braak stages I (Fig. 4A). Surprisingly, 3% of this population had Lewy pathology in the OF. Similarly, subjects with clinical symptoms of PD (Braak stages III–VI) were very likely to have Lewy pathology; 90% of patients had OBT pathology, 84% of patients AON pathology, 90% had pathology in the amygdala and 80% had hippocampal pathology. The only exception to this was the OF, a neocortical region, which showed a 64% likelihood of Lewy pathology in these later stages.
Fig. 4.
Relationship between Lewy pathology and PD. (A) When Lewy pathology in each olfactory region is compared with the diagnosis of PD, it is apparent that pathology in the olfactory bulb occurs prior to clinical diagnosis of PD. (B) Comparison of Lewy pathology with PD diagnosis shows that a diagnosis of PD becomes more likely if Lewy pathology is present in the higher olfactory regions.
The PM diagnosis of PD is dependent on the presence of Lewy bodies in brain stem nuclei such as the substantia nigra or substantial cell loss in these regions in a patient suffering from parkinsonian symptoms. Lewy body pathology in the AON (OR: 18.5, Pearson's χ2 = 43.661, P = 0.0000) and OBT (OR: 23, Pearson's χ2 = 14.288, P = 0.0001) were least likely to be associated with the diagnosis of PD. When Lewy pathology affected regions further along the olfactory pathway the likelihood of a PD diagnosis increased, so that 56% with amygdala pathology (OR: 36.62, Pearson's χ2 = 66.885, P = 0.0000), 66% with hippocampal (OR: 36.88, Pearson's χ2 = 90.219, P = 0.0000) and 86% with OF pathology (Fig. 4B) had a PD diagnosis (OR: 60.93, Pearson's χ2 = 90.391, P = 0.0000).
Discussion
Previous work has shown that Lewy pathology appears early in the olfactory bulb of patients with dementia (Braak et al. 2003). Here we describe Lewy pathology in five regions of the olfactory pathway; olfactory tract/bulb (OBT), the anterior olfactory nucleus (AON) near the insertion of the olfactory tract into the lateral olfactory gyrus, the region of olfactory projection to the orbito-frontal cortex (OF), the hippocampus (HI) and the amygdala (Am).
Results show that Lewy pathology is more prevalent than previously suggested and varies in distribution, with some olfactory areas more prone to damage than others. These results also suggest that Lewy pathology progresses along olfactory pathways, affecting more peripheral, lower-order regions first before progressing through to affect higher-order cortical regions last. Furthermore, when Lewy pathology is compared with Braak staging and PM diagnosis of PD these results further support the idea that olfactory Lewy pathology occurs prior to clinical symptoms of PD.
Historically, Lewy body pathology has been primarily described as a hallmark of PD. However, it is important to note that Lewy pathology is not confined to PD (Jellinger et al. 2002; McKeith, 2006). Our findings show that there is at least some degree of Lewy pathology in over one-third of subjects studied. This is higher than previously shown (Stevens et al. 2002; Rahkonen et al. 2003) but is not wholly unsurprising as Lewy bodies and neurites can be difficult to detect unless they are specifically looked for. It is possible that some subjects previously considered as having pure AD are actually cases of mixed Alzheimer and Lewy pathologies (Hansen et al. 1993). These studies would therefore not take into account any pathology that may be present in other susceptible regions, particularly the olfactory bulb.
Our finding that the olfactory bulb and tract is the most likely to exhibit Lewy pathology is consistent with those of Braak and colleagues (Braak et al. 2003, 2004), who have shown that Lewy pathology in the olfactory bulb occurs prior to clinical symptoms. In the cortical olfactory pathway the insertion of the olfactory tract into the olfactory cortex has a high incidence of pathology. This is slightly surprising as there is relatively little pathology in the OF. The high incidence of pathology in the region of the insertion of the olfactory tract might be due to its close relationship to the olfactory tract. It is the primary olfactory cortex and receives direct synaptic input from the olfactory bulb. It is therefore interesting that the OF is relatively spared, despite being only one further synaptic connection along the pathway. The reasons for this are intriguing.
The simplest explanation would be that some patients died before the orbitofrontal cortical pathology could develop. However, if we assume that the pathology can progress with equal ease through synapses, the hippocampus, which receives only indirect input from the olfactory tract, should be as spared as the OF. This is not the case.
In the limbic olfactory pathway the amygdala is more likely to be affected than the hippocampus. Again this difference in susceptibility may be due to the olfactory nucleus of the amygdala receiving a direct input from the olfactory tract while the hippocampus receives input only via an indirect route. The higher susceptibility of the hippocampus to damage compared with the OF might be due to its close connection with other regions of the brain heavily affected by neurodegeneration. Many subjects in this study also exhibited Alzheimer's-type pathology, which affects the amygdala and hippocampus in particular. Taken together these results further support the possibility that Lewy pathology is progressive in the olfactory system (Braak et al. 2003, 2004).
Why then are certain neurons apparently more susceptible to Lewy pathology than others? Very little is known about the causes of the selective vulnerability of a small proportion of neurons to alpha-synuclein accumulation even in regions where Lewy pathology is present. However, cortical pyramidal neurons in layers III and V are thought to be particularly susceptible (Wakabayashi et al. 1995) Indeed, there is still considerable debate over the vulnerability of different neuronal subpopulations to neurodegeneration in general (Attems et al. 2007). One possibility for this differential susceptibility is the morphological properties of affected neurons. Braak and colleagues postulate that the susceptibility of neurons to Lewy pathology, particularly in PD, is due to properties of axons and the level of myelination. In PD susceptible neuronal cells types tend to be projection neurons with long thin axons and are poorly myelinated (Braak et al. 2004). This might help to explain the preferential susceptibility of olfactory tract projection neurons to Lewy pathology, rather than the more robustly myelinated and short axoned interneurons of the OF.
A second possibility is that synaptic plasticity confers susceptibility to neurodegeneration of neurons. This is particularly attributable to hyperphosphorylated tau pathology in the hippocampus of Alzheimer's patients (Arendt, 2004) but may also be relevant to the olfactory pathways. The olfactory pathway is an intensely plastic region of the brain, particularly at the level of the olfactory bulb, while the OF has relatively rigid synapses (Arendt, 2004). Therefore, any link between plasticity and neurodegenerative susceptibility may also be relevant here. It is an interesting feature, however, that equally plastic neuronal pathways within the hippocampus show different vulnerability to different types of pathology (Iseki et al. 1998). The non-perforating route (of the perforant pathway) terminates in the CAII region of the hippocampus and has been shown to be particularly sensitive to degeneration in patients with DLB (Iseki et al. 1997, 1998).
A further possibility for this selective vulnerability could be related to neurotransmitter differences. It is commonly accepted that in PD the most vulnerable cell to degeneration is the dopaminergic cell. The main dopaminergic cell type in the olfactory system is the periglomerular cells of the olfactory bulb. It is therefore interesting that little or no Lewy pathology was observed within these layers. Indeed, a recent study has shown an increase in dopaminergic periglomerular cells in PD patients (Huisman et al. 2004) rather than a loss. Huisman and colleagues suggest that it is the inhibitory action of these periglomerular cells on the glomeruli that leads to anosmia/hyposmia rather than a loss of neuronal function due to neurodegeneration.
In the anterior olfactory nucleus, where Lewy pathology is particularly common, the pyramidal and interneurons are glutamatergic and GABAergic, respectively (Haberly, 1997). The olfactory cortex also receives noradrenergic, serotonergic and dopaminergic input from the brain stem (Haberly, 1997). At present it would be very difficult to envisage that the differences in neuronal vulnerability to Lewy pathology would be associated with specific neurotransmitter systems. A full immunohistochemical study to relate the different neurotransmitter systems with Lewy pathology in the olfactory pathways has yet to be performed.
The presence of Lewy pathology in the olfactory pathways prior to the appearance of clinical signs suggests there is a threshold to functional deficits. This study shows that the diagnosis of PD is more likely the further along the olfactory pathways are involved. It is known that olfactory function is lost early in neurodegenerative diseases such as Alzheimer's (Mesholam et al. 1998) and that there is a relationship between olfactory function and Lewy pathology (Doty et al. 1988; McShane et al. 2001; Olichney et al. 2005). Olfactory function may therefore be an indicator of Lewy pathology and a test for smell may be helpful in the early diagnosis of neurodegenerative diseases such as DLB.
The use of a smell test to diagnose specifically a disease such as DLB is complicated both by the loss of smell in AD and by environmental factors that could result in a loss of smell in a healthy individual. Therefore, a smell test may be helpful in addition to the consensus guidelines (McKeith, 2006) already employed in the diagnosis of dementia with Lewy bodies. An early diagnosis of DLB would be beneficial because it would allow treatment to be started earlier to help to slow down progression of the disease.
Another important consideration is that, although Lewy pathology may be progressive, it is difficult to predict the rate of progression. Indeed, in some subjects there may be little or no progression of Lewy pathology. Although we can accept that in severe cases of PD the pathology started in the olfactory system before the clinical signs have developed, it is not at all certain that all patients with LB pathology in the olfactory bulb/tract go on to develop full-blown PD if they live long enough. This may be the case in the two control subjects in this study who had evidence of Lewy pathology despite having no clinical symptoms. These may be examples of subjects where the pathology has either failed to progress or has only progressed to a stage undetected by current clinical procedures. In light of this, any possible smell test must only be used as an aid to diagnosis in addition to the clinical guidelines already in place to distinguish dementia with Lewy bodies from PD and AD.
Conclusion
The presence of Lewy pathology begins in the olfactory system prior to development of clinical symptoms of PD and as the pathology becomes more widespread the clinical diagnosis of PD becomes more likely. This raises the possibility of using a smell test as an early diagnosis of neurodegenerative diseases. Future studies will need to be performed to test whether there is an association of the level and extent of Lewy pathology with a loss of smell and the development of clinical features of neurodegenerative diseases such as PD and dementia with Lewy bodies.
Acknowledgments
We would like to thank all members of the Oxford Project to Investigate Memory and Ageing (OPTIMA). Without their help and the multiple collaborations this work would not have been possible. We are also grateful to all the patients, their families, carers and clinicians involved in this study. We are also grateful to former and present members of the group involved in sample preparations, especially Mrs Suzanne Litchfield and Dr Maria Smith. Finally we would like to thank Kevin Hardware for the illustrations used in Fig. 3. Ethical approval was granted by the Central Oxfordshire Research Ethics Committee. The study was supported by the Alzheimer's Society.
References
- Arendt T. Neurodegeneration and plasticity. Int J Dev Neurosci. 2004;22:507–514. doi: 10.1016/j.ijdevneu.2004.07.007. [DOI] [PubMed] [Google Scholar]
- Arima K, Ueda K, Sunohara N, et al. NACP/alpha-synuclein immunoreactivity in fibrillary components of neuronal and oligodendroglial cytoplasmic inclusions in the pontine nuclei in multiple system atrophy. Acta Neuropathol (Berl) 1998;96:439–444. doi: 10.1007/s004010050917. [DOI] [PubMed] [Google Scholar]
- Attems J, Quass M, Gartner W, et al. Immunoreactivity of calcium binding protein secretagogin in the human hippocampus is restricted to pyramidal neurons. Exp Gerontol. 2007;42:215–222. doi: 10.1016/j.exger.2006.09.018. [DOI] [PubMed] [Google Scholar]
- Braak E, Braak H. Silver staining method for demonstrating Lewy bodies in Parkinson's disease and argyrophilic oligodendrocytes in multiple system atrophy. J Neurosci Meth. 1999;87:111–115. doi: 10.1016/s0165-0270(98)00173-3. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K. Stages in the development of Parkinson's disease-related pathology. Cell Tissue Res. 2004;318:121–134. doi: 10.1007/s00441-004-0956-9. [DOI] [PubMed] [Google Scholar]
- Crowther RA, Daniel SE, Goedert M. Characterisation of isolated alpha-synuclein filaments from substantia nigra of Parkinson's disease brain. Neurosci Lett. 2000;292:128–130. doi: 10.1016/s0304-3940(00)01440-3. [DOI] [PubMed] [Google Scholar]
- Daniel SE, Hawkes CH. Preliminary diagnosis of Parkinson's disease by olfactory bulb pathology. Lancet. 1992;340:186. doi: 10.1016/0140-6736(92)93275-r. [DOI] [PubMed] [Google Scholar]
- Del Tredici K, Rub U, de Vos RA, Bohl JR, Braak H. Where does parkinson disease pathology begin in the brain? J Neuropathol Exp Neurol. 2002;61:413–426. doi: 10.1093/jnen/61.5.413. [DOI] [PubMed] [Google Scholar]
- Doty RL, Deems DA, Stellar S. Olfactory dysfunction in parkinsonism: a general deficit unrelated to neurologic signs, disease stage, or disease duration. Neurology. 1988;38:1237–1244. doi: 10.1212/wnl.38.8.1237. [DOI] [PubMed] [Google Scholar]
- Doty RL, Riklan M, Deems DA, Reynolds C, Stellar S. The olfactory and cognitive deficits of Parkinson's disease: evidence for independence. Ann Neurol. 1989;25:166–171. doi: 10.1002/ana.410250210. [DOI] [PubMed] [Google Scholar]
- Fujiwara H, Hasegawa M, Dohmae N, et al. alpha-Synuclein is phosphorylated in synucleinopathy lesions. Nat Cell Biol. 2002;4:160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- Gai WP, Yuan HX, Li XQ, Power JT, Blumbergs PC, Jensen PH. In situ an d in vitro study of colocalization and segregation of alpha-synuclein, ubiquitin, and lipids in Lewy bodies. Exp Neurol. 2000;166:324–333. doi: 10.1006/exnr.2000.7527. [DOI] [PubMed] [Google Scholar]
- Haberly LB. Olfactory cortex. In: Shepherd GM, editor. The Synaptic Organization of the Brain. New York: Oxford University Press; 1997. pp. 377–416. [Google Scholar]
- Hansen LA, Masliah E, Galasko D, Terry RD. Plaque-only Alzheimer disease is usually the lewy body variant, and vice versa. J Neuropathol Exp Neurol. 1993;52:648–654. doi: 10.1097/00005072-199311000-00012. [DOI] [PubMed] [Google Scholar]
- Hawkes CH, Shephard BC, Daniel SE. Is Parkinson's disease a primary olfactory disorder? QJM. 1999;92:473–480. doi: 10.1093/qjmed/92.8.473. [DOI] [PubMed] [Google Scholar]
- Huisman E, Uylings HB, Hoogland PV. A 100% increase of dopaminergic cells in the olfactory bulb may explain hyposmia in Parkinson's disease. Mov Disord. 2004;19:687–692. doi: 10.1002/mds.10713. [DOI] [PubMed] [Google Scholar]
- Iseki E, Li F, Odawara T, Kosaka K. Hippocampal pathology in diffuse Lewy body disease using ubiquitin immunohistochemistry. J Neurol Sci. 1997;149:165–169. doi: 10.1016/s0022-510x(97)05387-2. [DOI] [PubMed] [Google Scholar]
- Iseki E, Marui W, Kosaka K, Akiyama H, Ueda K, Iwatsubo T. Degenerative terminals of the perforant pathway are human alpha-synuclein-immunoreactive in the hippocampus of patients with diffuse Lewy body disease. Neurosci Lett. 1998;258:81–84. doi: 10.1016/s0304-3940(98)00856-8. [DOI] [PubMed] [Google Scholar]
- Jellinger KA, Seppi K, Wenning GK. Lewy bodies in patients presenting clinically with Alzheimer disease. J Alzheimers Dis. 2002;4:327–328. doi: 10.3233/jad-2002-4408. [DOI] [PubMed] [Google Scholar]
- Lewy FH. Paralysis Agitans I. Pathologische anatomie. In: Lewandowsky M, editor. Handbuch der Neurologie. Berlin: Springer; 1912. p. 920. [Google Scholar]
- Liposits Z, Kallo I, Hrabovszky E, Gallyas F. Ultrastructural pathology of degenerating ‘dark’ granule cells in the hippocampal dentate gyrus of adrenalectomized rats. Acta Biol Hung. 1997;48:173–187. [PubMed] [Google Scholar]
- McKeith IG. Consensus guidelines for the clinical and pathologic diagnosis of dementia with Lewy bodies (DLB): report of the Consortium on DLB International Workshop. J Alzheimers Dis. 2006;9:417–423. doi: 10.3233/jad-2006-9s347. [DOI] [PubMed] [Google Scholar]
- McShane RH, Nagy Z, Esiri MM, et al. Anosmia in dementia is associated with Lewy bodies rather than Alzheimer's pathology. J Neurol Neurosurg Psychiatry. 2001;70:739–743. doi: 10.1136/jnnp.70.6.739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mesholam RI, Moberg PJ, Mahr RN, Doty RL. Olfaction in neurodegenerative disease: a meta-analysis of olfactory functioning in Alzheimer's and Parkinson's diseases. Arch Neurol. 1998;55:84–90. doi: 10.1001/archneur.55.1.84. [DOI] [PubMed] [Google Scholar]
- Mezey E, Dehejia A, Harta G, Papp MI, Polymeropoulos MH, Brownstein MJ. Alpha synuclein in neurodegenerative disorders: murderer or accomplice? Nat Med. 1998;4:755–757. doi: 10.1038/nm0798-755. [DOI] [PubMed] [Google Scholar]
- Nolte J. Human Brain: an Introduction to its Functional Anatomy. Missouri: Mosby-Year Book; 1993. [Google Scholar]
- Ohm TG, Muller H, Braak H, Bohl J. Close-meshed prevalence rates of different stages as a tool to uncover the rate of Alzheimer's disease-related neurofibrillary changes. Neuroscience. 1995;64:209–217. doi: 10.1016/0306-4522(95)90397-p. [DOI] [PubMed] [Google Scholar]
- Olichney JM, Murphy C, Hofstetter CR, et al. Anosmia is very common in the Lewy body variant of Alzheimer's disease. J Neurol Neurosurg Psychiatry. 2005;76:1342–1347. doi: 10.1136/jnnp.2003.032003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R, McKeith I, Perry E. In: Introduction Dementia with Lewy Bodies. Perry R, McKeith I, Perry E, editors. Cambridge: Cambridge University Press; 1996. pp. 1–5. [Google Scholar]
- Price JL. Olfactory system. In: Paxinos G, editor. The Human Nervous System. New York: Academic Press; 1990. p. 979. [Google Scholar]
- Purves D, Fitzpatrick D, Augustine GJ, et al. Neuroscience. 3. Sunderland, MA: Sinauer Associates Inc; 2004. [Google Scholar]
- Rahkonen T, Eloniemi-Sulkava U, Rissanen S, Vatanen A, Viramo P, Sulkava R. Dementia with Lewy bodies according to the consensus criteria in a general population aged 75 years or older. J Neurol Neurosurg Psychiatry. 2003;74:720–724. doi: 10.1136/jnnp.74.6.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388:839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Stevens T, Livingston G, Kitchen G, Manela M, Walker Z, Katona C. Islington study of dementia subtypes in the community. Br J Psychiatry. 2002;180:270–276. doi: 10.1192/bjp.180.3.270. [DOI] [PubMed] [Google Scholar]
- Tsuboi Y, Wszolek ZK, Graff-Radford NR, Cookson N, Dickson DW. Tau pathology in the olfactory bulb correlates with Braak stage, Lewy body pathology and apolipoprotein epsilon4. Neuropathol Appl Neurobiol. 2003;29:503–510. doi: 10.1046/j.1365-2990.2003.00453.x. [DOI] [PubMed] [Google Scholar]
- Tu PH, Galvin JE, Baba M, et al. Glial cytoplasmic inclusions in white matter oligodendrocytes of multiple system atrophy brains contain insoluble alpha-synuclein. Ann Neurol. 1998;44:415–422. doi: 10.1002/ana.410440324. [DOI] [PubMed] [Google Scholar]
- Uchikado H, Lin WL, DeLucia MW, Dickson DW. Alzheimer disease with amygdala Lewy bodies: a distinct form of alpha-synucleinopathy. J Neuropathol Exp Neurol. 2006;65:685–697. doi: 10.1097/01.jnen.0000225908.90052.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakabayashi K, Hansen LA, Masliah E. Cortical Lewy body-containing neurons are pyramidal cells: laser confocal imaging of double-immunolabeled sections with anti-ubiquitin and SMI32. Acta Neuropathol (Berl) 1995;89:404–408. doi: 10.1007/BF00307643. [DOI] [PubMed] [Google Scholar]
- Wakabayashi K, Hayashi S, Kakita A, et al. Accumulation of alpha-synuclein/NACP is a cytopathological feature common to Lewy body disease and multiple system atrophy. Acta Neuropathol (Berl) 1998;96:445–452. doi: 10.1007/s004010050918. [DOI] [PubMed] [Google Scholar]