Abstract
The structure of the cardiac foramen ovale from 17 species representing six cetacean families, the Monodontidae, Phocoenidae, Delphinidae, Ziphiidae, Balaenidae and the Balaenopteridae, was studied using the scanning electron microscope. Eight white whale fetuses (Delphinapterus leucas) and a narwhal fetus (Monodon monoceros) represented the Monodontidae; one fetal and nine neonatal harbour porpoises (Phocoena phocoena) and a finless porpoise fetus (Neophocoena phocoenoides) represented the Phocoenidae; two white-beaked dolphin fetuses (Lagenorhynchus albirostris), four fetal and one neonatal Atlantic white-sided dolphins (Lagenorhynchus acutus), a Risso's dolphin fetus (Grampus griseus), two common bottle-nosed dolphin neonates (Tursiops truncatus), a female short-beaked common dolphin fetus (Delphinus delphis), four killer whale fetuses (Orcinus orca) and two long-finned pilot whale fetuses (Globicephala melas) represented the Delphinidae; two northern bottlenose whale fetuses (Hyperoodon ampullatus) represented the Ziphiidae; one bowhead whale fetus (Balaena mysticetus) represented the Balaenidae and five Common minke whale fetuses (Balaenoptera acutorostrata), one blue whale fetus (Balaenoptera musculus), nine fin whale fetuses (Balaenoptera physalus) and four humpback whale fetuses (Megaptera novaeangliae) represented the Balaenopteridae. The hearts of an additional two incompletely identified toothed and four baleen whale fetuses were also studied. In each species the fold of tissue derived from the cardiac septum primum and subtended by the foramen ovale had the appearance of a short tunnel or sleeve which was fenestrated at its distal end. In the toothed whales the tissue fold was tunnel-shaped with the interatrial septum as the floor whereas in baleen whales it was more sleeve-like. In toothed whales thin threads extended from the fold to insert into the interatrial septum whereas a network of threads covered the distal end of the sleeve in the baleen whales. Similar structures were present in the corresponding cardiac tissues of neonatal Hippopotamidae.
Keywords: fetus, heart, Hippopotamidae, Mysticeti, neonate, Odontoceti, whale
Introduction
The anatomy of the circulation of the adult cetacean is adapted to the aquatic environment in which the animal lives, and in a number of important respects is therefore different from that of terrestrial mammals (Pabst et al. 1999). Likewise, the physiology of the cetacean is modified to cope with the differences between species in such things as the time spent under water, the depths to which the animal dives in its search for prey and temperature regulation. Adjustments are made to cardio-respiratory function, reproduction, diet during pregnancy and thereafter, as well as to fetal and neonatal development (Gambell, 1968; Lockyer, 1984; Oftedal, 1997; Boyd et al. 1999; Butler, 2004).
Investigation of pre- and immediate postnatal development is technically difficult in sea mammals, and the relatively few studies that have been carried out have largely been undertaken on seal fetuses and neonates (see Elsner, 1999; Noren et al. 2004). For obvious practical reasons almost nothing is known about the biology of the cetacean fetus. Nevertheless, there is written evidence of early research into this topic, a good example being the dissection of the pregnant harbour porpoise (Phocoena phocoena) and its fetus performed as an investigative demonstration by Bartholin (1654). Further anatomical studies of fetuses were undertaken by Eschricht (1837, 1849, 1869) and Kükenthal (1893, 1914) and subsequent embryological studies have been carried out on the harbour porpoise by Müller (1920) and on the spotted dolphin (Stenella attenuata) by Sedmera et al. (2003).
The cardiovascular anatomy of the mammalian fetus differs in a number of important ways from that of the adult. Each of these is a direct result of gas exchange occurring in the placenta of the prenatal animal rather than in its lungs. Oxygenated blood flowing in the umbilical vein from the placenta passes through the liver via the ductus venosus into the caudal vena cava. It then passes from there into the left atrium through another specialization within the fetal circulation, the foramen ovale (Fig. 1). This opening from the terminal portion of the caudal vena cava allows blood to be shunted directly from the caudal vena cava into the left side of the heart (Amoroso et al. 1942; Franklin et al. 1942). The ductus arteriosus (Fig. 1) enables the deoxygenated blood, which is pumped from the right ventricle into the pulmonary trunk, to be shunted away from the unventilated lungs and into the descending aorta. At birth the placenta is abandoned, the lungs expand to take over the role of respiration, and the foramen ovale and ductus arteriosus normally close.
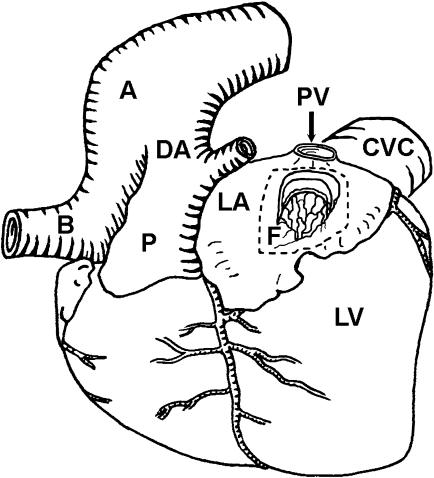
Fig. 1.
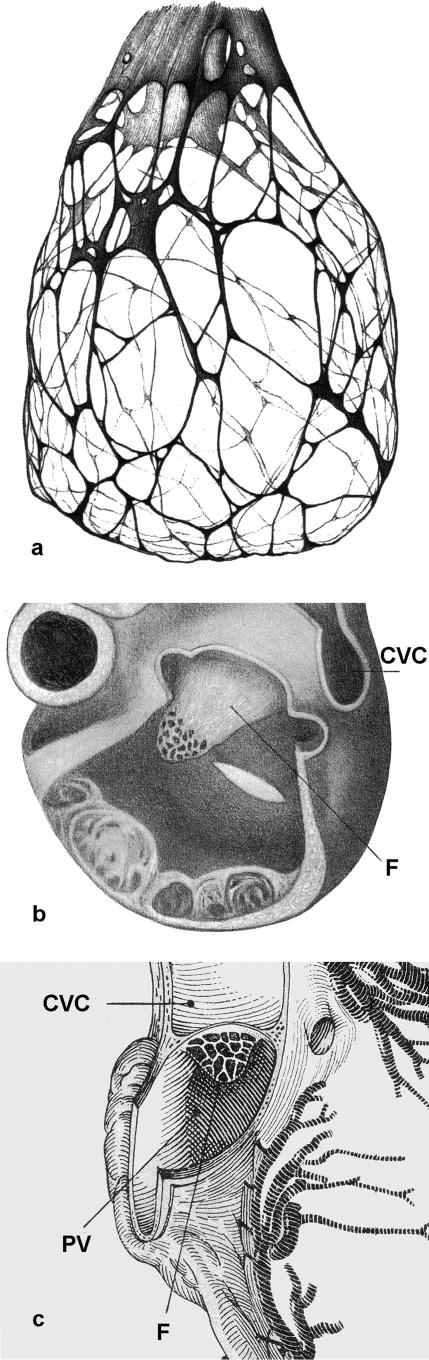
Schematic diagram of the heart of a cetacean fetus viewed from the left with a window cut into the left atrium. The relative positions of the aorta (A), left common carotid artery (B), ductus arteriosus (DA), pulmonary trunk (P), left atrium (LA), left ventricle (LV), caudal vena cava (CVC), pulmonary vein (PV) and the fold of tissue (F) representing the developed septum primum lying in the lumen of the left atrium are indicated. The latter is distal to the foramen ovale, which it obscures. Pericardial, pleural and lung tissues have been removed.
The anatomy of the closure of these shunts in the cetacean neonate was studied by Slijper (1936, 1961). The lumen of the ductus arteriosus appeared to be reduced by progressive constriction of the musculature in the intima media, and his studies suggested that complete anatomical closure of that vessel in cetaceans can take a number of years. In contrast, the available evidence indicates that closure of the foramen ovale is more rapid and is achieved in another way. A flap of tissue, derived from the embryonic septum primum of the heart, channels the blood flowing through the foramen ovale into the lumen of the left atrium. Shortly after birth this fold of tissue collapses under the increased volume of blood streaming into the left atrium from the pulmonary veins draining the now expanded lungs of the neonate (Amoroso et al. 1942; Franklin et al. 1942; Hong & Choi, 2000). Very little or nothing is known about the structure of this fold of tissue in the fetuses of most cetacean species. Only a few brief reports have been published (Knox, 1838; Jackson, 1845; Burmeister, 1869; Eschricht, 1869; Turner, 1870; Beauregard & Boulart, 1897; Schulte, 1916; Kernan & Schulte, 1918; Müller, 1920; Walmsley, 1938; Slijper, 1936, 1961). The aim of this paper is to bring together these scattered descriptions and place them in the context of a more systematic, scanning electron microscopic study of the left atrial tissues of a number of whale families. The nomenclature used to describe the cetacean species throughout this paper is that employed for the IUCN/SSC Action Plan (Reeves et al. 2003). The relative fetal and neonatal ages of the species studied were derived from analyses of fetal and neonatal cetacean sizes published by Risting (1928), Laws (1959), Lockyer (1981, 1984), Sampson (1989), Boyd et al. (1999) and Wells et al. (1999).
Materials and methods
We studied 65 hearts obtained from fetal and neonatal specimens representing both suborders of the Cetacea. From the suborder Odontoceti we examined hearts from representatives of four families: Monodontidae, Phocoenidae, Delphinidae and Ziphiidae. The suborder Mysticeti was represented by two families, Balaenidae and Balaenopteridae. All specimens came either from museum collections, following veterinary autopsies of stranded animals (Anon., 2005) or as stillbirths from zoological collections. The sources, and their abbreviations, were Dundee University, Scotland (DU); Kelvingrove Art Gallery and Museum, Glasgow, Scotland (KAGM); the McManus Galleries and Museum, Dundee, Scotland (MGM); the Museum für Naturkunde Berlin, Germany (MNB); the Natural History Museum, London, England (NHM); the National Museum of Natural History, Leiden, the Netherlands (NMNH); the Royal Museums of Scotland, Edinburgh, Scotland (RMS); the Royal Zoological Society of Antwerp, Belgium (RZSA); Sea Mammal Research Unit, St Andrews, Scotland (SMRU); and the University of Bergen Museum of Zoology, Norway (UBMZ). To give an indication of the relative size of each fetus or neonate, the length of the body (lip to tail notch) and the length of gestation of each species at birth are indicated in square brackets [L = mm; G = months] after the species name, and the length of the body of the fetus or neonate is indicated after each specimen number.
Suborder Odontoceti
Of the Monodontidae we studied eight white whale or beluga fetuses (Delphinapterus leucas) [L = 1500–1600 mm; G = ~14 months], four males (DU no. 2473a, L = 210 mm; RMS nos. PetMus65a, L = 250 mm and PetMus65b, L = 270 mm; MNB no. Vanhoffen 9.7. 1894, L = 500 mm), one female (DU no. 2473b, L = 300 mm), and three of unknown sex (DU nos. 2664b, L = 230 mm; 2664a, L = 350 mm; and NoNum, L unrecorded).
In addition we examined one male narwhal fetus (Monodon monoceros) [L = 1500–1600 mm; G = 15 months] (MNB no. 3666, L = 250 mm).
Of the Phocoenidae we studied ten harbour porpoises (Phocoena phocoena) [L = 750 mm; G = ~11 months] one fetus of unknown sex (KAGM no. Z1989-36-2, L = 280 mm), and nine neonates, two females (RMS nos. M1167/94, L = 750 mm and M1267/94, L = 770 mm), two males (RMS nos. M1034/94, L = 770 mm and M1102/94, L = 820 mm) and five of unknown sex (RMS nos. SW1990/57, L = 800 mm; SW1991/59, L = 940 mm; SW1991/73, L = 1000 mm; SW1990/106, L = 1250 mm; M204/95, juvenile, L unrecorded).
In addition we examined one female finless porpoise fetus (Neophocoena phocaenoides) [L = 800 mm; G = 11 months] (MNB no. 5987, L = 630 mm).
Of the Delphinidae we studied: two white-beaked dolphin fetuses (Lagenorhynchus albirostris) [L = 1150 mm; G = ?], one female (UBMZ no. 3526, L = 750 mm), and one male (UBMZ no. 1309: L = 1130 mm), five Atlantic white-sided dolphins (Lagenorhynchus acutus) [L = 1070–1220 mm; G = ~11 months], one female fetus (UBMZ no. 1178, L = 180 mm), three male fetuses (UBMZ nos. 2102, L = 220 mm; 1305, L = 630 mm and 1303, L = 900 mm), and one neonate of unknown sex (AAM644), one Risso's dolphin (Grampus griseus) [L = 1350–1660 mm; G = ~13–14 months], a female neonate (RMS no. M832/92, L = 1100 mm), two common bottle-nosed dolphins (Tursiops truncatus) [L = 840–1220 mm; G = 12 months], both female neonates (RZSA nos. 115/92 (6 days) and 356/89 (12 days)), one female short-beaked common dolphin fetus (Delphinus delphis) [L = 800–900 mm; G = 10–11 months] (SMRU no. MAFF.TRAWL/12/79, L = 270 mm), four killer whale fetuses (Orcinus orca) (L = 1800–2500 mm; G = ~17 months], two females (MNB no. 5641, L = 460 mm and UBMZ no. 1606, L = 525 mm), and two males (UBMZ nos. 1296, L = 860 mm, and 1292, L = 890 mm), and two male long-finned pilot whale fetuses (Globicephala melas) [L = 1770 mm; G = ~12–15 months] (NMNH no. 5.9.85, L = 350 mm and UBMZ no. 1280, L = 625 mm).
The family Ziphiidae was represented by two female northern bottlenose whale fetuses (Hyperoodon ampullatus) [L = 3500 mm; G = ~12 months] (MNB no. MUSBRES0, L = 220 mm and UBMZ no. 1141: L = 420).
Suborder Mysticeti
The family Balaenidae was represented by one male bowhead whale fetus (Balaena mysticetus) [L = 3500–5500 mm; G = 13–14 months] (RMS no. PETMUS6. 1965, L = 520).
Of the family Balaenopteridae we examined: five common minke whale fetuses (Balaenoptera acutorostrata) [L = 2600 mm; G = 10–11 months], two females (UBMZ nos. 1540, L = 355 mm and 1247, L = 930 mm) and three males (UBMZ nos. 4284, L = 240 mm; 1248, L = c. 600 mm (head removed), and 1259, L = 710 mm), one male blue whale fetus (Balaenoptera musculus) [L = 7000 mm; G = 12 months] (UBMZ no. 3276, L = 460 mm), nine fin whale fetuses (Balaenoptera physalus) [L = 6400 mm; G = 12 months], three females (RMS no. Z1939.18, L = 270 mm; SMRU nos. D1130, L = 320 mm and SGG131, L = 330 mm), and six males (SMRU [archived in NHM] – specimen nos. Jar800/SPR2409/D2101, L = 350 mm; Jar894/SPR113, L = 370 mm; Jar404/SPR112, L = 370 mm; Jar406, L = 420 mm; RMS no. Z1991.093, L = 440 mm; and SMRU no. SGG82, L = 600 mm) and four humpback whale fetuses (Megaptera novaeangliae) [L = 4000–5000 mm; G = 12 months], two females (MGM no. NH 1984/60, L = 310 mm; MNB no. MUS.BRES16, L = 500 mm) and two males (MNB no. MUS.BRES12, L = 170 mm, and MUS.BRES13, L = 290 mm).
The hearts of a further two Odontocete fetuses of incompletely identified genus were also studied: RMS no. Z. 1925.9.1302, L = 260 mm (possibly a common bottle-nosed dolphin (Tursiops truncatus)) and KAGM no. Z55-59, L = 320 mm (possibly a sperm whale (Physeter macrocephalus) [L = 3400–4900; G = 14–16 months]).
An additional four ‘baleen whales’ of unknown genus were examined, two female fetuses (KAGM no. Z49-78c, L = 520 mm and UBMZ no. ‘Large baleen whale’ [AAM406], L = 1500 mm) and two male fetuses (KAGM nos. Z49-78a, L = 370 mm, and Z49-78, L = 390 mm).
The caudal vena cava was dissected free of the diaphragm. The aorta, pulmonary arteries, pulmonary veins, lung tissue and suspensory ligaments were cut close to the heart. In several instances the foramen ovale was exposed by removing part of the roof of the right atrium (see Fig. 1). In all other cases the fold of tissue arising from the foramen ovale and projecting into the left atrium was exposed by removing the lateral wall of the mid section of the left auricle and, where necessary, part of the roof of the left atrium. In those instances where tissues were taken for electron microscopy, the interatrial septum was generally removed as part of the specimen together with the associated walls of the atrium. Otherwise the fold of tissue was observed in situ with the aid of a dissecting microscope. The removed specimens were first placed overnight in a solution of 3% gluteraldehyde in 0.1 m sodium cacodylate buffer (pH = 7.3), and then immersed for a second night in 2% guanidine hydrochloride and 2% tannic acid (Murakami et al. 1977). They were then postfixed in 2% osmium tetroxide in distilled water for 8 h. Dehydration in graded acetones was followed by critical point drying using carbon dioxide (Cohen, 1979). After they were mounted on aluminium stubs, the specimens were sputter coated with 20 µm gold/palladium (Echlin, 1975) and viewed in a scanning electron microscope (Phillips 505). Small pieces of the flap of tissue arising from the foramen ovale from five cetaceans [white whale (Vanhoffen 9.7 1894), harbour porpoise (M1034/94), Atlantic white-sided dolphin (AAM644), common minke whale (1248) and a baleen whale (AAM406) of unknown species] were also prepared for routine light and transmission electron microscopy.
The suboptimal conditions under which the material in museum collections was originally gathered, fixed (methods unknown) and preserved (in alcohol or 10% formalin) precluded any detailed study of the ultrastructure of the cardiac tissue. Nevertheless, in most specimens there was only superficial damage to the microscopic appearance of the hearts, and in the light of the age of many of them (often over 100 years old) they were surprisingly well preserved.
Results
General findings
There was a relatively thick, tough pericardium around the hearts of the larger cetacean fetuses and neonates. In all species and at all ages studied, the length of the caudal vena cava, between the exit of the ductus venosus from the liver and the entrance through the foramen ovale into the left atrium of the cetacean fetus, was relatively short.
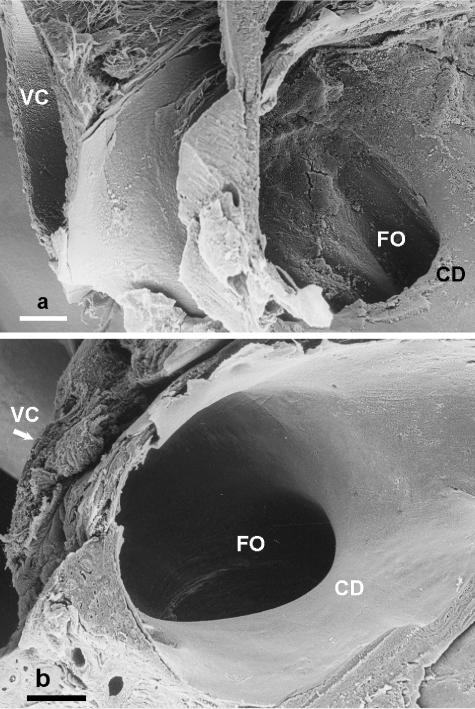
In all species, the flap of tissue subtended by the foramen ovale, when viewed from the terminal part of the caudal vena cava, had the appearance of a short tunnel or sleeve. Its entrance, the foramen ovale, was formed in part (caudally) by the tissues of the caudal vena cava and in part (cranially) by the wedge of atrial tissue termed the crista dividens (Fig. 2a,b). The siting of the pulmonary vein or veins in the roof of the right atrium seemed to be such that the drainage of this venous blood was directed to flow onto the length of the flap (Fig. 1). Within the left atrium, the wall of the interatrial septum was smooth, as was the proximal part of the roof of the atrium. The wall of the left auricle was thin and reinforced by prominent pectinate muscles.
Fig. 2.
(a) Scanning electron micrograph of the foramen ovale (FO) in a white whale (Delphinapterus leucas) fetus viewed from the right atrium, illustrating the curved edge of the crista dividens (CD) of the interatrial septum, and the caudal vena cava (VC). Scale bar = 1 mm. (b) Scanning electron micrograph of the foramen ovale (FO) in a humpback whale (Megaptera novaeangliae) fetus viewed from the right atrium, illustrating the curved edge of the crista dividens (CD) of the interatrial septum and the caudal vena cava (VC). Scale bar = 1 mm.
Observations on the Odontoceti (toothed whales)
Monodontidae
White whale (Delphinapterus leucas) (n = 8)
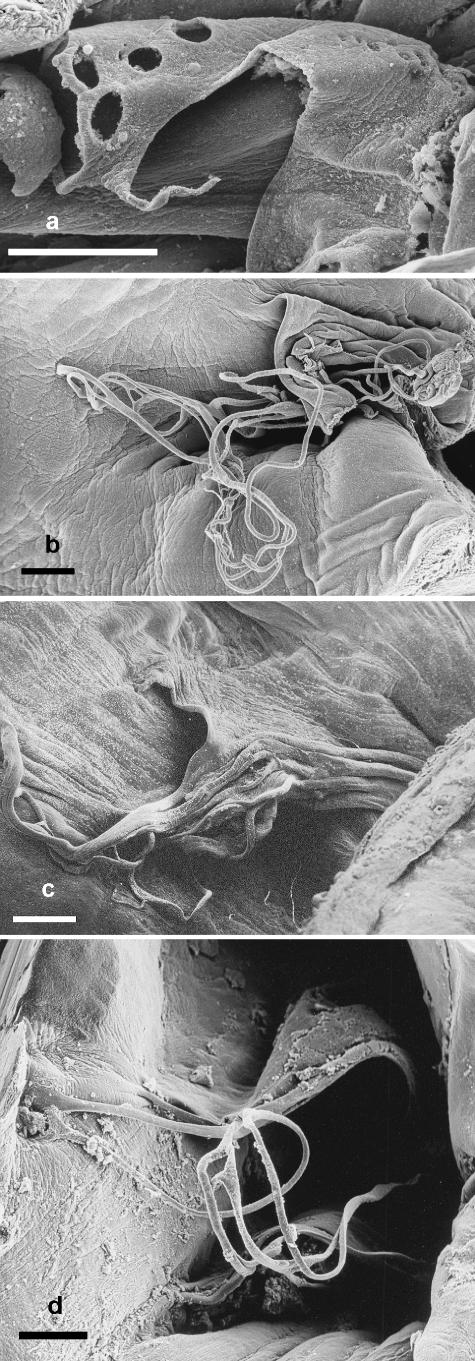
The fold of tissue formed a tunnel shape with the interatrial septum as the floor. The tissue fold was continuous in all but two of the fetuses, with the distal end of the flap being straight-edged. In all but one case, one or more thin threads ran from this edge to insert into the interatrial septum. The distal end of one of the folds of tissue, which was fenestrated, had a single long thread bordering a large opening on the side of the fold not lying under the pulmonary venous drainage (Fig. 3a). Several additional small openings were present proximal to this large opening. The other fenestrated fold of tissue showed several small openings. Branched fibrous tissue was seen underlying the endothelium of the tissue fold near the attachment of the leading edge to the interatrial septum.
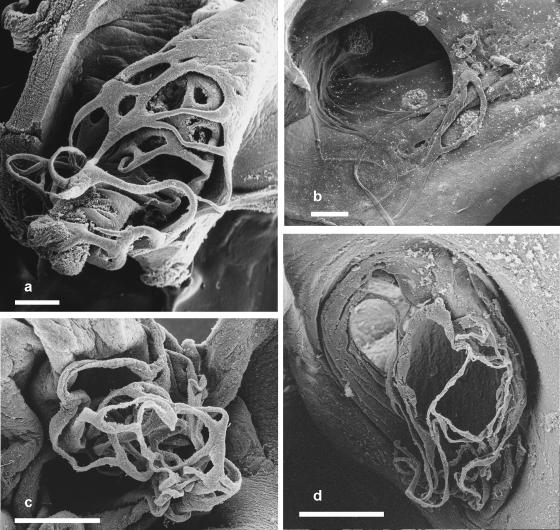
Fig. 3.
(a) Scanning electron micrograph of the fenestrations in the fold of tissue subtended by the foramen ovale of a white whale (Delphinapterus leucas) fetus. Scale bar = 1 mm. (b) Scanning electron micrograph of the left atrium of a harbour porpoise (Phocoena phocoena) fetus illustrating the loose network of threads at the distal end of the tunnel-like fold of tissue subtended by the foramen ovale, and their attachment to the interatrial septum. Scale bar = 1 mm. (c) Scanning electron micrograph of the left atrium of a neonatal harbour porpoise illustrating the apparently coalesced network of threads near the distal end of the tunnel-like fold of tissue subtended by the foramen ovale. Scale bar = 1 mm. (d) Scanning electron micrograph of the distal end of the tunnel-like fold of tissue in a finless porpoise (Neophocoena phocoenoides) fetus illustrating the network of threads and their attachment to the interatrial septum. Scale bar = 1 mm.
Narwhal (Monodon monoceros) (n = 1)
The sleeve-like fold of tissue appeared to be largely unfenestrated. The interatrial septum did not seem to be a component part of its construction. A network of branched threads of different thickness arose from the distal end of the sleeve and one thread was attached to the wall of the atrium.
Phocoenidae
Harbour porpoise (Phocoena phocoena) (n = 10)
The fold of tissue was in the form of a largely unfenestrated tunnel with the interatrial septum and the adjacent atrial wall forming the floor in all but one of the specimens (Fig. 3b). Those fenestrations that were present were situated on the side of the tunnel adjacent to the atrio-ventricular valve, and away from the pulmonary venous drainage. Threads and ribbons of tissue formed a network of variable size distal to these openings. Some threads were anchored to the atrial wall.
In one neonatal specimen the distal end of the flap demonstrated bulbous growth and knotting of what appeared to have been several adjacent threads (Fig. 3c). Other neonatal specimens demonstrated similar stages in the closure of the foramen ovale. In two of these the fold of tissue had contracted back over the opening and the threads which anchored the distal end of the fold to the atrial septum were thick and seemed to have coallesced with their neighbours. The tissue of the fold formed a plug over the foramen and the depression thus created represented a fossa ovalis. Short threads of tissue anchored the fold to the interatrial septum.
Finless porpoise (Neophocoena phocoenoides) (n = 1)
The unfenestrated flap of tissue was in the form of a tunnel with the interatrial septum acting as the floor (Fig. 3d). Thread-like attachments were made from the distal end of the tunnel to the interatrial septum. One of these threads branched to form a loose network, the long threads of which inserted onto the atrial walls beyond the end of the tunnel.
Delphinidae
White-beaked dolphin (Lagenorhynchus albirostris) (n = 2)
The fold of tissue is in the form of a tunnel which has the interatrial septum and adjacent atrial tissue as the floor. In one specimen, several small round openings of less than 1 mm in diameter were scattered over the surface of the tunnel. The other specimen showed no fenestrations. The distal end of the tunnel was partly covered in a loose network of threads. Several of these were attached to the atrial wall near the distal end of the tunnel. The fold of tissue showed concentrations of branched fibrous material beneath its endothelium.
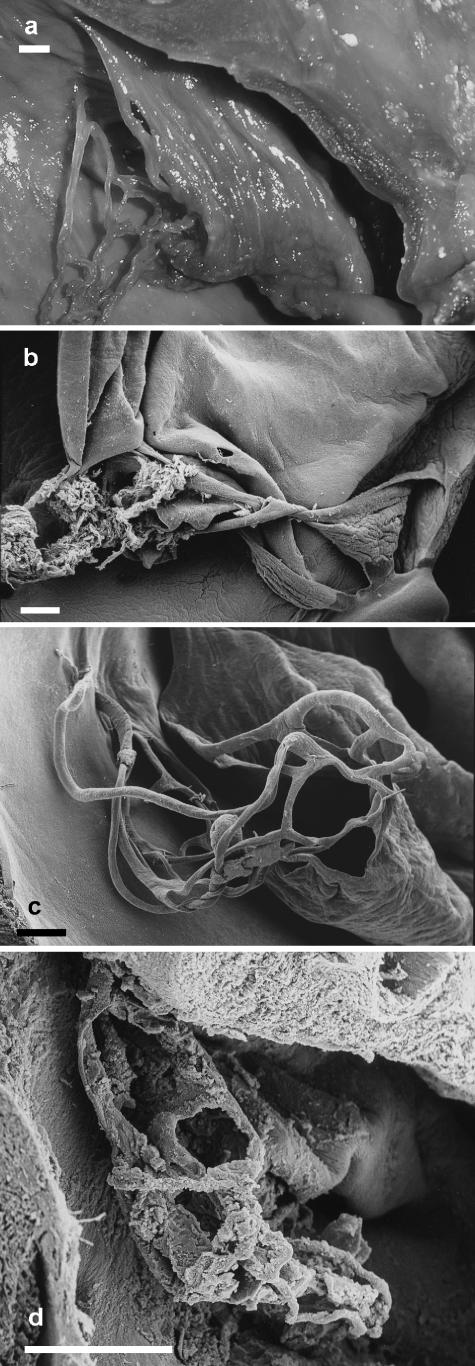
Atlantic white-sided dolphin (Lagenorhynchus acutus) (n = 5)
The fold was in the form of a largely unfenestrated tunnel (Fig. 4a). The interatrial septum provided the floor of the tunnel. Several small holes were present near where the proximal part of the fold attached to the interatrial septum and at the distal end of the fold. The wall of the fold suggested that aggregations of branched fibrous material were present beneath the endothelium. A loose network of threads arose from the distal end of the tunnel and projected into the lumen of the atrium. Some individual threads attached to the walls of the atrium.
Fig. 4.
(a) Photograph of the left atrium of an Atlantic white-sided dolphin (Lagenorhynchus acutus) neonate illustrating the network of threads at the distal end of the tunnel-like fold of tissue subtended by the foramen ovale. Scale bar = 1 mm. (b) Scanning electron micrograph of the distal end of the collapsed tube-like fold of tissue in the left atrium of a Risso's dolphin (Grampus griseus) fetus. Blood clots adhere to the flat ribbons of tissue making up the network at its distal end. Scale bar = 1 mm. (c) Scanning electron micrograph of the distal end of the tunnel-like fold of tissue in the heart of a common bottle-nosed dolphin (Tursiops truncatus) neonate. Scale bar = 1 mm. (d) Scanning electron micrograph of the network of threads at the distal end of the tunnel-like fold of tissue subtended by the foramen ovale in a short-beaked common dolphin (Delphinus delphis) fetus. Scale bar = 1 mm.
Risso's dolphin (Grampus griseus) (n = 1)
The fold was in the form of a long, wide tunnel, with the interatrial septum forming a wide floor (Fig. 4b). The fold was unfenestrated for most of its length. Flat straps and long threads enclosed large and small openings at the distal end of the fold on the side that was not adjacent to the pulmonary venous drainage. Three of these interconnected flat straps appeared to be anchored to the atrial wall behind the leading edge of the fold. The subendothelial tissues of the flap were branched and fibrous.
Common bottle-nosed dolphin (Tursiops truncatus) (n = 2)
The fold of tissue formed a tunnel-like structure, with the floor being provided by the interatrial septum, to a larger degree in the younger neonate (6 days old) than in the other (Fig. 4c). The fold was unfenestrated for most of its length. In the younger neonate a network, made up of flat straps and long threads, enclosed large and small openings in that part of the distal end of the flap which was hidden from the opening of the pulmonary venous drainage by the unfenestrated part of the fold. One or two threads were anchored to the interatrial septum. In the older specimen (12 days old), one large-diameter thread, arising from at least two others, attached the fold to the interatrial septum; the collapsed fold obscured further details of its origin. The leading edge of the fold was almost straight in both specimens, that of the older specimen having a somewhat ‘thickened’ appearance. The subendothelial layer of the fold in both specimens was branched and fibrous. The older specimen appeared to be ‘knotting’ its flap over the foramen ovale.
Short-beaked common dolphin (Delphinus delphis) (n = 1)
The unfenestrated fold of tissue formed a tunnel with the interatrial septum as its floor. A network of threads arose at its distal end (Fig. 4d). At least two of these threads anchored the network to the interatrial septum.
Killer whale (Orcinus orca) (n = 4)
The fold of tissue running into the left atrium from the foramen ovale was largely tunnel-shaped with the interatrial septum forming its floor. The last part of the fold was more sleeve-like with the interatrial septum not forming a component part; the remaining attachment to the interatrial septum was via some long threads from the straight-edged distal end of the fold. The side of the fold lying opposite the pulmonary venous drainage was unfenestrated. The distal end of the rest of the fold supported an open network of thin threads and ribbons of tissue.
Long-finned pilot whale (Globicephala melas) (n = 2)
The crista dividens in this specimen was represented by a blunt piece of atrial tissue rather than a sharply defined arc. The wide tunnel into the lumen of the left atrium was formed largely from the fold of the septum primum, with the interatrial septum as its floor. The fold was unfenestrated. The distal end was straight edged on the side under the pulmonary venous drainage. On the other side there were a few threads, the remains of which indicated that they had formed a network.
Ziphiidae
Northern bottlenose whale (Hyperoodon ampullatus) (n = 2)
The tissue fold in both fetuses was in the form of an unfenestrated tunnel with the interatrial septum providing the floor. The subendothelial tissue at the distal end of the fold in the older fetus was fibrous in appearance and resembled a coalesced network of threads. In one specimen the broken ends of two thick threads suggested that either one large thread or a network had been cut off during tissue preparation. No threads were seen attaching to the atrial wall.
Observations on the Mysticeti (baleen whales)
Balaenidae
Bowhead whale (Balaena mysticetus) (n = 1)
The fold of tissue was in the form of a tunnel with the interatrial septum as its floor for the first 5 mm of its path into the left atrium. Thereafter, the fold became a sleeve, the distal end of which was loosely covered by a network of threads of varying thicknesses and lengths (Fig. 5a). There appeared to be no threads from this network to the atrial wall. The wall of the fold showed evidence of branched fibrous material underlying the endothelium, and several small openings were present towards its distal end.
Fig. 5.
(a) Scanning electron micrograph illustrating the network of threads at the distal end of the fenestrated sleeve-like fold of tissue subtended by the foramen ovale in a bowhead whale (Balaena mysticetus) fetus. The sleeve has rolled upon itself. Scale bar = 1 mm. (b) Scanning electron micrograph illustrating the threads at the distal end of the unfenestrated tunnel-like fold of tissue from the foramen ovale in a north atlantic minke whale (Balaenoptera acutorostrata) fetus. Scale bar = 1 mm. (c) Scanning electron micrograph illustrating the network of threads at the distal end of the fenestrated sleeve-like fold of tissue subtended by the foramen ovale in a fin whale (Balaenoptera physalus) fetus. Scale bar = 1 mm. (d) Scanning electron micrograph illustrating the partially autolysed threads at the distal end of the fenestrated sleeve-like fold of tissue subtended by the foramen ovale in a humpback whale (Megaptera novaeangliae) fetus. Scale bar = 1 mm.
Balaenopteridae
Common minke whale (Balaenoptera acutorostrata) (n = 5)
The fold of tissue from the foramen ovale was attached to the wall of the septum which, together with part of the atrial wall, formed the floor of a tunnel (Fig. 5b). The unfenestrated interatrial septal part of the fold extended further into the atrium that the part adjacent to the atrial wall. As a consequence, the distal opening of the tunnel was wide and in the form of an arc. In one fetus a network of threads and ribbons of tissue, running in parallel to the distal end of the fold, created an open extension of the tunnel. In the other fetuses the distal edge subtended a network of interconnected threads and ribbons of tissue which extend the fold into the lumen of the atrium. In one of these, the interconnecting threads formed a network of fenestrations round a large central opening. That part of the fold adjacent to the interatrial septum was attached to the septum by threads arising from this network. The side of the fold nearest to the atrioventricular valve was fenestrated and was attached to the atrial wall by short ribbons of tissue.
Blue whale (Balaenoptera musculus) (n = 1)
The fenestrated fold of tissue terminated in a network of threads. The extent to which the interatrial septum may have contributed to its tunnel shape was not clear.
Fin whale (Balaenoptera physalus) (n = 9)
In two of the fetuses the fold of tissue arising from the caudal vena cava and the crista dividens was in the form of a tunnel with the interatrial septum forming the floor (Fig. 5c). In four of the fetuses this fold of tissue was in the form of a tunnel for part of its length, and thereafter it formed a short sleeve. In two fetuses the tissue fold was in the form of a sleeve over its entire length. Generally, the fold was fenestrated distally along about half its length, although in one fetus it was unfenestrated. Branching fibrous material was seen lying under the endothelium of the fold. There appeared to be few if any holes in that part of the fold lying below the pulmonary venous drainage. Where present in the smallest fetus, openings were of different sizes and were bordered largely by thin, flat straps of tissue. In all fetuses the distal end of the fold of tissue was covered in a loose network of threads. In the largest fetus, these threads and ribbons of tissue varied in thickness. In four fetuses one or more threads of tissue attached the sleeve or the network of threads to the interatrial septum.
Humpback whale (Megaptera novaeangliae) (n = 4)
A thin fold of tissue projected from the walls of the foramen ovale into the lumen of the left atrium and contributed towards the ‘tube-like’ appearance in all specimens. The anatomy of this fold of tissue was slightly different in each specimen. In one fetus the fold of tissue initially formed a tunnel with the interatrial septum as its floor. Distally the structure was that of a sleeve projecting into the lumen of the atrium. In the other two fetuses the fold of tissue had the form of a short sleeve. In all three fetuses an open network of threads covering its distal end (Fig. 5d). The thickness of the strands forming the network was likewise not uniform. Occasionally thin threads bisected openings bounded by much thicker strands of tissue. The surfaces of the latter, and the surfaces of the unfenestrated portion of the fold, showed undulations and wrinkles suggestive of infilled openings and fibrous subendothelial structures. The wall of the fold situated under the pulmonary venous drainage was unfenestrated.
Incompletely identified whales (n = 6)
Of the six specimens which lack definitive species identification, two have received provisional classification.
RMS no. Z. 1925.9.1302 was thought to be a common bottlenosed dolphin (Tursiops truncatus). Its tissue fold formed a tunnel with the interatrial septum as its floor. The wall of the tunnel was unfenestrated where it lay below the pulmonary venous drainage, but was fenestrated distally on the side closest to the atrio-ventricular valve. Tissue damage denied access to the distal end of the tissue fold.
KAGM no. Z55-59 was thought to be a sperm whale (Physeter catodon) fetus. Its foramen ovale opened into a sleeve-like fold of tissue, the distal end of which was covered with a network of threads, some of which were ribbon-like (Fig. 6a). No threads appear to attach to the walls of the atrium, and apart from the crista dividens, the interatrial septum did not contribute towards the structure of the sleeve. The subendothelial texture of the fold was fibrous, with what appeared to be the remains of threads embedded in its structure.
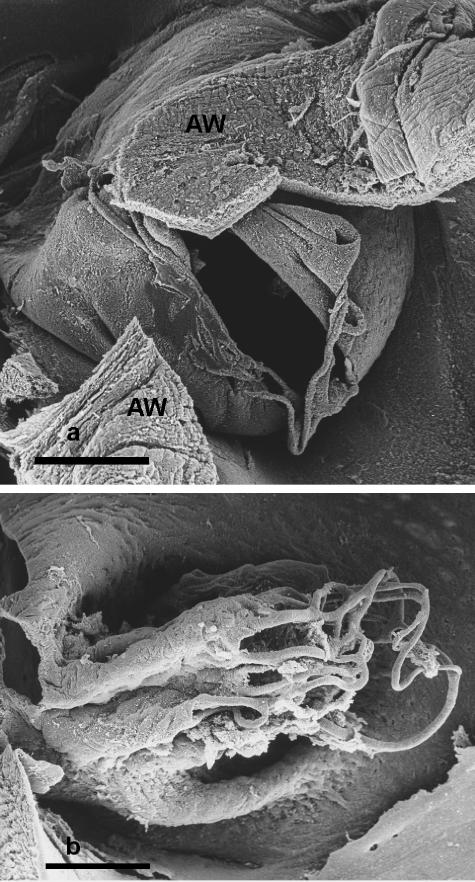
Fig. 6.
(a) Scanning electron micrograph illustrating the distal end of the fenestrated sleeve-like tissue fold subtended by the foramen ovale in the fetus of an incompletely identified species, possibly sperm whale (Physeter catodon). The tissues labelled AW are sections of atrial wall. Scale bar = 1 mm. (b) Scanning electron micrograph illustrating the network of threads at the distal end of the fenestrated sleeve-like tissue fold subtended by the foramen ovale in an unidentified ‘baleen whale’ fetus. Scale bar = 1 mm.
In each of the four ‘baleen’ whales the fold of tissue was in the form of a sleeve-like tube projecting from the foramen ovale into the left atrium (Fig. 6b). This sleeve was unfenestrated on the side beneath the pulmonary venous drainage, whereas both the distal end and the other side of the sleeve were fenestrated. The holes varied in size and were bounded by threads which varied in thickness. The subendothelial structure of the fold was fibrous in appearance. The walls of the fold terminated in a network of relatively short threads, none of which was seen attached to the walls of the atrium.
Light and electron microscopy
Distributed in the mesenchyme and connective tissue of specimens of the flap of tissue taken from the hearts of the younger cetacean fetuses (white whale, common minke whale, large baleen whale) was evidence suggestive of developing cardiomyocytes. Incompletely differentiated parallel arrays of fibrils were found in the cytoplasm around the centrally located nuclei of these specimens. The samples of the tissue flap taken from the neonates (harbour porpoise, Atlantic white-sided dolphin) clearly demonstrated centrally located strands and bundles of cardiac muscle bordered on either side, in the subendothelial matrix, by collagen fibres and fibroblasts.
Discussion
This investigation of a number of fetal and neonatal cetaceans has enabled a description to be given, for the first time, of the fold of tissue representing the septum primum which stretches from the foramen ovale into the left atrium of the hearts. The specimens collected represent a larger series of species and families of both the Odontoceti and the Mysticeti than has previously been undertaken. Where specimen availability made it possible there was also a correspondingly broad range in the ages of cetacean fetuses and neonates investigated. It is the first comparative analysis of these structures since the studies by Slijper (1936, 1961). The relatively good quality of tissue preservation shown by the museum specimens, and the availability of professionally collected and conserved animal stranding material (Anon., 2005) have enabled the tunnel-like or tubular shape of this fold of tissue to be visualized by scanning electron microscopy for the first time.
In each specimen, the thin fold of tissue projecting into the left atrium and subtended by the foramen ovale, when viewed from the terminal part of the caudal vena cava, had the appearance of a short tunnel or sleeve. The entrance was formed in part (caudally) by atrial tissue associated with the caudal vena cava and in part (cranially) by the wedge of interatrial tissue termed the crista dividens (Fig. 2). It was in the structure of this fold of tissue that differences were found between specimens.
In general, the walls of the fold in the Odontoceti were largely unfenestrated proximally and fenestrated distally, the latter usually becoming a loose network extending beyond the end of the fold (Figs 3 and 4). The fenestrations, when present, were not uniform in size, being predominantly small proximally and somewhat larger distally. They also tended to be located on the side of the fold that was not directly under the pulmonary venous return. The strands of tissue forming the distal network were not uniform in thickness, length or structure. Occasionally thin, round threads bisected openings bounded by much thicker ribbon-like strands of tissue. The surfaces of the latter, and the surfaces of the unfenestrated portion, often showed undulations and wrinkles suggestive of fibrous subendothelial structures and possibly of infilled previous fenestrations. Histological studies showed that strands of cardiac muscle were responsible for this appearance. In many of the toothed whales individual threads from the network inserted into the interatrial septum or the atrial wall.
Early reports of the fold of tissue, although much less detailed, gave descriptions which corresponded to this general picture. The interatrial septum in the Franciscana (Pontoporia blainvillei) [L = 700 mm; G = 10.5 months], a member of the Odontocete family Platanistidae, was described as being membranous, narrow and thin walled, and was perforated during fetal life (Burmeister, 1869). Another report, of a near-term dolphin fetus (possibly Stenellasp.) stated that an ‘exceedingly delicate membrane’ was found associated with the foramen ovale (Jackson, 1845). Although the fenestrated fold of tissue attached to the foramen ovale of the white-beaked dolphin (Lagenorhynchus albirostris) was depicted by Burne (Fraser, 1952), it is not possible to derive a detailed understanding of that structure from his illustration. Rather more information was given of a near term pigmy sperm whale (Kogia breviceps) fetus [L = ~1200 mm; G = ~11 months] by Kernan & Schulte (1918). They found that the fold of tissue extending from the foramen ovale into the left atrium seemed ‘adequate to effect complete closure were it not for its extensive fenestration, for in much of its extent it abounds in minute but close set perforations’.
It was apparent from the present study that the interatrial septum played a structural role as the ‘floor’ of the tunnel into the left atrium, the fold of tissue comprising its walls and roof. This was also clear from the illustration of the fold of tissue from the pigmy sperm whale (Kernan & Schulte, 1918). Only in the hearts of the killer whale and the specimen presumed to be a sperm whale was there evidence that it was the last part of the fold that became ‘sleeve-like’ (Fig. 6a).
The structure of the corresponding fold of tissue in the Mysticeti was somewhat different from that in the Odontoceti in that the interatrial septum played a lesser role. In most cases the fold of tissue was ‘sleeve-like’ for much or all of its pathway into the lumen of the left atrium. The distal end of this sleeve was also somewhat different in five of the fin whales, as well as the bowhead, humpback and ‘baleen’ whales in that the threads were restricted to a network attached to the fold rather than making any contact with the atrial tissue (Figs 5a,d and 6b). This confirmed early observations by Knox (1838), Eschricht (1869) and Schulte (1916). Knox (1838) reported that, when the heart of a bowhead whale fetus (720 mm) was opened, ‘the foramen ovale was found altogether peculiar, a membranous sac, the size of a full sized thimble, presenting at the bottom a delicate reticulated net-work, occupied its place, and projected into the left auricle’. Eschricht (1869) depicted this sack-shaped net (Fig. 7a).
Fig. 7.
(a) The network of threads at the end of the tubular fold of tissue subtended by the foramen ovale of a bowhead whale (Balaena mysticetus) fetus as depicted by Eschricht (1869). (b) The fenestrated thimble-shaped fold of tissue subtended by the foramen ovale of a sei whale (Balaenoptera borealis) fetus as depicted by Schulte (1916). (c) The fenestrations at the distal end of the tubular fold of tissue subtended by the foramen ovale of a fin whale (Balaenoptera physalis) fetus as depicted by Walmsley (1938).
The blue whale fetus studied by Turner (1870) was at a later stage of gestation (5944 mm long) than the fetus in the present study. He found that the ‘almost circular foramen readily admitting five extended digits ... Surrounding this opening and attached to its edge, a loose, membranous, annular fold, formed by a duplication of the endocardium was seen. When put on the stretch it projected into the auricle, and the projecting border was free and pierced with large fenestrae.’ A somewhat similar description was given of a slightly smaller fetus of the same species by Beauregard & Boulart (1897). The fold of tissue from the female fetus (375 mm) of a sei whale (Balaenoptera borealis) [L = ~4400 mm; G = ~11 months] dissected by Schulte (1916) was described as ‘highly peculiar in that it is attached in its entire circumference to the limbus and has no free edge. It protrudes in the left atrium as a long funnel or cornucopia with a fenestrated fundus’ (see Fig. 7b). This funnel extended ‘completely free into the left atrium and was not maintained in position by reticnacula (sic), save that near its base ventrally a very small fold, like a minute frenulum, connects it with the wall of the atrium.’ His illustration resembles the situation found in the different species of baleen whales of the present study (see Figs 5 and 6b). It also fits the description of a fin whale fetus about half-way through gestation (Walmsley, 1938) in which a thimble-shaped membranous sac, with a fenestrated distal part, was seen projecting into the cavity of the left atrium from the septal wall (see Fig. 7c).
Many of the specimens in the present study came from fetuses estimated from the available data to be at early to mid stages of gestation (Laws, 1959; Lockyer, 1981). Others were from animals either just before or after birth. This range of ages might have been expected to have some influence on the structures of the flap of tissue. However, few age-related changes of a macroscopic nature were observed between fetuses of the same species. Similar results were obtained from variously aged fetuses of domestic animal species, which suggested that relatively little change in the overall structure of the tissue fold took place in the course of gestation (Macdonald et al. 1988; Macdonald, 1988). However, studies in the rat have demonstrated that there is a spurt of growth in the length and thickness of the septum primum fold at the time of birth (Momma et al. 1992). We did not have available a comparable series of fetuses at the end of gestation. However, we did observe in older fetuses what appeared to be the partial in-filling of fenestrations, a degree of subendothelial fibrousness and corresponding histological changes in the structure of the tissue fold. At younger ages presumptive cardiac muscle cells were loosely distributed in the connective tissue. By the end of gestation bundles of cardiac cells lay below the subendothelial connective tissue. Other studies have similarly demonstrated cardiac muscle beneath the endothelium in the human, horse and sheep fetus (Patten, 1931; Leach, 1940; Macdonald et al. 1988), and in one experiment in which this fold of tissue was rapidly removed from the hearts of newborn lambs it could be observed to contract rythmically in vitro (Barclay & Franklin, 1938). We may expect therefore that this tissue fold in near-term cetacean fetuses contracts in synchrony with the atria against the sides of the volume of blood passing from the caudal vena cava through its lumen into the left atrium.
The usefulness of placental and other fetal membranes in the assessment of phylogenetic relationships above the family level has been recognized for some time (Mossman, 1937, 1953, 1987). The relative conservatism of morphology of fetal membranes, and the absence of significant differences in placental structures within families, can be explained by the concept that the growth and function of these structures are restricted to the environment of the uterus, and therefore not directly subject to the selective pressures of the external environment (Mossman, 1953, 1987). Similar arguments may be extended to an analysis of foramen ovale morphology, the physiological function of which is also restricted to the fetal period of development (Edelstone & Rudolph, 1979; Kiserud et al. 1992). There is consistency between cetaceans in the presence of threads at the distal end of the tissue fold. However, differences were apparent between the two suborders of the Cetacea in the extent to which these threads formed networks, were attached to the interatrial septum and walls of the atria, and the degree to which the interatrial septum contributed towards the formation of the passage way into the left atrium.
The general structure of the cardiac tissue fold of the whales also bore many similarities to those of the ruminants and equids (Patten, 1931; Leach, 1940; Macdonald et al. 1988). However, in particular, the developed remains of the cardiac septum primum of the Mysticete whales appeared to be very like those of the Hippopotamidae (Krahnert, 1942; Franklin et al. 1942; Macdonald, 1988) in which a proximally unfenestrated and distally fenestrated sleeve-like fold of tissue projects from the foramen ovale into the left atrium of both the common hippopotamus (Hippopotamus amphibius) and the pigmy hippopotamus (Choeropsis liberiensis), and in each a network of threads covers its distal end (Fig. 8a,b). Partial autolysis of the tissue fold from the pigmy hippopotamus revealed a similarly branched structure to the subendothelial strands of tissue which were approximately parallel in arrangement around the lumen of the fold. Recent molecular data have lent considerable support to the hypothesis that Cetatacea are phylogenetically nested within Artiodactyla as a sister taxa to Hippopotamidae (Arnason et al. 2004; Price et al. 2005). The traditional clustering of the hippopotamids with the suids (pigs) and tayassuids (peccaries) to form the Suiformes is not supported by the molecular data nor by the significant differences found between the hippos and the pig-like animals in the structure of this fold of cardiac tissue (Macdonald, 1988, 1994). The nomenclature used here for Hippopotamidae is that of Boisserie (2005).
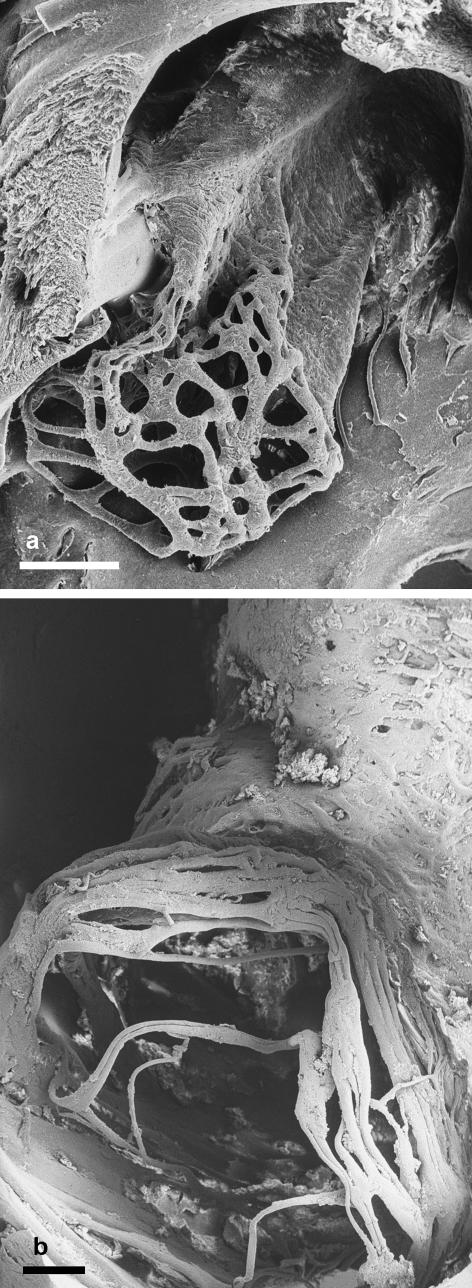
Fig. 8.
(a) The fenestrated sleeve-like fold of tissue subtended by the foramen ovale of a hippopotamus (Hippopotamus amphibius) fetus (crown–rump length = 20 cm) illustrating the network of threads at its distal end. Scale bar = 1 mm. (b) The partially autolysed fold of tissue from the foramen ovale of a pigmy hippo (Choeropsis liberiensis) neonate with the branched fibrous subendothelial tissue exposed. The threads at the distal end of the fold of tissue form a loose network. Scale bar = 1 mm.
The impact of the structure of the foramen ovale tissue fold on the physiology of the cetacean fetus can only be a matter of conjecture in the absence of experimental studies. The loose arrangement of the network of threads in young toothed whales would suggest that it presents little resistance to the flow of oxygenated blood into the left atrium. The relative closeness of the foramen ovale to the outlet from the ductus venosus also implies that the accelerated blood flow from the latter measured in other species may also play a role in the cetacean fetus (Edelstone & Rudolph, 1979; Kiserud et al. 1992). The maintenance of an adequate flow of oxygenated blood from the placenta to the left atrium of the rapidly growing older cetacean fetus could present a range of physiological hurdles not generally experienced by most non-diving land mammals. Past and current logistical difficulties have meant that the impacts of maternal diving, feeding and other behaviours on the cardiovascular system of the pregnant whale and her fetus largely remain to be studied. The application of recent techniques and relatively non-invasive technology may help to overcome these problems.
Following birth of the youngster the rapid volume flow of blood from the placenta through the ductus venosus and into the foramen ovale is terminated. Instead the lungs expand, blood flows from the right ventricle to the respiratory alveoli for re-oxygenation, and returns to discharge from the pulmonary veins onto the largely unfenestrated wall of the fold tissue subtended by the foramen ovale. This change in direction of the volume flow into the left atrium contributes to the collapse of the tissue fold against the interatrial septum and the covering of the foramen ovale. Slijper (1961) presented data for the porpoise and reviewed the small amount of available literature on the timing of closure of the foramen ovale in other neonatal cetaceans which together indicated that this takes place within about 10 days of birth. Closure is effected by the tissue fold hinging along the edge of the foramen adjacent to the terminal portion of the caudal vena cava (Kernan & Schulte, 1918). We found in neonatal porpoise that both bulbous growth and knotting of the distal end of the flap was associated with the closure of the foramen ovale. The threads which anchored the distal end of the fold to the atrial septum appeared to have coallesced with their neighbours and short threads of tissue anchored the fold to the interatrial septum. Schulte (1916) also commented that adhesions at the base of the valve may be seen as the initiation of a process of closure in the larger whales. In the horse and ox, knotting of the corresponding threads at the distal end of the tissue fold seems to be an integral part of the closure of the foramen ovale (Ottoway, 1944; Macdonald et al. 1988). A similar process appeared to be employed by the neonatal dolphins of the present study.
In conclusion, the shape of the fold of tissue which develops from the cardiac septum primum was somewhat similar in a range of cetacean species. In the toothed whales the tunnel-shaped tissue fold was fenestrated at its distal end and had the interatrial septum as the floor. In baleen whales the tissue fold was more sleeve-like with fenestrations at its distal end. In toothed whales thin threads extended from the fold to insert into the interatrial septum whereas a network of threads covered the distal end of the sleeve of the latter. Similar structures were present in the corresponding cardiac tissues of neonatal Hippopotamidae.
Acknowledgments
We would like to thank the curators of mammals and administrative staff of the following institutions for making available for study the material in their collections: Dundee City Museum; The University of Dundee, Scotland; Glasgow Museums and Art Galleries, Scotland; the Koninklijke Maatschappij voor Dierkunde van Antwerpen, Belgium; Dr Iain Boyd of the Sea Mammal Research Institute, St Andrews, Scotland; Dr Ingvar Byrkjedal of the University of Bergen Museum of Zoology, Norway; Dr Chris Smeenk and Marianne Addink of the Rijksmuseum van Natuurlike Historie, Leiden, the Netherlands; Dr Andrew Kitchener of the Royal Museum of Scotland; and Dr Renate Angermann of das Zoologisches Museum, Museum fur Naturkunde der Humboldt-Universität zu Berlin, Germany. Grateful thanks also to Mr Harry Ross of the Scottish Agricultural Colleges Veterinary Investigation Service for access to specimens from mammal strandings around the Scottish coasts, and to Dr Thijs Kuiken for access to specimens from mammal strandings around the English and Welsh coasts. We also gratefully acknowledge the technical assistance of Gunnar Langhelle, Steven Mitchell and Derek Pennman and the assistance of Colin Warwick in the preparation of illustrations and photography. Without the hospitality of Ute and ‘Achin Beermann and their family, the late Dr Hans Frädrich, Director of Berlin Zoo, and Dr Nini Yang, Director of the International Office of the University of Edinburgh, Beijing, this study would not have been completed. The project was supported by the Balloch Trust and the University of Edinburgh.
References
- Amoroso EC, Barclay AE, Franklin KJ, Prichard MML. The bifurcation of the posterior caval channel in the eutherian foetal heart. J Anat. 1942;76:240–247. [PMC free article] [PubMed] [Google Scholar]
- Anon . Out of the Blue: the UK Whale and Dolphin Stranding Scheme. London: Natural History Museum; 2005. [Google Scholar]
- Arnason U, Gullberg A, Janke A. Mitogenomic analyses provide new insights into cetacean origin and evolution. Gene. 2004;333:27–34. doi: 10.1016/j.gene.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Barclay AE, Franklin KJ. The time of functional closure of the foramen ovale in the lamb. J Physiol. 1938;94:256–258. doi: 10.1113/jphysiol.1938.sp003678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartholin T. Historiarum anatomicarum rariorum Centuriae II; historia XXV. Amstelodami: 1654. [Google Scholar]
- Beauregard H, Boulart R. Recherches anatomiques sur les Balænides. Nouv Arch Mus Hist Nat Paris 3rd Ser. 1897;9:95–112. [Google Scholar]
- Boisserie JR. The phylogeny and taxonomy of Hippopotamidae (Mammalia: Artiodactyla): a review based on morphology and cladistic analysis. Zool J Linn Soc. 2005;143:1–26. [Google Scholar]
- Boyd IL, Lockyer C, Marsh HD. Reproduction in marine mammals. In: Reynolds JE, Rommel SA III, editors. Biology of Marine Mammals. Washington, DC: Smithsonian Institution Press; 1999. pp. 218–286. [Google Scholar]
- Burmeister G. Descripcion de cuatro especies de delfinides de la costa Argentina en el océano atlántico. An Mus Nac Hist Nat B Aires. 1869;1:367–442. [Google Scholar]
- Butler PJ. Metabolic regulation in diving birds and mammals. Resp Physiol Neurobiol. 2004;141:297–315. doi: 10.1016/j.resp.2004.01.010. [DOI] [PubMed] [Google Scholar]
- Cohen AL. Critical point drying principles and procedures. Scanning Electron Microscopy. 1979;II:303–323. [Google Scholar]
- Echlin P. Splutter coating techniques for scanning electron microscopy. Scanning Electron Microsc. 1975;II:217–224. [Google Scholar]
- Edelstone DI, Rudolph AM. Preferential streaming of ductus venosus blood to the brain and heart in fetal lambs. Am J Physiol. 1979;237:H724–H729. doi: 10.1152/ajpheart.1979.237.6.H724. [DOI] [PubMed] [Google Scholar]
- Elsner R. Living in water: solutions to physiological problems. In: Reynolds JE, Rommel SA III, editors. Biology of Marine Mammals. Washington, DC: Smithsonian Institution Press; 1999. pp. 73–116. [Google Scholar]
- Eschricht DF. De organis, quae respirationi et nutritioni foetus mammalium inserviunt. Hafniae: JH Schultz; 1837. [Google Scholar]
- Eschricht DF. Zoologisch-anatomisch-physiologische Untersuchungen über die nordischen Walthiere. Leipzig: L. Voss; 1849. [Google Scholar]
- Eschricht DF. Ni Tavler til Oplysning af Hvaldyrenes Bygning. Det Kongelige Danske Videnskabernes Selskabs Skrifter, 5. Række, Naturvidenskabelig og Mathematisk Afdeling 9 B. 1. Kjobenhavn: Muhle; 1869. [Google Scholar]
- Franklin KJ, Amoroso EC, Barclay AE, Prichard MML. The valve of the foramen ovale and its relation to pulmonary vein entries. Vet J. 1942;98:29–41. [Google Scholar]
- Fraser FC. Handbook of R. H. Burne's Cetacean Dissections. London: British Museum (Natural History); 1952. [Google Scholar]
- Gambell R. Seasonal cycles and reproduction in sei whales of the Southern Hemisphere. Discovery Rep. 1968;35:31–134. [Google Scholar]
- Hong YM, Choi JY. Pulmonary venous flow from fetal to neonatal period. Early Human Dev. 2000;57:95–103. doi: 10.1016/s0378-3782(99)00058-4. [DOI] [PubMed] [Google Scholar]
- Jackson JBS. Dissection of a spermaceti whale and three other cetaceans. Boston J Nat Hist. 1845;5:137–171. [Google Scholar]
- Kernan JD, von Schulte HW. Memoranda upon the anatomy of the respiratory tract, foregut and thoracic viscera of a foetal Kogia breviceps. Bull Am Mus Nat Hist. 1918;38:231–267. [Google Scholar]
- Kiserud T, Eik-Nes SH, Blaas HG, Hellevik LR. Foramen ovale: an ultrasonographic study of its relation to the inferior vena cava, ductus venosus and hepatic veins. Ultrasound Obstet Gynec. 1992;2:389–396. doi: 10.1046/j.1469-0705.1992.02060389.x. [DOI] [PubMed] [Google Scholar]
- Knox FJ. Catalogue of anatomical preparations illustrative of the whale, particularly the Great northern rorqual (Balaena maximus borealis), now exhibiting in the pavilion, North College Street. Edinburgh: Neill; 1838. [Google Scholar]
- Krahnert R. Zur Anatomie des Flusspferdeherzen (Hippopotamus amphibius L. und Choeropotamus liberiensismort) Z Wiss Zool. 1942;155:317–342. [Google Scholar]
- Kükenthal W. Vergleichend anatomische und entwicklungsgeschichte Untersuchungen an Waltieren. Denkschr Med-Naturw Ges Jena. 1893;3:1–448. [Google Scholar]
- Kükenthal W. Untersuchungen an Walen (Zweiter Teil) Jenaische Z Naturw. 1914;51:1–122. [Google Scholar]
- Laws RM. The foetal growth rates of whales, with special reference to the Fin Whale, Balaenopter physalus. Discovery Rep. 1959;29:281–308. [Google Scholar]
- Leach EH. Footnote. In Franklin, K.J., Barclay, A.E. & Prichard, M.M.L. Some observations on the cardio-vascular system in the viable foetal lamb. J Anat. 1940;75:75–87. [PMC free article] [PubMed] [Google Scholar]
- Lockyer CH. Growth and energy budgets of large baleen whales from the Southern Hemisphere. FAO Fish Series. 1981;5 III:379–487. [Google Scholar]
- Lockyer CH. Review of Baleen Whale (Mysticeti) reproduction and implications for management. Rep Int Whaling Comm Spec Issue. 1984;6:27–50. [Google Scholar]
- Macdonald AA. Comparative anatomy of the foramen ovale in the Suina. Anat Embryol. 1988;178:53–57. doi: 10.1007/BF00305014. [DOI] [PubMed] [Google Scholar]
- Macdonald AA. The placenta and cardiac foramen ovale of the babirusa (Babyrousa babyrussa) Anat Embryol. 1994;190:489–494. doi: 10.1007/BF00235496. [DOI] [PubMed] [Google Scholar]
- Macdonald AA, Fowden AL, Ousey J, Silver M, Rossdale PD. The foramen ovale of the fetal and neonatal foal. Equine Vet J. 1988;20:255–260. doi: 10.1111/j.2042-3306.1988.tb01517.x. [DOI] [PubMed] [Google Scholar]
- Momma K, Ito T, Mori Y, Yamamura Y. Perinatal adaptation of the cardiovascular system. Early Human Dev. 1992;29:167–170. doi: 10.1016/0378-3782(92)90133-2. [DOI] [PubMed] [Google Scholar]
- Mossman HW. Comparative morphogenesis of the fetal membranes and accessory uterine structures. Carnegie Contr Embryol. 1937;26:129–246. doi: 10.1016/0143-4004(91)90504-9. [DOI] [PubMed] [Google Scholar]
- Mossman HW. The genital system and the fetal membranes as criteria for mammalian phylogeny and taxonomy. J Mammal. 1953;34:289–298. [Google Scholar]
- Mossman HW. Vertebrate Fetal Membranes. New Brunswick: Rutgers University Press; 1987. [Google Scholar]
- Müller HC. Zur entwicklungsgeschichte von Phocaena communis Less. Arch Naturgesch. 1920;86A(7):1–113. [Google Scholar]
- Murakami T, Yamamoto K, Itoshisha T, Irino S. Modified tannin-osmium conductive staining method for non-conductive S.E.M. specimens. Arch Histol Cytol. 1977;40:35–40. doi: 10.1679/aohc1950.40.35. [DOI] [PubMed] [Google Scholar]
- Noren SR, Cuccurullo V, Williams TM. The development of diving bradycardia in bottlenose dolphins (Tursiops truncates) J Comp Physiol B. 2004;174:139–147. doi: 10.1007/s00360-003-0398-9. [DOI] [PubMed] [Google Scholar]
- Oftedal OT. Lactation in whales and dolphins: evidence of divergence between baleen- and toothed-species. J Mamm Gl Biol Neopl. 1997;2:205–230. doi: 10.1023/a:1026328203526. [DOI] [PubMed] [Google Scholar]
- Ottoway CW. The anatomical closure of the foramen ovale in the equine and bovine heart: a comparative study with observations on fetal and adult states. Vet J. 1944;100:111–118. 130–134. [Google Scholar]
- Pabst DA, Rommel SA, McLellan WA. The functional morphology of marine mammals. In: Reynolds JE, Rommel SA III, editors. Biology of Marine Mammals. Washington, DC: Smithsonian Institution Press; 1999. pp. 15–72. [Google Scholar]
- Patten BM. The closure of the foramen ovale. Am J Anat. 1931;48:19–44. [Google Scholar]
- Price SA, Bininda-Emonds ORP, Gittleman JL. A complete phylogeny of the whales, dolphins and even-toed hoofed mammals (Cetartiodactyla) Biol Rev. 2005;80:445–473. doi: 10.1017/s1464793105006743. [DOI] [PubMed] [Google Scholar]
- Reeves RR, Smith BD, Crespo EA, Notarbartolo DI, Sciara G. Dolphins, Whales and Porpoises: 2002–2010 Conservation Action Plan for the World's Cetaceans. Gland, Switzerland and Cambridge, UK: IUCN/SSC Cetacean Specialist Group; 2003. [Google Scholar]
- Risting S. Whales and Whale Foetuses: Statistics of Catch and Measurements Collected from the Norwegian Whalers’ Association, 1922–25. Copenhagen: Host; 1928. [Google Scholar]
- Sampson DB. Foetal Growth in Southern Fin Whales. Portsmouth: Portsmouth Polytechnic Centre for Marine Resource Economics; 1989. [Google Scholar]
- Schulte H von W. Anatomy of a foetus of Balaenoptera borealis. Mem Am Mus Nat Hist NS. 1916;1:289–502. [Google Scholar]
- Sedmera D, Misek I, Klima M, Thompson RP. Heart development in the spotted dolphin (Stenella attenuata) Anat Rec A. 2003;273:687–699. doi: 10.1002/ar.a.10086. [DOI] [PubMed] [Google Scholar]
- Slijper EJ. Die Cetaceen Vergleichend-Anatomisch und Systematisch. 's-Gravenhage: Martinus-Nijhoff; 1936. [Google Scholar]
- Slijper EJ. Foramen ovale and ductus arteriosus botalli in aquatic mammals. Mammalia. 1961;25:528–570. [Google Scholar]
- Turner W. An account of the Great Finner-Whale, stranded at Longniddry. Part 1: The soft parts. Trans R Soc Edinb. 1870;26:197–251. [Google Scholar]
- Walmsley T. Some observations on the vascular system of a female fetal finback. Contrib Embryol. 1938;164:107–187. [Google Scholar]
- Wells RS, Boness DJ, Rathbun GB. Behavior. In: Reynolds JE, Rommel SA III, editors. Biology of Marine Mammals. Washington, DC: Smithsonian Institution Press; 1999. pp. 324–422. [Google Scholar]