Abstract
We cloned two hemoglobin genes from Arabidopsis thaliana. One gene, AHB1, is related in sequence to the family of nonsymbiotic hemoglobin genes previously identified in a number of plant species (class 1). The second hemoglobin gene, AHB2, represents a class of nonsymbiotic hemoglobin (class 2) related in sequence to the symbiotic hemoglobin genes of legumes and Casuarina. The properties of these two hemoglobins suggest that the two families of nonsymbiotic hemoglobins may differ in function from each other and from the symbiotic hemoglobins. AHB1 is induced, in both roots and rosette leaves, by low oxygen levels. Recombinant AHB1 has an oxygen affinity so high as to make it unlikely to function as an oxygen transporter. AHB2 is expressed at a low level in rosette leaves and is low temperature-inducible. AHB2 protein has a lower affinity for oxygen than AHB1 but is similar to AHB1 in having an unusually low, pH-sensitive oxygen off-rate.
Leghemoglobins, the first hemoglobins to be identified in higher plants (1), are found in the nodules of legumes where they are believed to transport oxygen to nitrogen-fixing endosymbiotic bacteria (2). Nodule-expressed hemoglobins also have been identified in Casuarina glauca, a nitrogen-fixing nonlegume (3). Collectively, these nodule-expressed hemoglobins are called “symbiotic hemoglobins” (4). In addition to symbiotic hemoglobins, these plants have another hemoglobin that shows only limited amino acid sequence similarity to the symbiotic hemoglobins. Genes encoding this type of hemoglobin also have been cloned from the nitrogen-fixing elm Parasponia andersonii (5, 6) and from plants that do not fix nitrogen, including monocots (6–8). These “nonsymbiotic” hemoglobins are typically expressed at low levels in root tissue. The Parasponia hemoglobin is unusual in that it appears to be bi-functional; it transports oxygen in nitrogen-fixing nodules and, like the nonsymbiotic hemoglobins, is expressed in root tissue (6). The function of nonsymbiotic hemoglobins in normal plant tissues has not been established. To examine the function of nonsymbiotic hemoglobins, we have cloned hemoglobins from Arabidopsis thaliana.
MATERIALS AND METHODS
Plant Growth and Stress Treatments.
Arabidopsis C24 ecotype seeds were surface sterilized, then grown on MS agar with 3% sucrose, 40–50 plants/plate, at 22°C (16/8 h light/dark cycle) until harvest. Plants used for AHB1 induction analysis were grown on agar without sucrose. Dehydration, chilling, and hypoxia treatments were performed as outlined in Dolferus et al. (9). All treatments, except chilling (24 h), were for 12 h unless specified. Sucrose treatment involved placing plantlets in MS with 1% sucrose overnight. RNA was extracted using the method of Logemann et al. (10) and separated on formaldehyde gels as described by Dolferus et al. (9); RNA gel blotting, hybridization, and posthybridization washes also were done as described by Dolferus et al. RNA loadings were calibrated with an Arabidopsis Ubiquitin riboprobe.
Library Screening, Subcloning, and Sequencing.
Arabidopsis genomic libraries (9- to 15-kb fraction of Sau3A-digested C24 genomic DNA in λEMBL4) were screened with random primed probes (11). Screening and subsequent subcloning involved standard conditions and DNA manipulation, as described in Ausubel et al. (11). Automated DNA sequencing, with both dye primer and dye terminator reactions, was performed according to company specifications (Perkin–Elmer).
Reverse Transcriptase-PCR Analysis.
Reverse transcriptase-PCR was carried out on first strand cDNA that was synthesized from RNA with Moloney murine leukemia virus reverse transcriptase as per company specifications (Promega). PCR was then performed with specific primers (100 nM) and standard PCR conditions (11). The resulting reverse transcriptase-PCR products were cloned and sequenced, confirming that the predicted introns are absent from the final transcript.
Southern Analysis.
Genomic DNA was extracted using the method of Taylor and Powell (12). Hybridization and post hybridization treatment was as described in Ausubel et al. (11).
Database Search.
Database searches were conducted through key word searching of the Expressed Sequence Tag database on the National Center for Biotechnology World Wide Web site (http://www.ncbi.nlm.nih.gov).
Expression, Purification, and Analysis Of Recombinant Proteins.
cDNA clones of AHB1 and AHB2 were fused to an efficient ribosomal binding site, as per Springer and Sligar (13), and inserted into the pET24(+) expression vector (Novagen). Escherichia coli, strain BL21(DE3), containing each expression vector was grown to OD600nM of 0.6, and then recombinant protein expression was induced with 1 mM isopropyl β-d-thiogalactoside. After 4 h at 37°C, cells were centrifuged and resuspended in 100 mM Tris⋅HCl (pH 8.0), sonicated, and cleared by centrifugation; 50 mg/ml hemin⋅HCl was added to the soluble fraction. Recombinant AHB1 protein was purified by 25–70% ammonium sulfate fractionation, resuspended in 20 mM Tris⋅HCl (pH 8.0), and dialyzed twice into 4 liters of the same buffer. Dialyzed extracts were concentrated (YM-10 membrane, Amicon) and passed over a cation exchange column (DE52 Whatman) in 20 mM Tris⋅HCl (pH 8.0). Red fractions were concentrated, adjusted to pH 6.0 with dilute acetic acid, and passed over an anion exchange column (CM52, Whatman) with 20 mM sodium phosphate buffer (pH 8.0). The red fraction was concentrated to 10 mg/ml and stored at −80°C. Recombinant AHB2 protein was purified as was described for AHB1, with the following modification: 25–90% ammonium sulfate fractionation. Elution from DE52 resin was achieved with 250 mM Tris⋅HCl, following 5 column volumes of 100 mM Tris⋅HCl wash buffer. After elution from CM52 resin, red fractions were adjusted to 25% ammonium sulfate saturation (pH 8.0) and passed over a phenol-Sepharose column (equilibrated with 20 mM Tris⋅HCl/25% ammonium sulfate) then eluted with 20 mM Tris⋅HCl/12.5% ammonium sulfate and concentrated to 10 mg/ml for storage. Rate constants for O2 and CO binding were measured using rapid mixing and laser photolysis methods (14).
RESULTS
A Nonsymbiotic Hemoglobin Gene from Arabidopsis.
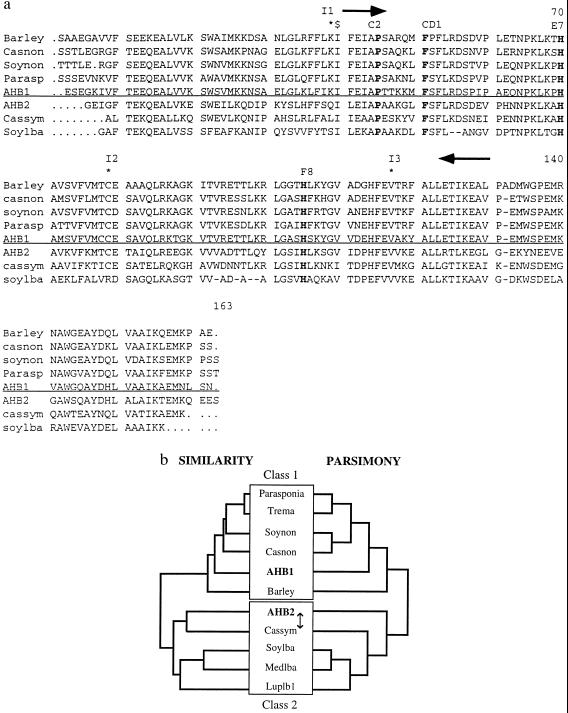
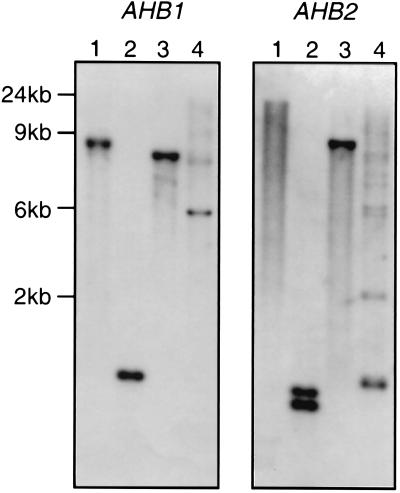
Using degenerate primers that amplified a nonsymbiotic hemoglobin gene fragment from soybean (7), we amplified a 459-bp fragment of a nonsymbiotic hemoglobin gene from Arabidopsis. This gene fragment was used to isolate a full-length hemoglobin gene, AHB1, with a predicted amino acid sequence that shows strong homology to the sequences of nonsymbiotic hemoglobins from other plants (Fig. 1 a and b), including predicted ORFs containing amino acid residues that are conserved in all plant hemoglobins [the proximal F8 and distal E7 histidines, phenylalanine CD1 and proline C2 (15)]. There are three introns in positions identical to the introns occurring in all other plant hemoglobins (Fig. 1a). Southern analysis, at high stringency, suggests this gene is present in single or low copy number in the Arabidopsis genome (Fig. 2).
Figure 1.
(a) Alignment of some plant hemoglobin protein sequences. Proximal and distal histidines, phenylalanine CD1, and proline C2 are shown in bold. The location of primers used to amplify AHB1 PCR fragment are shown as arrows. $ represents the 5′ end of the AHB2 cDNA (Clone ID 11161). Intron positions are marked with stars and labeled I1-I3. Class 1 and 2 hemoglobins are separated by the horizontal line. (b) Cladistic analysis of plant hemoglobins. The left tree is a similarity tree of plant hemoglobin protein sequences created using the Genetics Computer Group pileup program. Class 1 and 2 hemoglobins form two distinct clades. The right tree is one of the two most parsimonious trees (CI .855, RC .654) generated by an exhaustive search of all possible trees using the paup, Ver. 3.1.1 program. The two trees of maximum parsimony differed in whether they grouped the Casuarina symbiotic hemoglobin or AHB2 with the leghemoglobins (represented by the arrow). The bootstrap value for the basal branch of this tree is 100%. The two classes of plant hemoglobins are highlighted by boxes. Hemoglobins (and GenBank accession numbers): barley: barley nonsymbiotic hemoglobin (U01228); casnon: Casuarina nonsymbiotic hemoglobin (X53950); soynon: soybean nonsymbiotic hemoglobin (U47143); parasp: Parasponia hemoglobin (U27194); AHB1: This study (U94998); AHB2: this study (U94999); cassym: Casuarina symbiotic hemoglobin (77695); soylba: soybean leghemoglobin A (J01299); medslba: Medico sativa leghemoglobin A (X14311); luplb1: lupin leghemoglobin 1 (Y00401).
Figure 2.
Southern blots of AHB1 and AHB2. Arabidopsis (C24 ecotype) genomic DNA digested with BamHI, DraI, EcoRI, and XbaI (lanes 1, 2, 3, and 4, respectively) and hybridized with AHB1 and AHB2 cDNA probes. (Extra bands on the XbaI lanes are due to partial digestion.)
A Leghemoglobin-Like Gene from Arabidopsis.
We identified a second hemoglobin gene (AHB2) by searching the Arabidopsis Expressed Sequence Tag database. The single partial length cDNA showing homology to plant hemoglobins (clone ID 11161) does not correspond in sequence to the AHB1 gene, with only 69% identity at the nucleotide level. This gene, AHB2, has three introns in identical positions to those of other plant hemoglobins and, like AHB1, appears to be a single copy gene according to Southern blot data (DraI cuts within the coding sequence of AHB2, producing two bands in this lane; Fig. 2). When the amino acid sequence of AHB2 is aligned with other plant hemoglobin protein sequences, it appears to be more similar to the symbiotic hemoglobins of Casuarina and legumes than it does to nonsymbiotic plant hemoglobins (Fig. 1a). If the AHB2 protein sequence is used in a blast (basic local alignment search tool) search, it consistently detects symbiotic hemoglobins with higher blast scores than most nonsymbiotic hemoglobins. A similarity tree of plant hemoglobin protein sequences, constructed with the pileup function of the Genetics Computer Group package, also shows AHB2 to be more similar to the symbiotic hemoglobins of legumes and Casuarina (Fig. 1b). The same clade occurs in the two trees of maximum parsimony constructed using the paup program, Ver. 3.1.1, one of which is presented (Fig. 1b). The bootstrap value for the basal branch of this clade is high (100%), supporting the grouping of AHB2 with the symbiotic hemoglobins of legumes and Casuarina.
AHB1 Is Expressed in Root Tissue and Is Hypoxia-Inducible.
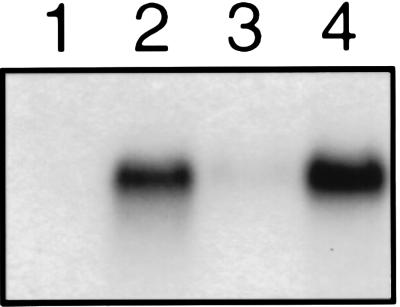
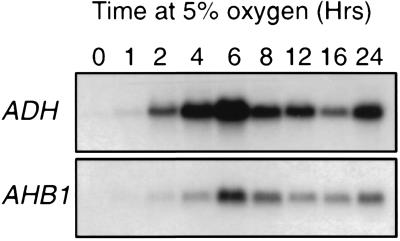
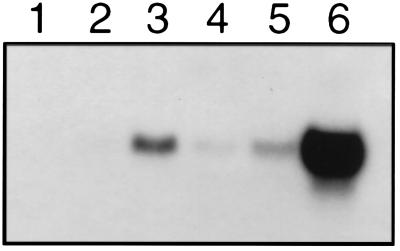
AHB1 expression, examined with RNA gel blots, is detectable at low levels in root tissue and is strongly induced in both the roots and rosette leaves of plants subjected to hypoxic conditions (Fig. 3). The time course of the AHB1 response to low oxygen levels is similar to that of the alcohol dehydrogenase gene (ADH), a well characterized hypoxic response gene (ref. 16; Fig. 4). The maximum level of AHB1 transcript was observed after 6–8 h of hypoxia treatment. Transcription of AHB1 is induced under a 5% oxygen environment, but stronger induction occurs at 0.1% oxygen (Fig. 5). A transcriptional response also was elicited by a 1% sucrose solution. Cold, dehydration, heat shock, oxidative stress, and wounding treatments did not affect AHB1 transcript levels.
Figure 3.
AHB1 expression in root and rosette tissues. Total RNA extracted from rosette leaves (lane 1), rosettes treated with 5% oxygen (lane 2), roots (lane 3), and roots treated with 5% oxygen (lane 4) hybridized with an AHB1 antisense riboprobe.
Figure 4.
Time course analysis of the AHB1 hypoxia response. Total RNA from whole plants treated with 5% oxygen for varying times hybridized with either AHB1 or ADH antisense riboprobes.
Figure 5.
AHB1 expression during environmental stress treatments. Total RNA from control plants (lane 1) or plants subjected to chilling (lane 2), 1% sucrose (lane 3), dehydration (lane 4), 5% oxygen (lane 5), or 0.1% oxygen (lane 6) hybridized with an AHB1 antisense riboprobe.
AHB2 Is Expressed in Rosette Leaves.
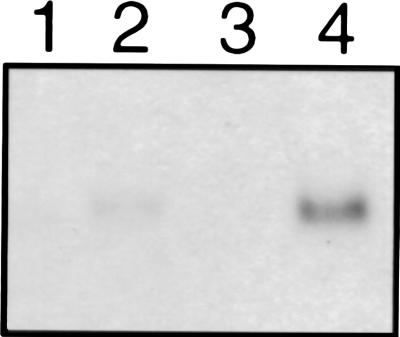
A low level of AHB2 expression was found in rosette leaves, but unlike AHB1, there was no detectable expression in root tissue. AHB2 expression was not affected by sucrose or hypoxia treatment, but low temperature did increase AHB2 transcript levels (Fig. 6). The low temperature response seems to be confined to the rosette leaves with no response evident in root tissue (Fig. 6). Wounding, heat shock, oxidative stress, and dehydration had no effect on the level of AHB2 transcript.
Figure 6.
Induction of AHB2 by low temperature. Total RNA from root tissue (lane1) or rosette leaves (lane 2) and root tissue after chilling treatment (lane 3) or rosette leaves after chilling treatment (lane 4) hybridized with an AHB2 antisense riboprobe.
Recombinant AHB1 and AHB2 Proteins Have Different Oxygen Binding Properties.
Recombinant AHB1 protein, expressed in E. coli, was purified, and the kinetics of O2 and CO binding were measured. AHB1 shows a remarkably high O2 affinity, with a P50 value equal to 1.6 nM at pH 7, 20°C (Table 1). Similar oxygen binding parameters have been observed for the nonsymbiotic hemoglobins of soybean and rice (R.A.W., unpublished data, and M. Hargrove, personal communication). Lowering the pH to 5 raises the P50 to 6.7 nM (Table 1), but this value is still smaller than that of any known plant cytochrome oxidase (17), making it unlikely that AHB1 could transport oxygen to these terminal oxidases. AHB1 also has a low O2 dissociation rate constant compared with that of soybean leghemoglobin A, a known oxygen transporter (Table 1). Thus, AHB1 would appear to be an oxygen-scavenging protein rather than an oxygen transporter.
Table 1.
Comparison of parameters* for O2 binding to recombinant hemoglobins at 20°C
| Protein | pH | P50, nM | kO2, s−1 | M KCO/KO2 |
|---|---|---|---|---|
| AHB1 | 7.0 | 1.6 | 0.12 | 2.0 |
| 5.0 | 6.7 | 0.38 | ||
| AHB2 | 7.0 | 130 | 0.14 | 26 |
| 5.0 | 2700 | 2.1 | ||
| Soy lbA† | 7.0 | 44 | 5.6 | 87 |
| 5.0 | 20 | 2.7 |
Where kO2 and k′O2 are the bimolecular dissociation and association rate constants for O2 binding, respectively. The dissolved O2 concentration at which hemoglobin is half saturated (P50) is equivalent to the dissociation equilibrium constant for O2 (K′O2 = kO2/k′O2). KO2 and KCO are the association equilibrium constants for O2 and CO, respectively. M is the partition coefficient expressing the relative affinities for O2 and CO.
Recombinant soybean leghemoglobin A.
In contrast, recombinant AHB2 has a much lower affinity for O2, with P50 values of 130 nM at pH 7 and 2,700 nM at pH 5 (Table 1). The dissociation rate constant is low at pH 7 but increases 20-fold at pH 5, approaching the value for soybean leghemoglobin A under the same acidic conditions. The low association rate constant for O2 binding to AHB2 appears to be caused by the reversible formation of a hexacoordinate complex (hemochrome) in deoxygenated AHB2. The imidazole side chain of the distal histidine (E7), the only polar residue in the distal pocket, may coordinate directly to the iron atom of the heme group, thereby inhibiting rapid O2 binding and facilitating O2 dissociation.
DISCUSSION
AHB1 is related to the nonsymbiotic hemoglobin genes present in other plants. The identification of this gene in Arabidopsis supports the idea that nonsymbiotic hemoglobin genes may be present in all plants (6). The expression pattern of AHB1 is similar to that of other nonsymbiotic hemoglobins; low levels of expression in root tissue are common to all nonsymbiotic hemoglobins (3–8), sucrose inducibility has been observed for the Casuarina nonsymbiotic hemoglobin (4), and the hypoxic induction of AHB1 resembles that of the barley hemoglobin (8).
AHB2 represents a new class of nonsymbiotic hemoglobin gene. It shows limited homology to the other nonsymbiotic hemoglobins and has a different pattern of gene expression. AHB2-like genes may be present in a wide range of plants. In support of this theory, we have cloned an AHB2 homologue from Brassica napus (R.A.W., unpublished data). We suggest that the AHB1-like “nonsymbiotic hemoglobin” gene family be referred to as class 1 plant hemoglobin genes and that AHB2-like genes be referred to as class 2 plant hemoglobin genes.
It has been suggested that symbiotic hemoglobin genes of legumes and Casuarina arose from gene duplication of a nonsymbiotic (class 1) hemoglobin gene in the ancestor of nitrogen-fixing plants (5, 6). The similarity between AHB2 and symbiotic hemoglobins suggests an alternative explanation; a class 2 hemoglobin gene may have been the direct ancestor of the symbiotic hemoglobin genes. In contrast, it appears that a class 1 hemoglobin has been recruited into symbiotic function in Parasponia nodules. The recruitment of either class 1 or class 2 plant hemoglobins into symbiotic roles seems to have been associated with changes in gene expression, leading to high levels of expression in nodules, and with alterations in the oxygen binding properties of the protein.
Leghemoglobins facilitate oxygen flux down an oxygen gradient without the involvement of other proteins. It is unlikely that either AHB1 or AHB2 acts as an oxygen transporter in this manner. The oxygen affinity of AHB1 is too high for release of oxygen to cytochrome oxidase, and the oxygen dissociation rates of both proteins are too slow for efficient oxygen transport. Instead, these proteins may act as oxygen scavengers, binding oxygen until interactions with other proteins occur. The eventual destination for bound oxygen could be mitochondrial metabolism or oxygen-consuming enzymatic reactions. It is also possible that AHB1 or AHB2 have unidentified catalytic properties. Analysis of transgenic plants that over- or underexpress AHB1 or AHB2, combined with cellular localization of these proteins, should lead to a better understanding of the functions of these and other plant hemoglobins.
Acknowledgments
We thank Janice Norman for her excellent technical assistance and Cyril Appleby for his helpful discussions.
Footnotes
References
- 1.Kubo, H. (1939) in Appleby, C. A. (1992) Sci. Prog. 76, 365–398.
- 2.Appleby C A. Annu Rev Plant Physiol. 1984;35:443–478. [Google Scholar]
- 3.Fleming A I, Wittenberg J B, Wittenberg B A, Dudman W F, Appleby C A. Biochim Biophys Acta. 1987;911:209–220. [Google Scholar]
- 4.Jacobsen-Lyon K, Jensen E Ø, Jorgensen J, Marcker K A, Peacock W J, Dennis E S. Plant Cell. 1995;7:213–223. doi: 10.1105/tpc.7.2.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Landsmann J, Dennis E S, Higgins T J, Appleby C A, Kortt A A, Peacock W J. Nature (London) 1986;324:166–168. [Google Scholar]
- 6.Bogusz D, Appleby C A, Landsmann J, Dennis E S, Peacock W J. Nature (London) 1988;331:178–180. doi: 10.1038/331178a0. [DOI] [PubMed] [Google Scholar]
- 7.Andersson C R, Jensen E Ø, Llewellyn D J, Dennis E S, Peacock W J. Proc Natl Acad Sci USA. 1996;93:5682–5687. doi: 10.1073/pnas.93.12.5682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.E. R. Taylor E R, Nie X Z, MacGregor A W, Hill R D. Plant Mol Biol. 1994;24:853–862. doi: 10.1007/BF00014440. [DOI] [PubMed] [Google Scholar]
- 9.Dolferus R, Jacobs M, Peacock W J, Dennis E S. Plant Physiol. 1994;105:1075–1087. doi: 10.1104/pp.105.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Logemann J, Schell J, Willmitzer L. Anal Biochem. 1987;163:16–20. doi: 10.1016/0003-2697(87)90086-8. [DOI] [PubMed] [Google Scholar]
- 11.Ausubel F M, Brenton R, Kingston R E, Moore D D, Siedman J G, Smyth J A, Struhl K. Current Protocols in Molecular Biology. New York: Wiley; 1994. [Google Scholar]
- 12.Taylor A, Powell B. BRL Focus. 1982;4:4. [Google Scholar]
- 13.Springer B A, Sligar S G. Proc Natl Acad Sci USA. 1997;84:8961–8965. doi: 10.1073/pnas.84.24.8961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hargrove M S, Barry J K, Bruckner E A, Berry M B, Phillips G N, Jr, Olson J S, Arrendo-Peter R, Dean J M, Klucas R V, Sarath G. J Mol Biol. 1997;266:1032–1042. doi: 10.1006/jmbi.1996.0833. [DOI] [PubMed] [Google Scholar]
- 15.Chang C, Meyerowitz E M. Proc Natl Acad Sci USA. 1986;83:1408–1412. doi: 10.1073/pnas.83.5.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bashford D, Chothia C, Lesk A M. J Mol Biol. 1987;196:199–216. doi: 10.1016/0022-2836(87)90521-3. [DOI] [PubMed] [Google Scholar]
- 17.Millar A H, Bergerson F J, Day D A. Plant Physiol Biochem. 1994;32:847–852. [Google Scholar]
- 18.Wittenberg J B, Wittenberg B A. Annu Rev Biophys Biophys Chem. 1990;19:217–241. doi: 10.1146/annurev.bb.19.060190.001245. [DOI] [PubMed] [Google Scholar]