Abstract
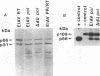
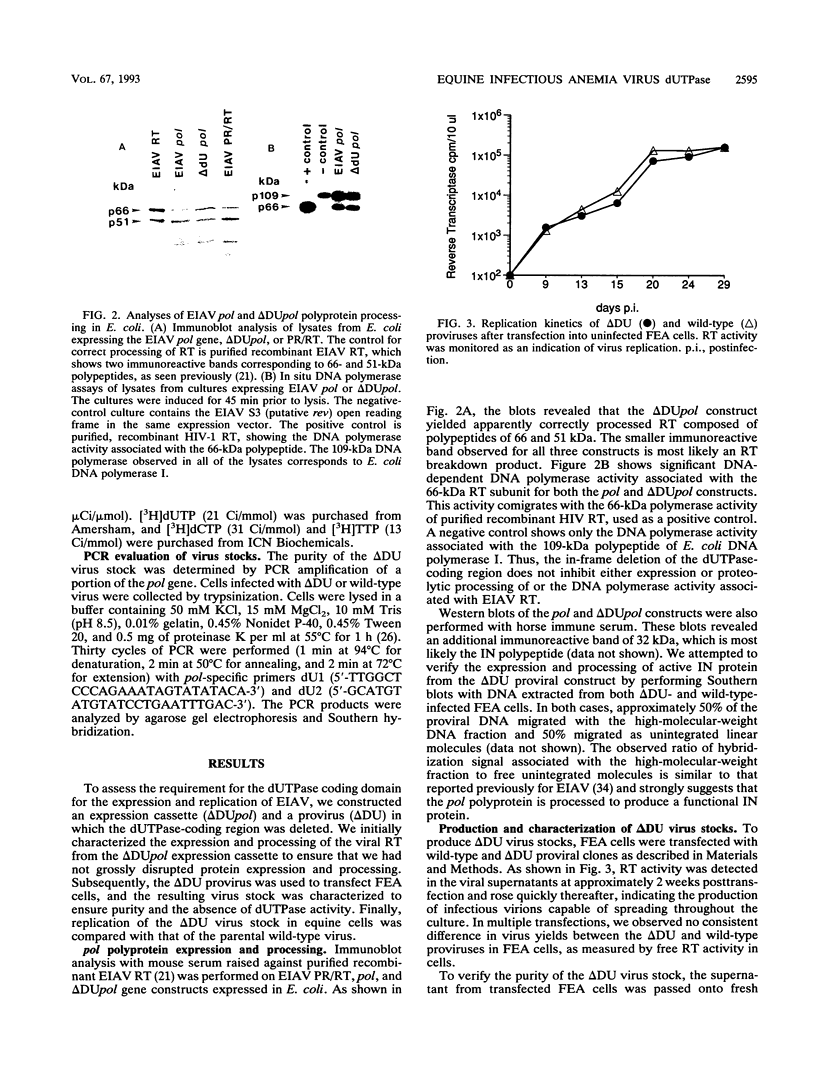
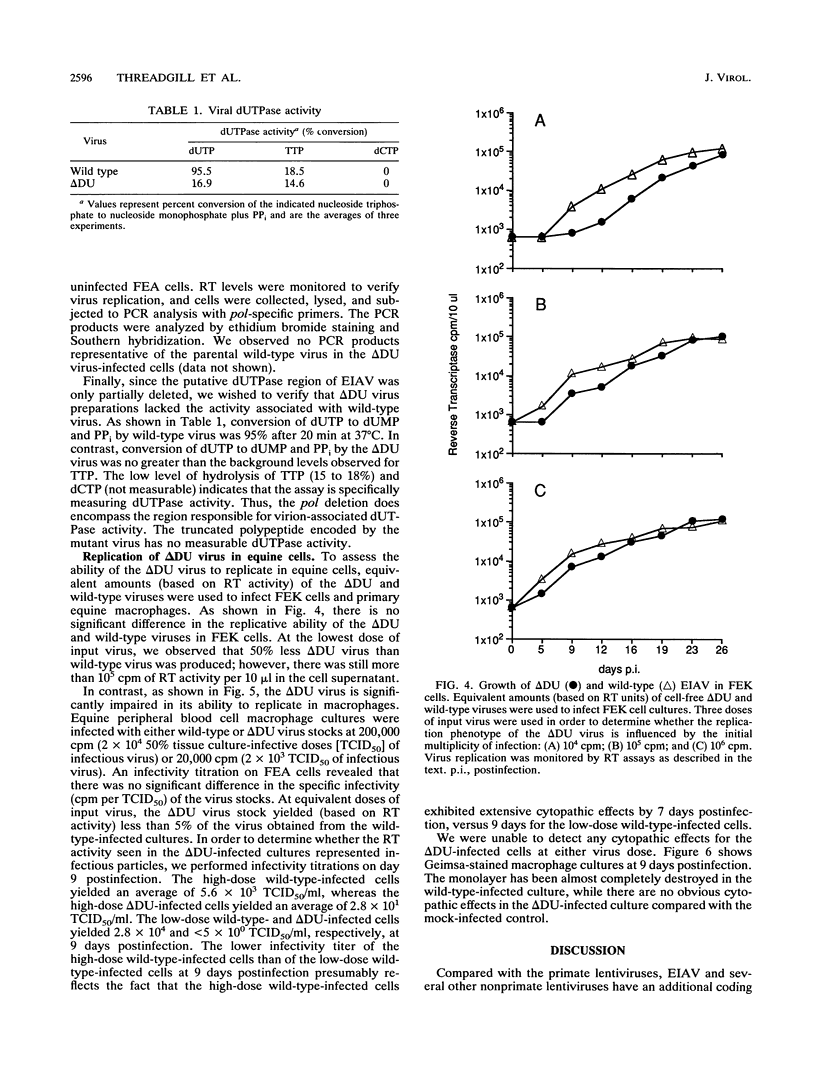
The putative dUTPase domain was deleted from the polymerase (pol) gene of equine infectious anemia virus (EIAV) to produce a recombinant delta DUpol Escherichia coli expression cassette and a delta DU proviral clone. Expression of the recombinant delta DUpol polyprotein yielded a properly processed and enzymatically active reverse transcriptase, as determined by immunoblot analysis and DNA polymerase activity gels. Transfection of delta DU provirus into feline (FEA) cells resulted in production of virus that replicated to wild-type levels in both FEA cells and fetal equine kidney cells. In contrast, the delta DU virus replicated poorly (less than 1% of wild-type levels) in primary equine macrophage cultures, as measured by reverse transcriptase assays. Preparations of delta DU virus contained negligible dUTPase activity, which confirms that virion-associated dUTPase is encoded in the pol gene region between the RNase H domain and integrase, as has been demonstrated previously for feline immunodeficiency virus (J. H. Elder, D. L. Lerner, C. S. Hasselkus-Light, D. J. Fontenot, E. Hunter, P. A. Luciw, R. C. Montelaro, and T. R. Phillips, J. Virol. 66:1791-1794, 1992). Our results suggest that virus-encoded dUTPase is dispensable for virus replication in dividing cells in vitro but may be required for efficient replication of EIAV in nondividing equine macrophages, the natural host cells for this virus.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERTANI L. E., HAEGGMARK A., REICHARD P. ENZYMATIC SYNTHESIS OF DEOXYRIBONUCLEOTIDES. II. FORMATION AND INTERCONVERSION OF DEOXYURIDINE PHOSPHATES. J Biol Chem. 1963 Oct;238:3407–3413. [PubMed] [Google Scholar]
- Barker D. E., Roizman B. Identification of three genes nonessential for growth in cell culture near the right terminus of the unique sequences of long component of herpes simplex virus 1. Virology. 1990 Aug;177(2):684–691. doi: 10.1016/0042-6822(90)90534-x. [DOI] [PubMed] [Google Scholar]
- Boyer P. L., Ferris A. L., Hughes S. H. Cassette mutagenesis of the reverse transcriptase of human immunodeficiency virus type 1. J Virol. 1992 Feb;66(2):1031–1039. doi: 10.1128/jvi.66.2.1031-1039.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedergren-Zeppezauer E. S., Larsson G., Nyman P. O., Dauter Z., Wilson K. S. Crystal structure of a dUTPase. Nature. 1992 Feb 20;355(6362):740–743. doi: 10.1038/355740a0. [DOI] [PubMed] [Google Scholar]
- Duker N. J., Grant C. L. Alterations in the levels of deoxyuridine triphosphatase, uracil-DNA glycosylase and AP endonuclease during the cell cycle. Exp Cell Res. 1980 Feb;125(2):493–497. doi: 10.1016/0014-4827(80)90145-7. [DOI] [PubMed] [Google Scholar]
- Elder J. H., Lerner D. L., Hasselkus-Light C. S., Fontenot D. J., Hunter E., Luciw P. A., Montelaro R. C., Phillips T. R. Distinct subsets of retroviruses encode dUTPase. J Virol. 1992 Mar;66(3):1791–1794. doi: 10.1128/jvi.66.3.1791-1794.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher F. B., Preston V. G. Isolation and characterisation of herpes simplex virus type 1 mutants which fail to induce dUTPase activity. Virology. 1986 Jan 15;148(1):190–197. doi: 10.1016/0042-6822(86)90414-9. [DOI] [PubMed] [Google Scholar]
- GREENBERG G. R., SOMERVILLE R. L. Deoxyuridylate kinase activity and deoxyuridinetriphosphatase in Escherichia coli. Proc Natl Acad Sci U S A. 1962 Feb;48:247–257. doi: 10.1073/pnas.48.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroir L. E., Deutsch W. A. Drosophila deoxyuridine triphosphatase. Purification and characterization. J Biol Chem. 1987 Jan 5;262(1):130–134. [PubMed] [Google Scholar]
- Gregersen J. P., Wege H., Preiss L., Jentsch K. D. Detection of human immunodeficiency virus and other retroviruses in cell culture supernatants by a reverse transcriptase microassay. J Virol Methods. 1988 Feb;19(2):161–168. doi: 10.1016/0166-0934(88)90159-0. [DOI] [PubMed] [Google Scholar]
- Hokari S., Hasegawa M., Sakagishi Y., Kikuchi G. Deoxyuridine triphosphate nucleotidohydrolase activity and its correlation with multiplication of erythroid cells in rat spleen. Biochem Int. 1987 May;14(5):851–857. [PubMed] [Google Scholar]
- Hokari S., Sakagishi Y. Purification and characterization of deoxyuridine triphosphate nucleotidohydrolase from anemic rat spleen: a trimer composition of the enzyme protein. Arch Biochem Biophys. 1987 Mar;253(2):350–356. doi: 10.1016/0003-9861(87)90188-3. [DOI] [PubMed] [Google Scholar]
- Hokari S., Sakagishi Y., Tsukada K. Enhanced activity of deoxyuridine 5'-triphosphatase in regenerating rat liver. Biochem Biophys Res Commun. 1982 Sep 16;108(1):95–101. doi: 10.1016/0006-291x(82)91836-8. [DOI] [PubMed] [Google Scholar]
- Hokari S., Takizawa A., Tanaka M., Sakagishi Y. Calf thymus deoxyuridine triphosphatase differs from rat spleen enzyme in molecular disposition. Biochem Int. 1989 Sep;19(3):453–461. [PubMed] [Google Scholar]
- Issel C. J., Coggins L. Equine infectious anemia: current knowledge. J Am Vet Med Assoc. 1979 Apr 1;174(7):727–733. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Le Grice S. F., Grüninger-Leitch F. Rapid purification of homodimer and heterodimer HIV-1 reverse transcriptase by metal chelate affinity chromatography. Eur J Biochem. 1990 Jan 26;187(2):307–314. doi: 10.1111/j.1432-1033.1990.tb15306.x. [DOI] [PubMed] [Google Scholar]
- Le Grice S. F., Panin M., Kalayjian R. C., Richter N. J., Keith G., Darlix J. L., Payne S. L. Purification and characterization of recombinant equine infectious anemia virus reverse transcriptase. J Virol. 1991 Dec;65(12):7004–7007. doi: 10.1128/jvi.65.12.7004-7007.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lirette R., Caradonna S. Inhibition of phosphorylation of cellular dUTP nucleotidohydrolase as a consequence of herpes simplex virus infection. J Cell Biochem. 1990 Aug;43(4):339–353. doi: 10.1002/jcb.240430406. [DOI] [PubMed] [Google Scholar]
- McGeoch D. J. Protein sequence comparisons show that the 'pseudoproteases' encoded by poxviruses and certain retroviruses belong to the deoxyuridine triphosphatase family. Nucleic Acids Res. 1990 Jul 25;18(14):4105–4110. doi: 10.1093/nar/18.14.4105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGuire T. C., Crawford T. B., Henson J. B. Immunofluorescent localization of equine infectious anemia virus in tissue. Am J Pathol. 1971 Feb;62(2):283–294. [PMC free article] [PubMed] [Google Scholar]
- McMahon A. P., Bradley A. The Wnt-1 (int-1) proto-oncogene is required for development of a large region of the mouse brain. Cell. 1990 Sep 21;62(6):1073–1085. doi: 10.1016/0092-8674(90)90385-r. [DOI] [PubMed] [Google Scholar]
- Nation M. D., Guzder S. N., Giroir L. E., Deutsch W. A. Control of Drosophila deoxyuridine triphosphatase. Existence of a developmentally expressed protein inhibitor. Biochem J. 1989 Apr 15;259(2):593–596. doi: 10.1042/bj2590593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardo E. G., Gutiérrez C. Cell cycle- and differentiation stage-dependent variation of dUTPase activity in higher plant cells. Exp Cell Res. 1990 Jan;186(1):90–98. doi: 10.1016/0014-4827(90)90214-u. [DOI] [PubMed] [Google Scholar]
- Perry S. T., Flaherty M. T., Kelley M. J., Clabough D. L., Tronick S. R., Coggins L., Whetter L., Lengel C. R., Fuller F. The surface envelope protein gene region of equine infectious anemia virus is not an important determinant of tropism in vitro. J Virol. 1992 Jul;66(7):4085–4097. doi: 10.1128/jvi.66.7.4085-4097.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power M. D., Marx P. A., Bryant M. L., Gardner M. B., Barr P. J., Luciw P. A. Nucleotide sequence of SRV-1, a type D simian acquired immune deficiency syndrome retrovirus. Science. 1986 Mar 28;231(4745):1567–1572. doi: 10.1126/science.3006247. [DOI] [PubMed] [Google Scholar]
- Rasty S., Dhruva B. R., Schiltz R. L., Shih D. S., Issel C. J., Montelaro R. C. Proviral DNA integration and transcriptional patterns of equine infectious anemia virus during persistent and cytopathic infections. J Virol. 1990 Jan;64(1):86–95. doi: 10.1128/jvi.64.1.86-95.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sellon D. C., Perry S. T., Coggins L., Fuller F. J. Wild-type equine infectious anemia virus replicates in vivo predominantly in tissue macrophages, not in peripheral blood monocytes. J Virol. 1992 Oct;66(10):5906–5913. doi: 10.1128/jvi.66.10.5906-5913.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonigo P., Barker C., Hunter E., Wain-Hobson S. Nucleotide sequence of Mason-Pfizer monkey virus: an immunosuppressive D-type retrovirus. Cell. 1986 May 9;45(3):375–385. doi: 10.1016/0092-8674(86)90323-5. [DOI] [PubMed] [Google Scholar]
- Spector R., Boose B. Development and regional distribution of deoxyuridine 5'-triphosphatase in rabbit brain. J Neurochem. 1983 Oct;41(4):1192–1195. doi: 10.1111/j.1471-4159.1983.tb09073.x. [DOI] [PubMed] [Google Scholar]
- Stoker N. G., Fairweather N. F., Spratt B. G. Versatile low-copy-number plasmid vectors for cloning in Escherichia coli. Gene. 1982 Jun;18(3):335–341. doi: 10.1016/0378-1119(82)90172-x. [DOI] [PubMed] [Google Scholar]
- Tye B. K., Nyman P. O., Lehman I. R., Hochhauser S., Weiss B. Transient accumulation of Okazaki fragments as a result of uracil incorporation into nascent DNA. Proc Natl Acad Sci U S A. 1977 Jan;74(1):154–157. doi: 10.1073/pnas.74.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whetter L., Archambault D., Perry S., Gazit A., Coggins L., Yaniv A., Clabough D., Dahlberg J., Fuller F., Tronick S. Equine infectious anemia virus derived from a molecular clone persistently infects horses. J Virol. 1990 Dec;64(12):5750–5756. doi: 10.1128/jvi.64.12.5750-5756.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willey R. L., Smith D. H., Lasky L. A., Theodore T. S., Earl P. L., Moss B., Capon D. J., Martin M. A. In vitro mutagenesis identifies a region within the envelope gene of the human immunodeficiency virus that is critical for infectivity. J Virol. 1988 Jan;62(1):139–147. doi: 10.1128/jvi.62.1.139-147.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M. V., Cheng Y. Human deoxyuridine triphosphate nucleotidohydrolase. Purification and characterization of the deoxyuridine triphosphate nucleotidohydrolase from acute lymphocytic leukemia. J Biol Chem. 1979 Apr 25;254(8):2897–2901. [PubMed] [Google Scholar]
- Williams M. V. Deoxyuridine triphosphate nucleotidohydrolase induced by herpes simplex virus type 1. Purification and characterization of induced enzyme. J Biol Chem. 1984 Aug 25;259(16):10080–10084. [PubMed] [Google Scholar]
- Williams M. V. Herpes simplex virus-induced dUTPase: target site for antiviral chemotherapy. Virology. 1988 Sep;166(1):262–264. doi: 10.1016/0042-6822(88)90171-7. [DOI] [PubMed] [Google Scholar]
- Williams M. V., Parris D. S. Characterization of a herpes simplex virus type 2 deoxyuridine triphosphate nucleotidohydrolase and mapping of a gene conferring type specificity for the enzyme. Virology. 1987 Feb;156(2):282–292. doi: 10.1016/0042-6822(87)90408-9. [DOI] [PubMed] [Google Scholar]