Abstract
The morphogenesis of tissues and organs requires dynamic changes in cells and in extracellular matrix components. It is known that various extracellular matrix molecules are of fundamental importance for gonad differentiation and growth. In the adult testis, the extracellular matrix represents an important component of the interstitium, participating in the transport of biologically active substances needed for the communication between different cellular components, as well as for the regulation of spermatogenesis and hormone production. The present study was designed in order to identify the proteoglycans biglycan, decorin and perlecan, as well as the glycosaminoglycan hyaluronan, during testis development in mouse embryos. Our data profile the chronology of testis differentiation, as well as the distribution of these extracellular matrix components during testis development in mice. We show that these extracellular matrix molecules are present early in the development of the gonads, suggesting that they play a role in gonad development. In addition, we found no decorin in the testicular cords. Furthermore, of the proteoglycans analysed, only biglycan was seen surrounding immature Sertoli cells and Leydig cell precursors in the testicular cords. This indicates that specific sets of extracellular matrix molecules are required in the various compartments of the developing gonad.
Keywords: gonad, hyaluronan, morphogenesis, mouse embryos, proteoglycans, testis development
Introduction
Mammalian gonadal development and sexual differentiation are complex processes that require the coordinated expression of specific sets of genes in a strictly spatiotemporal manner (Viger et al. 1998). Gonadal differentiation initiates when the primordial germ cells (PGCs) arrive at and populate the genital ridges. The differentiation of a testis or an ovary is what holds the key to sexual development, and is under control of the SRY gene present on the Y-chromosome (Gubbay et al. 1990; Sinclair et al. 1990; Koopman et al. 1991; Mackay, 2000; Koopman, 2001; Veitia et al. 2001). In the male, supporting cell lineages in the genital ridges are induced to differentiate as Sertoli cells. Immature Sertoli cells form clusters enveloping the PGCs, and both are confined to a common compartment designated the testicular cords (Koopman et al. 1990; Palmer & Burgoyne, 1991), which, in all mammals, will form the seminiferous tubules and epididymis in adult testis.
The PGCs constitute an embryonic cell line that gives rise to gametes in vertebrates. At a very early stage of embryonic development, PGCs originate outside the embryo proper and migrate by a well-defined extracellular matrix (ECM)-rich pathway to the genital ridges (Fujimoto et al. 1985; Garcia-Castro et al. 1997; Soto-Suazo et al. 2002).
The morphogenesis of tissues and organs requires dynamic changes in the ECM. In fact, it is known that the ECM plays a fundamental role in the differentiation of the testis (Pelliniemi et al. 1984). In the adult testis, the ECM is an interstitial component that is important for the transport of the biologically active substances needed for the communication between different cellular components, as well as for the regulation of spermatogenesis and hormone production (Ungefroren et al. 1995). During the postnatal development of rat testis, laminin, fibronectin and entactin-1 play important roles in the morphogenesis process (Weber et al. 2002). The different tissue compartments in the testis (the interstitial space, the blood vessel walls and the basement membrane of the seminiferous tubules) are particularly rich in ECM molecules, such as proteoglycans (Takaba et al. 1991).
The differentiating gonads consist of somatic cells that constitute the coelomic epithelium, mesenchymal tissue, mesonephric tissue and PGCs. In the mouse, the differentiation of the testis initiates at 10.5 days post-conception (dpc), the time at which the majority of PGCs are arriving at the genital ridges (Ginsburg et al. 1990; Gomperts et al. 1994; Molyneaux et al. 2001). At this time, PGCs acquire an elongated shape, exhibit pseudopodia and present an irregular outline under the electron microscope (Soto-Suazo & Zorn, 2005; Pereda et al. 2006). Histochemical detection of alkaline phosphatase activity can be used to identify PGCs (Clark & Eddy, 1975). However, the most precise method of identifying PGCs involves the use of monoclonal antibodies, such as the carbohydrate stage-specific embryonic antigens (SSEAs) α-SSEA-1, α-SSEA-3 and SSEA-4 (Fox et al. 1981; Shevinsky et al. 1982). At 12.5 dpc, the XY gonad presents clear morphological changes that are not apparent in the XX gonad (McLaren, 1983; Schmahl et al. 2000). At this stage the mouse testis presents large peripheral blood vessels and a newly forming testicular cords. As early as 13 dpc, the differentiation of testicular cords occurs rapidly. Over the following days (14 and 15 dpc), the testicular cords as well the surrounding basement membrane enlarge significantly. Embryonic testis development requires the morphogenesis of cords and the growth of their cell populations to allow organ formation. It is well accepted that the coordination of growth and differentiation of various cell types requires locally produced ECM components (Pelliniemi et al. 1984; Hadley et al. 1985, 1988; Zanchetta & Munari, 1987; Gelly et al. 1989; Santamaria et al. 1990; eeviewed by Luck, 1994; Mackay, 2000).
In early reports, structural components of the ECM were detected at different developmental stages in the differentiating rat gonad: fibronectin was detected in embryos from embryonic day (E)12 to E15 by Paranko et al. (1983); fibronectin and laminin were detected in embryos from E13 to E17 by Agelopoulou & Magre (1987); entactin-1 was detected in young adult male rats by Weber et al. (2002); type I and III collagen were detected in testis from the undifferentiated stage E12 until birth by Paranko (1987); type IV collagen was detected in rat gonads from 12 dpc to 20 dpc by Gelly et al. (1989); fibroblast growth factors and receptors have been detected in the gonads of postpartum rats (Cancilla et al. 2000); and transforming growth factor-alpha was detected in cultivated mature gonads of rats from 13 dpc until birth by Levine et al. (2000).
Proteoglycans and glycosaminoglycans are important for organogenesis and ECM assembly, creating distinctive profiles that affect many of the characteristics of the extracellular microenvironment in a variety of development systems (Toole, 1981, 1991). Due to their ability to bind growth factors, decorin and biglycan have been correlated with cell proliferation and differentiation (Yamaguchi et al. 1990; Ruoslahti & Yamaguchi, 1991). The glycosaminoglycan hyaluronan occupies large domains of the extracellular spaces. Because it is highly polyanionic and is hydrophilic in nature, hyaluronan can expand the extracellular space and create ‘paths of least resistance’ for cell migration (Toole & Trelstad, 1971; Browder et al. 1991; Toole, 1991). In addition, hyaluronan is believed to play an important role in controlling cell growth and differentiation during embryonic development (Toole, 2001). The proteoglycan versican, a member of the hyalectin family of molecules, binds to hyaluronan and to ECM compounds in a wide variety of tissues, creating loose, hydrated matrices during key events in development and disease. Versican also regulates cell adhesion, survival, proliferation and migration, as well as ECM assembly and determination of the cell phenotype (Wight, 2002).
Considering the importance of ECM molecules in organogenesis, the aim of the present study was to identify the proteoglycans biglycan, decorin and perlecan, as well as the glycosaminoglycan hyaluronan, during testis development. We report the chronology of testis differentiation, as well as the expression and distribution of these ECM components during testis development of mice.
Materials and methods
Tissue collection
Swiss female mice aged 2–3 months were used. The animals were housed in a light- and temperature-controlled environment (12-h light/dark cycle, 22 °C), with food and water available ad libitum. Females were caged overnight with breeding males and examined the next morning to check for a vaginal plug, which would indicate that copulation had occurred. The day on which a plug was identified was considered 0 dpc (Fujimoto et al. 1985). At least three animals per day were used at 12, 13, 14 and 15 dpc. The animals were deeply anaesthetized with single intraperitoneal injections of tribromoethanol (Avertin®; 0.025 mL/g body weight) and killed by cervical dislocation, after which the uteri were removed. On each day post-conception, five embryos were dissected from the uterus under a stereomicroscope and were then cut into two transverse sections. Embryos were fixed by immersion for 4 h in methacarn (absolute methanol/chloroform/glacial acetic acid; 6 : 3 : 1) with gentle stirring and were then washed in ethanol, also with gentle stirring, after which they were dehydrated and embedded in Paraplast (Oxford Labware, St. Louis, MO, USA) at 60 °C. National guidelines for laboratory animal care were followed, and all experiments were approved by the Institute of Biomedical Sciences Animal Ethics Committee (authorization number, 144/2002).
Immunoperoxidase staining
Embryos were cut on a microtome (Micron HM-200) into 5-µm sections, adhered to glass slides using 0.1% poly-l-lysine (Sigma, St Louis, MO, USA) and then dried at room temperature. Each of the succeeding steps was followed by thorough rinsing with phosphate-buffered saline (PBS).
Sections were treated with 3% H2O2 in PBS for 30 min to block endogenous peroxidase activity. All steps were performed in a humidified chamber, and care was taken to avoid drying out of sections. Non-specific staining was blocked by incubating the sections for 30 min with normal goat serum diluted 1 : 1 in PBS–10% bovine serum albumin. For each antibody, non-specific reactions were blocked by incubating the sections for 2 h with undiluted SuperBlock (Pierce, Rockford, IL, USA) blocking buffer.
For decorin and biglycan immunoreaction, sections were incubated for 1 h at 37 °C in 20 mm Tris-HCl, pH 6.0, containing 0.2 U/mL of chondroitinase ABC (Seikagaku Corp., Tokyo, Japan). For heparan sulfate proteoglycan (HSPG) immunoreaction, the sections were incubated for 1 h at 37 °C with 300 U mL−1 solution of bovine testis hyaluronidase (Sigma) in 0.1 m sodium acetate buffer.
Rabbit polyclonal antibodies against murine decorin (LF-113) and biglycan (LF-106) (Fisher et al. 1995), which recognize an epitope in the core protein of each of these macromolecules, were diluted to 1 : 2000 and 1 : 1000, respectively.
The rat anti-HSPG monoclonal antibody (A7L6; Chemicon International, Temecula, CA, USA), which reacts with core protein domain 4 of the perlecan proteoglycans (Folkvord et al. 1989), was diluted to 1 : 100 in PBS–0.3% Tween 20.
To avoid background staining, monoclonal antibodies were preconjugated with biotin as described by Soto-Suazo et al. (2002).
For hyaluronan detection, the sections were incubated with a biotinylated probe (kindly provided by Dr H. Nader, UNIFESP, São Paulo, Brazil) consisting of the link protein-like domain of the aggrecan core protein (Tengblad, 1980). The probe was diluted to 1 : 80 in PBS–0.3% Tween 20 in PBS–0.3% Tween 20. After 1 h at 37 °C, the probe was incubated overnight at 4 °C. For the detection of perlecan, slides were incubated with secondary antibodies (directly) with the hyaluronan probe or with rat heparan-sulfate monoclonal antibody, after which they were rinsed three times in PBS and incubated for 1 h at room temperature in the streptavidin/peroxidase complex (Vector). For the detection of decorin and biglycan, the sections were washed thoroughly with PBS followed by incubation for 1 h at room temperature with biotin-conjugated goat anti-rabbit IgG (Vector) diluted 1 : 1000 in PBS.
Peroxidase was visualized using 0.03% 3,3′-diaminobenzidine (Sigma) in PBS with 0.03% H2O2. The sections were counterstained with Mayer's haematoxylin. The specificity of immunolabelling was tested by omitting the primary antibody. The diversity of tissues present in each embryo was also very useful for evaluating the specificity of the antibodies. The samples were examined with a Nikon Eclipse E600 microscope. Images were captured using Image-Pro Plus software (Media Cybernetics, Silver Spring, MD, USA).
Results
Morphological analysis
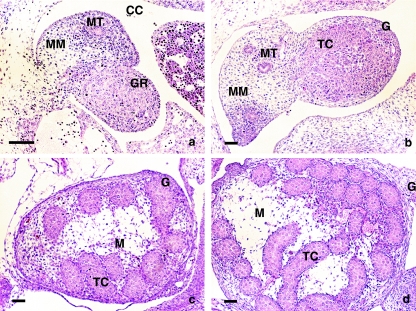
The developing gonad of the E12 embryos consists of loose mesenchymal tissue supported by the developing mesonephric tissue and covered by coelomic epithelium, which surrounds a mass of mesenchymal tissue and PGCs within the developing gonad (Schmahl et al. 2000). As development progresses, testicular cords are separated from the epithelium. Simultaneously, the epithelium and the outermost testicular cords become separated by mesenchymal tissue, which differentiates into the developing tunica albuginea. After E13, the testis rapidly increases its volume and becomes flattened. We found the gonad to contain germ and somatic cell lines arranged in cords that were connected to the mesonephros on the basal side of the gonad (Fig. 1a–d).
Fig. 1.
Morphological stages of testis development: (a) E12 embryo presenting morphologically undifferentiated gonads; (b) E13 embryo showing the developing testis; (c,d) E14 and E15 embryos presenting differentiated testicular cords. H&E staining. CC, coelomic cavity; G, gonad; GR, gonadal ridges; MT, mesonephric tubule; MM, mesonephric mesenchyme; M, mesenchyme of testis; TC, testicular cords. Scale bar, 50 µm.
At E14 and E15, the cells among the testicular cords were organized as an anastomosed network, forming structures in the shape of simple arches that were aligned generally perpendicular to the long axis of the testis. These arches were connected to the rete testis (Fig. 1c–d).
Immunohistochemical and histochemical analysis
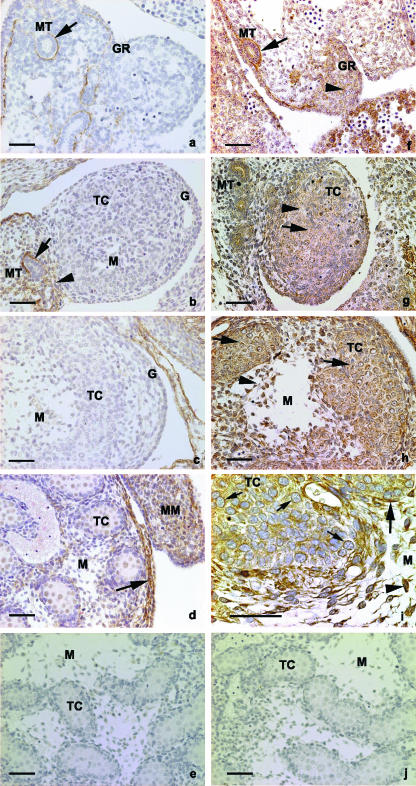
Biglycan, decorin, perlecan and hyaluronan were detected in the mesonephric–gonadal complex of mouse fetuses, as well as in growing testis, from E12 to E15 (Figs 2 and 3). During that same period, however, the immunoreaction for decorin was restricted to the basement membrane of the mesonephric tubule and the surrounding mesenchyme (Fig. 2a–d).
Fig. 2.
Immunoperoxidase for decorin: (a,b) E12 and E13 embryos, respectively, presenting immunoreaction in the basement membrane of the mesonephric tubule (arrow); (b) E13 embryo. Decorin is observed in the mesenchyme surrounding the mesonephric tubule (arrowhead); (c) E14 embryo presenting no immunoreaction for decorin in the gonad; (d) E15 embryo presenting immunoreactivity in the basement membrane of the coelomic epithelium (arrow) as well as in the mesenchyme of the mesonephros (MM). (e) Control section for decorin. (f–j) Immunoperoxidase for biglycan: (f) E12 embryo presenting immunoreaction in the mesenchyme of the genital ridges (arrowhead) and in the basement membrane of the mesonephric tubules (arrow); (g) gonad of an E13 embryo. Biglycan is present in the mesenchyme among the testicular cords (arrowhead) and in the basement membrane of the testicular cords (arrow); (h) E14 embryo. The immunoreaction is observed in the mesenchyme of the developing gonad (arrowhead); (i) E15 embryos. Strong immunoreactivity is seen in the cytoplasm of immature Sertoli cells and Leydig cell precursors of the testicular cords (small arrows, as well as within the mesenchymal cells (arrowhead). (j) Control section for biglycan. G, gonad; GR, gonadal ridges; MT, mesonephric tubule; M, mesenchyme of testis; TC, testicular cords; MM, mesonephric mesenchyme. Scale bar, 50 µm.
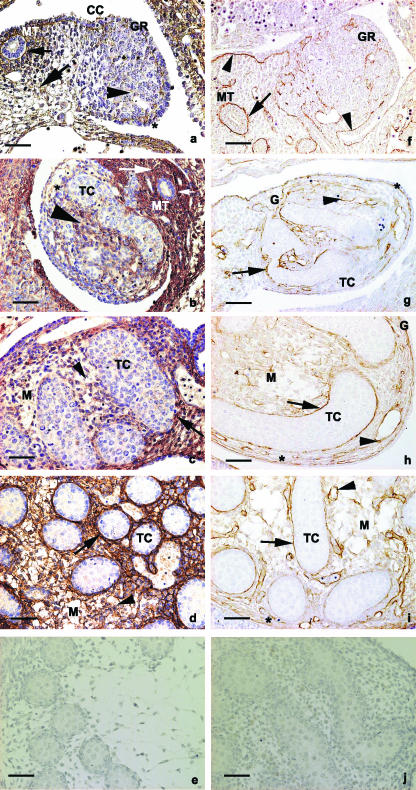
Fig. 3.
Reaction for hyaluronan: (a,b) E12 and E13 embryos, respectively. Hyaluronan is seen in the mesenchyme of the mesonephros (arrows) and in the region of the basement membrane of the mesonephric duct (small arrow). Note the reaction in the basement membrane of the coelomic epithelium (asterisk) and in the mesenchyme of the developing gonad (large arrowhead). Note that the immunoreaction is stronger at E13 than at E12; (c,d) E14 and E15 embryos, respectively. Hyaluronan is present in the basement membrane region (arrow) and in the mesenchyme of the testicular cords (arrowhead); (e) control section for hyaluronan. (f–j) Immunoperoxidase for perlecan: (f) gonads of E12 embryos. Perlecan is observed in the basement membranes of the mesonephric duct (arrow) and coelomic epithelium (arrowhead); (g–i) E13, E14 and E15 embryos, respectively. Perlecan is observed in the regions of the basement membranes of the testicular cords (arrows), blood vessels (arrowhead) and coelomic epithelium (asterisk). (j) Control section for perlecan. BV, blood vessel; G, gonad; GR, gonadal ridges; MT, mesonephric tubule; MM, mesonephric mesenchyme; M, mesenchyme of testis; TC, testicular cords; CC, coelomic cavity. Scale bar, 50 µm.
Morphologically undifferentiated gonads
In E12 mouse fetuses, the developing gonad consisted of the coelomic epithelium and the underlying mesenchyme, containing only a few mesonephric tubules. Biglycan, perlecam and hyaluronan were immunodetected in the basement membrane regions of the mesonephric–gonadal complex, whereas hyaluronan and biglycan were immunodetected in the mesenchyme of the gonadal ridge and mesonephros (Fig. 2f–l and Fig. 3a and 3f).
Developing testis
In E13 mouse fetuses, the gonadal ridge had thickened, and some mesonephric tubules were associated with the growing region. The testicular cords seemed to be connected to the surface epithelium. Perlecan, biglycan and hyaluronan were observed surrounding the differentiating testicular cords, the coelomic epithelium and the blood vessels, whereas biglycan and hyaluronan were observed in the mesenchyme lying between the testicular cords (Figs 2g and 3b,d,g). The pattern of immunoreaction was similar at E14 and E15, being especially pronounced at E15. Both testes contained elongated arch-like cords, which appeared as semicircular structures (Fig. 1c,d). The testicular cord basement membrane was immunoreactive to perlecam and biglycan antibodies, as well as presenting histochemical staining for hyaluronan (Figs 2h and 3c,d,h,i). The mesenchymes surrounding the testicular cords were immunoreactive for biglycan and histochemically reactive for hyaluronan (Figs 2f–I and 3b–d). Biglycan was observed in the testicular cords, surrounding the immature Sertoli cells and Leydig cell precursors, chiefly at E15. In E15 embryos in particular, the immunoreaction for biglycan was observed very close to the nuclei of germinal and mesenchymal cells, suggesting intracytoplasmic localization of biglycan in these cells (Fig. 3i).
Control sections were created by omitting the incubation with the primary antibodies. The control sections presented no immunoreactivity.
Discussion
The present study describes, for the first time, the distribution of decorin, biglycan, perlecan and hyaluronan in the developing gonads of mice. Importantly, although all of these molecules were present at all stages studied, the distribution of each varies among the different structures of the mesonephric–gonadal complex. Decorin was present exclusively in the basement membrane regions of the coelomic epithelium and mesonephric tubules, whereas perlecan, biglycan and hyaluronan were present in the basement membrane regions of the developing testicular cords. In addition, hyaluronan and biglycan were present in the mesenchyme surrounding the testicular cords. It is noteworthy that, of the three proteoglycans analysed, only biglycan was observed in the proximity of the testicular somatic cells, immature Sertoli cells and Leydig cell precursors.
To elucidate the complex relationships that form during gonadal development, variations in the ECM molecule profiles, such as those observed in our study, need to be correlated with the various morphological phases. At E12.5, the mouse testis is first distinguishable from the indifferent gonad due to the presence of a large peripheral blood vessel and newly forming testicular cords (McLaren, 1983). These cords consist of centrally organized PGCs surrounded by a layer of pre-Sertoli cells and an outer sheath of peritubular myoid cells, the Leydig cell precursors lying among them (McLaren, 1985). The tunica albuginea is fully formed by E15 (Mackay & Smith, 1989). A similar sequence of events in early testicular development is seen in rats (Magre & Jost, 1980) and rabbits (Wartenberg et al. 1991). The first event leading to testicular differentiation seems to be that PGCs cluster with certain somatic cells, the future Sertoli cells, thereby forming testicular cords (Byskov & Hoyer, 1994).
Epithelial–mesenchymal interactions clearly have a significant influence on the differentiation of testicular cords (Cunha et al. 1980, 1981; Bernfield et al. 1984). Virtually all basement membranes contain, as an intrinsic component, the multifaceted proteoglycan perlecan (Couchman & Ljubimov, 1989; Murdoch et al. 1994). We found that concentrations of perlecan in the basement membranes increased progressively during testicular cord differentiation. From E12 to E13, there is rapid cell proliferation, and this phenomenon requires the disruption of the coelomic basement membrane restricted to the gonadal ridges (Gelly et al. 1989). Discontinuity of the basement membrane surrounding the differentiating testicular cords occurs at E13. Interactions among mesenchymal cells, presumptive Sertoli cells and gonocytes are responsible for the synthesis of newly discontinuous basement membrane. It has been shown that, in vitro, the deposition of a complex matrix requires the presence of differentiated Sertoli and peritubular cells (Skinner et al. 1985). Soto-Suazo et al. (2002) suggested that, during testicular cord development, perlecan acts as a barrier that prevents the PGCs escaping the developing gonads, simultaneously facilitating PGC migration.
The fibroblast growth factor (FGF) family has various biological functions. Members of the FGF family can influence cell growth, cell differentiation and cell migration. In addition, basic FGF (bFGF) plays a role in angiogenesis and in tissue repair (Brucato et al. 2002). In rat testes, bFGF affects Leydig and Sertoli cell steroidogenesis (Laslett et al. 1995; Schteingart et al. 1999). It is known that the biological activity of bFGF is mediated by interaction with the high-affinity cell surface bFGF receptor. Moreover, interaction between bFGF and HSPGs such as perlecan and syndecan modulates FGF activity, as well as facilitating receptor binding and activation (Yayon et al. 1991; Iozzo, 1998).
It has been shown that HSPGs are present on the surface of immature rat Sertoli cells (Mounis et al. 1991; Brucato et al. 2001), and that these cells express syndecan1–4 mRNA (Brucato et al. 2000). During testicular development, the physiology of Sertoli cells changes dramatically. Cell proliferation first decreases and then ceases, allowing the establishment of the blood–testis barrier at approximately day 20 postpartum (Brucato et al. 2002). Oestradiol synthesis by the Sertoli cells in postpartum testes is stimulated by follicle-stimulating hormone under the regulation of FGF and HSPG molecules (Brucato et al. 2002). We therefore suggest that bFGF, syndecan and perlecan also stimulate the growth and differentiation of Sertoli cells and gonocytes during intrauterine testis development.
Decorin and biglycan belong to the family of the leucine-rich small proteoglycans. In the present study, decorin was not detected in the developing testicular cords, being seen exclusively in the basement membrane of coelomic epithelium and mesonephric tubules and only during their early development. By contrast, biglycan was detected in the basement membrane of developing testicular cords, as well as surrounding the immature Sertoli cells and Leydig cell precursors. This finding is in agreement with those of previous studies showing that decorin is absent from the gonads of E12 mouse embryos (Soto-Suazo et al. 2002). In addition, it indicates that biglycan plays a role in gonad development, whereas decorin does not.
In addition to playing structural roles (mainly in collagen fibrillogenesis), decorin and biglycan also bind transforming growth factor beta (TGF-β), thereby participating in the regulation of cell proliferation (Yamaguchi & Ruoslahti, 1988; Hildebrand et al. 1994). There are indications that the interaction between decorin and TGF-β is competitively inhibited by biglycan (Hocking et al. 1999). Decorin binding is thought to neutralize TGF-β biological activity. However, due to the reversibility of this interaction, decorin also acts as a local reservoir for TGF-β in tissues (Ruoslahti & Yamaguchi, 1991).
Studies involving rat Sertoli cells have demonstrated in vivo TGF-β immunoreactivity (Teerds & Dorrington, 1993) and in vitro TGF-β production (Skinner & Moses, 1989; Mullaney & Skinner, 1993). The strategic presence of biglycan in the basement membrane and in the pericellular region might facilitate the binding of the majority of the produced growth factors, preventing them from being diffused out of the testis cells (Ungefroren et al. 1995). In addition, the presence of biglycan in the ECM might be related to collagen fibrillogenesis, as has been observed in other models (Vogel et al. 1984; Vogel & Trotter, 1987; Uldbjerg & Danielsen, 1988; Hocking et al. 1998).
During organogenesis, a complex and intricate ECM network fills the space between the cells and provides a unique histoarchitecture for the pathway along which PGCs move. The ECM complex also provides strength and physical support for the tissues (Pereda et al. 2006). Therefore, migratory cells might utilize the space created by the special arrangement and composition of the ECM molecules, which offer less resistance to cell movement (Erickson, 1990; Browder et al. 1991). The genesis of these extracellular spaces has been associated with the accumulation of hyaluronan (Toole, 1981). In the hyaluronan-rich matrices, the stimulation of cell proliferation (Brecht et al. 1986) and migration (Toole, 1981) depends upon the matrix being hydrated and offering low resistance, thereby shielding cells from contact inhibition. The pericellular matrix expansion also requires interaction of versican, hyaluronan and CD44 (Wight, 2002). This macromolecular complex increases the viscoelastic nature of the pericellular matrix, creating a highly malleable extracellular environment that supports the cell shape change necessary for cell proliferation and migration (Toole, 1982, 2001; Lee et al. 1993).
According to Toole (2001), the characteristics of the ECM might be important for keeping cells separate and mobile, discouraging premature aggregation, adhesion and differentiation. In addition, hyaluronan interacts with the cell surface receptor CD44, thereby creating a similar extracellular environment during critical morphogenetic events such as cell movement and proliferation (Knudson & Knudson, 1993; Knudson et al. 1993). Furthermore, versican also participates in cell proliferation and contributes to the pericellular ECM expansion that is required for the proliferation and migration of cells (Evanko et al. 1999, 2001).
Considering these previous findings, we can conclude that the presence of hyaluronan in the interstitium and basement membrane regions of testicular cords plays a role in organ growth, which requires cell proliferation and migration for the establishment of a mature gonad.
The mechanism involved in the formation and differentiation of the testes and testicular cords remains unclear. However, structural studies have shown that the formation of a basement membrane and the presence of ECM molecules might be important for morphogenetic events and organ differentiation. It is known that mesenchymes condense around the testicular cords and that progressive differentiation of the basement membrane occurs after the fetal testosterone peak (Gondos, 1980). The ECM plays a crucial role in tissue formation during organogenesis and creates a barrier among androgen production by interstitial Leydig cells, intertubular Sertoli cells and PGCs (Weber et al. 2002). Within the lamina propria of the seminiferous tubules, the production of the ECM results from the cooperation of somatic Sertoli and peritubular cells (for a review, see Skinner, 1991). The presence of perlecan, biglycan and hyaluronan surrounding the Sertoli cells during all of the developmental stages observed in the present study suggests that these molecules play a role in the morphogenesis of the testis.
In summary, our results suggest that the presence of perlecan, biglycan and hyaluronan plays a role in gonadal development. In addition, we showed that decorin is absent from the testicular cords, whereas, of the proteoglycans analysed, only biglycan was observed surrounding the cells of the testicular cords. These data suggest that specific ECM molecules are required for testis development. Further studies involving knockout animals are needed in order to test this hypothesis.
Acknowledgments
This study was conducted as partial fulfillment of the requirements for the PhD degree of C.A.M. (advisor, T.M.Z.) and received financial support in the form of a fellowship from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES, Coordination of the Advancement of Higher Education). The authors are grateful to Mrs Cleusa M. R. Pellegrini for excellent technical assistance. We would also like to thank Dr Mauricio Soto-Suazo for helpful suggestions given during the development of this study. The antibodies against the decorin and biglycan proteoglycans were a generous gift from Dr L. W. Fisher, National Institute of Dental and Craniofacial Research (NIDOR, NIH, Bethesda, MD, USA). The hyaluronan probe was a gift from Dr Helena B. Nader and Dr Lucia Sampaio (Laboratory of Molecular Biology, Federal University of São Paulo, Brazil). The project was also supported by grants from the Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP, Foundation for the Support of Research in the state of São Paulo) and Conselho Nacional de Desenvolvimento Cient’fico e Tecnológico (CNPq, National Council for Scientific and Technological Development).
References
- Agelopoulou R, Magre S. Expression of fibronectin and laminin in fetal male gonads in vivo and in vitro with and without testicular morphogenesis. Cell Differ. 1987;21:31–36. doi: 10.1016/0045-6039(87)90445-3. [DOI] [PubMed] [Google Scholar]
- Bernfield M, Banerjee SD, Koda JE, Rapraeger AC. Remodeling of the basement membrane as a mechanism of morphogenesis tissue interaction. In: Trelstad RL, editor. The Role of Extracellular Matrix in Development. New York: Alan Liss; 1984. pp. 545–572. [Google Scholar]
- Brecht M, Mayer U, Schiosser E, Prehm P. Increased hyaluronate synthesis in required for fibroblast detachment and mitosis. Biochem J. 1986;239:445–450. doi: 10.1042/bj2390445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browder L, Erickson C, Jeffery W. Developmental Biology. 3. Chicago: Saunders College Publishing; 1991. [Google Scholar]
- Brucato S, Harduin-Lepers A, Godard F, Bocquet J, Villers C. Expression of glypican-1, syndecan-1 and syndecan-4 mRNAs protein kinase C-regulated in rat immature Sertoli cells by semi-quantitative RT-PCR analysis. Biochim Biophys Acta. 2000;1474:31–40. doi: 10.1016/s0304-4165(00)00006-4. [DOI] [PubMed] [Google Scholar]
- Brucato S, Fagnen G, Villers C, Bonnamy PJ, Langris M, Bocquet J. Biochemical characterization of integral membrane heparan sulfate proteoglycans in Sertoli cells from immature rat testis. Biochim Biophys Acta. 2001;1510:474–487. doi: 10.1016/s0005-2736(00)00378-3. [DOI] [PubMed] [Google Scholar]
- Brucato S, Bocquet J, Villers C. Cell surface heparan sulfate proteoglycans. Target and partners of the basic fibroblast growth factor in rat Sertoli cells. Eur J Biochem. 2002;269:502–511. doi: 10.1046/j.0014-2956.2001.02672.x. [DOI] [PubMed] [Google Scholar]
- Byskov AG, Hoyer PE. Embryology of mammalian gonads and ducts. In: Knobil E, Neill JD, editors. The Physiology of Reproduction. 2. New York: Raven Press, Ltd; 1994. pp. 487–540. [Google Scholar]
- Cancilla B, Davies A, Ford-Perriss M, Risbridger GP. Discrete cell- and stage-specific localisation of fibroblast growth factors and receptor expression during testis development. J Endocrinol. 2000;164:149–159. doi: 10.1677/joe.0.1640149. [DOI] [PubMed] [Google Scholar]
- Clark JM, Eddy EM. Fine structural observations on origin and association of primordial germ cells of the mouse. Dev Biol. 1975;47:136–155. doi: 10.1016/0012-1606(75)90269-9. [DOI] [PubMed] [Google Scholar]
- Couchman JR, Ljubimov AV. Mammalian tissue distribution of a large heparan sulfate proteoglycan detected by monoclonal antibodies. Matrix. 1989;9:311–321. doi: 10.1016/s0934-8832(89)80007-1. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Chung LWK, Shannon JM, Reese BA. Stromal–epithelial interactions in sex differentiation. Biol Reprod. 1980;22:19–42. doi: 10.1095/biolreprod22.1.19. [DOI] [PubMed] [Google Scholar]
- Cunha GR, Shannon JM, Neubauer BL, et al. Mesenchyme–epithelial interactions in sex differentiation. Hum Genet. 1981;58:68–77. doi: 10.1007/BF00284152. [DOI] [PubMed] [Google Scholar]
- Erickson C. Cell migration in the embryo and adult organism. Cell Biol. 1990;2:67–74. doi: 10.1016/s0955-0674(05)80033-x. [DOI] [PubMed] [Google Scholar]
- Evanko SP, Angello JC, Wight TN. Formation of hyaluronan and versican-rich pericellular matrix is required for proliferation and migration of vascular smooth muscle cells. Artherioscler Thromb Vasc Biol. 1999;19:1004–1013. doi: 10.1161/01.atv.19.4.1004. [DOI] [PubMed] [Google Scholar]
- Evanko SP, Johnson PY, Braun KR, Underhill CB, Dudhla J, Wight TN. Platelet-derived growth factor stimulates the formation of versican-hyaluronan aggregates and pericellular matrix expansion in arterial smooth muscle cells. Arch Biochem Biophys. 2001;394:29–38. doi: 10.1006/abbi.2001.2507. [DOI] [PubMed] [Google Scholar]
- Fisher LW, Stubbs JT, Young MF. Antisera and cDNA probes to human and certain animal model bone matrix noncollagenous proteins. Acta Orthop Scand. 1995;66:61–65. [PubMed] [Google Scholar]
- Folkvord JM, Viders D, Coleman-Smith A, Clark RA. Optimization of immunohistochemical techniques to detect extracellular matrix proteins in fixed skin specimens. J Histochem Cytochem. 1989;37:105–113. doi: 10.1177/37.1.2461979. [DOI] [PubMed] [Google Scholar]
- Fox N, Damjanov I, Martinez-Hernandez A, Knowel BB, Solter D. Immunohistochemical localization of the early embryonic antigen (SSEA-1) in postimplantation mouse embryos and fetal and adult tissues. Dev Biol. 1981;83:391–398. doi: 10.1016/0012-1606(81)90487-5. [DOI] [PubMed] [Google Scholar]
- Fujimoto T, Yoshinaga K, Kono I. Distribution of fibronectin on the migratory pathway of primordial germ cells in mice. Anat Rec. 1985;211:271–278. doi: 10.1002/ar.1092110307. [DOI] [PubMed] [Google Scholar]
- Garcia-Castro M, Anderson R, Heasman J, Wylie C. Interaction between germ cells and extracellular matrix glycoproteins during migration and gonad assembly in the mouse embryo. J Cell Biol. 1997;138:471–480. doi: 10.1083/jcb.138.2.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gelly JL, Richoux JP, Leheup BP, Grignon G. Immunolocalization of type IV collagen and laminin during rat gonadal morphogenesis and postnatal development of the testis and epididymis. Histochemistry. 1989;93:31–37. doi: 10.1007/BF00266844. [DOI] [PubMed] [Google Scholar]
- Ginsburg M, Snow MH, McLaren A. Primordial germ cells in the mouse embryo during gastrulation. Development. 1990;110:521–528. doi: 10.1242/dev.110.2.521. [DOI] [PubMed] [Google Scholar]
- Gomperts M, Wylie C, Heasman J. Primordial germ cell migration. Ciba Found Symp. 1994;182:121–134. doi: 10.1002/9780470514573.ch7. [DOI] [PubMed] [Google Scholar]
- Gondos B. Development and differentiation of the testis and male reproductive tract. In: Steinberger A, Steinberger E, editors. Testicular Development, Structure and Function. New York: Raven Press; 1980. pp. 3–20. [Google Scholar]
- Gubbay J, Collignon J, Koopman P, et al. A gene mapping to the sex-detemining region of the mouse Y chromosome is a member of a novel family of embryologically expressed genes. Nature. 1990;346:245–250. doi: 10.1038/346245a0. [DOI] [PubMed] [Google Scholar]
- Hadley MA, Byers SW, Suárez-Quian CA, Kleinmann HK, Dym M. Extracellular matrix regulates Sertoli cells differentiation, testicular cord formation, and germ cell development in vitro. J Cell Biol. 1985;101:1511–1522. doi: 10.1083/jcb.101.4.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadley MA, Byers SW, Suárez-Quian CA, Djakiew D, Dym M. In vitromodels of differentiated Sertoli cells structure and function in vitro. Cell Dev Biol. 1988;24:550–557. doi: 10.1007/BF02629090. [DOI] [PubMed] [Google Scholar]
- Hildebrand A, Romaris M, Rasmussen LM, et al. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor β. Biochem J. 1994;302:527–534. doi: 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocking AM, Shinomura T, McQuillan DJ. Leucine-rich repeat glycoproteins of the extracellular matrix. Matrix Biol. 1998;17:1–19. doi: 10.1016/s0945-053x(98)90121-4. [DOI] [PubMed] [Google Scholar]
- Hocking AM, Shonomura T, McQuillan DJ. Leucine-rich repeat glycoprotein of the extracellular matrix. Matrix Biol. 1999;17:1–19. doi: 10.1016/s0945-053x(98)90121-4. [DOI] [PubMed] [Google Scholar]
- Iozzo RV. Matrix proteoglycans: from molecular design to cellular function. Annu Rev Biochem. 1998;67:609–652. doi: 10.1146/annurev.biochem.67.1.609. [DOI] [PubMed] [Google Scholar]
- Knudson CB, Knudson W. Hyaluronan-binding proteins in development, tissue homeostasis, and disease. FASEB J. 1993;7:1233–1241. [PubMed] [Google Scholar]
- Knudson W, Bartnik E, Knudson CB. Assembly of pericellular matrices by COS-7 cells transfected with CD44 lymphocyte-homing receptor genes. Proc Natl Acad Sci USA. 1993;90:4003–4007. doi: 10.1073/pnas.90.9.4003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koopmann P, Gubbay J, Vivian N, Goodfelow P, Lovell-Badge R. Expression of a candidate sex-determining gene during mouse testis differentiation. Nature. 1990;348:450–452. doi: 10.1038/348450a0. [DOI] [PubMed] [Google Scholar]
- Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991;351:117–121. doi: 10.1038/351117a0. [DOI] [PubMed] [Google Scholar]
- Koopman P. Gonadal development: signals for sex. Curr Biol. 2001;11:R481–R483. doi: 10.1016/s0960-9822(01)00287-1. [DOI] [PubMed] [Google Scholar]
- Laslett AL, McFarlane JR, Hearn MTW, Risbridger GP. Requirement for heparan sulphate proteoglycans to mediate basic fibroblast growth factor (FGF-2) induced stimulation of Leydig cell steroidogenesis. J Steroid Biochem Molec Biol. 1995;54:245–250. doi: 10.1016/0960-0760(95)00138-p. [DOI] [PubMed] [Google Scholar]
- Lee GM, Johnstone B, Jacobson K, Caterson B. The dynamic structure of the pericellular matrix on living cells. J Cell Biol. 1993;123:1899–1907. doi: 10.1083/jcb.123.6.1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine E, Cupp AS, Miyashiro L, et al. Role of transforming growth factor-alpha and the epidermal growth factor receptor in embryonic rat testis development. Biol Reprod. 2000;62:477–490. doi: 10.1095/biolreprod62.3.477. [DOI] [PubMed] [Google Scholar]
- Luck MR. The gonadal extracellular matrix. In: Charlton HM, editor. Oxford Reviews of Reproductive Biology. Vol. 16. Oxford: Oxford University Press; 1994. pp. 33–85. [Google Scholar]
- Mackay S, Smith RA. Mouse gonadal differentiation in vitro in the presence of fetal calf serum. Cell Diff Dev. 1989;27:19–28. doi: 10.1016/0922-3371(89)90041-5. [DOI] [PubMed] [Google Scholar]
- Mackay S. Gonadal development in mammals at the cellular and molecular levels. Int Rev Cytol. 2000;200:47–99. doi: 10.1016/s0074-7696(00)00002-4. [DOI] [PubMed] [Google Scholar]
- Magre S, Jost A. The initial phases of testicular organogenesis in the rat. An electron microscopy study. Arch Anat Micr Morph Expér. 1980;69:297–318. [PubMed] [Google Scholar]
- McLaren A. Studies on mouse germ cells inside and outside the gonad. J Exp Zool. 1983;228:167–171. doi: 10.1002/jez.1402280203. [DOI] [PubMed] [Google Scholar]
- McLaren A. Relation of germ cell sex to gonadal development. In: Halvorson HO, Monroy A, editors. The Origen and Evolution of Sex. New York: Alan R. Liss; 1985. pp. 289–300. [Google Scholar]
- Molyneaux KA, Stallock J, Schaible K, Wylie C. Time-lapse analysis of living mouse germ cell migration. Dev Biol. 2001;240:488–498. doi: 10.1006/dbio.2001.0436. [DOI] [PubMed] [Google Scholar]
- Mounis A, Barbey P, Langris M, Bocquet J. Detergent-solubilized proteoglycans in rat testicular Sertoli cells. Biochim Biophys Acta. 1991;1074:424–432. doi: 10.1016/0304-4165(91)90095-x. [DOI] [PubMed] [Google Scholar]
- Mullaney BP, Skinner MK. Transforming growth factor β (β1, β2 and β3) gene expression and action during pubertal development of the seminiferous tubule: potential role at the onset of spermatogenesis. Mol Endocrinol. 1993;7:67–76. doi: 10.1210/mend.7.1.8446109. [DOI] [PubMed] [Google Scholar]
- Murdoch AD, Liu B, Schwarting R, Tuan RS, Iozzo RV. Widespread expression of perlecan proteoglycan in basement membranes and extracellular matrices of human tissues as detected by a novel monoclonal antibody against domain III and by in situ hybridization. J Histochem Cytochem. 1994;42:239–249. doi: 10.1177/42.2.7507142. [DOI] [PubMed] [Google Scholar]
- Palmer SJ, Burgoyne OS. In situ analysis of fetal, prepuberal and adult XX–XY chimeric mouse testes: Sertoli cells are predominantly, but not exclusively, XY. Development. 1991;112:265–268. doi: 10.1242/dev.112.1.265. [DOI] [PubMed] [Google Scholar]
- Paranko J, Pelliniemi LJ, Vaheri A, Foidart JM, Lakkala-Paranko T. Morphogenesis and fibronectin in sexual differentiation of rat embryonic gonads. Differentiation. 1983;23:S72–S81. doi: 10.1007/978-3-642-69150-8_13. [DOI] [PubMed] [Google Scholar]
- Paranko J. Expression of type I and III collagen during morphogenesis of fetal rat testis and ovary. Anat Rec. 1987;219:91–101. doi: 10.1002/ar.1092190115. [DOI] [PubMed] [Google Scholar]
- Pelliniemi LJ, Paranko J, Grund SK, Frojdman K, Foidart JM, Lakkala-Paranko T. Extracellular matrix in testicular differentiation. Ann NY Acad Sci. 1984;438:405–416. doi: 10.1111/j.1749-6632.1984.tb38302.x. [DOI] [PubMed] [Google Scholar]
- Pereda J, Zorn T, Soto-Suazo M. Migration of human and mouse primordial germ cells and colonization of developing ovary: an ultrastructural and cytochemical study. Micros Res Techn. 2006;69:386–395. doi: 10.1002/jemt.20298. [DOI] [PubMed] [Google Scholar]
- Ruoslahti E, Yamaguchi Y. Proteoglycans as modulators of growth factor activities. Cell. 1991;64:867–869. doi: 10.1016/0092-8674(91)90308-l. [DOI] [PubMed] [Google Scholar]
- Santamaria L, Martinez-Onsurbe P, Paniagua R, Nistal M. Laminin, type IV collagen, and fibronectin in normal and cryptorchid human testes. An immunohistochemical study. Int J Androl. 1990;13:135–146. doi: 10.1111/j.1365-2605.1990.tb00970.x. [DOI] [PubMed] [Google Scholar]
- Schmahl J, Eicher EM, Washburn LL, Capel B. Sry induces cell proliferation in the mouse gonad. Development. 2000;127:65–73. doi: 10.1242/dev.127.1.65. [DOI] [PubMed] [Google Scholar]
- Schteingart HF, Meroni SB, Capena DF, Pellizzari EH, Cigorraga SB. Effects of basic fibroblast growth factor and nerve growth factor on lactate production, c-glutamyl trans-peptidase and aromatase activities in cultured Sertoli cells. Eur J Endocrinol. 1999;141:539–545. doi: 10.1530/eje.0.1410539. [DOI] [PubMed] [Google Scholar]
- Shevinsky LH, Knowies BB, Damjanov I, Solter D. Monoclonal antibody to murine embryos defines a stage-specific embryonic antigen expression on mouse embryos and human teratocarcinoma cells. Cell. 1982;30:697–705. doi: 10.1016/0092-8674(82)90274-4. [DOI] [PubMed] [Google Scholar]
- Sinclair AH, Berta P, Palmer MS, et al. A gene from the human sex-determining region encodes a protein with homology to ta conserved DNA-binding motif. Nature. 1990;346:240–244. doi: 10.1038/346240a0. [DOI] [PubMed] [Google Scholar]
- Skinner MK, Tung PS, Fritz IB. Cooperativity between Sertoli cells and testicular peritubular cells in the production and deposition of extracellular matrix components. J Cell Biol. 1985;100:1941–1947. doi: 10.1083/jcb.100.6.1941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner MK, Moses HL. Transforming growth factor β gene expression and action in the seminiferous tubule: peritubular cell–Sertoli cell interactions. Mol Endocrinol. 1989;3:625–634. doi: 10.1210/mend-3-4-625. [DOI] [PubMed] [Google Scholar]
- Skinner MK. Cell–cell interactions in the testis. Endocr Rev. 1991;12:45–77. doi: 10.1210/edrv-12-1-45. [DOI] [PubMed] [Google Scholar]
- Soto-Suazo M, San Martin S, Ferro ES, Zorn TMT. Differential expression of glycosaminoglycans and proteoglycans in the migratory pathway of the primordial germ cells of the mouse. Histochem Cell Biol. 2002;118:69–78. doi: 10.1007/s00418-002-0414-2. [DOI] [PubMed] [Google Scholar]
- Soto-Suazo M, Zorn TMT. Primordial germ cells migration: morphological and molecular aspects. Anim Reprod. 2005;2:147–160. [Google Scholar]
- Takaba H, Nagai T, Hashimoto J, Yamamoto M, Miyake K. Identification of collagens in the human testis. Urol Int. 1991;46:180–183. doi: 10.1159/000282128. [DOI] [PubMed] [Google Scholar]
- Teerds KJ, Dorrington JH. Localization of transforming growth factor β1 and β2 during testicular development in the rat. Biol Reprod. 1993;48:40–45. doi: 10.1095/biolreprod48.1.40. [DOI] [PubMed] [Google Scholar]
- Tengblad A. Quantitative analysis of hyaluronate in nanogram amounts. Biochem J. 1980;185:101–105. doi: 10.1042/bj1850101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toole B. Glycosaminoglycans in morphogenesis. In: Hay E, editor. Cell Biology of Extracellular Matrix. New York: Plenum Press; 1981. pp. 259–289. [Google Scholar]
- Toole B. Developmental role of hyaluronate. Connect Tissue Res. 1982;10:93–100. doi: 10.3109/03008208209034409. [DOI] [PubMed] [Google Scholar]
- Toole B. Proteoglycans and hyaluronan in morphogenesis and differentiation. In: Hay E, editor. Cell Biology of Extracellular Matrix. 2. New York: Plenum Press; 1991. pp. 305–342. [Google Scholar]
- Toole B. Hyaluronan in morphogenesis. Cell Dev Biol. 2001;12:79–87. doi: 10.1006/scdb.2000.0244. [DOI] [PubMed] [Google Scholar]
- Uldbjerg N, Danielsen CC. A study of the interaction in vitro between type I collagen and a small dermatan sulfate proteoglycan. Biochem J. 1988;251:643–648. doi: 10.1042/bj2510643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungefroren H, Ergun S, Krull NB, Holstein AF. Expression of the small proteoglycans biglycan and decorin in the adult human testis. Biol Reprod. 1995;52:1095–1105. doi: 10.1095/biolreprod52.5.1095. [DOI] [PubMed] [Google Scholar]
- Veitia RA, Salas-Cortés L, Ottolenghi C, Pailhoux E, Cotinot C, Fellous M. Testis determination in mammals: more questions than answers. Mol Cell Endocrinol. 2001;179:3–16. doi: 10.1016/s0303-7207(01)00460-9. [DOI] [PubMed] [Google Scholar]
- Viger RS, Mertineit C, Trasler JM, Nemer M. Transcription factor GATA-4 is expressed in a sexually dimorphic pattern during mouse gonadal development and is a potent activator of the Müllerian inhibiting substance promoter. Development. 1998;125:2665–2675. doi: 10.1242/dev.125.14.2665. [DOI] [PubMed] [Google Scholar]
- Vogel KG, Paulsson M, Heinegard D. Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem J. 1984;223:587–597. doi: 10.1042/bj2230587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel KG, Trotter JA. The effect of proteoglycans on the morphology of collagen fibrils formed in vitro. Coll Relat Res. 1987;7:105–114. doi: 10.1016/s0174-173x(87)80002-x. [DOI] [PubMed] [Google Scholar]
- Wartenberg H, Kinsky I, Viebahn C, Schomolke C. Fine structural characteristics of testicular cord formation in the developing rabbit gonad. J Electron Microsc Techn. 1991;19:133–157. doi: 10.1002/jemt.1060190203. [DOI] [PubMed] [Google Scholar]
- Weber MA, Groos S, Aumüller G, Konrad L. Post-natal development of the rat testis: steroid hormone receptor distribution and extracellular matrix deposition. Andrologia. 2002;34:41–54. doi: 10.1046/j.1439-0272.2002.00465.x. [DOI] [PubMed] [Google Scholar]
- Wight TN. Versican: a versatile extracellular matrix proteoglycan in cell biology. Curr Op Cell Biol. 2002;14:617–623. doi: 10.1016/s0955-0674(02)00375-7. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Ruoslahti E. Expression of human proteoglycan in Chinese hamster ovary cells inhibits cell proliferation. Nature. 1988;336:244–246. doi: 10.1038/336244a0. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Mann DM, Ruoslahti E. Negative regulation of transforming growth factor-beta by the proteoglycan decorin. Nature. 1990;346:281–284. doi: 10.1038/346281a0. [DOI] [PubMed] [Google Scholar]
- Yayon A, Klagsbrun M, Esko JD, Leder P, Ornitz DM. Cell surface, heparin-like molecules are required for binding of basic fibroblast growth factor to its high affinity receptor. Cell. 1991;64:841–848. doi: 10.1016/0092-8674(91)90512-w. [DOI] [PubMed] [Google Scholar]
- Zanchetta R, Munari PF. Immunocytochemistry of the extracellular matrix (ECM) in normal human testis. In: Spera G, de Kretser DM, editors. Morphological Basis of Human Reproductive Function. New York: Plenum Press; 1987. pp. 55–58. [Google Scholar]