Abstract
The triangular fibrocartilage complex (TFCC) transmits load from the wrist to the ulna and stabilizes the distal radioulnar joint. Damage to it is a major cause of wrist pain. Although its basic structure is well established, little is known of its molecular composition. We have analysed the immunohistochemical labelling pattern of the extracellular matrix of the articular disc and the meniscal homologue of the TFCC in nine elderly individuals (age range 69–96 years), using a panel of monoclonal antibodies directed against collagens, glycosaminoglycans, proteoglycans and cartilage oligomeric matrix protein (COMP). Although many of the molecules (types I, III and VI collagen, chondroitin 4 sulphate, dermatan sulphate and keratan sulphate, the oversulphated epitope of chondroitin 6 sulphate, versican and COMP) were found in all parts of the TFCC, aggrecan, link protein and type II collagen were restricted to the articular disc and to entheses. They were thus not a feature of the meniscal homologue. The shift in tissue phenotype within the TFCC, from a fibrocartilaginous articular disc to a more fibrous meniscal homologue, correlates with biomechanical data suggesting that the radial region is stiff and subject to considerable stress concentration. The presence of aggrecan, link protein and type II collagen in the articular disc could explain why the TFCC is destroyed in rheumatoid arthritis, given that it has been suggested that autoimmunity to these antigens results in the destruction of articular cartilage. The differential distribution of aggrecan within the TFCC is likely to be reflected by regional differences in water content and mobility on the radial and ulnar side. This needs to be taken into account in the design of improved MRI protocols for visualizing this ulnocarpal complex of the wrist.
Keywords: aggrecan, triangular fibrocartilage complex, type II collagen, ulnocarpal complex
Introduction
The triangular fibrocartilage complex (TFCC) is a collection of anatomically related structures which interconnect the carpus and the distal ends of the radius and ulna. Schmidt (2004) refers to it as the ‘ulnocarpal complex’ in his authoritative account of the gross anatomy of the wrist and lists its components as the triangular (ulnocarpal) disc itself, a meniscal homologue, ligaments linking the ulna to the radius and to several carpal bones, the prestyloid recess and the tendon sheath of extensor carpi ulnaris. It is uniquely a hominid feature which ensures that the lower end of the ulna does not articulate directly with the carpal bones. Drobner & Hausman (1992) have suggested that such an isolation of the ulna was necessary to create the rotational ability needed for tree swinging or brachiation. The TFCC stabilizes the distal radioulnar joint and the ulnar side of the carpus, and transmits load from the wrist to the ulna. Its basic structure has been the subject of numerous studies (e.g. Benjamin et al. 1990; Bednar et al. 1991; Nakamura et al. 1996, 2001; Nakamura & Yabe, 2000) and its pathology has attracted considerable attention from hand surgeons. Damage to the TFCC is commonly reported, particularly in elderly individuals (Lee et al. 2004), and the presence of nociceptive fibres within the disc (Cavalcante et al. 2004) suggests that it is implicated in wrist pain.
Despite the clinical significance of the disc, we know little of its molecular composition. However, a better understanding of the distribution of extracellular matrix (ECM) molecules that are typical of cartilaginous or fibrous tissues would illuminate the relationship between the structure in different parts of the TFCC, their mechanical properties and their vulnerability to injury and/or degenerative change. Here we present an immunohistochemical evaluation of the articular disc and the meniscal homologue, using a wide spectrum of antibodies that have been employed in previous studies to explore the spectrum of tissues that are embraced by the term ‘fibrocartilage’ (for a review, see Milz et al. 2005).
Materials and methods
Tissue preparation
The radioulnocarpal wrist complex was removed from one limb in each of nine unembalmed cadavers within 48 h of death. The cadavers ranged in age from 69 to 96 years, and there were five males and four females. No history of wrist pain or pathology was available. The tissue was fixed for at least 2 days in 90% methanol at 4 °C and stored if necessary at –20 °C. Institutional ethics committee approval was not required for this study, as all tissue was obtained from bodies donated to the Department of Anatomy at Munich University for research purposes. After fixation, the complete wrist complex was cut into three pieces (palmar, central and dorsal – see Fig. 1) with a diamond band saw. The tissue was decalcified for several days in 5% EDTA, infiltrated overnight with 5% sucrose in phosphate-buffered saline (PBS) and cryosectioned in the coronal plane at 14 µm.
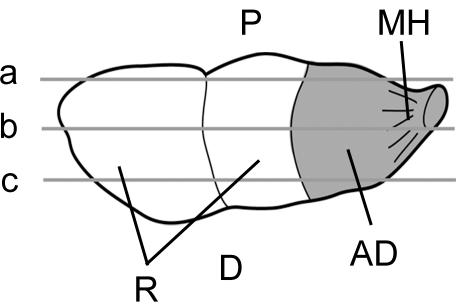
Fig. 1.
Diagrammatic representation of the articular disc (AD) and meniscal homologue (MH), seen from the carpal side. This shows the location of the sections taken from the palmar (a), central (b) and dorsal (c) regions of the TFCC for immunohistochemistry. Note that the disc and meniscal homologue cover the lower end of the ulna. D, dorsal aspect; P, palmar aspect; R, radius.
Immunohistochemical labelling
Details of the immunohistochemical procedures are similar to those used in our previous studies (e.g. Milz et al. 2006). Briefly, sections were labelled with a panel of monoclonal antibodies (Table 1) or stained with toluidine blue for general histological examination. The antibodies were directed against collagens (types I, II, III, VI), glycosaminoglycans (chondroitin 4 and 6 sulphates, dermatan sulphate and keratan sulphate), proteoglycans (aggrecan, link protein, versican and tenascin) and cartilage oligomeric matrix protein (COMP). Sections which were immunolabelled for aggrecan or link protein were treated with 10 mm dithiothreitol in 50 mm Tris-HCl, 200 mm sodium chloride, pH 7.4, for 2 h at 37 °C and then alkylated with 40 mm iodoacetamide in PBS for 1 h at 37 °C. The sections were subsequently incubated with chondroitinase AC (0.25 U mL−1, 37 °C; Sigma-Aldrich). Non-specific binding of the secondary antibody was reduced by blocking the sections with horse serum for 60 min. For control sections, the articular disc component of the TFCC alone was examined in sections where the primary antibody was either omitted or was replaced with an antibody against neurofilament protein. Antibody binding was detected using a Vectastain ABC ‘Elite’ avidin/biotin/peroxidase kit (Vector Laboratories) and the sections counterstained with Mayer's haematoxylin.
Table 1.
Primary monoclonal antibodies used for immunohistochemistry
| Antigen | Clone | Dilution | Pre-treatment | Source | Reference |
|---|---|---|---|---|---|
| Collagen I | Col I | 1 : 2000 | Hyal (1.5 IU mL−1) & ChABC (0.25 IU mL−1) | Sigma-Aldrich | none |
| Collagen II | CIICI | 1 : 6 | Hyal (1.5 IU mL−1) & ChABC (0.25 IU mL−1) | DSHB | Holmdahl et al. (1986) |
| Collagen III | FH7A | 1 : 4000 | Hyal (1.5 IU mL−1) & ChABC (0.25 IU mL−1) | Sigma-Aldrich | Olsen et al. (1993) |
| Collagen VI | 5C6 | 1 : 10 | Hyal (1.5 IU mL−1) & ChABC (0.25 IU mL−1) | DSHB | Hessle & Engvall (1984) |
| Keratan sulphate | 5D4 | 1 : 1500 | none | B. Caterson | Caterson et al. (1983) |
| Chondroitin 4 sulphate | 2B6 | 1 : 1500 | ChAC (0.25 IU mL−1) | B. Caterson | Caterson et al. (1985) |
| Chondroitin-4- & Dermatan-sulphate | 2B6 | 1 : 1500 | ChABC (0.25 IU mL−1) | B. Caterson | Caterson et al. (1985) |
| Chondroitin-6-sulphate | 3B3 | 1 : 150 | ChABC (0.25 IU mL−1) | B. Caterson | Caterson et al. (1985) |
| Chondroitin-6-sulphate (oversulphated) | 7D4 | 1 : 350 | none | B. Caterson | Caterson et al. (1990) |
| Cartilage oligomeric matrix protein (COMP) (rat) | HC484D1 | 1 : 20 | none | Serotec | none |
| Link protein | 9/30/8A4 | 1 : 10 | Reduction and alkylation then ChAC (0.25 IU mL−1) | B. Caterson | Calabro et al. (1992) |
| Aggrecan | 12/21/1C6 | 1 : 10 | Reduction and alkylation then ChAC (0.25 IU mL−1) | B. Caterson | Calabro et al. (1992) |
| Versican | 12C5 | 1 : 10 | ChAC (0.25 IU mL−1) | DSHB | Asher et al. (1991, 1995) |
| Tenascin | T2H5 | 1 : 100 | ChAC (0.25 IU mL−1) | Serotec | Verstraeten et al. (1992) |
| Neurofilaments | RT97 | 1 : 10 | none | DSHB | none |
ChAC, chondroitinase AC; ChABC, chondroitinase ABC; Hyal, hyaluronidase; DSHB, Developmental Studies Hybridoma Bank, University of Iowa (USA).
Results
The position of the articular disc and the meniscal homologue are shown in Fig. 2 and the results of the immunohistochemical survey summarized in Table 2. There was no sharp boundary between the disc and the homologue, which merged imperceptibly with each other. However, only the articular disc was characterized by fibrocartilage cells, which were most evident on its radial side (Fig. 2, inset). The central region of the disc (i.e. section ‘b’ in Fig. 1) attached to hyaline cartilage at the edge of the radius (Fig. 2), but the disc had a typical fibrocartilaginous enthesis in its dorsal and palmar regions (see Fig. 4d). In four of the nine specimens, there were central perforations in the disc.
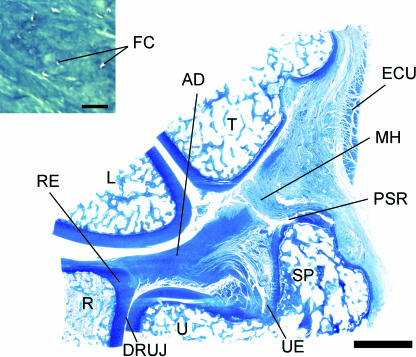
Fig. 2.
Macroscopic view of a coronal section through the wrist to show the position and components of the TFCC. The section passes through the central portion (‘b’ in Fig. 1) of the articular disc (AD). Note the presence of hyaline cartilage at the radial enthesis (RE) of the disc and the continuity of the disc with the meniscal homologue (MH) on the ulnar side. DRUJ, distal radioulnar joint; ECU, tendon sheath of extensor carpi ulnaris; L, lunate; PSR, prestyloid recess; R, radius; SP, styloid process of the ulna; T, triquetral; U, ulna; UE, ulnar enthesis of the articular disc. Scale bar = 5 mm. Inset: the fibrocartilaginous character of the articular disc. FC, fibrocartilage cells. Scale bar = 50 µm. Both sections are stained with toluidine blue.
Table 2.
A summary of the immunohistochemical labelling patterns in the different parts of the TFCC
| Articular disc | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Radial enthesis | Ulnar enthesis | Central portion | Meniscal homologue | |||||||||
| p | c | d | p | c | d | p | c | d | p | c | d | |
| Collagen I | + | + | + | + | + | + | + | + | + | + | + | + |
| Collagen II | + | + | + | + | + | + | + | + | + | 0 | 0 | 0 |
| Collagen III | + | + | + | + | + | + | + | + | + | + | + | + |
| Collagen VI | + | + | + | + | + | + | + | + | + | + | + | + |
| KS | + | 90 | + | + | 90 | + | + | 90 | + | + | 90 | + |
| C-4-S | + | + | + | + | + | + | + | + | + | + | + | + |
| C-4-S + DS | + | + | + | + | + | + | + | + | + | + | + | + |
| C-6-S | + | + | + | + | + | + | + | + | + | + | 90 | + |
| 7D4 | 70 | 80 | 50 | 70 | 80 | 70 | 80 | 60 | 70 | 30 | 30 | 40 |
| COMP | + | + | + | + | + | + | + | + | + | + | + | 90 |
| Link protein | + | + | + | + | + | + | 80 | + | + | 10 | 20 | 40 |
| Aggrecan | 90 | + | + | 90 | + | + | 80 | + | + | 10 | 30 | 30 |
| Versican | + | + | + | + | + | + | + | + | + | + | + | + |
| Tenascin | 80 | 90 | 80 | 80 | 90 | 90 | 90 | 90 | 90 | 90 | 90 | 80 |
The percentage of positively labelled regions [i.e. the dorsal (d), central (c) and palmar (p)] in the different cadavers is stated, as an indication of the generality of the findings between different individuals. A + sign indicates 100% labelling (i.e. it was seen in every cadaver in which that region of the TFCC was sectioned), but all other percentage values are stated as figures. The results indicate the agreed consensus of two of the authors. C-4-S, chondoitin 4 sulphate; C-6-S, chondoitin 6 sulphate; DS, dermatan sulphate; KS, keratan sulphate.
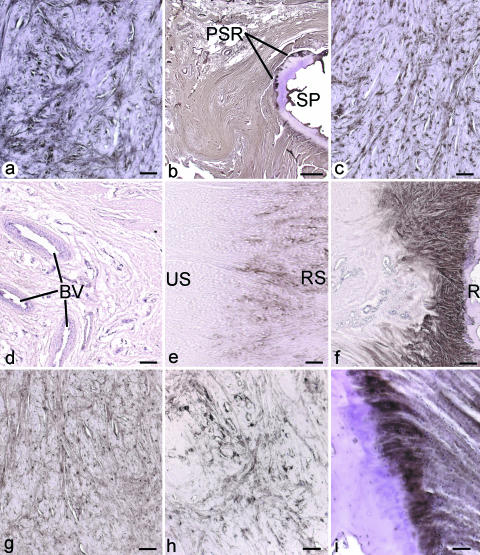
Fig. 4.
Immunohistochemical labelling for proteoglycans in the articular disc, its radial and ulnar entheses and in the meniscal homologue. (a–c) Strong labelling for aggrecan in (a) the palmar region of the disc – scale bar = 100 µm; (b) the radial enthesis from the central part of the disc – scale bar = 100 µm; (c) the ulnar enthesis (UE) from the palmar region of the disc – scale bar = 200 µm. (d) Versican labelling in the dorsal region of the radial enthesis (RE) of the disc. Note the absence of labelling (*) in the soft tissue immediately adjacent to the tidemark (TM). Scale bar = 100 µm. (e) Tenascin labelling in the central region of the radial enthesis of the disc. HC, hyaline cartilage. Scale bar = 100 µm. (f) COMP labelling in the meniscal homologue. Scale bar = 100 µm. (g) Strong labelling for COMP at the ulnar enthesis (UE) in the central region of the disc. Note the prominent fibrocartilage cells (FC). Scale bar = 20 µm. (h) COMP in the dorsal region of the articular disc, near the radial enthesis. Note the strong labelling in the immediate vicinity of blood vessels (BV). Scale bar = 40 µm. (i) A control section from the palmar side of the radial enthesis of the articular disc, incubated with antibody RT97. No staining was seen at the enthesis. Scale bar = 100 µm.
Immunohistochemistry
Collagens
Types I, III and VI collagen were found throughout both the disc and the meniscal homologue (Fig. 3a–c), but type II collagen was restricted to the disc itself, and to all entheses examined (Fig. 3e,f). Labelling for type II collagen was not a feature of the meniscal homologue (Fig. 3d,e). It was also absent around blood vessels that were occasionally seen invading the articular disc from its dorsal aspect near its radial enthesis (Fig. 3f).
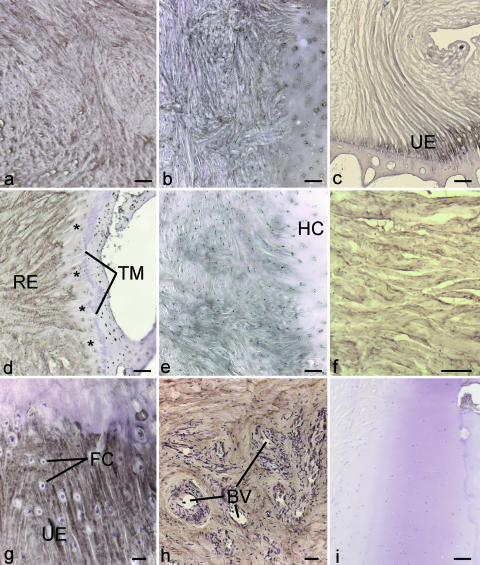
Fig. 3.
Immunohistochemical labelling for collagens and glycosaminoglycans. All figures except ‘i’ were from central sections through the disc (i.e. plane ‘b’ in Fig. 1). (a) Prominent labelling for type I collagen in the articular disc. Scale bar = 100 µm. (b) Prominent labelling for type I collagen in the meniscal homologue. PSR, prestyloid recess; SP, styloid process of the ulna. Scale bar = 1 mm. (c) Type VI collagen in the articular disc. Scale bar = 100 µm. (d) Absence of type II collagen in the meniscal homologue. BV, blood vessels. Scale bar = 200 µm. (e) Type II collagen in the articular disc in the region adjacent to the meniscal homologue. Note that labelling is present on the radial side (RS) of the disc, but absent on the ulnar side (US), i.e. towards the meniscal homologue. Scale bar = 200 µm. (f) Positive labelling for type II collagen at the radial enthesis of the articular disc. R, radius. Scale bar = 200 µm. (g,h) Labelling for chondroitin 6 sulphate (g) and its oversulphated epitope (h) from comparable regions of the articular disc. Scale bar for g = 100 µm and for h = 50 µm. (i) Labelling for chondroitin 4 sulphate at the ulnar enthesis of the articular disc, in a section which passes through its dorsal side (i.e. ‘c’ in Fig. 1). Scale bar = 50 µm.
Glycosaminoglycans
Chondroitin 4 sulphate, dermatan sulphate and keratan sulphate and the oversulphated epitope of chondroitin 6 sulphate (recognized by antibody 7D4) were found in all regions of the disc and meniscal homologue (Fig. 3g–i). Labelling for the oversulphated epitope was especially prominent in the radial part of the articular disc (Fig. 3h). Although labelling for chondroitin 6 sulphate was strong in the disc (Fig. 3g), it was reduced to a speckled distribution in the meniscal homologue. Indeed, in one specimen, labelling was totally absent.
Proteoglycans
Aggrecan was restricted to the radial portions of the disc and to entheses and was not a feature of the meniscal homologue (Fig. 4a–c). As with type II collagen, labelling for aggrecan was locally absent around invading blood vessels in the radial part of the articular disc. Link protein showed more or less the same labelling pattern as aggrecan, although there was weak, spot-like labelling in the dorsal region of the meniscal homologue in four specimens. In contrast to aggrecan, versican labelling was detected in all regions of the disc (Fig. 4d). However, in cadavers with markedly fibrocartilaginous entheses, labelling for versican (and also type I collagen) was locally absent at the hard/soft tissue interface (Fig. 4d). Tenascin labelling in the disc (Fig. 4e) was similar to that of versican, although there was inconsistent labelling in the meniscal homologue. COMP was present in the disc, the meniscal homologue and the superficial zone of neighbouring hyaline articular cartilage (Fig. 4f–h). The ECM around blood vessels invading the dorsal aspect of the disc was also positively labelled (Fig. 4h).
No labelling was detected in any control sections, either those in which the primary antibody was omitted or sections incubated with an antibody against an antigen (neurofilament proteins) not characteristic of cartilage (Fig. 4i).
Discussion
Although as its name suggests, the TFCC is commonly viewed as a fibrocartilaginous structure, our results show that it is an inhomogenous structure in which there is a shift in the tissue phenotype from its radial to its ulnar side. On the ulnar side of the TFCC, the meniscal homologue is fibrous, but on the radial side, the articular disc is fibrocartilaginous. It is thus the disc, rather than the meniscal homologue, which labels for type II collagen and aggrecan – typical markers of a cartilaginous phenotype (for a review, see Milz et al. 2005). The fibrocartilaginous character of a healthy disc and its avascularity are both functional requirements of the compression to which the disc is subject and this is probably why the disc heals poorly after injury or degenerative rupture (Thiru-Pathi et al. 1986; Bednar et al. 1991).
The articular disc of the TFCC is a stiff structure, the shape and position of which is relatively constant during pronation and supination (Pfirrmann et al. 2001; Makita et al. 2003). A high load is needed to tear it in vitro (Adams et al. 1996). We suggest that these mechanical characteristics stem in part from its aggrecan content. Aggrecan is a large, aggregating proteoglycan that is responsible for attracting large quantities of water into a tissue (e.g. articular cartilage and intervertebral discs), thus allowing it to resist compression (Roughley et al. 2006). The presence of this and other proteoglycans also relates to the finding of 7D4 labelling in the disc – an epitope of chondroitin 6 sulphate which has previously been linked to degenerative changes in articular cartilage (Caterson et al. 1990; Lin et al. 2004). It is thus pertinent to note that four of the nine TFCCs we examined had perforated discs.
The presence of aggrecan, type II collagen and other molecules typical of the cartilage phenotype could have a bearing on the involvement of the TFCC in rheumatoid arthritis (RA). There is now considerable evidence to suggest that an autoimmune response to these and other antigens in articular cartilage may contribute to the pathogenesis of this disease (Ronnelid et al. 1994; Kim et al. 1999; Li et al. 2000; Myers et al. 2001). This raises the possibility that one or more of the molecules that we have detected in the region of the articular disc are antigenic targets for autoimmunity in RA within the TFCC. Although we lack data on molecular changes in the articular disc of patients with RA, it is known that the wrist joint is one of the earliest joints to be affected by the disease (Hämäläinen et al. 1992) and that the TFCC is often destroyed (Ilan & Rettig, 2003).
Finally, diagnosing tears in vivo in the articular disc is challenging and many clinicians favour arthroscopy over magnetic resonance imaging (MRI) because of the low sensitivity of MRI machines for detecting smaller tears. However, as the quality of MRI machines in hospitals improves, MRI is likely to be increasingly favoured for diagnosing TFCC damage because of the invasive nature of arthroscopy. Because the signal intensity in MRIs reflects the proton content of the tissue, it may be useful for radiologists to be aware of regional differences in the water content and mobility (i.e. the chemical environment) of the TFCC. This is likely to relate to variations in the distribution of aggrecan that we have demonstrated here in different parts of the complex. Benjamin & Bydder (2007) have recently shown how the use of ultrashort TE (UTE) pulse sequences can enable radiologists to recognize fibrocartilage within ligamentous structures elsewhere in the body.
Note that a limitation of the present study is a lack of any detailed history of wrist pathology in the cadavers we used. Nevertheless, the study does provide the first detailed analysis of the molecular composition of the extracellular matrix of the TFCC. We have shown that regional variations in the distribution of molecules can be related to the mechanical function of the disc and have highlighted the presence of aggrecan and type II collagen, which could relate to the involvement of the TFCC in RA. Our study provides the foundation for future work directed at understanding the nature of the pathological processes affecting the TFCC.
Acknowledgments
The results of this study form part of the doctoral thesis of B.S. and are published by permission of the Medical Faculty of the Ludwig-Maximilians-Universität München. The monoclonal antibodies CIICI, 5C6, 12/21/1C6, 9/30/8A4, 12C5 and RT97 developed by Holmdahl & Rubin, Engvall, Caterson, Caterson, Asher and Wood, respectively, were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biological Sciences, Iowa City, IA 52242, USA.
References
- Adams BD, Samani JE, Holley KA. Triangular fibrocartilage injury: a laboratory model. J Hand Surg. 1996;21A:189–193. doi: 10.1016/S0363-5023(96)80099-2. [DOI] [PubMed] [Google Scholar]
- Asher RA, Perides G, Vanderhaeghen JJ, Bignami A. Extracellular matrix of central nervous system white matter: demonstration of an hyaluronate–protein complex. J Neurosci Res. 1991;28:410–421. doi: 10.1002/jnr.490280314. [DOI] [PubMed] [Google Scholar]
- Asher RA, Scheibe RJ, Keiser HD, Bignami A. On the existence of a cartilage-like proteoglycan and link proteins in the central nervous system. Glia. 1995;13:294–308. doi: 10.1002/glia.440130406. [DOI] [PubMed] [Google Scholar]
- Bednar MS, Arnoczky SP, Weiland AJ. The microvasculature of the triangular fibrocartilage complex: its clinical significance. J Hand Surg. 1991;16A:1101–1105. doi: 10.1016/s0363-5023(10)80074-7. [DOI] [PubMed] [Google Scholar]
- Benjamin M, Evans EJ, Pemberton DJ. Histological studies on the fibrocartilage complex of the wrist. J Anat. 1990;172:59–67. [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Bydder G. Magnetic resonance imaging of entheses using ultrashort TE (UTE) pulse sequences. J Magn Reson Imag. 2007;25:381–389. doi: 10.1002/jmri.20825. [DOI] [PubMed] [Google Scholar]
- Calabro A, Hascall VC, Caterson B. Monoclonal antibodies directed against epitopes within the core protein structure of the large aggregating proteoglycan (aggrecan) from the swarm rat chondrosarcoma. Arch Biochem Biophys. 1992;298:349–360. doi: 10.1016/0003-9861(92)90421-r. [DOI] [PubMed] [Google Scholar]
- Caterson B, Christner JE, Baker JR. Identification of a monoclonal antibody that specifically recognizes corneal and skeletal keratan sulphate. Monoclonal antibodies to cartilage proteoglycan. J Biol Chem. 1983;258:8848–8854. [PubMed] [Google Scholar]
- Caterson B, Christner JE, Baker JR, Couchman JR. Production and characterization of monoclonal antibodies directed against connective tissue proteoglycans. Fed Proc. 1985;44:386–393. [PubMed] [Google Scholar]
- Caterson B, Mahmoodian F, Sorrell JM, et al. Modulation of native chondroitin sulphate structure in tissue development and in disease. J Cell Sci. 1990;97:411–407. doi: 10.1242/jcs.97.3.411. [DOI] [PubMed] [Google Scholar]
- Cavalcante ML, Rodrigues CJ, Mattar R., Jr Mechanoreceptors and nerve endings of the triangular fibrocartilage in the human wrist. J Hand Surg. 2004;29A:432–435. doi: 10.1016/j.jhsa.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Drobner WS, Hausman MR. The distal radioulnar joint. Hand Clin. 1992;8:631–642. [PubMed] [Google Scholar]
- Hämäläinen M, Kammonen M, Lethimäki M. Epidemiology of wrist involvement of rheumatoid arthritis. In: Simmen BR, Hagena F-W, et al., editors. Rheumatology. The Wrist in Rheumatoid Arthritis. Basel: Karger; 1992. pp. 1–7. [Google Scholar]
- Hessle H, Engvall E. Type VI collagen. Studies on its localization, structure, and biosynthetic form with monoclonal antibodies. J Biol Chem. 1984;259:3955–3961. [PubMed] [Google Scholar]
- Holmdahl R, Rubin K, Klareskog L, Larsson E, Wigzell H. Characterization of the antibody response in mice with type II collagen-induced arthritis, using monoclonal anti-type II collagen antibodies. Arthritis Rheum. 1986;29:400–410. doi: 10.1002/art.1780290314. [DOI] [PubMed] [Google Scholar]
- Ilan DI, Rettig ME. Rheumatoid arthritis of the wrist. Bull Hosp Jt Dis. 2003;61:179–185. [PubMed] [Google Scholar]
- Kim HY, Kim WU, Cho ML, et al. Enhanced T cell proliferative response to type II collagen and synthetic peptide CII (255–274) in patients with rheumatoid arthritis. Arthritis Rheum. 1999;42:2085–2093. doi: 10.1002/1529-0131(199910)42:10<2085::AID-ANR8>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Lee DH, Dickson KF, Bradley EL. The incidence of wrist interosseous ligament and triangular fibrocartilage articular disc disruptions: a cadaveric study. J Hand Surg. 2004;29:676–684. doi: 10.1016/j.jhsa.2004.02.011. [DOI] [PubMed] [Google Scholar]
- Li NL, Zhang DQ, Zhou KY, et al. Isolation and characteristics of autoreactive T cells specific to aggrecan G1 domain from rheumatoid arthritis patients. Cell Res. 2000;10:39–49. doi: 10.1038/sj.cr.7290034. [DOI] [PubMed] [Google Scholar]
- Lin PM, Chen CT, Torzilli PA. Increased stromelysin-1 (MMP-3), proteoglycan degradation (3B3- and 7D4) and collagen damage in cyclically load-injured articular cartilage. Osteoarth Cart. 2004;12:485–496. doi: 10.1016/j.joca.2004.02.012. [DOI] [PubMed] [Google Scholar]
- Makita A, Nakamura T, Takayama S, Toyama Y. The shape of the triangular fibrocartilage during pronation and supination. J Hand Surg. 2003;28B:537–545. doi: 10.1016/s0266-7681(03)00136-0. [DOI] [PubMed] [Google Scholar]
- Milz S, Benjamin M, Putz R. Molecular parameters indicating adaptation to mechanical stress in fibrous connective tissue. Adv Anat Embryol Cell Biol. 2005;78:1–71. [PubMed] [Google Scholar]
- Milz S, Aktas T, Putz R, Benjamin M. Expression of extracellular matrix molecules typical of articular cartilage in the human scapholunate interosseous ligament. J Anat. 2006;208:671–679. doi: 10.1111/j.1469-7580.2006.00552.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers LK, Higgins GC, Finkel TH, et al. Juvenile arthritis and autoimmunity to type II collagen. Arth Rheum. 2001;44:1775–1781. doi: 10.1002/1529-0131(200108)44:8<1775::AID-ART313>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Yabe Y, Horiuchi Y. Functional anatomy of the triangular fibrocartilage complex. J Hand Surg. 1996;24B:22–26. doi: 10.1016/s0266-7681(96)80135-5. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Yabe Y. Histological anatomy of the triangular fibrocartilage complex of the human wrist. Ann Anat. 2000;182:567–572. doi: 10.1016/S0940-9602(00)80106-5. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Takayama S, Horiuchi Y, Yabe Y. Origins and insertions of the triangular fibrocartilage complex: a histological study. J Hand Surg. 2001;26B:446–454. doi: 10.1054/jhsb.2001.0562. [DOI] [PubMed] [Google Scholar]
- Olsen BJ, Ninomiya Y. Collagens. In: Kreis T, Vale R, editors. Guidebook to the Extracellular Matrix and Adhesion Proteins. Oxford: Oxford University Press; 1993. pp. 32–44. [Google Scholar]
- Pfirrmann CWA, Theumann NH, Chung CB, Botte MJ, Trundell DJ, Resnick D. What happens to the triangular fibrocartilage complex during pronation and supination of the forearm? Analysis of its morphology and diagnosic assessment with MR arthrography. Skeletal Radiol. 2001;30:677–685. doi: 10.1007/s002560100429. [DOI] [PubMed] [Google Scholar]
- Ronnelid J, Lysholm J, Engstrom-Laurent A, Klareskog L, Heyman B. Local anti-type II collagen antibody production in rheumatoid arthritis synovial fluid. Evidence for an HLA-DR4-restricted IgG response. Arthritis Rheum. 1994;37:1023–1029. doi: 10.1002/art.1780370707. [DOI] [PubMed] [Google Scholar]
- Roughley P, Martens D, Rantakokko J, Alini M, Mwale F, Antoniou J. The involvement of aggrecan polymorphism in degeneration of human intervertebral disc and articular cartilage. Eur Cell Mater. 2006;11:1–7. [PubMed] [Google Scholar]
- Schmidt H-M. Die Anatomie des ulnokarpalen Komplexes. Orthopäde. 2004;33:628–637. doi: 10.1007/s00132-004-0665-9. [DOI] [PubMed] [Google Scholar]
- Thiru-Pathi RG, Ferlic DC, Clayton ML, McClure DC. Arterial antomy of the triangular fibrocartilage of the wrist and its surgical significance. J Hand Surg. 1986;11A:258–263. doi: 10.1016/s0363-5023(86)80065-x. [DOI] [PubMed] [Google Scholar]
- Verstraeten AA, Mackie EJ, Hageman PC, et al. Tenascin expression in basal cell carcinoma. Br J Dermatol. 1992;127:571–574. doi: 10.1111/j.1365-2133.1992.tb14867.x. [DOI] [PubMed] [Google Scholar]