Abstract
Tadpoles of the Megophryidae, a South Asian family of litter frogs, are unique among anurans by virtue of their expanded caudal skeletons, which include supernumerary vertebral centra. The number of these vertebrae varies widely within the family, with tadpoles of Leptobrachella having as many as 30 and Leptolalax only five. Vertebral morphology is also quite variable, ranging from complete, perichordal centra to fragmentary ossifications. This variation in the caudal osteology of larval megophryids, however, is not manifested in the adult morphology. Post-metamorphic litter frogs have a typical anuran axial skeleton, invariably comprising eight presacral vertebrae, a single sacral vertebra and, postsacrally, the urostyle. To resolve this incongruity between life phases and to determine the precise metamorphic fate of supernumerary caudal vertebrae in megophryids, we examined metamorphic specimens from the genera Leptobrachella, Leptolalax, Ophryophryne and Megophrys. In all four, the caudal larval skeleton undergoes massive reduction, leaving only the coccyx and hypochord untouched. Caudal centra are apparently degraded by osteoclasts, which have not previously been implicated in vertebral remodelling during anuran metamorphosis. In Megophrys and Ophryophryne metamorphs, presacral centra also undergo resorption, consistent with an epichordal mode of centrum formation. The conservation of megophryid adult axial osteology in the face of extensive larval skeletal diversity reveals the role of metamorphosis in constraining anuran morphology.
Keywords: axial skeleton, developmental constraint, evolution, hypochord, metamorphosis, osteoclast, tadpole, urostyle
Introduction
A hallmark feature of frogs and toads compared with other vertebrates is their reduced axial skeleton. Among adult anurans, the vertebral column consists of no more than nine presacral vertebrae, a single sacral vertebra and, postsacrally, the urostyle (Trueb, 1973; Púgener, 2002). This elongate structure comprises, ventrally, the ossified hypochord and, dorsally, the coccyx, a fusion of 2–4 rudimentary neural arch elements (Branham & List, 1979; Ročková & Roček, 2005; Handrigan & Wassersug, 2007). The strict conservation of this axial morphology led Handrigan & Wassersug (2007) to propose it as the central character of the anuran Bauplan (body plan), which was established in the most archaic frog Prosalirus bitis nearly 200 million years ago (Shubin & Jenkins, 1995). While all extant frogs and toads converge on this morphology, tadpoles from one family, the Megophryidae, present a unique and dramatic exception – discrete, supernumerary vertebrae in their tails.
Griffiths (1956, 1963) was the first to note the expanded caudal skeleton of megophryid tadpoles, counting over a dozen extra vertebral centra in Megophrys major. Haas et al. (2006) subsequently reported up to 30 caudal vertebrae in Leptobrachella mjobergi larvae, and, most recently, Handrigan et al. (2007) noted the trait in tadpoles from three other megophryid genera, Ophryophryne, Leptolalax and Xenophrys. Within the family, there is marked variation in the osteology and ontogeny of the postsacral skeleton. In all cases, however, vertebral development appears to be accelerated relative to limb development and other ontogenetic events. As Handrigan et al. (2007) suggest, this heterochronic shift of axial development may have been an impetus for the homoplasic reappearance of caudal vertebrae in the family.
The presence of supernumerary caudal vertebrae in megophryid larvae can also be correlated with their unique riparian lifestyle (Haas et al. 2006; Handrigan et al. 2007). Megophryid tadpoles live in montane streams, wherein they occupy an array of microhabitats, depending on the genus. Large-bodied leptobrachine tadpoles (e.g. Leptobrachium, Scutiger), which bear no caudal centra, are typically found in the deep splash pools adjacent to streams. However, vermiform leptobrachines, such as Leptobrachella, are more exposed to water torrents, which they escape by burrowing into the rocky stream bed. Haas et al. (2006) suggest that an expanded caudal skeleton endows Leptobrachella with greater manoeuvrability needed to traverse tight spaces between the rocks. Megophryine tadpoles, such as Megophrys, Ophryophryne and Xenophrys, may also benefit from the enhanced flexibility afforded by supernumerary vertebrae as they are often found burrowing among the leafy substratum at the stream edge (Lathrop, 2003; Handrigan et al. 2007).
After metamorphosis, the utility of the tail and caudal skeleton of megophryids is apparently lost. Adult litter frogs, as their name implies, are typically found among the leaf litter and vegetation of the forest floor (Lathrop, 2003), where a tail – with or without a skeleton – would provide little benefit. Rather, the superfluous weight of the tail would probably impede motility, making megophryid metamorphs easy targets for predation (Wassersug & Sperry, 1977; Handrigan & Wassersug, 2007). Not surprisingly then, the postsacral skeleton of megophryids is greatly reduced in the adult compared with the larvae (Fig. 1; Griffiths, 1963; Púgener, 2002). A compact urostyle provides additional surfaces for the attachment of muscles that assist in symmetrical extension of the hind limbs during jumping, at the expense of undulatory locomotion (cf. Emerson, 1979, 1985). The transformation of the caudal vertebrae in megophryids to the adult caudal skeleton has not been previously explored.
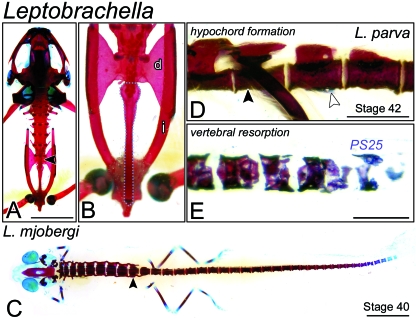
Fig. 1.
The postsacral skeleton of Leptobrachella. (A) An adult of Leptobrachella mjobergi (FMNH 222857) cleared and stained for bone (red) and cartilage (blue) shown in dorsal perspective. The presacral skeleton comprises eight imbricate vertebrae, each bearing transverse processes. The sacral vertebra (black arrowhead) is followed caudally by the urostyle. Scale bar, 5 mm. (B) Postsacral skeleton of A shown at higher magnification. The elongate urostyle (outlined in blue) is built from the fusion of the coccyx with the ossified hypochord. The sacral diapophyses (d) articulate with the pelvic ilia (i). (C) A Stage-40 metamorph of Leptobrachella mjobergi (FMNH 157998) shown in dorsal view. The postsacral skeleton comprises 29 discrete, perichordal centra. Scale bar, 3 mm. (D) Lateral view at level of the sacrum (black arrowhead) of Stage-42 Leptobrachella parva (FMNH 244079). A longitudinal ridge (white arrowhead) can be seen on the ventral face of the first postsacral centrum. This structure will probably contribute to the urostyle of the adult skeleton. Scale bar, 1 mm. (E) Close-up lateral view of most caudal centra of Leptobrachella parva metamorph (same specimen as in D). Vertebrae are porous and fragmented, suggestive of osteoclastic degradation. PS25, the 25th postsacral centrum. Scale bar, 1 mm.
Here we document the ultimate fate of these supernumerary caudal vertebrae in the megophryid genera Leptobrachella, Leptolalax, Megophrys and Ophryophryne. In all four, the larval tail skeleton undergoes massive reduction, with vertebrae apparently degraded by the action of osteoclasts, leaving only the coccyx and ossified hypochord intact. A similar phenomenon affects presacral centra in Megophrys and Ophryophryne, consistent with an epichordal mode of vertebral development and contrary to the perichordal type generally assigned to the Megophryidae (Griffiths, 1963; Kluge & Farris, 1969; Púgener, 2002). These dramatic changes in the megophryid axial skeleton reflect the differences in ecology and locomotion between larval and adult anuran phases. We suggest that metamorphosis is the single greatest developmental constraint on anuran morphology. Not only must cenogenetic adaptations be compatible with metamorphosis, but the transformation itself must be stringently executed, producing the conserved skeletal morphology of the adult.
Materials and methods
Metamorphic specimens of Leptobrachella, Leptolalax, Megophrys and Ophryophryne were obtained from various private and museum collections (see Table 1 for catalogue numbers); Xenophrys specimens were unavailable for study. In most cases, materials had been fixed in neutral-buffered formalin prior to long-term storage in 70% ethanol. Before processing, standard body measurements (total and snout–vent length) were taken using an electronic caliper (accurate to 0.01 cm) and specimens were staged according to Gosner (1960).
Table 1.
Megophryid specimens examined. Specimens were either cleared and stained for bone and cartilage (‘C & S’) or serially sectioned in paraffin (‘Histology’)
| Species | Gosne stage | Catalogue no. | Total length (mm) | Snout–vent length (mm) | Processing |
|---|---|---|---|---|---|
| Leptobrachella | |||||
| mjobergi | 40 | FMNH 157998 | 32.1 | 10.7 | C & S |
| Adult | FMNH 222857 | 18.7 | 18.7 | C & S | |
| parva | 42 | FMNH 244079 | 38.4 | 10.7 | C & S |
| Leptolalax pelodytoides | 40 | FMNH 254546.1 | 58.7 | 18.7 | C & S |
| 43 | FMNH 254546.2 | 53.9 | 21.0 | C & S | |
| 45 | ROM 42278 | 20.5 | 20.5 | C & S | |
| Megophrys | |||||
| boettgeri | 44 | A-30639.1 | 34.9 | 17.1 | C & S |
| 44 | A-30639.2 | 32.5 | 16.5 | C & S | |
| 44/45 | A-30639.3 | 35.7 | 15.3 | C & S | |
| lateralis | 43 | ROM 42365 | 47.1 | 14.7 | C & S |
| 45 | ROM 42366 | 31.7 | 16.0 | C & S | |
| 46 | ROM 42368 | 19.0 | 19.0 | C & S | |
| longipes | 31 | ZRC 1.4079 | 38.6 | 12.0 | Histology |
| montana | 44 | CAS 138409.1 | 28.5 | 12.9 | C & S |
| 44 | CAS 138409.2 | – | – | Histology | |
| 45 | CAS 138410 | 18.8 | 14.0 | C & S | |
| Ophryophryne microstoma | 42 | ROM 42344 | 22.8 | 8.8 | C & S |
| 43 | ROM 42345 | 15.7 | 10.9 | C & S |
A, American Museum of Natural History, New York, USA; CAS, California Academy of Science, San Francisco, USA; FMNH, Field Museum of Natural History, Chicago, USA; ROM, Royal Ontario Museum, Toronto, Canada; ZRC, Raffles Museum of Singapore.
Whole-mount bone-staining
Whole megophryid specimens were eviscerated and then cleared and stained with Alcian Blue and Alizarin Red following the protocol of Hanken & Wassersug (1981). In cases of poorly preserved or aged specimens, Alcian Blue staining was extended up to 7 days to maximize sulfated proteoglycan labelling.
Histology
Specimens for histology were decalcified in 10% EDTA for up to 10 days, dehydrated in an ethanol series, cleared in Histo-Clear II (National Diagnostics HS-202) and then embedded in paraffin. Serial sections were cut at a thickness of 5–10 µm and then mounted on slides subbed with Haupt's adhesive. Following overnight desiccation at 37 °C, sections were rehydrated in an ethanol series and stained by a Mallory's trichrome protocol. Briefly, sections were stained in 1% acid fuchsin for 15 s, the stain differentiated by two quick dips in fresh distilled H2O (dH2O) and then fixed by immersion in 1% phosphomolybdic acid (1–2 min). Following a quick dH2O rinse, sections were stained in Mallory's (0.5% aniline blue: 2% orange G) for 75 s, briefly rinsed and then dehydrated prior to two 10-min washes in xylene. The Mallory's trichrome stain rendered connective tissue and cartilage blue (aniline blue), muscle red to orange (orange G) and other tissues plus nuclei pink (acid fuchsin). A Zeiss Axiocam MRc digital camera was used to photograph all sectioned and whole-mount specimens.
Nomenclature
We apply the anatomical nomenclature and vertebral numbering system of Trueb (1973) throughout; to wit, trunk vertebrae are counted using Roman numerals, starting rostrally with I, the atlas, and continuing to IX, the sacral vertebra. For postsacral vertebrae (PS), we employ an Arabic numbering system, counting PS1, PS2 and so on for progressively more distal vertebrae. Finally, we define ‘coccyx’ as the fusion of rudimentary, postsacral neural arch elements. The coccyx combines with the ossified hypochord, located ventral to the notochord, to form a urostyle, the complete postsacral skeleton of the adult.
Results
Adult skeletal osteology
The adult axial skeleton of Leptobrachella mjobergi (FMNH 222857; Fig. 1A) comprises eight presacral vertebrae (Vertebrae I–VIII) followed caudally by a single sacral vertebra (IX). Centra are cylindrical in shape, but do not enclose a persistent notochord; Púgener (2002) diagnosed this morphology as ‘perichordal’. Presacral Vertebrae II–VIII bear transverse processes, which are particularly elongate on Vertebrae II–IV. Expanded diapophyses project laterally from the sacral vertebra to articulate with the pelvic ilia. The postsacral skeleton, or urostyle, is rod-like and comprises a caudally tapered coccyx ankylosed with the hypochord, which is itself closely associated with the sacral vertebra by a monocondylar articulation (Fig. 1B). For accounts of the adult skeletal osteology of other megophryid genera, including Megophrys, Scutiger and Leptobrachium, refer to Púgener (2002) and Griffiths (1963).
Leptobrachella
Presacral skeleton
At Stage 40, nearing the end of prometamorphosis (sensuHall & Larsen, 1998), the presacral skeleton of Leptobrachella mjobergi (FMNH 157998; Fig. 1C) is nearly complete. Vertebrae I–IX articulate at their zygapophyses and thumb-like transverse processes extend from Vertebrae II–IV. Presacral centra are more slender than those caudal to the sacrum. The presacral skeleton of a Stage-42 Leptobrachella parva (FMNH 244079) metamorph appears only slightly more developed: diapophyses extend from the sacral vertebrae and centra are more closely compacted.
Postsacral skeleton
Twenty-nine supernumerary centra are present in the tail of the Leptobrachella mjobergi metamorph (Fig. 1C). They are spool-like in appearance and, with the exception of PS28 and PS29, are all strongly stained with Alizarin Red. Rudimentary neural arch pedicels project dorsally from PS1 and PS2, but have not yet fused to form the coccyx. Ventrally, there is no evidence of a hypochord. In the Leptobrachella parva specimen, which is in metamorphic climax (sensuHall & Larsen, 1998), a longitudinal ridge is evident on the ventral surface of PS1, corresponding to the nascent hypochord (Fig. 1D). The neural arch pedicels of PS1 and PS2 nearly span the length of the underlying centra at their base. The most caudal postsacral centra appear fragmented and porous, suggesting that they may be undergoing osteoclastic degradation (Fig. 1E).
Leptolalax pelodytoides
Presacral skeleton
At Stage 40 (FMNH 254546.1), presacral vertebrae articulate at their zygapophyses and the intersegmental space between centra has nearly closed. Neural arch pedicels nearly span the full length of the associated centrum at their base (Fig. 2A). Finger-like transverse processes are found on Vertebrae II–IV, while diapophyses barely extend from the sacrum. By Stage 43 (FMNH 254546.2), the diapophyses are more prominent and have broadened and become rounded at their lateral face. They are further dilated at Stage 45 (ROM 42278), extending longitudinally to the level of neighbouring vertebrae, and articulate with the pelvic ilia. Presacral vertebrae at this stage are tightly apposed, separated only by cartilaginous intervertebral discs and appear imbricate from the dorsal perspective. Transverse processes extend from Vertebrae II–IX.
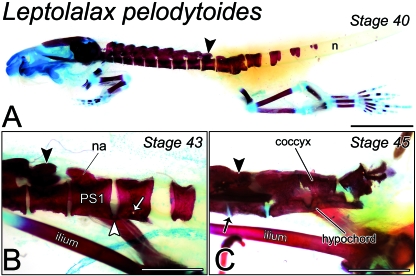
Fig. 2.
Remodelling of the postsacral skeleton of Leptolalax pelodytoides at metamorphosis. (A) Lateral view of a Stage-40 metamorph (FMNH 254546.1) cleared and stained for bone and cartilage. The presacral skeleton comprises eight closely articulated vertebrae. The sacrum (black arrowhead) is followed immediately by two longitudinally compacted centra and, more caudally, a perichordal centrum, which circumscribes the notochord (n), and three progressively more rudimentary dorso-lateral ossification pairs. Scale bar, 4 mm. (B) Lateral view of immediately postsacral vertebrae of a Stage-43 metamorph (FMNH 254546.2). Post-sacral Centrum 1 (PS1) is joined to PS2 at its caudal margin by an osseous bridge (white arrowhead). Both vertebrae are expanded ventrally, forming the hypochordal ridge. A small gap is visible between the ridge and the ventral surface of PS2 proper (white arrow), indicating an extra-vertebral origin for the hypochord. na, neural arch. Scale bar, 2 mm. (C) Lateral view of the caudal skeleton near the end of metamorphosis (Stage 45; ROM 42278). Intervertebral cartilage (black arrow) can be seen between the sacrum (black arrowhead) and adjacent vertebrae. Postsacral Vertebrae 1 and 2 are fused both dorsally and ventrally, forming the coccyx and hypochord, respectively. The remnants of PS3 and 4 are displaced dorsally. Scale bar, 2 mm.
Postsacral skeleton
At Stage 40, six supernumerary centra, the complete postsacral series, are present. PS1 and PS2 are tightly compacted, and a prominent, bulbous outgrowth is visible on the ventral surface of the former (Fig. 2A). This structure probably contributes to the urostyle. Postsacral Vertebra 3 is a perichordal ring, while PS4 is incomplete ventrally; PS5 and PS6 dorsally overlie the notochord as paired ossifications. These vertebrae are noticeably more developed at Stage 43: Postsacral Vertebra 4 has fused ventrally, and PS5 and PS6 each extend over the dorso-lateral surface of the notochord. More proximally, PS3 completely encircles the notochord, and PS1 and PS2 are joined ventro-medially by the hypochord, which also extends caudally into the intersegmental space between PS2 and PS3 (Fig. 2B). Consistent with an extravertebral origin for the hypochord, a small gap can be seen between it and the vertebral body of PS2.
Towards late metamorphic climax, at Stage 45, all caudal vertebrae have been longitudinally compacted into the coccyx and the notochord in the tail has degraded entirely. The remnants of PS1 and PS2 are closely articulated dorsally and fused completely as the hypochord ventrally (Fig. 2C). Laterally, both vertebrae show signs of degradation. Postsacral Vertebrae 4–6 are displaced dorsally and also show signs of degradation.
Megophrys
Presacral skeleton
In all species examined, spanning Stages 43–46, presacral centra show signs of osteoclastic degradation. This process progresses rostro-caudally, with those centra furthest from the sacrum appearing the most degraded. Coincident with this degradation, the notochord appears increasingly attenuate in later stages (Fig. 3A). At Stage 45 in M. montana (CAS 138410), Centra I–III persist only as flattened dorsal discs, consistent with the epichordal form (sensuPúgener, 2002). Closer to the sacrum, centra still encompass the notochord, but are noticeably degraded at their lateral and ventral aspects (Fig. 3A). In transverse section, this degraded bone has been infiltrated by multinucleate cells that resemble osteoclasts (CAS138409.2). By Stage 46 in M. lateralis (ROM 42368), the notochord has disappeared entirely along the length of the axial skeleton. Presacral centra, all now epichordal, are separated by cartilaginous intervertebral discs. The elongate transverse processes of Vertebrae II–IV, discernible from posthatching stages onwards, are now joined by stumpy processes on V–VIII. Diapophyses extend laterally from the sacral vertebra, having dilated considerably since Stage 43 (ROM 42365). Laminae are fused dorsally on all presacral vertebrae but the atlas.
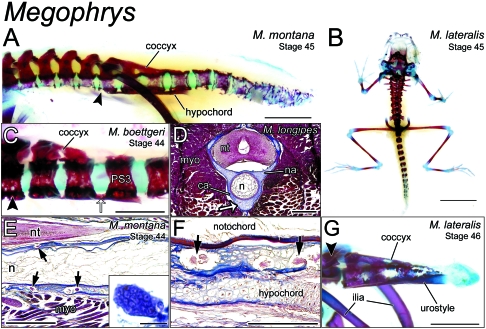
Fig. 3.
Remodelling of the postsacral skeleton of Megophrys at metamorphosis. (A) Lateral view of the axial skeleton (Vertebrae I–III not shown) of a Stage-25 Megophrys montana metamorph (CAS 138410). With the exception of the sacrum (black arrowhead) and those postsacral vertebrae in contact with the hypochord and coccyx, centra are fragmented and porous along the length of the vertebral column, suggestive of osteoclastic degradation. Scale bar, 2 mm. (B) A cleared-and-stained metamorph of Megophrys lateralis (Stage 45; ROM 42366) in dorsal perspective. Proximal postsacral centra are perichordal, whereas, more distally, centra comprise discrete ossification centres. Scale bar, 4 mm. (C) Close-up lateral view of the immediately postsacral vertebrae of a Megophrys boettgeri metamorph (Stage 44; A-30639.1). Postsacral Vertebrae 1–3 are joined ventrally by a longitudinal bridge (white arrow), which extends from the caudal margin of PS3. This structure will go on to form the hypochord in later stages. The dorsal surface of PS1 and 2 are fused as the coccyx. The hindlimb skeleton has been disarticulated from the sacrum (arrowhead), allowing for an unobstructed view. Scale bar, 1 mm. (D) Transverse cross-section at the level of PS1 of a Stage-31 tadpole of Megophrys longipes. The hypochordal ridge (white arrow) is clearly continuous with the perichordal tissue constituting the centrum (ca). myo, myotome; n, notochord; na, neural arch; nt, neural tube. Scale bar, 0.5 mm. (E) Parasagittal section through the sacral region of an M. montana metamorph (Stage 44; CAS 138409.2), showing multinucleate, osteoclast-like cells (arrows) embedded in peri-notochordal osseous tissue. Scale bar, 0.5 mm. Inset shows a close-up view of a multinucleate osteoclast in section. Scale bar, 50 µm. n, notochord; nt, neural tube; myo, myomere. (F) Sagittal section through the same specimen in E. Osteoclast-like cells are clearly visible in the peri-notochordal osseous tissue, but absent from the nascent hypochord, lying ventral. Scale bar, 0.25 mm. (G) Lateral view of the sacrum (black arrowhead) and caudal skeleton of a Stage-46 M. lateralis (ROM 42368) nearing the very end of metamorphosis. Postsacral Vertebrae 1–3 have been incorporated into the urostyle. All other supernumerary centra have been resorbed by this point. Scale bar, 2 mm.
Postsacral skeleton
Among the species examined, the number of postsacral vertebrae varies between 11 and 15. Generally, only the most proximal 5–7 are perichordal, completely encompassing the notochord, while more caudal centra comprise discrete dorsal and ventral plates or paired ossifications (Fig. 3B). As at presacral levels, postsacral vertebral centra are porous, suggestive of osteoclastic degradation. The sacrum and PS1–PS3 appear less eroded. These same vertebrae also show signs of contributing to urostyle development (Fig. 3A). At Stage 44 in M. boettgeri (A-30639.1; Fig. 3C), the postzygapophyses of PS1 are bridged with the adjacent neural arches of PS2 and, in turn, the dorsal surface of the vertebral body of PS3, forming the coccyx. Ventrally, the thickened floors of PS1–PS3 are closely apposed with the hypochord. Histological analysis of the sacral region of a Stage-31 M. longipes tadpole confirms that the two tissues are continuous with each other (Fig. 3D). At intervertebral levels, the hypochord can be seen as a discrete structure (Fig. 3A,C).
At Stage 45 in M. montana (CAS 138410), centra are completely fragmented and nearly impossible to distinguish from each other at the tail tip (Fig. 3A). More proximally, postsacral vertebrae are largely intact. Postsacral Vertebrae 4 and 5 have not yet fragmented and PS1–3 are porous only at their lateral surfaces. Histological examination of postsacral centra in a metamorph (Stage 44; CAS138409.2) reveals multinucleate, osteoclast-like cells occupying cavities in the perichordal tissue (Fig. 3E). In sagittal section, these cells are not found in the hypochordal tissue, which will contribute to the urostyle (Fig. 3F). In whole-mount, the hypochord is continuous with the floors of PS1–PS3, but discrete at intervertebral levels. Dorsally, the neural arches of the sacrum, PS1, and PS2 are bridged with PS3, forming paired, longitudinal ridges that constitute the coccyx (Fig. 3A,C). By Stage 46 in M. lateralis (ROM 42368) these thin bridges have broadened and constitute the coccyx. Postsacral Vertebrae 1–3 have collapsed laterally, while ventrally, they are continuous with a thickened, ossified hypochord. All other postsacral centra have been resorbed (Fig. 3G).
Ophryophryne microstoma
Presacral skeleton
In the Stage-42 metamorph (ROM 42344), all presacral vertebrae articulate at their zygapophyses. Finger-like transverse processes can be clearly seen on Vertebrae II–IV, while rounded diapophyses project laterally from the sacrum. Slender laminae are fused dorso-medially on Vertebrae III–VIII. Later in metamorphic climax (Stage 43; ROM 42345), laminae are also fused dorsally on the sacral vertebra. In the same specimen, transverse processes extend from Vertebrae II–VIII, and the sacral diapophyses are moderately dilated. Presacral centra have degraded at their dorsal and lateral faces coincident with the collapse of the notochord (Fig. 4A).
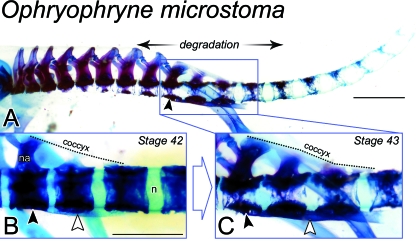
Fig. 4.
Remodelling of the postsacral skeleton of Ophryophryne microstoma at metamorphosis. (A) The postcranial skeleton of a Stage-43 metamorph (ROM 42345) shown in lateral perspective. Vertebral centra appear more degraded at distal levels relative to the sacrum (black arrowhead). The most rostral presacral centra are completely degraded at their lateral and ventral faces, consistent with the epichordal type described by Griffiths (1963). More caudally, including all postsacral vertebrae, centra are noticeably porous, but still encompass the notochord (i.e. perichordal type). Scale bar, 1.5 mm. (B) Lateral view of the sacrum (black arrowhead) and PS1–3 of a Stage-42 metamorph (ROM 42344). Dorsally, the coccyx (traced in broken line) joins the sacrum, PS1 and PS2. The hypochord (white arrowhead) can be clearly seen ventrally, spanning the sacrum to PS2. Vertebral centra enclose the notochord (n) and are not degraded. na, neural arch. Scale bar, 1 mm. (C) By Stage 43, presented here as a close-up of the sacral region of A, centra are degraded laterally and the coccyx (broken line) and hypochord (white arrowhead) have each thickened and now span the sacrum (black arrowhead) to PS3.
Postsacral skeleton
The postsacral skeleton at Stage 42 comprises 14 centra, the maximum number seen in O. microstoma. The immediately postsacral vertebrae present early signs of urostyle development. Slender cartilaginous bridges join the rudimentary neural arches of PS1 and PS2 to bumps on the dorsal surface of PS3. Ventrally, the sacrum, PS1 and PS2 are continuous with a cartilaginous hypochord, constituting the ‘hypochordal ridge’ (Fig. 4B). This structure is visible from posthatching stages onwards (Handrigan et al. 2007). At more caudal levels, centra are porous and in the early stages of resorption. The effects of this degradative process are more pronounced in the Stage-43 metamorph, with centra appearing lucent and unevenly ossified. In the same specimen, PS1–PS3 are bridged dorsally as the coccyx and ventrally with the hypochord; they are degraded at their lateral faces (Fig. 4C).
Discussion
Caudal vertebral fate at metamorphosis
Given that the megophryid adult postsacral skeleton is largely indistinguishable from that of other anurans (Fig. 1A; Griffiths, 1963; Púgener, 2002), we hypothesized that any supernumerary caudal vertebrae in the tadpole must either be resorbed with other tail structures or incorporated into the urostyle at metamorphosis. We have noted features of both modes in megophryid metamorphs. In Leptolalax pelodytoides, proximal tail centra appear to contribute to the nascent coccyx, dorsally, and the hypochord, ventrally (Fig. 2C). At more distal levels of the tail (PS1 and PS2), however, centra appear porous and fragmented, suggestive of resorption. Caudal centra have a similar appearance in Megophrys (Fig. 3A) and Ophryophryne (Fig. 4A) metamorphs, with distal centra more degraded than those closer to the sacrum. Histological examination of the caudal vertebrae of a Megophrys montana metamorph uncovered multinucleate, osteoclast-like cells occupying cavities in the cartilaginous perichordal tissue (Fig. 3E,F). The role of osteoclasts in remodelling the anuran axial skeleton at metamorphosis has been overlooked. Indeed, the only detailed studies of anuran osteoclasts relate to their function in tooth resorption or in the development of long bones (see Goode, 1967; Yaeger & Kraucunas, 1969; Shaw, 1988, 1989; Felisbino & Carvalho, 2001). To our knowledge, this is the first work to implicate osteoclasts in axial skeletal change during anuran metamorphosis.
The evidence for postsacral vertebral resorption is less direct for Leptobrachella, owing to the paucity of metamorphs from the genus in our sample. At Stage 42 in Leptobrachella parva, the most distal supernumerary centra appear porous and in the very earliest stages of resorption (Fig. 1E). Presumably, this degradative process extends to more proximal levels with the further progress of metamorphosis, but additional specimens, particularly those in late metamorphic climax, are needed to confirm this hypothesis. Similarly, metamorphic specimens are needed for Xenophrys, the fifth megophryid genus identified by Handrigan et al. (2007) to have tadpoles bearing extra vertebrae in their tails. Given the close phylogenetic affinities of Xenophrys with Megophrys and Ophryophryne (Frost et al. 2006), we predict that the axial skeleton undergoes dramatic reduction by osteoclastic degradation during metamorphosis in that genus.
Modes of centrum development in megophryids
A serendipitous outcome of this research was the observation of skeletal resorption of presacral vertebrae in Megophrys (Fig. 3A) and Ophryophryne (Fig. 4A) metamorphs. As in the tail, presacral centra in these specimens appear porous and fragmentary and have been infiltrated by osteoclast-like cells (data not shown). Unlike caudal centra, however, degradation is restricted to the ventral and lateral faces of the perichordal sheath – the dorsal arc is left intact (Figs 3A and 4A). This closely corresponds with a mode of epichordal centrum development described by Griffiths (1963): ‘A cartilaginous perichordal sheath is formed … but ossification is limited to the dorsal part of the cylinder and, at or after metamorphosis, the lateral and ventral perichordal walls are lost … The notochord also atrophies.’ Based on their early ossification pattern and the circum-notochordal morphology of larval vertebrae (Handrigan et al. 2007), however, megophryid centra clearly warrant the ‘perichordal’ label typically applied to them in the literature (Griffiths, 1963; Kluge & Farris, 1969; Púgener, 2002).
Variation in centrum ontogeny has previously been noted by Kluge & Farris (1969): ‘... there is a continuum of subtle change between [epichordal and perichordal modes]. The numerous levels of intermediacy suggest that the two categories cannot continue to be recognized …’ They added: ‘In the future studies that are obviously required to … assess the states of the continuum, we believe the critical developmental stages to be studied most intensively are those during and shortly after metamorphosis [when] the notochord and the ventral lateral walls of the perichordal tube disintegrate.’ Our study, with its focus on these critical developmental stages, clearly validates their assertion, revealing the perichordy–epichordy dichotomy to be an oversimplification of a developmentally labile process. Caudal vertebral development in megophryids also defies ready categorization; Handrigan et al. (2007) noted a range of ontogenetic modes even among closely related genera.
Dual origins for the hypochord in megophryids
In most anurans, the hypochord forms as a discrete, ossified rod under the notochord just caudal to the sacrum (Branham & List, 1979; Ročková & Roček, 2005; Handrigan & Wassersug, 2007, their fig. 3). In megophryids, however, the hypochord is only visible as a discrete structure at intervertebral levels. At vertebral levels, it is continuous with the ventral face of the vertebral body, forming a ‘hypochordal ridge’ (Figs 3A,C and 4B,C). The osseous tissue of immediately postsacral centra then appears to have been co-opted into forming the hypochord and ultimately contributing to the urostyle. At metamorphosis, this tissue is spared while all other portions of the vertebral body are resorbed (with the exception of dorsal tissues forming the coccyx). Thus, in megophryids at least, the ossified hypochord may have a dual origin – from the hypochord proper as well as the dorsal face of postsacral centra. This clarifies Griffiths's (1963) inaccurate description of the urostyle of Megophrys major as ‘a posterior, ventral outgrowth of the first postsacral intervertebral body.’ Furthermore, it corroborates the hypochord fate-mapping work of Shook et al. (2004), which evoked a mesodermal origin for the structure in anurans. Meanwhile, others (Lofberg & Collazo, 1997; Eriksson & Lofberg, 2000) have proposed an endodermal origin for the hypochord in the axolotl and zebrafish.
Ecological considerations
It is not known how the presence of caudal vertebrae affects the time it takes to resorb the tadpole tail. However, the process is likely to be protracted compared with normal pond tadpoles of similar size, owing to the presumed difficulty of degrading ossified tissue. This is in accordance with Downie et al.'s (2004) general prediction of longer metamorphic periods in cases of morphological specialization in the tadpole stage. A persistent tail would probably have locomotor consequences for the metamorphosing megophryid. Handrigan & Wassersug (2007) argue that jumping performance would be diminished, primarily by the added dead weight, making metamorphs easier targets for predation.
The possibility remains, however, that the postsacral skeleton of megophryids may not impede tail resorption whatsoever. Supernumerary caudal vertebrae, though strongly ossified in most species examined, appear quite thin and fragile in whole-mount. Histologically, they have the appearance of cartilage, with lacunae embedded in a fibrous collagen matrix. Presumably, such cartilaginous tissue would be far less difficult to resorb than osteoid bone. Furthermore, a short, persistent tail may represent no major mechanical handicap to megophryids, which are generally poor jumpers as adults and avoid predation by mimicking leaves on the forest floor (Lathrop, 2003). Experiments mirroring the tail amputation studies of Wassersug & Sperry (1977) would instruct as to the precise functional consequences of a retained tail to megophryid metamorphs.
Megophryid tadpoles are not unrivalled in their ability to remodel their axial skeleton at metamorphosis. Metamorphosing ‘tenuis’ larvae of the pearlfish Carapus homei can resorb up to 70 vertebrae on their way to becoming juveniles. Whereas it is not known how long or by what mechanism this massive remodelling process occurs, Parmentier et al. (2004) have correlated it with the pearlfish's unique lifestyle. They suggest that the additional vertebrae enable the parasitic larva to make its first entrance into the body cavity of its benthic host, the sea cucumber, via the anus. As with Leptobrachella and other burrowing megophryids, the additional vertebrae of C. homei probably allow for greater manoeuvrability in a resistant substrate (Haas et al. 2006; Handrigan et al. 2007).
Metamorphosis and anuran morphology
At metamorphosis, megophryids replace their unique caudal skeleton with the urostyle that characterizes all frogs and toads. This dramatic remodelling highlights the disparity between larval and adult phases with respect to habitat and locomotion, i.e. megophryid tadpoles burrow in streams, while adults live among leaf litter on the forest floor. The extreme morphological conservation of the adult anuran axial skeleton, even when preceded by diverse tadpole morphology, reveals the overarching role that developmental constraint has played in anuran evolution. We suggest that metamorphosis is the single greatest constraint on anuran morphology, affecting both larvae and adults alike. Cenogenetic adaptations in the larval phase must be compatible with metamorphosis to ensure a viable and reproductive adult. In the case of megophryids, caudal centra do not disrupt the characteristic progression of the metamorphic event – they are degraded pari passu with other tissues of the tail. The stringent regulation of metamorphosis itself also ensures that anurans will have a reproductive stage, but consequently seems to entrap them into their conservative postmetamorphic skeletal morphology.
Acknowledgments
We acknowledge the following people and institutions for the generous loan of specimens: Darrel Frost at the American Museum of Natural History, New York; Robert Murphy and Amy Lathrop at the Royal Ontario Museum, Toronto; Harold Voris, Robert Inger and Alan Resetar at the Field Museum of Natural History, Chicago; Kelvin Lim at the Raffles Museum of Singapore; and Bob Drewes and Jens Vindum at the California Academy of Sciences, San Francisco. We also thank Barb Banbury, Amy Lathrop, Richard Elinson and Brian K. Hall for helpful discussion or critical commentary on the manuscript. Special thanks to Alexander Haas for first introducing us to Leptobrachella mjobergi and for his invaluable input to the manuscript. This study was supported by grants from the Society for Integrative and Comparative Biology, Sigma-Xi, The Scientific Society, the Natural Sciences and Engineering Research Council of Canada, and the Patrick Lett Fund, Dalhousie University.
References
- Branham AE, List JC. Development of the urostyle during metamorphosis in five species of anurans. J Morph. 1979;159:311–330. doi: 10.1002/jmor.1051590303. [DOI] [PubMed] [Google Scholar]
- Downie JR, Bryce R, Smith J. Metamorphic duration: an under-studied variable in frog life histories. Biol J Linn Soc Lond. 2004;83:261–272. [Google Scholar]
- Emerson SB. The ilio-sacral articulation in frogs: form and function. Zool J Linn Soc. 1979;11:153–168. [Google Scholar]
- Emerson SB. Jumping and leaping. In: Hildebrand M, Bramble DM, Liem KF, Wake DB, editors. Functional Vertebrate Morphology. Cambridge: Belknap Press; 1985. pp. 58–72. [Google Scholar]
- Eriksson J, Lofberg J. Development of the hypochord and dorsal aorta in the zebrafish embryo (Danio rerio) J Morph. 2000;244:167–176. doi: 10.1002/(SICI)1097-4687(200006)244:3<167::AID-JMOR2>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Felisbino SL, Carvalho HF. Growth cartilage calcification and formation of bone trabeculae are late and dissociated events in the endochondral ossification of Rana catesbeiana. Cell Tissue Res. 2001;306:319–323. doi: 10.1007/s004410100446. [DOI] [PubMed] [Google Scholar]
- Frost DR, Grant T, Faivovich J, et al. The amphibian tree of life. Bull Am Mus Nat Hist. 2006;297:1–371. [Google Scholar]
- Goode RP. The regeneration of limbs in adult anurans. J Embryol Exp Morph. 1967;18:259–267. [PubMed] [Google Scholar]
- Gosner KL. A simplified table for staging anuran embryos and larvae with notes on identification. Herpetologica. 1960;16:183–190. [Google Scholar]
- Griffiths I. The status of Protobatrachus massinoti. Nature. 1956;177:342–343. [Google Scholar]
- Griffiths I. The phylogeny of the Salientia. Biol Rev Camb Philos Soc. 1963;38:241–292. doi: 10.1111/j.1469-185x.1963.tb00784.x. [DOI] [PubMed] [Google Scholar]
- Haas A, Hertwig S, Das I. Extreme tadpoles: the morphology of the fossorial megophryid larva, Leptobrachella mjobergi. Zoology. 2006;109:26–42. doi: 10.1016/j.zool.2005.09.008. [DOI] [PubMed] [Google Scholar]
- Hall JA, Larsen JH. Postembryonic ontogeny of the spadefoot toad, Scaphiopus intermontanus (Anura: Pelobatidae): skeletal morphology. J Morph. 1998;238:179–244. doi: 10.1002/(SICI)1097-4687(199811)238:2<179::AID-JMOR4>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- Handrigan GR, Haas A, Wassersug RJ. Bony-tailed tadpoles: the development of supernumerary caudal vertebrae in larval megophryids (Anura) Evol Dev. 2007;9:190–202. doi: 10.1111/j.1525-142X.2007.00149.x. [DOI] [PubMed] [Google Scholar]
- Handrigan GR, Wassersug RJ. The anuran Bauplan: a review of the adaptive, developmental, and genetic underpinnings of frog and tadpole morphology. Biol Rev Camb Philos Soc. 2007;82:1–25. doi: 10.1111/j.1469-185X.2006.00001.x. [DOI] [PubMed] [Google Scholar]
- Hanken J, Wassersug R. The visible skeleton. Func Photogr. 1981;44:22–26. [Google Scholar]
- Kluge AG, Farris JS. Quantitative phyletics and the evolution of anurans. Syst Zool. 1969;18:1–32. [Google Scholar]
- Lathrop A. Asian toadfrogs (Megophryidae) In: Duellman WE, editor. Amphibians. Grzimek's Animal Life Encyclopedia. 2. Vol. 6. Detroit: Gale Group; 2003. pp. 109–117. [Google Scholar]
- Lofberg J, Collazo A. Hypochord, an enigmatic embryonic structure: study of the axolotl embryo. J Morph. 1997;232:57–66. doi: 10.1002/(SICI)1097-4687(199704)232:1<57::AID-JMOR3>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- Parmentier E, Lecchini D, Vandewalle P. Remodelling of the vertebral axis during metamorphic shrinkage in the pearlfish. J Fish Biol. 2004;64:159–169. [Google Scholar]
- Púgener LA. The vertebral column and spinal nerves of anurans. University of Kansas; 2002. Doctoral dissertation. [Google Scholar]
- Ročková H, Roček Z. Development of the pelvis and posterior part of the vertebral column in the Anura. J Anat. 2005;206:17–35. doi: 10.1111/j.0021-8782.2005.00366.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw JP. A quantitative comparison of osteoclasts in the teeth of the anuran amphibian Xenopus laevis. Arch Oral Biol. 1988;33:451–453. doi: 10.1016/0003-9969(88)90203-8. [DOI] [PubMed] [Google Scholar]
- Shaw JP. A morphometric study of bone and tooth volumes in the pipid frog Xenopus laevis (Daudin), with comments on the importance of tooth resorption during normal tooth replacement. J Exp Zool. 1989;249:99–104. doi: 10.1002/jez.1402490117. [DOI] [PubMed] [Google Scholar]
- Shook DR, Majer C, Keller R. Pattern and morphogenesis of presumptive superficial mesoderm in two closely related species, Xenopus laevis and Xenopus tropicalis. Dev Biol. 2004;270:163–185. doi: 10.1016/j.ydbio.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Shubin NH, Jenkins FA. An Early Jurassic jumping frog. Nature. 1995;377:49–52. [Google Scholar]
- Trueb L. Bones, frogs, and evolution. In: Vial JL, editor. Evolutionary Biology of the Anurans: Contemporary Research on Major Problems. Columbia, MO: University of Missouri Press; 1973. pp. 65–132. [Google Scholar]
- Wassersug RJ, Sperry D. The relationship of locomotion to differential predation on Pseudacris triseriata (Anura: Hylidae) Ecology. 1977;58:830–839. [Google Scholar]
- Yaeger JA, Kraucunas E. Fine structure of the resorptive cells in the teeth of frogs. Anat Rec. 1969;164:1–13. doi: 10.1002/ar.1091640101. [DOI] [PubMed] [Google Scholar]