Abstract
Bone growth at the cranial sutures relies on proliferation of osteogenic progenitor cells and/or differentiation of osteoblasts. The current study was undertaken to assess these events in relation to suture growth and fusion. A total of 21 pigs, divided into three age groups (0.5–1.5 months, 3–4 months and 5–7 months), were used for immunohistochemical evaluation of cell proliferation (BrdU) and osteogenic differentiation (Cbfa1/Runx2) in the interfrontal and interparietal sutures. Proliferation and osteogenic differentiation were both more prominent near the bone fronts than in the central zone. With age, both proliferation and osteogenic differentiation diminished. Proliferation ceased on the endocranial (dura mater) side by the age of 3–4 months. Proliferation on the pericranial side was accompanied by active bone formation and initiation of suture fusion from this side. In conclusion, (1) decreased suture bone growth with age reflects decreased cell proliferation and probably also osteogenic differentiation, and (2) suture fusion occurs from the pericranial side where activity remains relatively high.
Keywords: cranial suture, immunohistochemistry, osteoblast differentiation, proliferation, suture fusion
Introduction
Cranial sutures are important growth sites for the braincase at early stages of life when the neurocranium is expanding rapidly (Baer, 1954; Moss, 1954; Mednick & Washburn, 1956; Duterloo & Enlow, 1970). Histologically, suture growth is characterized by continuous bone deposition along the margins, although the details vary among species and sutures (Manzanares et al. 1988; Opperman, 2000). When the neurocranium approaches adult size, suture growth gradually diminishes or ceases (Ten Cate et al. 1977). The remaining growth of the braincase is then mainly contributed by the pericranium (Mednick & Washburn, 1956; Moss & Young, 1960; Sun et al. 2004). At the cellular level, however, it is unclear whether the age-related decrease of bone formation activity is due to diminished cell proliferation or differentiation or both. In human calvarial bone, both phenomena occur (de Pollak et al. 1997), but it is not clear whether sutural cells show the same pattern.
Another important aspect for suture growth is fusion. Over the last decade, suture fusion has been extensively investigated using murid rodent models, and ample data demonstrate that the dura mater plays a critical role in regulating suture fusion (Opperman et al. 1993, 1995, 1998; Roth et al. 1996; Bradley et al. 1997; Kim et al. 1998; Greenwald et al. 2000; Rice et al. 2000). In murid rodents under normal conditions, only the posterior interfrontal suture fuses and this occurs at about the time of weaning. The posterior interfrontal suture is homologous with part of the human metopic suture. In addition, these two sutures share an anterior to posterior, endo- to ectocranial direction of assembly (Warren & Longaker, 2001) and of fusion (Warren & Longaker, 2001; Weinzweig et al. 2003). Given these similarities, it is reasonable to speculate that human metopic suture fusion is also governed by the dura mater.
For the sagittal (interparietal) suture, continued patency characterizes murid models, and this patency may also be controlled by the dura mater, which appears to have local specializations (Bradley et al. 1997; Greenwald et al. 2000). Unlike the murid sagittal suture, however, the human sagittal suture (and all human braincase sutures except the metopic) often fuses, with initial synostosis typically seen during the second decade of life (Todd & Lyon, 1924, 1925; Kokich, 1986; Manzanares et al. 1988). It is not clear what role, if any, is played by the dura mater in late fusing sutures such as the human sagittal suture. Rats and mice, in which no sutures fuse late (Warren & Longaker, 2001), do not provide suitable models for sagittal suture fusion. Thus, a large animal model is needed.
The pig braincase is a classic model for assessing the relative importance of sutural vs. pericranial growth of the skull (Brash, 1934; Mednick & Washburn, 1956), and in vivo suture and bone strains are uniquely well known for this species (Herring & Teng, 2000; Sun et al. 2004). The pig sagittal (interparietal) suture starts to fuse at 6–7 months of age (Sun et al. 2004), similar to the teenage years in humans. In this sense, the pig interparietal suture may be a better model to understand the fusion of the human sagittal suture.
Fusion of the pig sagittal suture is initiated from the ectocranial side (Sun et al. 2004). Although this is unlike the normal human condition, premature metopic and sagittal suture fusion in humans also suggest a significant involvement of the pericranial side, frequently manifested as an ectocranial ridge (reviewed by Cohen, 1993). Thus, a different mechanism involving the ectocranial periosteum may be possible and can be investigated in the pig.
Therefore, the current study was undertaken for two purposes. First, we wished to provide descriptive data on cell proliferation and differentiation in pig interparietal and interfrontal sutures and how they change with age. These data provide baseline information about sutures in a large animal model. Second, we wished to compare cellular activity between the ectocranial and endocranial regions to investigate a possible association with suture fusion.
Materials and methods
Animals and labelling
Samples from 21 pigs (Sus scrofa), including nine standard pigs and 12 Hanford strain miniature pigs (Charles River Laboratories, now available from Sinclair Research Farms, Columbia, MO, USA), were gathered after use in other studies. At the time of death, the nine standard pigs were 0.5–1.5 months (youngest group), five Hanfords were 3–4 months old (middle group) and seven Hanfords were 5–7 months old (oldest group). All animals received 5′-bromo-2′-deoxyuridine (BrdU) labelling (Sigma, St Louis, MO, USA). Animals in the youngest group, which were originally used for craniofacial periosteum studies (Ochareon, 2004), were injected 3 h before they were killed. The Hanford pigs, which were used for studies on suture mechanics and bone growth (Rafferty et al. 2003; Sun et al. 2004), were injected 2 days prior to being killed. Because of the differences in breed and procedure, comparison of BrdU labelling between the youngest and the other two groups was qualitative only (detailed below). All experimental procedures were approved by the University of Washington Institutional Animal Care and Use Committee.
Tissue processing and staining
Suture specimens were harvested 3 h after labelling in the youngest group and immediately after terminal mechanical experiments in the middle and oldest groups. These experiments involved the placement of strain gauges on cranial sutures and adjacent bones as well as masticatory muscle EMG recording (Sun et al. 2004). Pigs were deeply anaesthetized with halothane and nitrous oxide, and intracardiac (for smaller pigs) or carotid (for larger pigs) perfusion was performed with 3 L of heparinized 0.9% saline followed by 3 L of fixative (Prefer, Anatech Ltd, Battle Creek, MI, USA). The skinned whole skull was immersed in Prefer for further fixation. Two weeks later, specimen blocks of the posterior interfrontal and the anterior interparietal sutures were collected (Fig. 1A). The locations of specimens were adjacent to undecalcified specimens used to measure bone mineral apposition rate in our previous study (Sun et al. 2004). After 2 more weeks of fixation, the specimen blocks were decalcified with a mixture of 15% formic acid and 2.6% sodium formate. Completely decalcified specimens were embedded in paraffin and 5-µm-thick coronal sections were obtained.
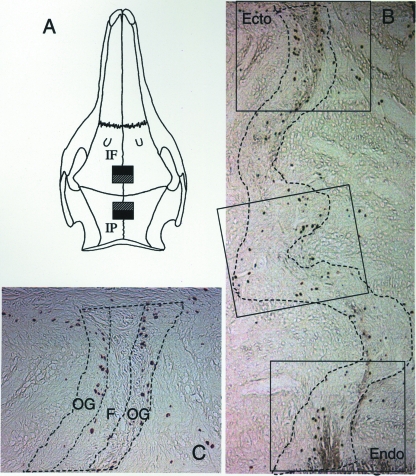
Fig. 1.
(A) Specimen collection from the posterior interfrontal (IF) and anterior interparietal (IP) sutures. Hatched boxes, decalcified specimens; filled boxes, undecalcified specimens. (B) Image capture (rectangles) included the ectocranial (Ecto) and endocranial (Endo) margins and every other field along the course of the suture. The suture margins are marked by broken lines. (C) Image analyses. The width of the suture space was equally divided (indicated by broken lines) into outer thirds (OG, osteogenic zones) and middle third (C, central zone). The density of positive cells (mm−2) in each zone was measured separately.
About three sections from each block were stained with haematoxylin and eosin for general histology. Another 4–6 sections were processed immunohistochemically to reveal cell proliferation or osteogenic differentiation. Sections were deparaffinized with the xylene substitute Clearene® (Surgipath Medical Industries, Inc., Richmond, IL, USA) and rehydrated. Endogenous peroxidase activity was blocked by incubation with 2% hydrogen peroxide at room temperature for 15 min. Antigen retrieval was then performed by incubation with 1% trypsin at 37 °C for 20 min. Endogenous biotin and non-specific binding proteins were blocked by incubation with a 1 : 1 mixture of avidin (Avidin/Biotin blocking kit, Vector Laboratories, Inc., Burlingame, CA, USA) and 1% bovine serum albumin (BSA, Vector Laboratories) at room temperature for 15 min, followed by incubation with a 1 : 1 mixture of biotin (Vector Laboratories) and 1% BSA at room temperature for 15 min. Sections were then reacted with either mouse monoclonal BrdU antibody (IgG1, working concentration 0.5 µg mL−1, Becton Dickinson Biosciences, San Jose, CA, USA) or rabbit polyclonal Cbfa-1 antibody (IgG, working concentration 2 µg mL−1, Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA) at 4 °C overnight. Negative control sections were reacted to mouse IgG1 isotype (for BrdU, Dako Corp., Carpenteria, CA, USA) or rabbit IgG (for Cbfa-1, Southern Biotechnology Associates Inc., Birmingham, AL, USA). The reaction was stopped by thorough washing with PBS. At room temperature, the sections were then treated with biotin-conjugated secondary antibody for 30 min, followed by incubation with avidin-labelled peroxidase for another 30 min. Both reagents were from the standard ABC kit (Vector Laboratories). Chromogen development was performed by means of the peroxidase substrate 3,3′-diaminobenzidine (DAB) (Vector Laboratories) for 2–6 min and stopped by thorough rinsing with water. For better differentiation of positive reactions, no counter-staining was applied. Sections were dehydrated, cleared and cover-slipped using Cytoseal™ 60 medium (Richard-Allan Scientific, Kalamazoo, MI, USA).
Image capture and analysis
For the BrdU reaction, two sections were analysed for each suture. Images showing the full width of the suture (10× or 20× objective) were captured with a digital camera (Coolsnap™ fx, Photometrics, Tucson, AZ, USA) using MetaVue software (Universal Imaging Corp., Downingtown, PA, USA). Captured images included the ectocranial and endocranial openings of the suture as well as every other image along its interior course (Fig. 1B). All captured images were named by randomly generated code to blind the analysis. For fusing sutures, only images from regions where the suture was still patent were analysed.
Quantification of BrdU-positive cells was performed in MetaVue. Suture width was first divided into three equal thirds using the caliper tool. The middle third was designated as the central zone while the outer two thirds, which resemble the osteogenic layer of periosteum, were designated as the osteogenic zone (Fig. 1C). BrdU-positive cells in each zone were counted and the number was divided by the area of that zone, resulting in a measurement defined as proliferative cell density. Measurements were first obtained separately for each region (ectocranial, interior or endocranial). For regions that had two or more images, the sum of the positive cells was divided by the sum of the areas to achieve the proliferative cell density of the entire region. Proliferative cell density for all regions combined (entire suture) was calculated by dividing the sum of positive cells by the sum of the areas.
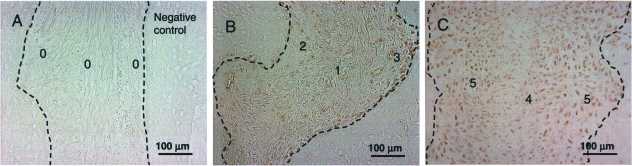
For Cbfa-1, serial images were captured and coded similarly. Unlike BrdU, only semi-quantitative analysis was performed. A scoring index was used (0, no reaction; 1, minimal reaction; 2, weak reaction, 3, moderate reaction; 4, strong reaction; 5, very strong reaction) to assess relative intensity for each image. Example images of different ranks are shown in Fig. 2. Each image was assessed simultaneously by two investigators (Sun and Lee), and the analysis was repeated after a 2-week interval. The average of the two assessments was used for the final analysis.
Fig. 2.
Examples of Cbfa-1 reaction scoring (see text). No counterstaining was used. Exposure time and white balancing values were kept constant during image capture. The mild colour inconsistency between images is due to differences in background staining between sections. The suture margins are indicated by broken lines. Reaction strength scores were assessed for the central and osteogenic zones separately. (A) Negative control; (B) interior of #304 (oldest group) interfrontal suture, and (C) interior of #313 (youngest group) interfrontal suture.
Results
Suture histology
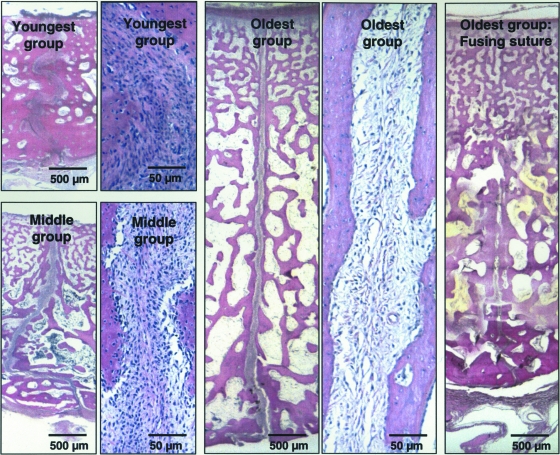
Haematoxylin and eosin-stained slides (Fig. 3) showed that with age, the interparietal sutures increased in thickness (from ectocranial surface to endocranial surface) and decreased in suture width, cellularity and the degree of interdigitation. The interfrontal sutures (not shown) had the same age-associated changes except that flattening of the suture surface was only seen at the ectocranial side in the oldest group (Sun et al. 2004). Generally the osteogenic zone appeared to be more cellular and less fibrous than the central zone. Suture fusion had occurred in three out of seven interparietal sutures of the oldest group and was initiated from the ectocranial side in all fusing sutures. Neither ectocranial nor endocranial ridging was observed in these animals.
Fig. 3.
Suture histology (H&E). All images are from the interparietal sutures with the upper side toward the ectocranial surface of the suture. Viewed under a 1× objective (scale bar 500 µm), suture thickness increased with age while suture width decreased. Viewed under a 10× objective (scale bar 50 µm), suture cellularity decreased substantially with age. Compared with the two older groups, the suture of the youngest group had more interdigitations. Suture fusion had started in some interparietal sutures of the oldest group. Note the suture gap has disappeared on the ectocranial side but is retained on the endocranial side.
Cell proliferation
Typical anti-BrdU-reacted suture images are shown in Fig. 4. No reaction was present in negative control sections. Qualitatively, the youngest and the middle age groups had stronger proliferative activity than the oldest group, and the size of the stained nuclei appeared to decrease with age.
Fig. 4.
BrdU reaction inside the suture. Magnification, exposure time and white balancing values were held constant. Suture margins are indicated by broken lines. Cell proliferation was relatively strong in the youngest and the middle age groups but diminished in the oldest age group (arrows). The size of positively stained nuclei appeared to decrease with age. In all three age groups, more positive cells were present in the osteogenic than in the central zone. Scale bar, 100 µm.
Comparisons of proliferative cell densities of whole sutures (all regions combined) are presented in Table 1. Regardless of age and suture, the osteogenic zones had higher density of proliferative cells than the central zones. The two sutures did not differ except that in the oldest group the interfrontal osteogenic zone had higher proliferative cell density than the interparietal osteogenic zone (paired t-test, P = 0.03). Proliferative activity demonstrated an overall decrease with age from the middle to the oldest group, significant for the interparietal suture. Due to the different timing of BrdU injection in the youngest group (3 h rather than 2 days before killing), counts from this group could not be compared statistically with the other groups. However, it is likely that more labelled cells would have been seen in this group had survival time been increased to 2 days.
Table 1.
Proliferative (BrdU+) cell density (mm−2) of sutures
| Animal ID | IF-Og | IF-C | IP-Og | IP-C |
|---|---|---|---|---|
| Youngest group | ||||
| 308 | 134 | 117 | 258 | 129 |
| 309 | 172 | 92 | 120 | 35 |
| 310 | 142 | 83 | 152 | 94 |
| 311 | 186 | 161 | 135 | 91 |
| 312 | 231 | 161 | 178 | 68 |
| 313 | 143 | 70 | 130 | 72 |
| 314 | 242 | 188 | 282 | 220 |
| 315 | 207 | 190 | 293 | 232 |
| 316 | 186 | 233 | ND | ND |
| Average | 182 | 144 | 194 | 118 |
| SD | 39 | 56 | 72 | 72 |
| OG vs. C (paired t-test): P | 0.020 | < 0.001 | ||
| IF vs. IP (paired t-test): P | 0.647 | 0.427 | ||
| Middle group | ||||
| 297 | 104 | 58 | 108 | 108 |
| 299 | 418 | 259 | 281 | 114 |
| 300 | 208 | 73 | 294 | 190 |
| 293 | 310 | 65 | 351 | 209 |
| 294 | 208 | 134 | 96 | 74 |
| Average | 260 | 114 | 258 | 155 |
| SD | 135 | 97 | 105 | 52 |
| OG vs. C (paired t-test): P | 0.028 | 0.056 | ||
| IF vs. IP (paired t-test): P | 0.615 | 0.717 | ||
| Oldest group | ||||
| 301 | 116 | 105 | 68 | 68 |
| 302 | 158 | 80 | 112 | 78 |
| 303 | 84 | 42 | 35 | 6 |
| 304 | 136 | 80 | 91 | 55 |
| 306 | 163 | 51 | 49 | 35 |
| 321 | 144 | 46 | 171 | 119 |
| 322 | 71 | 37 | 44 | 25 |
| Average | 124 | 63 | 81 | 55 |
| SD | 36 | 26 | 48 | 37 |
| OG vs. C (paired t-test): P | <0.001 | < 0.001 | ||
| IF vs. IP (paired t-test): P | 0.033 | 0.603 | ||
| Middle vs. oldest group (2-sample t-test): P | 0.077 | 0.224 | 0.047 | 0.027 |
IF, interfrontal suture; IP, interparietal suture; OG, osteogenic zone; C, central zone. ND, no data.
Regional (ectocranial, interior and endocranial) comparisons of proliferative cell densities are shown in Table 2. For the youngest and middle groups, no regional difference was found. For the oldest group, however, proliferation tended to be higher in the ectocranial region than in the interior and endocranial regions, although only the osteogenic zone of the interparietal suture reached statistical significance (anova, P = 0.01).
Table 2.
Regional differences of proliferative (BrdU+) cell density (mean ± SD, mm−2)
| IF-Og | IF-C | IP-Og | IP-C | |
|---|---|---|---|---|
| Youngest group (n = 9) | ||||
| Ecto | 203 ± 97 | 189 ± 129 | 215 ± 96 | 157 ± 114 |
| Interior | 166 ± 49 | 123 ± 70 | 179 ± 88 | 88 ± 58 |
| Endo | 207 ± 74 | 170 ± 70 | 196 ± 78 | 126 ± 81 |
| anova: P | 0.461 | 0.322 | 0.723 | 0.308 |
| Middle group (n = 5) | ||||
| Ecto | 266 ± 205 | 110 ± 104 | 300 ± 269 | 182 ± 211 |
| Inferior | 242 ± 111 | 123 ± 81 | 201 ± 79 | 124 ± 58 |
| Endo | 263 ± 170 | 112 ± 90 | 270 ± 179 | 103 ± 57 |
| anova: P | 0.970 | 0.972 | 0.713 | 0.623 |
| Oldest group (n = 7) | ||||
| Ecto | 221 ± 173 | 84 ± 67 | 237 ± 191 | 115 ± 126 |
| Interior | 107 ± 43 | 59 ± 37 | 70 ± 39 | 62 ± 48 |
| Endo | 69 ± 105 | 41 ± 81 | 35 ± 48 | 20 ± 22 |
| anova: P | 0.072 | 0.467 | 0.011 | 0.101 |
IF, interfrontal suture; IP, interparietal suture; OG, osteogenic zone; C, central zone.
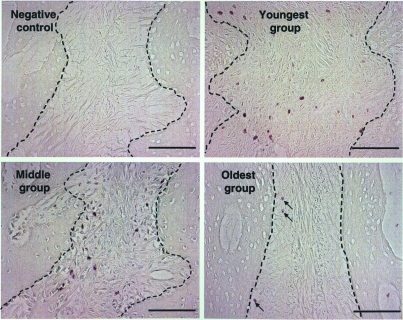
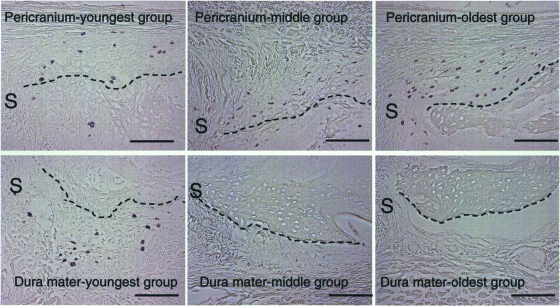
Perisutural (pericranium and dura mater) cell proliferation is shown in Fig. 5. Due to the placement of strain gauges on the ectocranial bone surface of some pigs during the terminal experiment, which necessitated pericranium reflection (Sun et al. 2004), only qualitative observations were made. The youngest group showed comparable levels of proliferative cells in the pericranium and the dura mater. With age, cell proliferation diminished in both the pericranium and the dura mater. However, this was far more dramatic in the latter. By the age of 3–4 months (middle group), cell proliferation on the dural side was almost completely inactivated, while the pericranial side still retained moderate proliferation even at the age of 5–7 months (oldest group).
Fig. 5.
Perisutural tissue cell proliferation. The suture (S) is on the right side of each image. Bone surfaces toward the pericranium (upper panel) or the dura mater (lower panel) are marked by broken lines. With age, BrdU-labelled cells remained numerous on the pericranium, but were almost completely absent on the dural side in the two older age groups. Scale bar, 100 µm.
Osteogenic differentiation
Cbfa-1 staining was satisfactory in 13 of 21 subjects. Scored data of the whole suture (regions combined) are shown in Table 3. Due to the limited sample size, the middle group was not included in the statistical analyses. As exemplified in Fig. 2(B,C), the Cbfa-1 reaction was always stronger in the osteogenic zone than in the central zone (Mann–Whitney U-tests for youngest and oldest groups, P = 0.003, 0.075). No difference was found between the interfrontal and interparietal sutures (P > 0.05). Comparison between the youngest and the oldest group showed that the younger animals had significantly stronger reactions, especially in the interparietal suture (Fig. 2). Although breed as well as age distinguished these groups, the fact that the few available values from the middle group were intermediate suggests that the difference in Cbfa-1 activity was a true age effect.
Table 3.
Cbfa-1 reaction semi-quantitative assessment (average score*)
| Animal ID | IF-Og | IF-C | IP-Og | IP-C |
|---|---|---|---|---|
| Youngest group | ||||
| 309 | 4.8 | 3.0 | 4.9 | 3.4 |
| 310 | 5.0 | 3.1 | 5.0 | 3.2 |
| 311 | 5.0 | 2.9 | 5.0 | 3.2 |
| 312 | ND | ND | 5.0 | 3.7 |
| 313 | 5.0 | 4.0 | 4.7 | 2.8 |
| 314 | 4.0 | 2.0 | 5.0 | 4.3 |
| Average | 4.8 | 3.0 | 4.9 | 3.4 |
| SD | 0.4 | 0.7 | 0.1 | 0.5 |
| OG vs. C (Mann–Whitney U-test): P | 0.011 | 0.003 | ||
| IF vs. IP (Mann–Whitney U-test): P | 0.672 | 0.200 | ||
| Middle group | ||||
| 299 | 4.5 | 2.1 | ND | ND |
| 300 | 3.7 | 1.8 | 4.0 | 1.0 |
| Average | 4.1 | 2.0 | 4.0 | 1.0 |
| SD | 0.6 | 0.2 | ||
| Oldest group | ||||
| 303 | 4.2 | 1.7 | 2.9 | 0.8 |
| 304 | 3.6 | 1.6 | 4.0 | 2.0 |
| 306 | 3.7 | 1.6 | 1.8 | 0.8 |
| 321 | ND | ND | 4.5 | 2.1 |
| 322 | 4.3 | 2.3 | 1.7 | 0.2 |
| Average | 4.0 | 1.8 | 3.0 | 1.2 |
| SD | 0.4 | 0.3 | 1.3 | 0.8 |
| OG vs. C (Mann–Whitney U-test): P | 0.020 | 0.075 | ||
| IF vs. IP (Mann–Whitney U-test): P | 0.327 | 0.323 | ||
| Youngest vs. oldest group (Mann–Whitney U-test): P | 0.046 | 0.027 | 0.005 | 0.006 |
Scores ranged from 0 (no reaction) to 5 (very strong). IF, interfrontal suture; IP, interparietal suture; OG, osteogenic zone; C, central zone; ND, no data.
Regional (ectocranial, interior and endocranial) comparisons of Cbfa-1 reactions are shown in Table 4. The only significant difference was the central zone of the interfrontal suture, in which osteogenic differentiation was less in the endocranial region than elsewhere (Kruskal–Wallis test, P = 0.04).
Table 4.
Regional differences in Cbfa-1 intensity (osteogenic differentiation) (score, mean ± SD)
| IF-Og | IF-C | IP-Og | IP-C | |
|---|---|---|---|---|
| Youngest group (n = 6) | ||||
| Ecto | 5.0 ± 0.0 | 3.3 ± 0.7 | 5.0 ± 0.0 | 3.7 ± 1.0 |
| Interior | 4.8 ± 0.4 | 2.8 ± 1.5 | 4.8 ± 0.4 | 2.7 ± 0.8 |
| Endo | 4.6 ± 0.8 | 2.9 ± 0.5 | 5.0 ± 0.0 | 3.6 ± 0.4 |
| Kruskal–Wallis test: P | 0.581 | 0.656 | 0.368 | 0.087 |
| Middle group (n = 2) | ||||
| Ecto | 4.3 ± 0.4 | 1.5 ± 1.4 | 5.0 | 2.0 |
| Inferior | 4.5 ± 0.7 | 2.8 ± 1.2 | 4.0 | 1.7 |
| Endo | 3.5 ± 0.7 | 1.5 ± 0.7 | 4.0 | 1.0 |
| Oldest group (n = 5) | ||||
| Ecto | 4.0 ± 1.2 | 2.0 ± 1.7 | 1.4 ± 1.9 | 0.3 ± 0.4 |
| Interior | 4.4 ± 0.4 | 2.4 ± 0.4 | 2.4 ± 1.7 | 1.1 ± 0.9 |
| Endo | 3.5 ± 0.7 | 1.0 ± 0.7 | 2.7 ± 1.2 | 0.8 ± 0.9 |
| Kruskal–Wallis test: P | 0.235 | 0.036 | NA | NA |
IF, interfrontal suture; IP, interparietal suture; OG, osteogenic zone; C, central zone. NA, only two subjects had ectocranial assessment because the other three had already fused ectocranially.
Discussion
Osteogenic vs. central zones
As reviewed by Cohen (1993), the number of suture layers varies according to different authors. In the present study, we arbitrarily divided each suture into three layers, a central zone sandwiched in between two osteogenic zones. This was based on our observation that a typical three-layered structure, as defined by Kokich (1986) using the human zygomaticomaxillary suture, was present in most of our suture sections.
However there were no sharp boundaries between zones, making quantification rather difficult, especially for BrdU. In this study, we employed a simple geometric criterion to define boundaries. Although objective and repeatable, this definition only approximated the true anatomical layers. This approximation makes the statistical comparison of osteogenic and central zones very conservative, because mixing of zones would add to the variability of the measurements. Our finding that significantly more proliferative cells were present in the osteogenic zone than in the central zone therefore is a real difference. This feature was shown in both the standard farm pigs and the Hanford minipigs, suggesting this is a common pattern for cranial suture growth among different strains of pigs, even though their general growth rate may be dramatically different (Swindle et al. 1994). This finding also agrees with Opperman et al. (1998), who reported more cell proliferation in the osteogenic zone of the rat coronal suture. Cell proliferation of these two zones may serve different functions. Proliferation in the osteogenic zone probably serves to replenish progenitor osteoblasts for the bone fronts, while central zone proliferation may contribute progenitor fibroblasts, endothelial cells, and a self-renewing mesenchymal cell population for the maintenance of the matrix.
Similar to BrdU, Cbfa-1 reaction was stronger in the osteogenic zone than in the central zone. As a key regulator for mesenchymal differentiation into the osteochondral lineage and for osteoblast maturation (Karsenty, 2000), it is not surprising to see more Cbfa-1 reaction along the bone fronts, where more committed osteogenic cells are present.
Difference between sutures and age
The interfrontal and interparietal sutures were similar in both cell proliferation and osteogenic differentiation. Although surprising with regard to the fact that the interparietal fuses earlier, this finding is consistent with our previous result that these sutures have the same mineral apposition rate (Sun et al. 2004). This similarity suggests that the two sagittal sutures can be considered parts of a single entity.
The comparison of cell proliferation between the middle and oldest group found that the latter had significantly decreased cell proliferation, suggesting that reduction of cell proliferation plays an important role in the diminishing of bone formation with age in these sutures (Sun et al. 2004). For cell differentiation, the comparison between the youngest group and the oldest group also suggested an age-associated decrease. However, due to the potential intrinsic growth difference between the two strains of pigs, further experiments will be needed to confirm that osteogenic differentiation is also involved.
Suture fusion
In murid rodents, the pericranium has been considered unimportant in regulating the fusion of sutures because its removal does not change suture fate (Moss, 1960; Opperman et al. 1994). In the pig interparietal suture, however, the pericranium is probably essential for suture fusion. First, fusion of the interparietal suture is initiated from the ectocranial side (Fig. 3). Second, cell proliferation and osteogenic differentiation are sustained in the ectocranial region of the suture and on the ectocranial bone surface, while on the endocranial side, proliferation diminishes and Cbfa-1 expression is down-regulated. That the process observed is normal fusion for this suture is further indicated by the absence of ridging, a manifestation of osteogenic activity that often accompanies premature synostosis (Cohen, 1993; Mooney et al. 1996; Weinzweig et al. 2003).
These findings raise three questions. Why does fusion begin ectocranially in the pig sagittal suture but endocranially in the murid posterior interfrontal and in human braincase sutures? What is the role of the dura mater in the pig? Is there a biomechanical explanation for these species difference?
Ectocranial fusion in pigs appears to be related to continuing osteogenic activity in the overlying periosteum as the skull thickens. This strong osteogenic potential also keeps bone formation active along the ectocranial suture margins. Both osteogenic cells and pro-osteogenic factors from the pericranium could easily migrate to the suture bone fronts. By contrast, due to the cessation of brain expansion, the suture gap width does not enlarge. Continuous bone formation at the ectocranial suture margins, then, gradually encroaches on the suture space and eventually bridges the two bone fronts. It is conceivable that the initial bridging would significantly immobilize the suture, enabling the fusion front to move down toward the endocranial side. This phenomenon has been reported in craniosynostoses, where suture fusion is often initiated at a single point and then spreads along the suture (Cohen, 1993). Alternatively, normal suture fusion in the pig may be analogous to the healing of a fracture, in which a callus bridges the fragments before the actual healing can occur.
We thus suggest that interparietal suture fusion in pigs is a byproduct of rapid ectocranial bone apposition that thickens the skull. The thickening is related to the very different shape of the brain and the outer skull in large-bodied ungulates. In adult animals, the bone interiors are entirely excavated by the frontal sinuses (Sisson & Hillmann, 1975). In rodents and primates, the outer and inner tables of the skull are much more parallel, cranial thickening and ectocranial osteogenesis are therefore much less, and the sutures are not overtaken by periosteal activity.
With regard to the dura mater, in pigs it is clear that the dura mater is much less osteogenic than the pericranium, especially in older animals. This differs from the rodent posterior frontal suture, where dura-produced osteogenic factors promote fusion (Greenwald et al. 2000). By contrast, our data are consistent with Opperman et al.'s (1995) finding in the rodent coronal suture that the dura mater can inhibit suture fusion, because the endocranial region did not fuse. Given the thickness of the braincase (Fig. 3), dural inhibitory signals could not easily diffuse to the ectocranial side to counteract the strong osteogenic activity of the periosteum. At this point we cannot determine whether the endocranial patency of the pig interparietal suture is a default stable condition until the bone bridging is completed or whether it reflects inhibitory signals arising from the dura mater or intrasuturally.
In either case, one might expect that cranial sutures of other thick-skulled, fast-growing species might also tend to fuse from the ectocranial side and that the fastest growing sites would fuse earliest. This might explain why fusion of sutures in pachycephalosaurs is limited to the fast-growing dome area (Goodwin et al. 1998; Goodwin & Horner, 2004). Whether these sutures fuse from the pericranial side is, however, not known.
Finally, a biomechanical role seems possible, but the important factor is probably skull thickening rather than suture fusion per se. The strong osteogenic potential of the ectocranial surface in the pig may be a species-specific result of mechanical demand. The pig braincase is attached by strong jaw and neck muscles and is heavily loaded during mastication (Herring & Teng, 2000) and possibly even more during digging and fighting. The outer table of the skull clearly reflects expansions related to these large muscle attachments. Although a thick-walled skull in Homo erectus may have served similar adaptive functions (Boaz & Ciochon, 2004), these were not retained in modern humans.
Taken together, this study suggests that large animal models with sutures that fuse after masticatory function is established may utilize different mechanisms than the well-studied sutures of murid rodents. Except for the metopic suture, human braincase sutures belong to the later fusion category (Todd & Lyon, 1924, 1925; Kokich, 1986; Manzanares et al. 1988). The relative importance of the dura mater and pericranium for fusion of these sutures remains to be established.
Acknowledgments
We thank Dr Kathy Rafferty for helping with the animal experiments, Dr Pannee Ochareon for providing some of the specimens and Ms Trisha Emry for the histological processing. The study was supported by PHS award DE 08513 from NIDCR.
References
- Baer MJ. Patterns of growth of the skull as revealed by vital staining. Hum Biol. 1954;26:80–126. [PubMed] [Google Scholar]
- Boaz NT, Ciochon RT. Headstrong hominids. Natural History. 2004;113:28–34. [Google Scholar]
- Bradley JP, Levine JP, McCarthy JG, Longaker MT. Studies in cranial suture biology: regional dura mater determines in vitro cranial suture fusion. Plast Reconstr Surg. 1997;100:1091–1099. doi: 10.1097/00006534-199710000-00001. [DOI] [PubMed] [Google Scholar]
- Brash JC. Some problems in the growth and developmental mechanics of bone. Edinburgh Med J N S. 1934;41:305–387. [PMC free article] [PubMed] [Google Scholar]
- Cohen MMJ. Sutural biology and the correlates of craniosynostosis. Am J Med Genet. 1993;47:581–616. doi: 10.1002/ajmg.1320470507. [DOI] [PubMed] [Google Scholar]
- Duterloo HS, Enlow DH. A comparative study of cranial growth in Homo and Macaca. Am J Anat. 1970;127:357–368. doi: 10.1002/aja.1001270403. [DOI] [PubMed] [Google Scholar]
- Goodwin MB, Bauchholtz EY, Johnson RE. Cranial anatomy and diagnossi of Stygimoloch spinifer (ornithischia: pachycephalosauria) with comments on cranial dispaly structures in agonistic behavior. J Vert Paleont. 1998;18:363–375. [Google Scholar]
- Goodwin MB, Horner JR. Cranial histology of Pachycephalosaurs (Ornithischia: Marginocephalia) reveals transitory structures inconsistent with head-butting behavior. Paleobiology. 2004;30:253–267. [Google Scholar]
- Greenwald JA, Mehara BJ, Spector JA, et al. Regional differentiation of cranial suture-associated dura mater in vivo and in vitro: implications for suture fusion and patency. J Bone Miner Res. 2000;15:2413–2430. doi: 10.1359/jbmr.2000.15.12.2413. [DOI] [PubMed] [Google Scholar]
- Herring SW, Teng S. Strain in the braincase and its sutures during function. Am J Phys Anthropol. 2000;112:575–593. doi: 10.1002/1096-8644(200008)112:4<575::AID-AJPA10>3.0.CO;2-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karsenty G. Role of Cbfa1 in osteoblast differentiation and function. Semin Cell Dev Biol. 2000;11:343–346. doi: 10.1006/scdb.2000.0188. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Rice DP, Kettunen PJ, Thesleff I. FGF-, BMP- and Shh-mediated signalling pathways in the regulation of cranial suture morphogenesis and calvarial bone development. Development. 1998;125:1241–1251. doi: 10.1242/dev.125.7.1241. [DOI] [PubMed] [Google Scholar]
- Kokich VG. The biology of sutures. In: Cohen MM Jr, editor. Craniosynostosis: Diagnosis, Evaluation and Management. New York: Raven Press; 1986. pp. 81–103. [Google Scholar]
- Manzanares MC, Goret-Nicaise M, Dhem A. Metopic sutural closure in the human skull. J Anat. 1988;161:203–215. [PMC free article] [PubMed] [Google Scholar]
- Mednick LW, Washburn SL. The role of the sutures in the growth of the brain case of the infant pig. Am J Phys Anthropol. 1956;14:175–191. doi: 10.1002/ajpa.1330140215. [DOI] [PubMed] [Google Scholar]
- Mooney MP, Smith TD, Burrows AM, et al. Coronal suture pathology and synostotic progression in rabbits with congenital craniosynostosis. Cleft Palate Craniofac J. 1996;33:369–378. doi: 10.1597/1545-1569_1996_033_0369_cspasp_2.3.co_2. [DOI] [PubMed] [Google Scholar]
- Moss ML. Growth of the calvaria in the rat: the determination of osseous morphology. Am J Anat. 1954;94:333–361. doi: 10.1002/aja.1000940302. [DOI] [PubMed] [Google Scholar]
- Moss ML. Inhibition and stimulation of sutural fusion in the rat calvaria. Anat Rec. 1960;136:457–467. doi: 10.1002/ar.1091360405. [DOI] [PubMed] [Google Scholar]
- Moss ML, Young RW. A functional approach to craniology. Am J Phys Anthropol. 1960;18:281–292. doi: 10.1002/ajpa.1330180406. [DOI] [PubMed] [Google Scholar]
- Ochareon P. Craniofacial periosteal cell capacities. Seattle: University of Washington; 2004. PhD thesis. [Google Scholar]
- Opperman LA, Sweeney TM, Redmon J, Persing JA, Ogle RC. Tissue interactions with underlying dura mater inhibit osseous obliteration of developing cranial sutures. Dev Dyn. 1993;198:312–322. doi: 10.1002/aja.1001980408. [DOI] [PubMed] [Google Scholar]
- Opperman LA, Persing JA, Sheen R, Ogle RC. In the absence of periosteum, transplanted fetal and neonatal rat coronal sutures resist osseous obliteration. J Craniofac Surg. 1994;5:327–332. doi: 10.1097/00001665-199411000-00012. [DOI] [PubMed] [Google Scholar]
- Opperman LA, Passarelli RW, Morgan EP, Reintjes M, Ogle RC. Cranial sutures require tissue interactions with dura mater to resist osseous obliteration in vitro. J Bone Miner Res. 1995;10:1978–1987. doi: 10.1002/jbmr.5650101218. [DOI] [PubMed] [Google Scholar]
- Opperman LA, Chhabra A, Nolen AA, Bao Y, Ogle RC. Dura mater maintains rat cranial sutures in vitro by regulating suture cell proliferation and collagen production. J Craniofac Genet Dev Biol. 1998;18:150–158. [PubMed] [Google Scholar]
- Opperman LA. Cranial sutures as intramembranous bone growth sites. Dev Dyn. 2000;219:472–485. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1073>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- de Pollak C, Arnaud E, Renier D, Marie PJ. Age-related changes in bone formation, osteoblastic cell proliferation, and differentiation during postnatal osteogenesis in human calvaria. J Cell Biochem. 1997;64:128–139. [PubMed] [Google Scholar]
- Rafferty KL, Herring SW, Marshall CD. Biomechanics of the rostrum and the role of facial sutures. J Morph. 2003;257:33–44. doi: 10.1002/jmor.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice DP, Aberg T, Chan Y, et al. Integration of FGF and TWIST in calvarial bone and suture development. Development. 2000;127:1845–1855. doi: 10.1242/dev.127.9.1845. [DOI] [PubMed] [Google Scholar]
- Roth DA, Bradley JP, Levine JP, McMullen HF, McCarthy JG, Longaker MT. Studies in cranial suture biology: Part II. Role of the dura in cranial suture fusion. Plast Reconstr Surg. 1996;97:693–699. doi: 10.1097/00006534-199604000-00001. [DOI] [PubMed] [Google Scholar]
- Sisson S, Hillmann DJ. Porcine osteology. In: Getty R, editor. Sisson and Grossman's The Anatomy of the Domestic Animals. Philadelphia, PA: W.B. Saunders Company; 1975. pp. 1231–1252. [Google Scholar]
- Sun Z, Lee E, Herring SW. Cranial sutures and bones: growth and fusion in relation to masticatory strain. Anat Rec. 2004;276A:150–161. doi: 10.1002/ar.a.20002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swindle MM, Smith AC, Laber-Laird K, Dungan L. Swine in Biomedical Research: Anagement and Models. Institute for Laboratory Animal Research 36; 1994. [Google Scholar]
- Ten Cate AR, Freeman E, Dickinson JB. Sutural development: structure and its response to rapid expansion. Am J Orthod. 1977;71:622–636. doi: 10.1016/0002-9416(77)90279-2. [DOI] [PubMed] [Google Scholar]
- Todd TW, Lyon DW. Endocranial suture closure, its progress and age relationship: Part I. Adult males of white stock. Am J Phys Anthropol. 1924;7:325–384. [Google Scholar]
- Todd TW, Lyon DW. Cranial suture closure: Its progress and age relationship. Part II. Ectocranial closure in adult males of white stocks. Am J Phys Anthropol. 1925;8:23–45. [Google Scholar]
- Warren SM, Longaker MT. Advances in murine cranial suture research. Internet J Plastic Surg. 2001;1:1. doi: 10.1097/00006534-200102000-00034. from http://www.ispub.com/ostia/index.php?xmlFilePath=journals/ijps/vol1n1/cranial.xml. [DOI] [PubMed] [Google Scholar]
- Weinzweig J, Kirschner RE, Farley A, et al. Metopic synostosis: Defining the temporal sequence of normal suture fusion and differentiating it from synostosis on the basis of computed tomography images. Plast Reconstr Surg. 2003;112:1211–1218. doi: 10.1097/01.PRS.0000080729.28749.A3. [DOI] [PubMed] [Google Scholar]