Abstract
The first systematic account of the neural crest in the human has been prepared after an investigation of 185 serially sectioned staged embryos, aided by graphic reconstructions. As many as fourteen named topographical subdivisions of the crest were identified and eight of them give origin to ganglia (Table 2). Significant findings in the human include the following. (1) An indication of mesencephalic neural crest is discernible already at stage 9, and trigeminal, facial, and postotic components can be detected at stage 10. (2) Crest was not observed at the level of diencephalon 2. Although pre-otic crest from the neural folds is at first continuous (stage 10), crest-free zones are soon observable (stage 11) in Rh.1, 3, and 5. (3) Emigration of cranial neural crest from the neural folds at the neurosomatic junction begins before closure of the rostral neuropore, and later crest cells do not accumulate above the neural tube. (4) The trigeminal, facial, glossopharyngeal and vagal ganglia, which develop from crest that emigrates before the neural folds have fused, continue to receive contributions from the roof plate of the neural tube after fusion of the folds. (5) The nasal crest and the terminalis-vomeronasal complex are the last components of the cranial crest to appear (at stage 13) and they persist longer. (6) The optic, mesencephalic, isthmic, accessory, and hypoglossal crest do not form ganglia. Cervical ganglion 1 is separated early from the neural crest and is not a Froriep ganglion. (7) The cranial ganglia derived from neural crest show a specific relationship to individual neuromeres, and rhombomeres are better landmarks than the otic primordium, which descends during stages 9–14. (8) Epipharyngeal placodes of the pharyngeal arches contribute to cranial ganglia, although that of arch 1 is not typical. (9) The neural crest from rhombomeres 6 and 7 that migrates to pharyngeal arch 3 and from there rostrad to the truncus arteriosus at stage 12 is identified here, for the first time in the human, as the cardiac crest. (10) The hypoglossal crest provides cells that accompany those of myotomes 1–4 and form the hypoglossal cell cord at stages 13 and 14. (11) The occipital crest, which is related to somites 1–4 in the human, differs from the spinal mainly in that it does not develop ganglia. (12) The occipital and spinal portions of the crest migrate dorsoventrad and appear to traverse the sclerotomes before the differentiation into loose and dense zones in the latter. (13) Embryonic examples of synophthalmia and anencephaly are cited to emphasize the role of the neural crest in the development of cranial ganglia and the skull.
Keywords: cardiac crest, cranial and spinal ganglia, epipharyngeal placodes, human embryo, hypoglossal crest
Introduction
What is now termed the neural crest was identified first in 1868 in the chick embryo by Wilhelm His, Senior, who designated it as Zwischenstrang, literally an ‘intermediate cord’ (His, 1868). It was detected independently in selachians a decade later by Balfour. The term neural crest (crista neuralis), appropriate particularly for a transient dorsal ridge on the neural tube in amphibians, received official recognition in the Nomina embryologica (1967, 1977). In German it is called Neuralleiste.
Much of the pioneer work on neural crest was in amphibians, and is associated historically with the name Hörstadius, whereas subsequent studies on derivatives of the crest have been undertaken particularly in avian embryos and are associated especially with LeDouarin and her group.
The mammalian neural crest (reviewed by Morriss-Kay et al. 1993) is now being investigated by labelling of crest cells either by WGA or by DiI (e.g. Fukiishi & Morriss-Kay, 1992; Serbedzija et al. 1992). More recently crest cells have been identified in transgenic mice, providing valuable information on the developing heart (Jiang et al. 2000), the face and teeth (Chai et al. 2000), and the vault of the skull (Jiang et al. 2002) as well as the neck and shoulder (Matsuoka et al. 2005).
The neural crest is characterized by a wide variety of derivatives, including notably spinal and cranial ganglia, as found by His. The origin, migration, and fate of the cells of the crest have been studied extensively in a variety of species. Furthermore, it is generally thought that the evolution of the neural crest was instrumental in that of vertebrates.
Cells of the neural crest are generally distinguishable by being relatively larger and more rounded than their neighbours, and they may occur in groups or form strands. There are distinct differences between the neural crest cells and the surrounding mesenchyme (Morriss-Kay et al. 1993), except in the transitional zone between the two groups (Baxter & Boyd, 1939). Consequently it has been possible to identify a number of components of the crest in the human by regular microscopy. A particularly striking example is provided by the emigrating crest cells at stage 12 (Fig. 4A), which give a ‘frightened hedgehog’ appearance to the optic vesicles (Bartelmez & Blount, 1954, Fig. 17). An important technical step (frequently omitted, however) is the construction of precise three-dimensional reconstructions.
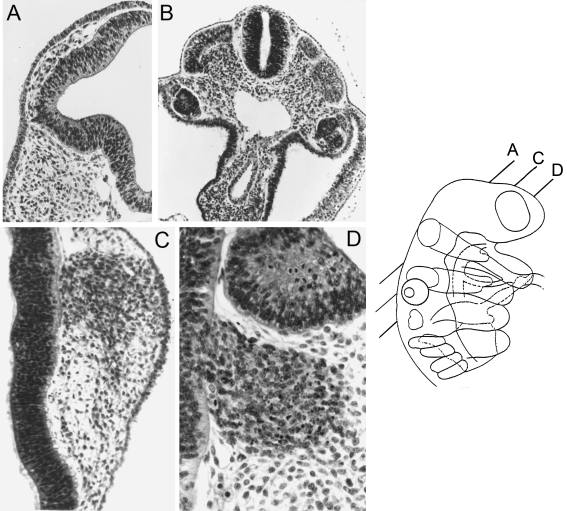
Fig. 4.
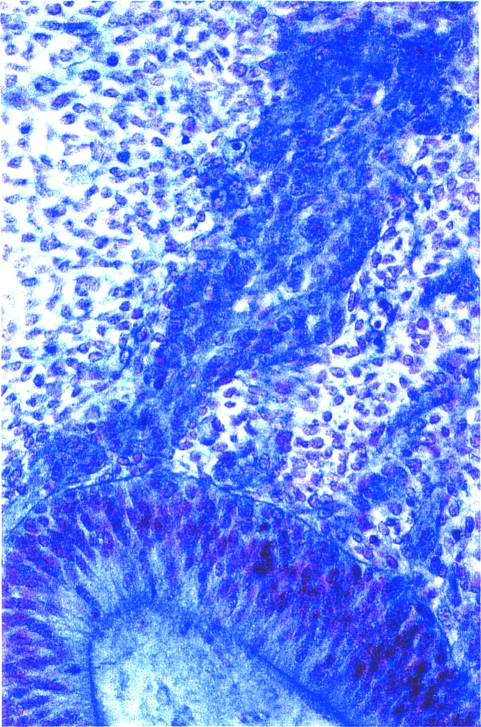
The neural crest at stage 12. The planes of sections of A, C, and D are shown in the key. Alum cochineal/haematoxylin and eosin. (A) Optic crest is clearly emigrating from the optic vesicle. (B) Spinal crest in the thoracic region. The roof cells of the neural tube resemble the crest cells that are visible between the tube and the dermatomyotomes. (C) The trigeminal ganglion is connected here to the surface ectoderm by cells believed to be emigrating from the latter. (D) The facial ganglion (seen here below the otic primordium) of the embryo shown in C. The cellular columns of the neural wall continue into the ganglion and a basement membrane is absent at this level.
Although considerable information can be obtained in the human concerning the origin of crest cells, only the very early migratory pathways can be detected in standard morphological examinations. Nevertheless, migration has been clarified in several regions, e.g. the hypoglossal cord (O’Rahilly & Müller, 1984) and the nasal crest (Müller & O’Rahilly, 2004).
It would be difficult to find a structure in the human that has not been studied systematically. Surprisingly the neural crest is such and the current investigation was undertaken to fill an important lacuna in the literature on human embryology. The main objectives were: (1) to provide a systematic account of the neural crest in the human embryo using standard histological techniques, including silver, and precise three-dimensional graphic reconstructions; (2) to search as far as possible for indications of the origin, early migratory pathways, and derivatives of the neural crest in the human material available; (3) to reassess the relationships of the neuromeres, the epipharyngeal placodes, and the pharyngeal arches to the neural crest in the human; (4) to present all data in terms of the established staging system; and (5) to indicate any noteworthy differences between the human findings and those reported in other mammals.
Material and methods
The neural crest and associated areas were investigated at stages 9–20 (approximately 31/2–7 weeks) in 185 serially sectioned human embryos (five of stage 9, 12 of 10, 18 of 11, 22 of 12, 20 of 13, 36 of 14, 26 of 15, 39 of 16, 4 of 17, and one each of stages 18–20), mostly from the Carnegie Collection. The sections, which included all three major planes, were stained with alum cochineal, haematoxylin and eosin, or azan. Silver impregnation (Bodian method) was used in eighteen instances. Sixty-two precise graphic reconstructions were prepared by the authors, using the point-plotting technique (basically similar to that in Gaunt & Gaunt, 1978). A new reconstruction at stage 10 was made of the embryo described and illustrated by Corner (1957), which accounts for some discrepancies in interpretation. The internationally accepted Carnegie staging system based on morphological criteria (O’Rahilly & Müller, 1987) was used throughout and the ages given are post-fertilizational. The imprecise term gestational is avoided (O’Rahilly & Müller, 2000).
Results
Stage 9 (c. l.5–2.5 mm)
Before any fusion of the neural folds has occurred, and in the absence of so-called cerebral vesicles, the prosencephalon, mesencephalon, and rhombencephalon are distinguishable.
In two out of five embryos (1–3 somitic pairs) some cells rostral to the otic disc are considered to represent mesencephalic neural crest (Fig. 1A).
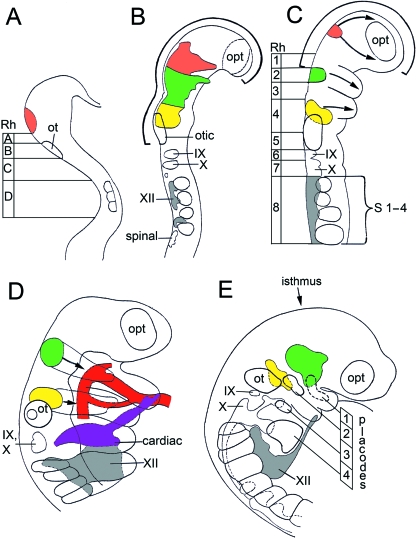
Fig. 1.
The neural crest in graphic reconstructions at stages 9 to 13. Right lateral views. The embryos are drawn at decreasing magnifications. (A) Mesencephalic crest (orange) has begun to form in stage 9. (B) Neural crest from the widely open rostral neuropore (thick curved black line) in stage 10. The preotic crest (mesencephalic, trigeminal, and facial components) is emigrating from the neural folds. The postotic and occipitospinal crest is present. (C) Trigeminal (green) and facial (yellow) ganglia have appeared in stage 11. Migration of the crest (arrows) continues. The uppermost arrow indicates a frontonasal component. Migration to pharyngeal arches 1 and 2 (arrows) is from closed neural tube. Rhombomeres 1, 3, and 5 are now seen to be crest-free. (D) The cardiac and hypoglossal crest at stage 12. The cardiac crest arises from Rh. 6 and Rh. 7 and migrates to the truncus arteriosus, which it surrounds. Hypoglossal crest at this stage occupies mainly pharyngeal arch 4. Trigeminal and facial crest migrate (arrows) into arches 1 and 2 respectively. (E) Placodal activity at stage 13. Placodes of pharyngeal arches 1–4 are indicated. The hypoglossal cord reaches pharyngeal arch 2. Colours: orange, mesencephalic; green, trigeminal; yellow, facial; grey, hypoglossal; violet, cardiac; red, aortic arches.
Stage 10 (c. 2–3.5 mm)
The neural folds begin to fuse and the optic sulcus appears. A longitudinal groove (sulcus cristae) is found on the medial aspect of each neural fold. The cranial neural crest is particularly evident at this stage. The mesencephalic and V to VII crest develops from the neural folds, whereas the IX to XII and the spinal crest comes from the roof of the closed neural tube. A careful reconstruction of the Corner embryo gave a distinct impression that the crest cells are more extensively dispersed than previously reported, and that the mesencephalic, trigeminal, and facial components form a continuous mass (Fig. 1B), thereby occupying the future sites of Rh. 1 and Rh. 3. Between the mesencephalon and the otic placode, crest cells clearly derived from the neural ectoderm develop at sites where the neural folds have not yet fused. Crest cells can be seen leaving the neural folds or the neural tube at sites where the basement membrane is interrupted (Fig. 2A). Mesencephalic crest is migrating towards the optic primordium. The facial and vagal crest is identifiable when 6 somites are present, the trigeminal and glossopharyngeal at 7, and the hypoglossal and spinal components begin to be visible at 10 somites. An unusual appearance is presented by the facial crest because a substantial portion of its cells is seen to be derived directly from the lateral wall of Rh.4 in the Corner embryo (Fig. 2B). Some cells emigrate from the otic plate at 9 somites. The glossopharyngeal and vagal crest appears early as two precursors in Rh. 6 and 7 during this stage (Fig. 1B). The hypoglossal crest begins to spread between the occipital somites (Figs 1B, 2C) and spinal crest extends along somite 5. Pharyngeal arch 1 is present and its maxillary process is visible.
Fig. 2.
Neural crest at stage 10. Alum cochineal. (A) Mesencephalic crest from the neural folds can be seen above. The crest cells are darker and more rounded than those of the mesoderm. The optic sulcus (arrow) is visible and the optic primordium is present. (B) Facial crest in stage 10 is emerging laterad from the wall of the neural groove. (C) Hypoglossal crest is migrating between the neural tube and the first somite. The planes of section are shown in the key. Figure 2B is taken from R. O’Rahilly and F. Müller, The Embryonic Human Brain, 3rd edn. Copyright ©, 2006, Wiley-Liss. Reprinted by permission of John Wiley and Sons, Inc.
Stage 11 (c. 2.5–4.5 mm)
Optic crest derived from the optic primordium is now visible. Mesencephalic crest is still emerging from the neural folds in a 13-somite embryo. Moreover, mesencephalic crest mixes with the optic crest (Fig. 1C) and contributes to the sheath of the optic vesicle. Some mesencephalic crest cells migrate towards the future frontonasal region. Crest cells clearly derived from the neural ectoderm are still emerging at sites where the neural folds have become fused since the previous stage. Pharyngeal arches 1 to 3 have developed, and mesencephalic and trigeminal cells migrate into pharyngeal arch 1. Trigeminal and facial ganglia are now recognizable as dense agglomerations of crest cells. Migration to pharyngeal arches 1 and 2 continues and a cellular otic sheath derived from the otic primordium begins to develop (Fig. 3). Crest-free zones are now found at levels Rh. 1, Rh. 3, and Rh. 5. Neural crest representing pia mater covers the surface of Rh. 8. Spinal crest is present in the cervical region (Table 1).
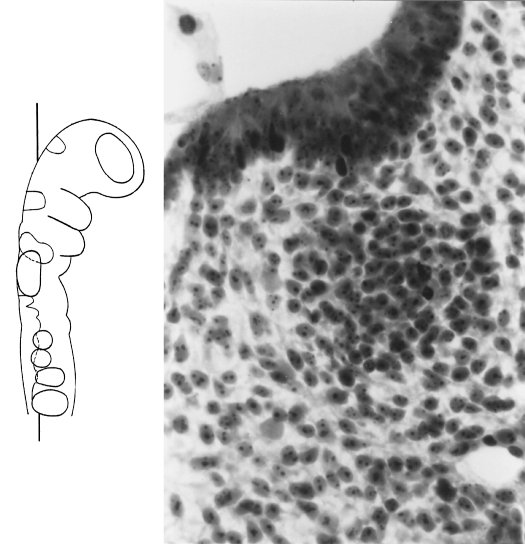
Fig. 3.
Otic crest at stage 11. The otic pit can be seen above and also cellular strands from its edge, especially lateral to (to the left of) the facial ganglion. Azan.
Table 1.
The caudal limit of the occipitospinal neural crest (abscissa) related to the number of somitic pairs (ordinate) in 19 staged embryos. Spinal ganglia are in phase with the somites, although not with the centra (O’Rahilly and Müller, 2003). According to Orts-Llorca (1934) the sacrococcygeal ganglia (arrow) arise from the neural tube by cellular bridges rather than from typical neural crest.
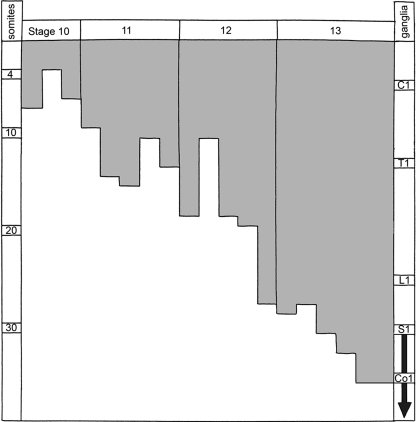 |
C, cervical; Co., coccygeal; L, lumbar; S, sacral; T, thoracic.
Stage 12 (c. 3–5 mm)
Optic crest is particularly evident at this stage (Fig. 4A). Neural crest representing pia mater surrounds the surface of the mesencephalon. Formation of neural crest proceeds at the levels of Rh. 2, 4, 6, and 7. The trigeminal ganglion contains some short nerve fibres and it continues to receive cells from the roof of Rh. 2. Some cells of the surface ectoderm join the trigeminal crest in which cellular débris can be observed (Fig. 4D). Trigeminal and facial crest continues to migrate into pharyngeal arches 1 and 2 respectively. The facial ganglion receives cells from the roof of Rh. 4 and also from its lateral walls (Fig. 4D), and epipharyngeal placodal contributions are evident. The otic vesicle is now related to Rh. 5 (Fig. 1D). Some of its migrating cells begin to form the vestibular ganglion. Glossopharyngeal and vagal ganglia are discernible, and migration continues from Rh. 6 and Rh. 7. A characteristically horizontal arrangement of the crest of the accessory nerve is observed in this stage. The hypoglossal crest of Rh. 8 does not form ganglia. It unites with myotomic cells of somites 1 to 4 in the hypoglossal cell cord and is visible in pharyngeal arch 4. The hypoglossal crest has not yet reached the lateral lingual swelling of pharyngeal arch 1. Some crest cells derived from Rh. 6 and Rh. 7 are in continuity ventrally with the hypoglossal crest. At this stage they proceed towards the truncus arteriosus via pharyngeal arches 1 and 2. This prolongation (Fig. 1D) is interpreted here as the cardiac neural crest. The spinal crest has migrated caudad (Table 1) and in cross-sections it can be seen to extend between the surface of the alar lamina of the neural tube and the dermatomyotomes of the somites (Figs 4B, 5A). The cells of the roof plate of the neural tube can resemble those of the crest that have already migrated laterad (Fig. 4B). Either three or four pharyngeal arches are present.
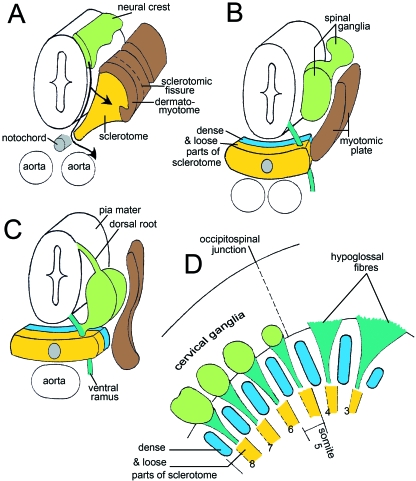
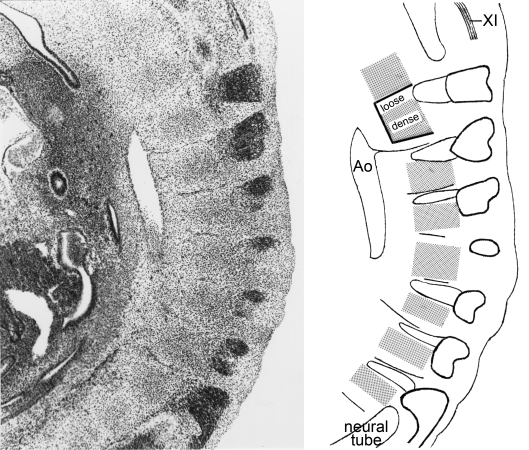
Fig. 5.
The development of the occipitospinal neural crest, based on photomicrographs of serial sections. (A) The spinal crest at stage 12 does not extend beyond the dermatomyotomes. The main (ventrolateral) migratory pathway detectable from sections is shown by an arrow. (B) The crest at stage 13 has given rise to spinal ganglia. The myotomic plates are found laterally, and ventrally the sclerotomes show dense and loose portions. Motor fibres penetrate the loose portions. (C) The neural tube at stage 15 is covered by pia mater, believed to be derived from the neural crest (Sensenig, 1951; O’Rahilly and Müller, 1986). Dorsal and ventral roots are forming a spinal nerve. (D) Lateral view of the occipitospinal transition at stage 15. Spinal ganglia and sclerotomes are in register. No ganglia form in the occipital part. Nerve fibres of XII pass through the loose moieties of the sclerotomes, as do also the spinal nerves. Occipital and spinal sclerotomes are in register. (The dense areas will develop into the lateral parts of the basioccipital and into the neural arches of the cervical vertebrae.)
Stage 13 (c. 4–6 mm)
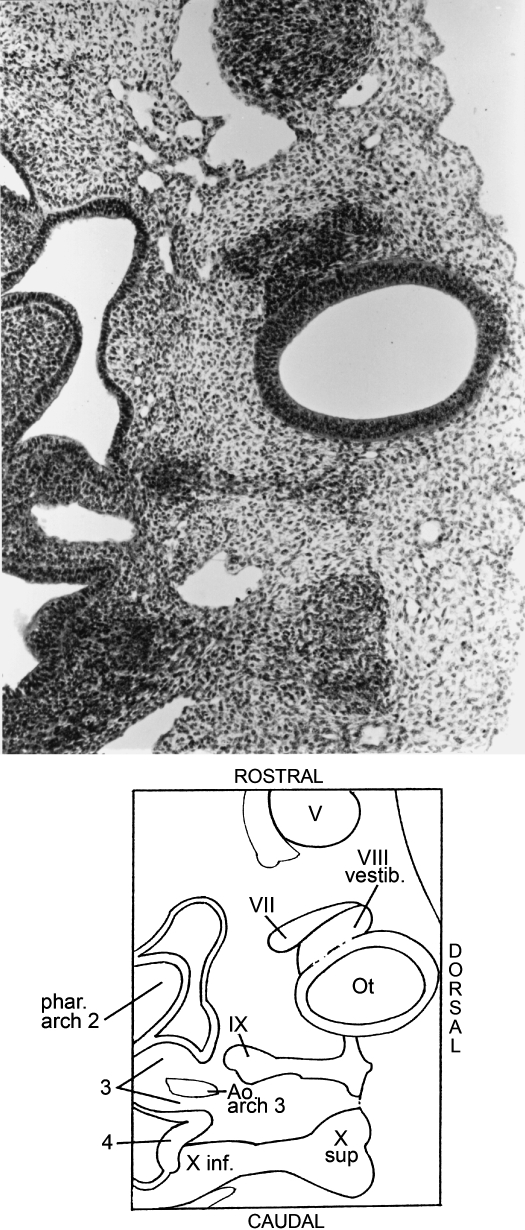
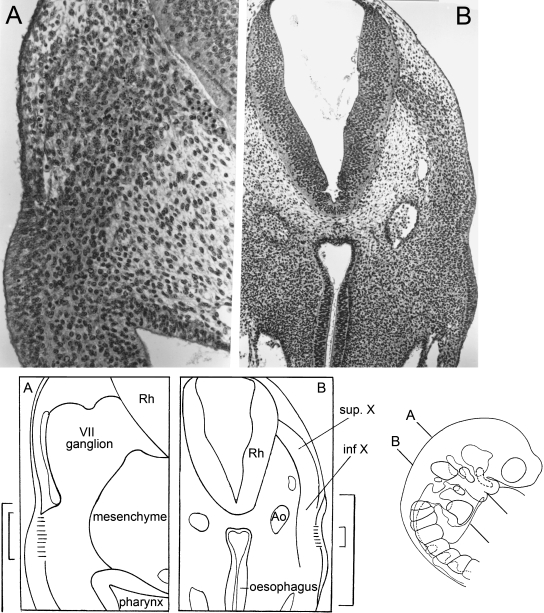
The terminalis-vomeronasal crest develops from the pluristratified epithelium of the nasal placodes. A slight indication of tripartite division of the trigeminal ganglion is found in a few embryos; the ophthalmic division is the clearest. The ectoderm over the trigeminal ganglion gives rise to cells that are added to the ganglion. This epipharyngeal placode, however, is not typical because it is not a restricted zone. Some crest cells can be seen in the roof of the newly formed isthmic neuromere (arrow in Fig. 1E) in a few embryos. The facial ganglion is traversed by afferent nerve fibres. The otic sheath is well developed around the otic vesicle.
In some embryos the glossopharyngeal and vagal ganglia are dividing into superior and inferior ganglia (Figs 1E, 6). Typical epipharyngeal placodes are clearly present at this stage. They contribute to the facial ganglion, to the inferior glossopharyngeal ganglion, and to the inferior vagal ganglion (Figs 7, 8). Neural crest of the accessory nerve is again evident. It reaches from the vagal crest uninterruptedly to the spinal crest (Figs 1E, 6). The hypoglossal cell cord migrates to pharyngeal arch 2 (Fig. 1E). Cells of the spinal crest migrate mediad to the somites and occupy an extensive surface. The developing spinal ganglia are still connected to each other (Fig. 6) and they lie alongside the medial part of the wall of the neural tube (Fig. 5B). The sclerotomes have adopted a horizontal position and develop loose rostral and dense caudal moieties. The newly developed ventral fibres of the spinal nerves pass through the loose parts.
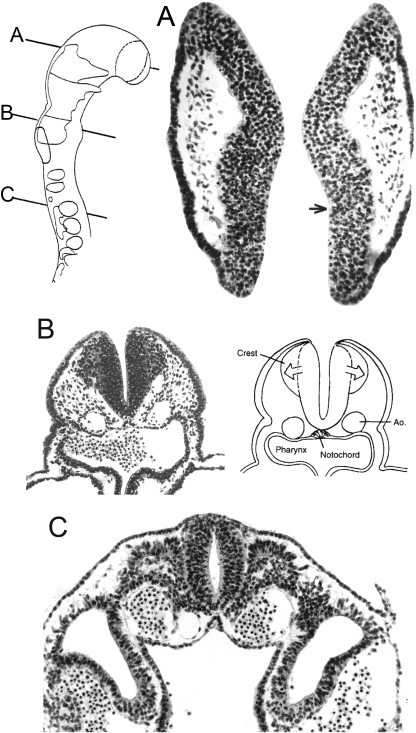
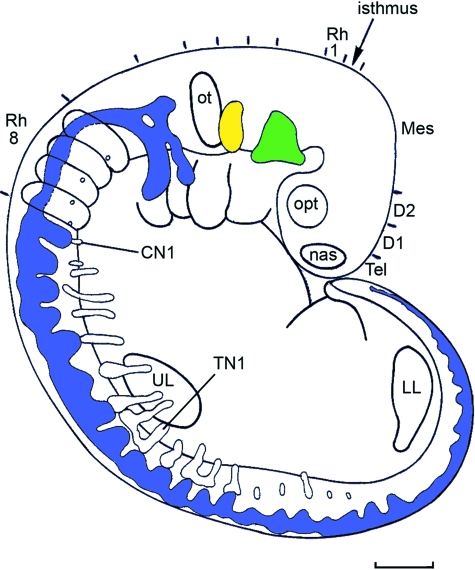
Fig. 6.
The postotic neural crest in stage 13 is still a continuous band. Superior and inferior ganglia of IX and X are present, as are also the cervical and some thoracic ganglia. The ventral spinal roots are included, but the hypoglossal crest is not shown. CN1, cervical nerve 1. LL, lower limb bud. TN1, thoracic nerve 1. UL, upper limb bud.
Fig. 7.
Cranial ganglia at stage 13 seen in a laterally situated sagittal, azan-stained section. The pharyngeal arches and the cranial ganglia show a segmental arrangement. Scale bar: 0.6 mm.
Fig. 8.
Placodal activity in stage 13. The planes of sections of A and B are shown in the key. The site of the epipharyngeal placodes is indicated in the key drawings here by brackets, the shorter of which marks the portion where the basement membrane is clearly interrupted. Haematoxylin and eosin. (A) Pharyngeal arch 2 containing facial crest migrating from the facial ganglion. The placode is recognizable by its much higher surface ectoderm compared with the regular ectoderm. Emigrating placodal cells can be observed where the basement membrane is interrupted. (B) Pharyngeal arch 4 with migrating vagal crest. The placode delivers cells to the crest. The future superior vagal ganglion is slightly less compact than the inferior, which adheres to the placode.
Stage 14 (c. 5–7 mm)
Nasal crest migrates from the nasal placodes towards but without reaching the telencephalon. The vestibular ganglion is clearly identifiable by its passage through efferent nerve fibres to the future utricle. The cervical sinus caudal to pharyngeal arch 2 is present. The accessory crest, seen already at the previous stage, is now penetrated by nerve fibres. The ventral fibres of the spinal nerves appear as if they were ‘squeezing’ their way through the loose portions of the sclerotomes, as best seen in sagittal sections (Fig. 9).
Fig. 9.
The relationship of spinal ganglia to sclerotomes in stage 14. Haematoxylin and eosin. In this laterally situated sagittal section the accessory nerve of one side can be seen in the upper right-hand corner. The ganglia extend over most of the medial myotomic surfaces, which are not seen here, that is, over the whole segment. The nerve fibres appear to be ‘squeezed’ through the loose rostral parts of the sclerotomes where also the blood vessels (horizontal dark streaks) are located (cf Fig. 5D). A sclerotome is outlined in the key drawing. Ao., aorta.
Stage 15 (c. 7–9 mm)
A nasal pit has appeared and the crest cells from its epithelium adhere to each other and form fascicles. Nasal crest begins to reach the telencephalic wall. Cells of the future cochlear ganglion are smaller than those of the vestibular. The hypoglossal cord penetrates the region of pharyngeal arch 1 and in some embryos arrives in the lateral lingual swelling. The cervical ganglia reach their typical ventral position (Fig. 5C). Pia mater is identifiable as a single layer of neural crest cells. Lateral views show the relationship of hypoglossal and cervical nerve fibres and loose sclerotomic compartments (Fig. 5D).
Stage 16 (c. 8–11 mm)
Between the nasal pit and the telencephalic wall is a relatively extensive mesenchymal zone, which neural crest cells and fibres have to traverse. Crest-derived olfactory fibres enter the region of the future olfactory bulb. The cochlear ganglion and cochlear fibres are present.
Stages 17 to 20 (c. 13–20 mm)
Vomeronasal and terminalis constituents develop from the nasal crest at the laterally situated nasal pit. A mesenchymal nasal septum shows no evident connection with the nasal crest. At stage 18 a vomeronasal ganglion has developed and its nerve fibres are accompanied by neurons derived from nasal crest cells. At stage 20 neural crest cells and/or neurons still emerge from the nasal epithelium and form cords. They migrate in the capillary-rich mesenchyme towards the base of the prosencephalon (Fig. 10).
Fig. 10.
Nasal crest is arising from the tip of the nasal pit at stage 20 and migrating rostrocaudad towards the basal region of the diencephalon. Azan. From R. O’Rahilly and F. Müller, ‘The Embryonic Human Brain,’ 3rd Ed. Copyright ©, 2006, Wiley-Liss. Reprinted by permission of John Wiley and Sons, Inc.
The neuromeres in the human have been described, named, and classified elsewhere (Müller & O’Rahilly, 1997). The authors’ current interpretation of the neural crest in the human is summarized in Table 2.
Table 2.
The origin and nomenclature of the components of the neural crest in the human, including their relationship to the neuromeres and their initial appearance, as well as the arrival of ganglia, related to somitic number and developmental stages
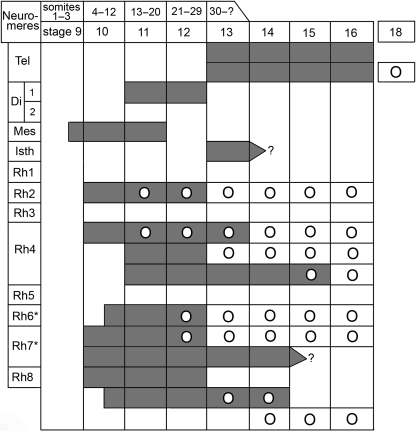 |
1Dark grey indicates neural crest. Circles denote ganglia. The glossopharyngeal and vagal concern the superior ganglia only.
*Rh. 6 and Rh. 7 give origin to the cardiac crest.
Discussion
The neural crest, a characteristic feature of vertebrates, is a transitory pluripotent structure, the cells of which are endowed with extensive migratory capacities. The migration of crest cells involves an epithelio-mesenchymal transition, whereby mesenchymal cells then become dispersed along specific migratory pathways and come to reside in their final position, frequently in compact clusters (e.g. Fig. 3), where they undergo cellular differentiation (Duband et al. 1995). In terms of recent studies of cell lineage, neural crest stem cells (as also neuronal-restricted and glial-restricted precursors) are ‘self-renewing, multipotential stem cells’ that can be distinguished from neuroepithelial cells by their ‘differentiation potential, cell surface markers, gene expression, and cytokine response’ (Kalyani & Rao, 1998, Fig. 4). The use of precursor cells in therapeutic strategies is being actively investigated (ibid.).
The pattern of emigration varies according to the species, the axial level, and the stage. In the human, for example, presumptive crest cells arise bilaterally from open neural folds at the neurosomatic junction, (e.g. Fig. 1B, mesencephalic and trigeminal components), but also in the median portion of the closed neural tube (e.g. Fig. 4B and Streeter, 1942, Fig. 5, xi). Additional sites include the lateral wall of the neural tube (Fig. 2B), ventral cells of the neural tube (Orts Llorca, 1934), and the neural ectoderm that will give rise to the retina (Fig. 4A).
It needs to be stressed that variations in relative timing occur between species. For example, a noticeable difference between murine and human development is the relatively more rapid appearance of the telencephalic evaginations in the mouse with 18–23 somites (corresponding to stages 11 and 12), whereas they are found in the human only at stages 14 and 15, which are essentially beyond the somitic period. Similarly, spinal ganglia are seen in the rat when the caudal neuropore is just closed, whereas in the human they have not even begun to appear when the caudal neuropore closes (at stage 12); even in stage 14 no completely isolated spinal ganglia are yet visible.
Terminology
In studies of the development of the cranial ganglia Veit's nomenclature (as used, for example, by Veit & Esch, 1922) is generally found: crista neuralis rostralis (craniale Kopfganglienleiste) and crista neuralis caudalis (caudale Kopfganglienleiste). This subdivision, however, is too limited in scope (O’Rahilly, 1965). In the present study some fourteen named topographical subdivisions of the crest were distinguished (Table 2), and the term neural crest had already been applied to most of them, e.g. the optic crest (Bartelmez & Blount, 1954). Moreover, eight of the subdivisions give rise to ganglia (small circles in Table 2).
The rostral crest, a term best avoided, is the combined mesencephalic and trigeminal crest. The term acousticofacial crest (primordium acustico-facialis) has given rise to considerable controversy, and justifiable doubts about its suitability have been expressed (Baxter & Boyd, 1939). Originally the mass so designated was thought to give rise to the geniculate and vestibulocochlear ganglia (e.g. Bartelmez, 1923; Bartelmez & Evans, 1926, in the human). Subsequently, however, the so-called acousticofacial crest of mammals is generally believed to be purely facial.
The ganglionic crest, a term now rarely used, emphasizes merely a later and regionally restricted aspect of the neural crest. In addition, it needs to be stressed that there is no evidence in the human that Neuralleiste and Ganglienleiste are ‘two completely different formations’ (Holmdahl, 1934) and no support exists for the triphasic development vigourously championed by Holmdahl (1934) on the basis of only three human embryos, namely, Neuralleiste, Indifferenzstadium, and Ganglienleiste.
The eight numbered rhombomeres (Fig. 1C) are preceded by four lettered precursors (Fig. 1A: Rh. A–D), frequently termed prorhombomeres. The precise interrelationship of the components of the two systems lacks agreement. The most recent view in the human is that A corresponds to Rh. 1–3, B to Rh. 4, C to Rh. 5–7, and D to Rh. 8 (Müller & O’Rahilly, 1997). A careful study of rodents, however, suggests that the A/B boundary corresponds to Rh. 2/3 rather than Rh. 3/4 (Ruberte et al. 1997). It should be mentioned that rhombomere 8 (Table 2), frequently ignored, has been illustrated in the mouse (Morriss-Kay & Wilkie, 2005, labelled OC in their Fig. 3H) as well as in the human.
Some older terms have been replaced by their current, official equivalents, e.g. dorsal root ganglion by spinal ganglion, jugular ganglia by superior glossopharyngeal and vagal ganglia, petrous or petrosal ganglion by inferior glossopharyngeal, and nodose ganglion by inferior vagal ganglion.
Previous investigations
Although excellent descriptive accounts of the neural crest have been published in such forms as Ambystoma (Northcutt & Brändle, 1995), and some limited information on primates is available (Müller & O’Rahilly, 1980; Peterson et al. 1996), no systematic account of the crest specifically in the human has appeared. The crest, however, was included in a valuable series of reconstructions (2–16 somites) by Bartelmez & Evans (1926) and supplemented by additional examples in staged embryos by Bartelmez & Dekaban (1962). Moreover, spinal crest in 57 staged embryos was described by Sensenig (1951, 1957). In contrast, only three embryos were examined by Holmdahl (1934) and only four (with no reconstructions) by Theiler (1949), and their earliest examples already possessed 28 and 25 somites respectively (stage 12).
The placodes associated with the cranial ganglia were regarded by Streeter (1942) as detached islands of specialized neurectodermal material. Their development in the human was studied by Van Campenhout (1948) in more than a dozen Carnegie embryos, the stages (11–16) of which are now known. It is to be stressed that in the human as in the mouse, but distinct from the chick embryo, emigration of the cranial neural crest takes place before closure of the rostral neuropore, and no accumulation of crest is found between the surface ectoderm and the roof plate of the neural tube.
Nasal and optic crest
The telencephalon is visible for the first time at stage 10 (O’Rahilly & Müller, 2006).
The nasal placodes or plates appear in the surface ectoderm at stage 11 (Müller & O’Rahilly, 2004). The term nasal placode is preferred to olfactory placode, which is said to be neurectodermal, but whether the placode and the telencephalon arise from adjacent cellular fields in the rostral part of the neural plate (Sternberg, 1927; Streeter, 1942) is not entirely clear in mammals. With the aid of immunohistochemistry, cells found leaving the nasal plate in a human embryo of stage 13 have been interpreted as pioneer cells, and those at stages 14 and 15 as olfactory receptor neurons that migrate to the prosencephalon (Bystron et al. 2006), i.e. to the future olfactory bulb.
The nasal crest appears last, when cranial neural crest elsewhere has ceased to form (at stage 13), and it persists longer than other components (at least to stage 23). Its derivatives include neurons of the olfactory nerve, glial cells for olfactory fibres, ganglionic cells for the vomeronasal and terminalis ganglia (Müller & O’Rahilly, 2004), and LHRH precursors (Schwanzel-Fukuda et al. 1996) that migrate to and become integrated in the basal hypothalamus.
The right and left optic primordia are first visible at stage 10 (O’Rahilly & Müller, 2006). The optic crest of the human, which was first observed at stage 12 by Blount, was described in considerable detail by Bartelmez & Blount (1954), who included excellent illustrations. These authors suggested that the sheath of the optic vesicle and cup, which is derived from the optic crest, produces the pigment cells of the uvea.
The forebrain in the human, with the exception of the optic region, was not found to contribute neural crest. Moreover, ‘no clear evidence has been found in Homo’ for a lateral crest primordium (Bartelmez, 1962) and it was not seen in the present study.
Comparative aspects
The nasal crest has been shown in the mouse to give origin to the nasal septum (Jiang et al. 2002, Fig. 1).The nasal placodes are derived from a progenitor tissue of the prosencephalon (Osumi-Yamashita et al. 1994). The placodes of the rabbit were studied in detail by Groth (1938, 1939).
Neural crest cells originate from the forebrain of the mouse and make a contribution to the frontonasal mesenchyme (Morriss-Kay et al. 1993), as do also cells of the mesencephalic crest (Osumi-Yamashita et al. 1994). At least in certain mammals, e.g. pig and the insectivore Hemicentetes (Bartelmez, 1960), as well as the rat (Bartelmez, 1962), an earlier source of crest from the forebrain develops from ‘lateral lips’ of the neural folds. Mesencephalic crest has been shown to contribute to the dental mesenchyme of the mandibular molars in the rat (Imai et al. 1996).
Preotic and otic crest
A preotic sulcus and a postotic concavity are not always readily identifiable in human embryos. One preotic example at stage 9 and one at stage 10 were at the A/B boundary, one at stage 11 and one at stage 12 were at the level of RH. 4, in association with the appearance of the facial ganglion. Four more probable or at least possible instances were noted in a series of 18 embryos. A postotic concavity was found in an embryo at stage 9 at the B/C boundary, and a likely example at stage 12 at the level of Rh. 5. In rodents, injection of DiI into the sulci indicates the level of Rh. 2/3 for the preotic sulcus and Rh. 5 for the otic sulcus (Ruberte et al. 1997).
The mesencephalic is the first identifiable crest in the human. It appears at stage 9 (3 somites) and is visible up to stage 11, when it spreads out towards the frontonasal region and the eye, where it mingles with the optic crest (Müller & O’Rahilly, 1986).
A study of 17 reconstructed embryos of stages 10 to 12 in the literature showed clearly that the mesencephalic and trigeminal components of the neural crest are separable entities, although they may occur as a combined mass in stage 10 and early in stage 11. During stage 12 the cells of the mesenchymal crest have emigrated, so that the rostral crest appears to be represented then by the trigeminal constituent only.
The skull is derived from neural crest, mesenchyme of other origin (e.g. prechordal plate, primitive node, axial mesoderm), and occipital sclerotomes (basioccipital, exoccipital, jugular process). The sclerotomic components can be followed up to at least stage 23.
The trigeminal crest has been detected by the alkaline phosphatase reaction at stage 11 (Mori, 1959, 13–14 somites). Short nerve fibres appear in the trigeminal ganglion at stage 12.
The trigeminal ganglion receives some contributions from the ectoderm (Fig. 4C), and this was observed in stage 13 by Ingalls (1907) and in three Carnegie embryos of stage 12 by Van Campenhout (1948). The relevant ectoderm, however, is not thickened, so that a functional more than a morphological placode seems to be involved.
An unusual finding was that a part of the facial crest arises directly from the lateral wall of the neural tube in one embryo of stage 10 (Fig. 2B) and this was observed also at stage 12 (Fig. 4D). It is considered to be a normal feature. The median, dorsal mass of presumed crest seen by Baxter & Boyd (1939, Text-figure 2D and Plates 1 and 2) in the ‘acoustico-facial neural crest primordia’ at stage 10, was thought by those authors to be a variation or possibly to be pathological, and nothing similar was observed in the present study.
In the current investigation, at stage 10 the otic crest of Rh.4 was identifiable. At stage 11 some otic cells are already being added to the original facial crest. They represent the beginning of the vestibular ganglion (Fig. 7). At stage 14 afferent fibres to the facial ganglion and transiting efferent fibres to the vestibular component of ganglion VIII enable a distinction to be made between the facial (geniculate) ganglion and the vestibular ganglion (Müller & O’Rahilly, 1988a). The vestibular ganglion may consist at least partly of otic crest (Politzer, 1956; O’Rahilly, 1963). At stage 15 the cochlear ganglion, which is probably derived entirely from the otic crest, is recognizable by its smaller cells (Müller & O’Rahilly, 1988b). Streeter (1906) believed that at least a part of the cochlear ganglion develops by budding from the vestibular ganglion. At stage 16 unequivocal identification of the cochlear ganglion is possible when cochlear nerve fibres appear abruptly (Streeter, ibid.; Müller & O’Rahilly, 1989a).
It should be mentioned that the otic primordium is not a stable point of reference because it undergoes a relative caudal shift in relation to the rhombomeres, and this has been plotted in stages 9 to 14 (Müller & O’Rahilly, 2003, Fig. 2).
Comparative aspects
The emigration of crest cells away from the neural epithelium in the cranial region in the mouse (Duband et al. Fig. 1C) is basically similar to that in the human. The mesencephalic crest arises early from the neural folds also in the rat (Adelmann, 1925; Morriss-Kay, 1981; Tan & Morriss-Kay, 1985; Serbedzija et al. 1992) and has been demonstrated in transgenic mouse embryos (Jiang et al. 2002). It extends to the telencephalon to become frontonasal mesenchyme (Tan & Morriss-Kay, 1985; Osumi-Yamashita et al. 1994), which forms inter alia the nasal and maxillary bones (Morriss-Kay, 2001). Although statements on the contributions of neural crest to the human skull must be regarded as provisional, the presumed elements, from extrapolation of observations in the mouse, include the frontal, nasal, zygomatic, squamous temporal, maxilla and mandible, and the hyoid (Morriss-Kay, 2001, Fig. 3B). Current reviews of craniofacial development that include some recent information on the neural crest in various species have been published (Evans & Francis-West, 2005).
The isthmic crest, which is found on the roof of the isthmic rhombomere, appears to be involved more with the development of the meninges than with that of the nucleus of the mesencephalic tract of the trigeminal nerve, as followed in mouse embryos (Widmer et al. 1998, who did not, however, mention crest cells specifically).
The trigeminal crest and its migration, as well as the formation of the trigeminal ganglion, seem to occur slightly earlier in the mouse, with reference to the number of somites present (Jiang et al. 2002). The caudal boundary of the trigeminal crest in the mouse forms the caudal border of the frontal bone and a small tongue of tissue between the parietal bones (Morriss-Kay & Wilkie, 2005).
That the vestibular ganglion may consist either partly or entirely of otic crest cells has been held by several authors, e.g. Groth (1938, 1939) in the rabbit, Batten (1958) in the sheep (one of his many studies in that species), and Deol (1967, 1970a, 1979b) in the mouse.
The origin of various cranial ganglia from crest at the rhombencephalic level has been studied in the mouse with the aid of the dye DiI (Serbedzija et al. 1992)
Postotic crest
Glossopharyngeal and vagal components are not yet separated from each other at stage 12. Segregation is present in stage 13 and development into superior and inferior ganglia begins at the same time. The continuation of the inferior ganglia extends into pharyngeal arches 3 and 4 (Figs 1E, 6).
The hypoglossal crest arises at the level of Rh. 8 and begins to develop during stage 10. It constitutes a continuous band at stage 11, but produces no ganglia. At stages 12–14 the crest joins myotomic cells (which are not yet myoblasts) from the occipital somites (namely, 1–4). The combined crest and myotomic cells are known as the hypoglossal cord, the development of which has been followed in the human (O’Rahilly & Müller, 1984). There is no evidence that the hypoglossal crest forms skeletal elements, although it does traverse the arches.
Publications on the occipitospinal region include accounts of the hypoglossal cord (O’Rahilly & Müller, 1984), the number of the occipital somites and the head-neck interface (Müller & O’Rahilly, 1994), and the migration of the postotic neural crest as well as comparisons with other species, such as mouse and pig (Müller & O’Rahilly, 2003).
Ganglionic material related to the rarely seen dorsal roots of the hypoglossal nerve is generally termed the ganglion of Froriep, who, however, failed to find either the dorsal roots or a ganglionic rudiment in the adult human. Moreover, there is no solid evidence for the existence of the ganglion in the human embryo (O’Rahilly & Müller, 1984). The first cervical ganglion, which is always small, can, in the absence of precise reconstructions, easily be misinterpreted as a Froriep ganglion.
The crest that arises from Rh. 6 and Rh. 7 was reconstructed previously (Müller & O’Rahilly, 1987, Fig. 6A,B). Because of its termination around the truncus arteriosus (Fig. 1D) it is interpreted here as the cardiac crest for the first time in the human.
Comparative aspects
Myotomic material forms the intrinsic lingual musculature, whereas neural crest gives rise to the associated connective tissue, as confirmed recently in transgenic mice (Matsuoka et al. 2005).
The cardiac crest of the rat arises in the occipital region between somites 1 and 2, but ‘the cardiac pathway is taken by only a small subpopulation of the occipital crest’ (Fukiishi & Morriss-Kay, 1992). Lineage studies in the mouse indicate that the entire conotruncal mesenchyme is of neural crest origin (Jiang et al. 2000). In a study of fibroblast growth factor-15 in the mouse, it has been suggested that ‘both mouse and human hindbrain/spinal cord enhancer exerts its activity during critical period of early development of the cardiac neural crest cells’ (Saitsu et al. 2006).
Pharyngeal arches and epipharyngeal placodes
It is believed that the pharyngeal arches and the neuromeres are related by the neural crest (Table 3).
Table 3.
The relationship between the neural crest, the rhombomeres, and the pharyngeal arches in the human
| Neural crest at stage 10 | Rhombomeres at stage 11 | Pharyngeal arches |
|---|---|---|
| Rh1 | 1 at stage 10 | |
| Trigeminal | Rh2 | |
| Rh3 | 2 at stage 11 | |
| Facial | Rh4 | |
| Rh5 | 3 at stages 11 &12 | |
| Glossopharyngeal | Rh6 | |
| Vagal* | Rh7 | 4–6 at stages 12 &13 |
| Hypoglossal | Rh8 |
And accessory crest at stage 12.
The epipharyngeal placodes or discs (epibranchial only in species with gills) are typically areas of thickened surface ectoderm that are a part of the ectodermal ring (O’Rahilly & Müller, 1985) and contribute cells to cranial ganglia. The placodes appear in stage 12 and are active in pharyngeal arches 2–4 in stages 12 and 13, and particularly so in arch 1 in stage 12 (Van Campenhout, 1948). This may be a reflection of differences in Hox gene expression found between arch 1 and the following arches (Vieille-Grosjean et al. 1997). Enhanced activity of alkaline phosphatase has been detected in the placode of arch 3 at stage 12 (Mori, 1965). The height of placodal development is attained before the appearance of the cervical sinus in stage 14. The placodal activity of pharyngeal arch 1 differs from that in arches 2–4 in that the ectodermal contribution appears earlier and is not a clearly localized thickened area (Fig. 4C).
Comparative aspects
The skeletal components of the pharyngeal arches are derived from mesoderm and rhombencephalic neural crest in the mouse (Trainor & Tam, 1995). Crest cells in the rat migrate from the mesencephalon and rhombomeres 1 and 2 to arch 1, from rhombomere 4 to arch 2, and from rhombomeres 6 and 7 to the more caudally situated arches (Tan & Morriss-Kay, 1985). Endoderm is also important and is involved in establishing and patterning the pharyngeal arches, as well as in inducing the formation of the epipharyngeal placodes.
Spinal crest
As the number of somitic pairs increases, the caudal limit of the spinal crest descends during each stage (Table 1). Approximately 19 primordia of spinal ganglia are present at stage 13, and 33 at stage 14. Subsequently, 30–35 ganglia are found from stage 15 to stage 23 (O’Rahilly & Müller, 2003, Fig. 7b).
Early agglomerations for future spinal ganglia (C1-T5) at stage 13 (Fig. 6) are still part of the uninterrupted cord formed by the spinal crest. Moreover, the accessory and spinal components are continuous at this stage. The development of isolated ganglia extends into stage 17. All the spinal ganglia are formed during secondary neurulation.
In distinction to the more rostral ganglia the sacral and coccygeal seem to arise directly from the neural tube by means of dorsal and/or ventral cellular bridges (Holmdahl, 1934; Orts Llorca, 1934).
In the truncal region the cells of the roof plate of the neural tube, interpreted as presumptive crest, resemble those migrating laterad as definitive neural crest (Fig. 4B). Newly emigrated crest cells in the median region, as shown in drawings, were not observed in the human, much less a median dorsal agglomeration ‘sitting on’ the neural tube.
In the human a migratory pathway between neural tube and sclerotomes (Fig. 5A, stage 12) is detectable before the development of dense and loose zones. In the next stage (Fig. 5B) the cervical sclerotomes develop loose rostral and dense caudal zones. Only the ventral fibres of the spinal nerves traverse the sclerotomes, which they do through the loose rostral zones (Fig. 5C). Other pathways would require special methods for their demonstration.
The enteric nervous system (neurons and glia) is derived from the neural crest (Timmermans et al. 2001), and it is generally accepted that vagal crest colonizes the entire intestine, sacral crest the ‘postumbilical’ gut, and truncal crest the oesophagus and stomach (as well as providing all of the sympathetic ganglia).
A participation of ‘neurendoderm’ has been proposed in the human duodenum and vermiform appendix (Van Campenhout, 1957).
Comparative aspects
Three main migratory pathways are generally described for the spinal crest: (1) ventrolateral between dermatomyotome and sclerotome; and later (2) ventromedial between sclerotome and neural tube, and (3) between surface ectoderm and dermatomyotome. These have been verified by DiI labelling in the mouse (Serbedzija et al. 1990). Crest that forms the spinal ganglia passes beneath the dermatomyotome and/or through the medial portion of the rostral halves of the sclerotomes according to a study with monoclonal antibody in the mouse (Kubota et al. 1996). It would seem that the spinal ganglia are produced earlier in the mouse than in the human, namely at the time of closure of the caudal neuropore (Nievelstein et al. 1993). Future pigment cells in the mouse take route No. 3 (Serbedzija et al. 1990, 1994). The mesenchyme around the notochord, as well as the caudal halves of the sclerotome, are inhibitory to migration.
Apart from contributing ganglia the spinal crest is believed to have an important role in the musculo-skeletal system, namely that somite-derived muscle fibres become attached to skeletal elements through connective tissue derived from the postotic neural crest (Matsuoka et al. 2005).
The sacral crest has been followed in the mouse by DiI labelling (Serbedzija et al. 1991).
A participation of endoderm in the formation of cranial ganglia has been proposed in the mouse (de Winiwarter, 1938) and in the rabbit (Coërs, 1946). Furthermore, a possible role of ‘neurendoderm’ has been considered for the developing pancreas in the cow (Van Campenhout, 1943).
Malformations
Reviews of craniofacial malformations in the light of embryology and murine genetics have been published. (Wilkie & Morriss-Kay, 2001; Morriss-Kay & Wilkie, 2005). A number of abnormal conditions have been related to the neural crest, such as the DiGeorge (velo-cardio-facial) sequence, in which the tissues of pharyngeal arches 3 and 4, including derivatives of the corresponding pharyngeal pouches, are affected. It has been proposed that a further series of anomalies is associated specifically with the postotic neural crest, constituting a group that includes, for example, Sprengel deformity and the Klipppel–Feil sequence (Matsuoka et al. 2005).
The term neurocristopathy is frequently used for abnormal conditions attributed to maldevelopment of the neural crest. Apart from a controversial hypothesis relating neural crest to peripheral neuropathy in limb anomalies caused by thalidomide, and which raised a number of embryological difficulties (Gardner & O’Rahilly, 1976), a few accepted examples will be mentioned.
A striking instance is neurofibromatosis (of von Recklinghausen) type 1, a polymorphic, genetic condition associated with neuroectodermal derivatives, including especially those of the neural crest.
A well known representative is congenital aganglionic megacolon (Hirschsprung disease), a neurocristopathy of the enteric nervous system characterized by absence or reduction of parasympathetic ganglion cells in the submucosal and myenteric plexuses of the hindgut. The condition is generally attributed to defective proliferation and migration of ganglion cells from the neural crest along vagal fibres proceeding to their normal destination in the wall of the large intestine.
Possible and/or probable examples of neural crest abnormalities in the human embryo have been reported, e.g. at 20 mm (Gruenwald, 1941).
Two instructive types of abnormal embryos (Müller & O’Rahilly, 1984, 1989b, 1990) are summarized here. In prospective anencephaly at stage 13 the cranial ganglia were well formed, although the rostral neuropore of the still satisfactorily developed brain was open from the middle of the midbrain rostrad. In an anencephalic embryo of stage 22 membranous cranial elements and the nasal septum were present, as was the pharyngeal skeleton, although the skull was deformed in the absence of a formative influence of the brain. Basioccipital components derived from sclerotomic material developed. It is concluded that the neural crest and its derivatives are well represented in anencephaly.
In contrast to the above, in prospective synophthalmia at stage 16 cranial ganglia were missing or reduced and at stages 19–21 skeletal structures were defective, as were derivatives of the pharyngeal arches. The nasal septum was absent. These features are known to develop from the neural crest, so that the crest and its derivatives are inadequate in synophthalmia.
Concluding remarks
This article brings to a close an extensive series of detailed morphological studies of development in numerous staged human embryos and conducted over a number of years. Because the successive investigations were based on the same series of accurately staged embryos (mostly from the Carnegie Collection) and because the precise three-dimensional reconstructions were prepared uniformly by one person (F.M.), the findings for any given structure or system can readily be compared with those for another to provide an integrated view of sequential developmental events. Although a few of the prenatal ages assigned to the stages have been altered over the years, the morphological staging system itself (as refined in 1987) has retained its validity intact and is essential for the scientific study of the human embryo.
Acknowledgments
It is a particular pleasure to thank Professor Gillian Morriss-Kay for providing valuable comments and helpful suggestions, as well as for the electronic preparation of the figures.
References
- Adelmann HB. The development of the neural folds and cranial ganglia of the rat. J Comp Neurol. 1925;39:19–171. [Google Scholar]
- Bartelmez GW. The subdivisions of the neural folds in man. J Comp Neurol. 1923;35:231–247. [Google Scholar]
- Bartelmez GW. Neural crest from the forebrain in mammals. Anat Rec. 1960;138:269–282. doi: 10.1002/ar.1091380308. [DOI] [PubMed] [Google Scholar]
- Bartelmez GW. The proliferation of neural crest from forebrain levels in the rat. Contrib Embryol Carnegie Instn. 1962;37:1–12. [Google Scholar]
- Bartelmez GW, Blount MP. The formation of neural crest from the primary optic vesicle in man. Contrib Embryol Carnegie Instn. 1954;35:55–71. [Google Scholar]
- Bartelmez GW, Dekaban AS. The early development of the human brain. Contrib Embryol Carnegie Instn. 1962;37:13–32. [Google Scholar]
- Bartelmez GW, Evans HM. Development of the human embryo during the period of somite formation, including embryos with 2 to 16 pairs of somites. Contrib Embryol Carnegie Instn. 1926;17:1–67. [Google Scholar]
- Batten EH. The origin of the acoustic ganglion in the sheep. J Anat. 1958;92:660–661. [PubMed] [Google Scholar]
- Baxter JS, Boyd JD. Observations on the neural crest of a ten-somite human embryo. J Anat. 1939;73:318–326. [PMC free article] [PubMed] [Google Scholar]
- Bystron I, Rakic P, Molnár Z, et al. The first neurons of the human cerebral cortex. Nature Neurosci. 2006;9:880–886. doi: 10.1038/nn1726. [DOI] [PubMed] [Google Scholar]
- Chai Y, Jiang X, Ito Y, et al. Fate of the mammalian neural crest during tooth and mandibular morphogenesis. Development. 2000;127:1671–1679. doi: 10.1242/dev.127.8.1671. [DOI] [PubMed] [Google Scholar]
- Coërs C. La formation des nerfs mixtes crâniens chez le lapin. Arch Biol. 1946;57:13–77. [Google Scholar]
- de Winiwarter H. Origine et développement du ganglion carotidien. Appendice: participation de l’hypoblast à la constitution des ganglion crâniens. Arch Biol. 1938;50:67–94. [Google Scholar]
- Deol MS. The neural crest and the acoustic ganglion. J Embryol Exp Morphol. 1967;17:533–541. [PubMed] [Google Scholar]
- Deol MS. The origin of the acoustic ganglion and effects of the gene dominant spotting (Wv) in the mouse. J Embryol Exp Morphol. 1970a;23:773–784. [PubMed] [Google Scholar]
- Deol MS. The relationship between abnormalities of pigmentation and of the inner ear. Proc Roy Soc London A. 1979b;175:201–217. doi: 10.1098/rspb.1970.0019. [DOI] [PubMed] [Google Scholar]
- Duband JL, Monier F, Delannet M, Newgreen D. Epithelium-mesenchyme transition during neural crest development. Acta Anat. 1995;154:63–78. doi: 10.1159/000147752. [DOI] [PubMed] [Google Scholar]
- Evans D, Francis-West P. Craniofacial development. J Anat. 2005;207:435–682. doi: 10.1111/j.1469-7580.2005.00482.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukiishi Y, Morriss-Kay GM. Migration of cranial neural crest cells to the pharyngeal arches and heart in rat embryos. Cell Tissue Res. 1992;268:1–8. doi: 10.1007/BF00338048. [DOI] [PubMed] [Google Scholar]
- Gardner E, O’Rahilly R. Neural crest, limb development, and thalidomide embryopathy. Lancet. 1976;1:635–637. doi: 10.1016/s0140-6736(76)90433-5. [DOI] [PubMed] [Google Scholar]
- Gaunt PN, Gaunt WA. Three Dimensional Reconstruction in Biology. Tunbridge Wells: Pitman Medical; 1978. New Edition. [Google Scholar]
- Groth W. Der Ursprung der Reichzellenneuroblasteme und ihre erste Entwicklung bis zur Ausbildung der Reichnervenanlage beim Kaninchen. Z mikr-anat Forsch. 1938;43:207–234. [Google Scholar]
- Groth W. Der Ursprung der Labyrinthplacode and des Ganglion statoacusticum im Vergleich zur Genese des Riechorgans beim Kaninchen. Z mikr-anat Forsch. 1939;45:393–442. [Google Scholar]
- Gruenwald P. Tissue anomalies of probable neural crest origin in a 20 mm human embryo with myeloschisis. Arch Pathol. 1941;31:489–500. [Google Scholar]
- His W. Untersuchungen über die erste Anlage des Wirbelthierleibes. Die erste Entwickelung des Hühnchens im Ei. Leipzig: Vogel; 1868. 237 pages and 12 Plates. [Google Scholar]
- Holmdahl D. Neuralleiste und Ganglienleiste beim Menschen. Z mikr-anat Forsch. 1934;36:137–178. [Google Scholar]
- Imai H, Osumi-Yamashita N, Ninomiya Y, Eto K. Contribution of early-emigrating midbrain crest cells to the dental mesenchyme of mandibular molar teeth in rat embryos. Dev Biol. 1996;176:151–165. doi: 10.1006/dbio.1996.9985. [DOI] [PubMed] [Google Scholar]
- Ingalls NW. Beschreibung eines menschlichen Embryos von 4.9 mm. Arch mikr Anat Entw. 1907;70:506–576. [Google Scholar]
- Jiang X, Iseki S, Maxson RE, et al. Tissue origins and interactions in the mammalian skull vault. Develop Biol. 2002;241:106–116. doi: 10.1006/dbio.2001.0487. [DOI] [PubMed] [Google Scholar]
- Jiang X, Rowitch DH, Soriano P, et al. Fate of the mammalian cardiac neural crest. Development. 2000;127:1607–1616. doi: 10.1242/dev.127.8.1607. [DOI] [PubMed] [Google Scholar]
- Kalyani AJ, Rao MS. Cell lineage in the developing neural tube. Biochem Cell Biol. 1998;76:1051–1068. [PubMed] [Google Scholar]
- Kubota Y, Morita T, Ito K. New monoclonal antibody (4E9R) identifies mouse neural crest cells. Develop Dynamics. 1996;206:368–378. doi: 10.1002/(SICI)1097-0177(199608)206:4<368::AID-AJA3>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Matsuoka T, Ahlberg PE, Kessaris N, et al. Neural crest origins of the neck and shoulder. Nature. 2005;436:347–355. doi: 10.1038/nature03837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori T. Histochemical studies on the distribution of alkaline phosphatase in early human embryos. II. Observations on an embryo with 13–14 somites. Arch Histol Japan. 1959;18:197–209. [Google Scholar]
- Mori T. Histochemical studies on the distribution of alkaline phosphatase in early human embryos. III. Embryos in Streeter's Horizon XII. Okajimas Folia Anat Jap. 1965;40:765–793. doi: 10.2535/ofaj1936.40.4-6_765. [DOI] [PubMed] [Google Scholar]
- Morriss-Kay G, Ruberte E, Fukiishi Y. Mammalian neural crest and neural crest derivatives. Ann Anat. 1993;175:501–507. doi: 10.1016/s0940-9602(11)80209-8. [DOI] [PubMed] [Google Scholar]
- Morriss-Kay GM. Growth and development of pattern in the cranial neural epithelium of rat embryos during neurulation. J Embryol Exp Morphol. 1981;65:225–241. [PubMed] [Google Scholar]
- Morriss-Kay GM. Derivation of the mammalian skull vault. J Anat. 2001;199:143–151. doi: 10.1046/j.1469-7580.2001.19910143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morriss-Kay GM, Wilkie AOM. Growth of the normal skull vault and its alteration in craniosynostosis: insights from human genetics and experimental studies. J Anat. 2005;207:637–653. doi: 10.1111/j.1469-7580.2005.00475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller F, O’Rahilly R. The early development of the nervous system in staged insectivore and primate embryos. J Comp Neurol. 1980;l93:74l–75l. doi: 10.1002/cne.901930311. [DOI] [PubMed] [Google Scholar]
- Müller F, O’Rahilly R. Cerebral dysraphia (future anencephaly) in a human twin embryo at stage l3. Teratology. 1984;30:l67–l77. doi: 10.1002/tera.1420300203. [DOI] [PubMed] [Google Scholar]
- Müller F, O’Rahilly R. The development of the human brain and the closure of the rostral neuropore at stage ll. Anat Embryol. 1986;l75:205–222. doi: 10.1007/BF00389597. [DOI] [PubMed] [Google Scholar]
- Müller F, O’Rahilly R. The development of the human brain, the closure of the caudal neuropore, and the beginning of secondary neurulation at stage l2. Anat Embryol. 1987;l76:4l3–430. doi: 10.1007/BF00310083. [DOI] [PubMed] [Google Scholar]
- Müller F, O’Rahilly R. The first appearance of the future cerebral hemispheres in the human embryo at stage l4. Anat Embryol. 1988a;177:495–511. doi: 10.1007/BF00305137. [DOI] [PubMed] [Google Scholar]
- Müller F, O’Rahilly R. The development of the human brain, including the longitudinal zoning in the diencephalon at stage 15. Anat Embryol. 1988b;179:55–71. doi: 10.1007/BF00305100. [DOI] [PubMed] [Google Scholar]
- Müller F, O’Rahilly R. The human brain at stage 16, including the initial evagination of the neurohypophysis. Anat Embryol. 1989a;179:551–569. doi: 10.1007/BF00315698. [DOI] [PubMed] [Google Scholar]
- Müller F, O’Rahilly R. Mediobasal prosencephalic defects, including holoprosencephaly and cyclopia, in relation to the development of the human forebrain. Am J Anat. 1989b;185:391–414. doi: 10.1002/aja.1001850404. [DOI] [PubMed] [Google Scholar]
- Müller F, O’Rahilly R. The development of anencephaly and its variants. Am J Anat. 1990;190:193–218. doi: 10.1002/aja.1001900302. [DOI] [PubMed] [Google Scholar]
- Müller F, O’Rahilly R. Occipitocervical segmentation in staged human embryos. J Anat. 1994;185:251–258. [PMC free article] [PubMed] [Google Scholar]
- Müller F, O’Rahilly R. The timing and sequence of appearance of neuromeres and their derivatives in staged human embryos. Acta Anat. 1997;158:83–99. [PubMed] [Google Scholar]
- Müller F, O’Rahilly R. Segmentation in staged human embryos: the occipitocervical region revisited. J Anat. 2003;203:297–315. doi: 10.1046/j.1469-7580.2003.00219.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller F, O’Rahilly R. Olfactory structures in staged human embryos. Cells Tissues Organs. 2004;178:93–116. doi: 10.1159/000081720. [DOI] [PubMed] [Google Scholar]
- Nievelstein RAJ, Hartwig NG, Vermeij-Keers C, et al. Embryonic development of the mammalian caudal neural tube. Teratol. 1993;48:21–31. doi: 10.1002/tera.1420480106. [DOI] [PubMed] [Google Scholar]
- Nomina embryologica Preliminary List. 1967. printed privately.
- Nomina embryologica . Included with the Nomina anatomica. 4. Amsterdam: Excerpta Medica; 1977. [Google Scholar]
- Northcutt RG, Brändle K. Development of branchiomeric and lateral line nerves in the axolotl. J Comp Neurol. 1995;355:427–454. doi: 10.1002/cne.903550309. [DOI] [PubMed] [Google Scholar]
- O’Rahilly R. The early development of the otic vesicle in staged human embryos. J Embryol Exp Morphol. 1963;11:741–755. [PubMed] [Google Scholar]
- O’Rahilly R. The optic, vestibulocochlear, and terminal-vomeronasal neural crest in staged human embryos. In: Rohen JW, editor. The Structure of the Eye 2nd Symposium. Stuttgart: Schattauer; 1965. pp. 557–564. [Google Scholar]
- O’Rahilly R, Müller F. The early development of the hypoglossal nerve and occipital somites in staged human embryos. Am J Anat. 1984;169:237–257. doi: 10.1002/aja.1001690302. [DOI] [PubMed] [Google Scholar]
- O’Rahilly R, Müller F. The origin of the ectodermal ring in staged human embryos of the first 5 weeks. Acta Anat. 1985;l22:l45–l57. [PubMed] [Google Scholar]
- O’Rahilly R, Müller F. The meninges in human development. J Neuropathol Exp Neurol. 1986;45:588–608. [PubMed] [Google Scholar]
- O’Rahilly R, Müller F. Developmental Stages in Human Embryos Including a Revision of Streeter's ‘Horizons’ and a Survey of the Carnegie Collection. Washington, D.C: Carnegie Institution 637; 1987. Publication. [Google Scholar]
- O’Rahilly R, Müller F. Prenatal ages and stages: measures and errors. Teratology. 2000;61:382–384. doi: 10.1002/(SICI)1096-9926(200005)61:5<382::AID-TERA10>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- O’Rahilly R, Müller F. Somites, spinal ganglia, and centra. Cells Tissues Organs. 2003;173:75–92. doi: 10.1159/000068948. [DOI] [PubMed] [Google Scholar]
- O’Rahilly R, Müller F. The Embryonic Human Brain. An Atlas of Developmental Stages. 3. Hoboken, New Jersey: Wiley-Liss; 2006. [Google Scholar]
- Orts Llorca F. Über die Entwicklung der caudalen Spinalganglien beim Menschen. Z Anat Entw. 1934;102:462–480. [Google Scholar]
- Osumi-Yamashita N, Ninomiya Y, Doi H, Eto K. The contribution of both forebrain and midbrain crest cells to the mesenchyme in the frontonasal mass of mouse embryos. Devel Biol. 1994;164:409–419. doi: 10.1006/dbio.1994.1211. [DOI] [PubMed] [Google Scholar]
- Peterson PE, Blankenship TN, Wilson DB, Hendrickx AG. Analysis of hindbrain neural crest migration in the long-tailed monkey (Macaca fascicularis. Anat Embryol. 1996;194:235–246. doi: 10.1007/BF00187134. [DOI] [PubMed] [Google Scholar]
- Politzer G. Die Entstehung des Ganglion acusticum beim Menschen. Acta Anat. 1956;26:1–13. [PubMed] [Google Scholar]
- Ruberte E, Wood HB, Morriss-Kay GM. Prorhombomeric subdivision of the mammalian embryonic hindbrain: is it functionally meaningful? Int J Dev Biol. 1997;41:213–222. [PubMed] [Google Scholar]
- Saitsu H, Shiota K, Ishibaschi M. Analysis of Fibroblast growth factor 15 cis-elements reveals two conserved enhancers which are closely related to cardiac outflow tract development. Mech Dev. 2006;123:665–673. doi: 10.1016/j.mod.2006.07.002. [DOI] [PubMed] [Google Scholar]
- Schwanzel-Fukuda M, Crossin KL, Pfaff DW. Migration of luteinizing hormone-releasing hormone (LHRH) neurons in early human embryos. J Comp Neurol. 1996;366:547–557. doi: 10.1002/(SICI)1096-9861(19960311)366:3<547::AID-CNE12>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Sensenig EC. The early development of the meninges of the spinal cord in human embryos. Contrib Embryol Carnegie Instn. 1951;34:145–157. [Google Scholar]
- Sensenig EC. The development of the occipital and cervical segments and their associated structures in human embryos. Contrib Embryol Carnegie Instn. 1957;36:141–151. [Google Scholar]
- Serbedzija GN, Bronner-Fraser M, Fraser SE. Vital dye analysis of cranial neural crest cell migration in the mouse embryo. Development. 1992;116:297–307. doi: 10.1242/dev.116.2.297. [DOI] [PubMed] [Google Scholar]
- Serbedzija GN, Bronner-Fraser M, Fraser SE. Developmental potential of trunk neural crest cells in the mouse. Development. 1994;120:1709–1718. doi: 10.1242/dev.120.7.1709. [DOI] [PubMed] [Google Scholar]
- Serbedzija GN, Burgan S, Fraser SE, Bronner-Fraser M. Vital dye labelling demonstrates a sacral neural crest cell contribution to the enteric nervous system of chick and mouse embryos. Development. 1991;111:857–866. doi: 10.1242/dev.111.4.857. [DOI] [PubMed] [Google Scholar]
- Serbedzija GN, Fraser SE, Bronner-Fraser M. Pathways of trunk neural crest cell migration in the mouse embryo as revealed by vital dye labelling. Development. 1990;108:605–612. doi: 10.1242/dev.108.4.605. [DOI] [PubMed] [Google Scholar]
- Sternberg H. Beschreibung eines menschlichen Embryo mit vier Ursegmentpaaren. Z Anat Entw. 1927;82:142–240. [Google Scholar]
- Streeter GL. On the development of the membranous labyrinth and the acoustic and facial nerves in the human embryo. Am J Anat. 1906;6:139–165. [Google Scholar]
- Streeter GL. Description of age group XI. Contrib Embryol Carnegie Instn. 1942;30:211–245. [Google Scholar]
- Tan SS, Morriss-Kay GM. The development and distribution of the cranial neural crest in the rat embryo. Cell Tissue Res. 1985;240:403–416. doi: 10.1007/BF00222353. [DOI] [PubMed] [Google Scholar]
- Theiler K. Studien zur Entwicklung der Ganglienleiste. II. Teil. Befunde zur Frühentwicklung der Ganglienleiste beim Menschen. Acta Anat. 1949;8:96–112. [PubMed] [Google Scholar]
- Timmermans J-P, Markwald RR, Litke LL. The enteric nervous system and its targets. Anat Rec. 2001;262:1–135. doi: 10.1002/1097-0185(20010101)262:1<::AID-AR1029>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Trainor PA, Tam PPL. Cranial paraxial mesoderm and neural crest cells of the mouse embryo. Development. 1995;121:2569–2582. doi: 10.1242/dev.121.8.2569. [DOI] [PubMed] [Google Scholar]
- Van Campenhout E. Le neurentoblaste pancréatique chez l’embryon de vache. Bull Acad Roy Méd Belgique. 1943;8:254–264. 6th series. [Google Scholar]
- Van Campenhout E. La contribution des placodes épiblastiques au développement des ganglions des nerfs crâniens chez l’embryon humain. Arch Biol (Liège. 1948;59:253–266. [PubMed] [Google Scholar]
- Van Campenhout E. La neurogenèse au niveau de l’appendice humain, embryonnaire et adulte. Acta Neuroveg. 1957;16:233–249. doi: 10.1007/BF01227255. [DOI] [PubMed] [Google Scholar]
- Veit O, Esch P. Untersuchung eines in situ fixierten, operativ gewonnenen menschlichen Eies der vierten Woche. Z Anat Entw. 1922;63:343–414. [Google Scholar]
- Vieille-Grosjean I, Hunt P, Gulisano M, et al. Branchial HOX gene expression and human craniofacial development. Develop Biol. 1997;183:49–60. doi: 10.1006/dbio.1996.8450. [DOI] [PubMed] [Google Scholar]
- Widmer CG, Morris-Wiman JA, Calhoun JC. Development of trigeminal mesencephalic and motor nuclei in relation to masseter muscle innervation in mice. Devel Brain Res. 1998;108:1–11. doi: 10.1016/s0165-3806(98)00009-1. [DOI] [PubMed] [Google Scholar]
- Wilkie AOM, Morriss-Kay GM. Genetics of craniofacial development and malformation. Nature Reviews. 2001;2:458–486. doi: 10.1038/35076601. [DOI] [PubMed] [Google Scholar]