Abstract
The Monodelphis oviduct can be divided into four anatomical segments: preampulla (comprising fimbriae and infundibulum), ampulla, isthmus with crypts and uterotubal junction. Ovaries are enclosed in a periovarial sac, the bursa, and in some specimens tubules of an epoophoron could be identified. In both structures non-ciliated cells develop small translucent vesicles, which accumulate in the cell apices and presumably produce fluid as often seen in the bursa and in the tubules of the epooophoron. These vesicles do not stain with Alcian blue or PAS. The same applies also to the non-ciliated cells of the fimbriae. The oviducal epithelium of ampulla and the surface epithelium of the isthmus consisting of ciliated and non-ciliated, secretory cells undergo considerable changes during the estrous cycle. Proestrus shows low numbers of ciliated cells, some are in the process of neo-ciliogenesis, non-ciliated cells carry solitary cilia and few remnant secretory granules from the previous cycle may be found. At estrus the amount of ciliated cells in ampulla and isthmus has increased, most non-cililated cells lost the solitary cilia, developed longer microvilli and formed numerous secretory granules in their cell apices. At postestrus secretory products, often surrounded by membranes, are extruded into the oviducal lumen and contribute towards egg coat formation. First signs of deciliation processes are apparent. Solitary cilia reappear.
At metestrus only few secretory cells are left with some secretory material. The lumen is often filled with shed cilia and cell apices. Proliferation of basal bodies within non-secretory cells indicate the formation of new ciliated cells.
The non-ciliated epithelial cells of the isthmic crypts form no secretory granules but accumulate a great number of translucent vesicles, which in contrast to the secretory granules do not stain with Alcian blue or PAS.
Keywords: epithelium, estrous cycle, marsupial, oviduct
Introduction
A considerable amount of information is available on the details of the surface morphology of the oviducts in a variety of eutherian species (Hunter, 1988; Jansen, 1995). By contrast, there is little information currently available about the oviduct of marsupials. Studies of marsupial reproduction, however, provide us with insights into the evolution of mammalian reproduction in general (Roberts et al. 1994; Roberts & Breed, 1996a, b; Renfree & Shaw, 1999; Selwood & Coulson, 2006; Renfree, 2006; Samollow, 2006). Monodelphis has become an intensively utilized laboratory-bred research marsupial, especially for studying reproductive biology.
Early descriptions of the oviduct stem from Andersen (1928) and Hartman (1923) depicting the tube of the opossum Didelphis virginiana. Recently two species have been described using ultrastructural techniques, Pseudocheirus (Armati-Gulson & Lowe, 1985) and Trichosurus (Arnold & Shorey, 1985). Aspects of the oviduct while enveloping the oocytes with mucoid layers have been studied in Sminthopsis crassicaudata (Breed et al. 1989) and in M. domestica (Philips & Fadem, 1987; Baggot & Moore, 1990), and reviewed in general by Selwood (2000).
In accordance with different anatomical, physiological and biochemical requirements, the oviduct is composed of distinct compartments. Their features comply with various necessities: to convey sperm with fertilizing capacities to encounter the oocyte, to recognize, nurture and transport the developing egg and to relay embryonic signals to distant organs. Different parts at different times contribute to these functions in response to endocrine, paracrine and neural signals.
The marsupialian zygote is transported rapidly through the oviduct accompanied by the formation of a mucoid coat and a shell membrane with the entry to the uterus. In all marsupials so far examined, cleavage starts after entering the uterus (Selwood, 2000; Zeller & Fryer, 2001), probably due to the shortness of the tubal passage.
Some authors divide marsupial oviducts only into two arbitrary halves: the ampullary segment, adjacent to the ovary, and the isthmic segment, adjacent to the uterus (Taggart, 1994; Bedford & Breed, 1994).
For the purpose of description, however, most authors divide the oviduct into four anatomical sections: (1) preampulla comprising fimbriae and infundibulum, (2) ampulla, (3) isthmus, and (4) the very end connecting the tube with the uterus, the utero-tubal junction (Van Blerkom & Motta, 1979). There are often no precise boundaries separating the segments. This classification is followed in the present study.
The oviducts vary considerably between species in length and in the degree of convolution. The mucosa lining of the oviduct consists of intricately folded, pseudostratified or simple epithelium with two types of cells, ciliated and interspersed non-ciliated cells. The non-ciliated cells are primarily secretory elements with glycoprotein-secreting capacity.
The oviducal epithelium is known to be responsive to estrogen and progesterone (Hunter, 1988), but as already described for Macaca nemestrina (Odor et al. 1980, 1983; Breed et al. 1989) the response of the epithelium to estrogen varies considerably from one area to another within a given segment in an individual animal and from one animal to another. These observations also apply to Monodelphis.
In Monodelphis the presence of an ovarian bursa is developed as a sac that encloses more or less completely the ovary and which consists of a peritoneal fold structure.
In some of the Monodelphis specimens epoophoron structures have been identified. With the decline of the mesonephros during postnatal reproductive differentiation in the female, the mesonephric or Wolffian duct and its tubules loose their primary function and regress (Mackay et al. 2004). This regression does not lead to a complete disappearance, however, some vestiges of the Wolffian duct persist, the epoophoron. It consists of a variable number of tubules and runs within the mesovarium from the hilus of the ovary towards the oviduct. Its tubules mostly end blind (Mossman & Duke, 1973; Kress & Millian, 1987).
The aim of this study is to report structural changes of the oviducal epithelium during the estrous cycle of Monodelphis and to correlate the findings with their possible oviducal functions and with observations obtained studying changes in the uterus and cervix (Wick & Kress, 2002a,b).
Material and methods
The animals used for this study were obtained from a breeding colony at the Biocenter of the University of Basel. Females were killed by the administration of an overdose of Vetanarcol (Veterinaria, Zürich, Switzerland) or with CO2 gas. The reproductive tract was fixed by perfusion or by immersion and processed immediately either for light, transmission or scanning electron microscopy.
A total number of 56 animals used were sexually mature and aged between 6 and 33 months (32 females were in metestrus, 5 females in pro-estrus, 10 females in estrus, 5 in early postestrus and 4 in late postestrus).
The estrous cycle stages were identified from vaginal histology by either paraffin or semithin sections and not from vaginal smears or the appearance of cornified epithelium in the urine. This procedure allows viewing the whole epithelium in the different vaginal areas than having to identify only shed cells.
Light microscopy: the oviducts were fixed in buffered formaldehyde, Bouin or Susa solution, embedded in paraffin wax for serial sections (5–7 µm) and stained with haematoxylin-eosin (HE) or periodic acid-Schiff stain (PAS). For identification of the cells which produce neutral or different acid glycoproteins, the sections were stained with Alcian blue (8GX Sigma A 3157) at pH 2.5.
Transmission electron microscopy: tissues were fixed either per perfusion or by immersion. The fixatives used were 2–2.5% glutaraldehyde and 1–2% paraformaldehyde in 0.1 M sodium cacodylate or 0.1 M Millonig buffer (Millonig, 1964 in Hayat, 1981) for 2 h at 4 °C. While placed in buffered glutaraldehyde, the different regions of the oviduct were divided (proximal part, close to the ovary, distal part adjacent to the uterus and uterotubal junction. Postfixation took place in 1% OsO4 for 2 h at 4 °C. Some material was fixed directly in 1% OsO4 for 2 h at 4 °C. After fixation, the tissues were rinsed in several changes of the corresponding buffer, dehydrated and embedded in Epon 812 (Balzers, Liechtenstein). Semithin sections were stained with phenylenediamin and ultrathin sections with uranyl acetate and lead citrate and viewed under a Philips 400 transmission electron microscope (Philips, Eindhoven, the Netherlands).
Scanning electron microscopy: for SEM a total of 10 animals were used. Fixation took place the same way as for transmission electron microscopy. While placed in buffered glutaraldehyde, the different regions of the oviduct were prepared.
After fixation the tissues were rinsed with several changes of the corresponding buffer, dehydrated in a graded series of acetone, critical point-dried in a Balzers CPD 30 and mounted on aluminium stubs. The specimens were coated with 17-nm gold. The blocs were viewed in a Philips XL 20 scanning electron microscope.
Results
Table 1 summarizes the presence of secretory granules and vesicles in the different oviducal segments during the estrous cycle and correlates light microscopical staining and electron microscopical structures.
Table 1.
Correlation between light-microscopical PAS/Alcian blue staining (LM) and electron microscopical (EM) structures of non-ciliated, secretory oviducal cells
| Oviducal segment | Proestrus | Estrus | Postestrus | Metestrus | |
|---|---|---|---|---|---|
| Fimbriae | LM | — | — | — | — |
| EM | sv + (+) | sv + (+) | sv + (+) | sv + (+) | |
| Ampulla | LM | + | +++ | ++ (+) | + (+) |
| EM | sg + | sg +++ | sg ++ (+) | sg + (+) | |
| Isthmus | |||||
| Surface epithelium | LM | — (+) | +++ | ++ (+) | — (+) |
| EM | sg — | sv, sg ++ | sv, sg + | − (+) | |
| Crypts | LM | — (+) | — (+) | — (+) | — |
| EM | sv − (+) | sv +++ | sv ++ | sv — (+) | |
| LM | — | — | — | — | |
| Epoophoron | |||||
| EM | sv ++ (+) | sv ++ (+) | sv ++ (+) | sv ++ (+) | |
Alcian blue and PAS more or less identical in LM staining intensity: — negative; + weak; ++ moderate; +++ strong (predominantly located in the apical cytoplasm).
EM structure: sv, small translucent vesicles; sg, secretory granules; — none, + few, ++/+++ numerous.
The preampulla forms tall slender folds or fimbriae (Fig. 1a), whose inner surfaces consist of ridges covered with epithelium. Some of these folds develop as a sac which encloses the ovary and forms a bursa ovarii (Fig. 1b). Bursae are highly variable in structure amongst the eutheria and marsupials observed (Mossman & Duke, 1973). In the case of Monodelphis it seems to form a complete sac around the ovary.
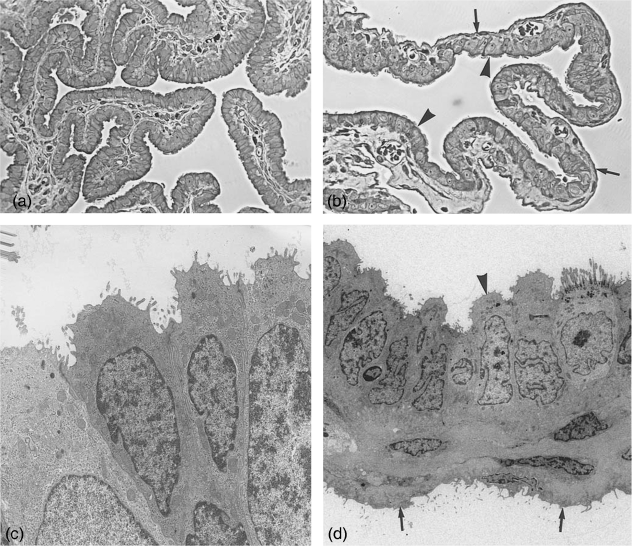
Fig. 1.
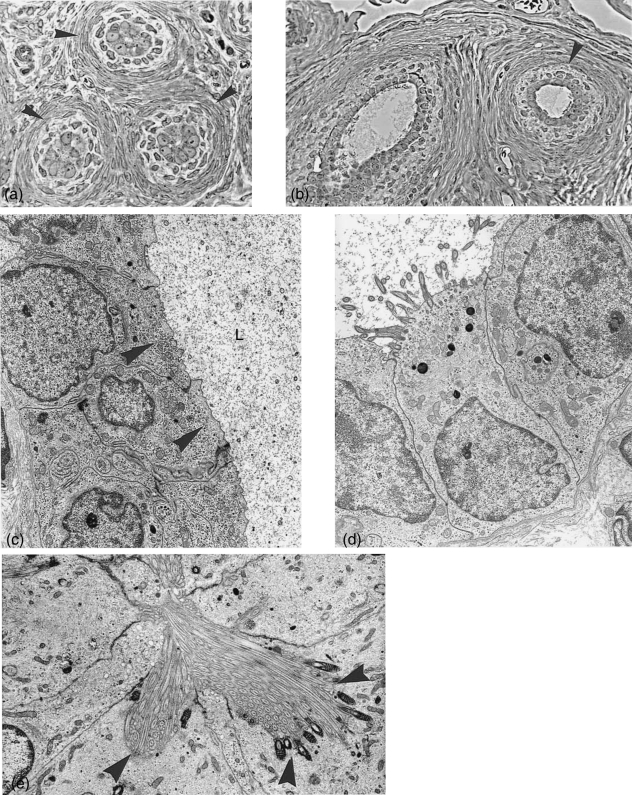
Fimbriae and bursa. (a) Semithin section through fimbrial folds at metestrus stage covered by ciliated and non-ciliated cells. ×245. (b) Semithin section through the bursa at estrus stage. Arrowheads, bursa epithelium with ciliated and non-ciliated cells; arrows, serosal epithelium. ×245. (c) Electronmicrograph of fimbrial epithelium at metestrus, depicting only non-ciliated cells. ×6100. (d) Bursa wall, with cuboidal bursal epithelium (arrowhead) and flat serosal epithelium (arrows) at metestrus. ×2300.
Light microscopy: the fimbriae, finger-like projections are covered by ciliated cells and in places by numerous non-ciliated cells. The non-ciliated cells appear negative in all stages of the estrous cycle after staining with Alcian blue and PAS (Fig. 2c). The transition into the infundibulum is not distinct; its structure resembles the appearance of the ampulla.
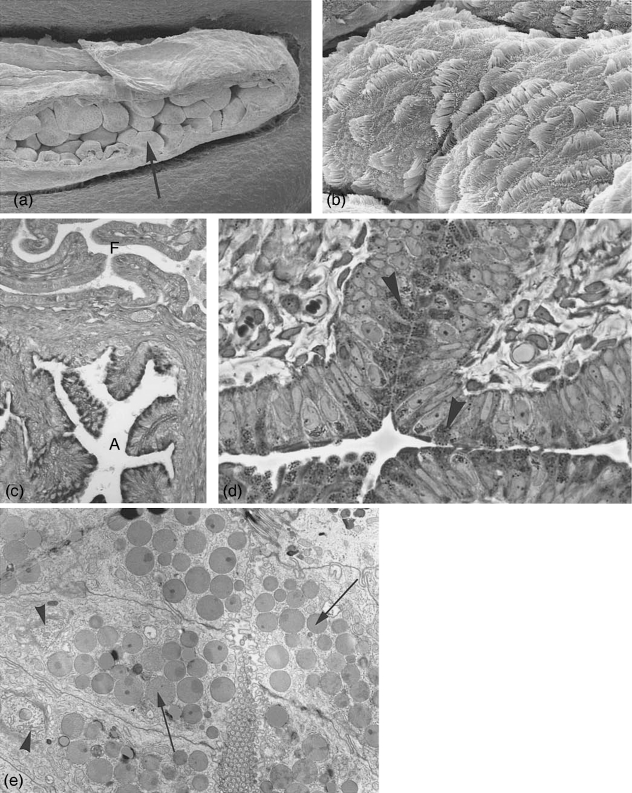
Fig. 2.
Ampulla at estrus. (a) Scanning electron microscopy. Overview of ampullar folds (arrow). ×560. (b) Folds covered with an epithelium consisting of ciliated and non-ciliated, secretory cells. ×7000. (c) Light microscopical section stained with PAS. Note the intensive positive reaction of the secretory cells in the ampulla (A) and the negative reaction of the fimbrial epithelium (F) where no staining products could be detected. ×145. (d) Semithin section with ampullar epithelium exhibiting non-ciliated, secretory cells, densely packed with secretory granules (arrowheads) at estrus. ×615. (e) Electronmicrograph of secretory cells at estrus, filled with secretory granules often of heterogeneous density (arrows) and highly active Golgi complexes (arrowheads). ×6400.
Electron microscopy: the fimbriae carry a columnar or pseudostratified epithelium, formed of ciliated cells and non-cilliated cells. Among the cilia microvilli are interspersed. Non-ciliated cells appear often in small groups and carry numerous microvilli on their apices (Fig. 1c). Golgi apparatus are small but actively producing small translucent vesicles which appear in the cell apex. These vesicles obviously indicate a secretory activity but do not stain with PAS or Alcian blue (Fig. 2c). Ribosomes, polyribosomes and rough endoplasmic reticulum, as well as occasional small lysosomes, are regular features. The amount of vesicles seems similar during all stages of the estrous cycle. Only a small number of ciliated cells exhibit deciliation during metestrus and occasional neo-ciliogenesis during proestrus.
The bursa is composed of three layers: (1) the bursal epithelium facing the ovary; (2) connective tissue layers; (3) the peritoneal or serosal epithelium (Fig. 1b,d).
The inner lining of the bursa, which faces the ovary, consists of cuboidal, predominantly non-ciliated cells with some ciliated cells interspersed (Fig. 1b,d). The non-ciliated cells display small Golgi complexes producing small translucent vesicles. The amount of vesicles differs from cell to cell and shows no direct correlation with the cycle stage. Small lysosomes and individual necrotic cells are also present. The outer epithelium, facing the peritoneal cavity, is formed by a flat, elongated serosal epithelium (Fig. 1d).
Ampulla
The ampulla, where fertilization occurs in most eutherian mammals and marsupials, passes at its end without sharp demarcation into the convoluted isthmus.
The term ampulla, which describes the thicker portion of the oviduct adjacent to the ovary in eutherian mammals, does not accurately describe the same shape in marsupials. In the brushtail possum and Antechinus oviduct, the ampulla is actually narrower in diameter than the isthmus (Arnold & Shorey, 1985; Taggart, 1994). In Monodelphis both segments appear about the same diameter, the ampulla with wider lumen, higher plicae but a thinner wall, the isthmus with narrower lumen, lower plicae but a thicker wall. The terms ampulla and isthmus will be used in this paper for clarity, as proposed by Sidhu et al. (1999).
The mucosa of the ampulla is deeply folded into a complex system of plicae (Fig. 2a). The folds continue throughout the ampulla and the upper isthmus although the folds gradually decrease in height and complexity.
Light microscopy: in proestrus, secretory cells only occasionally show a slightly positive staining reaction. In estrus (Fig. 2c,d) and postestrus (Fig. 3a) numerous distinct positive secretory granules within these cells stain, while in metestrus only a very reduced number of faintly staining secretory cells can be found.
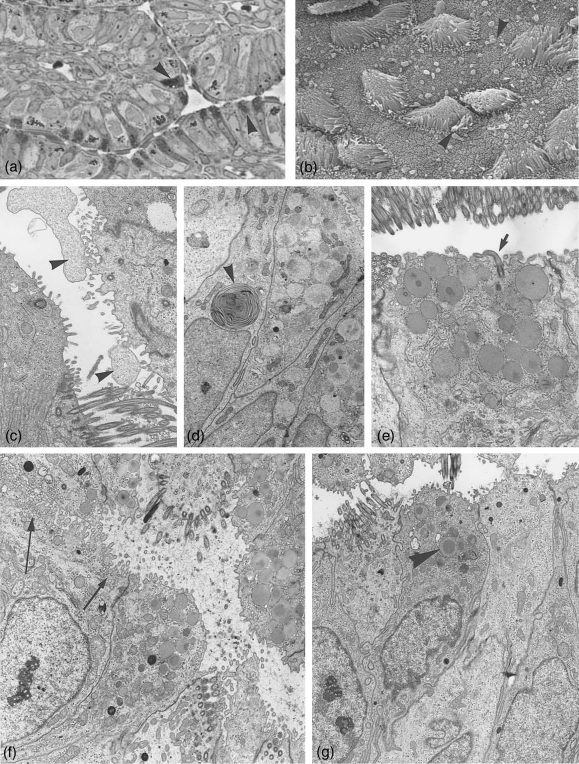
Fig. 3.
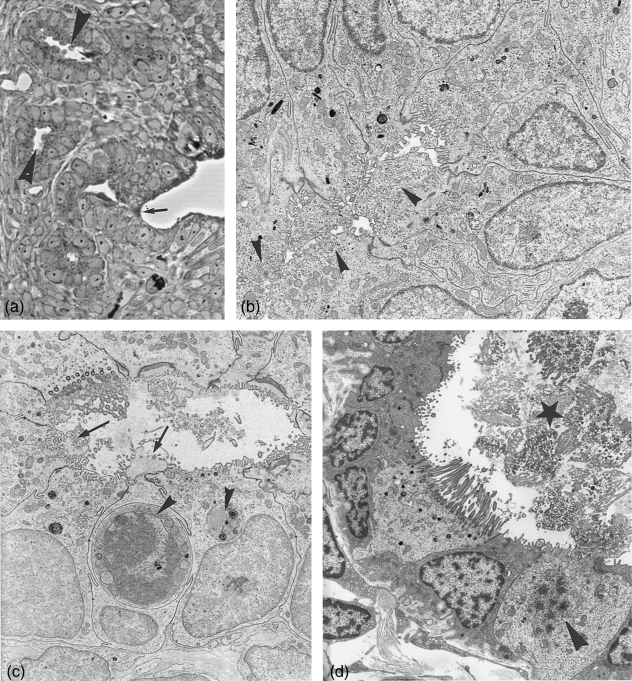
Ampulla at post-and metestrus. (a) Semithin section through ampullar epithelium at postestrus stage. The number of secretory active cells (arrowheads) and the amount of secretory granules per cell are reduced. ×500. (b) Scanning electronmicrograph showing postestrus stage with extruded secretory material (arrowheads), partially membrane bound after apocrine secretion. ×3200. (c–g) Electronmicrographs. (c) Non-ciliated cell with apocrine cytoplasmic exocytosis (arrowheads). ×3900. (d) Secretory products in degradation in form of whorls (arrowhead). ×4700. (e) Section through a secretory cell at postestrus still hosting numerous secretory granules, one cell carrying a solitary cilium (arrow). ×8000. (f) Some of the non-ciliated cells show apically numerous translucent small vesicles (arrows) beside few secretory granules. ×6400. (g) Ampullar epithelium at metestrus, occasionally a few remnant secretory granules are left (arrowhead). ×5000.
Electron microscopy: during each reproductive cycle the cytoplasm of the non-ciliated cells undergoes major structural changes. Ciliated and non-ciliated cells are alternating or sometimes found in small groups.
During the proestrus stage the non-ciliated cells dominate, carry mostly solitary cilia and contain only occasional few remnant secretory granules but a considerable amount of lysosomes.
In preparation for estrus, a succession of assembling steps for secretory granule formation in non-ciliated cells takes place and an increasing amount of cells with ciliogenesis can be observed.
During estrus ciliated cells are numerous (Fig. 2b) and contain highly active Golgi complexes, numerous mitochondria, a high density of free ribosomes and numerous electron-dense lysosomes. Occasionally ciliated cells can be observed still including a few secretory granules below the basal bodies of the cilia. This indicates a previous change from the secretory to the ciliated cell type as already described for the Monodelphis cervix (Wick & Kress, 2002b).
Most of the secretory cells exhibit during estrus supranuclear highly active Golgi complexes and an accumulation of numerous more or less electron-dense secretory granules up to diameters of 1.3 µm (Fig. 2d,e). Some of the secretory granules seem to form by the coalescing of small precursor vesicles. With the increase of the number of secretory granules, the nucleus is occasionally deplaced more basally. The cell apices are mostly dome-shape, carrying distinct microvilli covered with a thick glycocalyx.
During postestrus the whole ampular epithelium shows first signs of decomposition. Secretory cells (Fig. 3a) are in the process of exocytosis, the discharge products, secretory granules and cytoplasm are often seen with an encompassing membrane (Fig. 3b,c). Some secretion fixed in the tubal lumen is depicted in Figure 3(b). The remnant secretory granules show alterations, as more differing density within the same granule, more coalescing granules or formation of small and large whorls (Fig. 3d). Golgi complexes are still prominent. An increasing number of autophagocytotic vacuoles and aptototic processes become visible. Some cells develop new solitary cilia (Fig. 3e).
Besides the secretory granule containing cells, a number of non-ciliated cells with apices full of translucent small vesicles are present (Fig. 3f), probably producing a different product than cells forming secretory granules.
Ciliated cells contain numerous dense lysosomes and whorls as found in secretory cells at postestrus. Deciliation processes start, sometimes cilia are still attached to the cell but are already in dissolution.
In metestrus the ampullar lumen is full of cilia and pinched off cytoplasm in decomposition as found in the isthmus (Fig. 4d). The luminal content is disarranged and in a shambles. The material disintegrates into vesicle-like structures. A considerable amount of secretory granules may be left in secretory cells, while other cells appear empty (Fig. 3g). Apoptotic processes can be recognized and lysosomes are numerous. A massive de- and reconstruction is under way. The first signs of neo-ciliogenesis can be seen, the same time as in the isthmus (Fig. 4e).
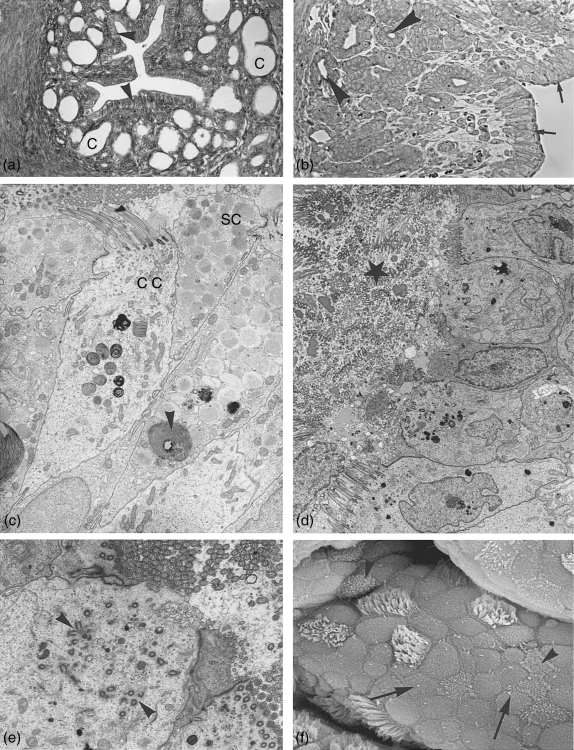
Fig. 4.
Isthmus. (a) Light microscopy: cross section through the isthmus region close to the uterus showing surface epithelium (arrowheads) and crypts (C). ×165. (b) Semithin section through the isthmus region with surface epithelium (arrows) and crypts (arrowheads). ×250. (c–f) Isthmus surface epithelium. (c) Electron microcoscope pictures of isthmus surface epithelium at postestrus stage with ciliated cell (cc) and non-ciliated secretory cells (sc) still containing numerous secretory granules. Degeneration processes (whorls) have started (arrowheads). ×4500. (d) Lumen at metestrus filled with massive deciliation and secretion products (star). Hardly any secretory granules are left in the secretory cells. ×3300. (e) At the same time ciliogenesis has started. Note the multiplication of basal bodies moving to the apical surface (arrowheads). ×6900. (f) Scanning electron microscope: isthmus surface at late metestrus/early proestrus showing numerous non-ciliated cells with solitary cilia (arrows) and ciliated cells exhibiting neo-ciliogenesis at different stages (arrowheads). ×7200.
Isthmus
In marsupials the isthmus is tortuous and has a wide lumen which narrows down at the entry of the uterus. By contrast to the ampulla the mucosa of the isthmus is less intensively folded, but the transition in the surface epithelium is difficult to detect. In the lower isthmus the elaborate folds give way to knob-like protrusions of the mucosa.
The isthmus is lined by a high columnar epithelium consisting of ciliated and secretory cells and exhibits crypt-like invaginations into the dense connective tissue (Figs 4a,b, 5a). In the upper isthmus the number of crypts is small and their depth not very pronounced, in the lower isthmus the number and depth of the crypts increase.
Fig. 5.
Isthmus crypts. (a) Semithin section, invagination of the surface epithelium (arrow) into crypts. Crypt epithelium consists at estrus mainly of secretory cells with bulging apices (arrowheads). ×310. (b) Electron microscopy: crypt cells at estrus. Secretion products exist in numerous translucent vesicles located in bulging cell apices (arrowheads). ×6120. (c) Crypt cells at late postestrus shedding apices and cilia (arrows). Small secretory vesicles can still be found in individual cell apices. Lysosomes and aptotosic cells are typical (arrowheads). ×4200. (d) Crypt at metestrus filled with shed cilia (star), cell with signs of neo-ciliogenesis (arrowhead). ×4300.
Light microscopy: while during proestrus only a few slightly positive secretory cells can be detected, in estrus many secretory cells appear positively stained in the surface epithelium, while staining in the crypts is rare. In postestrus a similar picture as in the estrus is found. In metestrus the picture changes; only secretory cell apices in the surface epithelium react slightly, while crypts react negatively in almost all cases. The picture is highly variable even within the same specimen.
Electron microscopy: in proestrus the surface epithelium shows areas where non-ciliated cells have apices carrying microvilli which are covered by a distinct glycocalyx. Some cells still contain large lysosomes, and secretory granules have not yet developed. Rough endoplasmic reticulum, free ribosomes and occasionally Golgi complexes and vesicles of different sizes are noticeable. Some cells show distinct ciliogenesis. The lateral walls are strongly interdigitated.
In estrus stage the surface epithelium consists of numerous secretory cells with dome-shaped apices and secretory granules which resemble those in the ampulla (Fig. 2e). Many ciliated cells contain numerous lysosomes and even secretory granules. Occasional cells with neo-ciliogenesis are still under way and a remnant sperm is still attached to the surface epithelium.
During postestrus extruded secretion and apocrine elimination of surface cell apices as shown for the ampulla (Fig. 3b) can be found fixed in the isthmus lumen. Through this elimination, cells contain different amount of secretory granules (Fig. 4c). Some non-ciliated surface cells contain only numerous small, clear vesicles as already observed in the ampulla (Fig. 3f).
The ciliated cells show the first signs of deciliation, the cilia and apices forming packets as seen in the cervix (Wick & Kress, 2002b).
During metestrus lots of detached cilia and a different amount of cell material in decomposition are characteristic in the isthmus lumen (Fig. 4d). The number of ciliated cells looks therefore reduced. Many of the secretory cells still contain apically numerous secretory granules. Towards the late metestrus, many surface cells still contain numerous secretory granules, while others don't. The deciliation process is still under way but occasionally neo-ciliation is already visible (Fig. 4e,f). Solitary cilia increase in number in late metestrus/early proestrus (Fig. 4f) and follow the same pattern as described in the uterus (Wick & Kress, 2002a).
Crypts in proestrus consist predominantly of cells with microvilli, numerous small lysosomes, and irregularly shaped apices with no obvious secretory granules but with small translucent vesicles.
During estrus, crypts exhibit fewer ciliated cells (unlike the uterine glands) (Fig. 5a,b). In contrast to the surface epithelium, non-ciliated, secretory cells produce and store no typical secretory granules, but the cells are crowded with small translucent vesicles and have dome-shaped apices (Fig. 5a,b). Highly active Golgi complexes seem to produce these vesicles. Extended interdigitations are visible between the cuboidal crypt cells, and individual solitary cilium can be found.
During postestrus the crypts (Fig. 5c) look very different from the isthmus surface epithelium. Only occasionally are ciliated cells present. Non-ciliated cells still contain a high number of small, clear vesicles in the cell apex. Exocytosis must occur but cannot be demonstrated. First signs of autophagic vacuoles, apoptosis and decomposition become obvious (Fig. 5c).
During metestrus crypts are lined by cuboidal cells mostly carrying microvilli containing a reduced number of small vesicles. The crypt lumen may be filled with some of the pushed-off cilia and cytoplasmic debris (Fig. 5d), as found in the isthmus lumen.
Uterotubal junction: the uterotubal junction connects the isthmus of the oviduct with the proximal part of the uterus. In metestrus and proestrus, when the uterus is of a fusiform shape, the uterotubal junction runs straight from the anterior extremety of the uterus into the twisting oviduct. In estrus, when the uterus becomes highly oedematous, the anterior uterine zone bends and the uterotubal junction lies at a right angle to the uterus.
In eutherians, it seems that the utero-tubal junction forms a barrier to sperm migration up the female tract (Hook & Hafez, 1968; Hunter, 1988). In marsupials, the timing and success of sperm transport in Didelphidae and Dasyuridae to the isthmic portion of the oviduct suggest that there is little if any barrier to sperm migration (Taggart, 1994).
The diameter of the lumen narrows, and the number of grooves and furrows is reduced, but some extend slightly into the uterine lumen and the surrounding wall is highly muscular. No special folds or sphincter could be observed.
The junction represents a transitional zone where structural characteristics of the mucosa of the isthmus and the uterus mingle.
The non-ciliated cells of the luminal epithelium of the junction resemble those of the uterine epithelium with no special staining properties of the secretory cells with regard to PAS or Alcian blue staining. Crypts are embedded in dense connective tissue while uterine glands are surrounded by loose connective tissue containing numerous macrophages (Wick & Kress, 2002a). The secretory material in the epithelial cells of the uterotubal junction and in some adjacent endometrial glands does not appear different from the morphological point of view and is said to give rise to the shell coat, the outer of the two extracellular coats which surround the oocyte and early embryos (Roberts et al. 1994; Selwood, 2000).
Epoophoron
The epoophoron is lined by cuboidal cells (Fig. 6a,b) which light microscopically show no reaction properties with PAS or Alcian blue.
Fig. 6.
Epoophoron. (a) Cross section (semithin) through epoophoron tubules with narrow lumen and distinct connective tissue layers (arrowheads) surrounding the epithelium. ×450. (b) Semithin sections through tubules with wider lumina lined with ciliated and non-ciliated cells. Both figures are taken from the same animal at metestrus stage. ×300. (c) Electron microscopy: non-ciliated epithelium lining the epoophoron tubules at metestrus, with numerous small vesicles at the cell apices (arrowheads) and secretion product in the lumen (L). ×6900. (d) A ciliated cell of the same tubule. ×6050. (e) A different type of ciliated cell, where cilia sit in deep invaginations with pronounced basal bodies (arrowheads). ×7100.
The tubules have been distinguished from other tubular structures by their surrounding layers of dense connective tissue (Fig. 6a,b). Typical smooth muscle cells could not be identified. The tubules are not of uniform width and the reconstruction of a possible connection with the bursa or the oviduct has not been attempted and is therefore not certain. The tubules comprise often a narrow hardly, visible lumen, but occasionally they are wider and filled with homogeneous ‘liquid’ or ‘debris’ (Fig. 6b,c). Tubules may have extended areas with no ciliated cells, other areas show both cell types, ciliated and non-ciliated (Fig. 6c,d). Their apices contain small Golgi complexes, occasionally coated vesicles, numerous ribosomes and small lysosomes and often numerous small vesicles (Fig. 6c) but no secretory granules. Solitary cilia are rare.
Some of the ciliated cells seem to form extra-long cilia sitting in a sort of pocket and forming deep roots (Fig. 6e) a type of cilia which has not been seen otherwise in the oviduct.
No clear cycle-related changes have been observed in the epoophoron.
Discussion
The ovaries of many mammals lie within a membranaceous sac, the bursa ovaricae which partly or completely isolates the ovary from the peritoneal cavity (Mossman & Duke, 1973). The function seems to lie in its aid in collection of oocytes by the oviduct and may be necessary for normal ovarian development (Martin et al. 1981). In the golden hamster Martin et al. (1981) and Nakatani et al. (1986) describe a bursa with an inner discontinuous bursal epithelial layer resting on a basal lamina that faces the ovary, while in Monodelphis the bursa is lined by a continuous cuboidal or cylindrical epithelium ciliated and non-ciliated, the latter accumulating numerous small electron-lucent vesicles. They presumably contribute to the bursal fluid which forms the microenvironment for the ovum when it leaves the follicle as described in rats (Shalgi et al. 1977). In marsupials the ovum traverses the bursa without cumulus oophorus cells. No noticeable changes in the bursal cells of Monodelphis have been found during the estrous cycle.
Only few papers have been devoted to the study of the epoophoron in general (Mossman & Duke, 1973), in insectivores (Feremutsch & Strauss, 1949; Kress & Milian, 1987) or in humans (Beltermann, 1964). Its function is still unknown, but secretory activities have been described in the above mentioned papers. The numerous electron-lucent vesicles in the epoophoron cells of Monodelphis indicate similar activities.
Solitary cilia have been described in the oviduct of humans (Hagiwara et al. 2002), in macaca (Rumery et al. 1978) and in rabbits (Odor & Blandau, 1985). In macaca and rabbits solitary cilia are prominent at late lutealphase or in ovariectomized animals. Experiments have shown that rising levels of estrogen suppress the formation of solitary cilia in the uteri (Tachi, 1984). Solitary cilia have been found in the uteri during postnatal development in mice (Plapinger, 1982) or Mongolian gerbils (Kress & Mardi, 1990). They disappeared at sexual maturity, in eutheria, solitary cilia are rarely seen in actively cycling animals.
It is of interest that in contrast to eutheria, the critical requirements for the formation of solitary cilia seem to be maintained in the oviduct and uteri during all stages of the cycling marsupial, but in varying numbers. In Monodelphis solitary cilia are most conspicuous and numerous in met- and proestrus, but less noticeable at estrus, when according to Harder & Jackson (1999) and Jackson & Harder (2000) estrogen level is highest.
The immotile solitary or primary cilia have previously been considered as ‘vestigial’ organelles. But they seem to be part of a surveillance system that may respond to discrete cellular activities and may be involved in mechanosensory and chemosensory activities (Wheatley, 2004; Praetorius & Spring, 2005).
The complex biochemical and physiological microenvironment of the oviduct is considered to be important in the successful survival and transport of gametes, fertilization and early embryonic development in mammals, including marsupials (Sidhu et al. 1999).
Rapid tubal transport is a characteristic of all the marsupial species investigated so far (Selwood, 2000). Such rapid transport implies a high synchronization of the events at ovulation and fertilization. The timing of mating and the transport of spermatozoa up the oviduct must ensure that spermatozoa reach the oocyte before mucin deposition.
In the dasyurids and didelphids examined, the upper segment in which fertilization occurs is as narrow or even narrower than the isthmus, a marked contrast to the swollen ampulla that generally characterizes the eutheria oviduct (Bedford, 1994, 1996). The absence of a cumulus oophorus is consistent with the narrow lumen of the upper oviduct in marsupials. The cumulus-free eggs, grown to a greater diameter than eutherian eggs, match the dimensions of the narrow region of the oviduct where fertilization occurs (Bedford, 2004).
Two types of cells are present in all the oviducal epithelium described so far, ciliated and non-ciliated, secretory cells. The ratio of these two cell types changes during the reproductive cycle under the influence of sexual hormones. The greatest density of fully developed ciliated cells is reached in the estrus period, influenced by estrogen. This phenomenon has been studied for eutheria in Macaca nemestrina (Rumery et al. 1978; Odor et al. 1980, 1983; Hagiwara et al. 2004) and rat (Reeder & Shirley, 1999) and for marsupials in the brush-tailed possum, Trichosurus (Arnold & Shorey, 1985). The general results correspond with the findings in Monodelphis.
In Monodelphis the oviducal epithelium undergoes distinct morphological changes, among which are the ciliation and deciliation process. Deciliation has been observed in late estrus and postestrus and occurs by shedding off cilia or by pinching off membrane bound cilia packets containing numerous cilia from the cell apices, as described for the rat oviduct by Reeder & Shirley (1999). In Monodelphis, early ciliogenesis begins in metestrus but is best seen in proestrus, by numerous newly formed precentrioles and basal bodies randomly arranged in the apical portion of, at that time, non-ciliated cells. Later, the basal bodies become aligned below the apical membrane and ciliary shafts are budding into the oviductal lumen (Fig. 4f).
Ciliation and deciliation of the oviducal epithelium constitute a continuous, hormonally controlled process throughout the estrous cycle (Quarmby, 2004). The present findings indicate that the process of deciliation is an ongoing one, that merely changes in rate.
Reeder & Shirley (1999) suggested that the epithelial cells in the rat oviduct may transform from one functional cell type to another, which is supported by the fact that ciliated and secretory cells alternately increase and decrease in number without evidence of much mitotic activity in either cell type. The appearance of secretory granules in ciliated cells and the phenomenon of deciliation and reciliation in the oviducal epithelium in M. domestica have led to the conclusion that these cells have the above mentioned potential, as found in the cervix also (Wick & Kress, 2002b). The ‘pinching off’ of whole clusters from the ciliated cells appears to be a rapid mechanism of deciliation, a process which can occur even when estrogen level is still high (Reeder & Shirley, 1999).
The non-ciliated cells are primarily secretory elements. In Monodelphis, according to the cycle stage and localization many secretory cells assume a goblet shape with the apical portion packed with granules; others develop only numerous electron-lucent vesicles.
M. domestica secretory granules of the oviduct mentioned so far (Philips & Fadem, 1987; Baggot & Moore, 1990) contain a slightly electron-dense centre surrounded by a less electron-dense region, these descriptions correspond with our findings.
Differences in the expression of secretory products in the infundibulum, ampulla and isthmus have been demonstrated in many eutherians (Jansen, 1995). These differences may partly show in a varying morphology of the secretory granules depending on the oviducal area as shown in macaca (Odor et al. 1983) or the golden hamster (El-Mestrah & Kan, 1999) where two types of secretory granules, some with internal lamellae-like structures, were found. The secretory granules in the Monodelphis oviduct did not exhibit morphological differences between ampulla and isthmus.
The oviduct fluid is a complex mixture of selective serum transudates and specific secretory products from the oviduct epithelium that differs among species (Sidhu et al. 1999). Several oviduct expressed proteins have been identified and characterized including the oviduct-specific estrogen-dependent glycoprotein (OGP) in several eutherian species (Buhi, 2002). Such an analysis is still lacking for marsupials. Electron-lucent vesicles found in numerous non-ciliated cells (Fig. 3f) may contribute towards this oviducal fluid.
In some mammals the glycoprotein and proteins secreted have been shown to associate with the ovulated ova and developing embryo (Abe 1994; Malette et al. 1995). In marsupials examined, in addition to the zona pellucida, a mucoid coat is acquired immediately after fertilization (Selwood, 2000; Zeller & Freyer, 2001; Bedford, 2004). In Monodelphis, deposition of the mucoid layer which consists of PAS-positive mucoproteins, begins in the ampulla during penetration of the zona by the sperm, and is secreted by the non-ciliated, secretory PAS-positive cells. Deposition is often asymmetrical (Baggot & Moore, 1990). The zygote is then transported rapidly through the oviduct accompanied by further formation of mucoid layers. A similar coat is known from rabbits and horses (Van Blerkom & Motta, 1979). A review about mucoid coats in marsupial is published by Selwood (2000).
Since mammalian sperm resides in the female genital tract for hours or days, the isthmic region of the oviduct appears to have a special responsibility for sperm. The storage has to maintain sperm viability and save energy. The interaction between sperm and the somatic cells of the oviduct has a synchronizing and coordinating effect to ensure the meeting of competent gametes (Töpffer-Petersen, 2002).
The isthmus displays a series of crypts that function as specialised prefertilization sperm storage site, a feature seen in some shrews and other mammals (Hunter et al. 1991; Bedford 1996, 2004; Bedford et al. 1997). The question of which cell type spermatozoa are attached remains open (Hunter et al. 1991); both possibilities, attachment to ciliated cells and attachment to secretory cells, have been described (Smith, 1998). In at least some of the marsupials it is thought that sperm is not attached to the epithelium in the pockets but simply trapped in mucus (Suarez, 2001).
In dasyurids and didelphid marsupials including M. domestica differentiated crypts in the isthmus of the oviduct house spermatozoa until ovulation (Breed et al. 1989; Rodger, 1991; Taggart & Temple-Smith 1991; Shimmin et al. 1999).
Breed et al. (1989) describe for Sminthopsis crassicornis that the isthmus surface lining contains ciliated and secretory cells, similar to the ampulla, while the isthmic crypts are lined by a different epithelium of low columnar non-ciliated cells with deeply staining apical granules. These cells are devoid of granules after sperm has left and the zygote passed. In S. crassicaudata sperm can be stored for three days (Selwood, 1987).
In M. domestica, however, where potential sperm storage time is short (Selwood et al. 1997), the secretory cells of the crypts were devoid of any secretory granules, but full of electron-lucent small vesicles, with few staining properties (Table 1). The chemical composition of this secretion product has yet to be established.
The distal part of the Monodelphis oviduct forming the utero-tubal junction shows isthmic crypts embedded in dense connective tissue mingled with the transition into proximal uterine epithelium and convoluted uterine glands which are surrounded by loose connective tissue and numerous macrophages as already mentioned by Wick & Kress (2002a).
The outermost envelope is the shell that encloses all marsupial embryos until prior to implantation. Various sites have been made responsible for the secretion of shell material, namely the lower oviduct and endometrial glands (Roberts et al. 1994). But it seems likely that the secretory cells responsible for shell formation reside solely in the endometrium (Selwood, 2000; Casey et al. 2002). From a morphological point of view, in Monodelphis crypts and uterine gland cells exhibit electron-lucent vesicles of similar appearance.
Conclusions
Further studies are needed to determine the exact chemical composition of the secretory granules and the content of the electron-lucent vesicles located in the isthmus and crypts, in order to evaluate their contribution in establishing the different marsupial egg coats in detail and for the maintenance of an appropriate sperm and conceptus environment.
The presence of solitary cilia at the apex of non-ciliated oviducal cells during most of the time of the cycle indicates a different hormonal situation in marsupials compared with eutherians. This long-ignored structure needs further research in order to reveal its possible functions.
Acknowledgments
The authors wish to thank Professor U.M. Spornitz for his support, Mrs M. Bäschlin and H. Schaller for skilful technical assistance with the light and electron microscopy and Mrs S. Hosch for secretarial assistance. Professor Lynne Selwood kindly helped with a critical reading of the manuscript. The study was supported by the Emilia Guggenheim-Schnurr Foundation, Basel.
References
- Abe H. Regional variations in the ultrastructural features of secretory cells in the rat oviductal epithelium. Anat Rec. 1994;240:77–85. doi: 10.1002/ar.1092400108. [DOI] [PubMed] [Google Scholar]
- Andersen DH. Comparative anatomy of the tubo-uterine junction. Histology and physiology in the sow. Am J Anat. 1928;42:255–305. [Google Scholar]
- Armati-Gulson P, Lowe J. Histology and scanning electron microscopy of the development of the reproductive tract of the common ringtail possum Pseudocheirus peregrinus Pseudocheiridae: Marsupialia. Aust Mammalogy. 1985;8:97–109. [Google Scholar]
- Arnold R, Shorey CD. Structure of the oviducal epithelium of the brushtailed possum Trichosurus vulpecula. J Reprod Fert. 1985;73:9–19. doi: 10.1530/jrf.0.0730009. [DOI] [PubMed] [Google Scholar]
- Baggot LM, Moore HD. Early embryonic development of the grey short-tailed opossum, Monodelphis domestica, in vivo and in vitro. J Zool Lond. 1990;222:623–639. [Google Scholar]
- Bedford JM. The contraceptive potential of fertilization: a physiological perspective. Human Reprod. 1994;9:842–858. doi: 10.1093/oxfordjournals.humrep.a138604. [DOI] [PubMed] [Google Scholar]
- Bedford JM. What marsupial gametes disclose about gamete function in eutherian mammals. Reprod Fert Develop. 1996;8:569–580. doi: 10.1071/rd9960569. [DOI] [PubMed] [Google Scholar]
- Bedford JM. Enigmas of mammalian gamete form and function. Biol Rev. 2004;79:429–460. doi: 10.1017/s146479310300633x. [DOI] [PubMed] [Google Scholar]
- Bedford JM, Philips DM, Mover-Lev H. Novel sperm crypts and behaviour of gametes in the fallopian tube of the white-toothed shrew, Crocidura russula Monacha. J exp Zool. 1997;277:262–273. doi: 10.1002/(sici)1097-010x(19970215)277:3<262::aid-jez7>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Bedford JM, Breed WG. Regulated storage and subsequent transformation of spermatozoa in the Fallopian tubes of an Australian marsupial, Sminthopsis crassicaudata. Biol Reprod. 1994;50:845–854. doi: 10.1095/biolreprod50.4.845. [DOI] [PubMed] [Google Scholar]
- Beltermann R. Elektronenmikroskopische Untersuchungen am Epoophoron des Menschen. Arch Gynäkol. 1964;200:275–284. doi: 10.1007/BF00670840. [DOI] [PubMed] [Google Scholar]
- Breed WG, Leigh CM, Bennett JH. Sperm morphology and storage in the female reproductive tract of the fat-tailed dunnart, Sminthopsis crassicaudata Marsupialia: Dasyuridae. Gamete Research. 1989;23:61–75. doi: 10.1002/mrd.1120230107. [DOI] [PubMed] [Google Scholar]
- Buhi WC. Characterization and biological roles of oviduct-specific, oestrogen-dependent glycoprotein. Reproduction. 2002;123:355–362. doi: 10.1530/rep.0.1230355. [DOI] [PubMed] [Google Scholar]
- Casey NP, Martinus R, Selwood L. Outer egg coats of the marsupial conceptus: secretion and protein composition. Mol Reprod Dev. 2002;62:181–194. doi: 10.1002/mrd.10073. [DOI] [PubMed] [Google Scholar]
- El-Mestrah M, Kan FWK. Ultrastructural and ultracytochemical features of secretory granules in the ampullary epithelium of the hamster oviduct. Anat Rec. 1999;255:227–239. doi: 10.1002/(SICI)1097-0185(19990601)255:2<227::AID-AR12>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- Feremutsch K, Strauss F. Beitrag zum weiblichen Genitaltrakt der madagassischen Centetinen. Rev Suisse Zool. 1949;56:2–106. [Google Scholar]
- Hagiwara H, Ohwada N, Takata T. Cell biology of normal and abnormal ciliogenesis in the ciliated epithelium. Int Rev Cytol. 2004;234:101–141. doi: 10.1016/S0074-7696(04)34003-9. [DOI] [PubMed] [Google Scholar]
- Hagiwara H, Harada S, Maeda S, Aoki T, Ohwada N, Takata K. Ultrastructural and immunohistochemical study of the basal apparatus of solitary cilia in the human oviduct epithelium. J Anat. 2002;200:89–96. doi: 10.1046/j.0021-8782.2001.00004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harder JD, Jackson LM. Opossums. In: Knobil E, Neill JD, editors. Encyclopedia of Reproduction. San Diego: Academic Press; 1999. pp. 515–523. [Google Scholar]
- Hartman CG. The oestrus cycle in the opossum. Am J Anat. 1923;32:353–421. [Google Scholar]
- Hayat MA. Fixation for Electron Microscopy. New York: Academic Press; 1981. p. 51. [Google Scholar]
- Hook SJ, Hafez ESE. A comparative anatomical study of the mammalian uterotubal junctions. J Morph. 1968;125:159–184. doi: 10.1002/jmor.1051250204. [DOI] [PubMed] [Google Scholar]
- Hunter RHF. Their Role in Fertility and Infertility. New York: Springer-Verlag, Berlin, Heidelberg; 1988. The Fallopian tubes. [Google Scholar]
- Hunter RHF, Fléchon B, Fléchon JE. Distribution, morphology and epithelial interactions of bovine spermatozoa in the oviduct before and after ovulation: a scanning electron microscope study. Tissue and Cell. 1991;23:641–656. doi: 10.1016/0040-8166(91)90020-t. [DOI] [PubMed] [Google Scholar]
- Jackson LM, Harder JD. Evidence for spontaneous postlactational estrus In gray short-tailed opossums Monodelphis domestica. Biol Reprod. 2000;62:1823–1827. doi: 10.1095/biolreprod62.6.1823. [DOI] [PubMed] [Google Scholar]
- Jansen RPS. Ultrastucture and histochemistry of acid mucus glycoproteins in the estrous mammal oviduct. Microscopy Res Tech. 1995;32:29–49. doi: 10.1002/jemt.1070320104. [DOI] [PubMed] [Google Scholar]
- Kress A, Millian J. The female genital tract of the shrew Crocidura russula. Adv Anat Embryol Cell Biol. 1987;101:1–76. doi: 10.1007/978-3-642-71479-5. [DOI] [PubMed] [Google Scholar]
- Kress A, Mardi L. Postnatal development of the Mongolian gerbil uterus. Acta Anat. 1990;137:234–240. doi: 10.1159/000146825. [DOI] [PubMed] [Google Scholar]
- Mackay S, Xie Q, Ullmann SL, Gilmore DP, Payne AP. Postnatal development of the reproductive system in the grey short-tailed opossum, Monodelphis domestica. Anat Embryol. 2004;208:121–133. doi: 10.1007/s00429-004-0386-1. [DOI] [PubMed] [Google Scholar]
- Malette B, Paquette Y, Merlen Y, Bleau G. Oviductins possess chitinase-and mucin-like domains: a lead in the search fort he biological function of these oviduct-specific zp-associating glycoproteins. Mol Reprod Dev. 1995;41:384–97. doi: 10.1002/mrd.1080410315. [DOI] [PubMed] [Google Scholar]
- Martin G, Sack M, Talbot P. The structure of bursae ovaricae surrounding the ovaries of the golden hamster. Anat Rec. 1981;201:485–498. doi: 10.1002/ar.1092010306. [DOI] [PubMed] [Google Scholar]
- Mossman WH, Duke KL. Comparative Morphology of the Mammalian Ovary. London: University of Wisconsin; 1973. [Google Scholar]
- Nakatani T, Shinohara H, Matsuda T. On the ovarian bursa of the golden hamster. II Intercellular connections in the bursal epithelium and passage of ferritin from the cavity into lymphatics. J Anat. 1986;148:1–12. [PMC free article] [PubMed] [Google Scholar]
- Odor D, Gaddum-Rosso LP, Rumery RE, Blandau RJ. Cyclic variations in the oviductal ciliated cells during the menstrual cycle and after estrogen treatment in the pig-tailed monkey, Macaca nemestrina. Anat Rec. 1980;198:35–57. doi: 10.1002/ar.1091980104. [DOI] [PubMed] [Google Scholar]
- Odor D, Gadum-Rosse P, Rumery RE. Secretory cells of the oviduct of the pig-tailed monkey, Macaca nemestrina, during the menstrual cycle and after estrogen treatment. Am J Anat. 1983;166:149–172. doi: 10.1002/aja.1001660203. [DOI] [PubMed] [Google Scholar]
- Odor L, Blandau RJ. Observations on the solitary cilium of rabbit oviductal epithelium: its motility and ultrastructure. Am J Anat. 1985;174:437–453. doi: 10.1002/aja.1001740407. [DOI] [PubMed] [Google Scholar]
- Philips DM, Fadem BH. The oocyte of a new world marsupial, Monodelphis domestica: structure, formation, and function of the enveloping mucoid layer. J exp Zool. 1987;242:363–371. doi: 10.1002/jez.1402420316. [DOI] [PubMed] [Google Scholar]
- Plapinger L. Surface morphology of uterine and vaginal epithelia in mice during normal postnatal development. Biol Reprod. 1982;26:961–972. doi: 10.1095/biolreprod26.5.961. [DOI] [PubMed] [Google Scholar]
- Praetorius HA, Spring KR. A physiological view of the primary cilium. Annu Rev Physiol. 2005;67:515–29. doi: 10.1146/annurev.physiol.67.040403.101353. [DOI] [PubMed] [Google Scholar]
- Quarmby LM. Cellular deflagellation. Int Rev Cytol. 2004;233:47–91. doi: 10.1016/S0074-7696(04)33002-0. [DOI] [PubMed] [Google Scholar]
- Reeder RL, Shirley B. Deciliation in the ampulla of the rat oviduct and effects of estrogen on the process. J exp Zool. 1999;283:71–80. [PubMed] [Google Scholar]
- Renfree MB. Society for reproductive biology founders lecture 2006 Life in the pouch: womb with a view. Reprod Fertil Dev. 2006;18:721–734. doi: 10.1071/rd06072. [DOI] [PubMed] [Google Scholar]
- Renfree MB, Shaw G. Marsupials. In: Knobil E, Neill JD, editors. Encyclopedia of Reproduction. San Diego: Academic press; 1999. pp. 104–114. [Google Scholar]
- Roberts CT, Breed WG. The marsupial shell membrane: an ultrastructural and immunogold localization study. Cell Tissue Res. 1996a;284:99–110. doi: 10.1007/s004410050570. [DOI] [PubMed] [Google Scholar]
- Roberts CT, Breed WG. Variation in ultrastructure of mucoid coat and shell membrane secretion of a dasyurid marsupial. Reprod Fertil Dev. 1996b;8:645–648. doi: 10.1071/rd9960645. [DOI] [PubMed] [Google Scholar]
- Roberts CT, Breed WG, Mayhofer G. Origin of the oocyte shell membrane of a dasyurid marsupial: an immunohistochemical study. J exp Zool. 1994;270:321–331. doi: 10.1002/jez.1402700311. [DOI] [PubMed] [Google Scholar]
- Rodger JC. Fertilzation of marsupials. In: Dunbar BS, O’Rand MG, editors. A Comparative Overview of Mammalian Fertilization. New York: Plenum Press; 1991. pp. 117–135. [Google Scholar]
- Rumery RE, Gaddum-Rosse P, Blandau RJ, Odor DL. Cyclic changes in ciliation of the oviductal epithelium in the pig-tailed macaque Macaca nemestrina. Am J Anat. 1978;153:345–366. doi: 10.1002/aja.1001530303. [DOI] [PubMed] [Google Scholar]
- Samollow PB. Status and applications of genomic resources for the gray short-tailed opossum, Monodelphis domestica, an American marsupial model for comparative biology. Aust J Zool. 2006;54:173–196. [Google Scholar]
- Selwood L. Embryonic development in culture of two dasyurid marsupials Sminthopsis crassicaudata Gould. and Sminthopsis macroura Spencer, during cleavage and blastocyst formation. Gamete Res. 1987;16:355–370. doi: 10.1002/mrd.1120160409. [DOI] [PubMed] [Google Scholar]
- Selwood L. Marsupial egg and embryo coats. Cells Tissues Organs. 2000;166:208–219. doi: 10.1159/000016733. [DOI] [PubMed] [Google Scholar]
- Selwood L, Robinson ES, Pederson RA, Vandeberg JL. Development in vitro of marsupials: a comparative review of species and a timetable of cleavage and early blastocyst stages of development in Monodelphis domestica. Int J Dev Biol. 1997;41:397–410. [PubMed] [Google Scholar]
- Selwood L, Coulson G. Marsupials as models for research. Aust J Zool. 2006;54:137–138. [Google Scholar]
- Shalgi R, Kaplan R, Kraicer PF. Proteins of follicular, bursal and ampullar fluids of rats. Biol Reprod. 1977;17:333–338. doi: 10.1095/biolreprod17.3.333. [DOI] [PubMed] [Google Scholar]
- Shimmin GA, Jones M, Taggart TA, Temple-Smith PD. Sperm transport and storage in the agile Antechinus, Antechinus agilis. Biol Reprod. 1999;60:1353–1359. doi: 10.1095/biolreprod60.6.1353. [DOI] [PubMed] [Google Scholar]
- Sidhu KS, Mate KE, Molina FC, Glazier AM, Rodger JC. Secretory proteins from the female reproductive tract of the brushtail possum Trichosurus vulpecula: binding to sperm and effects on sperm survival in vitro. Reprod Fertil Dev. 1999;11:329–336. doi: 10.1071/rd00010. [DOI] [PubMed] [Google Scholar]
- Smith TT. The modulation of sperm function by the oviductal epithelium. Biol Reprod. 1998;58:1102–1104. doi: 10.1095/biolreprod58.5.1102. [DOI] [PubMed] [Google Scholar]
- Suarez SS. Carbohydrate-mediated formation of the oviductal sperm reservoir in mammals. Cells Tissues Organs. 2001;168:105–112. doi: 10.1159/000016811. [DOI] [PubMed] [Google Scholar]
- Tachi S. Electron microscopic analysis of the effect of progesterone upon the hormone-sensitive solitari cilia and centriolar complexes in the luminal epithelium of the uterus of the ovariectomized-adrenalectomized rat. Am J Anat. 1984;169:45–58. doi: 10.1002/aja.1001690104. [DOI] [PubMed] [Google Scholar]
- Taggart DA. A comparison of sperm and embryo transport in the female reproductive tract of marsupial and eutherian mammals. Reprod Fertil Dev. 1994;6:451–472. doi: 10.1071/rd9940451. [DOI] [PubMed] [Google Scholar]
- Taggart DA, Temple-Smith PD. Transport and storage of spermatozoa in the female reproductive tract of the brown marsupial mouse, Antechninus stuartii Dasyuridae. J Reprod Fert. 1991;93:97–110. doi: 10.1530/jrf.0.0930097. [DOI] [PubMed] [Google Scholar]
- Töpfer-Petersen. Wagner EA, Friedrich J, Petrunkina A, Ekhlasi-Hundrieser M, Waberski D, Drommer W. Function of the mammalian oviductal sperm reservoir. J exp Zool. 2002;292:210–215. doi: 10.1002/jez.1157. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J, Motta P. Munich: Urban and Schwarzenberg; The cellular basis of mammalian reproduction. [Google Scholar]
- Wheatly D. Primary cilia: new perspectives. Cell Biol Int. 2004;28:75–77. doi: 10.1016/j.cellbi.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Wick R, Kress A. Ultrastructural changes in the uterine luminal and glandular epithelium during the oestrous cycle of the marsupial Monodelphis domestica (Grey short-tailed opossum) Cells Tissues Organs. 2002a;170:111–131. doi: 10.1159/000046185. [DOI] [PubMed] [Google Scholar]
- Wick R, Kress A. Ultrastructural changes in the cervical epithelium during estrous cycle of the marsupial Monodelphis domestica (Grey short-tailed opossum) Cells Tissues Organs. 2002b;171:162–176. doi: 10.1159/000063710. [DOI] [PubMed] [Google Scholar]
- Zeller U, Freyer C. Early ontogeny and placentation of the grey short-tailed opossum, Monodelphis domestica Didelphidae: Marsupialia: contribution to the reconstruction of the marsupial morphotype. J Zool Syst Evol Research. 2001;39:137–158. [Google Scholar]