Abstract
Kiwi (Apterygidae: Apteryx spp.) are traditionally assumed to detect their soil-dwelling invertebrate prey using their sense of smell. The unique position of the nares at the tip of the bill and the enlarged olfactory centres in the brain support this assumption. However, studies designed to show the importance of olfaction in prey-detection by Apteryx have provided equivocal results. Another family of probing birds, the Scolopacidae, detect their buried prey using specialised vibration and pressure-sensitive mechanoreceptors embedded in pits in the bill-tip. We found that aspects of the foraging patterns of Apteryx mantelli are like those of scolopacid shorebirds, suggesting that Apteryx may be using a similar prey-detection mechanism. We examined specimens of all five Apteryx species and conducted a morphological and histological examination of the bill of A. mantelli. We discovered that Apteryx possess an arrangement of mechanoreceptors within pits similar to that in Scolopacidae species and may therefore be able to localise prey using a similar vibrotactile sense. We suggest that this sense may function in conjunction with, or be dominant over, olfaction during prey-detection. The Apterygidae and the Scolopacidae are members of the two different super-orders of birds: the Paleognathae and the Neognathae, respectively. Therefore we cite the similar bill-tip anatomy of these two families as an example of convergent evolution across a deep taxonomic divide.
Keywords: foraging ecology, foraging sign, Grandry corpuscles, Herbst corpuscles, probing birds
Introduction
Probing is a foraging method used by many bird species. It involves inserting the bill into cracks in the bark of trees, between rocks, into flowers and vegetation or into the ground in search of food. Some groups of birds have developed special adaptations of the bill to facilitate lifestyles dominated by probing for prey. The best examples of these are the neognathous shorebirds in the family Scolopacidae (Charadriiformes). Members of this group possess variously elongated bills used for probing for invertebrates in coastal mudflats and sand. Most show a development of the bill-tip into a specialised tactile organ consisting of numerous mechanoreceptors (Herbst and Grandry corpuscles) embedded in pits in the bone (Bolze, 1968). This organ allows the birds to detect buried prey at some distance from the bill-tip by picking up vibrotactile and/or pressure cues in the substrate (Zweers & Gerritsen, 1997; Piersma et al. 1998; Nebel et al. 2005).
The nocturnal New Zealand Apteryx[kiwi; Apterygiformes: Apterygidae (Burbidge et al. 2003)] feed primarily upon invertebrates (85–95% of the diet, Reid et al. 1982; Kleinpaste, 1991; McLennan, 1991; Miles, 1995) sourced mostly from underground (Kleinpaste, 1991). While primarily forest dwelling birds, they forage in a wide variety of habitats ranging from alpine tussockland to farm pasture and sandy beaches. They are the only paleognathous birds to forage by probing and possess a long, slightly down-curved bill, which facilitates this activity. The five Apteryx species are also the only extant group of birds with the nares positioned at the tip of the bill. This unusual feature, combined with the ‘snuffling’ sound made by foraging Apteryx and the extensive development of the olfactory chamber and olfactory regions in the brain (Bang, 1971; Martin et al. 2007) has resulted in a common assumption that these birds find their prey using olfaction alone (Reid & Williams, 1975, Peat, 1990, McLennan, 1991).
Experiments carried out to ascertain the use of olfaction in prey-detection by Apteryx have produced mixed results. Benham (1906) and Wenzel (1968, 1971) reported that A. australis were very successful at finding buried odiferous artificial food using only their sense of smell. However, Wenzel's (1971) study suggests that A. australis were less successful at finding natural prey using smell alone. Strong (1911), Flinn (1995) and Jenkins (2001) failed to show that Apteryx are accurate in locating buried prey by smell, often probing areas containing no prey, or else being unable to detect the presence of prey before an area had been probed.
The morphology of the Apteryx olfactory system and the outcomes of some of the above experiments suggest that olfaction is well developed in the Apterygidae. When taken together, however, these studies fail to demonstrate convincingly that smell is the only, or even the primary, sense employed by Apteryx to find prey. Some authors specifically mention they suspect senses other than olfaction are involved in prey-detection. Haeusler (1923) hypothesized that the Apteryx bill was a highly sensitive organ of touch, observing that Apteryx invariably seized and ate food that came into direct contact with the bill-tip. Jenkins (2001) suggested that Apteryx use auditory and/or vibrotactile cues instead of olfaction.
Prey-detection mechanisms other than olfaction have not been systematically investigated in the Apterygidae. As probing birds, it seems likely they also use other mechanisms including remote-tactile systems similar to those seen in some members of the Scolopacidae (Gerritsen & Meiboom, 1986; Zweers & Gerritsen, 1997; Piersma et al. 1998). Parker (1891) described the presence of a bill-tip structure in Apteryx containing a honeycomb of small pits ‘in which are end-organs abundantly supplied by branches of the dorsal ramus of the orbitonasal nerve’, giving support to the above hypothesis.
Direct observation of foraging behaviour of wild Apteryx is difficult as they are nocturnal and forage mostly in forested areas. Most work on kiwi foraging, including the experiments mentioned above, has been carried out in captivity. In this paper we present, for the first time, ecological data showing that probe-hole patterns of wild Apteryx mantelli are similar to those of captive Calidris sandpipers (Scolopacidae). We also present a morphological and histological examination of the Apteryx bill-tip confirming that the Apterygidae possess a bill-tip organ similar to that of the Scolopacidae, and showing that this organ has distinct characteristics in Apteryx. To the best of our knowledge, this is the first description of the bill-tip organ of Apteryx since its brief mention by Parker (1891), and is also the first time that this sensory system has been described outside of the Scolopacidae.
In light of this finding, we discuss the need for a re-evaluation of prey-detection mechanisms, and an assessment of the relative importance of senses involved in prey-localisation, in Apteryx. We examine these findings as evidence of convergent evolution between the neognathous Scolopacidae and the paleognathous Apterygidae.
Materials and methods
Study species
Apteryx mantelli is the focal species of this study as it is the most readily accessible of the five species, both in terms of population distribution and archived material for histology. However, this species, like all other kiwi species, is endangered. Most specimens available to us are of young birds which have died in recent years and been retrieved for post-mortem as part of New Zealand's conservation efforts to save Apteryx species. Museum specimens of all five Apteryx species were examined to confirm that the morphology of the bill-tip organ is similar in all Apteryx.
Foraging pattern and probe-holes
This part of the study was carried out on Ponui Island, New Zealand (36°55′S, 175°11′E). Density of A. mantelli on this island is thought to be at least one bird/ha (I. Castro & S. Cunningham pers. obs.). Ponui Island supports a patchwork of different habitat types used by A. mantelli, including pasture, scrub (principally Coprosma spp. Leptospermum ericoides, and Pseudopanax crassifolius), thick Typha orientalis swamp, and regenerating Agathis australis and mixed broadleaf forest (Shapiro, 2006).
Foraging Apteryx leave behind distinctive signs in the form of ‘probe-holes’ created when the bird pushes its bill into the ground to capture buried prey. Foraging sign was investigated in Red Stony Hill Gully (RSHG; 35 ha), as a proxy for direct observation of the birds living in this area. RSHG supports vegetation types representative of the island as a whole, and sustains a population of at least 35 resident adult A. mantelli, plus sub-adult birds and non-residents which regularly visit the area. Adult Apteryx are territorial, and on Ponui, home ranges greatly overlap, particularly between neighbouring females and males (B. Ziesemann, pers. comm.; S. Cunningham pers. obs.).
Data on probe-hole characteristics and local distribution were collected from 154 searches in 1 m2 quadrants placed randomly throughout the gully over a five-month period. These data potentially suffer from pseudo-replication because it was impossible for us to assign probe-holes found to the individuals that made them. However, the high density and distribution of the birds in the area, combined with widely scattered random sampling sites, make it likely that many different individuals are represented in the sample (Fig. 1).
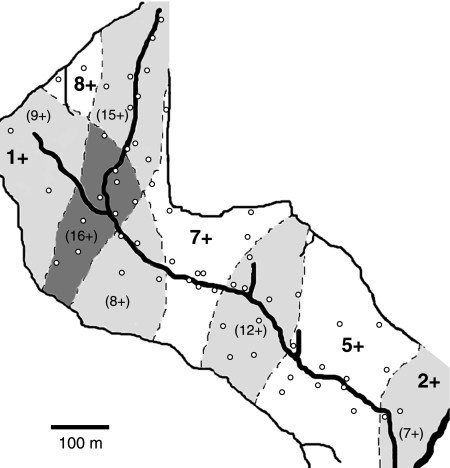
Fig. 1.
Schematic diagram of RSHG showing distribution of birds (estimated from encounters with 23 radio tagged individuals) and sampling sites. Open circles represent sampling quadrants. Thick lines represent the gully floor/streambed; thin lines represent the ridge-lines which bound the gully and define the study site. Dotted lines signify the generalised home range boundaries of Apteryx. Near neighbours with highly overlapping ranges have been grouped together, and their ranges are represented by shared boundary lines. The large numbers indicate the number of tagged individuals using each grouped range. Shaded areas represent areas of overlap between the ranges of neighbouring groups of birds, and the numbers in parentheses show the maximum number of birds using these shared areas (calculated by adding together the numbers of birds in each of the ranges which contribute to the shared area). The + sign indicates that an unknown number of untagged birds also use the area.
Probe-hole depth was measured to the nearest millimetre from the bottom of the probe-hole to the surface of the surrounding substrate. Width was measured across the mouth of the hole at its widest point. Angle of bill insertion was estimated by inserting a peg into the hole and measuring the angle between the peg and the surrounding substrate. At each site, the following measures were also taken: five measurements each to 50, 100 and 150 mm deep for soil penetrability (taken with a compression spring hand penetrometer; Landcare Research, Lincoln, New Zealand); three 20 mm core samples, taken to the same depths and analysed for soil gravimetric water content; percent canopy cover and percent ground cover (estimated); leaf litter depth (measured as the average of five depths taken by pushing a ruler through the litter to substrate surface); slope (measured with a clinometer; KOD, Korea); and aspect (direction of the slope, measured with a compass). Monthly rainfall data was supplied by NIWA (National Institute of Water and Atmospheric Research) from a weather station less than 15 km distance on neighbouring Waiheke Island. Data were not normally distributed, and were analysed using non-parametric tests (Spearman rank correlations).
Bill length data (measured from the cere to the bill-tip) was gathered from 36 A. mantelli from RSHG and two neighbouring gullies for comparison with data on probe-hole depths in RSHG.
Bill morphology
Bills of twenty Apteryx specimens (including two A. rowi, three each of A. haastii and A. owenii, five A. australis and seven A. mantelli specimens) from The Museum of New Zealand Te Papa Tongarewa, plus two further A. mantelli skulls from the Ecology Museum, Massey University, New Zealand, were examined under a light microscope to check for the presence and distribution of any pits similar to those found in the bills of probing Scolopacidae. Numbers of pits were counted, and the total bill length, length bearing sensory pits, and length of pre-maxilla overlap (sensory pad) were measured using Vernier callipers (Kincrome™; Carlton South, Australia).
Material for histological work was sourced from two A. mantelli (a one month old chick; a five month old juvenile female). These birds were archived in formalin for research purposes at Massey University, as part of the National Wildlife Mortality Database. Bills of these two birds were soaked in phenol to soften the keratin, decalcified using standard techniques (Thompson, 1966), routinely processed, and embedded in paraffin. Sagittal sections (3 µm) of the pre-maxilla and mandible of the chick were stained using Masson's trichrome and Haematoxylin & Eosin (after Luna, 1968); 5 µm thick sections were stained with Bielchowsky silver stain (after Bancroft & Stevens, 1982). Both 3 µm and 5 µm saggital sections from the bill-tips of the juvenile female were stained with Masson's trichrome and Bielchowsky silver stains respectively.
Bill-tip tissues remained very tough even after processing, and we were unable to obtain serial sections. Numbers of Herbst corpuscles per sensory pit were therefore estimated by multiplying up the number of corpuscles visible in 3 µm sections. Proportional measurements of three Herbst corpuscles from each specimen were taken from the shortest radius in cross-section, and were compared with the same measurements from pictomicrographs of Herbst corpuscles of other species presented in the literature.
Results
Foraging pattern and probe-holes
The average dimensions of probe-holes found were 43 mm deep (SD = 25 mm, n = 292) and 14 mm wide (SD = 8 mm, n = 293). The average angle of insertion of the A. mantelli bill into the ground while foraging was 69.5° (SD = 13.1°, n = 266). The overall distribution of probe-hole depths was skewed towards shallower holes (skewness = 1.66, Ryan-Joiner test for normality RJ = 0.932, P < 0.01). Most holes found were well below the maximum depth achievable by kiwi in the study population, based on bill length (Fig. 2).
Fig. 2.
Box plot of bill lengths of male and female A.mantelli on Ponui Island compared with depths of probe-holes found in Red Stony Hill Gully. Probe-holes deeper than maximum bill lengths of kiwi are opened by birds working to create a large hole into which the face is partially inserted (S. Cunningham pers. obs). ⊕ = mean length/depth. Boundaries of boxes indicate the interquartile range. Dividing line within each box indicates the median, whiskers indicate the range excluding outliers. Outliers are indicated by * and are calculated as data points falling beyond 1.5× the interquartile range outside the upper and lower quartiles.
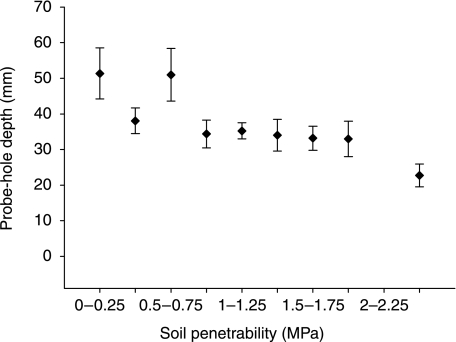
Probe-hole depth correlated significantly with both soil gravimetric water content (W) and soil penetrability (Table 1). Probe-holes deeper than 50 mm were only found where soil penetrability ≤ 0.75 MPa (Fig. 3). Deepest probe-holes occurred in soils with a gravimetric water content between W = 0.45 g g−1 and W = 0.9 g g−1.
Table 1.
Spearman rank correlations between soil parameters and probe-hole depths and density. W = soil gravimetric water content. MPa = Mega Pascal, * = significant at 5% level, ** = significant at 1% level
| Strength of correlation | ||||||
|---|---|---|---|---|---|---|
| Probe-hole depth | Probe-hole density | |||||
| Soil parameter | r | P | n | r | P | n |
| Penetrability (MPa) | −0.339 | 0.000** | 126 | 0.014 | 0.441 | 112 |
| W 0–50 mm | 0.149 | 0.052 | 120 | 0.251 | 0.010** | 85 |
| W 50–100 mm | 0.232 | 0.003** | 136 | 0.160 | 0.048* | 85 |
| W 100–150 mm | 0.245 | 0.003** | 120 | 0.166 | 0.064 | 85 |
Fig. 3.
Probe-hole depth and soil penetrability. Data presented are means ± 1 standard error. Soil penetrability data is averaged over three depths (0–50 mm, 50–100 mm, 100–150 mm). (Penetrability decreases with increasing values in MPa).
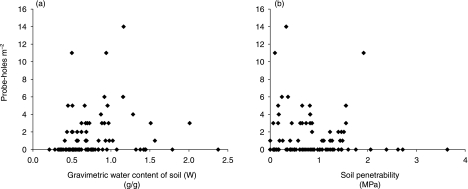
Probe-holes were found in clusters 67% of the time. Probe-hole density correlated significantly with soil gravimetric water content (W) (Table 1), and peaked between W = 0.5 g g−1 and W = 1.5 g g−1 in the top 50 mm of soil (Fig. 4a). There was no correlation between probe-hole density and increasing soil resistance (Table 1), however probe-holes were only found in quadrants where the force required to penetrate the top 50 mm of soil was less than 2 MPa (Fig. 4b).
Fig. 4.
Scatterplots of (a) soil gravimetric water content measured in the top 50 mm of soil and (b) soil penetrability measured in the top 50 mm of soil, against the number of probe-holes per m2 (penetrability decreases with increasing values in MPa).
Soil parameters were more important in explaining probe-hole depth and density than any other microhabitat variables measured. No other microhabitat variables correlated with probe-hole depth and except for canopy cover (r = 0.166, P = 0.020, n = 154), which itself correlated with soil water content below 100 mm (r = 0.225, P = 0.041, n = 83); no other microhabitat variables measured correlated with probe-hole density.
Bill-tip structure
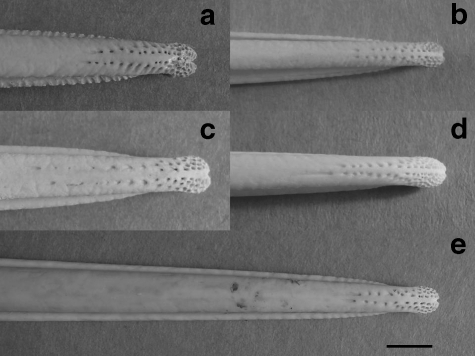
A similar pattern of pits, reminiscent of those seen in Scolopacidae bills, was found in the bill-tips of all twenty-two Apteryx museum specimens examined. Bill-tip structure was consistent across the five Apteryx species, with some interspecific variation in the number of pits present (Fig. 5, Table 2).
Fig. 5.
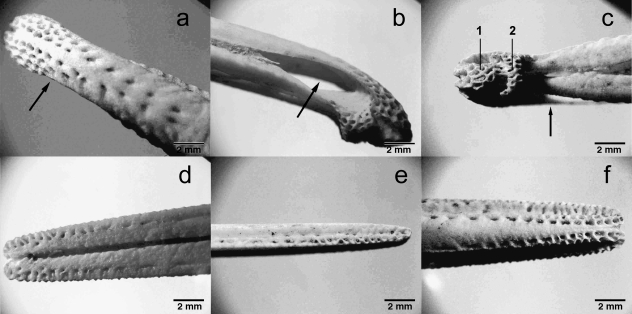
Pattern of pits in the bill-tip is similar across all Apteryx species. (a) A. australis, (b) A. owenii, (c) A. haastii, (d) A. rowi, (e) A. mantelli. Photographs to scale, scale bar = 5 mm.
Table 2.
Percentage of the bill length pitted and numbers of sensory pits in the Apteryx bill-tip and sensory pad. Data are presented as mean ± SD. Percentages are rounded to one decimal place, number of pits are rounded to the nearest whole number
| Species | n | % bill pitted | Sensory pad length as % bill length | Number of pits (pre-maxilla) | Number of pits (mandible) | Total number of pits | % of pits found in sensory pad |
|---|---|---|---|---|---|---|---|
| A. australis | 5 | 13.1% ± 0.9% | 3.6% ± 0.4% | 175 ± 31 | 140 ± 11 | 315 ± 23 | 24.9% ± 4.4% |
| A. haastii | 3 | 12.8% ± 2.7% | 3.6% ± 0.3% | 149 ± 19 | 118 ± 6 | 268 ± 17 | 26.1% ± 2.8% |
| A. mantelli | 9 | 12.1% ± 2.2% | 3.7% ± 0.6% | 125 ± 23 | 144 ± 34 | 269 ± 55 | 19.5% ± 3.4% |
| A. owenii | 3 | 12.1% ± 2.3% | 3.8% ± 0.1% | 122 ± 18 | 118 ± 20 | 240 ± 3 | 22.8% ± 5.9% |
| A. rowi | 2 | 13.2% ± 1.6% | 3.6% ± 0.2% | 204 ± 3 | 203 ± 11 | 407 ± 8 | 22.1% ± 1.5% |
| Averages/total n | 22 | 12.5% ± 1.8% | 3.7% ± 0.4% | 146 ± 35 | 142 ± 32 | 288 ± 58 | 22.3% ± 4.4% |
Pre-maxilla
The overlapping pre-maxilla bulges slightly ahead of the nares and is semi-circular in cross-section (Fig. 6a,b). Two parallel rows of small oval-shaped pits extend proximally from the nares along the dorsal surface of the bill-tip, to 12.5% ± 1.8% of the total bill length (Figs 6c, 7a, Table 2). All surfaces of the area forward of the nares are honeycombed with small polygonal pits placed broadly in rows and packed closely together (Fig. 7a–c). Additional pits fill any space between the rows large enough to accommodate them. Numbers of pits present in the bill-tips vary between species, with A. rowi having more pits than other Apteryx (Table 2).
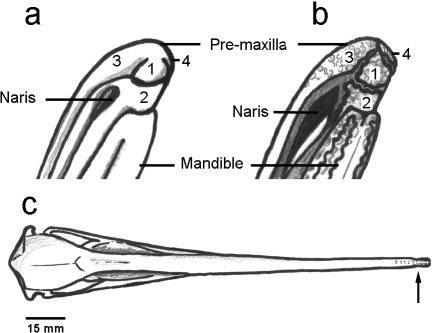
Fig. 6.
(a) Diagram of the intact tip of the Apteryx bill, lateral view rotated 45° to ventral. (b) Diagram of the tip of the Apteryx bill stripped of keratin and soft tissue, lateral view rotated 45° to ventral. The pre-maxilla significantly overlaps the mandible, forming a pre-oral sensory organ ‘sensory pad’ (made up of zones 1 and 2). Zones: 1 = ‘tactile disc’. 2 = ‘pre-oral area’. 3 and 4 = left and right dorsal zones. (c) Dorsal view of Apteryx skull – drawn from a female A. mantelli. The arrow indicates the position of the nares. The double row of dots leading back from the bill-tip indicates the extent to which the sensory pits run back along the bill.
Fig. 7.
Bill-tips of an adult female A. mantelli. Pre-maxilla (a) dorsal (b) lateral and (c) ventral views (in ventral view, the tactile disc (1) and pre-oral area (2) making up the bill-tip sensory pad can be seen). Arrows indicate position of nares. Mandible: (d) dorsal view (e) lateral view and (f) ventral view. Specimen from the Massey University Museum collection.
The tip of the pre-maxilla can be divided visually into several zones bounded by junctions in the keratin coat. These zones correspond directly to discrete areas in the morphology of the bone beneath (Fig. 6a,b). The overlapping ventral surface (we name ‘sensory pad’) is composed of two distinct zones, a central, circular ‘tactile disc’ and a semicircular ‘pre-oral zone’ (Figs 6, 7c). The dorsal bill surface is divided longitudinally into two zones by a narrow strip of un-pitted bone (Fig. 7a). We refer to these areas as ‘dorsal zones 3 and 4’ (Fig. 6).
Mandible
The mandible contains rows of pits extending proximally from the bill-tip on every surface (Fig. 7d–f). The dorsal surface of the mandible (inside the mouth of the living bird) has 1–2 rows of pits running along the outer edges (Fig. 7d).
The mandible has a narrow lateral groove along the length of the bill from base to tip. Pits are embedded in this groove towards the tip, and are also present in the raised bone on either side (Fig. 7e). Two further rows of pits run proximally along the edges of the ventral surface of the bill (Fig. 7f).
Histology
Sagittal sections through the pre-maxilla and mandible of A. mantelli show that the pits visible on the surface extend deeply into the bone, especially at the tip of the pre-maxilla (Fig. 8). The pits are occupied by Herbst corpuscles and Grandry corpuscle-like structures we have termed ‘terminal cell receptors’ following the nomenclature used by Gottschaldt (1985) and Wild (1990). These are embedded in a matrix of loose connective tissue and surrounded by nerve fibres (Fig. 9).
Fig. 8.
Longitudinal sections through the pre-maxilla and mandible of a 5-month-old female A. mantelli (×4). (a) Pre-maxilla (pointing left) showing deep forward facing pits at the tip and outward facing pits in the dorsal zone (examples marked P). (b) Mandible (pointing right) showing deep pits in the bone (examples marked P). Stain: Masson's trichrome. K = keratin layer. Images are oriented dorsal side up.
Fig. 9.
Numerous Herbst corpuscles embedded in the sensory pits of the mandible. (a) one-month-old chick ×10. Stain: Bielchowsky silver. (b) 5-month-old female A. mantelli ×40. Stain: Masson's Trichrome. Some examples of Herbst corpuscles labelled with arrows. (c, d) Terminal cell receptors (some examples labelled TCR) and Herbst corpuscles (H) from the mandible of a one-month-old A. mantelli chick (×100). In (d) Herbst corpuscle central axons (unstained) labelled with *; IC = inner core; arrows indicate outer zone. (c) Stain: Masson's trichrome. (d) Stain: Bielchowsky silver.
The ovoid Herbst corpuscles measure roughly 60 µm × 40 µm across, and are aligned with the long axes facing in various directions (Fig. 9d). Our estimates of the number per pit are very variable, ranging from 1–70, with an average of 23 ± 20 (n = 20 pits) in the five-month-old juvenile, and 20 ± 16 (n = 31 pits) in the month old chick. The corpuscles have reduced outer zones by comparison with pictomicrographs of Herbst corpuscles from bills of other birds (Table 3). The terminal cell receptors are smaller than the Herbst corpuscles and more variable in shape and size.
Table 3.
Proportion of total Herbst corpuscle width occupied by the inner core and outer zone in several species of bird. Data for A. mantelli specimens presented as mean ± SD (n). Refer to Fig. 9d for location of zones
| Proportion of total width | |||
|---|---|---|---|
| Species | Source | Inner core | Outer zone |
| Apteryx mantelli (juvenile female) | This study | 0.67 ± 0.41 (3) | 0.12 ± 0.11 (3) |
| Apteryx mantelli (month old chick) | This study | 0.51 ± 0.04 (3) | 0.19 ± 0.05 (3) |
| Philomachus pugnax | Bolze (1968) | 0.14 | 0.55 |
| Gallinago gallinago | Bolze (1968) | 0.27 | 0.53 |
| Calidris canutus | Piersma et al. (1998) | 0.20 | 0.60 |
| Anser sp. | Gottschaldt (1985) | 0.11 | 0.82 |
| Gallus sp. | Gottschaldt (1985) | 0.22 | 0.57 |
Discussion
The major findings of this paper are that the Apterygidae share some foraging patterns with, and possess analogous structures in the bill-tip to members of the family Scolopacidae. These findings suggest that the two families may share a similar prey-detection mechanism. The Apterygidae and the Scolopacidae are very distantly related groups, and the presence of a Scolopacidae-like bill-tip organ in Apteryx suggests convergent evolution of a complex sensory system across a deep taxonomic divide. We discuss each of these findings in more detail below.
Foraging patterns of Scolopacidae and Apterygidae
Prey burying depth, substrate conditions, and prey-detection strategies may all control the depth of insertion of the bill into the substrate by a probing bird. Scolopacids are affected by all of these factors under different conditions (Grant, 1984; Gerritsen & Meiboom, 1986; Mouritsen & Jensen, 1992; Nebel & Thompson, 2005).
Calidris sandpipers held in captivity employ repeated shallow ‘pecks’ to estimate prey density, followed by deeper probing to capture buried prey in trays presented to them (Gerritsen & Meiboom, 1986). This foraging results in an initial ratio of more shallow to fewer deep bill insertions into the substrate, which is reversed once the trays have been fully explored (Gerritsen & Meiboom, 1986). In the wild, these sandpipers use shallow pecks to capture shallowly buried or surface prey, and the ratio of pecks to probes may be determined by burying depth of prey rather than by prey-detection strategies (Nebel & Thompson, 2005). For example, Nebel & Thompson (2005) showed that Calidris mauri probe more often during hotter periods of the day – presumably in order to capture prey which has burrowed deeply to escape the heat.
Abiotic microhabitat conditions may also control where and how deeply tactile-foraging shorebirds may probe. Limnodromus griseus in an estuary environment will selectively probe more penetrable ripple crests rather than harder troughs in the sand, and use less than 20% of the average bill length in each probe (Grant, 1984). Calidris alpina foraging on mudflats in the Wadden Sea will probe significantly deeper and more often in areas of damper, more easily penetrable substrate (Mouritsen & Jensen, 1992).
A. mantelli in this study are also influenced by microhabitat conditions. They probe deeper on average in areas of more penetrable substrate, and choose damper substrate to probe in. Like L. griseus, they cluster their probe-holes and do not use the full capacity of the bill. The overall distribution of probe-holes found in this study was skewed towards holes much shallower than the total length of the Apteryx bill. Two possibilities may explain this pattern: prey at the study site might be buried shallowly, or Apteryx may be utilising shallower, less energetically costly probes in order to localise prey via remote-tactile cues – much like the captive sandpipers in Gerritsen & Meiboom's (1986) foraging trials.
Bill morphology and prey-detection mechanisms
Both the Apterygidae and the Scolopacidae show a complex arrangement of Herbst corpuscles and terminal cell receptors embedded within bony pits in the bill-tip. In Scolopacidae, this arrangement enables the birds to ‘remote-detect’ pressure or vibrational cues from buried invertebrate prey (Gerritsen & Meiboom, 1986; Zweers & Gerritsen, 1997; Piersma et al. 1998). The precise role of the Herbst corpuscles and terminal cell receptors in prey localisation, estimation of prey density, and prey-handling by these birds is unknown. It seems likely that the Herbst corpuscles are implicated in the location of prey because invertebrate-produced vibrations fall within their range of sensitivity, but outside that of terminal cell receptors (Zweers & Gerritsen, 1997).
The patterns of distribution and the orientation of bony pits, as well as the numbers and orientation of Herbst corpuscles within each pit, seem to be important for the functioning of the bill-tip organ and vary between species. For example, Piersma et al. (1998) showed that Calidris canutus are able to detect pressure disturbance patterns made by immobile bivalves in wet sand. Their histological examination of the C. canutus bill-tip showed that these birds possess neatly stacked Herbst corpuscles within forward facing ‘sensory’ pits (Piersma et al. 1998). Other calidrid sandpipers such as C. alpina, C. mauri and C. minutilla, have outward facing pits and use their bills to detect vibrations made by mobile invertebrates living beneath the surface of the substrate (Gerritsen & Meiboom, 1986; Nebel et al. 2005).
Apteryx species have outward, forward and even backward facing pits, suggesting that the Apterygidae may be able to use both vibrational and pressure cues when hunting for prey. The range of environmental conditions foraging Apteryx may encounter is large, as these birds occupy a diversity of habitats in a temperate zone experiencing climatic extremes. An ability to detect many types of tactile cues would be to the species' advantage. Further research will indicate the exact arrangement of mechanoreceptors within pits in the Apteryx bill and how they are used when foraging.
Some Scolopacidae species possess more Herbst corpuscles per pit than others (e.g. G. gallinago: 20–40 per pit; C. canutus, C. alpina: 15–35 per pit) (Bolze, 1968). It has been suggested that there is a positive relationship between the number of corpuscles in the pits and reliance of the species on probing while foraging (Bolze, 1968; Zweers & Gerritsen, 1997). This suggestion is strongly supported by Barbosa & Moreno's (1999) finding that an increased number of mechanoreceptors is highly correlated with tactile over visual foraging behaviour (Barbosa & Moreno, 1999). Our rough estimates of the total number of Herbst corpuscles present in each pit in the A. mantelli bill were very variable, ranging from 1–70 per pit. A. mantelli rely heavily on foraging by probing (Kleinpaste, 1991), therefore our finding of a large number of Herbst corpuscles in some parts of the A. mantelli bill is consistent with the studies mentioned above. More information is necessary to explain the variation in the number of corpuscles between pits in Apteryx.
The width of the outer zone of Herbst corpuscles is also known to vary between species: Herbst corpuscles found in the bills of Anser have a wider outer zone than those found in Gallus, for example (Gottschaldt, 1985). In Anser, the inner core reduces in diameter while the outer zone expands as the bird ages (Gottschaldt, 1985). Herbst corpuscles in the bills of the A. mantelli examined show a reduction of the outer zone compared with corpuscles found in the bills of other species. Examination of tissues from a larger sample including mature A. mantelli would help to clarify whether the reduced outer zone of Herbst corpuscles found is due to the age of the birds, or to a species-specific difference in structure.
The pre-maxilla of Apteryx overlaps the mandible to an extent not seen in other probing birds. The ventral surface of this area of overlap (sensory pad) is divided into two distinct zones, the pre-oral area and the tactile disc, an arrangement apparently unique to the Apterygidae. Around 20% of all the sensory pits present in Apteryx bill are found within this structure. At present, the specific function of this highly developed sensory pad area is unknown. Foraging Apteryx tend to move through their habitat gently tapping this pad on the ground (Haeusler, 1923; S. Cunningham pers. obs). If the pad is able to pick up vibrations made by buried invertebrates through the soil surface, then Apteryx may be using this behaviour to constantly update themselves on prey availability in the area without the necessity of probing.
As well as being involved in prey-detection, the sensory pad might be used by the bird for locomotor guidance. The visual fields in Apteryx include a narrow area of binocular vision in front of the head, beneath which is a blind area including the bill-tip (Martin et al. 2007). This means that while Apteryx may see ahead to guide themselves, they cannot see the bill-tip or the ground immediately around them. The bill-tip pad may therefore allow the bird to assess in detail terrain in its immediate vicinity, with this information added to less detailed visual information. Further investigation of the function of the pad is required before we can be sure these uses are feasible.
Convergent evolution between Scolopacidae and Apterygidae
Under current classification systems, all extant birds are divided into two super-orders, the Paleognathae and the Neognathae. Paleognathous birds include Apterygidae, while Scolopacidae belong to the super-order Neognathae (Perrins, 2004). Scolopacidae and Apterygidae are therefore separated by a wide taxonomic divide, and the similarities in bill-tip morphology between these groups are more likely to be due to convergent evolution than to any shared phylogenetic history.
Similarities between these groups go beyond just the bill-tip organ, suggesting that parallel evolutionary pathways from ancestral foraging mechanisms to the ‘remote-touch’ strategy may have been followed by both. Zweers & Gerritsen (1997) and Barbosa & Moreno (1999) hypothesised that the lengthening of the bill and increase in the number of mechanoreceptors in the bill-tip co-evolved with remote-tactile foraging in Scolopacidae: a pathway perhaps also followed by Apterygidae. The differences in bill structure (for example, the bill-tip sensory pad) between the groups may be due to the wider variety of habitats Apteryx exploit, differing phylogenies or subtly different sensory systems.
Further evidence of convergent evolution can be found in the brains of both groups of birds. The principal sensory trigeminal nucleus of the avian brain relays tactile information from the trigeminal system, including the bill-tip, to the telencephalic sensory end-station for this information (Dubbeldam, 1990). In Apteryx, this area is both large and histologically complex (Martin et al. 2007). Within the Scolopacidae, an enlarged principal sensory trigeminal nucleus is also a feature of the G. gallinago brain (Dubbeldam, 1990). C. alpina exhibits an expansion of the telencephalon also related to its specialised tactile foraging system, an expansion perhaps common to most probing Scolopacid species (Pettigrew & Frost, 1985).
Challenging assumptions about prey-detection in Apteryx
The presence of a shorebird-like bill-tip organ in the Apterygidae, combined with the specialisation and enlargement of areas of the Apteryx brain associated with the processing of tactile information (Martin et al. 2007), provides an alternate prey-detection mechanism for the Apterygidae which may be dominant over olfaction, or used in conjunction with this and/or other senses. This is exciting because it challenges the traditional assumption that Apteryx find their prey by smell alone.
The Apteryx bill is part of a wider sensory system which also includes mammal-like vibrissae present around the face and base of the bill, the olfactory sense, hearing and vision. Our understanding of Apteryx foraging will not be complete without investigating these other sensory systems and their interactions. Foraging trials based on those used by Floyd & Woodland (1981) and Montgomerie & Weatherhead (1997) to isolate the senses used in prey-detection in the Artamidae and Turdidae, are currently being undertaken with A. mantelli to discover the degree to which the Apterygidae rely on their bill-tip organ during foraging.
Acknowledgments
We thank Zdenek Halata, Fabiana Kubke and J. Martin Wild for help with the identification of Herbst corpuscles and terminal cell receptors in the kiwi bill-tip. We thank David Horne and John Dando for help with soil science, Pat Davey and Evelyn Lupton for creating the histology slides and Cleland Wallace for locating the kiwi skulls in the Massey University museum. We are grateful to Sandy Bartle, Alan Tennyson and Gillian Stone for access to Te Papa Tongarewa's bird collections; and to the Chamberlins for allowing us to work on their land. J. Martin Wild, Tom Jensen, and Murray Potter provided useful comments to earlier drafts of this paper and the comments of two anonymous referees greatly improved the manuscript.
References
- Bancroft JD, Stevens A. Theory and Practice of Histological Techniques. Edinburgh, New York: Churchill Livingstone; 1982. [Google Scholar]
- Bang BG. Functional anatomy of the olfactory system in 23 orders of birds. Acta Anat. 1971;79:1–71. doi: 10.1159/isbn.978-3-318-01866-0. [DOI] [PubMed] [Google Scholar]
- Barbosa A, Moreno E. Evolution of foraging strategies in shorebirds: an ecomorphological approach. Auk. 1999;116:712–725. [Google Scholar]
- Benham WB. The olfactory sense in Apteryx. Nature. 1906;74:222–223. [Google Scholar]
- Bolze G. Anordnung und Bau der Herbstchen Korperchen in Limicolenschnabeln im Zusammenhang mit der Nahrungsfindung. Zool Anz. 1968;181:313–355. [Google Scholar]
- Burbidge ML, Colbourne RM, Robertson HA, Baker AJ. Molecular and other biological evidence supports the recognition of at least three species of brown kiwi. Conserv Genet. 2003;4:167–177. [Google Scholar]
- Dubbeldam JL. On the functional interpretation of quantitative differences in forebrain organisation – the trigeminal and visual system in birds. Neth J Zool. 1990;40:241–253. [Google Scholar]
- Flinn R. Food detection by kiwi; can they really detect food items in the soil by smell alone? Palmerston North: Massey University; 1995. Research Report. [Google Scholar]
- Floyd RB, Woodland DJ. Localisation of soil-dwelling scarab larvae by the black-backed magpie, Gymnorhina tibicen (Latham) Anim Behav. 1981;29:510–517. [Google Scholar]
- Gerritsen AFC, Meiboom A. The role of touch in prey density-estimation by Calidris alba. Neth J Zool. 1986;36:530–561. [Google Scholar]
- Gottschaldt KM. Structure and function of avian somatosensory receptors. In: King AS, McLelland J, editors. Form and Function in Birds. London: Academic Press; 1985. pp. 375–461. [Google Scholar]
- Grant J. Sediment microtopography and shorebird foraging. Mar Ecol Prog Ser. 1984;19:293–296. [Google Scholar]
- Haeusler HR. Notes on the habits of the North Island Kiwi (Apteryx mantelli) Emu. 1923;22:175–179. [Google Scholar]
- Jenkins C. Use of olfaction in Northern Brown Kiwi Apteryx mantelli. Palmerston North: Massey University; 2001. MSc Thesis. [Google Scholar]
- Kleinpaste R. Kiwis in a pine forest habitat. In: Fuller E, editor. Kiwis: A monograph of the Apterygidae. Shrewsbury: Swan Hill; 1991. pp. 97–138. [Google Scholar]
- Luna LG. Manual of histologic staining methods of the Armed Forces Institute of Pathology. New York: McGraw-Hill; 1968. [Google Scholar]
- Martin GR, Wilson K-J, Wild JM, Parsons S, Kubke MF, Corfield J. Kiwi forego vision in the guidance of their nocturnal activities. PLosONE. 2:e198. doi: 10.1371/journal.pone.0000198. 10.1371/journal.pone.0000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLennan J. Brown Kiwis. In: Fuller E, editor. Kiwis: A monograph of the Apterygidae. Shrewsbury: Swan Hill; 1991. pp. 37–58. [Google Scholar]
- Miles J. Comparative ecology of northern brown kiwi (Apteryx australis mantelli) in Tongariro National Park and Tongariro Forest Park, central North Island. Palmerston North: Massey University; 1995. MSc Thesis. [Google Scholar]
- Montgomerie R, Weatherhead PJ. How robins find worms. Anim Behav. 1997;54:143–151. doi: 10.1006/anbe.1996.0411. [DOI] [PubMed] [Google Scholar]
- Mouritsen KN, Jensen KT. Choice of microhabitat in tactile foraging dunlins Calidris alpina: the importance of sediment penetrability. Mar Ecol Prog Ser. 1992;85:1–8. [Google Scholar]
- Nebel S, Jackson DL, Elner RW. Functional association of bill morphology and foraging behaviour in calidrid sandpipers. Anim Biol. 2005;55:235–243. [Google Scholar]
- Nebel S, Thompson GJ. Foraging behaviour of western sandpipers changes with sediment temperature: implications for their hemispheric distribution. EcolRes. 2005;20:503–507. [Google Scholar]
- Parker TJ. Observations on the anatomy and development of Apteryx. Phil Trans R Soc (Lond) 1891;182:25–134. [Google Scholar]
- Peat N. The Incredible Kiwi-A Wild South Book. Auckland: Random Century New Zealand; 1990. [Google Scholar]
- Perrins C, editor. The new encyclopaedia of birds. Oxford: Oxford University Press; 2004. [Google Scholar]
- Pettigrew JD, Frost BJ. A tactile fovea in the Scolopacidae? Brain Behav Evol. 1985;26:185–195. [PubMed] [Google Scholar]
- Piersma T, van Aelst R, Kurk K, Berkhoudt H, Maas LRM. A new pressure sensory mechanism for prey detection in birds: the use of principles of seabed dynamics? Proc R Soc Lond [Biol] 1998;265:1377–1383. [Google Scholar]
- Reid B, Ordish RG, Harrison M. An Analysis of the Gizzard Contents of 50 North Island Brown Kiwis, Apteryx australis mantelli, and Notes on Feeding Observations. NZ J Ecol. 1982;5:76–85. [Google Scholar]
- Reid B, Williams GR. The Kiwi. In: Kuschel G, editor. Biogeography and ecology in New Zealand. The Hague: D.W. Junk; 1975. pp. 301–330. [Google Scholar]
- Shapiro L. Diet overlap and potential competition between North Island brown kiwi chicks (Apteryx mantelli) and ship rats (Rattus rattus) for limited resources on Ponui Island, New Zealand. Palmerston North: Massey University; 2006. MSc Thesis. [Google Scholar]
- Strong RM. On the olfactory organs and sense of smell in birds. J Morphol. 1911;22:619–661. [Google Scholar]
- Thompson SW. Selected Histochemical and Histopathological Methods. Springfield: Charles C Thomas Publisher; 1966. [Google Scholar]
- Wenzel BM. Olfactory prowess of kiwi. Nature. 1968;220:1133–1134. doi: 10.1038/2201133a0. [DOI] [PubMed] [Google Scholar]
- Wenzel BM. Olfactory sensation in kiwi and other birds. Ann NY Acad Sci. 1971;188:183–193. doi: 10.1111/j.1749-6632.1971.tb13097.x. [DOI] [PubMed] [Google Scholar]
- Wild JM. Peripheral and central terminations of hypoglossal afferents innervating lingual tactile mechanoreceptor complexes in Fringillidae. J Comp Neurol. 1990;298:157–171. doi: 10.1002/cne.902980203. [DOI] [PubMed] [Google Scholar]
- Zweers GA, Gerritsen AFC. Transitions from pecking to probing mechanisms in waders. Neth J Zool. 1997;47:161–208. [Google Scholar]