Abstract
The functional requirements in muscle use are related to the fiber type composition of the muscles and the cross-sectional area of the individual fibers. We investigated the heterogeneity in the fiber type composition and fiber cross-sectional area in two muscles with an opposing function, namely the digastric and masseter muscles (n = 5 for each muscle) of adult male rats, by means of immunohistochemical staining according to their myosin heavy chain (MyHC) content. The digastric and masseter muscles were taken from Wistar strain male rats 10 weeks old. In the masseter six predefined sample locations were examined; in the digastric four. Most regions showed dominant proportions of type IIA and IIX fibers. However, both muscles also revealed a regional heterogeneity in their fiber type distribution. In the digastric, type I fibers were detected only at the central and deep areas of the anterior and posterior belly, respectively. Meanwhile, the peripheral area of the anterior belly contained a higher proportion of type IIB fibers. In the masseter, the type I fibers were absent. In the superficial masseter the distribution of IIA and IIB fibers was significantly different between the superior and inferior regions. In the deep masseter, regional differences were observed among all four examined areas, of which the posterolateral region contained the highest proportion of type IIB fibers. The cross-sectional areas of type IIB fibers were always the largest, followed by the type IIX and IIA fibers. Only a few differences in cross-sectional area of corresponding fiber types were detected between the various sites. In conclusion, the masseter and digastric muscles showed an obvious heterogeneity of fiber type composition and fiber cross-sectional area. Their heterogeneity reflects the complex role of the both muscles during function. This detailed description of the fiber type composition can serve as a reference for future studies examining the muscular adaptations after the onset of various diseases in the masticatory system.
Keywords: fiber types, heterogeneity, masticatory muscle, myosin heavy chain
Introduction
Skeletal muscles contain a mixture of fibers with different contractile properties, such as maximum force, contraction velocity and fatigability (Bottinelli et al. 1996). These fibers can be classified by their myosin heavy chain (MyHC) content using immunohistochemistry. Four major fiber types (I, IIA, IIX, and IIB), associated with the equally named myosin isoforms (Korfage et al. 2005a,b), have been identified in adult skeletal muscles of small mammals (Shiaffino & Reggiani, 1996; Pette & Staron, 1997). The fiber types have been associated with physiological properties of motor units, varying from type I muscle fibers, that are easily recruited, slow contracting and fatigue resistant, to the faster contracting type II fiber which can further be divided into type IIA, IIX and IIB fibers, that are easily recruited, slow contracting and fatigue resistant, to the more faster contracting fiber types (type IIA, IIX and IIB), of which type IIB is the least easily recruited, fastest and most fatigable (Larsson & Moss, 1993).
The fiber type composition of mammalian skeletal muscle fibers is influenced by several intrinsic factors, such as heredity, age and hormones (Baldwin & Haddad, 2001; Deschenes, 2004). Furthermore, muscle fibers can adapt their phenotypic properties to meet a wide range of functional demands (Sciaffino & Reggiani, 1996; Pette, 2002). In general, an increased mechanical load in a skeletal muscle is known to lead to the adaptation of this muscle towards a slower muscle phenotype (Pette & Vrbová, 1992; Caiozzo et al. 1996). The converse response has been observed when the muscle is subjected to a decreased mechanical load (Pette & Staron, 1997). From this it can be understood that a significant positive relationship exists between muscle use and the percentage of slow type fibers (Hensbergen & Kernell, 1997; van Wessel et al. 2005). These adaptive changes are reversible (Anderson & Aagaard, 2006).
The amount of force that a muscle fiber can produce depends not only on myosin isoform type, but also on its cross-sectional area (Maughan et al. 1983). This area increases with the amount of resistance that is experienced during contraction (McCall et al. 1996). Hence determination of the fiber type composition and cross-sectional area can be used to characterize the functional properties and requirements of a muscle.
The rat is currently the most used animal model for temporomandibular joint (TMJ) research (Herring, 2003). To fully understand the TMJ anatomy and function, and the possible changes due to experiments, a good knowledge of all important factors is essential. One of these factors is the muscle forces involved, as they are a significant source of the daily loading in the masticatory system. The daily loading by the masticatory muscles can vary regionally as both their activity and fiber type composition can be heterogeneous (Miller, 1991; Korfage et al. 2005a,b). Recently, the heterogeneity in the daily use of the rat's masseter and digastric muscles has been examined (Kawai et al. 2007). However, the heterogeneity in these muscles’ fiber type compositions has so far not been examined, although some data are available on the overall fiber type composition (Tuxen & Kirkeby, 1990; Cobos et al. 2001).
The aim of this study was to examine the heterogeneity in the fiber type composition and cross-sectional area in two jaw muscles of the rat with an opposing function, namely the digastric (a jaw opener) and the masseter (a jaw closer). The fiber types were characterized by their content of myosin heavy chain isoforms (MyHC), as identified with monoclonal antibodies (Bredman et al. 1990). The division of the digastric muscle in two separate bellies suggests a different function and thus a possible disparity in their fiber type composition. From the architectural complexity of the masseter, it can be expected that this muscle will show large regional differences in the distribution and cross-sectional area of the various fiber types.
Materials and methods
Ten-week-old male rats (n = 5) were killed by an overdose of pentobarbital (Nembutal, Sanofi Sante, Maassluis, The Netherlands), and their left masseter and digastric muscles were cut from their attachment sites after they had been exposed. For the histological analysis, the anterior and posterior bellies of the digastric were separated at their interconnecting tendon. These muscles were rapidly frozen in liquid-nitrogen-cooled isopentane and stored at −80 °C until required for further processing. The experimental procedure was approved by the Animal Care and Use Committee of the Medical School of the University of Amsterdam.
Immunohistochemistry
For each of the four muscle portions (anterior and posterior digastric, superficial and deep masseter) a series of transverse sections of 10 µm was cut with a cryomicrotome (Model CM1850, Leica Microsystems GmbH, Nussloch, Germany). With respect to masseter, at first deep masseter was cut, after that superficial masseter, because masseters were taken out as a mass. Cuts were obtained only from the belly of the muscles perpendicular to the main orientation of the muscle fibers at the middle part (Fig. 1). In this way most fibers were included in the analysis.
Fig. 1.
The jaw muscles in an adult rat. (A) Lateral view. The two lines indicate the level of sectioning for deep and superficial masseter. (B) Ventral view. The two lines indicate the level of sectioning for the anterior and posterior bellies of the digastric muscle.
After overnight fixation at −20 °C in a mixture of methanol-acetone-acetic acid-water (35 : 35 : 5 : 25) (Wessels et al. 1988), four consecutive sections were incubated with monoclonal antibodies raised against purified myosin (Bredman et al. 1991; Sant’Ana Pereira et al. 1995). Antibody 219-1D1 recognized MyHC-I, antibody 333-7H1 recognized MyHC-IIA, antibody 340-3B5 recognized all fast MyHC isoforms, antibody 332-3D4 recognized MyHC-IIA and MyHC-IIX, and antibody 249-5A4 recognized MyHC-cardiac-α myosin. This antibody panel could not distinguish hybrid fibers co-expressing MyHC-IIA and -IIX from pure fibers expressing only MyHC-IIA, and hybrid fibers co-expressing MyHC-IIX and -IIB from pure fibers expressing only MyHC-IIX. Therefore, we classified them either as type IIA (recognised by antibodies 333, 332 and 340), -IIX (recognised by antibodies 332 and 340), or -IIB fibers (recognised only by antibody 340). The specificity and characterization of these monoclonal antibodies against MyHC isoforms were demonstrated previously (Wessels et al. 1988; Sant'Ana Pereira & Moorman, 1994; Sant'Ana Pereira et al. 1995). Briefly, human ATPase-defined type I and type IIA muscle fibers reacted with antibodies against MyHC-I and MyHC-IIA, respectively, and human ATPase-defined type IIB muscle fibers contained a MyHC isoform that was homologous to the MyHC-IIX isoform of rodents. The indirect unconjugated immunoperoxidase technique (PAP-technique) was applied to detect the specific binding of the different antibodies. Nickel-DAB was used to visualize the staining (Hancock, 1982).
Sample method, fiber type classification and cross-sectional area measurements
In both digastric bellies a clear heterogeneity of the fiber distribution existed (Fig. 2). To fully examine this heterogeneity, in the anterior belly sample areas were located in the central and peripheral regions. In the posterior belly, sample regions were located in the deep and superficial regions. In the masseter, no clear heterogeneity was visibly present. In this muscle sample areas were regularly divided over the entire muscle cross-section. In the superficial masseter, two sample sites were chosen from its superior and inferior regions. In the deep masseter, sample locations were chosen at the anteromedial, anterolateral, posteromedial and posterolateral regions. Each sample area was photographed with a digital camera attached to a microscope. On average, each sample area consisted of 203 fibers, which were classified by means of the four consecutive incubated sections.
Fig. 2.
Overview of the anterior (A) and posterior (B) belly of the digastric incubated with a monoclonal antibody against MyHC-I. Higher magnifications of the central (C) and peripheral (D) area in the anterior digastric. Bar = 0.25 mm.
For the measurement of the cross-sectional areas, the fiber area outlines were drawn onto a transparent sheet; after that they were quantified by reading the drawn sheets, together with a grade mark for correction of enlargement, via a flat-bed scanner (Hewlett-Packard, Scanjet 4c, Roseville, CA, USA) into a personal computer. Fibers that were cut non-orthogonally were excluded from this analysis. A custom-made program computed the cross-sectional area of each muscle fiber from the reproduced image.
Statistical analysis
For each digastric and masseter sample location, the proportions and mean cross-sectional areas of the different fiber types were calculated for each animal (n = 5) at each muscle region. Then, mean and standard deviation values were determined. All the data were tested for normality of distribution (Kolmogorov-Smirnov test) and for uniformity (Bartlett's test). The differences in the proportion and fiber cross-sectional area between sample sites were tested by one-way analysis of variance with a post-hoc test (Bonferroni test). A probability of less than 0.05 for similarity of distribution was considered to be significantly different.
Results
Fiber type composition
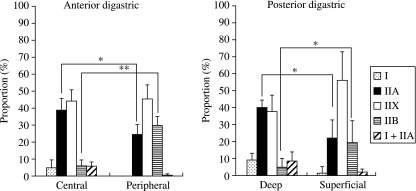
Digastric and masseter muscles showed regional heterogeneity in their fiber type composition (Fig. 2). In the anterior digastric, ca. 45% of the fibers were type IIX fibers irrespective of the intramuscular region (Fig. 3). For the other fiber types, the distribution was significantly different in the central and peripheral regions. Type I and type I + IIA fibers were detected only in the central part (each ∼5%), while the type IIB fibers made up 5 and 30% (P < 0.01) of the total for, respectively, the central and peripheral regions. At the central region, the proportion of type IIA fibers was significantly (P < 0.05) larger than that at the peripheral region. In the posterior digastric, the deep region contained a large proportion of type IIA (40%) and IIX (37%) fibers (Fig. 3). In the superficial region, these fiber types made up ca. 22% (P < 0.05) and 56% (not significant), respectively. Furthermore, the proportion of type IIB fibers was significantly (P < 0.05) larger at the superficial than at the deep region.
Fig. 3.
Proportion of fiber types in the anterior and posterior digastric. Error bars indicate a standard deviation. *Significance of difference between the values (P < 0.05); **significance of difference between the values (P < 0.01).
In the superficial masseter, the majority of fibers were of the IIX type, while almost no type I fibers were detected (Fig. 4). The proportion of type IIA fibers was larger (P < 0.05) in the inferior than in the superior region, while the proportion of type IIB fiber was larger (P < 0.05) in the superior region. In the deep masseter, regional differences were observed among all the four predefined areas. In the anterior region, almost all fibers were of the IIA or IIX types. However, the anterolateral region consisted of significantly more type IIX fibers (P < 0.01). The posterolateral part showed almost equal amounts of type IIA (31%), IIX (30%) and IIB (36%) fibers. In particular, the proportion of type IIB fiber was significantly larger (P < 0.05) than those in the other three parts. At the posteromedial part, the proportions of type IIX and IIA fibers were predominant (50% and 40%, respectively), of which the proportion of type IIX fibers was significantly (P < 0.05) larger than that in the posterolateral part. In none of the regions was MyHC-cardiac α detected.
Fig. 4.
Proportion of fiber types in the deep and superficial masseter. Error bars indicate a standard deviation. *Significance of difference between the values (P < 0.05); **significance of difference between the values (P < 0.01). (a) P < 0.05 compared with the proportions of type IIB fiber at the anteromedial, anterolateral and posterolateral regions.
Fiber cross-sectional areas
In general, for all examined sample sites, type IIB fibers were the largest (ca. 1450–2300 µm2), followed by the type IIX fibers (1040–1800 µm2).
In the anterior digastric, the cross-sectional areas of type IIA fibers were significantly (P < 0.05) larger in the central (624 ± 100 µm2) than in the peripheral region (527 ± 76 µm2) (Table 1). In the posterior digastric, the cross-sectional areas of type IIA and IIX fibers were significantly (P < 0.05) larger in the deep (916 ± 79 µm2 and 1330 ± 137 µm2) than in the superficial region (733 ± 163 µm2 and 1132 ± 153 µm2).
Table 1.
Fiber cross-sectional areas (µm2) of the digastric muscle mean ± SDa
For the masseter (Table 2), only the deep part showed differences in the cross-sectional area of the type IIA fibers. The largest difference was found in the medial region of this muscle part, where the fibers in the anteromedial region were much larger than those in the posteromedial region (1080 and 670 µm2, P < 0.01). No significant differences of the cross-sectional areas were detected between the superior and inferior areas.
Table 2.
Fiber cross-sectional areas (µm2) of the deep and superficial masseter muscle: mean ± SDa
Discussion
The functional capabilities of muscles can be determined by the size and MyHC isoforms content of their individual fibers. Previously, the global presence of fast and slow fiber types in the rat's jaw muscles was identified by ATPase analysis (Tuxen & Kirkeby, 1990), by immunohistochemistry (Cobos et al. 2001) and by the use of myosin gene expression at mRNA level (Arai et al. 2006). However, detailed quantitative measurements of their fiber type composition and cross-sectional area have not previously been conducted. The current study shows large regional differences in the fiber type composition and fiber size between various regions of the digastric and masseter muscles.
In our study, both bellies of the digastric contained type I fibers, which is consistent with previous studies (Hiraiwa, 1978; Cobos et al. 2001). However, these fibers appeared not to be homogeneous, as they were mainly found in the central area of the anterior belly and the deep area of the posterior belly. Also the distribution of the various fast fiber types in the digastric and masseter muscles showed marked differences. For instance, type IIB fibers were absent in the anterior region of the deep masseter.
These findings indicate heterogeneity in the fiber type compositions of both the digastric and masseter. This heterogeneity can be assumed to reflect the function of muscles and to verify the complex role of both muscles during function. The behavioral differences in muscle use can be related to the fiber type composition of the muscles. Slow muscles (containing predominantly MyCH-I) show a higher daily duty time and a larger daily number of bursts than fast muscles (Monster et al. 1978; Hensbergen & Kernell, 1997; Pette, 2002). We recently examined the daily jaw muscle activity in the adult rat by the use of a radio-telemetry system, and demonstrated that, compared to the superficial masseter, the anterior belly of the digastric muscle showed a higher duty time, a larger daily number of bursts and a longer average burst length (Kawai et al. 2007). This is also consistent with the absence of type I fibers in the masseter (Kawai et al. 2007).
The amount of aerobic, slow fibers may also be due to the fact that the digastric muscle plays an important role not only during the opening of the jaw, but also in stabilizing both the jaw and hyoid (Cobos et al. 2001), necessary for the positioning of the tongue. Furthermore, it is known that the rat and rabbit digastric can be activated even during closing acts (Weijs & Dantuma, 1975; Thomas & Peyton, 1983). This broad range of functions is also confirmed by the large number of low-level activities (> 100 000/day) in this muscle (Kawai et al. 2007). The findings of the presence of type I fibers in both digastric bellies, therefore, correspond to their role during various motor tasks. However, the heterogeneous distribution of these fibers suggests that this function is region specific in both digastric bellies.
In general, the masseter, as a jaw closer, plays an important role for the chewing power stroke. In rodents, the muscles contract symmetrically, alternating between muscle portions with a predominant forward and backward pulling component, resulting in a protraction and retraction of the jaw in addition to its elevation and depression (Langenbach & van Eijden, 2001). In rats, both the superficial and the deep masseter contribute to the power stroke during chewing. In addition, because the deep masseter has a more vertical orientation than the superficial masseter, the deep masseter is assumed to play an important role generating occlusal forces and keeping a mandiblar rest position. Meanwhile, the more horizontal orientation of the fibers in the superficial masseter suggests a role for jaw protrusion during the power stroke. This might involve fast and powerful muscle contractions, and especially in this muscle regions IIX and IIB type fibers were detected. In rabbits, MyHC-IIB fibers were not found in the masseter (Korfage et al. 2006). The frequency of chewing in rat mastication is faster than that in rabbit (Langenbach & van Eijden, 2001). In rats, therefore, the MyHC-IIB fibers in the digastric and masseter may contribute to a higher jaw velocity. The type IIB fibers are in the posterolateral region of the deep masseter, which shows a similar horizontal orientation of its fibers as in the superficial masseter. It could be suggested that this muscle region is involved in similar functions.
The type I + IIA fiber, which can be related to type IIC as classified by ATPase histochemistry, was defined as a skeletal fiber type that shows acid-stable and alkali-stable ATP-ase activity (Brooke & Kaiser, 1970). As for the aerobic capacity, type IIC fibers have an intermediate activity in comparison with pure type I and IIA fibers (Hintz et al. 1984). Immunohistochemically, type IIC fibers were detected only in the posterior parts (posterolateral and posteromedial) of deep masseter and in both digastric bellies. Previously, it has been reported that in the rat type IIC fibers fulfill a function which is similar to fibers that contain MyHC-cardiac α in the jaw muscles of man and rabbit (Kwa et al. 1995; Sciote & Kentish, 1996; Cobos et al. 2001). Furthermore, Kwa et al. (1995), using rabbits, demonstrated that the contraction velocity of pure MyHC-cardiac α fibers is intermediate to that of MyHC-I and MyHC-IIA. Therefore, considering the presently observed regional distribution, type I + IIA (IIC) fibers may have a similar function as the cardiac α fibers in the rabbit. It is not known why this latter type of fiber is not present in the rat.
As an animal model, rats have been used for various experiments addressing the functioning and adaptive capacities of the jaw system. Rats have the advantage of being small and easy to feed, and it is relatively simple to acquire inbred-strain rats which standardize the experimentation. The current study provides a detailed description of the normal digastric and masseter muscle contractile capabilities. This is essential in the evaluation of the relationship between the muscle activity and an experimentally induced pathological alteration, and could therefore provide valuable information in the prevention of diseases.
Acknowledgments
We are grateful to Jan Harm Koolstra for his constructive criticism. This research was supported in part by a grant (No. 18592235) for Science Research from the Ministry of Education, Science and Culture, Japan.
References
- Anderson JL, Aagaard P. Myosin heavy chain IIX overshoot in human skeletal muscle. Muscle Nerve. 2006;23:1095–1104. doi: 10.1002/1097-4598(200007)23:7<1095::aid-mus13>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Arai C, Ohnuki Y, Umeki D, Hiroshita A, Saeki Y. Effects of clenbuterol and cyclosporin a on the Myosin heavy chain mRNA level and the muscle mass in rat masseter. J Physiol Sci. 2006;56:205–209. doi: 10.2170/physiolsci.RP002206. [DOI] [PubMed] [Google Scholar]
- Baldwin KM, Haddad F. Plasticity in skeletal, cardiac, and smooth muscle. Invited review: effects of different activity and inactivity paradigms on myosin heavy chain gene expression in striated muscle. J Appl Physiol. 2001;90:345–357. doi: 10.1152/jappl.2001.90.1.345. [DOI] [PubMed] [Google Scholar]
- Bottinelli R, Canepari M, Pellegrino MA, Reggiani C. Force-velocity properties of human skeletal muscle fibres: myosin heavy chain isoforms and temperature dependence. J Physiol. 1996;495:573–586. doi: 10.1113/jphysiol.1996.sp021617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredman JJ, Weijs WA, Moorman AFM, Brugman P. Histochemical and functional fibre typing of the rabbit masseter muscle. J Anat. 1990;168:31–47. [PMC free article] [PubMed] [Google Scholar]
- Bredman JJ, Wessels A, Weijs WA, Korfage JAM, Soffers CAS, Moorman AFM. Demonstration of ‘cardiac-specific’ myosin heavy chain in masticatory muscles of human and rabbit. Histochem J. 1991;23:160–170. doi: 10.1007/BF01046587. [DOI] [PubMed] [Google Scholar]
- Brooke MH, Kaiser KK. Muscle fiber types: how many and what kind? Archs Neurol. 1970;23:369–379. doi: 10.1001/archneur.1970.00480280083010. [DOI] [PubMed] [Google Scholar]
- Caiozzo VJ, Haddad F, Baker MJ, Baldwin KM. Influence of mechanical loading on myosin heavy-chain protein and mRNA isoform expression. J Appl Physiol. 1996;80:1503–1512. doi: 10.1152/jappl.1996.80.5.1503. [DOI] [PubMed] [Google Scholar]
- Cobos AR, Segade LAG, Fuentes I. Muscle fibre types in the suprahyoid muscles of the rat. J Anat. 2001;198:283–294. doi: 10.1046/j.1469-7580.2001.19830283.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deschenes MR. Effects of aging on muscle fibre type and size. Sports Med. 2004;34:809–824. doi: 10.2165/00007256-200434120-00002. [DOI] [PubMed] [Google Scholar]
- Hancock MB. A serotonin-immunoreactive fiber system in the dorsal columns of the spinal cord. Neurosci Lett. 1982;31:247–252. doi: 10.1016/0304-3940(82)90028-3. [DOI] [PubMed] [Google Scholar]
- Hensbergen E, Kernell D. Daily durations of spontaneous activity in cat's ankle muscles. Exp Brain Res. 1997;115:325–332. doi: 10.1007/pl00005701. [DOI] [PubMed] [Google Scholar]
- Herring SW. TMJ anatomy and animal models. J Musculoskelet Neuro Inter. 2003;3:391–394. [PMC free article] [PubMed] [Google Scholar]
- Hintz CS, Coyle EF, Kaiser KK, Chi MMY, Lowry OH. Comparison of muscle fiber typing by quantitative enzyme assays and by myosin ATPase staining. J Histochem Cytochem. 1984;32:655–660. doi: 10.1177/32.6.6202737. [DOI] [PubMed] [Google Scholar]
- Hiraiwa T. Histochemical properties of masticatory muscles of growing rat and of mature mammals. Comp Biochem Phys. 1978;59A:231–238. [Google Scholar]
- Kawai N, Tanaka E, Langenbach GEJ, et al. Daily jaw muscle activity in freely moving rats measured with radio-telemetry. Eur J Oral Sci. 2007;115:15–20. doi: 10.1111/j.1600-0722.2007.00424.x. [DOI] [PubMed] [Google Scholar]
- Korfage JAM, Koolstra JH, Langenbach GEJ, van Eijden TMGJ. Fiber-type composition of the human jaw muscles-(Part 1) Origin and functional significance of fiber-type diversity. J Dent Res. 2005a;84:774–783. doi: 10.1177/154405910508400901. [DOI] [PubMed] [Google Scholar]
- Korfage JAM, Koolstra JH, Langenbach GEJ, van Eijden TMGJ. Fiber-type composition of the human jaw muscles-(Part 2) Role of hybrid fibers and factors responsible for inter-individual variation. J Dent Res. 2005b;84:784–793. doi: 10.1177/154405910508400902. [DOI] [PubMed] [Google Scholar]
- Korfage JAM, van Wessel T, Langenbach GEJ, Ay F, van Eijden TMGJ. Postnatal transitions in myosin heavy chain isoforms of the rabbit superficial masseter and digastric muscle. J Anat. 2006;208:743–751. doi: 10.1111/j.1469-7580.2006.00562.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwa SHS, Weijs WA, Juch PJW. Contraction characteristics and myosin heavy chain composition of rabbit masseter motor units. J Neurophysiol. 1995;73:538–549. doi: 10.1152/jn.1995.73.2.538. [DOI] [PubMed] [Google Scholar]
- Langenbach GEJ, van Eijden TMGJ. Mammalian feeding motor patterns. Amer Zool. 2001;41:1338–1351. [Google Scholar]
- Larsson L, Moss RL. Maximum velocity of shortening in relation to myosin isoform composition in single fibres from human skeletal muscles. J Physiol. 1993;472:595–614. doi: 10.1113/jphysiol.1993.sp019964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maughan RJ, Watson JS, Weir J. Strength and cross-sectional area of human skeletal muscle. J Physiol. 1983;33:37–49. doi: 10.1113/jphysiol.1983.sp014658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall GE, Byrnes WC, Dickinson A, Pattany PM, Fleck SJ. Muscle fiber hypertrophy, hyperplasia, and capillary density in college men after resistance training. J Appl Physiol. 1996;81:2004–2012. doi: 10.1152/jappl.1996.81.5.2004. [DOI] [PubMed] [Google Scholar]
- Miller AJ. Craniomandibular Muscles: Their Role in Function and Form. Boca Raton, FL: CRC Press; 1991. [Google Scholar]
- Monster AW, Chan H, O'Connor D. Activity patterns of human skeletal muscles: relation to muscle fiber type composition. Science. 1978;200:314–317. doi: 10.1126/science.635587. [DOI] [PubMed] [Google Scholar]
- Pette D. The adaptive potential of skeletal muscle fibers. Can J Appl Physiol. 2002;27:423–448. doi: 10.1139/h02-023. [DOI] [PubMed] [Google Scholar]
- Pette D, Staron RS. Mammalian skeletal muscle fiber type transitions. Int Rev Cytol. 1997;170:143–223. doi: 10.1016/s0074-7696(08)61622-8. [DOI] [PubMed] [Google Scholar]
- Pette D, Vrbová G. Adaptation of mammalian skeletal muscle fibers to chronic electrical stimulation. Rev Physiol Pharmacol. 1992;120:116–183. doi: 10.1007/BFb0036123. [DOI] [PubMed] [Google Scholar]
- Sant'Ana Pereira JAA, Moorman AFM. Do type IIB fibres of human muscle correspond to the IIX/D or to the IIB of rats? J Physiol. 1994;479:161–162. [Google Scholar]
- Sant'Ana Pereira JAA, Wessels A, Nijtmans L, Moorman AFM, Sargeant AJ. New method for the accurate characterization of single human skeletal muscle fibres demonstrates a relation between mATPase and MyHC expression in pure and hybrid types. J Muscle Res Cell Motil. 1995;16:21–34. doi: 10.1007/BF00125307. [DOI] [PubMed] [Google Scholar]
- Sciaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: Gene regulation and functional significance. Physiol Rev. 1996;76:371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- Sciote JJ, Kentish JC. Unloaded shortening velocities of rabbit masseter muscle fibres expressing skeletal or alpha-cardiac myosin heavy chains. J Physiol. 1996;492:659–667. doi: 10.1113/jphysiol.1996.sp021335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuxen A, Kirkeby S. An animal model for human masseter muscle: histochemical characterization of mouse, rat, rabbit, cat, dog, pig, and cow masseter muscle. J Oral Maxillofac Surg. 1990;48:1063–1067. doi: 10.1016/0278-2391(90)90290-i. [DOI] [PubMed] [Google Scholar]
- van Wessel T, Langenbach GEJ, Korfage JAM, et al. Fiber type composition of rabbit jaw muscles is related to their daily activity. Eur J Neurosci. 2005;22:2783–2791. doi: 10.1111/j.1460-9568.2005.04466.x. [DOI] [PubMed] [Google Scholar]
- Wessels A, Vermeulen JLM, Moorman AFM, Becker AE. Immunohistochemical detection of myosin heavy chain isoforms in large sections of whole human hearts. In: Carrero U, editor. Sarcomeric and Non-sarcometric Muscles: Basic and Applied Research Prospects for the 90s. Padova: Unipress; 1988. pp. 311–316. [Google Scholar]