Abstract
This study set out to determine whether the fat pad at the attachment of the Achilles tendon has features enabling it to function as an immune organ and a mechanosensory device, and to be a source of pain in insertional tendon injuries. Sections for histology and immunohistochemistry were cut from the Achilles tendon enthesis organ of 1 day old, 1 month, 4 month and 24 month old rats. For fluorescence and peroxidase immunohistochemistry, cryosections were labelled with primary antibodies directed against PGP9.5, substance P, neurofilament 200, calcitonin gene related peptide, CD68, CD36, myeloid related protein 14, actin and vinculin. The fat pad contained not only adipocytes, but also fibrous tissue, mast cells, macrophages, fibroblasts and occasional fibrocartilage cells. It was richly innervated with nerve fibres, some of which were likely to be nociceptive, and others mechanoreceptive (myelinated fibres, immunoreactive for neurofilament 200). The fibres lay between individual fat cells and in association with blood vessels. In marked contrast, the enthesis itself and all other components of the enthesis organ were aneural at all ages. The presence of putative mechanoreceptive and nociceptive nerve endings between individual fat cells supports the hypothesis that the fat pad has a proprioceptive role monitoring changes in the insertional angle of the Achilles tendon and that it may be a source of pain in tendon injuries. The abundance of macrophages suggests that the adipose tissue could have a role in combating infection and/or removing debris from the retrocalcaneal bursa.
Keywords: Achilles tendon, adipose tissue, entheses, innervation, spondyloarthropathies
Introduction
Adipose tissue is a conspicuous, though greatly neglected, component of entheses and enthesis organs (Benjamin et al. 2004b). It occurs not only within the enthesis itself, but also in the angle which the tendon/ligament makes with the bone (‘insertional angle fat’; Benjamin et al. 2004b). Elsewhere in tendons and ligaments, its presence is often dismissed as a sign of degeneration, but Benjamin et al. (2004b) have proposed a number of functions for adipose tissue at entheses. These include facilitating movement between tendon fascicles, and between the tendon or ligament and bone, dissipating stress concentration at attachment sites, and sensory perception. Kager's fat pad, which lies at the insertion of the human Achilles tendon, is one of the largest and most distinctive regions of adipose tissue associated with any enthesis. In man, it is also known as the retromalleolar fat pad (Canoso et al. 1988) and it fills the triangular space (Kager's triangle) in the posterior region of the ankle, between the tendon of flexor hallucis longus (FHL) anteriorly, the Achilles tendon posteriorly and the superior border of the calcaneus inferiorly (Ly & Bui-Mansfield, 2004). Even though the triangle itself is a well-known radiological landmark, and radiolucency of the retrocalcaneal recess in MRI images is used to rule out retrocalcaneal bursitis (Canoso et al. 1988), the structure and functions of the fat pad have remained largely unstudied. Recently however, Theobald et al. (2006) have shown that the fat has three distinctive parts, which they named according to the structures with which each is closely associated – i.e. a large superficial Achilles-associated region, a deep FHL-associated region, and a calcaneal bursal wedge that protrudes into the retrocalcaneal bursa. These authors confirmed and extended the earlier pioneering work of Canoso et al. (1988) which showed that the bursal wedge moves into the retrocalcaneal bursa during plantar flexion and out again during dorsiflexion. Both Theobald et al. (2006) and Canoso et al. (1988) have suggested that these movements minimise pressure changes within the bursa that might otherwise occur. Canoso et al. (1988) have likened the bursal wedge of fat to a freely moveable spacer that permits the tendon and bone to move apart, without creating excessive surface tension, and have made the important observation that its bursal movements are compromised in patients with seronegative spondyloarthropathy (SpA). The fat pad movements are thought to be promoted by the secretion of hyaluronan-rich synovial fluid by the fibroblast-like lining cells of its covering synovial membrane (Canoso et al. 1983).
We believe that the bursal wedge of fat has other functions that have yet to be properly investigated. (1) It could monitor the changing insertional angle which the Achilles tendon makes with the calcaneus as the foot moves and be a source of pain in insertional tendinopathies. (2) As adipose tissue is known to have immunological functions (Fantuzzi, 2005), Kager's fat pad could have an immunoprotective role for the retrocalcaneal bursa – removing debris which is known to be produced by wear and tear of its lining tissues (Rufai et al. 1995). It could also represent a source of inflammatory cells seen in SpA – conditions which commonly target the Achilles tendon.
The purpose of the present study is to provide further information on the structure of the bursal wedge of fat associated with the Achilles tendon in order to improve our understanding of the significance of such insertional angle fat. There is a comparable region of adipose tissue associated with the retrocalcaneal bursa of the rat (Rufai et al. 1996) and because of the need to examine the fat at different developmental stages, we have used this species for the current work. Our findings emphasise the importance of considering fat as a significant tissue at entheses and an integral part of many ‘enthesis organs’ (Benjamin et al. 2004a,b).
Materials and methods
Routine histology
White male Wistar rats aged 4 months were obtained from accredited commercial suppliers; 24 month old rats were bred in house at Cardiff University. The left ankle was removed from 3 rats at each age and fixed in 4% paraformadehyde (PFA) in 0.1 M phosphate buffer (PB) for 3–5 days, decalcified in 5% nitric acid, dehydrated in graded alcohols and embedded in paraffin wax. Serial sagittal sections were cut at 8 µm throughout each block and sections stained with Masson's trichrome, haematoxylin & eosin, van Gieson's elastic stain, or toluidine blue.
Immunohistochemistry
Rats aged 1day, 1 month, 4 months and 24 months old were obtained as above, and the ankle region dissected and fixed in 4% PFA for up to 4 h. Three animals were used at each age – one limb per animal. Specimens were subsequently washed in 0.1 M PB and soaked in 10% sucrose in PB overnight at 4 ºC. The tissue was then frozen in Serotec cryoprotectant (RA Lamb Medical Supplies; Eastbourne, UK) onto a cryostat chuck. Sagittal sections were cut at 10 µm on a cryostat (OFT5000; Bright Instrument Co. Ltd, Huntingdon, UK) and collected on Histobond slides (RA Lamb Medical Supplies). All labeling was carried out in a humidified chamber at room temperature unless otherwise stated.
Immunofluorescence
Sections were rehydrated with 0.1 M PB and incubated with 20% swine or goat serum for 1 h, prior to incubation with the primary antibodies – see Table 1. Following several washes with PB, the sections were incubated with the secondary antibody, swine anti-rabbit FITC (Dako UK, Ely, Cambridgeshire, UK) or goat anti-mouse FITC (Dako UK) (following pre-incubation with 1% normal rat serum (Invitrogen, Paisley, UK) for 1h at 4 °C) for 1 h, washed in PB and mounted under coverslips using Vectorshield containing DAPI to label cell nuclei (Vector Labs, Peterborough, UK). The tendon component of the enthesis organ acted as a convenient internal positive control for the actin and vinculin antibodies (Ralphs et al. 2002), and the base of the rat bladder was used as a positive control for the neuronal antibodies (Yokokawa et al. 1985; Yokokawa et al. 1986; Wakabayashi et al. 1998; Khan et al. 1999). For negative controls, representative sections were treated with 0.1 M PB, rabbit or mouse immunoglobulins (IgG; Dako UK) in place of the primary antibody.
Table 1.
List of primary antibodies used along with pretreatments, dilutions and sources. M – monoclonal antibody, P – polyclonal antibody
| Antibody | M/P | Unmasking treatment | Dilution/ Concentration | Source | Reference |
|---|---|---|---|---|---|
| Rabit anti-PGP 9.5 | P | None | 1:400 | Ultraclone, Isle of Wight, UK | Doran et al. (1983) |
| Rabbit anti-substance P | P | None | 1:2000 | Biomol, Exeter, UK | Hoyle et al. (1998) |
| Rabbit anti-calcitonin gene related peptide (ARP296/1) | P | None | 1:500 | Biomol, Exeter, UK | Skofitsch & Jacobowitz (1985) |
| Rabbit anti-neurofilament 200 | P | None | 1:2000 | Sigma-Aldrich, Poole, UK | Nakamura et al. (1987) |
| Mouse anti-hVin1 (binds to vinculin) | M | None | 10 µg/mL | Sigma-Aldrich, Poole, UK | Goncharova et al. (1992) |
| Alexa 488- conjugated phalloidin (for filamentous actin) | N/A | None | 1 U/mL | Molecular Probes, Chemicon Europe Ltd., Chandlers Ford, UK | |
| Mouse anti-COL-1 (for collagen I) | M | Hyaluronidase 30 min at 37 °C | 1:2000 | Sigma-Aldrich, Poole, UK | Mayne (1988) |
| Mouse anti-CD68 (macrophage marker for lysosome-associated antigens) | M | None | 1:400 | AbD Serotec, Oxford, UK | Damoiseaux et al. (1994) |
| Mouse anti-CD36 (UA009) (leucocyte marker) | M | None | 1:10 | Hycult biotechnology, Uden, NL | |
| Mouse anti-S100A9 (1C10) – for myeloid-related protein 14 | M | None | 1:200 | Abnova, Stratech Scientific Ltd., Newmarket, UK | |
| Rabbit IgG Normal Fraction. Negative control | N/A | None | 5 µg/mL | Dako, Ely Cambridgeshire, UK | |
| Mouse IgG1 Negative control | N/A | None | 5 µg/mL | Dako, Ely, Cambridgeshire, UK | |
| Mouse IgG2a Negative control | N/A | None | 5 µg/mL | Dako, Ely Cambridgeshire, UK |
Immunoperoxidase
Sections were rehydrated with 0.1 M PB and, where necessary, antigen retrieval procedures were performed (Table 1). Endogenous peroxidase activity was blocked with 3% hydrogen peroxide in distilled H2O for 10 min. Following washing, 20% goat, horse or swine serum was applied to the sections for 1 h, depending on the species in which the secondary antibody was raised (Table 1). All primary antibodies (Table 1) were incubated on the sections in PB, containing 0.1% Tween 20 (Sigma-Aldrich, Gillingham, UK), overnight at 4 °C. Following washing, the appropriate biotinylated secondary antibody was applied for 1 h at room temperature (following pre-incubation with 1% normal rat serum for 1 h at 4 °C) and an avidin-biotin complex (Vector Labs) applied for 30 min, following a further wash in PB. Sections were washed yet again and developed either with NovaRED or DAB substrate kits (Vector Labs). The greater omentum of 4 month rats was used as a positive control for CD68, CD36, and myeloid related protein 14 antibodies (Lagasse & Weissman, 1992; Biewenga et al. 1995). As above, negative control sections were incubated with 0.1 M, Mouse IgG1 (Dako, Cambridgeshire, UK), Mouse IgG2a (Dako, Cambridgeshire, UK), or rabbit IgG (Dako, Cambridgeshire, UK) in place of the primary antibody.
Results
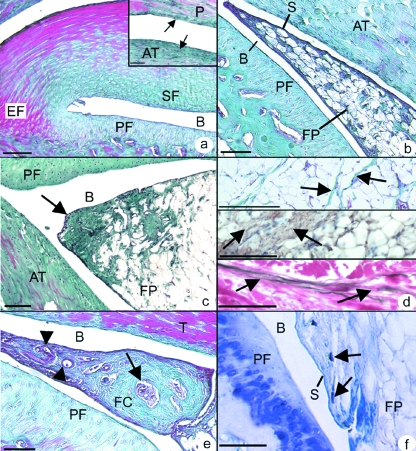
The basic structure of the enthesis organ associated with the rat Achilles tendon is similar to that previously described in man (Benjamin & McGonagle, 2001). Briefly, the tendon attachment is associated with 3 different fibrocartilages – an enthesis fibrocartilage (EF) at the tendon-bone junction, a sesamoid fibrocartilage within the deep surface of the tendon, and an opposing periosteal fibrocartilage covering the calcaneus (Fig. 1a). Further regions of fibrocartilage were also observed both near the superficial surface of the Achilles tendon and in the opposing tendon of plantaris (Fig. 1a– inset). The sesamoid and periosteal fibrocartilages form the walls of the retrocalcaneal bursa into which protrudes the tip of the synovial-covered wedge of adipose tissue (Fig. 1b) that is the focus of the current study. There were no marked differences between the fat pad of the sexually mature (4 months) and aged (24 months) rats, other than a tendency for the tip of the fat pad to become more fibrous with age (Fig. 1c).
Fig. 1.
(a) The Achilles enthesis organ in a 4 month old rat showing the enthesis (EF), sesamoid (SF) and periosteal fibrocartilages (PF). SF and PF form the walls of the retrocalcaneal bursa (B) into which the fat pad protrudes more proximally. Masson's trichrome. Scale bar = 200 µm. Inset: opposing regions of the Achilles (AT) and plantaris (P) tendons. The surface of both is fibrocartilaginous (arrows). Masson's trichrome. Scale bar = 100 µm. (b) The synovial-covered tip (S) of the fat pad (FP) protrudes into the retrocalcaneal bursa. The enthesis itself is located beyond the top left corner of the figure. 4 month rat. Masson's trichrome. Scale bar = 100 µm. (c) The tip of the fat pad (arrow) becomes more fibrous in the 24 month old rat. Masson's trichrome. Scale bar = 100 µm. (d – top) Fibrous strands (arrows) run between groups of adipocytes within the fat pad. 4 month old rat. Masson's trichrome. Scale = 200 µm. (d – middle) Fibrous strands within the fat pad immunolabelled for type I collagen (arrows). 4 month old rat. Scale bar = 100 µm. (d – bottom) Elastic fibres (arrows) in the fat pad within the fibrous strands. 4 month old rat. Van Gieson's elastic stain. Scale bar = 50 µm. (e) A bony nodule (arrow) within the tip of the fat pad of a 4 month old rat, surrounded by a region of fibrocartilage (FC) and prominent blood vessels near the tip of the pad (arrow heads) Masson's trichrome. Scale bar = 100 µm. (f) Mast cells (arrows) present within the fat pad in close association with the synovial lining (S). Toluidine blue. Scale bar = 10 µm.
Cellular composition
The fat pad was composed of unilocular adipocytes, separated by small bundles of elastic fibres and type I collagen fibres (Fig. 1d). The fat pad was anchored to the walls of the bursa by fibrous strands. Small nodules of fibrocartilage were occasionally seen near the tip of the fat pad and in one 4 month rat, the central core of this fibrocartilage contained bone (Fig. 1e).
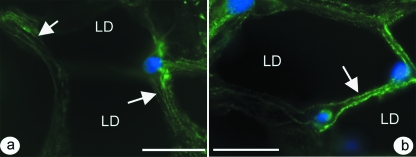
Mast cells were readily identifiable in the fat pad by the metachromasia of their granules in toluidine blue stained sections, and some lay close to blood vessels, nerves or the synovial membrane (Fig. 1f). Immunolabeling for actin with alexa488-conjugated phalloidin demonstrated the presence of filamentous actin within the cytoplasm of adipocytes and resident fibroblasts (Fig. 2a). In addition, speckled labelling for vinculin was seen both in adipocytes and in the occasional fibroblasts present between the fat cells (Fig. 2b).
Fig. 2.
(a) Adipocytes within the fat pad immunolabelled with alexa488-conjugated phalloidin. The array of actin filaments is associated with the peripheral cytoplasm (arrow). Note that the region containing the central lipid droplet (LD) in each fat cell is black. Scale bar = 20 µm. (b) Vinculin labeling in the adipocyte cytoplasm (arrow). Scale bar = 20 µm. In all cases, the nuclei have been counterstained blue with DAPI and the illustrations are from 4 month old rats.
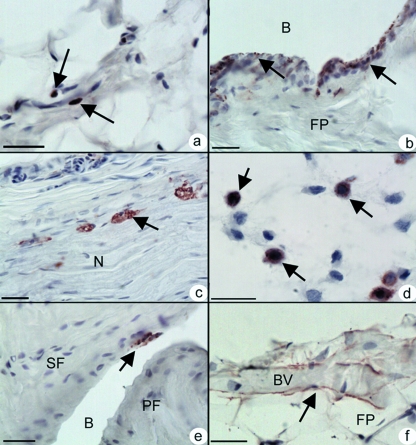
In rats of all ages, CD68 positive macrophages were identifiable within the fat pad (Fig. 3a), though the number varied greatly between animals. Such cells were rarely seen within the synovial membrane of the neonatal rat, but the number increased with age (Fig. 3b). Many of the CD68 macrophages were closely associated with blood vessels and the connective tissue of large nerve bundles (Fig. 3c). Neonates had a particularly large number of positive cells in relation to the size of the developing fat pad (Fig. 3d). In 24 month old rats, positive cells were present not only in the fat pad, but also on the surface of the sesamoid and periosteal fibrocartilages in the retrocalcaneal bursa (Fig. 3e). In contrast, there were very few positive cells labeling at any age with CD36 (not shown). Myeloid related protein 14 (MRP14) expression was most commonly detected in cells adjacent to blood vessels and in their endothelium – particularly in 24 month old rats (Fig. 3f).
Fig. 3.
The cell composition of the fat pad (a) CD68 positive cells (arrows) within the fat pad of a 4 month old rat. Scale bar = 20 µm (b) The abundance of CD68 positive cells (arrows) in the synovial lining of the fat pad in a 4 month old rat, contrasts with the rare finding of these cells in the synovium of neonates (not shown). B – Bursa. Scale bar = 20 µm. (c) CD68 positive cells (arrows) associated with a bundle of nerves (N) in the fat pad. 4 month old rat. Scale bar = 20 µm (d) The presumptive fat pad in the neonate. Note the large population of CD68 positive cells (arrows) that are present before pronounced differentiation of the adipocytes themselves. Scale bar = 20 µm. (e) CD68 positive cells (arrow) on the surface of the sesamoid fibrocartilage (SF) in a 4 month old rat. PF – Periosteal fibrocartilage. (f) Labelling for myeloid-related protein 14 that is associated with blood vessels (BV), is particularly prominent in 24 month old rats. Scale bar = 20 µm. All sections have been counterstained with Mayer's haematoxylin.
Innervation
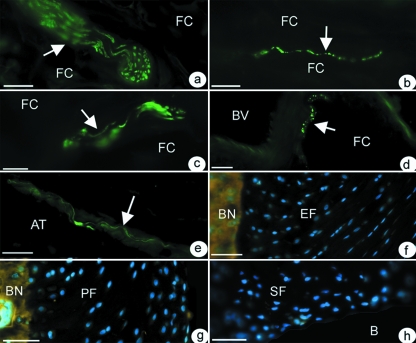
Immunohistochemical labeling of rat tissue with the general nerve marker – PGP 9.5 – showed that the fat pad was the only component of the enthesis organ which was innervated at all ages (Fig. 4a–d). Thus, no nerve fibres (sensory, motor or autonomic) were present in any of the fibrocartilages associated with the Achilles tendon in any animal (Fig. 4f–h). Neither was the acellular bursal lining of the sesamoid and periosteal fibrocartilages positive for PGP 9.5. Blood vessel-associated nerve fibres were, however, present in the paratenon. Some of these nerves labeled with antibodies against substance P and CGRP (indicating that they were nociceptive), others labeled with antibody neurofilament 200 (suggesting mechanoceptors; Fig 4e). No nerve fibres were present in the region where the Achilles tendon was in contact with plantaris.
Fig. 4.
The innervation of the fat pad (a–d). Nerve fibres are arrowed that are immunoreactive to (a) PGP 9.5 – in a large bundle of nerve fibres within the fat. FC – fat cells. Scale bar = 30 µm (b) Substance P – in a naked nerve fibre running between adjacent fat cells. Scale bar = 20 µm (c) Neurofilament 200 in a medium-sized bundle of nerve fibres in the fat. Scale bar = 30 µm (d) CGRP – in close association with a large blood vessel (BV). Scale bar = 20 µm (e) Nerve fibres in the epitenon labelled with neurofilament 200. AT – Achilles tendon. Scale bar = 30 µm. In contrast to the fat pad, the enthesis (f), periosteal (g) and sesamoid (h) fibrocartilages (EF, PF, and SF respectively) are all devoid of nerve fibres and thus all fail to label with the pan neurofilament marker PGP 9.5. In the absence of any neuronal labelling in f–h, the cell nuclei are counterstained blue with DAPI to show the tissue itself. B, bursa; BN, bone. Scale bars for f–h = 50 µm. All sections were from 4 month old rats.
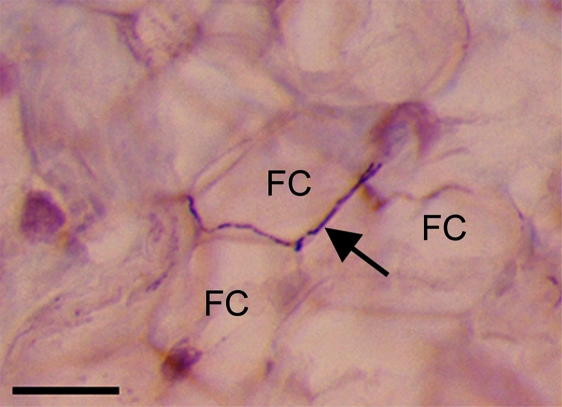
The nerve fibres/bundles within the fat pad were more common in the proximal region than at the tip. They typically lay between adjacent fat cells (Fig. 5), though some were present within and beneath the synovial membrane and where the synovium was reflected onto the tendon or the periosteum. All fibres within the fat pad were immunoreactive to PGP 9.5 (Fig. 4a). Many (but not all) of the nerve fibres innervating the fat pad (both blood-vessel associated and ‘free’) contained 200 kD neurofilaments (Fig. 4c). A qualitative evaluation suggested that the number of fibres immunoreactive to PGP 9.5 and neurofilament 200 increased with age up to 4 months, but that the number of positive fibres was less in 24 month old animals. Vessel-associated and ‘free’ nerve fibres immunoreactive to CGRP (Fig. 4d) and substance P (Fig. 4b) were also present in the fat pad at all ages studied and labeling here also increased with development up to 4 months of age. However, peptidergic fibres (i.e. substance P or CGRP-containing fibres) did not become less abundant in aged rats. In all animals, NF200 nerve fibres were more abundant within the fat pad than those immunoreactive to either substance P or CGRP.
Fig. 5.
A CGRP-positive fibre (arrow) running between adjacent fat cells (FC) in a 4 month old rat. Scale bar = 30 µm. Counterstained with Mayer's haematoxylin.
Discussion
Previous studies have highlighted the biomechanical importance of Kager's fat pad and particularly its calcaneal bursal wedge (Canoso et al. 1988; Theobald et al. 2006). The bursal wedge fails to move into the retrocalcaneal bursa in patients with SpA (Canoso et al. 1988). Our present results point to further structural features of the fat pad, which not only reflect its mechanical role but also support the hypothesis that it has additional functions. The co-localisation of actin filaments and vinculin in its adipocytes indicates the presence of focal contacts, which mechanically link actin microfilaments in the cell cytoplasm to the extracellular matrix via the transmembrane linkers – integrins. As focal contacts are known to be important in mechanosensitive cell signaling (Bershadsky et al. 2003; D’Addario et al. 2003), they are likely to link the fibrous components of the fat pad to the adipocytes, thus integrating the fibro-adipose nature of the tissue. The occasional signs of fibrocartilage differentiation suggest that the fat is subject to some degree of compression and the fibrous strands point to a degree of resilience that it must have against tensile loading. Hence, like the fat pad in the heel, Kager's pad could act as a shock absorber (Jahss et al. 1992). We conclude that it is likely that the fat contributes to dissipating stress away from the tendon-bone interface, like other depots of ‘insertional angle fat’ (Benjamin et al. 2004b). It is thus truly an integral part of the Achilles tendon enthesis organ (Benjamin et al. 2004a).
Our study shows that the normal Achilles enthesis is not innervated. Despite an exhaustive search for nerve fibres in rats of all ages, none was found within the enthesis, sesamoid or periosteal fibrocartilages. This was paralleled by an absence of blood vessels in these tissues and recalls the avascular and aneural nature of articular cartilage. The lack of nerve fibres is likely to reflect the relatively high levels of compression to which the fibrocartilages are subject, although the factors responsible for this are unknown. However, aggrecan may be important in accounting for the absence of nerves, as one of its major glycosaminoglycans (chondroitin sulphate) is known to act as an axonal growth inhibitor in the central nervous system (Johnson et al. 2002) and it is present in enthesis organ fibrocartilages (Waggett et al. 1998; Milz et al. 2005). It is a key molecule associated with compression-tolerance (Milz et al. 2005) and it also inhibits endothelial cell adhesion and migration (Johnson et al. 2005). Hence, vessel and nerve ingrowth reported previously in the entheses of elderly human Achilles tendons (Benjamin et al. 2007) may follow degenerative changes in the fibrocartilage, which are common in older people (Rufai et al. 1995). However, this does not explain why nerve fibres were also absent at birth, before the fibrocartilage had started to differentiate (Rufai et al. 1992).
In contrast to the aneural nature of enthesis organ fibrocartilages, the fat pad contains a large number of nerve fibres, including putative mechanoreceptors and nociceptors. The former could be important in monitoring movements of the fat pad during plantar and dorsi-flexion. Because of the intimate association between fat cells and their nerve fibres, the slightest deformation of the adipose tissue accompanying foot movements could trigger action potentials in the proprioceptive fibres. Equally, nociceptive fibres could be stimulated by any abnormal loading of the tissue. This could occur for example when fluid accumulates in patients with retrocalcaneal bursitis and the fat pad is thus subject to abnormal compression (Canoso, 1998). It is interesting to note that Hoffa's fat pad also contains nociceptive fibres and that an increased number occurs in ‘jumper's knee’ (Witonski & Wagrowska-Danielewicz, 1999). The number of mechanoreceptive fibres within the Achilles fat pad varies with age – increasing during growth up to sexual maturity, and then decreasing with old age. However, an age-related decrease in peptidergic fibres was not obvious and this is in accordance with the findings of Bergman et al. (1999) that mechanoreceptors are preferentially affected by age in comparison to nociceptive/peptidergic fibres. Finally, the presence of mast cells within the fat pad suggests the possibility of neurogenic inflammation in association with Achilles-related pain (McQueen, 1999). Peptidergic fibres may also play a vasodilatory role, affecting the blood vessels with which they are so closely associated. This can lead to tissue oedema and inflammation under conditions of tissue damage causing pain (McQueen, 1999).
CD68 labeling demonstrated the presence of a striking number of macrophages. These cells may be compared to macrophages demonstrated previously in the greater omentum. This too is a fatty synovial fold and one which provides a route for macrophages to enter the peritoneal cavity (Krist et al. 1995). If macrophages can pass from Kager's pad into the retrocalcaneal bursa, they could play a role in combating infection and/or removing cell debris. Macrophages that we identified on the surface of the bursal wall fibrocartilages may also be important. In man, we have previously demonstrated that debris is generated as a result of wear and tear of the lining periosteal and sesamoid fibrocartilages (Rufai et al. 1995). Fat at entheses could also be a source of the macrophages present in patients with SpA (McGonagle et al. 2002). It is interesting to note that along with the Achilles tendon, other entheses commonly affected in SpA are also associated with significant quantities of fat at their entheses – e.g. the proximal and distal attachments of the patellar tendon and the calcaneal enthesis of the plantar aponeurosis. Fat may thus be an important immune organ at healthy attachment sites, but also one that may be implicated in autoimmune or autoinflammatory conditions.
In conclusion, our study presents new data relating to the innervation and cellular composition of fat at the attachment of the Achilles tendon, which highlights the possibility of several novel functions of the fat pad. Our findings reinforce the importance of considering fat when trying to make sense of insertional disorders affecting the Achilles tendon.
Acknowledgments
We wish to thank Dr Jim Ralphs for supplying the vinculin and actin antibodies. Hannah Shaw is an Anatomical Society of Great Britain and Ireland studentship holder.
References
- Benjamin MHT, Suzuki D, Redman S, Emery P, McGonagle D. Microdamage and altered vascularity at the enthesis-bone interface provides an anatomic explanation for bone involvement in the HLA-B27 associated spondyloarthrides and allied disorders. Arthritis Rheum. 2007;56:224–233. doi: 10.1002/art.22290. [DOI] [PubMed] [Google Scholar]
- Benjamin M, McGonagle D. The anatomical basis for disease localisation in seronegative spondyloarthropathy at entheses and related sites. J Anat. 2001;199:503–526. doi: 10.1046/j.1469-7580.2001.19950503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin M, Moriggl B, Brenner E, Emery P, McGonagle D, Redman S. The ‘enthesis organ’ concept: Why enthesopathies may not present as focal insertional disorders. Arthritis Rheum. 2004a;50:3306–3313. doi: 10.1002/art.20566. [DOI] [PubMed] [Google Scholar]
- Benjamin M, Redman S, Milz S, Buttner A, et al. Adipose tissue at entheses: the rheumatological implications of its distribution. A potential site of pain and stress dissipation? Ann Rheum Dis. 2004b;63:1549–1555. doi: 10.1136/ard.2003.019182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergman E, Fundin BT, Ulfhake B. Effects of aging and axotomy on the expression of neurotrophin receptors in primary sensory neurons. J Comp Neurol. 1999;410:368–386. doi: 10.1002/(sici)1096-9861(19990802)410:3<368::aid-cne2>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Bershadsky AD, Balaban NQ, Geiger B. Adhesion-dependent cell mechanosensitivity. Annu Rev Cell Dev Biol. 2003;19:677–695. doi: 10.1146/annurev.cellbio.19.111301.153011. [DOI] [PubMed] [Google Scholar]
- Biewenga J, van der Ende MB, Krist LF, Borst A, Ghufron M, van Rooijen N. Macrophage depletion in the rat after intraperitoneal administration of liposome-encapsulated clodronate: depletion kinetics and accelerated repopulation of peritoneal and omental macrophages by administration of Freund's adjuvant. Cell Tissue Res. 1995;280:189–196. doi: 10.1007/BF00304524. [DOI] [PubMed] [Google Scholar]
- Canoso JJ. The premiere enthesis. J Rheumatol. 1998;25:1254–1256. [PubMed] [Google Scholar]
- Canoso JJ, Liu N, Traill MR, Runge VM. Physiology of the retrocalcaneal bursa. Ann Rheum Dis. 1988;47:910–912. doi: 10.1136/ard.47.11.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canoso JJ, Stack MT, Brandt KD. Hyaluronic acid content of deep and subcutaneous bursae of man. Ann Rheum Dis. 1983;42:171–175. doi: 10.1136/ard.42.2.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Addario M, Arora PD, Ellen RP, McCulloch CA. Regulation of tension-induced mechanotranscriptional signals by the microtubule network in fibroblasts. J Biol Chem. 2003;278:53090–53097. doi: 10.1074/jbc.M309027200. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JG, Dopp EA, Calame W, Chao D, MacPherson GG, Dijkstra CD. Rat macrophage lysosomal membrane antigen recognized by monoclonal antibody ED1. Immunology. 1994;83:140–147. [PMC free article] [PubMed] [Google Scholar]
- Doran JF, Jackson P, Kynoch PA, Thompson RJ. Isolation of PGP 9.5, a new human neurone-specific protein detected by high-resolution two-dimensional electrophoresis. J Neurochem. 1983;40:737–742. doi: 10.1111/j.1471-4159.1983.tb08124.x. [DOI] [PubMed] [Google Scholar]
- Fantuzzi G. Adipose tissue, adipokines, and inflammation. J Allergy Clin Immunol. 2005;115:911–919. doi: 10.1016/j.jaci.2005.02.023. quiz 920. [DOI] [PubMed] [Google Scholar]
- Goncharova EJ, Kam Z, Geiger B. The involvement of adherens junction components in myofibrillogenesis in cultured cardiac myocytes. Development. 1992;114:173–183. doi: 10.1242/dev.114.1.173. [DOI] [PubMed] [Google Scholar]
- Hoyle CH, Chakrabarti G, Pendleton NP, Andrews PL. Neuromuscular transmission and innervation in the urinary bladder of the insectivore Suncus murinus. J Auton Nerv Syst. 1998;69:31–38. doi: 10.1016/s0165-1838(98)00002-2. [DOI] [PubMed] [Google Scholar]
- Jahss MH, Michelson JD, Desai P, et al. Investigations into the fat pads of the sole of the foot: anatomy and histology. Foot Ankle. 1992;13:233–242. doi: 10.1177/107110079201300502. [DOI] [PubMed] [Google Scholar]
- Johnson WE, Caterson B, Eisenstein SM, Hynds DL, Snow DM, Roberts S. Human intervertebral disc aggrecan inhibits nerve growth in vitro. Arthritis Rheum. 2002;46:2658–2664. doi: 10.1002/art.10585. [DOI] [PubMed] [Google Scholar]
- Johnson WE, Caterson B, Eisenstein SM, Roberts S. Human intervertebral disc aggrecan inhibits endothelial cell adhesion and cell migration in vitro. Spine. 2005;30:1139–1147. doi: 10.1097/01.brs.0000162624.95262.73. [DOI] [PubMed] [Google Scholar]
- Khan MA, Dashwood MR, Thompson CS, Mumtaz FH, Morgan RJ, Mikhailidis DP. Time-dependent up-regulation of neuronal 5-hydroxytryptamine binding sites in the detrusor of a rabbit model of partial bladder outlet obstruction. World J Urol. 1999;17:255–260. doi: 10.1007/s003450050142. [DOI] [PubMed] [Google Scholar]
- Krist LF, Eestermans IL, Steenbergen JJ. Cellular composition of milky spots in the human greater omentum: an immunochemical and ultrastructural study. Anat Rec. 1995;241:163–174. doi: 10.1002/ar.1092410204. [DOI] [PubMed] [Google Scholar]
- Lagasse E, Weissman IL. Mouse MRP8 and MRP14, two intracellular calcium-binding proteins associated with the development of the myeloid lineage. Blood. 1992;79:1907–1915. [PubMed] [Google Scholar]
- Ly JQ, Bui-Mansfield LT. Anatomy of and abnormalities associated with Kager's fat Pad. Am J Roentgenol. 2004;182:147–154. doi: 10.2214/ajr.182.1.1820147. [DOI] [PubMed] [Google Scholar]
- Mayne R. Preparation and applications of monoclonal antibodies to different collagen types. Clin Biochem. 1988;21:111–115. doi: 10.1016/s0009-9120(88)80098-5. [DOI] [PubMed] [Google Scholar]
- McGonagle D, Marzo-Ortega H, O’Connor P, et al. Histological assessment of the early enthesitis lesion in spondyloarthropathy. Ann Rheum Dis. 2002;61:534–537. doi: 10.1136/ard.61.6.534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McQueen DS. Inflammatory pain and the joint. Basel: Birkhanser; 1999. [Google Scholar]
- Milz S, Benjamin M, Putz R. Molecular parameters indicating adaptation to mechanical stress in fibrous connective tissue. Adv Anat Embryol Cell Biol. 2005;178:1–71. [PubMed] [Google Scholar]
- Nakamura T, Kawahara H, Miyashita H, Watarai K, Takagi M, Tachibana S. Cross reactive identification of types 1 and 2C fibers in human skeletal muscles with monoclonal anti-neurofilament (200 kd) antibody. Histochemistry. 1987;87:39–45. doi: 10.1007/BF00518722. [DOI] [PubMed] [Google Scholar]
- Ralphs JR, Waggett AD, Benjamin M. Actin stress fibres and cell-cell adhesion molecules in tendons: organisation in vivo and response to mechanical loading of tendon cells in vitro. Matrix Biol. 2002;21:67–74. doi: 10.1016/s0945-053x(01)00179-2. [DOI] [PubMed] [Google Scholar]
- Rufai A, Benjamin M, Ralphs JR. Development and ageing of phenotypically distinct fibrocartilages associated with the rat Achilles tendon. Anat Embryol (Berl) 1992;186:611–618. doi: 10.1007/BF00186984. [DOI] [PubMed] [Google Scholar]
- Rufai A, Ralphs JR, Benjamin M. Structure and histopathology of the insertional region of the human Achilles tendon. J Orthop Res. pp. 585–593. [DOI] [PubMed]
- Rufai A, Ralphs JR, Benjamin M. Ultrastructure of fibrocartilages at the insertion of the rat Achilles tendon. J Anat. pp. 185–191. [PMC free article] [PubMed]
- Skofitsch G, Jacobowitz DM. Calcitonin gene-related peptide: detailed immunohistochemical distribution in the central nervous system. Peptides. 1985;6:721–745. doi: 10.1016/0196-9781(85)90178-0. [DOI] [PubMed] [Google Scholar]
- Theobald P, Bydder G, Dent C, Nokes L, Pugh N, Benjamin M. The functional anatomy of Kager's fat pad in relation to retrocalcaneal problems and other hindfoot disorders. J Anat. 2006;208:91–97. doi: 10.1111/j.1469-7580.2006.00510.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waggett AD, Ralphs JR, Kwan AP, Woodnutt D, Benjamin M. Characterization of collagens and proteoglycans at the insertion of the human Achilles tendon. Matrix Biol. 1998;16:457–470. doi: 10.1016/s0945-053x(98)90017-8. [DOI] [PubMed] [Google Scholar]
- Wakabayashi Y, Maeda T, Tomoyoshi T, Kwok YN. Increase of growth-associated protein-43 immunoreactivity following cyclophosphamide-induced cystitis in rats. Neurosci Lett. 1998;240:89–92. doi: 10.1016/s0304-3940(97)00933-6. [DOI] [PubMed] [Google Scholar]
- Witonski D, Wagrowska-Danielewicz M. Distribution of substance-P nerve fibers in the knee joint in patients with anterior knee pain syndrome. A preliminary report. Knee Surg Sports Traumatol Arthrosc. 1999;7:177–183. doi: 10.1007/s001670050144. [DOI] [PubMed] [Google Scholar]
- Yokokawa K, Sakanaka M, Shiosaka S, Tohyama M, Shiotani Y, Sonoda T. Three-dimensional distribution of substance P-like immunoreactivity in the urinary bladder of rat. J Neural Transm. 1985;63:209–222. doi: 10.1007/BF01252026. [DOI] [PubMed] [Google Scholar]
- Yokokawa K, Tohyama M, Shiosaka S, et al. Distribution of calcitonin gene-related peptide-containing fibers in the urinary bladder of the rat and their origin. Cell Tissue Res. 1986;244:271–278. doi: 10.1007/BF00219202. [DOI] [PubMed] [Google Scholar]