Abstract
Leftward volume asymmetry of the pars opercularis and pars triangularis may exist in the human brain, frequently referred to as Broca's area, given the functional asymmetries observed in this region with regard to language expression. However, post-mortem and magnetic resonance imaging (MRI) studies have failed to consistently identify such a volumetric asymmetry. In the present study, an analysis of the asymmetry of sulco-gyral anatomy and volume of this anterior speech region was performed in combination with an analysis of the morphology and volume asymmetry of the planum temporale, located within the posterior speech region, in 50 healthy subjects using MRI. Variations in sulcal anatomy were documented according to strict classification schemes and volume estimation of the grey matter within the brain structures was performed using the Cavalieri method of stereology. Results indicated great variation in the morphology of and connectivity between the inferior frontal, inferior precentral and diagonal sulci. There were significant inter-hemispheric differences in the presence of (1) the diagonal sulcus within the pars opercularis, and (2) horizontal termination of the posterior Sylvian fissure (relative to upward oblique termination), both with an increased leftward incidence. Double parallel inferior precentral sulci and absent anterior rami of the Sylvian fissure prevented stereological measurements in five subjects. Therefore volumes were obtained from 45 subjects. There was a significant leftward volume asymmetry of the pars opercularis (P = 0.02), which was significantly related to the asymmetrical presence of the diagonal sulcus (P < 0.01). Group-wise pars opercularis volume asymmetry did not exist when a diagonal sulcus was present in both or neither hemispheres. There was no significant volume asymmetry of the pars triangularis. There was a significant leftward volume asymmetry of the planum temporale (P < 0.001), which was significantly associated with the shape of the posterior Sylvian fissure as a unilateral right or left upward oblique termination was always associated with leftward or rightward volume asymmetry respectively (P < 0.01). There was no relationship between volume asymmetries of the anterior and posterior speech regions. Our findings illustrate the extent of morphological variability of the anterior speech region and demonstrate the difficulties encountered when determining volumetric asymmetries of the inferior frontal gyrus, particularly when sulci are discontinuous, absent or bifid. When the intrasulcal grey matter of this region is exhaustively sampled according to strict anatomical landmarks, the volume of the pars opercularis is leftward asymmetrical. This manuscript illustrates the importance of simultaneous consideration of brain morphology and morphometry in studies of cerebral asymmetry.
Keywords: Broca's area, cerebral cortex, diagonal sulcus, inferior frontal gyrus, language
Introduction
It has often been assumed that leftward structural asymmetry of the pars opercularis and pars triangularis exists in the human brain, given the specialisation of these cortical regions for language expression, the left cerebral hemisphere's dominance for language processing, and the leftward asymmetry of posterior speech regions, notably the planum temporale. However, unlike asymmetry of the planum temporale, asymmetry of the frontal operculum is not a robust finding. Furthermore, the relationship between the cortical asymmetries of the anterior and posterior speech regions is unknown. The present study sought to assess the asymmetry of the sulco-gyral anatomy (i.e. morphology) and volume (i.e. morphometry) of the pars opercularis and pars triangularis, and the relationship of these measures to the volume of the planum temporale. Simultaneous analysis of the volume, morphology and variability of the brain regions historically associated with unilateral hemispheric function may provide a structural basis for the between-human variability observed during language processing.
The pars opercularis and pars triangularis are frequently referred to as Broca's area of the cerebral cortex. By definition, Broca's area is the region of the cerebral cortex that mediates expressive speech, and damage to which causes expressive aphasia of the type Paul Broca observed in the nineteenth century (Broca, 1861a, 1861b, 1863, 1865). Based on assessment of gross macroscopic neuroanatomy, Broca maintained that the crucial region for this human function was the posterior region of the left third frontal convolution, corresponding to the left pars opercularis in particular, and also the pars triangularis. However, given that the definition of Broca's area is a clinico-functional one, and not an anatomical one, it is clear that this ‘area’ cannot be confined to the convolutions of the inferior frontal gyrus alone as expressive aphasia can occur in the absence of lesions to this region, and there is a widespread topology associated with Broca's aphasia (Mohr et al. 1978; Mohr, 1979; Tramo et al. 1988; Alexander et al. 1990). Nevertheless, the pars opercularis and pars triangularis are cortical regions crucial for the production of speech as shown by a wealth of functional neuroimaging, electrical stimulation and lesion studies. This functional and clinical literature has given impetus to anatomical studies of the asymmetry of this area.
Leftward asymmetry of the posterior regions of the inferior frontal gyrus is not a robust finding. The literature generally indicates that in humans, (1) cytoarchitectonic studies frequently report leftward asymmetry of area 44 and/or area 45 (Hayes & Lewis, 1993, 1995; Amunts et al. 1999, 2003; Uylings et al. 2005, 2006), which are the closest – but not corresponding – cellular sub-regions to the pars opercularis and pars triangularis respectively, (2) presence of the diagonal sulcus in the pars opercularis is more frequently observed in the left hemisphere relative to the right, although no significant hemispheric asymmetry has been demonstrated (Galaburda, 1980; Ono et al. 1990; Tomaiuolo et al. 1999), (3) post-mortem studies reveal a leftward asymmetry of the posterior regions of the inferior frontal gyrus only when the intra-sulcal anatomy is quantified in conjunction with the exterior convexity as opposed to the exterior convexity alone (Wada et al. 1975; Falzi et al. 1982; Albanese et al. 1989), and (4) there are great between-study discrepancies in the reporting of asymmetry of the posterior regions of the inferior frontal gyrus in MRI studies (Foundas et al. 1995, 1996, 1998, 2001a, 2001b; Tomaiuolo et al. 1999; Good et al. 2001; Watkins et al. 2001; Luders et al. 2004; Hervé et al. 2006; Knaus et al. 2006; Hammers et al. 2007). All studies have sought to determine leftward asymmetry of the anterior speech region given the functional lateralisation of this area, but many have failed to do so. The reasons for this are likely to be due to differential methodological applications, anatomical definitions, and sample sizes.
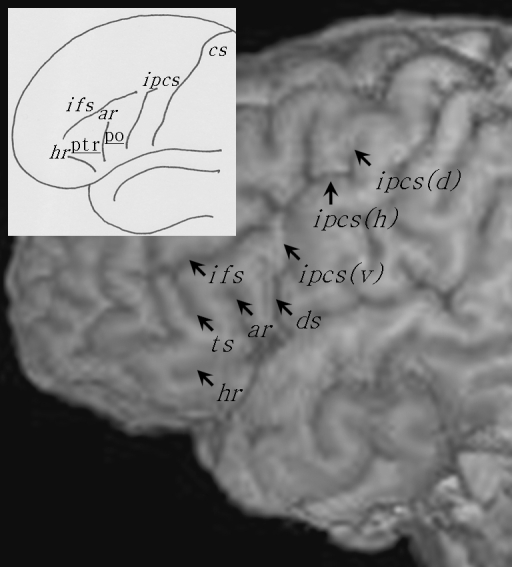
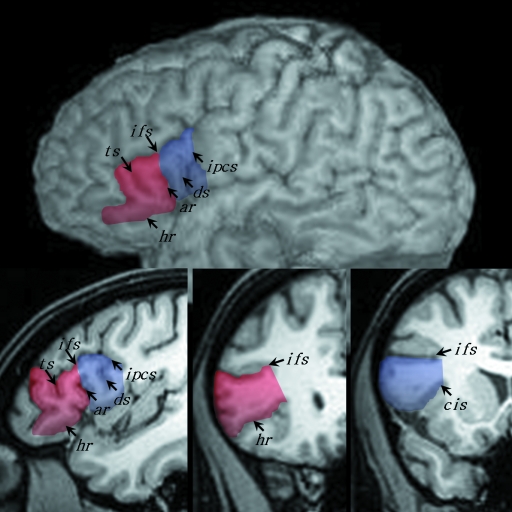
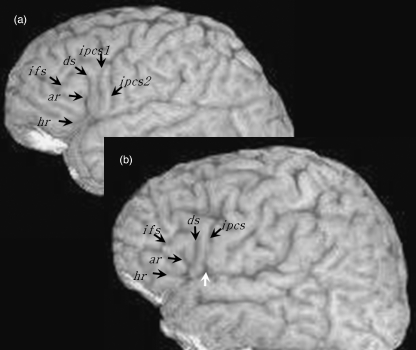
One advantage of the present study is with regard to the combined analysis of inter-hemispheric asymmetry of volume (i.e. morphometry) and sulcal anatomy (i.e. morphology). Figure 1 illustrates the external sulcal anatomy defining the pars opercularis and pars triangularis. The pars opercularis (po) is demarcated caudally from the ventral precentral gyrus by the ventral segment of the inferior precentral sulcus [ipcs(v)], dorsally from the middle frontal gyrus by the inferior frontal sulcus (ifs) and rostrally from the pars triangularis (ptr) by the anterior ascending ramus of the Sylvian fissure (ar). The pars triangularis is demarcated caudally from the pars opercularis by the anterior ascending ramus of the Sylvian fissure, dorsally from the middle frontal gyrus by the inferior frontal sulcus, and rostro-ventrally from the pars orbitalis by the anterior horizontal ramus of the Sylvian fissure (hr). The pars orbitalis lies between the anterior horizontal ramus of the Sylvian fissure and the lateral orbital sulcus. Within the pars opercularis there is occasionally a sulcus present called the diagonal sulcus (ds), which is clearly distinguishable from the anterior ascending ramus of the Sylvian fissure. More frequently there is a sulcus present within the pars triangularis called the triangular sulcus (ts). These sulcal contours are generally considered to demarcate the pars opercularis and pars triangularis from surrounding cortex (Duvernoy, 1999; Petrides, 2006), and are used as anatomical boundaries to estimate gyral volume of these regions (Wada et al. 1975; Falzi et al. 1982; Albanese et al. 1989; Tomaiuolo et al. 1999). There is however great inter-individual variability in the shape, length, and number of these sulcal contours which gives rise to great variability in size, surface area and volume of the pars opercularis and pars triangularis. Therefore, volume estimation of these variably shaped convoluted structures is more complex than of brain structures with relatively little between-individual morphological variation, such as deep subcortical nuclei. In the present study, we sought to estimate volume and determine volume asymmetries of the pars opercularis and pars triangularis, with simultaneous documentation of morphological variability of the sulcal contours defining these regions. In this light, we aim to provide a combined morphological and morphometric analysis of cerebral asymmetry of the pars opercularis and pars triangularis, which will enable us to evaluate the impact of anatomical variability on quantitative measurements.
Fig. 1.
The major sulcal contours defining the pars opercularis (po) and pars triangularis (ptr) of the inferior frontal gyrus. The main image shows the sulcal contours from the lateral convexity of a brain studied in the present investigation. The insert presents the major sulcal contours defining the pars opercularis and pars triangularis schematically (connections and precise sulcal morphology do not necessarily correspond to the main image). The pars opercularis is the region of cortex located between the ventral segment of the inferior precentral sulcus [ipcs(v)], the inferior frontal sulcus (ifs) and the anterior ascending ramus of the Sylvian fissure (ar). The pars triangularis is the region of cortex located between the anterior ascending ramus, inferior frontal sulcus and the anterior horizontal ramus of the Sylvian fissure (hr). In this example, a diagonal sulcus (ds) is present, and connects to the ventral segment of the inferior precentral sulcus. A triangular sulcus (ts) is also present, and connects to the inferior frontal sulcus. The inferior precentral sulcus consists of three segments, the ventral segment, a horizontal segment [ipcs(h)] and a dorsal vertical segment [ipcs(d)]. Cs, central sulcus.
Using a mathematically unbiased stereological technique, strict anatomical definitions and combined morphological and volumetric analyses on magnetic resonance (MR) images, the following research questions were addressed in the present study. Firstly, is there a significant hemispheric asymmetry in the morphology (i.e. variability in continuity, connections and presence of sulci) of the pars opercularis and pars triangularis? This study sought to document the anatomy of the sulcus lying within the pars opercularis (the diagonal sulcus) and the sulci that define the pars opercularis and pars triangularis (inferior precentral sulcus, inferior frontal sulcus, ascending ramus of the Sylvian fissure, and horizontal ramus of the Sylvian fissure) in the left and right cerebral hemispheres. Secondly, are there volume asymmetries of the grey matter of the pars opercularis and pars triangularis, and do such asymmetries relate to variability of the sulcal anatomy? The intra-sulcal grey matter was quantified within the pars opercularis and pars triangularis using the Cavalieri method of modern design stereology. Thirdly, is the asymmetry of the anterior speech region related to the asymmetry of posterior speech regions? To answer this, the volume of the planum temporale was also determined using stereological methods. Finally, are these regional asymmetries related to the more gross fronto-occipital torque asymmetry? Fronto-occipital torque is the typical frontal-rightward and occipital-leftward brain asymmetry that appears like an anti-clockwise twist around the longitudinal fissure (Bear et al. 1986; Toga & Thompson, 2003).
Methods
Subjects
Twenty females and 30 males of mean (± SD) age 45.2 ± 14.1 years were investigated, all of whom were neurologically and psychiatrically healthy. Subjects were right (n = 37; 13 females, 24 males) or left (n = 13; 7 females, 6 males) handed following testing with the Edinburgh Handedness Inventory (EHI, Oldfield 1971). The study had local research ethics committee approval. All volunteers gave signed informed consent and were medically screened prior to scanning.
Scanning protocol
T1-weighted images of the brain were obtained for each subject using a 1.5T SIGNA whole body MR imaging system (GE Medical Systems, Milwaukee, WI, USA). A spoiled gradient echo (SPGR) pulse sequence (TE = 9 ms, TR = 34 ms, flip angle = 30°) produced 124 coronal T1-weighted images with a FOV of 20 cm. Each image refers to a contiguous section of tissue of 1.6-mm thickness. Acquisition time was 13 min and 56 s for a 1 NEX scan. Image dimensions were 256 × 256 × 124 voxels of side 0.781 mm × 0.781 mm × 1.6 mm.
Assessment of morphology
The morphology of the sulcal contours defining and within the pars opercularis and pars triangularis was assessed using a combination of multiple orthogonal MR sections and rendered surfaces of the cerebral hemispheres using MRIcro (http://www.mricro.com, Chris Rorden, University of South Carolina, Columbia, SC, USA). It is crucial to refer to orthogonal sections for assessment of sulcal morphology as the full extent of intrasulcal anatomy cannot be appreciated from the surface of the brain alone. For example, studies have illustrated the presence of submerged gyri within the depths of the inferior precentral sulcus that can only be determined through examination of the fundus of sulci (Tomaiuolo et al. 1999; Germann et al. 2005). In particular, Germann et al. (2005) state that ‘Because superficial associations between folds are frequent, it is necessary to follow the relations between these three dimensional sulci in their depth to establish true connections’ (p. 335), and ‘... the fundi of two adjacent sulci may remain separated by a sulcal bridge even though they may appear to be connected on the surface of the brain’ (p. 336). Therefore the morphology of, and connections between sulci were exhaustively assessed using multiple viewing angles. The morphology of five sulci (or rami) was assessed.
Inferior frontal sulcus
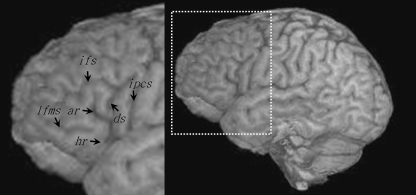
The length, continuity and connections of the inferior frontal sulcus are extremely variable between brains. It has been reported that the inferior frontal sulcus extends rostrally until approximately the midportion of the dorsal edge of the pars triangularis (Eberstaller, 1890), which has been confirmed in subsequent investigations (Petrides & Pandya, 2004). Furthermore, as Petrides & Pandya (2004) note, ‘the term “inferior frontal sulcus” has been used loosely to include additional sulci, such as the sulcus radiatus and the lateral frontomarginal sulcus’ (p. 954). It is therefore important to reliably identify the inferior frontal sulcus, and its segments, from adjacent and/or adjoining anterior frontal sulci. The sulcus radiatus [or radiate sulcus of Eberstaller (Petrides & Pandya, 2004)] and lateral frontomarginal sulcus are two relatively short sulci that are typically located anterior and ventral to the inferior frontal sulcus and the triangular sulcus, and although these sulci may give the impression of an additional anterior segment of the inferior frontal sulcus (Ono et al. 1990), they do not constitute an extension of the inferior frontal sulcus as they can be clearly differentiated by virtue of examination of the sections of the frontal lobe (Petrides & Pandya, 2004). Figure 2 illustrates the location of the lateral frontomarginal sulcus, which may be confused with the inferior frontal sulcus.
Fig. 2.
Location of the lateral frontomarginal sulcus (lfms) with respect to the major sulcal contours that define the pars opercularis and pars triangularis. This sulcus may give the impression of an anterior extension of the inferior frontal sulcus, but navigation through orthogonal sections clearly differentiates the two sulci. ar, anterior ascending ramus; ds, diagonal sulcus; hr, anterior horizontal ramus; ifs, inferior frontal sulcus; ipcs, inferior precentral sulcus.
The inferior frontal sulcus, in particular the posterior most regions, is easily identified in the human brain. It is identified as the first ventral horizontal frontal sulcus extending from the inferior precentral sulcus (either connected or separated by a bridge of cortex), lying immediately dorsal to the anterior ascending ramus of the Sylvian fissure. When the inferior frontal sulcus is continuous in its extent it ordinarily terminates at approximately the midportion of the dorsal edge of the pars triangularis (Eberstaller, 1890; Petrides & Pandya, 2004). When it is discontinuous and composed of two segments [but see Ono et al. 1990 who maintained that up to four segments could be identified, but may have included the sulcus radiatus and the lateral frontomarginal sulcus as an anterior segment of the inferior frontal sulcus (Petrides & Pandya, 2004)], the anterior segment is more difficult to distinguish from anterior frontal sulci, particularly from the surface of the brain, but is reliably achieved using assessment of the intrasulcal connectivity on orthogonal MR sections. We carefully distinguished the anterior inferior frontal sulcus from other anterior frontal sulci (e.g. sulcus radiatus/lateral frontomarginal sulcus/ventral intermediate frontal sulcus). Furthermore, despite the potential misclassification of the posterior most region of the inferior frontal sulcus as the horizontal segment of the inferior precentral sulcus when both sulci appear to align from the surface of the brain (Germann et al. 2005), the positioning of the ventral (vertical) segment of the inferior precentral sulcus and intrasulcal assessment using orthononal sections enabled reliable differentiation between the posterior inferior frontal sulcus and inferior precentral sulcus.
Finally, there are three primary forms of connections between the posterior inferior frontal sulcus and the ventral (or horizontal) segment of the inferior precentral sulcus (Ono et al. 1990; Germann et al. 2005): (1) a true connection refers to the inferior frontal sulcus flowing fully into the inferior precentral sulcus; (2) a superficial connection is a connection on the surface of the hemisphere, but a submerged bridge of cortex interrupts this connection; (3) no connection. Furthermore, a true connection may be divided into two further categories that are defined based on the morphology of the inferior frontal sulcus (Ono et al. 1990). A ‘true long’ connection refers to the anastomosis of the inferior precentral sulcus and a long continious inferior frontal sulcus, and a ‘true short’ connection refers to the anastomosis of the inferior precentral sulcus and a short discontinuous inferior frontal sulcus. An example of a true long connection can be seen in Fig. 1 and no connection in Fig. 2.
Inferior precentral sulcus
The precentral sulcus is located immediately anterior to and roughly parallel to the central sulcus, and marks the division between the precentral and three frontal gyri (superior, middle and inferior). It most typically consists of two major vertical sulci (a superior and inferior sulcus), which themselves are frequently comprised of multiple vertical and horizontal segments (Ono et al. 1990; Germann et al. 2005). Like the inferior frontal sulcus, the precentral sulcus exhibits a great deal of inter-individual morphological variation in shape and connections (see Germann et al. 2005 and Ono et al. 1990 for analysis of variability of the precentral sulcus). The inferior precentral sulcus alone may comprise three segments including a dorsal, transverse and ventral segment (Germann et al. 2005). The ventral most region of the inferior precentral sulcus, which marks the posterior most border of the pars opercularis and is thus of primary interest in the present study, is easily identified as the ventral segment of the first descending sulcus immediately anterior to the central sulcus. The inferior precentral sulcus may occasionally flow into the Sylvian fissure (Ono et al. 1990), although this was questioned in early anatomical studies (Eberstaller, 1890; Cunningham, 1892). The sulcus may rarely be bifid in the human (Ono et al. 1990) and in the great ape (Sherwood et al. 2003) brain. All references to the inferior precentral sulcus in the present study refer to the ventral segment that forms the posterior border to the pars opercularis.
Anterior ascending ramus of the Sylvian fissure
The anterior ascending ramus of the Sylvian fissure, which marks the division between the pars opercularis and pars triangularis, is a deep vertical ramus rising up from the Sylvian fissure into the inferior frontal gyrus, ordinarily located approximately where the anterior temporal lobe turns downwards to form the temporal pole. The anterior ascending ramus is located anterior to the diagonal sulcus, when present, and infrequently may be submerged within the inferior precentral sulcus (Tomaiuolo et al. 1999; Petrides & Pandya, 2004; Petrides, 2006), which requires three-dimensional navigation through the intrasulcal anatomy to demarcate the pars opercularis since this structure cannot be visualised from the cortical surface (Tomaiuolo et al. 1999).
Anterior horizontal ramus of the Sylvian fissure
The anterior horizontal ramus of the Sylvian fissure, which demarcates the pars triangularis from the more ventrally located pars orbitalis, appears like a continuation of the Sylvian fissure in the lateral-orbital frontal lobe, located approximately where the temporal lobe ends. There are several patterns of the anterior horizontal ramus, including (1) a positioning over the lateral surface, along the orbital margin or over the orbital surface, (2) sharing a common trunk with the anterior ascending ramus, but more commonly has a separate origin and (3) infrequently may be absent (Ono et al. 1990).
Diagonal sulcus
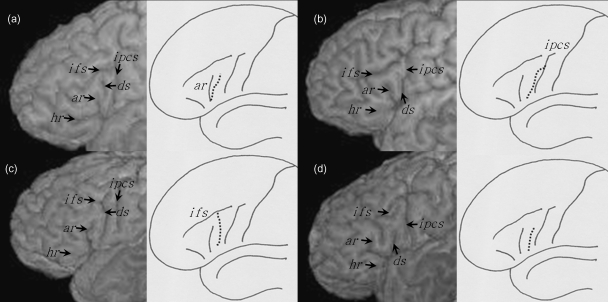
The diagonal sulcus is identified as a clear sulcus lying on the inferior frontal gyrus between the inferior precentral sulcus and the anterior ascending ramus of the Sylvian fissure. The morphology of this sulcus does not constitute a uniform appearance, as it occasionally merges with the anterior ascending ramus of the Sylvian fissure or extends from the inferior precentral sulcus or inferior frontal sulcus. In some cases the diagonal sulcus does not merge with any of the surrounding sulci and abuts with the Sylvian fissure. These four configurations of the diagonal sulcus are shown in Fig. 3, both schematically and from the surface of three-dimensionally rendered cerebral hemispheres.
Fig. 3.
The four connections of the diagonal sulcus. The connections are shown in subjects studied in the present investigation (left) and are also schematically illustrated (right). (a) Connection with the ascending horizontal ramus of the Sylvian fissure. (b) Connection with the inferior precentral sulcus. (c) Connection with the inferior frontal sulcus. (d) No connection with surrounding sulci. ar, anterior ascending ramus; ds, diagonal sulcus; hr, anterior horizontal ramus; ifs, inferior frontal sulcus; ipcs, inferior precentral sulcus.
The following sulcal features were recorded for each cerebral hemisphere studied:
inferior frontal sulcus: continuous (one segment) or discontinuous (two or more segments); connection with the inferior precentral sulcus: long connection (where the inferior precentral sulcus anastomoses with a continuous, uninterrupted inferior frontal sulcus), short connection (where the inferior precentral sulcus anastomoses with a discontinuous, interrupted inferior frontal sulcus), superficial connection (where a connection is apparent from the surface of the brain, but a submerged bridge of cortex separates the sulci) or no connection
inferior precentral sulcus (ventral most region): single or dual; connection or no connection with Sylvian fissure
anterior ascending ramus of the Sylvian fissure: present or absent
anterior horizontal ramus of the Sylvian fissure: present or absent; common or separate origin from the anterior ascending ramus
diagonal sulcus: present or absent; connection to inferior precentral sulcus, inferior frontal sulcus or anterior ascending ramus, or no connection to these sulci.
We also assessed the morphological variability of the posterior Sylvian fissure by visualising the general direction of fissure termination. Results were documented according to a simple categorisation scheme, namely horizontal termination or upward oblique termination. These two fissure configurations are shown in Fig. 4. A more exhaustive analysis of the bifurcation patterns of the posterior Sylvian fissure is provided elsewhere (Ono et al. 1990; Ide et al. 1996).
Fig. 4.
Termination of the posterior Sylvian fissure: horizontal (left) and upward oblique (right).
Stereological measurement
Scan re-orientation
MR images were transferred to a SUN Ultra 10 workstation running NRIA software (Brain Behaviour Laboratory, University of Pennsylvania, Philadelphia, PA, USA) to allow viewing, reslicing, and resizing datasets according to multiple planes. Firstly, NRIA was used to perform a one-stage reformatting process in which the data were interpolated to isotropic voxels and reformatted to a prescribed plane via single matrix transformation. The 256 × 256 × 124 acquired voxels of side 0.781 mm × 0.781 mm × 1.6 mm were linearly interpolated to 256 × 256 × 256 cubic voxels of side 0.781 mm.
All images were realigned perpendicular to the bicommissural plane. On a sagittal section closest to midline, a line was drawn connecting the anterior commissure and posterior commissure so that both structures could be viewed on the same axial section. To correct for variation in anterior to posterior roll, an axial plane along the bi-commissural axis was taken. A coronal plane, orthogonal to the longitudinal fissure was viewed at the point where the orbital cavities were at maximum cross-sectional area. From that coronal plane, a further adjustment to the axial plane was taken along the superior most aspect of the orbital cavities to correct for side-to-side pitch. To correct for deviations from sagittal midline, a plane taken through the longitudinal fissure of the corrected axial plane was taken. Images were rotated so that the bicommissural axis was positioned at zero degrees (i.e. horizontal).
Half of the scans were randomly selected and flipped from left to right, and the identity of each case was coded. Thus, the experimenters were blind to both the laterality and identity of each case. We did not perform measurements on MR images automatically transferred to a standardised reference space, given the effects of automated normalisation on regional brain morphology. In particular, previous studies have shown that linear and in particular non-linear normalisation algorithms may cause an expansion of regional brain tissue volume (Hammers et al. 2003; Keller, 2004). In particular, it has been recently shown that application of spatial normalisation increases the volume of the entire inferior frontal gyrus, and alters the directional asymmetry of this region from leftward in native MR scans to rightward in normalised images (Hammers et al. 2007).
Delineation of regions of interest
Pars opercularis and pars triangularis
The delineation of the pars opercularis and pars triangularis followed the anatomical definitions described by others (Duvernoy, 1999; Tomaiuolo et al. 1999; Petrides & Pandya, 2004; Petrides, 2006). The sulcal contours defining these regions were clearly visible on high-resolution T1-weighted MR images (Fig. 5). The pars opercularis (shown in blue in Fig. 5) was demarcated caudally from the precentral gyrus by the inferior precentral sulcus (ipcs), dorsally from the middle frontal gyrus by the inferior frontal sulcus (ifs) and rostrally from the pars triangularis by the anterior ascending ramus of the Sylvian fissure (ar). The pars triangularis (shown in red in Fig. 5) was demarcated caudally from the pars opercularis by the anterior ascending ramus of the Sylvian fissure, dorsally from the middle frontal gyrus by the inferior frontal sulcus, and rostro-ventrally by the anterior horizontal ramus of the Sylvian fissure (hr).
Fig. 5.
The sulcal contours defining the pars opercularis (blue) and pars triangularis (red) on MRI. Orthogonal sagittal (bottom left) and coronal (bottom middle/right) sections are shown in conjunction with a lateral rendering of the cerebral hemisphere in the same individual. ar, anterior ascending ramus; cis, circular insular sulcus; ds, diagonal sulcus; hr, anterior horizontal ramus; ifs, inferior frontal sulcus; ipcs, inferior precentral sulcus; ts, triangular sulcus.
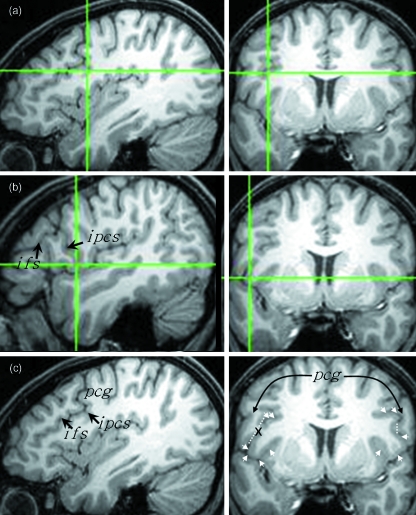
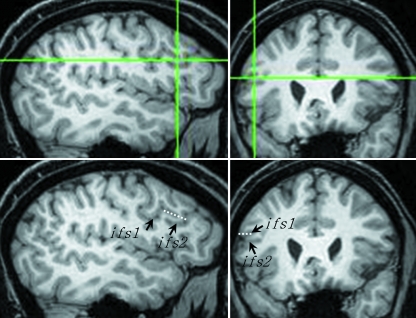
The entire grey matter of the pars opercularis and pars triangularis was measured on coronal images. White matter was not measured. To aid this process, the sulcal contours that define these structures were first marked on the realigned orthogonal coronal, sagittal and axial sections using BrainVoyager software (http://www.Brainvoyager.com, Brain Innovation, Maastricht, The Netherlands). The posterior most region of the pars opercularis was first delineated. Markers were placed deep within the inferior precentral sulcus to delineate the posterior operculum from the adjoining precentral gyrus using sagittal and coronal sections (Fig. 6). On coronal sections, the precentral gyrus appears to overlap laterally the deeper operculum and then the two structures merge; navigation using the orthogonal sections reliably differentiated these two structures thus providing a posterior limit for measurements of the pars opercularis. The inferior frontal sulcus was marked on sagittal sections to denote the superior border of the pars opercularis and pars triangularis. When the inferior frontal sulcus was discontinuous, markers ‘connecting’ the discontinuous segments were drawn, following the pattern and direction of the sulcus, to serve as the dorsal border and to eliminate any dorsally located cortex not comprising the inferior frontal gyrus (Fig. 7). A sagittal section of the Sylvian fissure and its anterior horizontal ramus were marked to delineate the inferior border of the pars opercularis and pars triangularis. Tracing of the anterior horizontal ramus followed the trajectory of the ramus. The pars opercularis and pars triangularis were demarcated by placing markers within the anterior ascending ramus. The dorsomedial (inferior frontal sulcus) and ventromedial (circular insular sulcus, anterior horizontal ramus) limits of the pars opercularis and pars triangularis were not marked. The contours of these sulci were sufficiently viewed on coronal sections for point counting.
Fig. 6.
Defining the posterior most region of the pars opercularis. The same coronal section is shown on the right (a–c) indicating the last section that the frontal operculum can be visualised in this particular right pars opercularis (on the left side of image). The crosshairs indicate the last section on which the dorsomedial (a) and ventrolateral (b) operculum can be visualised, immediately anterior to the inferior precentral sulcus (see corresponding sagittal sections). (c) is the same sagittal and coronal sections as (a) without the crosshairs. Markers were placed to separate the deep operculum from the laterally overlapping precentral gyrus (pcg), which are indicated by the arrows on the coronal section. The broken white line indicates the point of separation. ifs, inferior frontal sulcus; ipcs, inferior precentral sulcus.
Fig. 7.
Connecting the segments of a discontinuous inferior frontal sulcus. The orthogonal sagittal and coronal sections at the top show the point at which a bridge of cortex interrupts the sulcus (crosshairs). The same sections below indicate the two segments of the inferior frontal sulcus (ifs1, ifs2) and the point at which these segments are connected (broken white line).
Planum temporale
The planum temporale is a triangular region lying caudal to Heschl's gyrus on the supratemporal plane within the Sylvian fissure. The posterior boundary, following the method of Anderson et al. (1999), was where the Sylvian fissure turned to ascend in a more vertical plane or, if it remained horizontal, at its termination. The medial boundary was the deepest extent of the Sylvian fissure, and the anterior boundary was Heschl's sulcus. In those cases where there was more than one Heschl's gyrus, or it bifurcated at any point along its length, the posterior gyrus was included in the planum temporale, following the recommendations of Kim et al. (2000).
Stereological parameters
Unbiased estimates of the volume of the pars opercularis, pars triangularis and planum temporale were obtained using design-based stereology. This involved efficient application of appropriate point counting procedures (Gundersen & Osterby, 1981; Gundersen & Jensen, 1987; Gundersen et al. 1999) with in-house developed software (Easymeasure: Puddephat (1999); contact corresponding author for software information). This program displayed scans in a coronal orientation, with a square grid array of probe points superimposed in random position. Those points which lie over the structure of interest were indicated with a mouse-click. The volume of the structure concerned was calculated from the total number of points counted. Sectioning and point counting intensities were optimised to achieve a coefficient of error for the volume of less than 5% (Roberts et al. 2000).
Pars opercularis and pars triangularis
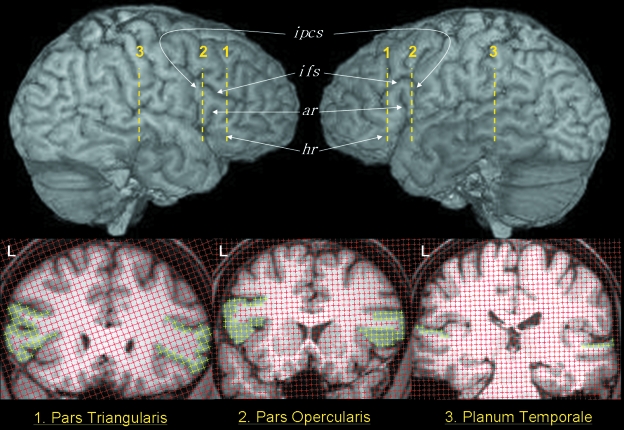
Separation between the grid array test points used for point counting of the pars opercularis and pars triangularis was 0.234 cm (i.e. 3 pixels). The slice interval was every section for the pars opercularis and every second section for the pars triangularis. The difference in slice interval between structures was due to the fact that the pars triangularis is usually longer (and thus larger) than the pars opercularis. An illustration of point counting within the pars opercularis and pars triangularis is presented in Fig. 8. Time taken to measure each structure was approximately 10 minutes per structure (×4 per brain, therefore 40 min per brain), after image realignment and region-of-interest demarcation that lasted approximately 20 min per brain. Therefore, total time taken for measurement of the pars opercularis and pars triangularis in the present study was approximately one hour per subject.
Fig. 8.
Point counting for stereological analysis of the pars triangularis (1), pars opercularis (2) and planum temporale (3). The approximate locations of the coronal sections are indicated on the lateral renderings of the same brain. The section areas of the three structures are indicated by the yellow points in the left and right hemispheres. ar, anterior ascending ramus; hr, anterior horizontal ramus; ifs, inferior frontal sulcus; ipcs, inferior precentral sulcus.
Planum temporale
For the planum temporale, the grid size was set to 0.312 cm (i.e. 4 pixels). At the outset of the study, slice interval for the planum temporale measures was every section. However, with progression of the study, and monitoring of the resultant coefficients of error, this proved to be unduly labour intensive. From this point forward, the slice interval was changed to every other section. It should be noted that due to the nature of the stereology system of measurement, this would have resulted in only alterations in the variance of the measurements. The unbiasedness of the stereological estimator would not have been affected. The mean coefficient of error was always less than 5% for both measurement protocols for the planum temporale, and was therefore deemed to be acceptable. An illustration of point counting within the planum temporale is presented in Fig. 8. Time taken for measurement of the planum temporale was approximately 10 min per structure (×2 per brain, therefore 20 min per brain).
Automated analysis of fronto-occipital torque
The fronto-occipital torque was calculated using the automated technique of Barrick et al. (2005). Briefly, after automated segmentation of grey and white matter (using SPM2, http://www.fil.ion.ucl.ac.uk/spm, Wellcome Department of Imaging Neuroscience, London, UK) and hemisphere extraction (Maes et al. 1999) a series of fully automated algorithms estimated the volume of the grey and white matter in both cerebral hemispheres, and for each parcellated lobe. Automatically determined asymmetry of the combined volume of grey and white matter within the frontal lobe (typically rightward) and occipital lobe (typically leftward) was used to determine brain torque.
Statistical analyses
McNemar's test was applied to test whether both the incidence of the diagonal sulcus and horizontal termination of the Sylvian fissure were associated with side of cerebral hemisphere. Multivariate analysis of variance (MANOVA) was used to analyse inter-hemispheric differences in the volume of the pars opercularis, pars triangularis and planum temporale and to test whether the measurements were influenced by handedness. In particular, we used a two-way nested MANOVA where the structures (pars opercularis/pars triangularis/planum temporale) were regarded as a three-dimensional array. The factors were side (left/right) and handedness (right/left). Between-hemisphere volume asymmetry of the three brain structures were calculated from the right (R) and left (L) volumes using a standard asymmetry formula [(R – L)/(R + L) × 100], where negative values indicate a larger leftward asymmetry (and vice versa for positive values), and the larger the value, the greater the asymmetry. Pearson's correlation coefficients (with multiple-comparisons correction) were used to investigate the relationships between the asymmetry coefficients of the pars opercularis, pars triangularis, planum temporale and fronto-occipital torque.
Results
Morphology
All results from analysis of sulcal morphology are provided in Table 1, and are provided separately for the left and right hemispheres.
Table 1.
Sulcal variability observed in the present study. ar, anterior ascending ramus; hr, anterior horizontal ramus; ifs, inferior frontal sulcus; ipcs, inferior precentral sulcus; sf, Sylvian fissure
| Left Hem | Right Hem | Total | ||
|---|---|---|---|---|
| Inferior frontal sulcus | continuous | 54% (27) | 50% (25) | 52% (52) |
| discontinuous | 46% (23) | 50% (25) | 48% (48) | |
| ipcs connection: long | 38% (19) | 32% (16) | 35% (35) | |
| ipcs connection: short | 28% (14) | 30% (15) | 29% (29) | |
| ipcs connection: superficial | 20% (10) | 18% (9) | 19% (19) | |
| ipcs connection: none | 14% (7) | 20% (10) | 17% (17) | |
| Inferior precentral sulcus | single | 96% (48) | 96% (48) | 96% (96) |
| bifid | 4% (2) | 4% (2) | 4% (4) | |
| no connection with sf | 84% (42) | 78% (39) | 81% (81) | |
| connection with sf | 16% (8) | 22% (11) | 19% (19) | |
| Anterior ascending ramus | present | 98% (49) | 100% (50) | 99% (99) |
| absent | 2% (1) | 0% (0) | 1% (1) | |
| Anterior horizontal ramus | present | 98% (49) | 98% (49) | 98% (98) |
| absent | 2% (1) | 2% (1) | 2% (2) | |
| ar/hr separate origin | 66% (33) | 70% (35) | 68% (68) | |
| ar/hr common trunk | 30% (15) | 28% (14) | 29% (29) | |
| Diagonal sulcus | present | 52% (26) | 20% (10) | 36% (36) |
| absent | 48% (24) | 80% (40) | 64% (64) | |
| ar connection | 8% (4) | 4% (2) | 6% (6) | |
| ipcs connection | 14% (7) | 4% (2) | 7% (9) | |
| ifs connection | 8% (4) | 2% (1) | 5% (5) | |
| no connection | 22% (11) | 10% (5) | 13% (16) | |
| Sylvian fissure | horizontal | 70% (35) | 34% (17) | 52% (52) |
| upward oblique | 30% (15) | 66% (33) | 48% (48) |
Inferior frontal sulcus
The inferior frontal sulcus was found to be a continuous straight sulcus in 52% of all brains, with no notable inter-hemispheric differences. The remainder (48%) consisted of a discontinuous inferior frontal sulcus, comprising no more than two segments. We observed that when the inferior frontal sulcus was a continuous straight sulcus, the anterior end typically terminated half way between the anterior ascending and anterior horizontal rami of the Sylvian fissure (i.e. the midportion of the dorsal edge of the pars triangularis). Such an example is provided in Fig. 1. However, when the inferior frontal sulcus was interrupted by a bridge of cortex and was thus discontinuous, the termination of the anterior end of the sulcus was sometimes located soon after the anterior ascending ramus of the Sylvian fissure, or may have extended beyond the triangular sulcus, but never reaching the anterior horizontal ramus of the Sylvian fissure. There was a long connection between the posterior inferior frontal sulcus and inferior precentral sulcus in 38% left and 32% right hemispheres. There was a short connection in 28% left and 30% right hemispheres, a superficial connection in 20% left and 18% right hemispheres, and no connection in 14% left and 20% right hemispheres.
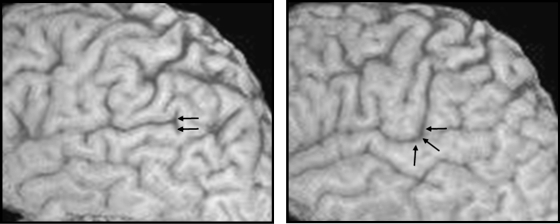
Inferior precentral sulcus
The ventral inferior precentral sulcus was a single sulcus in 96% left and 96% right hemispheres. There were dual parallel inferior precentral sulci in 4% left and 4% right hemispheres. One example of dual parallel inferior precentral sulci is provided in Fig. 9(a). The ventral inferior precentral sulcus had no direct connection with the Sylvian fissure in 84% left and 78% right hemispheres. A connection was observed in 16% left and 22% right hemispheres, and in all cases this referred to a connection on lateral sections of the brain but never medially as the bridge of cortex connecting the frontal operculum and precentral gyrus was always present. Therefore, this may be considered a superficial connection. An example of a connection between the inferior precentral sulcus and Sylvian fissure is provided in Fig. 9(b).
Fig. 9.
Rare features of the inferior precentral sulcus. (a) Double parallel inferior precentral gyrus. (b) Ventral inferior precentral sulcus connecting with the Sylvian fissure on the surface of the brain (white arrow). This connection is lateral/superficial, given that the posterior frontal operculum always connects with the precentral gyrus more medially. ar, anterior ascending ramus; ds, diagonal sulcus; hr, anterior horizontal ramus; ifs, inferior frontal sulcus; ipcs, inferior precentral sulcus.
Anterior ascending ramus of the Sylvian fissure
The anterior ascending ramus was identified in 99% of all hemispheres examined.
Anterior horizontal ramus of the Sylvian fissure
The anterior horizontal ramus could be identified in 98% of all hemispheres examined. The anterior ascending and horizontal rami had a separate origin in 66% left and 70% right hemispheres. Both rami had a common trunk in 30% left and 28% right hemispheres. An example of a separate origin can be seen in Fig. 3(b–d) and of a common trunk in Fig. 3(a).
Diagonal sulcus
We observed the presence of a diagonal sulcus in 52% left and 20% right hemispheres. There was no observable diagonal sulcus in 48% left and 80% right hemispheres. Given the large difference between the presence of the diagonal sulcus in the left and right hemispheres, we applied McNemar's statistical test to determine whether this was a significant difference. Results showed that the diagonal sulcus was present significantly more frequently in the left than the right hemisphere (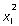 = 12.9, P < 0.001). The cell frequencies for the presence of the diagonal sulcus are provided in Table 2.
= 12.9, P < 0.001). The cell frequencies for the presence of the diagonal sulcus are provided in Table 2.
Table 2.
Cell frequencies for the presence of the diagonal sulcus in the 50 right and 50 left hemispheres
| Right Hem | |||
|---|---|---|---|
| Yes | No | ||
| Left Hem | Yes | 6 (12%) | 20 (40%) |
| No | 4 (8%) | 20 (40%) | |
The four sulcal configurations involving the diagonal sulcus detailed in the methods section were observed in the 36 diagonal sulci (26 left, 10 right) identified in the present study. In particular, a connection with the anterior ascending ramus of the Sylvian fissure was observed in 8% left and 4% right hemispheres, a connection with the inferior precentral sulcus in 14% left and 4% right hemispheres, a connection with the inferior frontal sulcus in 8% left and 2% right hemispheres, and no connection with local sulci in 22% left and 10% right hemispheres.
Posterior Sylvian fissure termination
A horizontally terminating posterior Sylvian fissure was identified in 70% left and 34% right hemispheres. An upward oblique terminating posterior Sylvian fissure was identified in 30% left and 66% right hemispheres. Given the large difference in termination type in the left and right hemispheres, we applied McNemar's statistical test to determine whether this was a significant difference. Results indicated that there was a significant left-greater-than-right difference in frequency of a horizontal termination, and a significant right-greater-than-left difference in frequency of upward oblique termination (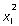 = 9.63, P = 0.002). The cell frequencies for the posterior Sylvian fissure are provided in Table 3.
= 9.63, P = 0.002). The cell frequencies for the posterior Sylvian fissure are provided in Table 3.
Table 3.
Cell frequencies for the presence of an upward oblique termination of the Sylvian fissure in the 50 right and 50 left hemispheres
| Right Hem | |||
|---|---|---|---|
| Yes | No | ||
| Left Hem | Yes | 8 (16%) | 7 (14%) |
| No | 25 (50%) | 10 (20%) | |
Stereological measurement
Prior to measurement of the study cohort (SSK = all pars opercularis and pars triangularis measurements; JRH = all planum temporale measurements), inter-rater reliability of cortical volumes was analysed using intraclass correlation coefficients (ICC) (Bartko, 1966). The volumes of the left and right pars opercularis, pars triangularis and planum temporale were estimated in 10 subjects by two different raters for analyses of inter-rater reliability (pars opercularis and pars triangularis measurements contrasted between SSK and Jack Taylor; planum temporale measurements contrasted between JRH and SSK). Reliability studies were administered prior to the design of the present study, and were performed on a cohort of healthy controls using identical techniques including MR imaging acquisition parameters and quantification methods. ICC's indicated that inter-rater variability was approximately 14% of the total data variability for all structures (ICC ≥ 0.86). Table 4 indicates the biological coefficient of variation and the inter-rater coefficient of variation (CV) for the pars opercularis, pars triangularis and planum temporale (CV is defined as the standard deviation divided by the mean of the variable).
Table 4.
Results from inter-rater reliability analyses
| Structure | Intraclass Correlation Coefficient | Biological CV | Inter-rater CV |
|---|---|---|---|
| Left pars opercularis | 0.86 | 32% | 5% |
| Right pars opercularis | 0.86 | 29% | 4% |
| Left pars triangularis | 0.94 | 35% | 2% |
| Right pars triangularis | 0.89 | 29% | 3% |
| Left planum temporale | 0.96 | 31% | 1% |
| Right planum temporale | 0.96 | 24% | 1% |
Exclusion of cases for morphometry
The morphology of the sulcal contours defining the pars opercularis and pars triangularis described above prevented delineation of these structures for volumetric measurement in five of the 50 brains for the following reasons: absent anterior ascending ramus (one brain; no anterior/posterior boundary for the pars opercularis and pars triangularis respectively), absent horizontal ramus (two brains; no anterior boundary for the pars triangularis), and double parallel inferior precentral sulcus (two brains; no posterior boundary for the pars opercularis). Therefore analysis of pars opercularis and pars triangularis volume was performed in 45 brains.
Volume asymmetry
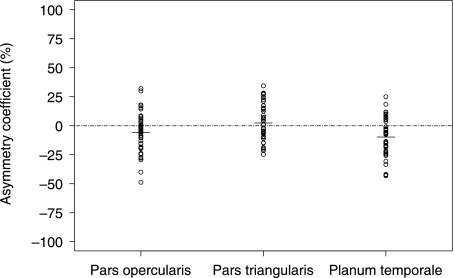
Table 5 indicates the descriptive results for the volume of the pars opercularis, pars triangularis and planum temporale, and reveals a large range in structure volume for the left and right pars opercularis and pars triangularis. Statistical analysis revealed significant effects of hemisphere side (right/left, F3,41 = 6.89, P < 0.01) but not of handedness (F3,41 = 0.45, P = 0.72). To elucidate the significant effects from the main analysis, we used paired samples t-tests. Results indicated significantly greater volume of the left pars opercularis (t44 = 2.47, P = 0.02) and planum temporale (t44 = 4.2, P < 0.001) relative to the right. There was no significant difference between the volume of the left and right pars triangularis (t44 = −0.74, P = 0.46). The distributions of asymmetry coefficients for all structures are presented in Fig. 10. The pars opercularis was leftward asymmetrical in 31 (69%) cases, the pars triangularis in 19 (42%) cases and the planum temporale in 35 (78%) cases. Symmetry of the pars opercularis, pars triangularis and planum temporale was found in 2 (5%), 6 (13%) and 2 (5%) cases respectively. The remaining cases were right asymmetrical.
Table 5.
Descriptive statistics for structure volume in the left and right hemisphere. Volumes are cm3
| Left mean | Left range | Right mean | Right range | |
|---|---|---|---|---|
| Pars opercularis | 6.86 | 3.21–11.23 | 6.08 | 3.02–10.46 |
| Pars triangularis | 6.94 | 3.54–14.72 | 7.65 | 3.59–15.34 |
| Planum temporale | 3.90 | 1.95–6.42 | 3.14 | 1.50–5.56 |
Fig. 10.
Asymmetry coefficients (%) for the pars opercularis, pars triangularis and planum temporale.
Relationships between measures
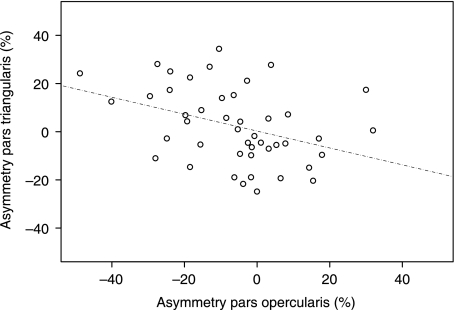
There were no significant positive correlations between the asymmetry coefficients. There was a significant negative correlation between the asymmetry coefficients of the pars opercularis and pars triangularis (r = −0.418, P = 0.01). This relationship is presented in Fig. 11. There were trends towards negative correlations between the asymmetry coefficients of the pars opercularis and planum temporale (r = −0.262, P = 0.08), and a positive correlation between pars opercularis asymmetry and fronto-occipital torque (torque mean value = 38.44; range = 12.19–69.67; all brains showing a combined rightward frontal and leftward occipital asymmetry, which is indicated by the positive torque values) (r = 0.314, P = 0.08). There were no other significant correlations between asymmetry coefficients (P > 0.08).
Fig. 11.
Scatterplot of pars opercularis asymmetry against pars triangularis asymmetry. Negative values of pars opercularis asymmetry are associated with positive values of pars triangularis asymmetry, and vice versa.
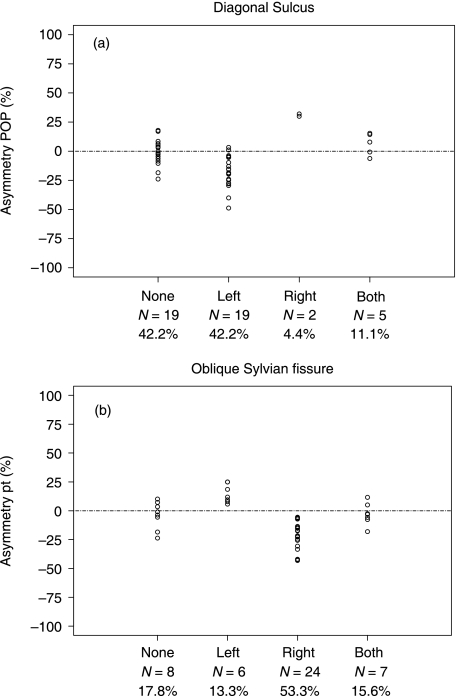
A two-way analysis of variance was applied to test the association between presence of diagonal sulcus and pars opercularis volume asymmetry, where the factors were right side and left side (both with levels presence/no presence) (Fig. 12a). The presence of a diagonal sulcus in the left hemisphere was significantly associated with negative (leftward) volume asymmetry of the pars opercularis (F1,41 = 15.9, P < 0.01). Moreover, the presence of diagonal sulcus in the right hemisphere was significantly associated with positive asymmetry of the pars opercularis (F1,41 = 28.7, P < 0.01) (although the sample size was very small in this group). The mean values (SD) corresponding to Fig. 12(a)[i.e. mean volume asymmetry coefficient of the pars opercularis in subjects groups with a diagonal sulcus in (i) neither hemisphere, (ii) only the left hemisphere, (iii) only the right hemisphere and (iv) both hemispheres] were –1.1 (10.4) for neither hemisphere, –18.1 (13.6) for the left hemisphere, 31.0 (1.4) for the right hemisphere, and 6.1 (9.5) for both hemispheres. Negative values indicate leftward volume asymmetry. No right/left interaction effects were observed (F1,41 = 0.69, P = 0.46).
Fig. 12.
Distribution of pars opercularis volume (pop) asymmetry relative to the presence of a diagonal sulcus (12a) and planum temporale volume (pt) asymmetry relative to presence of upward oblique Sylvian fissure termination (12b). Note that in contrast to the data presented in Tables 2 and 3, the data presented here are for the 45 subjects in whom structure volumes could be estimated. Negative values are leftward volume asymmetry. Asymmetry coefficients are broken down in subgroups: none = morphological feature not present in either hemisphere, left = present only in left hemisphere, right = present only in right hemisphere, both = present in both hemispheres. These figures indicate that leftward or rightward volume asymmetry of the pars opercularis is related to the presence of the diagonal sulcus in the left or right hemisphere respectively (top), and that leftward or rightward planum temporale asymmetry is driven by the presence of an upward oblique Sylvian fissure in the right or left hemisphere respectively (bottom).
An analogous statistical analysis was used to test whether planum temporale volume asymmetry was influenced by the posterior morphology of the Sylvian fissure (Fig. 12b). Results indicated that brains that exhibited an upward oblique termination in the right hemisphere showed a negative (i.e. leftward) volume asymmetry of the planum temporale (F1,41 = 17.0, P < 0.01). Furthermore, an upward oblique termination in the left hemisphere was significantly associated with a positive (rightward) volume asymmetry of the planum temporale (F1,41 = 19.3, P < 0.01). The mean values (SD) corresponding to Fig. 12(b) (as in Fig. 12a, but of planum temporale volume asymmetry and upward oblique termination of the Sylvian fissure) were –4.0 (11.9) for neither hemisphere, 13.0 (7.3) for the left hemisphere, –19.4 (11.8) for the right hemisphere, and –3.0 (9.5) for both hemispheres. Negative values indicate leftward volume asymmetry. No right/left interaction effects were observed (F1,41 = 0.001, P = 0.94).
Auxiliary analysis of the diagonal sulcus
We have presented evidence of a significant leftward asymmetry of the presence of the diagonal sulcus in the pars opercularis. Our results are consistent with previous research insomuch that a left-greater-than-right incidence has been demonstrated (Galaburda, 1980; Ono et al. 1990; Tomaiuolo et al. 1999), but our findings represent the most lateralised presence of the diagonal sulcus in the literature. Given that this lateralisation is significantly related to the observed leftward asymmetry of the pars opercularis, we performed an auxiliary analysis of the presence of the diagonal sulcus in a separate cohort of 50 brains.
We documented the presence of a diagonal sulcus on MR images from 50 healthy subjects matched for age and sex with the primary analysis [21 females, 29 males; mean (± SD) age 43.7 ± 12.7 years]. All images were acquired from the same MRI scanner using identical MR sequences and software. The investigator (SSK) was blind to identification and hemisphere side (half the images were flipped from left to right, encoded and anonymised, as in the primary analysis). We found that the diagonal sulcus was present in 29/50 (58%) left and 20/50 (40%) right hemispheres. This included 20 only in the left hemisphere, 11 only in the right hemisphere, nine bilaterally and ten in no hemispheres. McNemar's statistical test revealed no significant leftward asymmetry of the diagonal sulcus in the auxiliary sample (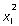 = 2.61, P = 0.11). In total, we observed a diagonal sulcus in 53/100 (53%) left and 27/100 (27%) right hemispheres.
= 2.61, P = 0.11). In total, we observed a diagonal sulcus in 53/100 (53%) left and 27/100 (27%) right hemispheres.
Discussion
The first objective of this study was to determine the morphological variability and asymmetry of the sulcal anatomy defining the pars opercularis and pars triangularis. It was found that the inferior frontal sulcus was almost as likely (48% of cases) to be discontinuous as a continuous straight sulcus (52%) in both hemispheres. When the inferior frontal sulcus was discontinuous, it consisted of no more than two segments. Most frequently, the posterior inferior frontal sulcus connected with the inferior precentral sulcus either fully or superficially; no connection was observed in 14% left and 20% right hemispheres. The inferior precentral sulcus most frequently did not reach the Sylvian fissure, but there was a lateral (superficial) connection in 16% left and 22% right hemispheres. The observation of double parallel inferior precentral sulci and absent anterior rami in 4% of brains prevented measurements of the pars opercularis and pars triangularis as these sulcal contours define the borders of the gyral structures. Significant inter-hemispheric differences in the presence of a diagonal sulcus and horizontal termination of the posterior Sylvian fissure were observed, both with a leftward asymmetry. In auxiliary analyses, a leftward asymmetry of the diagonal sulcus was again demonstrated, but not significantly so.
The second objective was to estimate the asymmetry of the volume of grey matter within the pars opercularis, pars triangularis, and planum temporale using stereology, and to relate asymmetries to inter-hemispheric differences in sulcal contours. Further to obtaining the expected leftward asymmetry of the planum temporale, we also found a significant leftward asymmetry of the pars opercularis, but a more subtle asymmetry in the latter. There was no significant difference between the volume of the left and right pars triangularis. Volume asymmetry of the pars opercularis was significantly related to the presence of a diagonal sulcus within the pars opercularis: leftward volume asymmetry of the pars opercularis was found in almost all brains (17/19) where a diagonal sulcus was only present in the left hemisphere, and rightward volume asymmetry was found in both brains (2/2) where a diagonal sulcus was only present in the right hemisphere. Group-wise pars opercularis volume asymmetry did not exist when a diagonal sulcus was present in both or neither hemispheres. In a similar manner, planum temporale volume asymmetry was determined by the termination of the posterior Sylvian fissure: all brains with an upward oblique termination in only the right hemisphere had leftward volume asymmetry (24/24) and all brains with this termination only in the left hemisphere had rightward volume asymmetry (6/6).
The third objective was to explore the relationship between asymmetry of the anterior and posterior speech regions. We found no significant relationship between the volume asymmetries of the pars opercularis and planum temporale, or the pars triangularis and planum temporale.
Finally we explored the relationship between asymmetry of the language cortical structures and the more gross morphological asymmetry of fronto-occipital torque. There were no significant relationships.
Before we discuss the biological implications of the above results we highlight pertinent methodological issues.
Methodological considerations
The Cavalieri method of modern design stereology is frequently applied in neuroscience to quantify neuronal density on a microscopic level (Gundersen & Osterby, 1981; Pakkenberg et al. 2003; Schmitz & Hof, 2005). The principles of stereology have additionally been applied to study regional brain volumes on MR images for scientific research (Sheline et al. 1996; Mackay et al. 1998; Roberts et al. 2000; Howard et al. 2003) and in clinical settings (Mackay et al. 2000; Keller et al. 2002a, 2002b; Salmenpera et al. 2005), with validation against post-mortem specimens (Doherty et al. 2000; Garcia-Finana et al. 2003; Jelsing et al. 2005). Point counting on MR images in conjunction with design-based stereology has been found to be at least as precise as thresholding and tracing methods and clearly superior in terms of speed and validity (Doherty et al. 2000), with excellent levels of inter- and intra-rater reliability (Mackay et al. 1998, 2000; Doherty et al. 2000; Keller et al. 2002a, 2002b). To date, applications include volume estimation of individual brain regions such as medial temporal lobe structures including the hippocampus, amygdala and parahippocampal gyrus (Mackay et al. 1998, 2000; Keller et al. 2002a, 2002b; Salmenpera et al. 2005), infratentorial structures including brainstem, cerebellum and upper cervical spinal cord (Edwards et al. 1999; Liu et al. 1999), and cerebral lobar and hemisphere volumes (Sheline et al. 1996; Mackay et al. 2000; Roberts et al. 2000; Howard et al. 2003). The present study has demonstrated reliability in the repeatability of measurements of the pars opercularis, pars triangularis and planum temporale using stereological methods.
In the present study, the pars opercularis was bordered caudally by the inferior precentral sulcus, dorsally by the inferior frontal sulcus, ventrally by the Sylvian fissure, and rostrally by the anterior ascending ramus. The pars triangularis was bordered caudally by the anterior ascending ramus, dorsally by the inferior frontal sulcus and rostro-ventrally by the anterior horizontal ramus. These sulcal contours are generally considered to demarcate the pars opercularis and pars triangularis of the inferior frontal gyrus (Duvernoy, 1999; Tomaiuolo et al. 1999; Petrides & Pandya, 2004; Petrides, 2006). However, we have demonstrated considerable variability in the sulcal contours defining these regions. This variability affected volumetric measurements of the pars opercularis and pars triangularis in some subjects. In particular, the morphology of the inferior frontal sulcus caused the greatest problems during demarcation in the present study. The inferior frontal sulcus was frequently discontinuous, which required the rater to adjoin the discontinued segments by following the trajectory of the sulci and marking a connection between the segments (Fig. 7). This method effectively cuts through the bridge of cortex that connects the inferior frontal gyrus with the middle frontal gyrus, thus causing an interruption of the inferior frontal sulcus. This method of demarcation is not ideal but is the only way to salvage volumetric measurements of the pars opercularis or pars triangularis in the large proportion of brains with discontinuous inferior frontal sulci without reference to cytoarchitectonic borders. An alternative measurement technique that would not require demarcation of the inferior frontal sulcus – and thus avoids morphological issues regarding the inferior frontal sulcus – is one described by Eckert et al. (2003). This study performed pars triangularis measurements by tracing the contours of the anterior ascending ramus and anterior horizontal ramus on consecutive sagittal sections. Rather than point counting within the grey matter pars triangularis which requires visualisation of the inferior frontal sulcus as the dorsal boundary as performed in the present study, the authors manually outlined the length of the two rami and estimated the size of the pars triangularis based on the length of the rami. For this form of measurement, a discontinuous inferior frontal sulcus does not pose a problem as the inferior frontal sulcus is not used for measurements. However, this method does not provide an estimation of grey matter volume within the pars opercularis and pars triangularis. Other methods of analysis that are not compromised by discontinuous, bifid, variable and sometimes absent sulcal contours include a nominal categorisation scheme of morphology (i.e. documentation of the shape of sulcal contours; Clark & Plante, 1998; Billingsley et al. 2003) or cytoarchitectonic analysis (Hayes & Lewis, 1993, 1995; Amunts et al. 1999, 2003; Uylings et al. 2005, 2006).
The work by Amunts et al. (1999) indicated that macroscopic sulcal contours do not consistently correspond to cytoarchitectonic borders in humans, and that there is great inter-individual variability in the regional cellular morphology of inferior frontal gyrus. Sherwood et al. (2003) have shown that this is also applicable to great apes. It is true that area 44, for example, is consistently located in the opercular region of the inferior frontal gyrus (Amunts et al. 1999). However, the anterior border of area 44 is occasionally located close to, or within, the diagonal sulcus (Amunts et al. 2004), and therefore the anterior and adjacent area 45 impinges on the pars opercularis given that the anterior border of area 44 is not the anterior ascending ramus of the Sylvian fissure. The cytoarchitectonic boundaries of area 44 and area 45 have been found to lie at different regions within the banks of sulci surrounding the pars opercularis and pars triangularis, and the boundaries of area 44 and area 45 may lie at different regions of cortex in different people (Amunts et al. 1999; Amunts & Zilles, 2006). Thus it is unwise to maintain that pars opercularis and area 44, pars triangularis and area 45, and pars orbitalis and area 47 have a one-to-one correspondence, and we therefore maintain a distinction between the leftward asymmetry of the pars opercularis observed in this study and the leftward asymmetry of area 44 previously reported (Amunts et al. 1999, 2003; Uylings et al. 2006). However, whilst we maintain a fundamental difference in the leftward asymmetry of the pars opercularis and area 44, it is difficult to ignore the fact that both asymmetries are of the posterior most region of the inferior frontal gyrus, corresponding to the region of the cerebral cortex Paul Broca attributed to the lateralised expression of speech.
Given the unreliable macroscopic-microscopic anatomical correlation within the inferior frontal gyrus, we included the ventral grey matter deep within the operculum in measurements of the pars opercularis – despite that this area is apparently cytoarchitectonically similar to insular cortex (von Economo & Koskinas, 1925; von Economo & Horn, 1930; Tomaiuolo et al. 1999) – as this region of the inferior frontal gyrus is crucial for speech production (Price, 2000). As the ventral region of the pars opercularis was not sampled in the study of Tomaiuolo et al. (1999), this may explain the larger volumes of the pars opercularis obtained in the present study. We decided not to base gyral measurements on cytoarchitectonic borders given the lack of anatomical correlation.
Unlike some post-mortem studies of the inferior frontal gyrus (Wada et al. 1975; Falzi et al. 1982), quantitative measurements of the posterior regions of the inferior frontal gyrus should be performed separately for the pars opercularis and pars triangularis. Results obtained from the present study show that these structures have reciprocal asymmetries, where left-greater-than-right asymmetry in one structure correlates with right-greater-than-left asymmetry in the other. Hence, if both structures are measured together, any differences between subject groups of interest may well be annulled.
Morphology
Inferior frontal sulcus
There were three main findings from analysis of the inferior frontal sulcus: (i) it was almost as likely to be discontinuous as a continuous straight sulcus, given that a bridge of cortex connecting the inferior frontal gyrus to dorsally located cortex was observed in 48% of the sample; (ii) when discontinuous, it consisted of no more than two distinct segments; and (iii) the posterior region of the inferior frontal sulcus connected (either fully or superficially) with the inferior precentral sulcus in the vast majority of cases, as no connection was observed in 14% left and 20% right hemispheres.
When we contrast our findings to those of Ono et al. (1990), we find a relatively consistent reporting of discontinuous inferior frontal sulci. We reported a discontinuous sulcus in 46% left and 50% right hemispheres, whilst Ono et al. report an incidence in 40% left and 56% right hemispheres. However, an important difference emerges between our study and that of Ono et al. with regards to the division of the inferior frontal sulcus into its constituent segments. We report that there were no more than two clearly identifiable segments of the discontinuous inferior frontal sulcus, while Ono et al. report that it may be interrupted into three segments in 28% left and 8% right hemispheres, and even four segments in 4% (i.e. one) right hemisphere. However, inspection of the classification of an inferior frontal sulcus that had been suggested to be constituted of three segments (Ono et al. 1990; p. 54) indicates that the presence of the lateral frontomarginal sulcus and an almost horizontal caudal extension, possibly representing the sulcus radiatus, was we suggest misclassified as an anterior segment of the inferior frontal sulcus. As we state in the methods section of this paper, Petrides & Pandya (2004) warn that inclusion of the lateral frontomarginal sulcus and sulcus radiatus as an anterior and orbital extension of the inferior frontal sulcus is anatomically inaccurate: ‘The tendency to treat these distinct sulci as rostral extensions of the inferior frontal sulcus introduces considerable confusion in the identification of the frontal sulci. For instance, we observed that, although in some cases the sulcus radiatus and the lateral frontomarginal sulci may anastomose and appear to join superficially the rostral end of the inferior frontal sulcus, these three sulci could be clearly separated by careful examination of sections of the frontal lobe.’ (Petrides & Pandya, 2004; p. 954). The identification of the segments of the inferior frontal sulcus in the present study was carefully performed using orthogonal sections of the intrasulcal anatomy which allowed us to distinguish between the inferior frontal sulcus and other anterior frontal sulci.
We observed a long connection between the posterior inferior frontal sulcus and inferior precentral sulcus in 38% left and 32% right hemispheres, a short connection in 28% left and 30% right hemispheres, a superficial connection in 20% left and 18% right hemispheres, and no connection in 14% left and 20% right hemispheres. There is between-study variation in the reporting of no connection between the inferior frontal sulcus and the inferior precentral sulcus. Cunningham (1892) reported no connection in 32.6% of the adult brains examined, Eberstaller (1890) in 24%, and Ono et al. (1990) in 12%. Cunningham (1892) suggested that the union between the inferior frontal and inferior precentral sulci may be deferred until after birth, as he reported an increased incidence of no connection between the sulci in foetal hemispheres. In particular, no connection was observed in 41.7% of full-term fetuses and in 50.2% of 8-month fetuses.
Inferior precentral sulcus
Early anatomical studies reported no connection between the ventral inferior precentral sulcus and the Sylvian fissure (Cunningham, 1892; Eberstaller, 1890). As maintained by Cunningham, ‘In many cases the vertical limb of the inferior precentral sulcus is shortened by a deficiency in its lower part, but it never, of itself, elongates so as to cut its way into the fissure of Sylvius’ (pp. 247–8). Cunningham and Eberstaller suggested that an indirect connection may exist when the inferior precentral sulcus is connected with the diagonal sulcus or the inferior transverse furrow of Rolando which connect with the Sylvian fissure. Conversely, Giacomini (1882) reported that the inferior precentral sulcus anastomoses with the anterior limb of the Sylvian fissure in 68% of brains, but both Cunningham and Eberstaller reconciled this finding by suggesting that Giacomini may have confused the inferior precentral sulcus with the diagonal sulcus. More recent work has reported that the inferior precentral sulcus directly adjoins the Sylvian fissure in 20% of left and 28% of right hemispheres (Ono et al. 1990). By virtue of their illustrations, Clark & Plante (1998) suggested that the inferior precentral sulcus does connect with the Sylvian fissure, but this feature is not discussed nor is the incidence reported. The results reported by Ono et al. are consistent with the findings obtained in the present study, as we observed a connection between the ventral most point of the inferior precentral sulcus in 16% of left and 22% of right hemispheres (Fig. 9b). The conflicting findings between Ono et al. (1990) and Cunningham (1892) cannot be attributed to sample size as results were based on 50 cerebral hemispheres in both studies. It is therefore crucial to clearly define what aspect of the inferior precentral sulcus and Sylvian fissure are connected. In the present study a connection referred to the absence of a bridge of cortex connecting the frontal operculum with the precentral gyrus on lateral sections. The frontal operculum always connects with the precentral gyrus, but in the cases we report a connection between the inferior precentral sulcus and Sylvian fissure, it does so medially and therefore may be considered a superficial or lateral connection.
We also report that the inferior precentral sulcus consisted of two parallel segments in 4% of brains studied (Fig. 9a). This is identical to that of Ono et al. (1990). The presence of a double inferior precentral sulcus will crucially affect volume quantification of the pars opercularis. If the inferior precentral sulcus consists of two parallel segments, it is impossible to delineate the posterior border of the pars opercularis, and therefore measurements must be abandoned. Sherwood et al. (2003) made this point with regards to the definition of the posterior pars opercularis in the great apes. Bifurcation of the inferior precentral sulcus caused the abandonment of pars opercularis measurements in two subjects in the present study. Tomaiuolo et al. (1999) were also forced to abandon measurements in two subjects due to the morphology of the inferior precentral sulcus, although they maintained that this was because the inferior precentral sulcus could not be reliably identified, and therefore was not necessarily due to bifurcation.
Anterior ascending and horizontal rami of the Sylvian fissure
The morphology (i.e. length, shape and depth) of the anterior ascending and horizontal rami of the Sylvian fissure will directly affect the volume of the pars triangularis, as these rami represent the posterior and anterior border respectively of the pars triangularis. The anterior ascending ramus is most frequently deeper and more substantial than the anterior horizontal ramus. Rarely are these rami reported to be absent in the human brain. In particular, Foundas et al. did not report absent anterior rami in their samples (Foundas et al. 1995, 1996, 1998). This may have been due to the smaller samples sizes in these studies, although a study from the same research group also did not report absent anterior rami in a sample of 96 hemispheres (Knaus et al. 2006). Tomaiuolo et al. (1999) reported absence of the anterior ascending ramus in 1.9% (2/104) hemispheres. There was no reporting of the anterior horizontal ramus given that the study was exclusively concerned with the pars opercularis (Tomaiuolo et al. 1999). Ono et al. (1990) reported a relatively increased incidence of absent anterior horizontal rami. In particular, there was no anterior horizontal ramus in 16% left and 8% right hemispheres. These findings therefore contrast with Knaus et al. (2006) who report no absent rami in a cohort almost double the size of Ono et al. This may be reconciled by the suggestion that the anterior ascending and horizontal rami do not always reach the lateral surface of the cerebrum (Eberstaller, 1890; Foundas et al. 1998) and therefore may be overlooked through analysis of the cortical surface alone, as performed by Ono et al. In the present study, all judgements on sulcal contours were carefully made using orthogonal sections, and there was no anterior ascending ramus in 1% of hemispheres and no anterior horizontal ramus in 2% of hemispheres. These results are comparable to those of Tomaiuolo et al. who also used orthogonal sections for decisions regarding the absence of the anterior ascending ramus (Tomaiuolo et al. 1999).
Diagonal sulcus
We reported a statistically significant left-greater-than-right frequency of the diagonal sulcus in our primary sample. In particular, a diagonal sulcus was present in 52% left and 20% right hemispheres. The average frequency of 36% reported in our primary sample is comparable to that of Galaburda (1980) and in particular Tomaiuolo et al. (1999). Previous studies have generally reported a left-greater-than-right incidence of the diagonal sulcus, but not to the extent of our primary sample. In particular, Galaburda (1980) reported the presence of a diagonal sulcus in 26.5% of left hemispheres and 12.75% of right hemispheres, Ono et al. (1990) in 72% left and 64% right hemispheres, and Tomaiuolo et al. (1999) in 34% left and 32% right hemispheres. When viewing the images of the ten subjects studied by Amunts et al. (2004, p. 45), a diagonal sulcus appears exclusively in the left hemisphere in two brains (20%), bilaterally in four brains (40%) and was not present in either hemisphere in four brains (40%). Given that we were surprised by the significantly lateralised effect in our primary sample, we decided to perform an auxiliary analysis of the presence of the diagonal sulcus in a separate cohort of brains, matched for sex, age and number, and assessed using identical methods to the primary sample. We found that 58% left and 40% right hemispheres had a diagonal sulcus, giving an overall total of 53% left and 27% right hemispheres. Therefore, a left-greater-than-right incidence was again reported, but not to the extent of the primary sample. It appears that the incidence of 20% of right hemispheres having a diagonal sulcus in the primary sample is unusually low with respect to (1) the left hemisphere in the same sample and (2) the left and right hemispheres in the auxiliary sample. However, it is still higher than the previously reported 12.75% by Galaburda (1980). The documentation of the presence of a diagonal sulcus – like assessment of all other sulcal contours and volumetric measurements – was carefully performed using orthogonal sections and the investigator (SSK in all cases) was blind to subject identification and, importantly, hemisphere side. There was therefore no observer bias. Interestingly, the reported incidence of 58% and 40% in the left and right hemispheres respectively in the auxiliary analysis of the present study is almost identical to the 60% and 40% indicated by Amunts et al. (2004).
Posterior Sylvian fissure
We report a significant inter-hemisphere difference in the shape of the posterior portion of the Sylvian fissure, with a greater incidence of horizontal termination in the left hemisphere and a greater incidence of upward oblique termination in the right hemisphere. The asymmetrical trajectory of the Sylvian fissure was one of the first neuroanatomical asymmetries to be described (Cunningham, 1892; Eberstaller, 1884, 1890). In addition to a more frequent horizontal termination, the Sylvian fissure in the left hemisphere has also been reported to have a gentler slope than the right hemisphere (Geschwind & Levitsky, 1968; Toga & Thompson, 2003). In the present study, we have shown that inter-hemispheric differences in the trajectory of the posterior Sylvian fissure are directly related to volume asymmetry of the planum temporale. We report that when an upward oblique termination is observed in the (1) right or (2) left cerebral hemisphere (with the contralateral hemisphere having a horizontal termination), volume asymmetry of the planum temporale will be (1) leftward or (2) rightward, respectively. The posterior border of the planum temporale in the present study, following Anderson et al. (1999), was at the point where the Sylvian fissure turns obliquely upward or at its horizontal termination. Given that the Sylvian fissure of the left hemisphere terminates horizontally more often than the right hemisphere, it is not surprising that the left planum temporale is longer and greater in volume compared to the right when the posterior boundary described in the present study is utilised.
Volume asymmetry
The pars opercularis and pars triangularis are intimately associated with expressive language functions, as demonstrated by numerous lesion, electrical stimulation and functional neuroimaging studies. As leftward asymmetry of expressive language function is usually seen in these structures in the majority of individuals, leftward asymmetry of structure volume may also be expected. The present study found a significant leftward volume of the pars opercularis, adding to the weight of evidence for an asymmetry of the posterior inferior frontal gyrus from post-mortem gyral (Falzi et al. 1982; Albanese et al. 1989), cytoarchitectonic (Amunts et al. 1999, 2003; Uylings et al. 2006) and MRI (Foundas et al. 1998, 2001a) studies of humans. Other studies (Wada et al. 1975; Tomaiuolo et al. 1999; Good et al. 2001; Watkins et al. 2001; Luders et al. 2004; Hervé et al. 2006) have failed to observe a leftward asymmetry of the pars opercularis or pars triangularis. The differing results are likely to be the result of differential methodological applications, in particular region-of-interest boundary definitions, or perhaps as we have shown in the present study, variability in the lateralised presence of the diagonal sulcus. There was no population-based asymmetry of the pars triangularis in the current cohort, which has been found in MRI tracing studies (Foundas et al. 1995, 1996, 1998, 2001b). Whilst we have discussed the lack of direct correspondence between the anatomical boundaries defining the pars opercularis and area 44, it is notable that our findings of the posterior most region of the inferior frontal gyrus (i.e. pars opercularis) being leftward asymmetrical and no asymmetry of the middle region (i.e. pars triangularis) is consistent with the inter-hemispheric cytoarchitectonic findings of area 44 (posterior most region) and area 45 (anterior to area 44) reported by Amunts & Zilles (2006): ‘Volumes of left BA 44 were always larger than those of the right hemisphere … In contrast to BA 44, the volumes of BA 45 did not differ significantly between the hemispheres’ (p. 25). However, other research has demonstrated a leftward asymmetry of area 45 (Hayes & Lewis, 1993, 1995).
One interesting finding from the present study was the relationship between volume asymmetry of the pars opercularis and morphological asymmetry of the diagonal sulcus. We are unaware of any other research reporting this association. The results from our primary analysis indicated that the presence of a diagonal sulcus within the left or right pars opercularis will heavily influence directional volumetric asymmetry of this region of cortex. This finding is intuitive given that the presence of an additional sulcus within the pars opercularis causes the observer to sample more intrasulcal grey matter within this region. However we did not observe a significant leftward lateralisation of the diagonal sulcus in the auxiliary analysis, although there was still a left-greater-than-right incidence. This raises the question whether a volume asymmetry of the pars opercularis would have been observed in the auxiliary sample. Given the association between volume asymmetry of the pars opercularis and presence of the diagonal sulcus in the primary sample, we might expect a non-significant volume asymmetry in the auxiliary sample.
Leftward asymmetry of the planum temporale reported in the present study is consistent with a large volume of literature showing the same asymmetry (see Shapleske et al. 1999, but see also Harasty et al. 2003). It was hypothesized that there would be a positive correlation between the asymmetries of the planum temporale and the frontal operculum, given (1) their crucial role in language reception and expression respectively in the language dominant hemisphere, (2) the functional (Papathanassiou et al. 2000; Matsumoto et al. 2004) and structural (Catani et al. 2002, 2005; Petrides & Pandya, 1998; Parker et al. 2005; Petrides, 2006) connectivity between these regions of cortex, and (3) the leftward asymmetry of the white matter tracts connecting these regions (Buchel et al. 2004; Nucifora et al. 2005; Parker et al. 2005; Barrick et al. 2007). However, no significant correlation was observed between the asymmetry coefficients of these two regions, despite the significant leftward volume asymmetry of the pars opercularis and planum temporale.
Conclusions
The present study reports a detailed analysis of the morphological and morphometric asymmetry of the pars opercularis and pars triangularis of the inferior frontal gyrus. We show that the morphology of the sulcal contours defining these two regions are extremely variable between individuals, particularly in terms of sulcus continuity and connectivity. The rare presentation of bifid or absent sulci/rami prevent quantitative assessment of the pars opercularis and pars triangularis as anterior or posterior anatomical boundaries cannot be defined. We also report that the diagonal sulcus appears more frequently in the left cerebral hemisphere. When the sulcal contours permit reliable delineation of the intrasulcal anatomy of the pars opercularis and pars triangularis, only the pars opercularis, the posterior most convolution of the inferior frontal gyrus, exhibits a leftward volume asymmetry. This region of cortex corresponds precisely to the region Paul Broca primarily associated with the leftward lateralisation of expressive speech. This manuscript demonstrates that quantification of asymmetry of the anterior speech region is a complex endeavour given the large inter-individual morphological variability, and illustrates the importance of simultaneous consideration of brain morphology and morphometry in studies of cerebral asymmetry.
Acknowledgments
We thank Jack Taylor at the School of Health Sciences, University of Liverpool for assistance with inter-rater studies of the pars opercularis and pars triangularis, and Professor Andrew Mayes and Dr Enis Cezayirli for providing access to their sample of MR images. We also thank the radiographers and nursing staff at MARIARC for assistance during MRI scanning. SSK is grateful for the support offered by Professor Timothy Crow at SANE POWIC, Department of Psychiatry, Warneford Hospital, University of Oxford.
References
- Albanese E, Merlo A, Albanese A, Gomez E. Anterior speech region. Asymmetry and weight-surface correlation. Arch Neurol. 1989;46:307–310. doi: 10.1001/archneur.1989.00520390073019. [DOI] [PubMed] [Google Scholar]
- Alexander MP, Naeser MA, Palumbo C. Broca's area aphasias: aphasia after lesions including the frontal operculum. Neurology. 1990;40:353–362. doi: 10.1212/wnl.40.2.353. [DOI] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Burgel U, Mohlberg H, Uylings HB, Zilles K. Broca's region revisited: cytoarchitecture and intersubject variability. J Comp Neurol. 1999;412:319–341. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Ditterich A, Zilles K. Broca's region: cytoarchitectonic asymmetry and developmental changes. J Comp Neurol. 2003;465:72–89. doi: 10.1002/cne.10829. [DOI] [PubMed] [Google Scholar]
- Amunts K, Weiss PH, Mohlberg H, et al. Analysis of neural mechanisms underlying verbal fluency in cytoarchitectonically defined stereotaxic space – the roles of Brodmann areas 44 and 45. Neuroimage. 2004;22:42–56. doi: 10.1016/j.neuroimage.2003.12.031. [DOI] [PubMed] [Google Scholar]
- Amunts K, Zilles K. A multimodal analysis of structure and function in Broca's region. In. In: Grodzinsky Y, Amunts K, editors. Broca's region. New York: Oxford University Press; 2006. pp. 17–30. [Google Scholar]
- Anderson B, Southern BD, Powers RE. Anatomic asymmetries of the posterior superior temporal lobes: a postmortem study. Neuropsychiatry Neuropsychol Behav Neurol. 1999;12:247–54. [PubMed] [Google Scholar]
- Barrick TR, Lawes IN, Mackay CE, Clark CA. White matter pathway asymmetry underlies functional lateralization. Cereb Cortex. 2007;17:591–8. doi: 10.1093/cercor/bhk004. [DOI] [PubMed] [Google Scholar]
- Barrick TR, Mackay CE, Prima S, et al. Automatic analysis of cerebral asymmetry: an exploratory study of the relationship between brain torque and planum temporale asymmetry. Neuroimage. 2005;24:678–691. doi: 10.1016/j.neuroimage.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Bartko JJ. The intraclass correlation coefficient as a measure of reliability. Psychol Rep. 1966;19:3–11. doi: 10.2466/pr0.1966.19.1.3. [DOI] [PubMed] [Google Scholar]
- Bear D, Schiff D, Saver J, Greenberg M, Freeman R. Quantitative analysis of cerebral asymmetries. Fronto-occipital correlation, sexual dimorphism and association with handedness. Arch Neurol. 1986;43:598–603. doi: 10.1001/archneur.1986.00520060060019. [DOI] [PubMed] [Google Scholar]
- Billingsley RL, Slopis JM, Swank PR, Jackson EF, Moore BD 3rd. Cortical morphology associated with language function in neurofibromatosis, type I. Brain Lang. 2003;85:125–139. doi: 10.1016/s0093-934x(02)00563-1. [DOI] [PubMed] [Google Scholar]
- Broca P. Nouvelle observation aphémie produite par un lésion de la moité postérieure des deuxième et troisième circonvolutions frontales. Bulletins de la Socie ‘te’ Anatomique. 1861a;6:398–407. [Google Scholar]
- Broca P. Remarques sur le Siége de la Faculté du Langage Articulé, Suivies d’une Observatoin d’aphémie (Perte de la Parole) Bulletin de la Société Anatomique de Paris. 1861b;6:330–357. [Google Scholar]
- Broca P. Localisation des fonctions cérébrales. Siège du langage articulé. Bulletins de la Société d’Anthropologie. 1863;4:200–204. [Google Scholar]
- Broca P. Sur le siège de la faculté du langage articule. Bulletins de la Société d’Anthropologie. 1865;6:377–393. [Google Scholar]
- Buchel C, Raedler T, Sommer M, Sach M, Weiller C, Koch MA. White matter asymmetry in the human brain: a diffusion tensor MRI study. Cereb Cortex. 2004;14:945–951. doi: 10.1093/cercor/bhh055. [DOI] [PubMed] [Google Scholar]
- Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. Neuroimage. 2002;17:77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- Catani M, Jones DK, Ffytche DH. Perisylvian language networks of the human brain. Ann Neurol. 2005;57:8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Clark MM, Plante E. Morphology of the inferior frontal gyrus in developmentally language-disordered adults. Brain Lang. 1998;61:288–303. doi: 10.1006/brln.1997.1864. [DOI] [PubMed] [Google Scholar]
- Cunningham D. Contribution to the surface anatomy of the cerebral hemispheres. Dublin: Royal Irish Academy; 1892. [Google Scholar]
- Doherty CP, Fitzsimons M, Holohan T, et al. Accuracy and validity of stereology as a quantitative method for assessment of human temporal lobe volumes acquired by magnetic resonance imaging. Magn Reson Imaging. 2000;18:1017–25. doi: 10.1016/s0730-725x(00)00185-5. [DOI] [PubMed] [Google Scholar]
- Duvernoy H. The human brain. Surface, blood supply and three-dimensional sectional anatomy. New York: Springer; 1999. [Google Scholar]
- Eberstaller O. Zur Oberflächenanatomie der Grosshirnhemisphären. Wien Med Bl. 1884;7:479–482. [Google Scholar]
- Eberstaller O. Das Stirnhirn. Wien and Leipzig: Urban and Schwarzenberg; 1890. [Google Scholar]
- Eckert MA, Leonard CM, Richards TL, Aylward EH, Thomson J, Berninger VW. Anatomical correlates of dyslexia: frontal and cerebellar findings. Brain. 2003;126:482–494. doi: 10.1093/brain/awg026. [DOI] [PubMed] [Google Scholar]
- Edwards SG, Gong QY, Liu C, et al. Infratentorial atrophy on magnetic resonance imaging and disability in multiple sclerosis. Brain. 1999;122(Pt 2):291–301. doi: 10.1093/brain/122.2.291. [DOI] [PubMed] [Google Scholar]
- Falzi G, Perrone P, Vignolo LA. Right-left asymmetry in anterior speech region. Arch Neurol. 1982;39:239–240. doi: 10.1001/archneur.1982.00510160045009. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Bollich AM, Corey DM, Hurley M, Heilman KM. Anomalous anatomy of speech-language areas in adults with persistent developmental stuttering. Neurology. 2001a;57:207–215. doi: 10.1212/wnl.57.2.207. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Eure KF, Luevano LF, Weinberger DR. MRI asymmetries of Broca's area: the pars triangularis and pars opercularis. Brain Lang. 1998;64:282–296. doi: 10.1006/brln.1998.1974. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Leonard CM, Gilmore RL, Fennell EB, Heilman KM. Pars triangularis asymmetry and language dominance. Proc Natl Acad Sci USA. 1996;93:719–722. doi: 10.1073/pnas.93.2.719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foundas AL, Leonard CM, Heilman KM. Morphologic cerebral asymmetries and handedness. The pars triangularis and planum temporale. Arch Neurol. 1995;52:501–508. doi: 10.1001/archneur.1995.00540290091023. [DOI] [PubMed] [Google Scholar]
- Foundas AL, Weisberg A, Browning CA, Weinberger DR. Morphology of the frontal operculum: a volumetric magnetic resonance imaging study of the pars triangularis. J Neuroimaging. 2001b;11:153–159. doi: 10.1111/j.1552-6569.2001.tb00026.x. [DOI] [PubMed] [Google Scholar]
- Galaburda AM. Broca's region: anatomic remarks made a century after the death of its discoverer. Rev Neurol (Paris) 1980;136:609–616. [PubMed] [Google Scholar]
- Garcia-Finana M, Cruz-Orive LM, Mackay CE, Pakkenberg B, Roberts N. Comparison of MR imaging against physical sectioning to estimate the volume of human cerebral compartments. Neuroimage. 2003;18:505–516. doi: 10.1016/s1053-8119(02)00021-6. [DOI] [PubMed] [Google Scholar]
- Germann J, Robbins S, Halsband U, Petrides M. Precentral sulcal complex of the human brain: morphology and statistical probability maps. J Comp Neurol. 2005;493:334–56. doi: 10.1002/cne.20820. [DOI] [PubMed] [Google Scholar]
- Geschwind N, Levitsky W. Human brain: left-right asymmetries in temporal speech region. Science. 1968;161:186–187. doi: 10.1126/science.161.3837.186. [DOI] [PubMed] [Google Scholar]
- Giacomini C. Varietà delle circonvoluzioni cerebrali dell’ uomo. Loescher, Turin: [Google Scholar]
- Good CD, Johnsrude I, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. Cerebral asymmetry and the effects of sex and handedness on brain structure: a voxel-based morphometric analysis of 465 normal adult human brains. Neuroimage. 2001;14:685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB. The efficiency of systematic sampling in stereology and its prediction. J Microsc. 1987;147:229–263. doi: 10.1111/j.1365-2818.1987.tb02837.x. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Jensen EB, Kieu K, Nielsen J. The efficiency of systematic sampling in stereology–reconsidered. J Microsc. 1999;193:199–211. doi: 10.1046/j.1365-2818.1999.00457.x. [DOI] [PubMed] [Google Scholar]
- Gundersen HJ, Osterby R. Optimizing sampling efficiency of stereological studies in biology: or ‘do more less well!’. J Microsc. 1981;121:65–73. doi: 10.1111/j.1365-2818.1981.tb01199.x. [DOI] [PubMed] [Google Scholar]
- Hammers A, Allom R, Koepp MJ, et al. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum Brain Mapp. 2003;19:224–247. doi: 10.1002/hbm.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammers A, Chen CH, Lemieux L, et al. Statistical neuroanatomy of the human inferior frontal gyrus and probabilistic atlas in a standard stereotaxic space. Hum Brain Mapp. 2007;28:34–48. doi: 10.1002/hbm.20254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harasty J, Seldon HL, Chan P, Halliday G, Harding A. The left human speech-processing cortex is thinner but longer than the right. Laterality. 2003;8:247–60. doi: 10.1080/13576500244000175. [DOI] [PubMed] [Google Scholar]
- Hayes TL, Lewis DA. Hemispheric differences in layer III pyramidal neurons of the anterior language area. Arch Neurol. 1993;50:501–505. doi: 10.1001/archneur.1993.00540050053015. [DOI] [PubMed] [Google Scholar]
- Hayes TL, Lewis DA. Anatomical specialization of the anterior motor speech area: hemispheric differences in magnopyramidal neurons. Brain Lang. 1995;49:289–308. doi: 10.1006/brln.1995.1035. [DOI] [PubMed] [Google Scholar]
- Hervé PY, Crivello F, Perchey G, Mazoyer B, Tzourio-Mazoyer N. Handedness and cerebral anatomical asymmetries in young adult males. Neuroimage. 2006;29:1066–1079. doi: 10.1016/j.neuroimage.2005.08.031. [DOI] [PubMed] [Google Scholar]
- Howard MA, Roberts N, Garcia-Finana M, Cowell PE. Volume estimation of prefrontal cortical subfields using MRI and stereology. Brain Res Brain Res Protoc. 2003;10:125–138. doi: 10.1016/s1385-299x(02)00202-7. [DOI] [PubMed] [Google Scholar]
- Ide A, Rodriguez E, Zaidel E, Aboitiz F. Bifurcation patterns in the human sylvian fissure: hemispheric and sex differences. Cereb Cortex. 1996;6:717–725. doi: 10.1093/cercor/6.5.717. [DOI] [PubMed] [Google Scholar]
- Jelsing J, Rostrup E, Markenroth K, et al. Assessment of in vivo MR imaging compared to physical sections in vitro– a quantitative study of brain volumes using stereology. Neuroimage. 2005;26:57–65. doi: 10.1016/j.neuroimage.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Keller SS. University of Liverpool; Quantitative magnetic resonance image analysis studies of brain morphology in patients with temporal lobe epilepsy in a large clinical database) PhD Thesis. [Google Scholar]
- Keller SS, Mackay CE, Barrick TR, Wieshmann UC, Howard MA, Roberts N. Voxel-based morphometric comparison of hippocampal and extrahippocampal abnormalities in patients with left and right hippocampal atrophy. Neuroimage. 2002a;16:23–31. doi: 10.1006/nimg.2001.1072. [DOI] [PubMed] [Google Scholar]
- Keller SS, Wieshmann UC, Mackay CE, Denby CE, Webb J, Roberts N. Voxel based morphometry of grey matter abnormalities in patients with medically intractable temporal lobe epilepsy: effects of side of seizure onset and epilepsy duration. J Neurol Neurosurg Psychiatry. 2002b;73:648–55. doi: 10.1136/jnnp.73.6.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JJ, Crespo-Facorro B, Andreasen NC, et al. An MRI-based parcellation method for the temporal lobe. Neuroimage. 2000;11:271–288. doi: 10.1006/nimg.2000.0543. [DOI] [PubMed] [Google Scholar]
- Knaus TA, Bollich AM, Corey DM, Lemen LC, Foundas AL. Variability in perisylvian brain anatomy in healthy adults. Brain Lang. 2006;97:219–232. doi: 10.1016/j.bandl.2005.10.008. [DOI] [PubMed] [Google Scholar]
- Liu C, Edwards S, Gong Q, Roberts N, Blumhardt LD. Three dimensional MRI estimates of brain and spinal cord atrophy in multiple sclerosis. J Neurol Neurosurg Psychiatry. 1999;66:323–330. doi: 10.1136/jnnp.66.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luders E, Gaser C, Jancke L, Schlaug G. A voxel-based approach to gray matter asymmetries. Neuroimage. 2004;22:656–664. doi: 10.1016/j.neuroimage.2004.01.032. [DOI] [PubMed] [Google Scholar]
- Mackay CE, Roberts N, Mayes AR, Downes JJ, Foster JK, Mann D. An exploratory study of the relationship between face recognition memory and the volume of medial temporal lobe structures in healthy young males. Behav Neurol. 1998;11:3–20. doi: 10.1155/1998/285061. [DOI] [PubMed] [Google Scholar]
- Mackay CE, Webb JA, Eldridge PR, Chadwick DW, Whitehouse GH, Roberts N. Quantitative magnetic resonance imaging in consecutive patients evaluated for surgical treatment of temporal lobe epilepsy. Magn Reson Imaging. 2000;18:1187–1199. doi: 10.1016/s0730-725x(00)00220-4. [DOI] [PubMed] [Google Scholar]
- Maes F, Van Leemput K, DeLisi L, Vandermeulen D, Suetens P. Quantification of cerebral grey and white matter asymmetry from MRI, lecture notes in computer science. In. In: Taylor C, Colchester A, editors. Proceedings 2nd International Conference on Medical Image Computing and Computer-Assisted Intervention – MICCAI’99. Cambridge: Springer; 1999. pp. 348–357. [Google Scholar]
- Matsumoto R, Nair DR, LaPresto E, et al. Functional connectivity in the human language system: a cortico-cortical evoked potential study. Brain. 2004;127:2316–2330. doi: 10.1093/brain/awh246. [DOI] [PubMed] [Google Scholar]
- Mohr JP. Broca's area and Broca's aphasia. In. In: Whitaker H, Whitaker HA, editors. Studies in neurolinguistics. New York: Elsevier; 1979. [Google Scholar]
- Mohr JP, Pessin MS, Finkelstein S, Funkenstein HH, Duncan GW, Davis KR. Broca aphasia: pathologic and clinical. Neurology. 1978;28:311–24. doi: 10.1212/wnl.28.4.311. [DOI] [PubMed] [Google Scholar]
- Nucifora PG, Verma R, Melhem ER, Gur RE, Gur RC. Leftward asymmetry in relative fiber density of the arcuate fasciculus. Neuroreport. 2005;16:791–794. doi: 10.1097/00001756-200505310-00002. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ono M, Kubik S, Abernathey CD. Atlas of the cerebral sulci. New York: Thieme, Stuttgart; 1990. [Google Scholar]
- Pakkenberg B, Pelvig D, Marner L, et al. Aging and the human neocortex. Exp Gerontol. 2003;38:95–99. doi: 10.1016/s0531-5565(02)00151-1. [DOI] [PubMed] [Google Scholar]
- Papathanassiou D, Etard O, Mellet E, Zago L, Mazoyer B, Tzourio-Mazoyer N. A common language network for comprehension and production: a contribution to the definition of language epicenters with PET. Neuroimage. 2000;11:347–57. doi: 10.1006/nimg.2000.0546. [DOI] [PubMed] [Google Scholar]
- Parker GJ, Luzzi S, Alexander DC, Wheeler-Kingshott CA, Ciccarelli O, Lambon Ralph MA. Lateralization of ventral and dorsal auditory-language pathways in the human brain. Neuroimage. 2005;24:656–666. doi: 10.1016/j.neuroimage.2004.08.047. [DOI] [PubMed] [Google Scholar]
- Petrides M. Broca's area in the human and nonhuman primate brain. In: Grodzinsky Y, Amunts K, editors. Broca's region. New York: Oxford University Press; 2006. pp. 31–46. [Google Scholar]
- Petrides M, Pandya DN. Association fiber pathways to the frontal cortex from the superior temporal region in the rhesus monkey. J Comp Neurol. 1988;273:52–66. doi: 10.1002/cne.902730106. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. The frontal cortex. In. In: Paxinos G, Mai JK, editors. The human nervous system. San Diego: Elsevier Academic Press; 2004. pp. 950–972. [Google Scholar]
- Price CJ. The anatomy of language: contributions from functional neuroimaging. J Anat. 2000;197(Pt 3):335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puddephat M. The Magnetic Resonance and Image Analysis Research Centre (MARIARC) Liverpool: University of Liverpool; 1999. Computer interface for convenient application of stereological methods for unbiased estimation of volume and surface area: studies using MRI with particular reference to the human brain. [Google Scholar]
- Roberts N, Puddephat MJ, McNulty V. The benefit of stereology for quantitative radiology. Br J Radiol. 2000;73:679–97. doi: 10.1259/bjr.73.871.11089458. [DOI] [PubMed] [Google Scholar]
- Salmenpera T, Kononen M, Roberts N, Vanninen R, Pitkanen A, Kalviainen R. Hippocampal damage in newly diagnosed focal epilepsy: a prospective MRI study. Neurology. 2005;64:62–68. doi: 10.1212/01.WNL.0000148643.36513.2A. [DOI] [PubMed] [Google Scholar]
- Schmitz C, Hof PR. Design-based stereology in neuroscience. Neuroscience. 2005;130:813–831. doi: 10.1016/j.neuroscience.2004.08.050. [DOI] [PubMed] [Google Scholar]
- Shapleske J, Rossell SL, Woodruff PW, David AS. The planum temporale: a systematic, quantitative review of its structural, functional and clinical significance. Brain Res Brain Res Rev. 1999;29:26–49. doi: 10.1016/s0165-0173(98)00047-2. [DOI] [PubMed] [Google Scholar]
- Sheline YI, Black KJ, Lin DY, et al. Stereological MRI volumetry of the frontal lobe. Psychiatry Res. 1996;67:203–214. doi: 10.1016/0925-4927(96)02831-4. [DOI] [PubMed] [Google Scholar]
- Sherwood CC, Broadfield DC, Holloway RL, Gannon PJ, Hof PR. Variability of Broca's area homologue in African great apes: implications for language evolution. Anat Rec A Discov Mol Cell Evol Biol. 2003;271:276–285. doi: 10.1002/ar.a.10046. [DOI] [PubMed] [Google Scholar]
- Toga AW, Thompson PM. Mapping brain asymmetry. Nat Rev Neurosci. 2003;4:37–48. doi: 10.1038/nrn1009. [DOI] [PubMed] [Google Scholar]
- Tomaiuolo F, MacDonald JD, Caramanos Z, et al. Morphology, morphometry and probability mapping of the pars opercularis of the inferior frontal gyrus: an in vivo MRI analysis. Eur J Neurosci. 1999;11:3033–3046. doi: 10.1046/j.1460-9568.1999.00718.x. [DOI] [PubMed] [Google Scholar]
- Tramo MJ, Baynes K, Volpe BT. Impaired syntactic comprehension and production in Broca's aphasia: CT lesion localization and recovery patterns. Neurology. 1988;38:95–98. doi: 10.1212/wnl.38.1.95. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Jacobsen AM, Zilles K, Amunts K. Left-right asymmetry in volume and number of neurons in adult Broca's area. Cortex. 2006;42:652–658. doi: 10.1016/s0010-9452(08)70401-5. [DOI] [PubMed] [Google Scholar]
- Uylings HB, Malofeeva LI, Bogolepova IN, Jacobsen AM, Amunts K, Zilles K. No postnatal doubling of number of neurons in human Broca's areas (Brodmann areas 44 and 45)? A stereological study. Neuroscience. 2005;136:715–728. doi: 10.1016/j.neuroscience.2005.07.048. [DOI] [PubMed] [Google Scholar]
- von Economo C, Horn L. Über Windungsrelief, Masse und Rindenarchitektonik der Supratemporalfläche, ihre individuellen und ihre Seitenunterschiede. Zeitschrift für Neurologie und Psychiatrie. 1930;130:678–757. [Google Scholar]
- von Economo C, Koskinas G. Die Cytoarchitektonik der Hirnrinde des erwachsenen Menschen. Berlin: Springer; 1925. [Google Scholar]
- Wada JA, Clarke R, Hamm A. Cerebral hemispheric asymmetry in humans. Cortical speech zones in 100 adults and 100 infant brains. Arch Neurol. 1975;32:239–246. doi: 10.1001/archneur.1975.00490460055007. [DOI] [PubMed] [Google Scholar]
- Watkins KE, Paus T, Lerch JP, et al. Structural asymmetries in the human brain: a voxel-based statistical analysis of 142 MRI scans. Cereb Cortex. 2001;11:868–877. doi: 10.1093/cercor/11.9.868. [DOI] [PubMed] [Google Scholar]