Abstract
Cultured cells are dramatically affected by the micro-environment in which they are grown. In this study, we have investigated whether HepG2 liver cells grown in three dimensional (3-D) cultures cope more effectively with the known cytotoxic agent, methotrexate, than their counterparts grown on traditional two dimensional (2-D) flat plastic surfaces. To enable 3-D growth of HepG2 cells in vitro, we cultured cells on 3-D porous polystyrene scaffolds previously developed in our laboratories. HepG2 cells grown in 3-D displayed excellent morphological characteristics and formed numerous bile canaliculi that were seldom seen in cultures grown on 2-D surfaces. The function of liver cells grown on 3-D supports was significantly enhanced compared to activity of cells grown on 2-D standard plasticware. Unlike their 2-D counterparts, 3-D cultures were less susceptible to lower concentrations of methotrexate. Cells grown in 3-D maintained their structural integrity, possessed greater viability, were less susceptible to cell death at higher levels of the cytotoxin compared to 2-D cultures, and appeared to respond to the drug in a manner more comparable to its known activity in vivo. Our results suggest that hepatotoxicity testing using 3-D cultures might be more likely to reflect true physiological responses to cytotoxic compounds than existing models that rely on 2-D culture systems. This technology has potential applications for toxicity testing and drug screening.
Keywords: 3-D cell growth, cell culture, drug screening, function, HepG2 cells, liver, polymer scaffold, toxicity, ultra-structure
Introduction
It has been proposed that engineering the cell culture micro-environment to create growth conditions that more accurately mimic the in vivo behaviour of cells is an essential step for improving the predictive accuracy of the drug discovery process (Bhadriraju & Chen, 2002). The design of three dimensional (3-D) in vitro models to develop new pharmacological treatments is instrumental to achieving this objective. Evidence suggests that modification of cell growth conditions can radically influence the behaviour of cells in response to chemical reagents (Sun et al. 2006).
Culturing cells in Petri dishes and flasks and encouraging them to grow and adopt a phenotype typical of their counterparts in vivo is challenging. Many important discoveries have come from studying cell proliferation, differentiation and function in vitro, however, the two-dimensional (2-D) environment cells’ experience on flat tissue culture plastic is far removed from the complexities of 3-D tissues within the body. In tissues, cells connect to each other as well as to the extra-cellular matrix (ECM). Receptor complexes on the surface of cells facilitate interactions with their neighbours, with the ECM and other exogenous factors, to enable cells to interpret the multitude of biochemical and physical cues from the immediate environment. Given this intricate mechanical and biochemical interplay, important biological properties may be missed if cells are studied within a 2-D culture system. Furthermore, although 2-D cultures are convenient for practical reasons when routinely growing cells in vitro, they do impose highly unnatural geometric and mechanical pressures on many types of cells.
It is now recognised that the context in which a cell is grown matters and changing the environment can radically alter the behaviour of cells. In different cell culture applications, it has been shown that the growth and function of cells as multi-cellular 3-D structures is significantly different to their growth as conventional 2-D monolayer cultures (Schmeichel & Bissell, 2003). The lack of dorsal anchorage points in 2-D cultures affects the balance between cells spreading or retracting, creating an unnatural stimulatory environment for the organization of lamellipodia, stress fibres and focal adhesions. This imbalance causes the cells to spread out in an extreme manner. For example, the spatial arrangement of ECM receptors in 2-D cultures is mainly concentrated on the ventral surface, whereas in 3-D cultures they are spread over the entire surface. Experiments have shown that growing cancerous breast cells in 3-D culture systems resulted in changes in patterns of gene expression and other biological activities that more closely reflect the activities of cells in living tissues (Weaver et al. 1997; Wang et al. 1998). Cell differentiation is also responsive to changes in culture environment, for example, fibroblasts responded to their 3-D surroundings by increasing their rate of proliferation and migration, and assumed asymmetrical morphologies more characteristic of fibroblasts in living tissues (Cukierman et al. 2001). Furthermore, culturing human pluripotent stem cells on 3-D scaffolds can markedly influence cell behaviour (Levenberg et al. 2003; Hayman et al. 2004) by offering growing tissues the opportunity to exploit additional space and form alternative interactions with neighbouring cells, interactions that are restricted in cultures grown as 2-D monolayers.
As a consequence of these findings, there is a requirement for novel in vitro culture systems that more authentically represent the cellular environment of the respective native tissues which in turn will help advance our understanding of complex biological phenomena. Demand in this area has created interest in fabricating materials that support 3-D cell growth to enhance cell function in ways that resemble tissues in vivo. One of the early most successful approaches has been seeding and culturing cells on biodegradable polymers such as poly(glycolic acid), poly(lactic acid) and their copolymers poly(lactic-co-glycolic acid) (Mikos et al. 1993). This family of polymers has much potential and they have successfully been implanted to treat various conditions such as repair of articular cartilage (Temenoff & Mikos, 2000). Gradual degradation of implanted biopolymers is an important feature of a scaffold to aid the integration of the cells they carry with existing host tissues. However, improper storage of such polymers can render them useless due to degradation and changes in their properties that can directly influence the quality and reproducibility of results. Furthermore, biodegradation itself is not necessarily a feature that is required of a polymer to create a 3-D environment that can be routinely used for investigating cell differentiation in vitro.
As an alternative strategy, hydrogels containing materials such as agarose, collagen or fibrin have successfully been employed to provide a 3-D environment for numerous cell types, including neurons, bone and cartilage (Blackshaw et al. 1997; Sakiyama et al. 1999; Yu et al. 1999; Fisher et al. 2004; Park et al. 2005). Such gels can be readily engineered to tailor their characteristics toward preferential cell growth and enhancement of cell development. Examples of such tailoring include the incorporation of biologically active molecules such as laminin into the gel (Yu et al. 1999) and altering physical parameters of the scaffold such as charge (Dillon et al. 1998). Hydrogels have multiple applications for tissue repair and have also been shown to promote tissue regeneration (Blackshaw et al. 1997). Although hydrogel technology provides a useful system for 3-D cell growth, the general everyday use of such gels for cell culture may be restricted by various practical issues including gel preparation, storage and variability.
We have previously reported that an inert solid scaffold that can be supplied pre-fabricated, sterile and ready to use would offer several advantages including reproducibility, robustness, stability and less preparation time (Hayman et al. 2004; Bokhari et al. 2007). Solid but highly porous 3-D matrices can be created by polymerisation in high internal phase emulsions (HIPEs) as we have previously described (Barbetta et al. 2000; Cameron & Barbetta, 2000; Krajnc et al. 2002). To prepare polymers by this technique, one (or more) monomer(s), and a cross linker, is (are) present in the continuous, or non-droplet, phase of the emulsion. Curing of the monomer(s) occurs around the dispersed phase droplets, which create voids in the resulting material (Cameron & Sherrington, 1996; Cameron, 2005; Zhang & Cooper, 2005). Volume contraction occurs on conversion of monomer to polymer, which ensures that each void is connected to all of its neighbours by interconnecting windows resulting in an open cell solid foam (Cameron et al. 1996). In addition, we have previously shown that the user can tailor and closely control the structure of the material in line with its intended purpose. This includes, for example, the development of customised scaffolds for the culture of different cell types that require alternative growth conditions (Carnachan et al. 2006). More recently, we have produced thin membranes of the polystyrene scaffold to enable the free entry of cells into the internal structure of the polymer. Our aim is to develop this technology and produce a user-friendly cell culture device that can be routinely employed for 3-D cell growth in vitro (Bokhari et al. 2007).
To demonstrate the potential application of this technology for the drug discovery process, we evaluated the structure and function of HepG2 cells cultured on 3-D porous polystyrene supports in the presence of a well known hepatotoxic drug. The human HepG2 cell line is derived from a hepatic carcinoma and is a well recognised model system frequently used to investigate liver cell function in vitro. In essence, HepG2 cells share many of the properties of anchorage-sensitive hepatocytes (Bouma et al. 1989), including secretion of differentiated gene products (Rollier et al. 1993), plasma membrane polarity (Maurice et al. 1988), and growth factor regulation of cell mobility (Tajima et al. 1992). Since the liver is the main organ involved in metabolism and the processing of xenobiotics, HepG2 liver cell lines are often used as tools to screen for the toxicity of drugs and test compounds that may affect liver cell function (Mersch-Sundermann et al. 2004). Therefore, the objective of this study was to investigate the short-term effects of exposing cells to methotrexate (MTX) by examining HepG2 cell structure and function in response to this cytotoxin. Being an antifolate, MTX is widely used in the treatment of malignant tumours and other diseases. Hepatotoxicity is a common complication and can lead to irreversible liver damage (Wu et al. 1983) which is normally characterised by fatty infiltration, inflammation, cellular necrosis and fibrosis (Barak et al. 1984). Accordingly, MTX is a useful candidate to assess liver toxicity in cultures of HepG2 cells grown on our 3-D polystyrene substrate compared to standard planar polystyrene surfaces.
Materials and methods
Preparation of PolyHIPE scaffolds
Detail concerning the preparation and fabrication of styrene-based PolyHIPE scaffolds has been described previously (Akay et al. 2004; Hayman et al. 2004, 2005). The polystyrene polymer was subsequently sectioned into 120 µm thick membranes and adhered to the base of polystyrene cups similar to those often used as well-inserts for cell culture as previously described (Bokhari et al. 2007). The prototype device was briefly immersed and sterilised using 70% ethanol (v/v) and washed three times with sterile phosphate buffered saline.
Culture of HepG2 cells
Human HepG2 hepatic carcinoma cells were purchased from the American Type Culture Collection (ATCC, Manassas, VC, USA). HepG2 cells were cultured at 37°C in 5% CO2 in growth medium [Dulbecco's modified Eagle medium (D-MEM, Gibco/BRL, Carlsbad, CA, USA) supplemented with 10% (v/v) foetal calf serum (FBS, Gibco/BRL), 100 µg mL−1 penicillin and 10 µg mL−1 streptomycin (Gibco/BRL)]. Cells were passaged every 5–7 days. Suspensions of HepG2 cells were produced from confluent cultures using trypsin/EDTA solution and cell concentration determined using a hemocytometer. HepG2 cells were seeded at equal densities either directly into wells of a standard 6-well plate (Nunc, Roskilde, Denmark) or into modified well-inserts mounted with the polymer and located in wells of a 6-well plate. Growth medium was changed every 3–4 days or as required.
Methotrexate (MTX) toxicity studies
Cells were seeded on 2-D and 3-D surfaces in triplicate and left to settle and adhere for 24 h. The medium was then changed and replaced with medium containing different concentrations of MTX (no MTX (vehicle alone, control), 8 µm, 31 µm, and 125 µm). Cells were subsequently incubated for 1, 3, 7 or 10 days, after which cultures were sampled and assayed for cell number/viability and levels of albumin and transglutaminase were determined.
Determination of cell viability
The number of viable cells was determined using a commercially available colorimetric assay (MTT assay; Promega, Madison, WI, USA) based on Mosmann's original method for measuring cell activity involving the conversion of a tetrazolium salt into a blue formazan product detectable by a spectrophotometer (570 nm) (Mosmann, 1983). The assay was performed according to the manufacturer's instructions on HepG2 cells cultured on 2-D and 3-D substrates for various periods under alternative growth conditions.
Metabolic activity of HepG2 cells
Levels of albumin were determined using a commercially available kit (Bioassay systems, Hayward, CA, USA) based on an established method that utilizes bromocresol green which forms a coloured complex specifically with albumin that is detectable at 620 nm. Known quantities of human albumin were used to establish the standard curve. Specific levels of albumin secretion were normalised to total protein levels (as determined by a standard Bradford assay).
Enzymatic assay of transglutaminase
Tissue transglutaminase is a cross-linking enzyme which has recently been suggested to play a role in the formation of fibrotic lesions in experimental settings. The leakage of this enzyme is often used as a marker for in vitro toxicity testing and its presence indicates damage to cell membranes. Several in vivo and in vitro experimental model systems show a direct relationship between the expression and activity of tissue transglutaminase, suppression of cell growth and programmed cell death (Piacentini et al. 1991; Melino et al. 2000; Ohtake et al. 2006). The level of transglutaminase was analysed by means of a quantitative enzymatic assay (Sigma, Poole, UK) as previously described (Folk & Cole, 1966).
Sample preparation for scanning electron microscopy (SEM) and transmission electron microscopy (TEM)
To visualise cells by SEM, cultures grown on 2-D or 3-D substrates were fixed in 2% paraformaldehyde and 2.5% glutaraldehyde in Sorenson's phosphate buffer for 60 min at room temperature (RT). Samples were then rinsed in 0.1 m phosphate buffer and immersed in 1% OsO4 (aq.) solution for 60 min, then dehydrated in 50%, 70%, 95% and 100% ethanol for 5 min, four times for each respective ethanol change. Samples of fixed cells grown on 2-D and 3-D substrates were then cut into smaller pieces (approximately 25 mm2), mounted on specimen holders and dried from CO2 at 38 °C at 1200 psi. Specimens were subsequently sputter coated with a 7 nm layer of chromium and analysed using a Hitachi S5200 SEM (Wokingham, UK).
To visualise cells by TEM, cells grown on 3-D substrates were fixed and treated as described above for SEM. However, subsequent to dehydration and being cut into small pieces, samples were embedded in resin (Araldite CY212, Agar Scientific, Stansted, UK) for 60 min at 37 °C and then placed into pyramidal moulds at 60 °C overnight. For the preparation of cultures grown on 2-D surfaces, cells were fixed in 2% paraformaldehyde and 2.5% glutaraldehyde in Sorenson's phosphate buffer for 60 min at RT. Cells were then scraped from tissue culture plastic and pelleted at 453 g for 10 min. Pelleted cells were subsequently washed in 0.1 m phosphate buffer and immersed in 1% OsO4 (aq.) solution for 60 min, then dehydrated in 50%, 70%, 95% and 100% ethanol for 5 min for each ethanol wash. The dehydrated cell pellets were then soaked in resin (Araldite CY212) for 60 min at 37 °C. When solidified, ultra thin sections of the resin embedded material were produced and subsequently analysed using a Hitachi H7600 TEM.
Statistical analysis
At least three independent replicates were performed for each experiment. The Mann Whitney U test was used to analyse data for statistical significance (at the 5% level of significance or greater).
Results
Morphological characteristics of HepG2 cells grown on alternative substrates
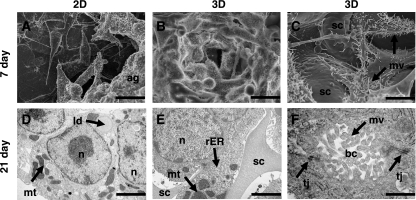
Significant differences in the appearance of HepG2 cells cultured either on 2-D or 3-D substrates were revealed using SEM (Fig. 1). Cultures grown for 7 days on 2-D polystyrene surfaces formed flat extended structures. Cells grown in 2-D were generally heterogeneous and disorganised in their appearance. After 14 days, cells cultured on tissue culture plastic started to cluster and form aggregates. Regions of HepG2 cultures grown for 14–21 days appeared unhealthy, some cells were rounding up and others were disintegrating (data not shown). Cultures grown on 3-D scaffolds spread across and into the structure of the material. Cells initially clustered into colonies of closely packed cells within the substance of the polymer, resembling small multi-cellular aggregates. This was indicative of the greater attachment and interaction between adjacent cells growing on the scaffold. After 7 days, HepG2 cells grown in 3-D appeared more homogeneous than their 2-D counterparts. We purposely set up some cultures at lower seeding densities to see how individual cells attached to the scaffold and extended across voids (Fig. 1). These images showed how cells grown in 3-D can maximise their surface area by interacting with adjacent cells and the incubation medium. In addition, imaging of individual cells at higher magnification showed that cells grown in 3-D consistently exhibited a significantly greater number of microvilli compared to cells cultured on 2-D surfaces.
Fig. 1.
Scanning (SEM, A–C) and transmission (TEM, D–F) electron micrographs showing examples of HepG2 cells cultured on 2-D (A, D) and 3-D (B, C, E, F) polystyrene substrates for either 7 (A–C) or 21 days (D–F). SEM showed that HepG2 cells cultured on 2-D surfaces were significantly more heterogeneous in structure (A) compared to cells grown on 3-D surfaces (B). Cells seeded at a lower density allowed visualisation of individual cell structure grown on 3-D scaffolds (sc) (C). HepG2 cells developed complex 3-D shapes and interactions with neighbouring cells. Higher magnification images revealed the expression of large numbers of microvilli (mv) on the surface of cells (C). TEM showed that hepatocytes grown on 2-D plastic contained typical cellular structures, for example, nuclei (n), mitochondria (mt), and lipid droplets (ld) (D). Images of HepG2 cells cultured on polystyrene scaffolds showed how the cells grow in close association with the polymer, completely surrounding struts of the scaffold (sc) as shown (E). Hepatocytes grown in 3-D also displayed an array of typical cellular organelles such as nuclei (n), mitochondria (mt), rough endoplasmic reticulum (rER) (E). Higher magnification TEM showed the formation tight junction (tj) complexes between adjacent cells (F). The space formed in between cells resembled bile canaliculi (bc) into which projected microvilli (mv). Scale bars: A, B 25 µm; C 12 µm; D 4 µm; E 2 µm; F 500 nm.
The ultra-structure of HepG2 cells cultured on different substrates was examined by TEM (Fig. 1). In general, cells grown on either 2-D or 3-D substrates contained a range of organelles typical of most mammalian cells, including mitochondria, nuclei, endoplasmic reticulum and lipid droplets. Preparations of 3-D cultures showed how cells grew around and in close association with the polystyrene support. These cells exhibited numerous morphological features typical of the liver tissue, nuclear membranes appeared to be normal, and mitochondria displayed structural variances within the normal range. The smooth and the rough endoplasmic reticulum, the Golgi complex and the glycogen content showed no structural abnormalities. Overall, the ultra-structure of HepG2 cells grown on 3-D scaffolds supports their ability to perform normal metabolic activity. Occasionally peroxisomal clusters, which are cell organelles found within mammalian liver and kidney, were observed as previously noted (Bokhari et al. 2007). Liver peroxisomes are normally present in differentiated hepatocytes and are responsible for the β-oxidation of the side chain of cholesterol in the course of bile acid synthesis (Beier & Fahimi, 1992). In native liver tissue and cell aggregates, hepatocytes possess polarity with two or three basal surfaces facing the sinusoid while adjacent cells form the bile canaliculi (Abu-Absi et al. 2002). Bile canaliculi are known to be rich in microvilli and components of bile metabolised in the cells are normally secreted into the canaliculus. Micrographs of cells grown in 3-D revealed adjacent hepatocytes often shared channels demarcated by tight junctions and lined with microvilli (Fig. 1). In contrast, such structures were seldom seen in cultures grown on 2-D surfaces.
Effects of methotrexate on cells grown in 2-D and 3-D
HepG2 cells grown in 2-D and 3-D formats were treated with various concentrations of MTX to evaluate their tolerance to a well known cytotoxin. Following each treatment period, cultures were then studied for biochemical (Fig. 2) and morphological (Figs 3 and 4) changes.
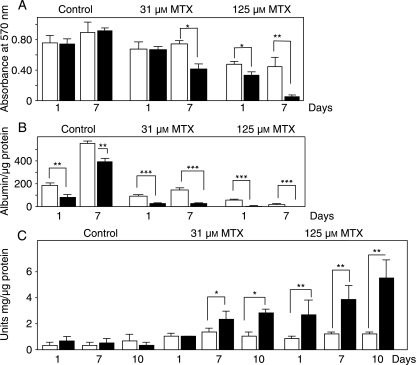
Fig. 2.
Functional activity of HepG2 cells grown on 2-D (solid bars) and 3-D (open bars) substrates when challenged by the cytotoxin, methotrexate (MTX). Data show cells were treated either with vehicle alone (control), or 31 µm MTX, or 125 µm MTX for up to 10 days. (A) Measure of cell viability using MTT assay. (B) Determination HepG2 cell metabolic activity by measurement of albumin secretion into the culture medium. Albumin levels were normalised to the total amount of protein per well. (C) Assessment of cell damage as determined by transglutaminase activity. Enzyme levels were normalised to the total amount of protein per well. For each experiment (A–C), cells were seeded at 1 × 106 cells/well. Data represent the mean ± SEM for three independent repeats. Significance is denoted by *P < 0.05, **P < 0.01 and ***P < 0.001 using the Mann Whitney U test.
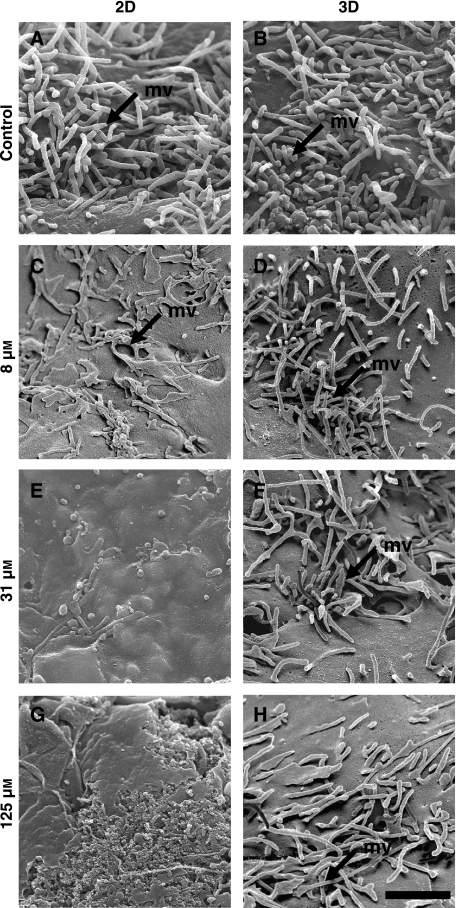
Fig. 3.
Scanning electron micrographs showing the effect of methotrexate (MTX) on the surface structure of HepG2 cells. Image panels show HepG2 cells were cultured on 2-D (A, C, E, G) and 3-D (B, D, F, H) substrates, treated with either vehicle [control, no MTX, (A, B)], 8 µm (C, D), 31 µm (E, F), or 125 µm (G, H) MTX. Note that microvilli (mv) on the cell surface are clearly visible in both control cultures (A, B) and cells exposed to low concentrations of MTX (C, D) when grown on either 2-D (A, C) or 3-D (B, D) substrates. At higher concentrations of the cytotoxin, cells grown on 2-D substrates possessed very few microvilli (E) and the cell surface showed evidence of breaking up at the maximum levels of MTX tested (G). In contrast, HepG2 cells grown in 3-D and exposed to increasing levels of MTX remained intact and exhibited large numbers of microvilli (F,H) although some flattening of microvilli was noted at higher concentrations (H). Scale bar: A–H 1 µm.
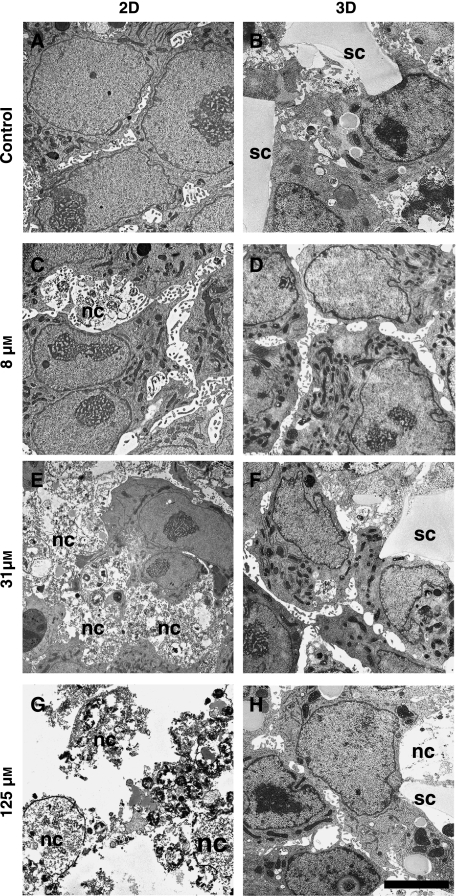
Fig. 4.
The effect of methotrexate (MTX) on the ultra-structure of HepG2 cells. TEM micrographs show cultured cells on 2-D (A, C, E, G) and 3-D (B, D, F, H) substrates, treated with either vehicle [control, no MTX, (A, B)], 8 µm (C, D), 31 µm (E, F), or 125 µm (G, H) MTX. Images of control cultures show the normal structure of cells corresponding to the growth substrate (A, B). The majority of cells grown on flat tissue culture plastic and exposed to 8 µm MTX possessed near normal cellular architecture although a few necrotic cells were identified (C, nc). Increasing concentrations of MTX resulted in the destruction of the vast majority of cells grown on 2-D substrates (E, G). Nuclear membranes had disintegrated and organelles normally found in healthy cells could be identified. There was an increased presence of large vacuolar spaces and membranous bodies resembling autophagolysosomes. In contrast, HepG2 cells grown on 3-D scaffolds maintained their structure and only a small number of necrotic cells (nc) were identified in cultures exposed to the 125 µm MTX (H). Scale bars A–H 3 µm.
We have previously shown that the materials used in this study are biocompatible and able to support the long term growth of cultured cells (Bokhari et al. 2007). As part of this study, cell viability was also assessed in the absence of the cytotoxic agent, methotrexate (control, Fig. 2). Viable cells were successfully cultured on both substrates for up to 21 days. Over time the MTT assay revealed that cell viability was significantly greater when grown in 3-D (data not shown). Cells grown on scaffolds have a greater surface area on which to attach and grow, compared to planar surfaces, where space per cell is restricted therefore this difference has been taken into account and values were normalised for in vitro assays where possible. Figure 2A illustrates cell viability after 1 and 7 days treated with varying concentrations of MTX. Treatment of HepG2 monolayers with MTX resulted in a gradual increase of absorbance at 15 µm MTX after 24 h but absorbance levels started to drop at 7 days (data not shown). With increasing MTX concentrations, the viability of cells grown on 2-D surfaces was visibly reduced especially at the higher levels of the cytotoxin. In 3-D cultures, sensitivity to MTX was not evident in the lesser concentrations of MTX; only at 62 µm MTX was there a significant decrease in absorbance levels compared to control values. This pattern was also seen in 3-D cultures grown for 7 days, whereas cells grown in monolayers for 7 days showed a sharp decrease in absorbance levels at 125 µm MTX concentrations. These results imply that cells cultured on 2-D surfaces under these conditions remain viable for a shorter period compared to cells cultured on 3-D scaffolds.
The metabolic activity of HepG2 cells was assessed by determination of the level of albumin secretion. In agreement with our earlier work (Bokhari et al. 2007), time courses of albumin secretion over 21 days showed significantly higher albumin concentrations in cultures grown on 3-D surfaces compared to cells grown in 2-D indicating that the 3-D environment is more conducive to cell function (Fig. 2 and data not shown). Data values were normalised to account for any differences in cell number. The metabolic activity of HepG2 cells was significantly reduced by increasing concentrations of MTX (Fig. 2B). Cells grown in 2-D were sensitive to the lowest concentrations of MTX tested (8 µm), whereas this level did not significantly influence albumin secretion by cells grown in 3-D (data not shown). However, increased levels of MTX (15.6 µm) did begin to reduce albumin secretion by cells cultured on scaffolds. Throughout cytotoxic challenge, higher levels of albumin secretion were noted in cells grown on 3-D plastic compared to 2-D materials. These data illustrate that hepatocyte cell function was impaired in the presence of MTX in a dose-dependent manner and cells grown in 3-D appeared more tolerant to the cytotoxin.
Measurement of transglutaminase was performed as a test for HepG2 cell toxicity in response to increasing concentrations of MTX. We examined the effects of MTX on transglutaminase levels in 2-D and 3-D cultures after 1, 7 and 10 days exposure to the cytotoxin (Fig. 3C). In control cultures, where there was no addition of MTX, levels of transglutaminase were found to be minimal and at similar levels. With increasing concentrations of the drug, such as 31 µm MTX, 2-D cultures secreted significantly higher levels of transglutaminase which increased in a dose dependent manner unlike cells grown in 3-D culture. These differences were statistically significant in all 2-D cultures at 7 and 10 days compared to their 3-D counterparts. In the 3-D cultures, increasing the concentration of MTX did not cause a significant increase in transglutaminase levels although there was a gradual rise in enzyme levels secreted into the culture medium at higher concentrations of the drug. These data further suggest that cells on 3-D porous materials are more tolerant to increasing levels of cytotoxin challenge.
Scanning and transmission electron micrographs demonstrated concentration dependent changes in cell structure subsequent to treatment with MTX. Representative examples of such morphological changes are illustrated in Figs 3 and 4. Normal, healthy HepG2 cells express numerous microvilli on their cell surface. When challenged with MTX, cells grown on 2-D surfaces gradually lost their microvilli in a dose dependent manner, whilst cells grown on scaffolds continued to express this structural feature (Fig. 3). In response to increasing levels of MTX, the surface of HepG2 cells grown as monolayers, first decreased the numbers of microvilli, then became flattened, and then started to disintegrate. No such changes were observed to the structure of HepG2 cells grown on 3-D scaffolds, although the microvilli of cells grown in the highest concentration of MTX tested (125 µm), did begin to show signs of flattening.
Further examination by TEM revealed ultra-structural changes to cells challenged by the cytotoxin (Fig. 4). Cells grown on 2-D surfaces possessed healthy morphology with prominent nuclei and nucleoli in control cultures. Following treatment with 8 µm MTX, cells with good nuclear architecture remained visible in 2-D cultures although areas of cellular necrosis were also evident. Hepatocyte 2-D cultures treated with increasing levels of MTX showed marked cytotoxicity and most cells became necrotic at high levels of MTX. Features such as endoplasmic reticulum de-granulation and the presence of ribosomal ghosts were observed. Furthermore, the granular cytoplasm generally lacked an organised structure and clumped chromatic granules were dispersed throughout the nucleus. Sub-cellular evidence of apoptosis was also observed; the plasma membrane was seen to rupture and a marked amount of vacuolation occured, possibly reflecting presence of lipid droplets and cellular degeneration. Cell shrinkage was also obvious, as well as a loss of cell-to-cell contact followed by formation of apoptotic bodies (autophagolysosomes) and cell death. At the highest concentration, ‘ghosts’ of cellular remains were observed, indicating that cells grown in 2-D exposed to higher levels of MTX had undergone an advanced stage of cell death.
In contrast, HepG2 cells grown in 3-D culture and exposed to MTX were significantly more resistant to the effects of the cytotoxin. The ultra-structure of cells treated with lower concentrations of MTX possessed normal organelles in their cytoplasm [rough endoplasmic reticulum (RER), ribosomes, mitochondria and lipid droplets]. The nuclei displayed normal heterochromatin and nucleoli. These features were well preserved throughout most of the concentrations of MTX tested. However, in some cells incubated with 125 µm of MTX, the nuclear membrane had an irregular morphology and other sub-cellular features, such as mitochondria, appeared to be slightly abnormal. It is likely therefore, that at higher concentrations of MTX, cells in 3-D cultures are starting to undergo changes similar to those experienced by cells cultured in 2-D as seen in significantly lower concentrations of the cytotoxin.
Discussion
The development of model systems that enable 3-D cell growth has revealed some limitations of traditional 2-D cell culture. In a living organism, cells are in contact with their neighbours and the extra cellular matrix. Several investigators have attempted to recreate this environment in the cell culture laboratory and have shown that the culture of cells in a 3-D space is more physiologically relevant, compared with cells cultured as a monolayer (Cukierman et al. 2001; Beningo et al. 2004; Witte & Kao, 2005). Designing 3-D cell culture models which more closely represent living tissues in the body, is widely acknowledged as an approach to advance basic research into health and disease and enable more accurate assessment of cell activity in vitro. We have previously reported the development of apparatus for 3-D cell growth (Bokhari et al. 2007). In this study, we demonstrate a specific application for this technology as a tool for more accurate assessment of drug activity by comparing the growth and function of HepG2 cells on 2-D and 3-D plastic substrates during cytotoxic challenge.
When HepG2 cells were grown on 2-D surfaces, they predominately grew as flat mono-layers and sometimes formed small multi-cellular masses. As a consequence such cultures appeared untidy, heterogeneous and probably contained cells displaying a range of activities. It is well known that culturing hepatocytes in conventional monolayer formats leads to cells adopting flat morphologies, with rare and short-lived bile canalicular-like structures (Awata et al. 1998; Berthiaume et al. 1996). When hepatocytes are cultured on adhesive surfaces, they self-assemble into 3-D, compacted aggregates called spheroids (Landry et al. 1985; Koide et al. 1990). Hepatocyte spheroids sustain viability for extended culture periods and maintain high levels of liver-specific functions, including albumin production and urea synthesis. In this study, ‘spheroid’-like cells were found to be present predominately in 3-D cultures and the surfaces of such cells were covered with numerous microvilli. The close apposition of HepG2 cells in 3-D culture as reported herein closely resembles the functional spheroids according to earlier reports which show tight cell-cell contacts (Awata et al. 1998). Several groups have interpreted the presence of these junctional complexes and bile canalicular structures with microvilli protruding into the lumen, as an indication that the hepatocytes have established membrane polarity (Peshwa et al. 1996). Collectively, these data indicate that 3-D cell growth promotes the differentiation of hepatocytes and their ability to resemble more closely their counterparts in vivo.
Albumin production is often employed as a marker of hepatocyte metabolic activity in vitro and is generally recognised as an indicator of liver-specific functions (Ranucci et al. 2000; Kane et al. 2006). Earlier work on the synthesis of albumin reported the rate to be 4 µg (min.mg DNA)−1 (Morgan & Peters, 1971) which is understood to be several fold greater than the rate of albumin secretion by cultured hepatocytes. It has previously been shown that cultures of hepatocytes on 2-D substrates produce lower levels of albumin (Ise et al. 1999). In agreement with this earlier report, we have found in this study that albumin levels were higher in the medium of 3-D cultures than in that of monolayer cultures. It was reasoned that the formation of hepatocyte aggregates not only increases cell viability, but also maintains functional activity under 3-D culture conditions.
The culture of hepatocytes in 2-D systems is routinely used for studying the toxicity of hepatotoxins. However, their usefulness in vitro is limited due to cell de-differentiation as evidenced by, for example, the reduction in protein secretion such as albumin and transferin. This limits many in vitro hepato-toxicity studies to studying the acute effects of drugs (Walker et al. 2000). A possible limitation of using 2-D culture systems for toxicological studies is that they may lead to over estimation of drug toxicity as a consequence of the absence of 3-D cell organisation. Indeed, cells grown in 2-D environments possess an abnormal architecture and are typically under stress, making them less tolerant to trauma, whereas cells growing in 3-D adopt structures resembling their native counterparts and are therefore less susceptible to environmental pressures. Despite several groups beginning to look at 3-D culture systems for hepatocytes, little has been reported in the literature on the effects of toxins on liver cell morphology and function when grown in a 3-D configuration. Accordingly, this report is topical and timely since it provides evidence of a new and useful approach to evaluate compound toxicity in a hepatocyte 3-D culture system.
Methotrexate (MTX) is an antifolate agent that acts mainly by inhibiting dihydrofolate reductase which in turn leads to decreased purine synthesis and consequently to decreased DNA synthesis, which ultimately results in the activation of programmed cell death (apoptosis). The concentrations of MTX used in this study were selected to mimic in part those employed in clinical settings. In high dose MTX treatments, the peak level of the cytotoxin in plasma is approximately 100 µm (Aquerreta et al. 2002). In this study, we have demonstrated that MTX itself is toxic to hepatocyte cells, even at relatively low doses on 2-D surfaces. This was also noted in a previous study where low dosage of MTX (10 µm) induced apoptosis on lymphocytic cell lines (Herman et al. 2005). HepG2 cells grown on 3-D surfaces, however, were able to tolerate higher concentrations of MTX. In contrast to cells grown on 2-D plastic, the viability, structure and function of 3-D cultures was not significantly influenced by low level MTX exposure. At the higher concentrations of MTX, cells grown on scaffolds were susceptible to cytotoxic challenge. These results suggest that toxicity studies using the 3-D cell culture system described herein, are more likely to provide realistic physiological responses to xenobiotic compounds than studies using 2-D culture models.
Concluding remarks
Our results suggest that testing drugs on liver cells using our 3-D culture system may be more likely to reflect true physiological responses to cytotoxic compounds than existing models that rely on 2-D culture models. Developing hepatocyte-based 3-D in vitro models for pharmacotoxicology is a valuable technology. In vitro assays that can provide more accurate data on compound activity will contribute to improving the efficiency of drug discovery, reducing drug development costs, and may contribute to reducing the number of animals used in research.
Acknowledgments
The authors would like to thank Christine Richardson and Emma-Jane Newton for their help with the SEM and TEM. This work was partly supported by the Engineering and Physical Sciences Research Council (GR/T24043).
References
- Abu-Absi SF, Friend JR, Hansen LK, Hu WS. Structural polarity and functional bile canaliculi in rat hepatocyte spheroids. Exp Cell Res. 2002;274:56–67. doi: 10.1006/excr.2001.5467. [DOI] [PubMed] [Google Scholar]
- Akay G, Birch MA, Bokhari MA. Microcellular polyHIPE polymer supports osteoblast growth and bone formation in vitro. Biomaterials. 2004;25:3991–4000. doi: 10.1016/j.biomaterials.2003.10.086. [DOI] [PubMed] [Google Scholar]
- Aquerreta I, Aldaz A, Giraldez J, Sierrasesumaga L. Pharmacodynamics of high-dose methotrexate in pediatric patients. Ann Pharmacother. 2002;36:1344–1350. doi: 10.1345/aph.1A446. [DOI] [PubMed] [Google Scholar]
- Awata R, Sawai H, Imai K, Terada K, Senoo H, Sugiyama T. Morphological comparison and functional reconstitution of rat hepatic parenchymal cells on various matrices. J Gastroenterol Hepatol. 1998;13(Suppl):S55–S61. [PubMed] [Google Scholar]
- Barak AJ, Tuma DJ, Beckenhauer HC. Methotrexate hepatotoxicity. J Am Coll Nutr. 1984;3:93–96. doi: 10.1080/07315724.1984.10720041. [DOI] [PubMed] [Google Scholar]
- Barbetta A, Cameron N, Cooper S. High internal phase emulsions (HIPEs) containing divinylbenzene and 4-vinylbenzyl chloride and the morphology of the resulting PolyHIPE materials. Chemical Communications. 2000:221–222. [Google Scholar]
- Beier K, Fahimi HD. Application of automatic image analysis for quantitative morphological studies of peroxisomes in rat liver in conjunction with cytochemical staining with 3–3′-diaminobenzidine and immunocytochemistry. Microsc Res Tech. 1992;21:271–282. doi: 10.1002/jemt.1070210404. [DOI] [PubMed] [Google Scholar]
- Beningo KA, Dembo M, Wang YL. Responses of fibroblasts to anchorage of dorsal extracellular matrix receptors. Proc Natl Acad Sci USA. 2004;101:18024–18029. doi: 10.1073/pnas.0405747102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthiaume F, Moghe PV, Toner M, Yarmush ML. Effect of extracellular matrix topology on cell structure, function, and physiological responsiveness: hepatocytes cultured in a sandwich configuration. Faseb J. 1996;10:1471–1484. doi: 10.1096/fasebj.10.13.8940293. [DOI] [PubMed] [Google Scholar]
- Bhadriraju K, Chen CS. Engineering cellular microenvironments to improve cell-based drug testing. Drug Discov Today. 2002;7:612–20. doi: 10.1016/s1359-6446(02)02273-0. [DOI] [PubMed] [Google Scholar]
- Blackshaw SE, Arkison S, Cameron C, Davies JA. Promotion of regeneration and axon growth following injury in an invertebrate nervous system by the use of three-dimensional collagen gels. Proc Biol Sci. 1997;264:657–661. doi: 10.1098/rspb.1997.0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bokhari M, Carnachan RJ, Cameron NR, Przyborski SA. Growth of hepatocyte cell line on inert three dimensional polystyrene scaffolds enhances cell function. Biochem Biophys Res Commun. 2007;354:1095–1100. doi: 10.1016/j.bbrc.2007.01.105. [DOI] [PubMed] [Google Scholar]
- Bouma ME, Rogier E, Verthier N, Labarre C, Feldmann G. Further cellular investigation of the human hepatoblastoma-derived cell line HepG2: morphology and immunocytochemical studies of hepatic-secreted proteins. In Vitro Cell Dev Biol. 1989;25:267–275. doi: 10.1007/BF02628465. [DOI] [PubMed] [Google Scholar]
- Cameron N. High internal phase emulsion templating as a route to well-defined porous polymers. Polymer. 2005;46:1439–49. [Google Scholar]
- Cameron N, Barbetta A. The influence of porogen type on the porosity, surface area and morphology of poly(divinylbenzene) PolyHIPE foams. Journal of Materials Chemistry. 2000;10:2466–2472. [Google Scholar]
- Cameron N, Sherrington D. High internal phase emulsions (HIPEs)-Structure, properties and use in polymer preparation. Biopolymers Liquid Crystalline Polymers Phase Emulsion. 1996;126:163–214. [Google Scholar]
- Cameron N, Sherrington D, Albiston L, Gregory D. Study of the formation of the open cellular morphology of poly(styrene/divinylbenzene) polyHIPE materials by cryo-SEM. Colloid and Polymer Science. 1996;274:592–595. [Google Scholar]
- Carnachan RJ, Bokhari M, Przyborski SA, Cameron NR. Tailoring the morphology of emulsion-templated porous polymers. Soft Matter. 2006;2:608–616. doi: 10.1039/b603211g. [DOI] [PubMed] [Google Scholar]
- Cukierman E, Pankov R, Stevens DR, Yamada KM. Taking cell-matrix adhesions to the third dimension. Science. 2001;294:1708–1712. doi: 10.1126/science.1064829. [DOI] [PubMed] [Google Scholar]
- Dillon GP, Yu X, Sridharan A, Ranieri JP, Bellamkonda RV. The influence of physical structure and charge on neurite extension in a 3D hydrogel scaffold. J Biomater Sci Polym Ed. 1998;9:1049–1069. doi: 10.1163/156856298x00325. [DOI] [PubMed] [Google Scholar]
- Fisher JP, Jo S, Mikos AG, Reddi AH. Thermoreversible hydrogel scaffolds for articular cartilage engineering. J Biomed Mater Res A. 2004;71:268–274. doi: 10.1002/jbm.a.30148. [DOI] [PubMed] [Google Scholar]
- Folk JE, Cole PW. Transglutaminase: mechanistic features of the active site as determined by kinetic and inhibitor studies. Biochim Biophys Acta. 1966;122:244–64. doi: 10.1016/0926-6593(66)90066-x. [DOI] [PubMed] [Google Scholar]
- Hayman MW, Smith KH, Cameron NR, Przyborski SA. Enhanced neurite outgrowth by human neurons grown on solid three-dimensional scaffolds. Biochem Biophys Res Commun. 2004;314:483–488. doi: 10.1016/j.bbrc.2003.12.135. [DOI] [PubMed] [Google Scholar]
- Hayman MW, Smith KH, Cameron NR, Przyborski SA. Growth of human stem cell-derived neurons on solid three-dimensional polymers. J Biochem Biophys Methods. 2005;62:231–240. doi: 10.1016/j.jbbm.2004.12.001. [DOI] [PubMed] [Google Scholar]
- Herman S, Zurgil N, Deutsch M. Low dose methotrexate induces apoptosis with reactive oxygen species involvement in T lymphocytic cell lines to a greater extent than in monocytic lines. Inflamm Res. 2005;54:273–280. doi: 10.1007/s00011-005-1355-8. [DOI] [PubMed] [Google Scholar]
- Ise H, Takashima S, Nagaoka M, Ferdous A, Akaike T. Analysis of cell viability and differential activity of mouse hepatocytes under 3D and 2D culture in agarose gel. Biotechnology Letters. 1999;21:209–213. [Google Scholar]
- Kane BJ, Zinner MJ, Yarmush ML, Toner M. Liver-specific functional studies in a microfluidic array of primary Mammalian hepatocytes. Anal Chem. 2006;78:4291–4298. doi: 10.1021/ac051856v. [DOI] [PubMed] [Google Scholar]
- Koide N, Sakaguchi K, Koide Y, et al. Formation of multicellular spheroids composed of adult rat hepatocytes in dishes with positively charged surfaces and under other nonadherent environments. Exp Cell Res. 1990;186:227–235. doi: 10.1016/0014-4827(90)90300-y. [DOI] [PubMed] [Google Scholar]
- Krajnc P, Brown J, Cameron N. Monolithic scavenger resins by amine functionalizations of poly(4-vinylbenzyl chloride-codivinylbenzene) PolyHIPE materials. Organic Letters. 2002;4:2497–2500. doi: 10.1021/ol026115k. [DOI] [PubMed] [Google Scholar]
- Landry J, Bernier D, Ouellet C, Goyette R, Marceau N. Spheroidal aggregate culture of rat liver cells: histotypic reorganization, biomatrix deposition, and maintenance of functional activities. J Cell Biol. 1985;101:914–923. doi: 10.1083/jcb.101.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levenberg S, Huang NF, Lavik E, Rogers AB, Itskovitz-Eldor J, Langer R. Differentiation of human embryonic stem cells on three-dimensional polymer scaffolds. Proc Natl Acad Sci USA. 2003;100:12741–6. doi: 10.1073/pnas.1735463100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurice M, Rogier E, Cassio D, Feldmann G. Formation of plasma membrane domains in rat hepatocytes and hepatoma cell lines in culture. J Cell Sci. 1988;90(Pt 1):79–92. doi: 10.1242/jcs.90.1.79. [DOI] [PubMed] [Google Scholar]
- Melino G, Candi E, Steinert PM. Assays for transglutaminases in cell death. Methods Enzymol. 2000;322:433–472. doi: 10.1016/s0076-6879(00)22042-9. [DOI] [PubMed] [Google Scholar]
- Mersch-Sundermann V, Knasmuller S, Wu XJ, Darroudi F, Kassie F. Use of a human-derived liver cell line for the detection of cytoprotective, antigenotoxic and cogenotoxic agents. Toxicology. 2004;198:329–340. doi: 10.1016/j.tox.2004.02.009. [DOI] [PubMed] [Google Scholar]
- Mikos AG, Sarakinos G, Leite SM, Vacanti JP, Langer R. Laminated three-dimensional biodegradable foams for use in tissue engineering. Biomaterials. 1993;14:323–330. doi: 10.1016/0142-9612(93)90049-8. [DOI] [PubMed] [Google Scholar]
- Morgan EH, Peters T., Jr The biosynthesis of rat serum albumin. V. Effect of protein depletion and refeeding on albumin and transferrin synthesis. J Biol Chem. 1971;246:3500–3507. [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Ohtake Y, Maruko A, Abe S, Fukumoto M, Ohkubo Y. Effect of retinoic acid-induced transglutaminase on cell growth in regenerating liver. Biomed Res. 2006;27:75–80. doi: 10.2220/biomedres.27.75. [DOI] [PubMed] [Google Scholar]
- Park Y, Sugimoto M, Watrin A, Chiquet M, Hunziker EB. BMP-2 induces the expression of chondrocyte-specific genes in bovine synovium-derived progenitor cells cultured in three-dimensional alginate hydrogel. Osteoarthritis Cartilage. 2005;13:527–536. doi: 10.1016/j.joca.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Peshwa MV, Wu FJ, Sharp HL, Cerra FB, Hu WS. Mechanistics of formation and ultrastructural evaluation of hepatocyte spheroids. In Vitro Cell Dev Biol Anim. 1996;32:197–203. doi: 10.1007/BF02722946. [DOI] [PubMed] [Google Scholar]
- Piacentini M, Autuori F, Dini L, et al. ‘Tissue’ transglutaminase is specifically expressed in neonatal rat liver cells undergoing apoptosis upon epidermal growth factor-stimulation. Cell Tissue Res. 1991;263:227–235. doi: 10.1007/BF00318764. [DOI] [PubMed] [Google Scholar]
- Ranucci CS, Kumar A, Batra SP, Moghe PV. Control of hepatocyte function on collagen foams: sizing matrix pores toward selective induction of 2-D and 3-D cellular morphogenesis. Biomaterials. 2000;21:783–793. doi: 10.1016/s0142-9612(99)00238-0. [DOI] [PubMed] [Google Scholar]
- Rollier A, DiPersio CM, Cereghini S, et al. Regulation of albumin gene expression in hepatoma cells of fetal phenotype: dominant inhibition of HNF1 function and role of ubiquitous transcription factors. Mol Biol Cell. 1993;4:59–69. doi: 10.1091/mbc.4.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakiyama SE, Schense JC, Hubbell JA. Incorporation of heparin-binding peptides into fibrin gels enhances neurite extension: an example of designer matrices in tissue engineering. Faseb J. 1999;13:2214–2224. doi: 10.1096/fasebj.13.15.2214. [DOI] [PubMed] [Google Scholar]
- Schmeichel KL, Bissell MJ. Modeling tissue-specific signaling and organ function in three dimensions. J Cell Sci. 2003;116:2377–2388. doi: 10.1242/jcs.00503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun T, Jackson S, Haycock JW, MacNeil S. Culture of skin cells in 3D rather than 2D improves their ability to survive exposure to cytotoxic agents. J Biotechnol. 2006;122:372–381. doi: 10.1016/j.jbiotec.2005.12.021. [DOI] [PubMed] [Google Scholar]
- Tajima H, Matsumoto K, Nakamura T. Regulation of cell growth and motility by hepatocyte growth factor and receptor expression in various cell species. Exp Cell Res. 1992;202:423–431. doi: 10.1016/0014-4827(92)90095-p. [DOI] [PubMed] [Google Scholar]
- Temenoff JS, Mikos AG. Review: tissue engineering for regeneration of articular cartilage. Biomaterials. 2000;21:431–440. doi: 10.1016/s0142-9612(99)00213-6. [DOI] [PubMed] [Google Scholar]
- Walker TM, Rhodes PC, Westmoreland C. The differential cytotoxicity of methotrexate in rat hepatocyte monolayer and spheroid cultures. Toxicol In Vitro. 2000;14:475–485. doi: 10.1016/s0887-2333(00)00036-9. [DOI] [PubMed] [Google Scholar]
- Wang F, Weaver VM, Petersen OW, et al. Reciprocal interactions between beta1-integrin and epidermal growth factor receptor in three-dimensional basement membrane breast cultures: a different perspective in epithelial biology. Proc Natl Acad Sci USA. 1998;95:14821–14826. doi: 10.1073/pnas.95.25.14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver VM, Petersen OW, Wang F, et al. Reversion of the malignant phenotype of human breast cells in three-dimensional culture and in vivo by integrin blocking antibodies. J Cell Biol. 1997;137:231–245. doi: 10.1083/jcb.137.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witte RP, Kao WJ. Keratinocyte-fibroblast paracrine interaction: the effects of substrate and culture condition. Biomaterials. 2005;26:3673–3682. doi: 10.1016/j.biomaterials.2004.09.054. [DOI] [PubMed] [Google Scholar]
- Wu GY, Wu CH, Stockert RJ. Model for specific rescue of normal hepatocytes during methotrexate treatment of hepatic malignancy. Proc Natl Acad Sci USA. 1983;80:3078–3080. doi: 10.1073/pnas.80.10.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Dillon GP, Bellamkonda RB. A laminin and nerve growth factor-laden three-dimensional scaffold for enhanced neurite extension. Tissue Eng. 1999;5:291–304. doi: 10.1089/ten.1999.5.291. [DOI] [PubMed] [Google Scholar]
- Zhang H, Cooper A. Synthesis and applications of emulsion-templated porous materials. Soft Matter. 2005;1:107–113. doi: 10.1039/b502551f. [DOI] [PubMed] [Google Scholar]