Abstract
In this paper we elucidate the pattern of initiation of the first teeth and the pattern of tooth replacement on the dentary of wild Atlantic salmon (Salmo salar L.), throughout nearly all stages of its life cycle, using serially sectioned heads and jaws, cleared and stained animals, and X-rays. The dentary teeth are set in one row. Tooth germs appear around hatching, first in odd positions, followed by even positions. From position 8 further backwards, teeth are added in adjacent positions. The first replacement teeth appear in animals of about 30 mm fork length. On the dentary of early life stages (alevins and fry), every position in the tooth row holds a functional (i.e. attached and erupted) tooth and a replacement tooth. The alternating pattern set up anteriorly in the dentary by the first-generation teeth changes in juveniles (parr) whereby teeth are in a similar functional (for the erupted teeth) or developmental stage (for the replacement teeth) every three positions. This pattern is also observed in marine animals during their marine life phase and in both sexes of adult animals prior to spawning (grilse and salmon), but every position now holds either a functional tooth or a mineralised replacement tooth. This is likely due to the fact that replacement tooth germs have to grow to a larger size before mineralisation starts. In the following spring, the dentary tooth pattern of animals that have survived spawning (kelts) is highly variable. The abundance of functional teeth in post-spawning animals nevertheless indicates that teeth are not lost over winter.
We confirm the earlier reported lack of evidence for the existence of an edentulous life phase, preceding the appearance of so-called breeding teeth during upstream migration to the spawning grounds, and consider breeding teeth to be just another tooth generation in a regularly replacing dentition. This study shows how Atlantic salmon maintains a functional adaptive dentition throughout its complex life cycle.
Keywords: dentition, odontogenesis, salmon life cycle, salmon skeleton, tooth replacement
Introduction
In tooth-bearing non-mammalian vertebrates, teeth fulfil a wide range of biological roles. They are used in food uptake and processing, to grasp prey, withholding prey from escape, or sometimes to bite pieces from larger prey. They also serve in defence, and even in courtship and copulatory behaviour. Unlike in mammals, teeth are continuously replaced during life. A dentition where this replacement proceeds in some coordinated fashion evidently offers an advantage given that it can continuously fulfil its biological role and contribute to the fitness of the organism (Huysseune & Sire, 1998).
How the coordinated replacement of teeth is achieved in an animal with a highly complex life cycle, such as the Atlantic salmon (Salmo salar L.), has not yet been elucidated, despite the fact that Atlantic salmon is a particularly well studied species (Fleming, 1996; Marschall et al. 1998). Atlantic salmon are carnivorous, ram-feeding fish with a life cycle that is considered one of the most intriguing in vertebrate zoology (Jones, 1959; Fleming, 1996). It involves hatching and juvenile life in freshwater, growth and maturation at the sea, and return to the animals' home river for spawning. Unlike Pacific salmon (genus Oncorhynchus) where all animals die after spawning, many individuals of Atlantic salmon (genus Salmo) survive spawning and can become repetitive spawners, going back to sea and returning to their home rivers many times in their life (Moore et al. 1995; Groot, 1996). The return of salmon to freshwater for spawning, and the laborious upstream migration, goes along with drastic skeletal alterations, affecting the scales, the vertebral column and almost all skull bones, both in structure and in shape (Tchernavin, 1938; Persson et al. 1998; Witten & Hall, 2001; Zazzo et al. 2006). Especially striking is the development of a hook at the tip of the lower jaw in males (called a kype) (Tchernavin, 1937, 1938; Darwin, 1877; Witten & Hall, 2001, 2002, 2003), a structure viewed as a secondary sexual character that likely contributes to the establishment of a dominance hierarchy among males at the spawning grounds (Darwin, 1877; Järvi, 1990).
In addition to substantial changes of the skull bones, maturing salmon of both sexes have been reported to develop a complete new set of teeth, so-called breeding teeth, during their upstream spawning migration. The development of these breeding teeth is reported to follow a toothless period (Tchernavin, 1937, 1938). In an earlier report, we examined the supposed existence of such a toothless stage (Witten et al. 2005). We have not been able to find an edentulous stage and have come to the conclusion that this observation can be explained by the considerable proliferation of the oral mucosa, concealing the teeth prior to the breeding period, and the use of inappropriate maceration techniques (Witten et al. 2005).
Here, we expand our data set to cover nearly all life stages of Atlantic salmon, from hatching up to repetitive spawning, in order to attempt to answer the following questions. (1) In what order is the initial set of teeth (the so-called first-generation teeth) established? (2) How is this tooth pattern maintained or possibly altered during the different life phases of Atlantic salmon? And (3) what changes affect the dentition in the most critical of all life phases: post-spawning survival? We have restricted our observations to the dentary. Data on the rainbow trout (Berkovitz 1978) suggest that patterning could be different on the upper jaws.
Our data should shed light not only on how the Atlantic salmon maintains a functional adaptive dentition throughout its complex life history, but also how the tooth patterns that we identify comply to current ideas about patterning of teeth in osteichthyans (cf. Huysseune & Witten, 2006).
Material and methods
All examined life stages of wild Atlantic salmon (see Table 1 for life stages and terms), were obtained from the population in the Miramichi River (New Brunswick, Canada) between 1999 and 2004 under licence and with support from the Canadian Department for Fisheries and Oceans (DFO) in Moncton, New Brunswick.
Table 1.
Terms used for life stages of Atlantic salmon
| Alevin: | Post-hatching, non-feeding stage with yolk sac. |
| Fry: | First feeding stage after the yolk sac has been resorbed. |
| Parr: | Juvenile salmon that stay between one and four years in freshwater. In their first year they are called 0+, in their second year 1+ and in their third year 2+. At the Miramichi river, parr can be assigned to year classes based on fork length (long term DFO data). |
| Marine animals: | In the present paper: juvenile animals that have completed the process of smoltification (transition to seawater) and that will become sexually mature later, connected to the anadromous spawning migration. |
| Grilse: | Animals that return to their home river for spawning for the first time, after spending one winter in the sea (1SW = one sea winter salmon). Grilse in the Miramichi river have commonly a fork length below 63 cm. |
| Salmon: | Animals that return to their home rivers for spawning for the first time after spending more than one winter at sea (MSW = multi sea winter salmon). Also animals that have survived the first spawning and return to their home rivers for repeated spawning, after spending one or more ‘winters’ (years) in the sea. The fork length of salmon in the Miramichi river is above 63 cm. |
| Kelts: | Animals that have survived spawning (as grilse or as salmon), have stayed over winter in the river and return to the sea in the spring. Kelts are also called ‘black salmon’. |
Eggs, alevins and fry were obtained from wild animals that were spawned at the Miramichi Salmon Conservation Centre (South Esk) in October 2001, 2002 and 2003 in the context of a restocking program. Eggs, alevins and fry were incubated under semi-natural conditions, in ambient water. Development from fertilisation to the beginning of hatching lasted about 165 days, equalling a relative age of 280 τsaccording to the developmental table of Gorodilov (1996) (τs being based on the temperature-dependent time to develop one somite). Samples were collected monthly (± 48 h) starting 4 weeks after fertilisation and ending four months after hatching, at which time the animals reached parr size. In addition, for the first two months after hatching, samples were taken weekly. All specimens were plastic-embedded and a total of nine specimens (ranging from 16.2 mm TL to 48.4 mm FL) were used to prepare serial histological sections from the head. Length is provided as total length (TL = snout to tail) and, with the development of a forked caudal fin (around 24 mm TL), as fork length (FL = snout to indentation of the caudal fin).
Twelve parr, juveniles in their first (0+), second (1+) and third (2+) fall after hatching (FL between 50.0 and 111.4 mm) were collected in October 1999 and 2002 at tributaries to the Miramichi River, Catamaran and Rocky Brook, respectively. These specimens were examined by X-ray, by clearing and staining and by serial sectioning.
Eleven actively feeding, sexually immature marine animals (FL between 24.6 cm and 32.1 cm, five males, six females) were obtained in November 2001 from the Labrador sea, in connection with a fisheries research survey by the Department of Fisheries and Oceans St. John's (Newfoundland). The specimens were X-rayed and subsequently used for clearing and staining.
Sexually mature animals on their upstream spawning migration were collected during the fall runs, September 1999 and 2000 in the Miramichi River at Red Bank, ~1 month prior to spawning. The animals were caught in traps used for the Red Bank (First Nation) food fishery. Eleven grilse (FL between 54.2 cm and 61.6 cm, eight males, three females) and five salmon (FL between 66.0 cm and 79.3 cm, two males, three females), caught in this program, were examined by X-ray and serial sectioning. Twelve kelts (FL between 51.4 cm and 78.7 cm, six males and six females) were also collected at Red Bank in April 2000 and 2001.
Scales from all adult animals (grilse, salmon, kelts) were analysed according to the Atlantic salmon scale reading guidelines (Shearer, 1992) to determine the animals’ life history as part of a continuous DFO monitoring program (Chaput et al. 2000). The data were used to confirm the status of the adult animals (first year spawners or repetitive spawners).
Histology and whole mount staining
Histological examinations were used to determine the status of tooth development (initiation and morphogenesis, cytodifferentiation, attachment) and resorption. Eggs, alevins and fry were fixed in buffered glutaraldehyde (Witten, 1997), embedded in Technovit 7100 (Heraeus Kulzer, Wehrheim, Germany), serially sectioned into 2 µm sagittal sections and stained with toluidine blue. Alternatively, some specimens were fixed in a mixture of glutaraldehyde and paraformaldehyde, embedded in epon according to standard procedures (cf. Huysseune & Sire, 1992), serially sectioned into 1 or 2 µm transverse sections, and stained with toluidine blue. Larger specimens (parr, grilse, salmon, kelts) were fixed in buffered 10% formaldehyde and, subsequent to X-raying, decalcified in 10% EDTA and embedded in paraffin. Serial, sagittal sections were stained with Masson's Trichrome, described for salmon in detail by Witten & Hall (2002). Clearing and staining was done according to standard procedures (Witten et al. 2000; Gillis et al. 2006).
Radiology
X-rays of entire heads (smaller animals) or of the separated lower jaws (larger animals) were taken using a portable ‘Mini X-ray HF80+’ machine (Mini X-ray inc., Northbrock, IL, USA) and ‘Kodak Industrex M Film Ready Pack II’ with a speed of 50 ASA (Kodak Industry, Eastman Kodak Company, Rochester, NY, USA), without any screen. The settings of the X-ray unit were 70 kV, 15 mA, 2 sec exposure time for larger animals and 50 KV for smaller stages. The distance between the X-ray cathode and the film was 40 cm. Radiographs were manually developed with Kodak chemicals and analysed using a stereo microscope.
Pattern of tooth replacement
To reconstruct the dentitions from serially sectioned jaws, sections of the dentary were first drawn using a drawing mirror attached to the microscope. Next, every tooth was assigned a number, its presence checked throughout the series by overlaying the drawings, and its developmental stage identified. Seven stages could be distinguished: (1) tooth germs in morphogenesis stage, (2) early cytodifferentiation stage, (3) late cytodifferentiation stage, or (4) undergoing attachment; (5) young functional teeth (attached recently), (6) fully attached, mature functional teeth, or (7) advanced functional teeth undergoing resorption.
Cleared and stained specimens were examined using a stereomicroscope. Teeth in successive positions were drawn using a drawing mirror attached to the microscope, and corresponding developmental stages were noted. The following four stages could be distinguished: young (small) replacement tooth germs, advanced (large) replacement tooth germs, functional teeth (either young or mature), and advanced functional teeth showing indications of resorption.
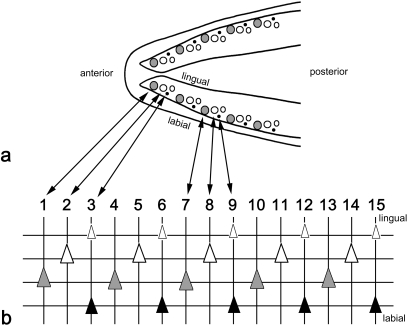
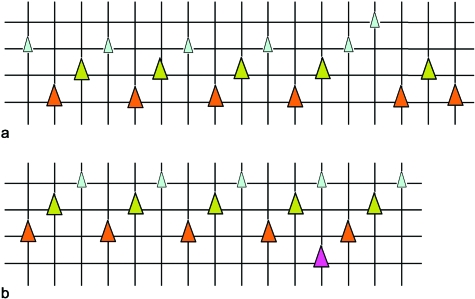
To reconstruct the dentition from the X-rays, the positions of the teeth on the horizontal X-ray were transposed onto transparent sheets, and complemented with information on their developmental stage read from (enhanced) lateral views. Teeth on the X-rays could be identified as either young tooth germs, as advanced replacement teeth, or as functional teeth (attached to the underlying bone). In addition, small tooth tips could be identified as remains of functional teeth in an advanced state of resorption. A semi-schematic representation of the dentition of adult Atlantic salmon is shown in Figure 1. All teeth that succeed each other in a given position constitute a tooth family.
Fig. 1.
Schematic representation of lower jaw dentition in adult Atlantic salmon. Semi-schematic drawing of the lower jaw dentition in an adult salmon (a) and schematic representation of the left lower jaw dentition (b) as identifiable in radiographs (see e.g. Fig. 5d). In (a), large grey circles represent functional teeth, large open circles advanced replacement teeth, and small open circles young replacement teeth. Remains of functional teeth in resorption are represented by small black circles. In (b), vertical lines connect teeth that are in a similar position in the tooth row and, because of their epithelial connection and developmental association, belong to the same tooth family. Horizontal lines connect teeth in a similar stage of development: young replacement teeth (small white triangles), advanced replacement teeth (large white triangles), functional teeth (large grey triangles), and teeth in resorption (black triangles). Teeth are initiated close to the oral epithelial surface; as they differentiate, they grow downwards and move from a more lingual to a more labial position. Anterior to the left, lingual to the top, labial to the bottom of the scheme.
Results
In all stages examined from alevins to repetitive spawners, the teeth on the dentary occur in a single row. Functional teeth occupy more marginal (labial) positions, replacement teeth more medial (lingual) positions; replacement teeth are placed slightly posterior with respect to their predecessor (cf. Fig. 1). Teeth are initiated close to the oral epithelial surface; as they differentiate, they grow towards the bone and move from a more lingual to a more labial position.
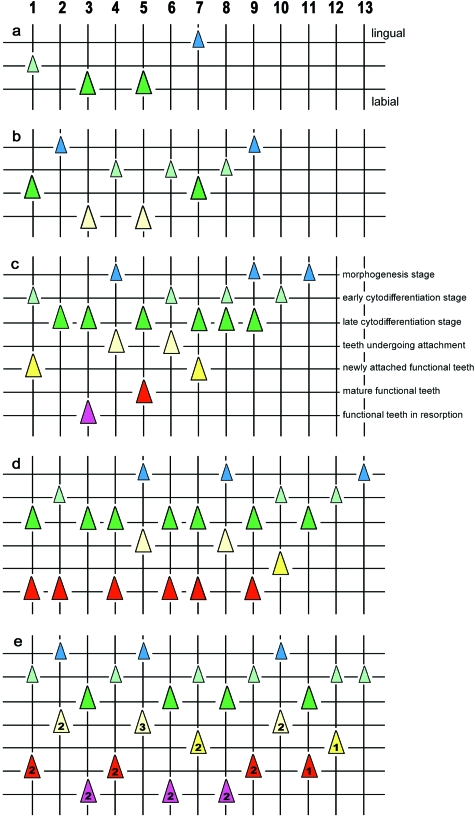
Alevins and fry (Fig. 2)
Fig. 2.
Tooth pattern in alevins and fry. Graphical representation of the order of tooth development on the dentary in alevin and fry stage wild Atlantic salmon. a: 18.1 mm TL, b: 22.7 mm TL, c: 31.1 mm FL; d: 32.6 mm FL; e: 48.4 mm FL. Vertical lines represent (numbered) tooth positions (= tooth families); horizontal lines connect teeth in a similar stage of development. Lingual to the top, labial to the bottom of each figure. Teeth form a linear series, from position 1 (anteriormost position on the dentary) to 13. Up to around 22.7 mm TL, all teeth are first-generation teeth, but they differ in developmental stage, reflecting the time at which they were initiated. Four stages are distinguished: initiation and morphogenesis stage (small blue triangles), early cytodifferentiation stage (small light green triangles), late cytodifferentiation stage (large dark green triangles), tooth undergoing attachment (large light yellow triangles). Functional teeth are attributed to one of the following three states of functionality: newly attached functional teeth (large dark yellow triangles), mature functional teeth (large red triangles), functional teeth undergoing resorption (large purple triangles). Between 22.7 mm TL and 31.1 mm FL, replacement teeth appear (second-generation teeth), the developmental stage of which corresponds largely to the state of functionality of the predecessor. They will replace the first-generation teeth and be succeeded themselves by third-generation teeth. In (e) most of the functional teeth belong to the second generation (labeled ‘2’), the tooth in position 5 already belongs to the third generation (labeled ‘3’), whereas the functional teeth in the last three positions still belong to the first generation. Further explanation related to the pattern: see text.
The first teeth observed are in early hatchlings (alevins) (16.2 mm TL), when the dentary bones are already ossifying (data not shown). The pattern of teeth is bilaterally symmetrical. A linear series of four tooth germs is found, ranging in developmental stage from morphogenesis to early cytodifferentiation. Comparison to older stages reveals that these teeth are located in positions 1, 3, 5 and 7. The most developed germs, showing the onset of matrix deposition, are in positions 3 and 5. The most rostral tooth germ (position 1) is in early cytodifferentiation stage, the most caudal germ (position 7) is in morphogenesis stage.
A similar situation is observed in a specimen of 18.1 mm TL, but all tooth germs are slightly advanced with respect to the former specimen (Fig. 2a). The germs in position 3 and 5 are still the most advanced; the most caudal germ (position 7) still shows the weakest development.
In an animal of 22.7 mm TL, the row has increased to nine positions, all of them with one germ (Fig. 2b). The tooth germs in positions 1, 3, 5 and 7 are in late cytodifferentiation stage, with the teeth in positions 3 and 5 showing the onset of attachment. The tooth germs in positions 2, 4, 6 and 8 are less advanced, position 2 being the weakest developed. Likewise, the germ in position 9 is still in morphogenesis phase.
In an animal of 31.1 mm FL, the row has increased to 11 positions (Fig. 2c). Replacement tooth germs are now present for the first time. Well attached, functional teeth are found in positions 1, 3, 5, and 7, and are associated with replacement tooth germs in early or late cytodifferentiation stage. The teeth in positions 2, 4, 6 and 8 have just attached or are about to do so, and (except in position 2) are associated with a tooth germ that is in morphogenesis or at most in early cytodifferentiation stage. Positions 9, 10 and 11 show first-generation tooth germs in a decreasing stage of development, with only position 9 showing evidence for the anlage of a successor.
In an animal of 32.6 mm FL the row has increased to 13 positions (Fig. 2d). Positions 1 to 10 all possess a functional tooth and a replacement tooth. Like in the previous animal, the most caudal positions (11, 12 and 13) have no functional teeth and show tooth germs in a decreasing stage of development. A detailed analysis shows that the youngest functional tooth is in position 3 and is still on its way to attachment (it is the second-generation tooth at this position, the first tooth having been lost). This tooth is accompanied by an initiation stage successor (not shown). The teeth in positions 5 and 8 are in the process of attachment and are associated with a morphogenesis stage replacement tooth. Old functional teeth (identifiable on histological sections by their thick dentine walls and a loosely organised pulp cavity with a network of stellate cells) are present in positions 1, 4, 7 and 9, and somewhat younger but fully attached functional teeth (identifiable by their highly cellular pulp cavity) are in positions 2, 6, and 10. Old functional teeth are associated with late cytodifferentiation stage successors, younger functional teeth usually with early cytodifferentiation stage successors.
Finally, in an animal of 48.4 mm FL, the row still consists of 13 positions (Fig. 2e). Except for the caudalmost position, all contain a functional and a replacement tooth. Functional teeth undergoing resorption are present in positions 3, 6 and 8; they are associated with successors in an advanced stage of development. Mature, well attached teeth are present in positions 1, 4, 9 and 11, and young functional teeth in positions 7 and 12, each of them associated to a replacement tooth in early cytodifferentiation stage (the successor in position 11 being slightly older). Teeth in the process of attachment are present in positions 2, 5 and 10, and are associated with a morphogenesis stage tooth germ. In other words, a sequence of decreasing age of the functional teeth (e.g. a tooth in resorption, a mature tooth, and an attaching tooth) is repeatedly found from anterior to posterior (e.g. positions 3 to 5, 8 to 10, 11 to 13). A slight developmental delay in positions 7 and 8 (7 slightly younger than expected, 8 not yet completely resorbed and its successor not attaching yet) could easily explain the seeming ‘break’ in this pattern. Such a repetitive pattern is already observed in an animal of 40.0 mm FL (data not shown). The developmental stage of the replacement tooth is furthermore in agreement with the state of functionality (i.e. age) of the predecessor. For example the successor is in late cytodifferentiation stage (almost attaching) if the predecessor is in resorption, it is in early to late cytodifferentiation stage when the predecessor is mature, or the successor has just passed morphogenesis stage if the predecessor has recently attached. This repetitive pattern of three consecutive positions with different stages of maturity of the functional tooth, and of development of the successor, is also found in the following life stages of the animal.
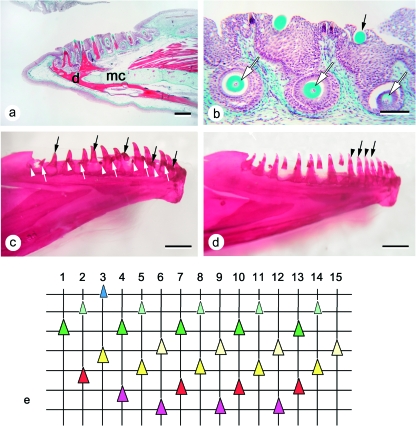
Parr (Fig. 3)
Fig. 3.
Tooth pattern in parr. (a) Representative sagittal section from the dentary of a 0+ parr. d: dentary bone; mc: Meckel's cartilage. Bar = 0.5 mm. (b) Larger magnification of three successive tooth positions in the same jaw as shown in (a). Although this can hardly be appreciated from a single section, the three replacement teeth (white arrows) are in a decreasing level of differentiation from left (anterior) to right (posterior), and they replace functional teeth that are of decreasing age (from a tooth in resorption (left, not visible on picture) to a tooth that has just attached (right, black arrow)). Bar = 100 µm. (c) Example of left dentary dentition (medial view) in a cleared and stained female parr specimen (111.4 mm FL). The tooth row is composed of 16 loci. The three types of arrows indicate teeth in a similar stage: functional teeth (black arrows), advanced replacement teeth (white arrows), and young replacement teeth (white arrowheads). Among the functional teeth indicated, some are in the process of attachment. Similar stages occur every third position in the tooth row. Note that posterior positions are slightly delayed compared to anterior positions. Bar = 1 mm. (d) Example of perturbed replacement pattern in a male parr specimen (108.5 mm FL), cleared and stained. Left dentary, medial view. The teeth indicated by an arrow and an arrowhead represent a succession of two, rather than three teeth, of diminishing age (compare with the regular pattern shown in c). Bar = 1 mm. (e) Graphical representation of the dentary dentition partly shown in (a). Vertical lines represent (numbered) tooth positions (= tooth families). Horizontal lines connect teeth in a similar stage of development (for replacement teeth) or functionality (for functional teeth). Stages distinguished and color codes used similar as in Figure 2. The oldest functional teeth are shown at the bottom of the chart (labial side of the dentary), youngest replacement tooth germs at the top (lingual side of the dentary). In the anterior part of the dentition, the functional tooth in position 4 is slightly advanced in comparison to the teeth in positions 7, 10 and 13, since resorption has started already. In the adjacent (more anterior) positions, the functional tooth in position 3 is advanced in comparison to the teeth in positions 6, 9 and 12, since it is already attaching, and the functional tooth in position 2 is advanced in comparison to the teeth in positions 5, 8, 11 and 14, since it is already well attached and has entered a regular functional phase. In line with the advanced state of these functional teeth, their successors are also more advanced in their development. E.g. in position 3, a tooth is found in morphogenesis stage.
The number of tooth positions on the parr specimens examined varies between 14 and 17 positions, or tooth families. Left and right jaw can differ in a number of tooth positions. Observed maximum numbers of tooth positions are 15 in 0+ parr, 16 in 1+ parr and 17 in 2+ parr. There is always a functional tooth and a replacement tooth in a single position (Fig. 3a,b), except for the caudalmost position, where a replacement tooth is often lacking. In addition, there is never more than one replacement tooth for a functional tooth. In cleared and stained specimens, replacement teeth can be present in the absence of a functional tooth, but this replacement tooth is then usually in an advanced developmental stage and likely to attach soon (Fig. 3c). Conversely, a replacement tooth can exceptionally appear to be lacking along the row, but in this case the functional tooth usually shows obvious signs of recent attachment (Fig. 3d). It should be remembered at this point that cleared and stained specimens do not show the presence of unmineralised tooth germs.
The pattern of tooth replacement in parr (as exemplified by a serially sectioned dentary of a 0+ parr, where all tooth germs can be identified, cf. Fig. 3a), is pictured diagrammatically in Fig. 3e. From this figure, it appears that in most parts of the tooth row, functional teeth are in a similar state of their replacement cycle (newly attached, fully functional, or in resorption) every third position. Thus, three successive positions (from anterior to posterior in the row) are taken by a functional tooth undergoing resorption, a fully attached functional tooth, and a newly attached, young, functional tooth, respectively (Fig. 3a,b,e, positions 6–8, 9–11, 12–14). Functional teeth that share the same state in their replacement cycle also have replacement teeth that share the same developmental stage. Thus a newly attached tooth has a replacement tooth that shows incipient matrix deposition; associated with a fully functional tooth is a replacement tooth where matrix deposition has well advanced, and finally, a functional tooth in resorption has a replacement tooth that is completing its basis, prior to the formation of attachment bone.
Although this pattern emerges as the general pattern for the parr examined (e.g. it is found on both sides in six of the eight cleared and stained parr specimens) (Fig. 3c), there is a general trend for the more posterior part of the tooth row to be slightly out of phase with respect to the anterior positions: in contrast to the anterior part of the tooth row, functional teeth in the more posterior positions often show heavy wear and/or resorption. Nevertheless, the fact that they correspond to positions, more anteriorly, where the functional tooth has disappeared already, suggests that the posterior part of the tooth row is slightly delayed compared to the more anterior part. This is observed both on the serially sectioned and cleared and stained jaws.
Two of the eight stained and cleared parr specimens (one male, one female) showed slight deviations from this repetitive pattern of three successive teeth in the row, of diminishing age. For instance, a replacement tooth could be found to be delayed, or conversely advanced, in its development compared to what could be expected on the basis of the pattern, resulting in similar stages of functional and replacement teeth every second, rather than third, position (e.g. Fig. 3d). Also, a pattern could be found with similar stages of development every fourth position (not shown).
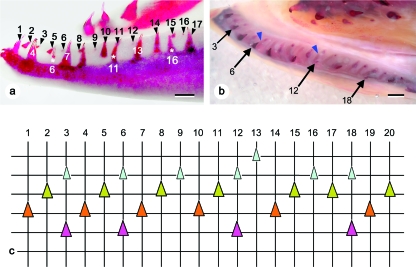
Marine specimens (Fig. 4)
Fig. 4.
Tooth pattern in marine animals. (a) Female. Lateral view of the left dentary, with tooth positions numbered. Due to the anterior inward curvature of the dentary, position 3 appears to lie caudal to position 4. Note the retention of a tooth undergoing resorption in position 6, 11 and 16 (white asterisks). Bar = 1 mm. (b) Male. Dorsal view of the left dentary. The epithelial connection between functional teeth and their successors is well visible (blue arrowheads). Functional teeth are located labially, young replacement teeth lingually. Arrows indicate old functional teeth that are retained in the presence of young replacement teeth (positions 3, 6, 12 and 18). Bar = 1 mm. (c) Graphical representation of the dentition on the dentary of marine animals, as exemplified by the left dentary of the animal shown in Fig. 4b. Vertical lines represent (numbered) tooth positions. Horizontal lines connect teeth in a similar stage of development (for replacement teeth) or functionality (for functional teeth). Lingual to the top, labial to the bottom of the chart. Four stages of tooth development can be distinguished on cleared and stained animals: young replacement teeth (small light green triangles), advanced replacement teeth (large dark green triangles), functional teeth (whether newly attached or mature) (large orange triangles), and functional teeth undergoing resorption (large purple triangles). Note that sets of three teeth in descending order of development succeed each other along the tooth row (positions 1–3, 4–6, etc.). This pattern is interrupted by an extra tooth germ in position 13, and an apparent loss of a tooth between positions 16 and 17. Due to the retention of a functional tooth together with a young replacement tooth (positions 3, 6, 12 and 18), functional teeth appear to be irregularly distributed and separated by variable numbers of replacement teeth.
X-rays of both male and female specimens reveal that, in contrast to the parr stage, every single position now often holds only one tooth: either a functional tooth (whether old and in resorption, fully attached and functional, or young and newly attached), or a tooth germ of variable size. Successions of three teeth of descending age (a functional tooth, an advanced replacement tooth and a young replacement tooth) in three successive antero-posterior positions do occur, but functional teeth are also often separated by a different number of tooth germs (none, or one instead of two). The cleared and stained lower jaws of three of these animals provide more details (Fig. 4a,b): successions of three teeth of descending age do occur frequently, but the youngest of the tooth germs is often associated with a functional tooth showing wear or resorption (Fig. 4b,c). This results in functional teeth being adjacent to each other (i.e. in neighbouring positions), or spaced by one tooth germ only. Occasionally a series of four teeth of descending age, or some other irregularity, is observed.
In comparison to the later freshwater stages, the teeth appear more slender, with a narrower basis, and are less recurved.
Grilse (Figs 5, 6)
Fig. 5.
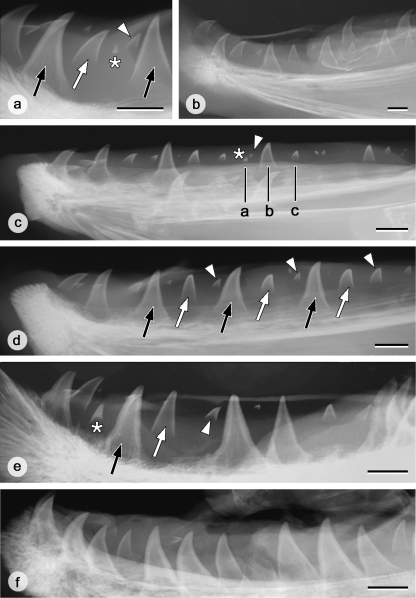
Tooth pattern on the left lower jaw in grilse, salmon and kelts. (a) Characteristics of teeth on X-rays as exemplified by a male grilse. A tooth germ in formation (white arrowhead) can be clearly distinguished from the remnants of a functional tooth in resorption (white asterisk): tooth tips in resorption are more heavily mineralised and they do not possess a V-shaped edge to the dentine as in developing teeth. Attached functional teeth are indicated by black arrows, an advanced replacement tooth by a white arrow. Bar = 10 mm. (b) X-ray of the left dentary of a male grilse. The graphical representation of the tooth pattern corresponding to this dentary is shown in Fig. 6a. Bar = 10 mm. (c) X-ray of the left dentary of a female grilse. In this animal, more posterior positions appear to be advanced with respect to anterior positions. The three black lines (a, b, c) indicate three consecutive positions that, if the pattern were continued from the front, would have been constituted by a functional tooth, a more advanced and a younger replacement tooth. Instead, the functional tooth has undergone resorption, leaving only the tooth tip (white asterisk), together with an early replacement tooth (white arrowhead). The next positions are similarly advanced. Together this creates the impression that, more anteriorly, four instead of three adjacent positions show decreasing developmental stages. Bar = 10 mm. (d) X-ray of the left dentary of a female salmon. Note the repetition of functional teeth (black arrows), advanced replacement teeth (white arrows), and young replacement teeth (white arrowheads) along the row. Bar = 10 mm. (e) X-ray of the left dentary of a male kelt. A pattern of three consecutive teeth of diminishing developmental stage is still recognizable in some parts of the tooth row (cf. black arrow, white arrow, white arrowhead); elsewhere adjacent teeth have attached. An asterisk indicates the tip of a tooth in resorption. Bar = 10 mm. (f) X-ray of the left dentary of a female kelt. Note that almost all teeth are attached; developing replacement teeth are rare. Bar = 10 mm.
Fig. 6.
Graphical representation of the dentition in representative grilse and salmon. Vertical lines represent tooth positions. Positions are shown from anterior (left) to posterior (right) but are not numbered because of the uncertain presence of anterior positions. In addition, not all positions at the rear end of the row are figured. Horizontal lines connect teeth in a similar stage of development (for replacement teeth) or functionality (for functional teeth). Lingual to the top, labial to the bottom of each chart. (a) Graphical representation of the dentition on the left dentary of a male grilse (cf. Fig. 5b). Legend and color codes as in Figure 4. (b) Graphical representation of part of the dentition on the left dentary of a female salmon (cf. Fig. 5d). Legend and color codes as in Figure 4.
Grilse possess approximately 20 to 25 teeth or tooth germs on each dentary. On X-rays replacement tooth germs can be recognized by their inverted V-shape and an unmineralised area between the mineralised tooth base and the supporting bone. Frequently, tiny but heavily mineralised tooth tips are observed very close to (i.e. in the same position as, but slightly rostral to) young developing tooth germs. They differ nevertheless in their morphological characteristics from these germs (Fig. 5a). In contrast to the tip of a developing tooth germ, these tips have no lighter central zone, and are heavily mineralised throughout. They are therefore interpreted as the remains of functional teeth after considerable resorption has occurred. This confirms observations on sections where functional teeth in an advanced stage of resorption (with only tooth tips persisting) could be observed in the close vicinity of developing tooth germs. Similarly, observations on sections revealed that well attached functional teeth are connected to a young replacement tooth germ with very little, unmineralised matrix, making the germ likely unnoticeable on X-rays.
Male grilse
An X-ray of the left dentary of a representative male grilse is shown in Figure 5b. Mapping this dentition on a chart yields the picture shown in Figure 6a. As can be observed from the X-ray, and read from this figure, only one tooth or tooth germ is visible in each position. In addition, a functional tooth is seen in every third position, whereas the intermediate positions are taken by tooth germs in decreasing stages of development. Among the specimens studied, some variation could be seen with respect to this common pattern, mostly at the front and the rear of the tooth row (cf. also Figs 5b, 6a). Variations ranged from a lacking functional tooth, or a replacement tooth being either younger or older than expected according to the pattern described above. In some cases, but not consistently, three adjacent positions were found to be advanced with respect to this pattern. However, the variations observed were minor with respect to the overall pattern that emerged, namely repetitive sequences of three teeth of decreasing age. The increased variation observed both at the rear and at the front end of the tooth row might be at least partly artefactual since the X-rays are difficult to interpret in both areas [rostrally because of the curvature of the jaw, caudally because of overlap with the hard tissues (and teeth) of the tongue].
Remarkably, sometimes two functional teeth could be seen in adjacent positions. Closer observation however revealed that one of these teeth was much more advanced, as indicated by the heavily mineralised base, whereas the other was clearly younger. The presence, more posteriorly along the row, of tooth tips undergoing resorption in positions expected to be taken by functional teeth, indicates that the more rostral sequences of three teeth were slightly delayed in their development (or conversely much more advanced, having already entered the next replacement cycle) with respect to more caudal ones, thus explaining the presence of ‘old’ functional teeth, and well advanced tooth germs in adjacent positions.
The pattern was not necessarily similar in both dentaries of the same animal. For instance, in some specimens the dentition on one dentary could be in a stage where several functional teeth were close to being resorbed or were undergoing resorption, whereas this was not the case on the contralateral dentary.
Female grilse
The pattern observed was similar to the pattern observed in the male grilse: one tooth or tooth germ in every position, being in a similar stage of functionality or development every three positions. In the rear of the tooth row, sequences of three teeth could be advanced with respect to those more in the front (Fig. 5c). Here, one in every three positions contained a tooth tip in resorption (accompanied by a tooth germ in development) instead of a functional tooth, as in the corresponding more anterior positions.
Pre-spawning salmon (Figs 5, 6)
No obvious differences were observed between males and females. An X-ray of a representative dentary is shown in Figure 5d and a graphical representation of this dentition is shown in Figure 6b. Clearly, the pattern described for the grilse also holds for the salmon. Briefly, repetitive sequences of three teeth or tooth germs were found, with similar stages of development every third position: a functional tooth, an advanced tooth germ caudal to this tooth, and a young tooth germ in the position caudal to this. Only minor variations were observed, such as the lack of a functional tooth, or a more advanced state of development of two adjacent positions in the row.
Post-spawning kelts (Fig. 5)
The spring animals that survive spawning display a highly variable dentition, both among males and females, as well as in single individuals (right versus left dentary). In some animals, repetitive sequences of three teeth of diminishing developmental stage could still be recognized, despite some variation in the state of development of individual teeth and/or a break in these sequences (i.e. sequences of three teeth of diminishing age separated by one or two teeth) (Fig. 5e). In others, the pattern was not recognizable at all. This could be related to the fact that more teeth than usual were attached, a situation more often found in males, but also occurring in females (cf. Fig. 5f). Alternatively, almost no attached functional teeth were left. Replacement teeth in spring animals were present but appeared to be more rare. In contrast, tips of teeth having undergone resorption appeared to be much more frequent. The teeth that were attached showed a heavily mineralised basis, and the surface of the tooth was less smooth than in spawning animals.
Discussion
To our knowledge, this is the first study that examines the tooth pattern throughout nearly all life stages in an animal with a complex life history, involving both freshwater and marine phases. The only other studies that have examined how the initial tooth pattern is set up in a teleost, and whether and how this relates to the pattern of tooth replacement, have been conducted for the zebrafish (Danio rerio) (Huysseune et al. 1998; Van der heyden & Huysseune, 2000; Van der heyden et al. 2001), and for the rainbow trout (Oncorhynchus mykiss), a close relative of Atlantic salmon (Berkovitz & Moore, 1974, 1975; Berkovitz, 1977, 1978; Fraser et al. 2004). However, when comparing our data with those published on trout, we should bear in mind that studies on farmed, inbred and often triploid rainbow trout (introduced into European waters as a foreign species) may not always be comparable with studies on wild Atlantic salmon. Farmed salmonids, especially triploids, often develop severe skeletal malformations that affect the skull and the axial skeleton (Madsen et al. 2000; Sadler et al. 2001); whether they also affect the dentition is not known.
We will first discuss the initial establishment of the tooth pattern (first-generation teeth), and how this pattern is maintained or altered during the subsequent life stages of Atlantic salmon. We will then briefly comment on the supposed development of breeding teeth (for a more exhaustive treatment of this aspect, see Witten et al. 2005). Finally, we discuss the changes that affect the dentition in the most critical of all adult life phases: that of post-spawning survival.
Setting up and maintaining the dentary tooth pattern in Atlantic salmon
The observations on the establishment, maintenance and modifications of the tooth pattern on the dentary in the different life stages of Atlantic salmon can be summarized as follows: The very first teeth in the salmon dentition appear around hatching and develop in odd positions, one tooth developing probably prior to the others. In some animals this is likely the tooth in position 3, as judged from its early resorption (Fig. 2c); in others it is more likely position 5, given its replacement ahead of that in position 3 (Fig. 2d). Next, tooth germs develop in the even positions, first positions 4 and 6, followed by 2 and 8. After these eight teeth have appeared in alternate positions (first odd, then even), first-generation teeth continue to be added in adjacent positions at the back of the dentary (positions 9, 10, etc.). Can this pattern be linked to potential space being created by the growing jaw? Several observations argue against this possibility. The first teeth on the salmon dentary (between odd positions) are initiated at twice the distance necessary for the initiation of adjacent teeth further back in the row, whilst their size is approximately similar. In the Mexican tetra (Astyanax mexicanus) too, the first teeth formed on the dentary and premaxillary are widely spread out (Trapani et al. 2005), making spacing through jaw growth probably not required to add teeth. Furthermore, the dentary bone in Atlantic salmon is present at the stage when the first odd-positioned teeth are forming, and given that bone cannot grow interstitially, spacing due to jaw growth to accomodate teeth in the intermediate (even) positions is unlikely. On the other hand, the addition of teeth in adjacent positions at the rear end of the row might well be linked to extension of the jaw posteriorly.
Surprisingly, the successors of the first-generation teeth do not develop in the same sequence. Rather, they are initiated in an order that will establish a repetitive pattern, whereby teeth are initiated simultaneously every third position along the tooth row, and whereby three adjacent positions are taken by teeth of decreasing age (cf. Fig. 1). Thus, one finds repetitive sequences of an old functional tooth with an advanced replacement tooth, a younger functional tooth with a young replacement tooth, and a functional tooth in attachment associated with an initiation or morphogenesis stage replacement tooth. This pattern does, however, not appear to be too rigorous among the fry specimens studied and deviations from the pattern are frequently observed. Deviations may be explained by the fact that the anterior first-generation teeth (up to position 8) are set up in an alternating pattern whereas the posterior teeth (from position 9 backwards) are added in adjacent positions. Furthermore, replacement teeth which initiate their development simultaneously along the row cannot all belong to the same tooth generation, given that the founder in each position developed at different times. For example the second-generation tooth in position 2 develops almost simultaneously with the third-generation tooth in position 5.
The high percentage of parr dentaries with a pattern that conforms completely with this repetitive pattern of three teeth of diminishing age indicates that this pattern has been stabilised in parr. Replacement teeth continue to develop already at attachment stage of the predecessor, so that, like in fry, every position along the tooth row holds both a functional tooth and its successor. The pattern observed in the parr seems to be maintained for a considerable time, since it is also found in the marine life stage, and in the upstream migrating animals (grilse and salmon). However, from the marine stage onwards, each position often holds either a functional tooth or a (mineralised) replacement tooth.
From this general picture, two major shifts in patterning seem to occur. The first one involves the switch from alevin (post-hatching, non-feeding) to fry/parr (feeding), i.e. from a dentition where (first-generation) teeth are initiated in alternate positions, to a pattern where (replacement) teeth are initiated simultaneously every third position in the row. The second one occurs after parr stage and involves the shift that leads to the presence of only one mineralised tooth or tooth germ at every position.
Berkovitz (1977, 1978) and Fraser et al. (2004) examined the establishment of the tooth pattern on the dentary in another salmonid fish, the rainbow trout (Oncorhynchus mykiss) and found that the teeth are set up in alternating positions, first odd, then even, with one tooth forming prior to all others, a situation similar to that described here for Atlantic salmon. Berkovitz & Moore (1974, 1975) also examined tooth replacement in rainbow trout, but neither of these two papers reports exactly how the initial pattern is turned into the replacement pattern. We have previously elaborated on possible developmental models describing how the initial pattern of alternating tooth initiation could be set up and turned subsequently into the replacement patterns observed (Huysseune & Witten, 2006; in particular their Fig. 1b) and we refer to this paper for a lengthy discussion. From our observations, it appears that establishment of the replacement tooth pattern is achieved by synchronising the formation of first-generation teeth in new positions to the formation of replacement teeth in older positions. Such a synchronisation may have been the outcome of an evolutionary selection process, given that regular replacement patterns are viewed as advantageous in terms of organismal fitness (Trapani et al. 2005).
Reinterpreting the results of Berkovitz & Moore (1974, 1975) on the trout, it is clear that the pattern they observe is exactly the same as the one we observe in Atlantic salmon. For example in the specimen labeled B1 by Berkovitz & Moore (1974), functional teeth are of approximately the same age every three positions, and two teeth are of decreasing age in between these functional teeth. Yet, whereas we emphasize lines connecting teeth that develop simultaneously, Berkovitz & Moore (1974, 1975) and Berkovitz (2000) emphasized the lines linking alternate teeth (and called them replacement waves). According to Berkovitz (2000), replacement waves that sweep through alternate positions provide a means to ensure that exfoliation in one series leaves the teeth in the adjacent series functional, thus securing retention of what may be considered a functional dentition.
In accordance with Berkovitz & Shellis (1978) and Smith (2003) we have previously argued that replacement tooth development is probably under local control, i.e. that each tooth family controls its own rate of replacement tooth formation through factors intrinsic to the odontogenic cells, rather than replacement being controlled through a field effect (i.e. by a gradient formed by a diffusible substance), and that the replacement pattern naturally follows from the establishment of the first-generation teeth (Huysseune & Thesleff, 2004; Huysseune & Witten, 2006; Huysseune, 2006). We propose that the same occurs in salmon: the first-generation teeth, and especially their successors (second-generation teeth) set the stage for a replacement pattern whereby an old functional tooth, a younger functional tooth, and a tooth in the process of attachment, occupy three successive positions in the tooth row.
Although the pattern described is generally fairly regular in all life stages except in kelts (but see below), variations do occur (see e.g. Fig. 6a). One of the common deviations of the pattern is that a number of adjacent positions in the posterior part of the tooth row is slightly out of phase compared to adjacent positions more anteriorly. Thus, in the anterior sets of three teeth the functional tooth is often not yet undergoing resorption or, more likely, its predecessor has disappeared already, in contrast to more posterior positions, where functional teeth are distinctly worn off, or undergo resorption. This more common deviation could be the result from the differences in how the pattern is set up initially in the anterior positions (positions 1 to 8) versus more posterior positions (9 and further backwards). Other types of variations also occur and can easily be explained if one assumes that the formation of the replacement tooth is under local control, rather than resulting from a field effect. Local control over the replacement process could also explain why bilateral symmetry is rarely observed and phase displacements between the two dentaries are often apparent (but see Huysseune & Witten, 2006; and Huysseune, 2006, for more lengthy discussions).
The second apparent change in the pattern during the salmon's life cycle involves the switch that seems to occur after the parr stage. Unlike parr, where every position holds two teeth (a functional tooth and a replacement tooth), the juveniles captured in sea and the mature animals that re-enter freshwater (grilse and salmon) have only one mineralised tooth or tooth germ in every position, and a functional tooth only every third position (in marine animals sometimes a functional and replacement tooth are still found at the same position). In our view, this switch does not imply a change in the pattern, but can easily be explained in two possible ways: either the replacement tooth is initiated at a later moment in the replacement cycle, or, given the larger size to which the tooth has to grow, it takes more time to develop mineralised matrix. The frequent observation, on X-rays of grilse and salmon, of a tooth tip resulting from advanced resorption, in association with a young tooth germ, is in support of this second interpretation. Similarly, observations on sections from adult specimens confirm the presence of unmineralised tooth germs associated with functional teeth, and of more advanced replacement teeth close to tooth tips surrounded by osteoclasts (see Witten et al. 2005). Interestingly, similar to what has been reported in several lissamphibians (Shaw 1989), the old functional tooth is apparently completely, or nearly completely resorbed.
Are there so-called breeding teeth?
At no time in the life cycle have we observed indications for an edentulous stage, suggested in the literature to precede the appearance of so-called breeding teeth during upstream migration to the spawning grounds (Tchernavin, 1937, 1938). Arguments against the presence of an edentulous stage are that the pattern remains unchanged between marine stages, and grilse and salmon, and that tooth tips in resorption (i.e. remnants of functional teeth) are found in the dentitions of grilse and salmon, caught close to their spawning grounds. That teeth in upstream migrating fish display a different shape is not unusual, given that tooth shape changes are common in teleosts exhibiting continuous tooth replacement (Huysseune, 1995; Wautier et al. 2001, 2002; Sire et al. 2002; Streelman et al. 2003; Vandervennet et al. 2006). Based on the analysis of exchange rates of oxygen isotopes of biogenic apatite in pacific salmon teeth, Zazzo et al. (2006) reached a similar conclusion regarding the absence of an edentulous stage. Thus, tooth formation and replacement continue uninterrupted in mature salmon during the animals’ upstream spawning migration. Nevertheless, what is possible is that during the transition of the marine to the freshwater stage, functional teeth attach through an incompletely mineralised tooth basis, or their growth is slowed down or arrested in an even less advanced developmental stage. Many methods used to clean the jaws involve removal of all soft tissues, washing away all tooth germs as well as teeth in the process of attachment or poorly attached teeth (less mineralised attachment bone), leaving the impression of a seemingly edentulous jaw. In addition to this, we should note that upstream migrating animals possess a thickened oral mucosa, through which the tips of the functional teeth hardly penetrate. This not only conceals the teeth present, but might also require a more drastic method to wash the soft tissues away while preparing the jaw skeleton.
Life stages between the immature marine stage and the mature freshwater grilse stage could shed further light on the precise characteristics of the dentition at this moment in the life cycle. Unfortunately, such material is at present lacking.
What happens during winter?
Adult salmon do not feed during the entire freshwater period (prior to and after spawning) and animals that survive spawning and stay in the river over the winter continue to starve until the following spring (Booth et al. 1999; Witten & Hall, 2003). Fat reserves in post-spawning Atlantic salmon decrease by 80% and protein reserves decrease by 20% (Jonsson et al. 1997). Male kelts from the studied population stop somatic growth, lose about 40% weight and resorb anterior parts of their kype skeleton at the tip of the lower jaw (Witten & Hall, 2003; Witten et al. 2004). Thus, it is not surprising that we find the winter at the spawning grounds to have a severe impact on the dentition as well. Whereas the fall animals (both grilse and salmon) display a fairly consistent pattern with teeth in a similar stage every three positions (pattern explained above), such a pattern is only rarely recognisable in post-spawning (spring) animals. In contrast, animals sometimes display many more teeth that are attached. Developing replacement teeth are more rare and remnants of teeth in resorption are more frequent. Given the stop of somatic growth in overwintering animals and the severe starvation they suffer, a functional explanation for extended tooth resorption and reduced development of new germs could relate to mineral and protein deficiency that also triggers salmon scale resorption (Persson et al. 1998; Witten et al. 2005). It is, however, more difficult to reconcile such an interpretation with the observation of more attached teeth. Assuming, however, that the entire cycle of tooth replacement slows down, the basis of those teeth that are present and not shed apparently continues to mineralise, likely a rather passive than active process. As a consequence, teeth that have persisted throughout the winter look firmly attached to the jaws on X-rays.
Given that kelts can become repetitive spawners (i.e. salmon), which have been shown to display a regular replacement pattern, surviving animals must restore a regular pattern of tooth replacement during their marine life that follows the freshwater period. It is tempting to speculate that those spring animals that still show an identifiable pattern reflect their better chance to survive the starvation period and will become repetitive spawning animals. The fact that male kelts do not completely resorb their kype and remodel proximal parts of the kype in regular bone tissue that elongates the lower jaw, led Witten & Hall (2003) to speculate that the animals not only survive the winter, but prepare themselves already for the next spawning season to be equipped with a larger kype and an elongated lower jaw as an enhanced secondary sexual character. At present, and despite successful measures by the DFO-Moncton to protect and to recover wild Atlantic salmon in the Miramichi River, this type of wild material (kelts, salmon) is too rare to substantiate such a line of thinking, nor can we test whether there is a connection between the tooth pattern of an animal and its condition factor.
Concluding remarks
Despite its complex life cycle, Atlantic salmon is capable of maintaining a dentition where teeth are continuously replaced in a coordinated fashion. The initial tooth pattern set up by the first-generation teeth is thereby converted into a pattern that characterizes all life stages, from parr over marine and freshwater stages to post-spawning animals. This study has allowed us to increase our basic knowledge about the mechanisms of development and patterning of the vertebrate dentition. Furthermore, understanding of the biological function of the tooth alterations in Atlantic salmon helps us to close an important gap in our knowledge of the biology of this endangered species and thus to contribute to the conservation and sustainable management of Atlantic salmon stocks.
Acknowledgments
We thank our colleagues Michael E. Chadwick, Gerald J. Chaput, David S. Moore, Joe Shaesgreen, John Hayward, and Peter Hardie from DFO-Moncton and Mark Hambrook from the Miramichi Salmon Conservation Centre. This study would not have been possible without their advise and their restless efforts to protect Atlantic salmon in the Miramichi River. We also greatly acknowledge the help of Lawrence Taylor, and Andrew Gillis and the members of the Hall lab (Dalhousie University). Mieke Soenens and Barbara De Kegel are gratefully acknowledged for expert technical assistance. AH acknowledges grants from the Bijzonder Onderzoeksfonds (Ghent University) 011B2396 and 011V1203, and the Fonds voor Wetenschappelijk Onderzoek-Vlaanderen 3G0159.05. BKH acknowledges funding from Natural Sciences and Engineering Research Council of Canada. EPW acknowledges funding from the Canadian-German Science and Technology Cooperation and from the ‘Deutsche Forschungsgemeinschaft’. This paper was made within the frame of the European COST Action B23-Oral facial development and regeneration.
References
- Berkovitz BKB. The order of tooth development and eruption in the rainbow trout (Salmo gairdneri) J exp Zool. 1977;201:221–226. [Google Scholar]
- Berkovitz BKB. Tooth ontogeny in the upper jaw and tongue of the rainbow trout (Salmo gairdneri) J Biol Buccale. 1978;6:205–215. [PubMed] [Google Scholar]
- Berkovitz BKB. Tooth replacement patterns in non-mammalian vertebrates. In: Teaford M, Smith M, Ferguson M, editors. Development, Function and Evolution of Teeth. Cambridge: Cambridge University Press; 2000. pp. 186–200. [Google Scholar]
- Berkovitz BKB, Moore MH. A longitudinal study of replacement patterns of teeth on the lower jaw and tongue in the rainbow trout Salmo gairdneri. Archs oral Biol. 1974;19:111–119. doi: 10.1016/0003-9969(74)90239-8. [DOI] [PubMed] [Google Scholar]
- Berkovitz BKB, Moore MH. Tooth replacement in the upper jaw of the rainbow trout (Salmo gairdneri) J exp Zool. 1975;193:221–234. doi: 10.1002/jez.1401930211. [DOI] [PubMed] [Google Scholar]
- Berkovitz BKB, Shellis RP. A longitudinal study of tooth succession in piranhas (Pisces: Characidae), with an analysis of the tooth replacement cycle. J Zool, Lond. 1978;184:545–561. [Google Scholar]
- Booth RK, McKinley RS, Ballantyne JS. Plasma non-esterified fatty acid profiles in wild Atlantic salmon during their freshwater migration and spawning. J Fish Biol. 1999;55:260–273. [Google Scholar]
- Chaput G, Moore D, Hayward J, Shaesgreen J, Dubee B. Ottawa: Canadian Stock Assessment Secreteriat; Stock status of Atlantic Salmon (Salmo salar) in the Miramichi River, 1999; pp. 1–5. Ottawa: Research Document 2000/004. [Google Scholar]
- Darwin CD. The Descent of Man, Selection in Relation to Sex. 2. London: John Murray; 1877. [Google Scholar]
- Fleming IA. Reproductive strategies of Atlantic salmon: ecology and evolution. Rev Fish Biol Fish. 1996;6:379–416. [Google Scholar]
- Fraser GJ, Graham A, Smith MM. Conserved deployment of genes during odontogenesis across osteichthyans. Proc R Soc Lond B. 2004;271:2311–2317. doi: 10.1098/rspb.2004.2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillis JA, Witten PE, Hall BK. Chondroid bone and secondary cartilage contribute to apical dentary growth in juvenile Atlantic salmon. J Fish Biol. 2006;68:1133–1143. [Google Scholar]
- Gorodilov YN. Description of the early ontogeny of the Atlantic salmon, Salmo salar, with a novel system of interval (state) identification. Environ Biol Fishes. 1996;47:109–127. [Google Scholar]
- Groot C. Salmonid life histories. In: Pennell W, Barton BA, editors. Principles of salmonid culture. Developments in Aquaculture and Fisheries Science 29. Amsterdam: Elsevier; 1996. pp. 97–230. [Google Scholar]
- Huysseune A. Phenotypic plasticity in the lower pharyngeal jaw dentition of Astatoreochromis alluaudi (Teleostei: Cichlidae) Archs oral Biol. 1995;40:1005–1014. doi: 10.1016/0003-9969(95)00074-y. [DOI] [PubMed] [Google Scholar]
- Huysseune A. Formation of a successional dental lamina in the zebrafish (Danio rerio): support for a local control of replacement tooth initiation. Int J Dev Biol. 2006;50:637–643. doi: 10.1387/ijdb.052098ah. [DOI] [PubMed] [Google Scholar]
- Huysseune A, Sire JY. Development of cartilage and bone tissues of the anterior part of the mandible in cichlid fish: a light and TEM study. Anat Rec. 1992;233:357–375. doi: 10.1002/ar.1092330304. [DOI] [PubMed] [Google Scholar]
- Huysseune A, Thesleff I. Continuous tooth replacement: the possible involvement of epithelial stem cells. BioEssays. 2004;26:665–671. doi: 10.1002/bies.20039. [DOI] [PubMed] [Google Scholar]
- Huysseune A, Sire JY. Evolution of patterns and processes in teeth and tooth-related tissues in non-mammalian vertebrates. Eur J oral Sci. 1998;106:437–481. doi: 10.1111/j.1600-0722.1998.tb02211.x. [DOI] [PubMed] [Google Scholar]
- Huysseune A, Witten PE. Developmental mechanisms underlying tooth patterning in continuously replacing osteichthyan dentitions. J Exp Zool. 2006;306B:204–215. doi: 10.1002/jez.b.21091. [DOI] [PubMed] [Google Scholar]
- Huysseune A, Van der heyden C, Sire JY. Early development of the zebrafish (Danio rerio) pharyngeal dentition (Teleostei, Cyprinidae) Anat Embryol. 1998;198:289–305. doi: 10.1007/s004290050185. [DOI] [PubMed] [Google Scholar]
- Järvi T. The effects of male dominance, secondary sexual characteristics and female mate choice on the mating success of male Atlantic salmon Salmo salar. Ethology. 1990;84:123–132. [Google Scholar]
- Jones JW. The Salmon. New York: Harper & Brothers; 1959. p. 192. [Google Scholar]
- Jonsson NB, Jonsson B, Hansen LP. Changes in proximate composition and estimates of energetic costs during upstream migration and spawning in Atlantic salmon. Salmo salar. J Anim Ecol. 1997;66:425–436. [Google Scholar]
- Madsen L, Arnberg J, Dalsgaard I. Spinal deformities in triploid all-female rainbow trout (Oncorhynchus mykiss) Bull Eur Assoc Fish Pathol. 2000;20:206–208. [Google Scholar]
- Marschall EA, Quinn TR, Roff DA, Hutchings JA, Metcalfe NB, Bakke TA, Saunders RL, Poff NL. A framework for understanding Atlantic salmon (Salmo salar) life history. Can J Fish Aquat Sci. 1998;55(Suppl. 1):48–58. [Google Scholar]
- Moore DS, Chaput GJ, Pickard PR. The effect of fisheries on the biological characteristics and survival of mature Atlantic salmon (Salmo salar) from the Miramichi river. Can Spec Publ Fish Aquat Sci. 1995;123:229–247. [Google Scholar]
- Persson P, Sundell K, Björnsson BT, Lundqvist H. Calcium metabolism and osmoregulation during sexual maturation of river running atlantic salmon. J Fish Biol. 1998;52:334–349. [Google Scholar]
- Sadler J, Pankhurst PM, King HR. High prevalence of skeletal deformity and reduced gill surface area in triploid Atlantic salmon (Salmo salar L.) Aquaculture. 2001;198:369–386. [Google Scholar]
- Shaw JP. A morphometric study of bone and tooth volumes in the pipid frog Xenopus laevis (Daudin), with comments on the importance of tooth resorption during normal tooth replacement. J Exp Zool. 1989;249:99–104. doi: 10.1002/jez.1402490117. [DOI] [PubMed] [Google Scholar]
- Shearer WM. Atlantic Salmon Scale Reading Guidelines. Copenhagen: ICES cooperative research report 188; 1992. [Google Scholar]
- Sire JY, Davit-Beal T, Delgado S, Van der heyden C, Huysseune A. First-generation teeth in nonmammalian lineages: Evidence for a conserved ancestral character? Microsc Res Techn. 2002;59:408–434. doi: 10.1002/jemt.10220. [DOI] [PubMed] [Google Scholar]
- Smith MM. Vertebrate dentitions at the origin of jaws: when and how pattern evolved. Evol Dev. 2003;5:394–413. doi: 10.1046/j.1525-142x.2003.03047.x. [DOI] [PubMed] [Google Scholar]
- Streelman JT, Webb JF, Albertson RC, Kocher TD. The cusp of evolution and development: a model of cichlid tooth shape diversity. Evol Dev. 2003;5:600–608. doi: 10.1046/j.1525-142x.2003.03065.x. [DOI] [PubMed] [Google Scholar]
- Tchernavin V. Skulls of salmon and trout. A brief study of their differences and breeding changes. Salmon and Trout Magazine. 1937;88:235–242. [Google Scholar]
- Tchernavin V. Changes in the salmon skull. Trans Zool Soc Lond. 1938;24:103–185. [Google Scholar]
- Trapani J, Yamamoto Y, Stock DW. Ontogenetic transition from unicuspid to multicuspid oral dentition in a teleost fish: Astyanax mexicanus, the Mexican tetra (Ostariophysi: Characidae) Zool J Linn Soc. 2005;145:523–538. [Google Scholar]
- Van der heyden C, Huysseune A. Dynamics of tooth formation and replacement in the zebrafish (Danio rerio) (Teleostei, Cyprinidae) Dev Dyn. 2000;219:486–496. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1069>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Van der heyden C, Wautier K, Huysseune A. Tooth succession in the zebrafish (Danio rerio) Archs Oral Biol. 2001;46:1051–1058. doi: 10.1016/s0003-9969(01)00064-4. [DOI] [PubMed] [Google Scholar]
- Vandervennet E, Wautier K, Verheyen E, Huysseune A. From conical to spatulate: intra-and interspecific changes in tooth shape in closely related eretmodine cichlids (Teleostei; Cichlidae: Eretmodini) J Morphol. 2006;267:516–525. doi: 10.1002/jmor.10418. [DOI] [PubMed] [Google Scholar]
- Wautier K, Van der heyden C, Huysseune A. A quantitative analysis of pharyngeal tooth shape in the zebrafish (Danio rerio, Teleostei, Cyprinidae) Archs Oral Biol. 2001;46:67–75. doi: 10.1016/s0003-9969(00)00091-1. [DOI] [PubMed] [Google Scholar]
- Wautier K, Huysseune A, Verheyen E. Tooth shape differences analyzed by biometric and morphometric approaches: a case study on two morphologically very similar lacustrine cichlid species. Conn Tiss Res. 2002;43:103–108. doi: 10.1080/03008200290000826. [DOI] [PubMed] [Google Scholar]
- Witten PE. Enzyme histochemical characteristics of osteoblasts and mononucleated osteoclasts in a teleost fish with acellular bone (Oreochromis niloticus, Cichlidae) Cell Tissue Res. 1997;287:591–599. doi: 10.1007/s004410050782. [DOI] [PubMed] [Google Scholar]
- Witten PE, Hall BK. The salmon kype. How does it grow? What's its purpose? Does it disappear after spawning? Atl Salm J. 2001;50:36–39. [Google Scholar]
- Witten PE, Hall BK. Differentiation and growth of kype skeletal tissues in anadromous male Atlantic Salmon (Salmo salar) Int J Dev Biol. 2002;46:719–730. [PubMed] [Google Scholar]
- Witten PE, Hall BK. Seasonal changes in the lower jaw skeleton in male Atlantic salmon (Salmo salar L.): remodelling and regression of the kype after spawning. J Anat. 2003;203:435–450. doi: 10.1046/j.1469-7580.2003.00239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witten PE, Villwock W, Peters N, Hall BK. Bone resorption and bone remodeling in juvenile carp (Cyprinus carpio) J Appl Ichthyol. 2000;16:254–261. [Google Scholar]
- Witten PE, Rosenthal H, Hall BK. Mechanisms and consequences of the formation of a kype (hook) on the lower jaw of male Atlantic salmon (Salmo salar L.) Mitt hamb zool Mus Inst. 2004;101:149–156. [Google Scholar]
- Witten PE, Hall BK, Huysseune A. Are breeding teeth in Atlantic salmon a component of the drastic alterations of the oral facial skeleton? Archs oral Biol. 2005;50:213–217. doi: 10.1016/j.archoralbio.2004.10.018. [DOI] [PubMed] [Google Scholar]
- Zazzo A, Smith GR, Patterson WP, Dufour E. Life history reconstruction of modern and fossil sockeye salmon (Oncorhynchus nerka) by oxygen isotopic analysis of otoliths, vertebrae, and teeth: Implication for paleoenvironmental reconstructions. Earth Planet Sci Lett. 2006;249:200–215. [Google Scholar]