Abstract
The anatomy, histology and ultrastructure of the thymus of a dipnoan, the Australian lungfish, Neoceratodus forsteri, was studied by light and transmission electron microscopy. The thymic tissue showed clear demarcation into a cortex and medulla with ample vascularization. Large cells including foamy and giant multinucleated cells with periodic acid Schiff/Alcian blue positive staining properties were localized mainly in the medulla. The major cellular components were epithelial cells and lymphoid cells. The epithelial cells were classified by location and ultrastructure into six sub-populations: capsular cells, cortical and medullary reticular cells, perivascular endothelial cells, intermediate cells, nurse-like cells and Hassall-like corpuscles. Myoid cells were found mainly in the cortico-medullary boundary and medulla. Macrophages and secretory-like cells were also present. These findings will provide a base of knowledge about the cellular immune system of lungfish.
Keywords: anatomy, Australian lungfish, electron microscopy, Hassall's corpuscles, histology, myoid cells, Neoceratodus forsteri, thymus
Introduction
The thymus plays an important role in hosting and providing a suitable microenvironment for the development and production of functionally competent T lymphocytes. With its unique structure and function, the thymus has been a subject of intensive study for more than a century.
Phylogenetically, the first appearance of a true thymus is in elasmobranches where it is located dorsal and medial to the gill arches (Beard, 1903). Aganthans appear to be devoid of a thymus, although hagfish do have lymphoid infiltration within the pharyngeal velar muscle (Riviere et al. 1975) and larval lampreys in the pharyngeal epithelium (Page & Rowley, 1982; Ardavin & Zapata, 1988). The morphology of the thymus from a wide variety of jawed vertebrates has been studied, including elasmobranches (Zapata, 1980; Luer et al. 1995), teleosts (Zapata, 1981; Chilmonczyk, 1992; Romano et al. 1999a; Danilova et al. 2004; Bowden et al. 2005; Xie et al. 2006), amphibians (Sherill et al. 1972; Rafael, 1990), reptiles (Bockman & Winborn, 1967; Bianchi et al. 1990; Saad & Zapata, 1992), birds (Kendall, 1980; Fonfría et al. 1982) and mammals (Smith et al. 1958; Haelst, 1967; Bodey et al. 1987; Suster & Rosai, 1990).
A unique feature of the thymus is its cellular organization. Thymic epithelium shows sub-population heterogeneity in both the capsule and the thymic interstitial components, which in turn form the three-dimensional framework of the thymus. Thymic epithelial cell sub-populations are distinguished by their location and function, which can vary even between different groups of jawed vertebrates. For example, only one type of thymic epithelial cell has been recognized in teleosts, such as Rutilus rutilus (Zapata, 1981) and Stcyases sanguineus(Gorgollon, 1983), whereas by using immunohistochemistry, epithelial cells in the thymus of rainbow trout, Salmo gairdneri, were differentiated into seven sub-populations (Castillo et al. 1990). In rat thymus, De Waal & Rademakers (1997) described four broad classes of epithelial cells using immunohistochemistry, which they were able to further sub-divide into four sub-populations of cortical epithelial cells and six sub-populations of medullary epithelial cells based on ultrastructure. Functionally, this heterogeneity is believed to be vital for the maturation of T lymphocytes in the thymus (Chilmonczyk, 1992).
Another interesting cellular component of the thymus is the myoid cell, which exhibit striation characteristic of skeletal muscles. Since their first description (Mayer, 1888), myoid cells have been found to vary considerably due to cell turnover in the thymus in different vertebrates (Bockman & Winborn, 1967; Raviola & Raviola, 1967; Töró et al. 1969; Nakamura et al. 1986; reviewed in Saad & Zapata, 1992).
The only major group in which the thymus has not been extensively examined is sarcopterygian fish, including lungfish. Lungfish have an interesting phylogenic position. Increasingly, molecular evidence indicates that lungfish are the closest extant group of fish to tetrapods (Zardoya & Meyer, 1997; Joss, 1998). A recent molecular study utilized the DNA sequences of recombination activating genes, RAG 1 and RAG 2, from all three families of lungfish, including the Australian lungfish Neoceratodus forsteri, as phylogenic markers to elucidate the phylogeny of lungfish (Brinkmann et al. 2004). However, molecular information on the immune system of lungfish in general and on their thymus in particular is lacking. Our knowledge is limited to the structure of their immunoglobulins (Marchalonis, 1969; Ota et al. 2003; Ohta & Flajnik, 2006) and the fact that the thymus of the South American lungfish Lepidosiren paradoxa persists in adults (Mimura & Mimura, 1977). More recent studies have addressed the morphology of granulocytes in L. paradoxa (Bielek & Strauss, 1993) and white blood cells in N. forsteri (Ward, 1969; Hine et al. 1990a,b). The current study is the first detailed report addressing the anatomy, morphology and ultrastructure of the Australian lungfish thymus.
Materials and methods
Animals and tissue collection
Lungfish were raised in the Australian Lungfish Breeding Facility at Macquarie University, Sydney, Australia. Juveniles 2–4 years old (weights ranging from 250 to 300 g) were used to study the morphology, anatomy, histology and ultrastructure of the thymus. The fish were taken from large fibreglass holding tanks immediately prior to being anaesthetized in Clove oil (Sigma, St Louis, MO, USA) (1–3 mL in 5 L water for 7–10 min according to lungfish size) and euthenased by spinal severance (following approval by the Macquarie University Animal Ethics Committee, permit number 2006/015). The gill cavity was washed with tap water to remove any detritus and the thymus was immediately dissected. For light microscopy, samples of thymus were fixed in 0.1 m phosphate buffered 10% formalin and processed by standard histological procedures to obtain wax sections of 4–6 µm thickness. The sections were stained either with haematoxylin/eosin or with alcoholic periodic acid Schiff (PAS)/Alcian blue stain, counterstained with eosin. Control slides were treated with 100 units 1 mL−1 salivary amylase (Sigma) for 2 h and a further 24 h at 37 °C in 0.1 m phosphate buffer prior to PAS/Alcian blue staining.
For electron microscopy, thymic tissues were dissected and directly fixed in a combination of 4% paraformaldehyde, 3% gluteraldehyde and 0.3 msucrose in 0.1 mPIPES buffer (pH 7.2), followed by postfixation in 1% osmium tetroxide. Fixed tissues were dehydrated in a graded series of ethanol, infiltrated and subsequently embedded in LR-white resin. Semi-thin and ultra-thin sections were obtained using a Reichert Ultracut (Leica, Vienna, Austria) ultramicrotome. Semi-thin sections (1 µm thickness) were stained with methylene blue, whereas ultra-thin sections (50–60 nm thickness) were stained with saturated aqueous uranyl acetate and Reynold's lead citrate. Sections were examined using a Philips CM10 transmission electron microscope (Amsterdam, the Netherlands).
Results
Morphology and anatomy of the thymus
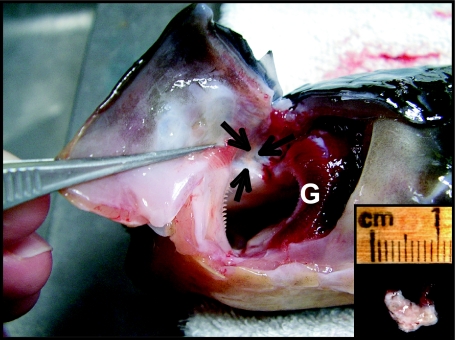
In juvenile N. forsteri, the thymus is a bilateral organ that lies anterior and dorsal to the most anterior gill arch. In some lungfish it extends ventrally to lie inside the opercular cartilage (inner thymus), delimited by connective tissue (Fig. 1). When freshly dissected, the thymus is a pale yellowish colour and measures 4–8 mm × 4 mm × 2 mm in lungfish weighing 250–300 g. The size varies between individual lungfish (3–12 mm × 3–5 mm × 1–3 mm) depending on fish length.
Fig. 1.
Lateral view of the branchial region of the Australian lungfish showing the anatomical dorsal location of the thymus (arrows) anterior to the most anterior gill arch (G). Insert shows a whole dissected gland.
Light microscopy
Thymus glands used in this study were sampled in different seasons. Notably, no histological differences were observed between any of these glands.
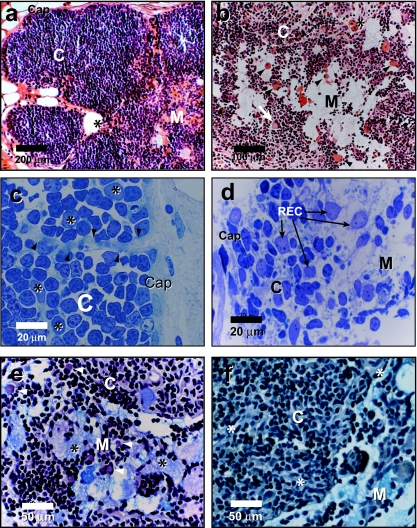
The thymus of N. forsteri is encapsulated within a single layer of epithelium delimited by connective tissue and partitioned into lobules that incompletely subdivide the thymus (Fig. 2a). Thymic tissue is tightly packed with lymphoid cells, which contain characteristically deeply stained nuclei and scant cytoplasm. The cellular components are demarcated into cortical and medullary areas, with thymocytes dense in the cortex and sparse in the medulla (Fig. 2a,b). Boundaries between the cortex and medulla are not sharply defined (Fig. 2b). The cortex is compartmentalized into lobules separated by trabeculae projecting from the capsular epithelium (Fig. 2c). Trabeculae may be composed of cords of epithelial cells, capillaries or fibrocytes. Cortical and medullary epithelial cells form a network with their cytoplasmic processes that increase the area of interaction with thymocytes and support the movement of lymphoid cells (Fig. 2d).
Fig. 2.
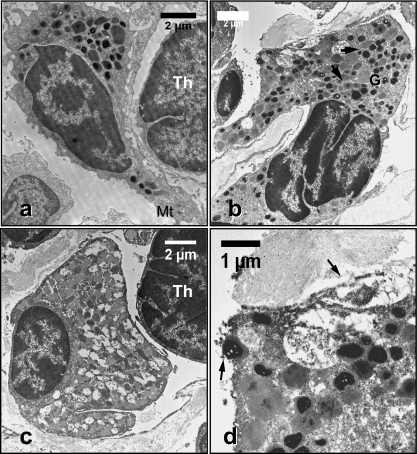
Histology of the thymus of N. forsteri. (a, b) Paraffin-embedded cross-sections of thymus stained with haematoxylin/eosin. The thymus is demarcated into a cortex (C) and medulla (M) and delimited by a capsule (Cap in a). Note the presence of myoid cells in the medulla, and the cortico-medullary boundary (arrow heads in b). Giant multinuclear cell is indicated by an arrow in b) and blood vessels by an asterix in (a) and (b). (c, d) LR white-embedded semi-thin sections of thymus stained with methylene blue showing trabeculae (arrows heads in c) originating from the capsule (Cap). Cortical reticular epithelial cells are indicated by an asterix. In (d), REC form a meshwork with their cytoplasmic processes in the cortex (C) and medulla (M). (e, f) Paraffin-embedded cross-section of thymus stained with PAS/Alcian blue. Note the Alcian blue positively stained material in the medulla (M) interstitium and the cytoplasm of foamy cells (* in e). Cells stained positively with PAS are indicated by arrowheads. (f) Tissue section treated with salivary amylase as a control. Cortex (C). Thymic blood vessels (*).
In contrast to the cortex, the medulla had relatively few cells of both the lymphoid and epithelial type (Fig. 2b,d). Numerous myoid cells were mostly located at the cortico-medullary boundary and in the medullary region (Fig. 2b). Haematoxylin/eosin-stained sections had large unstained medullary areas (Fig. 2a,b). However, adjacent sections showed the same area to stain positively for mucin with PAS/alcoholic Alcian blue. Alcian blue-positive mucin was found both within cells and in the inter-cellular matrix (Fig. 2e). Interestingly, mucin in the medulla varied in acidity as indicated by staining patterns (Fig. 2e). Large and foamy, round or irregularly shaped cystic epithelial cells and mucus cells were also found in the medulla. They had peripheral single nuclei or were multinucleated, and contained large vacuoles or granules filling their cytoplasm (Fig. 2e). These cells were positively stained with Alcian blue. Cells staining positively with PAS were also localized mainly in the medulla, but a few were present in the cortex (Fig. 2e). Analysis of serial sections which had been treated with salivary amylase revealed a negative PAS reaction in most of the cortical PAS-positive cells but not in others (Fig. 2f).
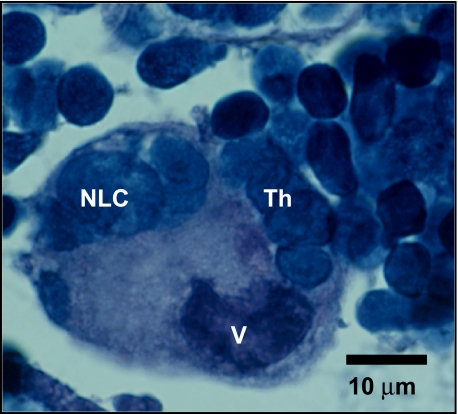
Numerous blood vessels penetrated the thymus (Fig. 2a,b,f), usually through trabeculae. They were separated from the thymic tissue by a single layer of epithelial cells. No lymph vessels were observed in the thymus. In both the cortex and medulla, cells including macrophages with varied cytoplasmic, granular and vacuolar contents were also observed. In light microscopy and semi-thin sections, thymic nurse cells were also observed containing numerous thymocytes with apparent vacuolar content (Fig. 3).
Fig. 3.
Paraffin-embedded cross-section of thymus cortico-medullary area stained with haematoxylin/eosin. Nurse-like cell (NLC) is indicated. Note the aggregation of thymocytes (Th) and the prominent vacuolar content (V).
Ultrastructure
Lymphoid cells were the predominant cellular component of N. forsteri thymus, varying in size from 4 to 9 µm (Fig. 4). Despite their size variation, these thymocytes share a characteristic feature of scant cytoplasm with a large nucleus, confirming the light microscope observation. The nuclei of thymocytes were darker (more electron dense) than the nuclei of other thymic cells. They were mostly non-lobed and roughly round in shape with heterochromatic chromatin, condensed and localized to the middle and inner side of the nuclear envelope. The nuclear envelope of some thymocytes showed indentations with some pores. Prominent nucleoli were also observed. The scant cytoplasm of thymocytes contained few mitochondria but abundant free ribosomes. Lymphoid cells with sizes exceeding 10 µm are described as lymphoblasts. Some are smaller than thymocytes when viewed under transmission electron microscopy (Fig. 5b). This discrepancy is due to the plane of the section. These cells, characterized by euchromatic or slightly heterochromatic nuclei, had a lower nuclear/cytoplasmic ratio than thymocytes. They also frequently had prominent nucleoli and were most commonly found in the cortex (Fig. 4a). They were characterized by irregularities in their cytoplasmic membranes and by their sparse endoplasmic reticulum and small electron-lucent vesicles (Fig. 4a). Frequently, both large and small thymocytes were observed to be in close contact with cytoplasmic processes from reticular epithelial cells (Fig. 4) and other cellular components of the thymus, such as myoid cells (Fig. 6) and macrophages (Fig. 8).
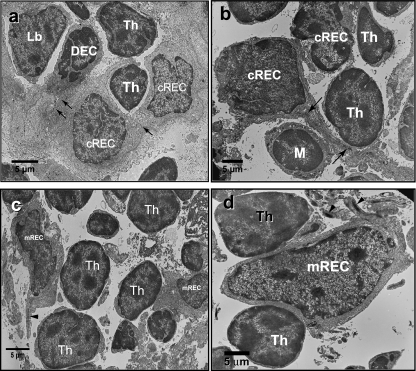
Fig. 4.
Electron micrographs of the thymus of N. forsteri. (a) The thymic cortex showing a cluster of cortical reticular epithelial cells (cREC) joined by desmosomes (arrows). Lymphoblast (Lb) and thymocytes (Th) can be observed in contact with cREC and dark epithelial cells (DEC). (b) Long bundles of tonofilaments in the cytoplasmic process of a cREC (arrows). Note the contact between the cREC and both a thymocyte (Th) and a macrophage (M). (c, d) mREC in contact with thymocytes (Th). Note the arrangement of tonofilaments in thick bundles in the cytoplasm of mREC.
Fig. 5.
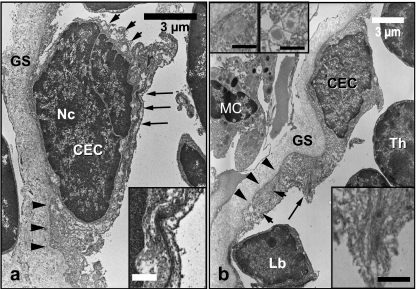
Electron micrographs of CEC. CEC are lying on the basal lamina membrane (arrowheads) and limited by ground substance material (GS). Bundles of tonofilaments (long arrows) are arranged along the cytoplasm membrane and both electron-lucent and centrally electron-dense vesicles (small arrows) can be seen. There is a prominent nucleolus (Nc) in the CEC in (a). A mast-like cell (MC), lymphoblast (Lb) and thymocyte (Th) can be seen in (b). Inserts to the lower right (a) and (b) show tonofilaments. Inserts to the upper left in (b) show vesicles. All insert scale bars = 1 µm.
Fig. 6.
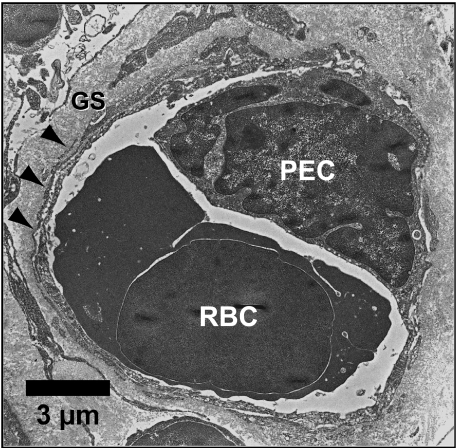
Electron micrograph showing cross-section of a blood capillary containing a red blood cell (RBC) next to a perivascular epithelial cell (PEC) surrounded by the basal lamina (arrowheads) and ground substance (GS).
Fig. 8.
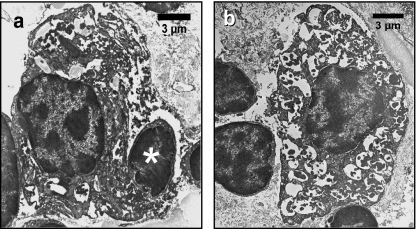
Two forms of macrophages found in N. forsteri thymus. (a) Activated macrophage in the cortico-medullary boundary with a necrotic lymphocyte (*) in the initial stage of degradation in the cytoplasm and extremely dilated cytoplasmic membranes. (b) Phagocytic cell in the medulla with vacuoles containing heterogeneous material.
Thymic epithelial cells were also characterized in the N. forsteri thymus based on their location and ultrastructure. Six main sub-populations of thymic epithelial cells were distinguished: capsular, cortical and medullary reticular epithelial cells, perivascular endothelial cells, intermediate epithelial cells, nurse-like cells and Hassall-like corpuscles.
Reticular epithelial cells (REC)
These cells were scattered throughout both the cortex and medulla forming a mesh with their dendrite-like cytoplasmic processes (Fig. 2d). These processes elongate to increase the contact area with lymphoid cells, which were frequently observed in the vicinity of REC. The REC varied in size and in their electron densities and will be referred to as pale and dark epithelial cells (Fig. 4a). Pale REC were observed in both the cortex and medulla. Cortical REC varied in size from 8 to 12 µm. They were irregular in shape and had long slender cytoplasmic processes that on occasion elongated to enclose thymocytes (Fig. 4a,b). A voluminous euchromatic nucleus was often located eccentrically. Dense chromatin was associated with the nuclear envelope. A prominent nucleolus was observed at the centre of the nucleus. The cytoplasm contained mitochondria that were variable in size. Short profiles of smooth endoplasmic reticulum were observed along with free ribosomes in the cytoplasm. Tonofilaments were prominent, measuring ≈ 12–25 nm in diameter and forming bundles of diameter ≈ 40–250 nm in the cytoplasmic processes (Fig. 4b). Both electron-lucent and floccular vacuoles with diameters ≈ 200–700 nm were also observed. Adjacent REC were connected by desmosomes most frequently in the cortex (Fig. 4a).
Dark REC were observed rarely and only in the cortex (Fig. 4a). In contrast to pale REC, dark REC did not have extended cytoplasmic processes. The nucleus was eccentric with high levels of electron-dense heterochromatic chromatin. Additionally, the mitochondria were distinctive with well developed tubular cristae. No vacuoles were observed in the cytoplasm.
Medullary REC were observed with both euchromatic and heterochromatic large voluminous irregular-shaped nuclei (Fig. 4c,d). The cytoplasm possessed few small mitochondria, a Golgi complex, short profiles of rough endoplasmic reticulum, tonofilaments and vesicles. The tonofilaments were abundant in the cytoplasm (≈ 12 nm in diameter) and formed distinctive keratinized bundles near to both the cytoplasmic membrane and the nuclear envelope (≈ 200–800 nm). Vesicles were variable in size (≈ 250–500 nm) and had electron lucent contents.
Capsular epithelial cells (CEC)
These single-layered epithelial cells were located in the outer area of the thymus and have spindle-like to ovoid shapes. CEC were delimited by a moderately dense basal lamina (≈ 100 nm in thickness) and covered with a wide band of amorphous ground substance (Fig. 5a,b). The nuclei of a CEC could be euchromatic or heterochromatic with condensed heterochromatin close to the nuclear envelope. The nucleolus was prominent. Their variably electron-dense cytoplasm had prominent tonofilaments (≈ 12 nm in diameter) arranged in long, keratinized bundles (≈ 40 nm in diameter) found along the cytoplasmic membrane and nuclear envelope with a length varying from 2.5–4 µm (Fig. 5 inserts). Furthermore, the cytoplasm contained well-developed rough endoplasmic reticulum, a few small mitochondria, abundant free ribosomes, vesicles with electron lucent content and vesicles with electron-moderate density arranged centrally (≈ 200–1000 nm in diameter) (Fig. 5b). CEC were connected to each other by desmosomes.
Perivascular endothelial cells (PEC)
These cells were observed as a single layer of cells that surrounded the thymic blood capillaries with their long cytoplasmic processes. They were bound by a basal lamina and ground amorphous substance which separated them from the thymic milieu by cytoplasmic extensions that penetrated the basal lamina and perivascular space (Fig. 6). PEC had low to median nuclear and cytoplasmic electron-density. The nucleus was irregular with some lobulation and was either hetero- or euchromatic with the chromatin condensing near the nuclear envelope. Contained within the cytoplasm of PEC were mitochondria of differing size, profiles of smooth endoplasmic reticulum and a few free ribosomes. Tonofilaments were not scattered throughout the cytoplasm but tended to form small bundles. Many electron lucent vacuoles (≈ 50–650 nm in diameter) were observed. Furthermore, granules with diameters ranging from 0.5–1.0 µm and contents of moderate electron density were observed.
Intermediate epithelial cells (IEC)
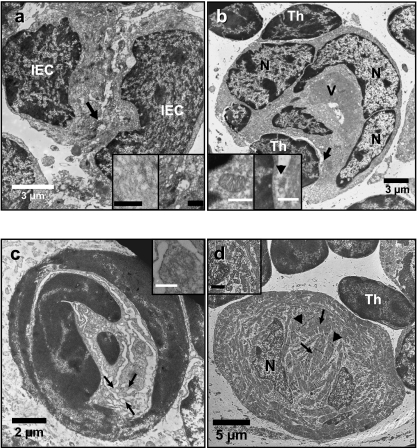
A population of epithelial cells with a medium-pale electron density and irregular euchromatic nuclei containing 1–2 prominent nucleoli was frequently observed in the cortex, but rarely in the medulla (Fig. 7a). These cells had the characteristic features of IEC, such as amorphous cytoplasm with prominent microtubules, small mitochondria, few free ribosomes scattered throughout the cytoplasm, short profiles of smooth endoplasmic reticulum, inconspicuous bundles of filaments adjacent to the cytoplasmic membrane and vacuoles with contents ranging from electron lucent to medium electron density. In contrast to REC, IEC tended to aggregate and form cell-cell desmosome junctions as evidenced by the observation of tonofilament aggregations near the cytoplasmic membranes of IEC adjacent cells (Fig. 7a inserts).
Fig. 7.
Electron micrographs of the thymus of N. forsteri. (a) Two intermediate epithelial cells (IEC) joined by a desmosome (arrow and magnified in the right insert) and containing filamentous material in the cytoplasm (left insert). Inserts scale bars = 0.5 µm. (b) A multinucleated (N) nurse-like cell internalizing a thymocyte (Th). Note the large vacuole in the cytoplasm (V). Right insert magnifies the area pointed by arrow clarifying one of the points of contact with the internalized thymocyte (arrowhead). Left insert shows well developed mitochondria in the cytoplasm of NLC. Inserts scale bars = 1 µm. (c) A Hassall-like corpuscle with dilated membranes (arrows) and degenerated mitochondria (insert). Insert scale bar = 0.5 µm. (d) A myoid cell in N. forsteri thymus. Note the irregular arrangement of myofibrils. Z-lines (arrowheads) and M-lines (arrows) are conspicuous. Clusters of rod-shaped mitochondria occur between myofibrils (insert). Inserts scale bar = 1 µm.
Thymic Nurse-like cells (NLC)
This type of epithelial cells with moderate to pale electron density was restricted to the cortico-medullary boundary of the thymus (Fig. 7b). Their size ranged between 23 and 35 µm. NLC contained thymocytes that were completely surrounded by the cytoplasm of the NLC. NLC had many large, irregular-shaped, euchromatic nuclei with their chromatin condensed around the nuclear envelope and a prominent nucleolus. The cytoplasm contained large rod-shaped mitochondria (≈ 1.0 µm in length and ≈ 0.5 µm in diameter) with well developed cristae of mixed lamellar and tubular arrangement (Fig. 7b, left inset). Short profiles of smooth endoplasmic reticulum were also observed. Ribosomes and filamentous cytoplasmic structures in NLC were scant. NLC contained a large and single vacuolar inclusion (≈ 12 µm in diameter) of moderately electron dense heterogeneous material that appeared to contain digested cytoplasmic organelles such as mitochondria (Fig. 7b). Interestingly, these cells had no cell-cell tight junctions (i.e. desmosomes). Areas of contact between NLC and internalized thymocytes were also observed (Fig. 7b, right insert).
Hassall-like corpuscles (HLC)
In the thymus of juvenile N. forsteri, structures resembling Hassall's corpuscles were observed in both the deep cortex and medulla (Fig. 7c). The size of these HLC was approximately 8–20 µm in diameter. Mostly they consisted of 2–3 epithelial cells with heterochromatic nuclei aggregated in a corpuscle arrangement. The cytoplasmic debris contained mitochondria and filamentous material, dilated membranes of endoplasmic reticulum and a Golgi complex. Interestingly, HLC were devoid of keratinized structures. The cell membrane and nuclear envelope appeared ‘hazy’ and was apparently undergoing degeneration.
Myoid cells
Myoid cells were large cells (≈ 30 µm) with apparent high metabolic activity as evidenced by their euchromatic nuclei and well developed, rod-shaped mitochondria (≈ 200 nm in diameter and > 1.5 µm in length) (Fig. 7d). Many free ribosomes were scattered in the cytoplasm. Myofibrils arranged haphazardly and many glycosomes (≈ 30 nm) scattered in between the myofibrils were the most prominent constituents of the cytoplasm. Electron diffuse vacuoles were found between the myofibrils. No cell-cell junctions were observed linking myoid cells to other cells in the N. forsteri thymus, although thymocytes were usually observed adjacent to myoid cells.
Macrophages
Macrophages ranging from 12–17 µm were scattered throughout both the cortex and medulla. The nucleus of this cell type was peripheral and often euchromatic with 1–3 prominent nucleoli. The chromatin was condensed toward the nuclear envelope. Two subpopulations of macrophages were detected: activated and non-activated. The cytoplasm of activated macrophages (Fig. 8a) contained extensive heterogeneous, apparently degenerating, material such as multivesicular inclusions, electron-dense bodies and even phagocytosed thymocytes. A well developed Golgi complex, large mitochondria, and dilated profiles of smooth endoplasmic reticulum adjacent to the nuclear envelope were also observed. Confirming the light microscopy observations, these cells were found in the medulla, and co-occurred with the large foamy cells that showed positive Alcian blue staining. Multinucleated giant cells with several peripherally arranged nuclei occurred in the same location (Fig. 2b). In contrast to activated macrophages, non-activated macrophages had microtubules and intermediate filaments in their cytoplasm (Fig. 8b). Moreover, vesicles containing floccular and median peripheral electron dense material were also present in these cells.
Secretory-like cells (SLC)
This population of cells had a distinct membrane-bound granular content with variable electron density. The nucleus was heterochromatic with chromatin condensed toward the nuclear envelope and there were prominent nucleoli. Depending on the granular size and content, two subpopulations of SLC were observed. The first subpopulation had mast cell properties with sizes varying between 7 to 10 µm (Fig. 9a,b). Their nuclei were large and could be oval, irregular or lobular in shape. The granules were variable in their size and electron density. Some granules were homogeneous and highly electron dense. They varied in size from 0.2–0.7 µm in diameter (Fig. 9a). Other granules were heterogeneous in that their content varied in electron density from moderate to high and sizes ranged from 0.3–1 µm in diameter (Fig. 9b). The moderately electron-dense granules are more electron dense in their centres. These cells also had some relatively large granules (≈ 1 µm in diameter) with a thread-like content. The cytoplasm of these SLC contained well developed mitochondria, a well developed Golgi complex, limited profiles of rough endoplasmic reticulum and many free ribosomes. Based on their similarities in granular content and cytoplasmic content, both cells in Fig. 9a and b may correspond to two different phases of maturity of the same cell lineage. The second sub-population of SLC had more than one type of granule (≈ 0.25–0.5 µm in diameter) with homogeneous contents varying from electron lucent to moderate electron density (Fig. 9c). Additionally, electron-lucent granules had floccular contents. Notably, granules of the same content tended to fuse and some of them could be observed adjacent to the cytoplasmic membrane (Fig. 9d). Distinct profiles of rough endoplasmic reticulum were also observed with many free ribosomes.
Fig. 9.
Electron micrographs showing sub-populations of secretory-like cells (SLC) in the N. forsteri thymus. (a) Mast-like cell with electron-dense granules. Mitochondria (Mt). (b) SLC with granules of moderate to lucent electron densities. Note the presence of granules with moderate electron density and electron-dense centres (arrows). Golgi complex (G). (c) SLC with heterogeneous granules. Thymocyte (Th). (d) Close-up of secretory granules (arrows) from (b).
Discussion
This study presents the first detailed observations on the anatomy, histology and ultrastructure of the thymus of the lungfish, N. forsteri. The thymus of N. forsteri shares the general features present in the thymus of all jawed vertebrates. In juvenile lungfish, the thymus is generally located superficially (sometimes more deeply) dorsolateral to the first gill arch. This anatomical location is similar to that described by many investigators in other fish species, both elasmobranch (Zapata, 1980; Luer et al. 1995) and teleost (Gorgollon, 1983; Chilmonczyk, 1992; Romano et al. 2000; Wyffels et al. 2005).
Thymus histology in N. forsteri does not appear to be affected by seasonal changes. Seasonal variations in thymic histology and function have been observed in gobiid fishes (Tamura & Homma, 1977) but not in trout (Tatner & Manning, 1983) or catfish (Ellsaesser et al. 1988).
The thymus of N. forsteri is lobulated, which again is a characteristic shared with many other fish such as the elasmobranches, Raja eglanteria, Raja clavata and Carcharhinus plumbeus (Zapata, 1980; Luer et al. 1995), and to a lesser extent in teleosts (Chilmonczyk, 1992). Lobulation, however, does appear to be a more general pattern for thymic structure in tetrapods, as it has also been found in reptiles (Saad & Zapata, 1992) and, with a more discrete pattern of lobulation, in birds (Kendall, 1980). The thymus of N. forsteri is also clearly demarcated into a cortex and medulla. Although this histological arrangement is not such a common pattern in teleosts (Chilmonczyk, 1992), it is commonly present in elasmobranches (Fange & Sundell, 1969; Zapata, 1980; Pulsford et al. 1984; Luer et al. 1995; Wyffels et al. 2005) and many tetrapods (Bockman & Winborn, 1967; Rafael, 1990; Suster & Rosai, 1990; Saad & Zapata, 1992; Schuurman et al. 1997).
In the thymus of N. forsteri, vascularization was observed in the parenchyma with cytoplasmic extensions penetrating the basal lamina, and a perivascular space separating the vessels from the thymic parenchyma. Bearman et al. (1975) have also reported the presence of similar perivascular spaces, and Weiss (1963) found similar cytoplasmic extensions through the basal lamina in the mouse thymus. Our observations indicate that the intra-vascular content is isolated from the thymic milieu by perivascular endothelial cells (PEC), as indicated by the continuity of PEC that surround the blood vessels. This suggests the presence of a thymic blood barrier in N. forsteri. However, a previous scanning electron microscope study on rat thymus found interruptions in the PEC basal lamina, suggesting that the thymic blood barrier may have some sort of permeability (Hwang et al. 1974). Furthermore, Chilmonczyk (1983) observed fenestrated endothelial cell cytoplasm and a discontinuous layer of epithelial cells surrounding the thymic capillaries of rainbow trout. By contrast, our transmission electron microscope observations on N. forsteri thymic vasculature found no interruptions in either the PEC or the basal lamina. Other studies which support a structurally complete thymic–blood barrier are those by Bearman et al. (1975) on human thymus, and numerous other studies on reptiles (reviewed by Saad & Zapata, 1992), the elasmobranch, Scyliorhinus canicula L. (Pulsford et al. 1984), and the teleosts R. rutilus (Zapata, 1981) and Siniperca chuatsi (Xie et al. 2006). Bearman et al. (1975) suggested that the discrepancy between the transmission and the scanning electron microscopy observations pertaining to a thymic–blood barrier could be due to the two-dimensional nature of transmission electron microscopy. Confirmation of this interpretation is provided by the continuity of thymic blood vessels in N. forsteri when transmission electron microscope images of different planes of thymic vessels are considered (data not shown).
As in all of the jawed vertebrates studied, the basic cellular component of N. forsteri was the epithelial cells that make the framework for the maturation of T lymphocytes, the latter being the other prominent constituent. The ultrastructural characteristics of thymocytes observed in N. forsteri, i.e. thin rim cytoplasm and few mitochondria, are similar to those observed in many other species (Murray et al. 1965; Bockman & Winborn, 1967; Kendall, 1980; Chilmonczyk, 1992). These similarities extend also to the lymphocytes observed in the blood of both the lungfish N. forsteri (Ward, 1969; Hine et al. 1990b) and Lepidosiren paradoxa (Ribeiro et al. 2007). Other thymic cellular components are macrophages, myoid cells and mast cells (Murray et al. 1965; Fange & Sundell, 1969; Gorgollon, 1983; Chilmonczyk, 1992). This framework is limited by the capsule from which trabeculae or septa penetrate into and compartmentalize the thymic parenchyma. Similar histological patterns have been described by many investigators for many different species (Kendall, 1981; Rafael, 1990; Chantanachookhin et al. 1991; Saad & Zapata, 1992).
Our findings indicate the presence of PAS and Alcian blue positively stained cells and material in the inter-cellular matrix predominantly in the medulla. The presence of this material suggests that these cells have a dynamic secretory function with the presence of glycosylated mucoid moieties in their secretions. These secretions could be mediators of a breakdown process taking place mainly in the medulla, as they are observed in association with foamy cells. These latter cells have been proposed to be phagocytic in other species (Loewenthal & Smith, 1952; Henery, 1966). Interestingly, the persistence of PAS positively stained material after digestion with salivary amylase has distinguished two types of epithelial cells in the thymic cortex by their different glycosylation pathways. The presence of cytoplasmic material stained by PAS/Alcian blue has been reported previously in the Loricariidean catfish, Harttiasp. (Savino & Santa-Rosa, 1982) and in rainbow trout, S. gairdneri(Chilmonczyk, 1983). Moreover, Loewenthal & Smith (1952) demonstrated an increase in the numbers of PAS positively stained large foamy cells in the mouse thymus as an indication of aging. Since our study looked only at juvenile lungfish, we cannot comment on any age-dependent feature, but we can say that in juvenile lungfish there is an acidic mucin moiety that is mainly restricted to the medulla.
The N. forsteri thymus showed the presence of a peri-epithelial ground substance surrounding the basal lamina of both CEC and PEC. Such ground substance surrounding PEC has been reported in human thymic vasculature (Bearman et al. 1975), rat thymus (Haelst, 1967) and in the teleost Siniperca chuatsi (Xie et al. 2006). The function of the peri-epithelial ground substance is still unknown. Kameya & Watanabe (1965) proposed that these structures may function as a barrier. Haelst (1967) has demonstrated that the ground substance present between rat thymic basal membranes contains collagen fibres. Moreover, Karttunen (1987) has shown that the thymic basement membranes themselves undergo dilation in patients with myasthenia gravis and the perivascular space was most prominent in young adults. The same investigator has shown that perivascular basement membranes are composed of laminin and type IV collagen. In addition to the PEC, the presence of such ground substance outside the basal lamina of CEC in the thymus of N. forsteri could play an important role in controlling the thymic microenvironment and the trafficking of thymocytes, as it does in lymph nodes (Fossum & Ford, 1985).
Ultrastructural analysis of epithelial cells in the thymus of N. forsteri revealed the presence of several subpopulations, based on their location, morphology and ultrastructure. There are general common features present in all thymic epithelial cell sub-populations, such as the irregularity and the lobulation of the nucleus, and the presence of tonofilaments. Although there is no universal nomenclature and classification of epithelial cell types in the thymus, many authors have observed the zonation of the thymus into cortex and medulla in many different species and have also reported the presence of epithelial sub-populations restricted to particular areas, including a layer of connective tissue or epithelium limiting the thymus [designated in this work as capsular epithelial cells (CEC)] and cortical and medullary reticular epithelial cells (cREC and mREC, respectively) (Bockman & Winborn, 1967; Kendall, 1980; Pulsford et al. 1984; Rafael, 1990; Saad & Zapata, 1992; Vicente et al. 1996; De Waal & Rademakers, 1997; Romano et al. 1999a). The morphology and ultrastructure of CEC in the lungfish thymus is similar to that in sharpsnout seabream, Diplodus puntazzo (Romano et al. 1999a), Cyprinus carpio (L.) (Romano et al. 1999b) and the rat (De Waal & Rademakers, 1997). Although the presence of tonofilament bundles in CEC is not a major observation in other species, the presence of distinct bundles of tonofilaments in the cytoplasm of CEC, cREC and mREC is a distinctive feature of these types of epithelial cells in the thymus of N. forsteri. The precise function of the tonofilaments is not clear, but their position in the cytoplasm suggests that they are mainly associated with tension resistance and maintaining the three-dimensional structure of epithelial cells (Bozzola & Russell, 1999). This, in turn, is important for maintaining the three-dimensional shape of the thymus and supporting maturation of the T lymphocytes.
Intermediate epithelial cells (IEC) were mainly localized in the cortex, whereas they have been reported by De Waal & Rademakers (1997) to be localized in both the cortex and medulla of rat thymus. IEC are believed to contribute mainly to the formation of thymic nurse cells (Kendall, 1991; Boyd et al. 1993), which in N. forsteri are restricted to the cortico-medullary border. Transitional forms of IEC that would mediate the formation of NLC have not been observed in the thymus of N. forsteri.
Thymic nurse cells (TNC) in general are multicellular complexes of epithelial and lymphoid cells in which the thymocytes are completely enclosed by vacuoles in the cytoplasm of the epithelial cells (Flano et al. 1996; Pezzano et al. 2001). Functionally, TNC are believed to provide an isolated micro-environment for thymocyte-positive selection (Romano et al. 1999a; Xie et al. 2006). Since their first description in 1980 (Wekerle et al. 1980), TNC have attracted the interest of many developmental immunologists and have now been observed in many species. In mammals they have a cortical localization (reviewed by De Waal & Rademakers, 1997), whereas they occur in the inner zone of the trout thymus (Flano et al. 1996) and are localized to the cortico-medullary boundary in sharpsnout seabream, D. puntazzo (Romano et al. 1999a). Ultrastructurally, and in agreement with our observation, Wekerle et al. (1980) showed the presence of multinucleated TNS in mice, and Flano et al. (1996) have shown the presence of different types of vacuolation in trout TNC. Our observation of both large vacuoles that contain cellular debris and the signs of lysis in internalized thymocytes suggests that the NLC observed in N. forsteri thymus could be sites of digestion and clearance of thymic, developmentally unwanted thymocytes. This is in agreement with the other observations that TNC may function in the clearance of apoptotic thymocytes to prevent accumulation of cellular debris (Aguilar et al. 1994; Hiramine et al. 1996; Pezzano et al. 2001).
Hassall's corpuscles have been observed previously in the thymus of tetrapods (Haelst, 1967; Kendall, 1980, 1981). Many investigators have proposed that they function to accumulate unwanted epithelial cells and thymocytes that have combined with Hassall's corpuscle-centric macrophages (Blau, 1967; Kendall, 1981; Fishelson, 2006). In the thymus of elasmobranches, Zapata (1980) reported the absence of Hassall's corpuscles in R. clavata and Torpedo marmorata. Moreover, Luer et al. (1995) observed structures resembling Hassall's corpuscles in sexually mature R. eglanteria and C. plumbeus. In the teleost thymus, Hassall's corpuscles are rarely found (reviewed in Gorgollon, 1983;Romano et al. 1999a). However, the presence of primitive structures resembling Hassall's corpuscles formed by apposition of two or more hypertrophied medullary epithelial cells has been observed in some teleosts (Hafter, 1952; Gorgollon, 1983). A recent report indicates a significant increase in numbers of Hassall's corpuscles in the thymus of cardinal fish (Apogonidae, Teleostei) as a result of environmental pollution (Fishelson, 2006). The same investigator suggests that they could be used as pathological biomarkers for environmental stress. In contrast, Saad & Zapata (1992) state that Hassall's corpuscles are lacking in the reptilian thymus and they explain their presence in lower vertebrates as clusters of medullary epithelial cells or modified epithelial cysts. When adding the latter observation to those for teleosts, the Hassall-like corpuscles (HLC) in N. forsterithymus could be explained as primitive or newly forming HLC. This is because the HLC we observed are small in size, devoid of keratinization and lack thymocytes and macrophages in their centres, unlike the human thymus where Hassall's corpuscles are large and composed of keratinized epithelial cells and thymocytes (Suster & Rosai, 1990). The presence of HLC could be related to age, so that more complex Hassall's corpuscles may occur in the thymus of older N. forsteri,which have not yet been examined.
Since their first description in frog thymus (Mayer, 1888), myoid cells have long been known to be widely distributed in the thymus of vertebrates (Raviola & Raviola, 1967; Sugimura, 1972; Kendall, 1980; Saad & Zapata, 1992; Bowden et al. 2005). The presence of myoid cells in the cortico-medullary border and the medullary region of the N. forsteri thymus supports the ontogenic and morphologic similarities between lower and higher vertebrates. Like elasmobranches (Zapata, 1980), myoid cells in N. forsteriare devoid of cell–cell junctions such as desmosomes. In N. forsteri, myoid cells seem to be present only in their mature morphology. Origins of myoid cells have been discussed in many previous reports. Their ontogeny could be intrathymic, as evidenced by desmosomes connecting immature myoid cells to REC in some species (Henery, 1966; Raviola & Raviola, 1967; Töró et al. 1969). On the other hand, thymic myoid cells might have a separate origin. A study using quail-chick chimeras has shown that the neural crest is the origin of myoid cells in birds (Nakamura et al. 1986). Morphological and ultrastructural observations on the thymic myoid cells of N. forsteri favours the latter proposal but it still needs to be supported by further studies addressing the ontogeny of thymic myoid cells. A number of myoid cells were observed to be in contact with thymocytes in the thymus of N. forsteri, which may indicate a role in thymocyte maturation. Their exact functional significance remains a matter of speculation given that they are neither connected to myoneural junctions nor possess direct neuromuscular contacts (Töró et al. 1969; Chilmonczyk, 1992).
It is well established that macrophages are a common component of the thymus in all vertebrates (Kendall, 1981; Pulsford et al. 1991; Chilmonczyk, 1992; Saad & Zapata, 1992). In addition to their regulatory roles they mainly function in eliminating incompetent, apoptotic cells and cellular debris in the thymic milieu (Pulsford et al. 1991). The activated population of macrophages observed in this study of the N. forsteri thymus seems to be correlated to lipid-laden foamy cells similar to those observed by Loewenthal & Smith (1952) in mouse thymus. Loewenthal & Smith (1952) showed that these cells react positively with PAS stain as do the macrophages in the lungfish thymus. Milićević et al. (1987) observed that the cytoplasmic inclusions of cortico-medullary macrophages contain polysaccharides, which would explain their PAS positivity. Martin (1981) on the other hand found them to contain abundant enzymes (Milićević & Milićević, 1985). Kendall (1981) proposed that the numbers of macrophages may be correlated with age-related involution of the thymus. Additionally, Milićević et al. (1987) suggested that the presence of dilated, vacuolar cytoplasmic inclusions, which in turn mimic accumulations of endogenous material, confirm that these cells could have a thymocyte regulatory role in addition to their phagocytic function. This is in agreement with our findings that the cytoplasm of activated macrophages possesses a markedly dilated Golgi complex and endoplasmic reticulum. Multinucleated giant cells observed in the N. forsteri thymus have also been observed in sole (teleost) thymus (Pulsford et al. 1991), the thymus of the amphibian, Bufo calamita (Barrutia et al. 1989), cultures derived from human thymic tissue and in age-related involuting thymuses (reviewed in Pulsford et al. 1991). It is well known that macrophages under the influence of certain culture conditions can differentiate into several types of cells including multinucleated giant cells (McInnes & Rennick, 1988). Peritoneal macrophages from N. forsteri(Hine et al. 1990b) and exudate macrophages from L. paradoxa (Ribeiro et al. 2007) have been reported to have many membrane projections, a large cytoplasm to nucleus ratio and vacuoles. This observation would indicate that the thymic macrophages in N. forsteri may have a role in the inflammation response.
The presence of mast cells and excretory-like cells is common in the vertebrate thymus and is mainly related to thymic endocrine function (Ledouarin & Jotereau, 1981; Chilmonczyk, 1983; Saad & Zapata, 1992). Moreover, results of a study on chicken embryonic thymus have shown that the thymus is a site of mast cell development with a medullary localization (Crivellato et al. 2005). Taken together, the observation of PAS-positive cells and the ultrastructural observation of secretory-like cells (Fig. 9d) strongly suggests the presence of an endocrine function for the N. forsterithymus. These secretory-like cells have not been identified frequently in teleosts (Zapata, 1981), although some authors observed vacuolated thymic epithelial cells or PAS-positive cytoplasm content in the teleost thymus (reviewed in Zapata, 1981; Savino & Santa-Rosa, 1982). The observation of different patterns of thymic secretory-like cells in N. forsteri supports the idea that these cells may represent different sub-populations with the possibility of different regulatory functions (Rafael, 1990).
The presence of HLC, multinuclear giant cells and foamy cells in the thymus of juvenile N. forsteri (2–4 year old lungfish) could be signs of the beginning of an age-related involution process. However, the domination of both connective and adipose tissue, usually associated with a decrease in lymphoid cell numbers in other fishes (reviewed in Sailendri & Muthukkaruppan, 1975; Chilmonczyk, 1992), was not observed in any of the lungfish used in this study. Nevertheless, lungfish are a long-lived species (mature at 15–25 years, with a life span that may exceed 100 years) and an older lungfish may show adipose tissue domination in its thymus. Thymus involution is phylogenically consistent (Manning, 1981). Usually the thymus grows rapidly in young animals to reach its maximum function and size during sexual maturation. After that, the thymus starts involuting slowly without completely disappearing (reviewed in Torroba & Zapata, 2003). In contrast, in some long-lived species, such as carp and some primitive sharks, the thymus does not appear to involute at all (Good et al. 1966). Since N. forsteri is a very long-lived species, it is likely that it could have an age-persistent thymus. This has been observed in the South American lungfish, L. paradoxa(Mimura & Mimura, 1977).
The above discussion of the thymus of the Australian lungfish, Neoceratodus forsteri, is aimed at providing a basic framework for further investigation. It will be important to make similar observations on older fish to resolve the question of age-related involution. It will also be important to determine when the thymus first starts to develop and to define its ontogenic trajectory. Following this work, it may be possible to determine the function of each of the cell types described here in order to better understand how the thymus of higher vertebrates has evolved.
Acknowledgments
The helpful suggestions of Nicole Vella from the microscopy unit in the Department of Biological Sciences/Macquarie University are highly appreciated. This work was supported by a Department of Biological Sciences/Macquarie University postgraduate scholarship.
References
- Aguilar LK, Aguilar-Cordova E, Cartwright J, Belmont JW., Jr Thymic nurse cells are sites of thymocyte apoptosis. J Immunol. 1994;152:2645–2651. [PubMed] [Google Scholar]
- Ardavin CF, Zapata A. The pharyngeal lymphoid tissue of lampreys. A morpho-functional equivalent of the vertebrate thymus? Thymus. 1988;11:59–65. [PubMed] [Google Scholar]
- Barrutia MG, Torroba M, Fernandez MJ, Vicente A, Zapata AG. Macrophages and epithelial cells of the thymus gland. An ultrastructural study in the natterjack, Bufo calamita. Tissue Cell. 1989;21:69–81. doi: 10.1016/0040-8166(89)90022-0. [DOI] [PubMed] [Google Scholar]
- Beard J. The origin and histogenesis of the thymus in Raja batis. Zool Jahrb Abt Anat Ontogenie Tiere. 1903;17:403–480. [Google Scholar]
- Bearman RM, Bensch KG, Levine GD. The normal human thymic vasculature: An ultrastructural study. Anat Rec. 1975;183:485–497. doi: 10.1002/ar.1091830402. [DOI] [PubMed] [Google Scholar]
- Bianchi F, Giannessi F, Dolfi A, Lupetti M. Lympho-epithelial interactions in the turtle Chrysemys scrypta elegans. Anat Rec. 1990;227:104–110. doi: 10.1002/ar.1092270112. [DOI] [PubMed] [Google Scholar]
- Bielek E, Strauss B. Ultrastructure of the granulocytes of the South American lungfish, Lepidosiren paradoxa: Morphogenesis and comparison to other leucocytes. J Morphol. 1993;218:29–41. doi: 10.1002/jmor.1052180103. [DOI] [PubMed] [Google Scholar]
- Blau JN. The dynamic behaviour of Hassall's corpuscles and the transport of particulate matter in the thymus of the guinea-pig. Immunology. 1967;13:281–292. [PMC free article] [PubMed] [Google Scholar]
- Bockman DE, Winborn WB. Electron microscopy of the thymus in two species of snakes, Crotalus atrox and Lampropeltis getulus. J Morphol. 1967;121:277–293. doi: 10.1002/jmor.1051210403. [DOI] [PubMed] [Google Scholar]
- Bodey B, Calvo W, Prummer O, Fliedner TM, Borysenko M. Development and histogenesis of the thymus in dog. A light and electron microscopical study. Dev Comp Immunol. 1987;11:227–238. doi: 10.1016/0145-305x(87)90023-1. [DOI] [PubMed] [Google Scholar]
- Bowden TJ, Cook P, Rombout JHWM. Development and function of the thymus in teleosts. Fish Shellfish Immunol. 2005;19:413–427. doi: 10.1016/j.fsi.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Boyd RL, Tucek CL, Godfrey DI, Izon DJ, Wilson TJ, Davidson NJ, et al. The thymic microenvironment. Immunol Today. 1993;14:445–459. doi: 10.1016/0167-5699(93)90248-J. [DOI] [PubMed] [Google Scholar]
- Bozzola JJ, Russell LD. Electron Microscopy: Principles and Techniques for Biologists. Sundbury, Massachusetts: Jones and Bartlett Publishers; 1999. [Google Scholar]
- Brinkmann H, Venkatesh B, Brenner S, Meyer A. Nuclear protein-coding genes support lungfish and not the coelacanth as the closest living relatives of land vertebrates. Proc Natl Acad Sci USA. 2004;101:4900–4905. doi: 10.1073/pnas.0400609101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castillo A, Razquin BE, Lopez-Fierro P, Alvarez F, Zapata A, Villena AJ. Enzyme-and immuno-histochemical study of the thymic stroma in the rainbow trout, Salmo gairdneri, Richardson. Thymus. 1990;15:153–166. [PubMed] [Google Scholar]
- Chantanachookhin C, Seikai T, Tanaka M. Comparative study of the ontogeny of the lymphoid organs in three species of marine fish. Aquaculture. 1991;99:143–150. [Google Scholar]
- Chilmonczyk S. The thymus of the rainbow trout (Salmo gairdneri) light and electron microscopic study. Dev Comp Immunol. 1983;7:59–68. doi: 10.1016/0145-305x(83)90055-1. [DOI] [PubMed] [Google Scholar]
- Chilmonczyk S. The thymus in fish: Development and possible function in the immune response. Ann Rev Fish Dis. 1992;2:181–200. [Google Scholar]
- Crivellato E, Nico B, Battistig M, Beltrami CA, Ribatti D. The thymus is a site of mast cell development in chicken embryos. Anat Embryol. 2005;V209:243–249. doi: 10.1007/s00429-004-0439-5. [DOI] [PubMed] [Google Scholar]
- Danilova N, Hohman VS, Sacher F, Ota T, Willett CE, Steiner LA. T cells and the thymus in developing zebrafish. Dev Comp Immunol. 2004;28:755–767. doi: 10.1016/j.dci.2003.12.003. [DOI] [PubMed] [Google Scholar]
- De Waal EJ, Rademakers LHPM. Heterogeneity of epithelial cells in the rat thymus. Microsc Res Tech. 1997;38:227–236. doi: 10.1002/(SICI)1097-0029(19970801)38:3<227::AID-JEMT4>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Ellsaesser CF, Bly JE, Clem LW. Phylogeny of lymphocyte heterogeneity: The thymus of the channel catfish. Dev Comp Immunol. 1988;12:787–799. doi: 10.1016/0145-305x(88)90053-5. [DOI] [PubMed] [Google Scholar]
- Fange R, Sundell G. Lymphomyeloid tissues, blood cells and plasma proteins in Chimaera monstrosa(Pisces, Holocephali) Acta Zoolog Stockholm. 1969;50:155–168. [Google Scholar]
- Fishelson L. Cytomorphological alterations of the thymus, spleen, head-kidney, and liver in cardinal fish (Apogonidae, Teleostei) as bioindicators of stress. J Morphol. 2006;267:57–69. doi: 10.1002/jmor.10385. [DOI] [PubMed] [Google Scholar]
- Flano E, Alvarez F, Lopez-Fierro P, Razquin BE, Villena AJ, Zapata AG. In vitro and in situ characterization of fish thymic nurse cells. Dev Immunol. 1996;5:17–24. doi: 10.1155/1996/14738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonfría J, Barrutia MG, Villena A, Zapata A. Ultrastructural study of interdigitating cells in the thymus of the spotless starling, Sturnus unicolor. Cell Tissue Res. 1982;225:687–691. doi: 10.1007/BF00214813. [DOI] [PubMed] [Google Scholar]
- Fossum S, Ford WL. The organization of cell populations within lymph nodes, their origins, life history and functional relationship. Histopathology. 1985;9:469–499. doi: 10.1111/j.1365-2559.1985.tb02830.x. [DOI] [PubMed] [Google Scholar]
- Good RA, Finstad J, Pollara J, Gabrielson AE. Morphologic studies on the evolution of the lymphoid tissues among the lower vertebrates. In: Smith RT, Miescher PA, Good RA, editors. Phylogeny of Immunity. Gainesville, FL: University of Florida Press; 1966. pp. 149–168. [Google Scholar]
- Gorgollon P. Fine structure of the thymus in the adult cling fish Sicyases sanguineus(Pisces, Gobiesocidae) J Morphol. 1983;177:25–40. doi: 10.1002/jmor.1051770103. [DOI] [PubMed] [Google Scholar]
- Haelst U. Light and electron microscopic study of the normal and pathological thymus of the rat. 1. The normal thymus. Cell Tissue Res. 1967;77:534–553. doi: 10.1007/BF00319347. [DOI] [PubMed] [Google Scholar]
- Hafter E. Histological age changes in the thymus of the teleost, Astyanax. J Morphol. 1952;90:555–581. [Google Scholar]
- Henery K. Mucin secretion and striated muscle in the human thymus. Lancet. 1966;i:183–185. doi: 10.1016/s0140-6736(66)90703-3. [DOI] [PubMed] [Google Scholar]
- Hine PM, Lester RJG, Wain JM. Observations on the blood of the Australian lungfish, neoceratodus-forsteri Klefft. 1. Ultrastructure of granulocytes, monocytes and thrombocytes. Aust J Zool. 1990a;38:145–154. [Google Scholar]
- Hine PM, Lester RJG, Wain JM. Observations on the blood of the Australian lungfish, Neoceratodus forsteriKlefft 2. Enzyme cytochemistry of blood-cells, peritoneal-macrophages and melanomacrophages. Aust J Zool. 1990b;38:145–154. [Google Scholar]
- Hiramine C, Nakagawa T, Miyauchi A, Hojo K. Thymic nurse cells as the site of thymocyte apoptosis and apoptotic cell clearance in the thymus of cyclophosphamide-treated mice. Lab Invest. 1996;75:185–201. [PubMed] [Google Scholar]
- Hwang W, Ho T, Luk S, Simon G. Ultrastructure of the rat thymus. A transmission, scanning electron microscope, and morphometric study. Lab Invest. 1974;31:473–487. [PubMed] [Google Scholar]
- Joss JMP. Are lungfish neotenic? Clin Exp Pharmacol Physiol. 1998;25:733–735. doi: 10.1111/j.1440-1681.1998.tb02286.x. [DOI] [PubMed] [Google Scholar]
- Kameya T, Watanabe Y. Electron microscopic observations on human thymus and thymoma. Acta Pathol Jpn. 1965;15:223–246. doi: 10.1111/j.1440-1827.1965.tb00005.x. [DOI] [PubMed] [Google Scholar]
- Karttunen T. Basement membrane proteins and reticulin in a normal thymus and the thymus in myasthenia gravis. Virchows Arch. 1987;V411:245–252. doi: 10.1007/BF00735030. [DOI] [PubMed] [Google Scholar]
- Kendall M. Functional anatomy of the thymic microenvironment. J Anat. 1991;177:1–29. [PMC free article] [PubMed] [Google Scholar]
- Kendall MD. Avian thymus glands: A review. Dev Comp Immunol. 1980;4:191–209. doi: 10.1016/s0145-305x(80)80023-1. [DOI] [PubMed] [Google Scholar]
- Kendall MD. The cells of the thymus. In: Kendall MD, editor. The Thymus Gland. London: Academic Press; 1981. pp. 63–83. [Google Scholar]
- Ledouarin NM, Jotereau FV. The ontogeny of the thymus. In: Kendall MD, editor. The Thymus Gland. London: Academic Press; 1981. pp. 37–62. [Google Scholar]
- Loewenthal LA, Smith C. Studies on the thymus of the mammal. IV. Lipid-laden foamy cells in the involuting thymus of the mouse. Anat Rec. 1952;112:1–15. doi: 10.1002/ar.1091120102. [DOI] [PubMed] [Google Scholar]
- Luer CA, Walsh CJ, Bodine AB, Wyffels JT, Scott TR. The elasmobranch thymus: Anatomical, histological, and preliminary functional characterization. J Exp Zool. 1995;273:342–354. [Google Scholar]
- Manning MJ. A comparative view of the thymus in vertebrates. In: Kendall MD, editor. The Thymus Gland. London: Academic Press; 1981. pp. 7–21. [Google Scholar]
- Marchalonis JJ. Isolation and characterization of immunoglobulin-like proteins of the Australian lungfish (Neoceratodus forsteri. Aust J Exp Biol Med Sci. 1969;47:405–419. doi: 10.1038/icb.1969.46. [DOI] [PubMed] [Google Scholar]
- Martin DWJ. Glycoproteins, proteoglycans and glycosaminoglycans. In: Martin DWJ, Mayes PA, Rodwell VW, editors. Harper's Review of Biochemistry. Los Altos: Lange Medical Publications; 1981. pp. 430–445. [Google Scholar]
- Mayer S. Zur Lehre von der Schilddruse und Thymus bei den Amphibien. Anat Anz. 1888;3:97–103. [Google Scholar]
- McInnes A, Rennick DM. Interleukin 4 induces cultured monocytes/macrophages to form giant multinucleated cells. J Exp Med. 1988;167:598–611. doi: 10.1084/jem.167.2.598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milićević NM, Milićević Z. Naphthol AS D chloroacetate esterase-positive macrophages in the cortico-medullary zone of the normal rat thymus. Virchows Arch (Cell Pathology) 1985;50:193–198. doi: 10.1007/BF02889901. [DOI] [PubMed] [Google Scholar]
- Milićević NM, Milićević Z, Colic M, Mujović S. Ultrastructural study of macrophages in the rat thymus, with special reference to the cortico-medullary zone. J Anat. 1987;150:89–98. [PMC free article] [PubMed] [Google Scholar]
- Mimura OM, Mimura I. Timo de Lepidosiren paradoxa (Fitz. 1836) Peixe Dipnoico. Bol Fisiol Anual Univ São Paulo. 1977;1:29–38. [Google Scholar]
- Murray RG, Murray A, Pizzo A. The fine structure of the thymocytes of young rats. Anat Rec. 1965;151:17–39. doi: 10.1002/ar.1091510104. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Nakano KE, Yasuda M. The ontogeny of thymic myoid cells in the chicken. Dev Growth Differ. 1986;28:185–190. doi: 10.1111/j.1440-169X.1986.00185.x. [DOI] [PubMed] [Google Scholar]
- Ohta Y, Flajnik M. IgD, like IgM, is a primordial immunoglobulin class perpetuated in most jawed vertebrates. PNAS. 2006;103:10723–10728. doi: 10.1073/pnas.0601407103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota T, Rast JP, Litman GW, Amemiya CT. Lineage-restricted retention of a primitive immunoglobulin heavy chain isotype within the Dipnoi reveals an evolutionary paradox. PNAS. 2003;100:2501–2506. doi: 10.1073/pnas.0538029100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page M, Rowley AF. A morphological study of pharyngeal lymphoid accumulation in larval lampreys. Dev Comp Immunol. 1982;2(Suppl):35–40. [Google Scholar]
- Pezzano M, Samms M, Martinez M, Guyden J. Questionable thymic nurse cell. Microbiol Mol Biol Rev. 2001;65:390–403. doi: 10.1128/MMBR.65.3.390-403.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pulsford A, Fange R, Zapata AG. The thymic microenvironment of the common sole, Solea solea. Acta Zool. 1991;72:209–216. [Google Scholar]
- Pulsford A, Morrow WJW, Fange R. Structural studies on the thymus of the dogfish, Scyliorhinus caniculaL. J Fish Biol. 1984;25:353–360. [Google Scholar]
- Rafael A. Thymus of Rana perezi: Presence of interdigitating cells. J Morphol. 1990;204:305–312. doi: 10.1002/jmor.1052040308. [DOI] [PubMed] [Google Scholar]
- Raviola E, Raviola G. Striated muscle cells in the thymus of reptiles and birds: An electron microscopic study. Am J Anat. 1967;121:623–645. doi: 10.1002/aja.1001210311. [DOI] [PubMed] [Google Scholar]
- Ribeiro MLDS, DaMatta RA, Diniz JAP, de Souza W, do Nascimento JLM, de Carvalho TMU. Blood and inflammatory cells of the lungfish Lepidosiren paradoxa. Fish Shellfish Immunol. 2007;23:178–187. doi: 10.1016/j.fsi.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Riviere HB, Cooper EL, Reddy AL, Hildemann WH. In search of the hagfish thymus. Amer Zool. 1975;15:39–49. [Google Scholar]
- Romano N, Ceccariglia S, Abelli L, Mazzini M, Mastrolia L. Lymphomyeloid organs of the Antarctic fish Trematomus nicolai and Chionodraco hamatus(Teleostei: Notothenioidea): a comparative histological study. Polar Biol. 2000;V23:321–328. [Google Scholar]
- Romano N, Fanelli M, Del Papa GM, Scapigliati G, Mastrolia L. Histological and cytological studies on the developing thymus of sharpsnout seabream, Diplodus puntazzo. J Anat. 1999a;194:39–50. doi: 10.1046/j.1469-7580.1999.19410039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano N, Taverne-Thiele AJ, Fanelli M, et al. Ontogeny of the thymus in a teleost fish, Cyprinus carpioL.: developing thymocytes in the epithelial microenvironment. Dev Comp Immunol. 1999b;23:123–137. doi: 10.1016/s0145-305x(98)00053-6. [DOI] [PubMed] [Google Scholar]
- Saad A, Zapata A. Reptilian thymus gland: an ultrastructural overview. Thymus. 1992;20:135–152. [PubMed] [Google Scholar]
- Sailendri K, Muthukkaruppan V. Morphology of lymphoid organs in a cichlid teleost, Tilapia mossambica(Peters) J Morphol. 1975;147:109–121. doi: 10.1002/jmor.1051470108. [DOI] [PubMed] [Google Scholar]
- Savino W, Santa-Rosa GL. The thymus gland in the loricariidean catfish Harttia sp. Dev Comp Immunol. 1982;6:375–380. doi: 10.1016/s0145-305x(82)80021-9. [DOI] [PubMed] [Google Scholar]
- Schuurman H-J, Kuper CF, Kendall MD. Thymic microenvironment at the light microscopic level. Microsc Res Tech. 1997;38:216–226. doi: 10.1002/(SICI)1097-0029(19970801)38:3<216::AID-JEMT3>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Sherill KC, Volpe EP, Ronald RC. Ultrastructure of the developing thymus of the leopard frog (Rana pipiens. Cell Tissue Res. 1972;V127:323–346. doi: 10.1007/BF00306877. [DOI] [PubMed] [Google Scholar]
- Smith C, Wharton TJ, Gerhardt AM. Studies on the thymus of the mammal. XI. Histochemical studies of thymus, spleen and lymph node in normal and irradiated mice. Anat Rec. 1958;131:369–387. doi: 10.1002/ar.1091310302. [DOI] [PubMed] [Google Scholar]
- Sugimura M. Myoid cells in the calf's thymus. Jpn J Vet Res. 1972;20:1–6. [PubMed] [Google Scholar]
- Suster S, Rosai J. Histology of the normal thymus. Am J Surg Pathol. 1990;14:284–303. doi: 10.1097/00000478-199003000-00010. [DOI] [PubMed] [Google Scholar]
- Tamura E, Homma Y. Histological changes in the organs and tissues of the gobiid fishes throughout their life-span. VIII–seasonal changes in the thymus of four species of gobies. Bull Jpn Soc Sci Fish. 1977;43:963–974. [Google Scholar]
- Tatner MF, Manning MJ. Growth of the lymphoid organs in rainbow trout Salmo gairdnerifrom 1 to 15 months of age. J Zool Lond. 1983;199:503–520. [Google Scholar]
- Töró I, Oláh I, Röhlich P, Virágh SZ. Electron microscopic observations on myoid cells of the frog's thymus. Anat Rec. 1969;165:329–341. doi: 10.1002/ar.1091650302. [DOI] [PubMed] [Google Scholar]
- Torroba M, Zapata AG. Aging of the vertebrate immune system. Microsc Res Tech. 2003;62:477–481. doi: 10.1002/jemt.10409. [DOI] [PubMed] [Google Scholar]
- Vicente A, Varas A, Sacedón R, Zapata AG. Histogenesis of the epithelial component of rat thymus: An ultrastructural and immunohistological analysis. Anat Rec. 1996;244:506–519. doi: 10.1002/(SICI)1097-0185(199604)244:4<506::AID-AR9>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Ward JW. Hematological studies on the Australian lungfish, Neoceratodus forsteri. Copeia. 1969;1969:633–635. [Google Scholar]
- Weiss L. Electron microscopic observations on the vascular barrier in the cortex of the thymus of the mouse. Anat Rec. 1963;145:413–437. doi: 10.1002/ar.1091450306. [DOI] [PubMed] [Google Scholar]
- Wekerle H, Ketelsen UP, Ernst M. Thymic nurse cells. Lymphoepithelial cell complexes in murine thymuses: morphological and serological characterization. J Exp Med. 1980;151:925–944. doi: 10.1084/jem.151.4.925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wyffels JT, Walsh CJ, Luer CA, Bodine AB. In vivo exposure of clearnose skates, Raja eglanteria, to ionizing X-radiation: acute effects on the thymus. Dev Comp Immunol. 2005;29:315–331. doi: 10.1016/j.dci.2004.08.005. [DOI] [PubMed] [Google Scholar]
- Xie HX, Nie P, Zhang YA, Sun BJ, Sun J, Yao WJ, et al. Histological and cytological studies on the developing thymus of mandarin fish Siniperca chuatsi(Perciformes: Teleostei) J Appl Ichthyol. 2006;22:125–131. [Google Scholar]
- Zapata A. Ultrastructure of elasmobranch lymphoid tissue. I. thymus and spleen. Dev Comp Immunol. 1980;4:459–472. doi: 10.1016/s0145-305x(80)80048-6. [DOI] [PubMed] [Google Scholar]
- Zapata A. Lymphoid organs of teleost fish. I. Ultrastructure of the thymus of Rutilus rutilus. Dev Comp Immunol. 1981;5:427–436. doi: 10.1016/s0145-305x(81)80055-9. [DOI] [PubMed] [Google Scholar]
- Zardoya R, Meyer A. Molecular phylogenetic information on the identity of the closest living relative(s) of land vertebrates. Naturwissenschaften. 1997;84:389–397. doi: 10.1007/s001140050415. [DOI] [PubMed] [Google Scholar]