Abstract
This paper reports on the development of an entirely new intestinal smooth muscle cell (ISMC) culture model using rat neonates for use in pharmacological research applications. Segments of the duodenum, jejunum and ileum were obtained from Sprague-Dawley rat neonates. The cell extraction technique consisted of ligating both ends of the intestine and incubating (37 ºC) in 0.25% trypsin for periods of 30–90 min. Isolated cells were suspended in DMEM-HEPES, plated and allowed to proliferate for 7 days. Cell culture quality was assessed via a series of viability tests using the dye exclusion assay. In separate experiments, tissues were exposed to trypsin for varying durations and subsequently histological procedures were applied. Cell purification techniques included differential adhesion technique for minimizing fibroblasts. Selective treatments with neurotoxin scorpion venom (30 µg mL−1) and anti-mitotic cytosine arabinoside (6 µm) were also applied to purify respectively ISMC and myenteric neurones selectively. The different cell populations were identified in regard to morphology and growth characteristics via immunocytochemistry using antibodies to smooth muscle α-actin, α-actinin and serotonin-5HT3 receptors. Based on both viability and cell confluence experiments, results demonstrated that intestinal cells were best obtained from segments of the ileum dissociated in trypsin for 30 min. This provided the optimum parameters to yield highly viable cells and confluent cultures. The finding was further supported by histological studies demonstrating that an optimum incubation time of 30 min is required to isolate viable cells from the muscularis externae layer. When cell cultures were treated with cytosine arabinoside, the non-neuronal cells were abolished, resulting in the proliferation of cell bodies and extended neurites. Conversely, cultures treated with scorpion venom resulted in complete abolition of neurones and proliferation of increasing numbers of ISMC, which were spindle-shaped and uniform throughout the culture. When characterized by immunocytochemistry, neurones were stained with antibody to 5HT3 receptors but not with antibodies to α-smooth muscle actin and α-actinin. Conversely, ISMC were stained with antibodies to α-smooth muscle actin and α-actinin but not with antibody to 5HT3 receptors. The present study provides evidence that our method of dissociation and selectively purifying different cell populations will allow for pharmacological investigation of each cell type on different or defined mixtures of different cell types.
Keywords: cell culture, intestinal smooth muscle cells, neuronal cells, rat neonates
Introduction
Studies of stimulus-contraction coupling of the smooth muscle cells have been largely performed on intact tissue. Although considerable information about the function of these tissues has been reported, results obtained from the intact tissue normally emerge from the complex network of organized smooth muscle cells into a multicellular unit rather than from the individual properties of the contractile cells themselves.
Thus, cell culture has become a valuable technique for studies of physiological properties of muscle cells (Lieberman et al. 1987). When specific cells are extracted from primary tissues and cultured, cells usually propagate, expand and divide into replicates assuming a uniform constitution (Freshney, 2005). Experiments in the cultivation of different cell populations from the small intestine, however, have proven difficult. For intestinal smooth muscle cells (ISMC) this is primarily related to the loss in phenotypic characteristics (Thyberg et al. 1985). Muscle cells are highly differentiated, which is evident from the large proportion of contractile filaments within the cell, and thus must undergo a process referred as ‘phenotypic modulation’ for mitosis and an increase in the cell number to occur (Chamley-Campbell et al. 1979; Owens, 1989). There is evidence that such a proliferation process leads to a decrease in the number of contractile proteins and hence change in the phenotypic properties of cells, resulting in a loss of the contractile response (Chamley-Campbell et al. 1979).
Furthermore, the complex layered nature of the gastrointestinal tract makes it difficult to acquire pure preparations of individual cell types. These difficulties are further compounded by the limited availability of adult animal species from which successful ISMC cultures can be established (Bitar & Makahlouf, 1986; Benham et al. 1986; Oishi et al. 2000). The increasing difficulty in obtaining viable proliferating cells with increasing age is mainly due to the onset of differentiation, the increase in fibrous connective tissue and the decrease in undifferentiated proliferating cells (Freshney, 2005). Conversely, embryonic or neonatal tissue disperses more readily and gives a higher number of proliferating cells compared to adult tissue (Freshney, 2005). Moreover, it has been reported that the phenotype of cultured neonatal smooth muscle cells is very stable in terms of its contractile profile (Yamashita et al. 1994) and less damaged by the dissociation process than is that of ISMC obtained from adult tissue (Chlopcikova et al. 2001).
However, when dealing with neonatal tissue, techniques for cellular dissociation require the support of advanced optical equipment because of the small size of the tissues (Smirnov et al. 1992; Frings et al. 2000). Although enzymatic and mechanical techniques of disaggregation usually give better yields in neonatal tissue due to its softness compared with adult tissue (Schaffer et al. 1997), this can be disadvantageous as cell cultures grown with this technique are more representative of the whole tissue (Freshney, 2005). Nevertheless, such limitations in the disaggregation of cells from neonatal tissue can be minimized if methods are employed to monitor the gradual dissociation from a specific region of the intestine. Furthermore, monitoring of morphological features of cultures combined with cell sorting through differential adhesion (Glazier & Graner, 1993) or selective treatments of ISMC (Blennerhassett & Lourenssen, 2000) and myenteric neurones (Blennerhassett & Lourenssen, 2000) may provide the parameters necessary for acquisition of highly pure cell preparations from neonatal tissue.
Although many of these initial concerns have been largely overcome, it remains a challenge to develop a cell culture model consisting entirely of a single cell type that can be used subsequently as a reliable tool for studies of basic pharmacological properties of the cells. The aim of this study is to develop a cell culture model to establish and characterize three main cell populations from the intestine using a combination of differing enzymatic dissociation times, cell viability testing and histological techniques to determine the optimum parameters for cell dissociation of different cell types from primary tissue. This study also aims at developing selective purification techniques to obtain specific cell populations from different intestinal layers and to characterize fully the individual cell types via immunocytochemistry studies.
Materials and methods
Cell culture technique
Cells were isolated from the small intestine of 1–4-day-old Sprague-Dawley rat neonates. The animals were killed by cervical dislocation of the neck. Segments of the duodenum, jejunum and ileum were removed and ligated at both ends using cotton thread. These segments were exposed to 0.25% porcine trypsin and 0.1% EDTA at varying dissociation times of 30, 45, 60, 75 and 90 min at 37 °C. After trypsinization a cell suspension was collected and centrifuged at 450 g for 5 min. The supernatant was discarded, leaving a pellet of cells that were then suspended in DMEM-HEPES supplemented with 10% fetal calf serum (FCS); 100 U mL−1 penicillin and 100 µg mL−1 streptomycin solution; 2 mm l-glutamine; 2.5% rat serum (RS); 0.2% amphotericin B. Cells were cultivated in 25 cm2 cell culture flasks at 37 °C and the medium was changed at 48-h intervals.
Cell confluence and cell viability experiments
Preliminary experiments were designed to investigate the main cell culture parameters (dissociation time, source of the tissue and animal age) required to optimize the cell culture techniques, and thus allow the collection of the maximum number of viable cells from the primary tissue. To examined this, cells were isolated from the different intestinal regions (duodenum, jejunum and ileum, n = 16), and the optimal dissociation time per intestinal region was determined by experiments involving collection of cells after trypsinization for 30, 45, 60, 75 and 90 min (n = 4). This later experiment was subsequently repeated for tissue derived from animals that were 1, 2, 3 and 4 days old (n = 4).
For each preparation, isolated cells were monitored for cell growth, and cultures were assessed for their ability to reach confluence within a 7-day period. In this study a cell culture was considered confluent when the entire cell culture surface was completely covered by well spread cells. This was assessed qualitatively. To assess the optimal dissociation technique quantitatively, the percentage of viable cells for each of these preparations was also measured by the trypan blue exclusion assay. Optimal parameters for cell culture were subsequently determined on the basis of which dissociation time, intestinal region and neonatal age range yielded the largest number of viable cells and cell cultures.
Histology
Segments of the duodenum, jejunum and ileum were incubated in 0.25% trypsin for 15, 30, 45, 60, 75 and 90 min. Segments were rinsed in Hanks’ balanced salt solution and fixed in 10% buffered formaldehyde (Sigma, St Louis, MO, USA) and left overnight. Preparations were then processed by dehydration in serial alcohols (70%, 80%, 90% each for 3 h and in 100% three times each for 2 h). This was followed by impregnation in Histoclear (Agar Scientific, Essex, UK) I (1 h) and Histoclear II and III (for 2 h each). The tissues were infiltrated by pure wax (two changes of wax, each for 2 h) and then embedded in fresh wax. Blocks were labelled and stored at 4 °C. For sectioning, wax blocks were removed individually from the refrigerator and immediately attached to a pre-cooled microtome. Sections were cut at a thickness of 5–7 µm; these were floated in a preheated water-bath (30 °C) and picked up singly on slides pre-coated with poly-l-lysine (Menzel-Glaser, Braunschweig, Germany). The slides were left to air dry at room temperature until stained.
After deparaffination (two changes of Histoclear each for 5 min) and hydration in descending grades of alcohols (two changes of 100%, 70%, and tap water each for 5 min), slides were stained with Herovici's picropolychrome (Herovici, 1963). Tissue sections were stained progressively from the nucleus (black) by incubation for 5 min each in two solutions, A and B. Solution A consisted of 0.25 g celestine blue, 2.5 g iron alum, dissolved and boiled for 3 min in 50 mL of distilled water. Subsequent to this, solution A was supplemented with 10 mL cold glycerol. Solution B consisted of 100 mL 5% aqueous solution of aluminium sulphate heated to boiling point, to which a further 50 mL of 1% of alcoholic solution of haematoxylin was added over 5 min and the resultant solution boiled for a further 3 min. After cooling, 100 mL of distilled water, 10 mL 4% aqueous FeCl3 and 1 mL HCl was added. Subsequently, the cytoplasmic stain (yellow-green) was performed by incubation of the sections for 2 min in solution C, where solution C consisted of a mixture of 0.25 g metanil yellow, 60 mL distilled water, and 5 drops acetic acid. This was followed by incubation for 2 min in solution D (mixture of 60 mL distilled water and 5 drops of acetic acid) and a further 2 min incubation in solution E (a mixture of 60 mL distilled water and 2 drops of saturated aqueous LiCO3). The sections were finally stained with connective tissue stain (red and blue) by incubation for 2 min in solution F (a mixture of a solution 1 : 0.05 g methyl blue in 50 mL distilled water and solution 2 : 0.1 g of acid fuchsin in 50 mL of saturated aqueous picric acid). After mixing solution 1 and solution 2, the resultant solution was supplemented with 10 mL of glycerol and 0.5 mL of saturated aqueous LiCO3. Between applications of solutions A–F the sections were washed for 2 min in running water. After the final incubation in solution F the sections were incubated for a further 2 min in 1% acetic acid.
Lastly, tissue sections were dehydrated in serial alcohol solutions (1 min each), incubated in Histoclear for 2 min each, mounted on glass slides using Histomount (Agar Scientific) and examined using a Nikon Eclipse 80i bright field microscope.
Isolation of intestinal smooth muscle cells and myenteric neurones
Following 30 min trypsinization, viable cells were successfully isolated from the primary tissue, plated onto plastic cell culture flasks and the medium changed after 48 h. These cultures consisted predominantly of ISMC, neuronal cells and fibroblasts.
In subsequent experiments these cells were purified through a differential adhesion technique, which minimized the number of fibroblasts from the culture. This was obtained by plating the suspension of mixed population of cells into plastic cell culture flasks and incubation for 30 min at 37 °C. During this time, fibroblast-type cells adhered to the surface (Yaffe, 1968). The remaining non-adherent cells were removed, plated onto a second cell culture flask and monitored until reaching confluence. Cell preparations consisted mainly of ISMC and neuronal cells and were called ‘cocultures’.
In some cultures, neurones were purified through selective removal of non-neuronal cells by pretreatment with antimitotic cytosine arabinoside (6 µm) following 24 h plating. Treatment with cytosine arabinoside containing media was carried out for 3 days; this was then replaced with normal medium and neuronal cells were allowed to proliferate for another 4 days.
ISMC purification was obtained primarily through the differential adhesion technique for 30 min to minimize the number of fibroblasts in culture. The remaining non-adherent cells were removed with the medium into a second flask and differential adhesion was applied for 5 h. Subsequently, the medium was aspirated, replaced by fresh medium and cells were allowed to proliferate for 24 h. Cells in culture were then treated with scorpion venom Maurus palmatus (30 µg mL−1) containing media for 24 h before being replaced with normal media. The cell population now comprised mostly intestinal smooth muscle cells, which were allowed to proliferate and reach confluence. Cells were then passaged and plated at a 1 : 2 dilution and allowed to proliferate for 24 h before being exposed again to a second treatment with scorpion venom Maurus palmatus (30 µg mL−1). Following this treatment, ISMC were uniform throughout the culture with no apparent neurones.
Immunocytochemistry
At confluence, cocultures and purified cell populations of neurones and ISMC were grown on glass coverslips overnight, fixed in 2% formaldehyde for 5 min and kept in phosphate buffered saline (PBS) until used for immunostaining experiments.
Dual-label immunocytochemistry with α-smooth muscle actin and 5HT3 receptor antibodies was carried out to verify the nature of cells growing in the cocultures and moreover to confirm the purity of newly attained ISMC and myenteric neurones in culture. Briefly, cells were made permeable by incubation in PBS containing 0.1% Triton X-100 for 15 min. Slides were then bathed in PBS containing 2% goat serum for 30 min before incubation for 60 min with α-smooth muscle actin [1 : 100 in PBS–1% bovine serum albumin (BSA)] at room temperature. This followed incubation with TRITC-labeled secondary antibody (1 : 30 in PBS–1% BSA) for 60 min at room temperature. Slides were subsequently incubated with the neuronal marker 5HT3 receptor (5 µg mL−1) antibody for 120 min at room temperature followed by incubation with fluorescein isothiocyanate (FITC)-labeled secondary antibody (1 : 80 in PBS–1% BSA) for 60 min at room temperature. Incubations were preceded by three washes with PBS.
Antibodies to α-actinin were also used to confirm the nature of the purified ISMC in culture. Slides were incubated with anti α-actinin antibodies (1 : 400 PBS–1% BSA) for 60 min at room temperature followed by incubation in anti-mouse secondary antibody labeled with Alexa Fluor (1 : 400 PBS–1% BSA) for 60 min at room temperature. Both incubations were preceded by three washes with PBS.
Negative controls were carried out in which primary antibodies were replaced with PBS. Preparations were mounted using DAPI (4′,6′-diamidino-2-phenylindole). DAPI is known to form fluorescent complexes with natural double-stranded DNA and is therefore useful for marking the cell nucleus (Sulston et al. 1983). Stained samples were observed with a Nikon Eclipse 80i fluorescence microscope, and images were digitally captured with sop (act-2u) software. Digital recombination of images using adobe photoshop was applied for the dual labelling of neurones and ISMC.
Compounds
Tissue culture reagents, trypsin-EDTA, formaldehyde, Triton X-100, BSA, antibodies, DAPI, cytosine arabinoside, scorpion venom, glycerol, aluminium sulphate, haematoxylin, HCl, acetic acid, celestine blue, metanil yellow, methyl blue, picric acid, and acid fuchsin were purchased from Sigma. Aqueous FeCl3, aqueous LiCO3, and iron alum were purchased from Alfa Aesar (Karlsruhe, Germany). Histoclear and Histomount were purchased from Agar Scientific. Poly-l-lysine was obtained from Menzel-Glaser. Glass coverslips and microscope slides were obtained from ULTIMA (Packard Ltd, Illinois, USA).
Statistical analysis
Comparative results were given as means ± SE. The significance of the differences was tested by one-way anova followed by the Bonferroni adjustment test.
Results
Enzyme treatment and dissociation
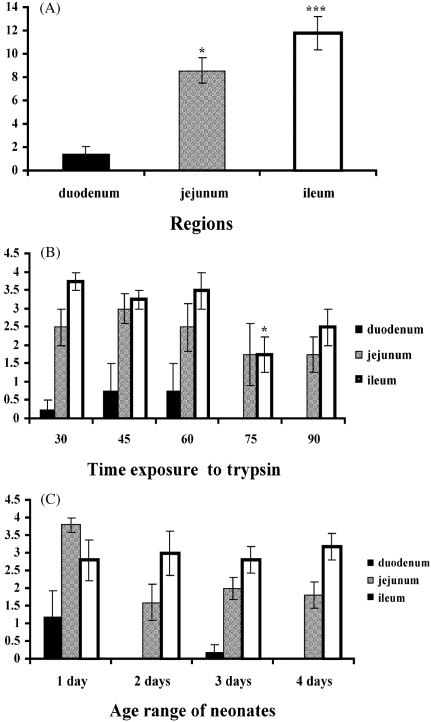
Intestinal cell preparations harvested from segments of both the jejunum and the ileum yielded significantly (P < 0.05 and P < 0.001, respectively) higher numbers of confluent cultures compared with the duodenum (Fig. 1A). Lower trypsinization times of 30, 45 and 60 min yielded higher numbers of confluent cultures in segments of the duodenum, jejunum and ileum compared to longer trypsinization times of 75 and 90 min (Fig. 1B).
Fig. 1.
Intestinal cell cultures obtained from the duodenum, jejunum and ileum reaching confluence (A). Intestinal cell cultures harvested at varying trypsin dissociation times taken from different intestinal regions reaching confluence (B). Intestinal cell cultures taken from different age range of neonates reaching confluence (C). Each point represents the mean ± SE; n = 16 (A) n = 4 (B,C) *P < 0.05, **P < 0.01 and ***P < 0.001 taken as significant differences in the group.
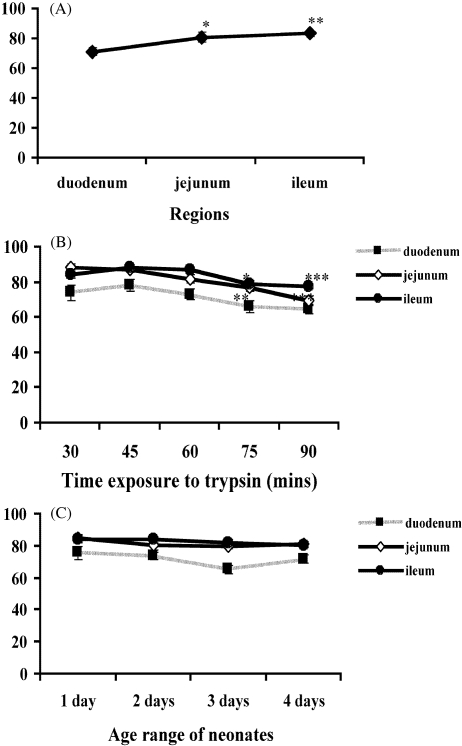
The cell viability assay also demonstrated that segments from both the jejunum and the ileum yielded a significantly (P < 0.05 and P < 0.01, respectively) higher percentage of viable cells compared with the duodenum (Fig. 2A). Similarly, shorter trypsin incubation times of 30, 45 and 60 min resulted in a higher percentage of viable cells (Fig. 2B). Further dissociations at 75 and 90 min led to significant (P < 0.05 and P < 0.001, respectively) reduction in the percentage of viable cells from the jejunum, and significant (P = 0.001 and P < 0.001, respectively) reduction in viability of cells harvested from the ileum (Fig. 2B).
Fig. 2.
Percentage of viable cells obtained from the duodenum, jejunum, and ileum (A). Percentage of viable cells harvested at varying trypsin dissociation times taken from different intestinal regions (B). Percentage of viable cells taken from different age range of neonates (C). Each point represent the mean ± SE; n = 16 (A,B) n = 4 (C). *P < 0.05, **P < 0.01 and ***P < 0.001 taken as significant differences in the group.
However, no significant differences in cell viability or number of cultures reaching confluence were observed in relation to the age range of neonates (Figs 1C and 2C).
Results obtained for the different intestinal regions, the varying trypsin dissociation times and age range of neonates, clearly demonstrate that extraction of cells from the ileum gives better yield and higher viability than did cell preparations from the jejunum and the duodenum.
Histology
Histology was used to identify the regions of the intestine from which cells were being harvested in relation to the different trypsinization times (Fig. 3A). Following 30 min exposure to trypsin, cells from the serosa and part of the muscularis externae were extracted (Fig. 3C). Trypsinization for 45 min enabled harvesting of cells from the entire muscularis externae (Fig. 3d). Further trypsinization led to complete removal of the submucosal layers (Fig. 3E–G).
Fig. 3.
(A) Section from intact jejunum and the distribution of the respective layer following Herovici's staining. (1) serosa, (2) muscularis externae [longitudinal smooth muscle cells (SMC)], (3) myenteric plexus, (4) muscularis externae (circular SMC), (5) submucosa, (6) villi. Section from the jejunum following incubation with the dissociation enzyme trypsin for 15 min (B), 30 min (C), 45 min (D), 60 min (E), 75 min (F) and 90 min (G). Scale bar = 100 µm.
Results obtained from cell viability and preliminary cell culture experiments in correlation with those from histological studies suggest that trypsinization for 30 min provides the optimum parameters for harvesting of sufficient numbers of viable ISMC and neurones.
Differential adhesion – morphological description of cell types
Morphologically, cell populations isolated from the neonatal intestine consisted of ISMC, neurones and fibroblasts. Thus, techniques for purifying cells were adopted to separate different cell types and yield pure cultures of individual cell types.
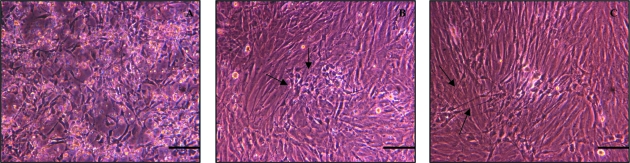
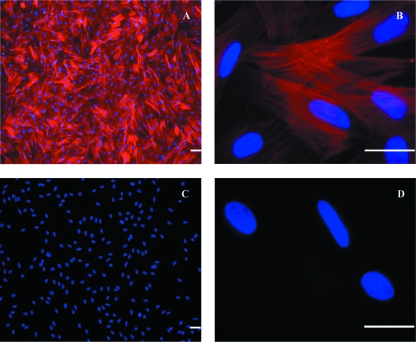
The differential adhesion technique was primarily applied to minimize the number of fibroblasts in culture. Cell attachment times were determined via time-lapse video microscopy, in which a frame was captured every 15 min. Monitoring of these cultures showed that fibroblasts attached first to the surface (after approximately 30 min), followed by smooth muscle cells (1–5 h); lastly, a major number of clusters of neurones started adhering after 5 h. The differential adhesion technique for depletion of fibroblasts resulted in cocultures of ISMC and neurones with uniform distribution of myenteric neurones among the ISMC (Fig. 4A–C).
Fig. 4.
Coculture of intestinal preparations at day 1 showing ISMC-like and rounded clusters of neurones (A). By day 2, ISMC developed into relatively uniform layer throughout the culture with intervening areas of characteristically developed myenteric neurones (arrows) (B). By day 5, ISMC were arranged in multilayered regions with intervening neurones showing extending neurites (arrows) from the myenteric neurones in coculture with ISMC. Scale bar = 100 µm.
Purified ISMC
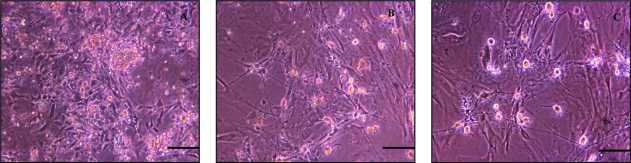
For purified ISMC cultures, the differential adhesion technique was again employed primarily for fibroblast removal, followed by incubation for 5 h, allowing attachment of sufficient numbers of ISMC. The differential adhesion technique considerably decreased the numbers of neurones in culture, although sparse clusters of neurones were observed throughout the culture of ISMC (Fig. 5B). Morphological monitoring clearly demonstrated that treatment with scorpion venom resulted in an increase in the proportion of ISMC in cultures (Fig. 5C) compared to untreated cultures. Furthermore, treatment with scorpion venom (30 µg mL−1) induced a selective dose-dependent neurotoxicity without affecting ISMC. It was also observed that single dose treatment was not sufficient to abolish completely the presence of neurones. We hypothesized that the presence of neurones in cultures obtained from neonatal intestine is inevitable, as neuronal activity is extremely high at this stage of tissue development. Thus, upon confluence, cells were passaged and exposed to a second dose of scorpion venom (30 µg mL−1), which subsequently led to multilayered regions of spindle-shaped ISMC with no apparent neurones (Fig. 5D).
Fig. 5.
Cells at day 1 (A) and following differential adhesion to minimize numbers of neurones and fibroblasts resulted in cultures of ISMC-like and few clusters of neurones (arrows) (B). At day 2, the neurotoxicity following pre-treatment with scorpion venom (30 µg mL−1) increased proliferation of ISMC (C). Incubation with second dose of scorpion venom resulted in cultures displaying multilayered spindle-shaped cells, which were uniform throughout the culture (D). Scale bar = 100 µm.
Purified myenteric neurones
For purified neuronal cultures, accessory cells were removed by a single treatment with antimitotic cytosine arabinoside (6 µm), resulting in a complex network of pure neurones by day 7 (Fig. 6A–C).
Fig. 6.
(A) At day 1, mixed populations of cells in culture. Following treatment with cytosine arabinoside (6 µm) non-neuronal cells are removed and neurones start to develop processes (B). By day 7, the neuronal cells have enlarged cell bodies with neurites that extended throughout the culture (C). Scale bar = 100 µm.
Immunocytochemistry
Dual-label immunocytochemistry on cocultures showed clusters of myenteric neurones adjacent to ISMC, which were identified by positive staining with antibodies to serotonin-5HT3 receptors and α-smooth muscle actin, respectively (Fig. 7A). Purified ISMC cultures stained positive for α-smooth muscle actin, displaying multilayered regions of actin filaments, and negative for antibodies raised to 5HT3 receptors, confirming the absence of neurones (Fig. 8A–B). Similarly, purified myenteric neurones stained positive for anti-5HT3 receptor antibodies in the large cell bodies and extended neurites, but negatively for α-smooth muscle actin (Fig. 9A). Additional immunocytochemistry included staining with anti α-actinin antibodies, which also stained positive on the ISMC cultures, equally showing multilayered regions of actinin filaments (Fig. 10A,B).
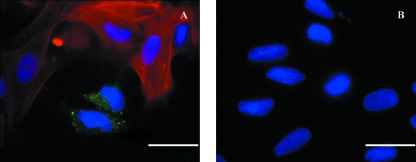
Fig. 7.
Coculture preparations with dual-label staining directed to 5HT3 receptors (green) and µ-smooth muscle actin (red) antibodies for characterization of neurones and ISMC respectively. DAPI mount was used for labeling cell nucleus (blue). Positive diffusion of the 5HT3 receptors antibody within the neuronal cell bodies opposed by neighbouring cells identified as ISMC (A). Negative controls showed no staining to both primary antibodies (B). Scale bar = 25 µm.
Fig. 8.
ISMC preparations with dual-label staining directed to 5HT3 receptors (green) and α-smooth muscle actin (red) antibodies for confirmation of the nature of the purified ISMC. DAPI mount was used for labeling cell nucleus (blue). Uniform ISMC actin filaments stained positive throughout the culture (A,B). Negative labeling to 5HT3 receptors antibody confirmed the absence of the neurones in the preparation (A,B). Negative controls showed no staining to both primary antibodies (C,D). Scale bar = 25 µm.
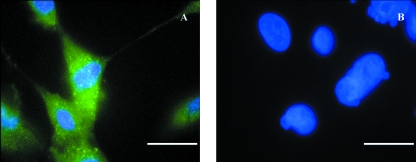
Fig. 9.
Neuronal preparations with dual-label staining directed to 5HT3 receptors (green) and α-smooth muscle actin (red) antibodies for confirmation of the nature of the purified neurones. DAPI mount was used for labeling cell nucleus (blue). Neuronal cell bodies and extended neurites stained positive to 5HT3 receptors antibody (A). Negative immunoreactivity to α-smooth muscle actin antibody confirms the absence of the ISMC in the preparation (A). Negative controls showed no staining to both primary antibodies (B). Scale bar = 25 µm.
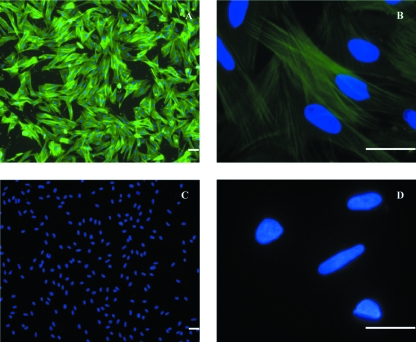
Fig. 10.
ISMC preparations stained with smooth muscle actinin antibodies (green) for confirmation of the nature of the purified ISMC. DAPI mount was used for labeling of cell nucleus (blue). Uniform ISMC actin filaments stained positive throughout the culture (A,B). Negative controls showed no staining to primary antibodies (C,D). Scale bar = 25 µm.
Discussion
The cell dissociation method reported in the present study provide evidence that the regions of the intestine from which cells are isolated, as well as the length of time allowed for trypsinization, influences the viability and growth rate of cells in culture. In support of these studies, histological procedures also established that an increase in trypsinization times leads to gradual dissociation from the outermost layers into the inner layers of the intestine. This suggests that this method can be used to determine the estimated time required for isolation of single cell type populations from specific intestine layers.
Previous studies performed on the isolation of ISMC from neonatal tissue reported the use of dissection under sophisticated optical control. The techniques involved the removal of the entire muscularis externae layer with the support of an advanced microscope and fine forceps (Frings et al. 2000) or specific removal of the longitudinal muscle layer with the help of stereomicroscopes (Smirnov et al. 1992). The method developed here, however, consisting simply of tying both ends of the intestine prior to dissociation, provides an inexpensive technique for isolation of intestinal cells from neonatal tissue, which can be adjusted for isolation of specific intestinal layers. Although this may also be achieved via microdissection techniques, microdissection systems are expensive, unavailable to many researchers, and isolation of large numbers of intestinal cells such as presented here would prove extremely time consuming or even impracticable in complex tissues (Kwapiszewska et al. 2004).
In an attempt to separate and culture different cell types, the differential adhesion technique was used, which minimized the number of fibroblastic cells in culture as well as allowed separating large number of neurones from ISMC. The differential adhesion technique has already been shown to be a useful method of separating fibroblasts and myogenic cells (Richler & Yaffe, 1970; Gareth et al. 1990; Rando & Blau, 1994; Qu et al. 1998).
Further selective purification of ISMC was attained through treatment with scorpion venom Maurus palmatus. According to numerous morphological (Vachon, 1952) as well as some biochemical (Goyffon & Kovoor, 1978) characteristics, scorpion venoms are divided into two groups: buthoids and chactoids. The buthoids, due their relevant medical importance, have been widely studied and their toxicity targets the excitation of axonal membranes by modification of their ionic conductance (Romey et al. 1975; Catterall, 1980; Pelhate & Zlotkin, 1982). The chactoids, including scorpio Maurus palmatus, have been poorly investigated as they were thought to be medically non-significant (Goyffon & Kovoor, 1978). On the basis of this information, scorpion venom Maurus palmatuswas assessed for purity and the most prominent characteristic present in the toxin components was the co-operativity between the two toxin units (paralytic fraction and the lethal factor), which when investigated through neurophysiological studies showed interaction with insect axonal preparations and extremely low toxicity compared with other buthoid venoms. Results also revealed that scorpion Maurus palmatus possesses co-operative interaction and blocking/suppressing effects on the ionic conductance for both potassium and sodium ions. It seems that the disturbance of the electrolyte balance in both ions affects cell membrane integrity and nerve transmission through interference with the conductance of axons (Lazarovici et al. 1982; Possani et al. 1999). Thus, treatment in culture with this toxin caused selective abolition of neurones, which when compared with untreated cultures contained relatively higher numbers of ISMC but otherwise identical cultures (Blennerhassett & Lourenssen, 2000) and no disturbance to ISMC population. Moreover, it has been postulated that the presence of neurones in vitro might correlate with the decrease in serum availability in culture resulting in decreased proliferation of ISMC (Blennerhassett & Lourenssen, 2000). Hence, removal of the neurones may increase the availability of serum proteins and promote an increase in the proliferation of ISMC.
Myenteric neurones were purified through antimitotic treatment with cytosine arabinoside. Pretreatment with this drug has been primarily used to purify neurones derived from dorsal root or superior cervical ganglia (Ecclestoni et al. 1989; Blennerhassett & Lourenssen, 2000) as it inhibits DNA replication of non-neuronal cells (Harrington & Perrino, 1995). In the present study a single treatment with cytosine arabinoside resulted in the complete removal of accessory cells (ISMC and fibroblasts) and rapid proliferation of myenteric neuronal cells bodies with extended neurites.
Further, attempts were made to examine the purity of the cultures using immunocytochemistry. Such experiments on cocultures confirmed the presence of both ISMC and neuronal cell populations. Antibodies to α-smooth muscle actin displayed a uniform distribution of ISMC among positively stained myenteric neurones to antibodies raised to 5-HT3 receptors. Anti-smooth muscle α-actin antibodies represent a useful tool for the study and characterization of smooth muscle in culture (Skalli et al. 1986). Receptors to 5HT3, on the other hand, are expressed in the enteric nervous system (Richardson et al. 1985;Mawe et al. 1986; Johnson & Heinemann, 1995) and play important roles in the enteric physiology stimulating neuronal activity. Recent studies on isolated myenteric neurones obtained from the mice small intestine have shown immunoreactivity to antibodies raised to 5HT3 receptors in neurones, labeling both neurites and cell bodies (Liu et al. 2002). Thus, in the present studies 5HT3receptors antibodies was used as a marker for myenteric neurones. In such experiments the purified myenteric neurones showed uniform expression of 5HT3 receptors within the cell bodies extending to the processes and no immunoreactivity to α-smooth muscle actin. Furthermore, purified ISMC cultures showed uniform expression and distribution of α-smooth muscle actin and no expression of 5HT3 receptors. Additionally, purified ISMC also displayed intense diffuse labeling in the cytoskeleton of the majority of cells following staining with antibodies raised to α-actinin. Distribution of α-actinin in single isolated muscle cells filaments has been revealed to be present in high concentration throughout the cytoplasm (Fay et al. 1983).
Although this work shows that ISMC and myenteric neurones can be readily dissociated from the neonatal intestine, the use of these cells in viable pharmacological assays still needs to be confirmed. However, studies performed on ISMC and myenteric neurones isolated from newborn rats have revealed that such preparations possess the ability to re-aggregate after 1 day in culture (Schaffer et al. 1997; Frings et al. 2000), and that neonatal ISMC contract in culture at frequencies similar to the ISMC in the intact tissue (Schultheiss & Diener, 1999; Frings et al. 2000). There is also evidence that cultured neonatal ISMC respond to cholinergic agonists and show inhibition of contraction by selective antagonists (Frings et al. 2000). Thus, it is highly likely that neonatal ISMC may be used in the development of new cell-based pharmacological assays.
Acknowledgments
The authors would like to acknowledge the University of Bradford and the Institute of Pharmaceutical Innovation for the provision of laboratory space and for supporting this research.
References
- Benham CD, Bolton TB, Lang RJ, Takewaki T. Calcium-activated potassium channels in single smooth muscle cells of rabbit jejunum and guinea-pig mesenteric artery. J Physiol. 1986;371:45–67. doi: 10.1113/jphysiol.1986.sp015961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitar KN, Makahlouf GM. Measurement of the function in isolated single smooth muscle cells. Am J Physiol Gastrointest Liver Physiol. 1986;250:G357–G360. doi: 10.1152/ajpgi.1986.250.3.G357. [DOI] [PubMed] [Google Scholar]
- Blennerhassett MG, Lourenssen S. Neural regulation of intestinal smooth muscle growth in vitro. Am J Physiol Gastrointest Liver Physiol. 2000;279:G511–G519. doi: 10.1152/ajpgi.2000.279.3.G511. [DOI] [PubMed] [Google Scholar]
- Catterall WA. Neurotoxins that act on voltage-sensitive sodium channels in excitable membranes. Annu Rev Pharmacol Toxicol. 1980;20:15–43. doi: 10.1146/annurev.pa.20.040180.000311. [DOI] [PubMed] [Google Scholar]
- Chamley-Campbell J, Campbell GR, Ross R. The smooth muscle cell in culture. Physiol Rev. 1979;59:1–61. doi: 10.1152/physrev.1979.59.1.1. [DOI] [PubMed] [Google Scholar]
- Chlopcikova S, Psotova J, Miketova P. Neonatal rat cardiomyocites – A model of morphological, biochemical and electrophysiologocal characteristics of the heart. Biomed Papers. 2001;145:49–55. [PubMed] [Google Scholar]
- Eccleston PA, Bannerman PG, Pleasure DE, Winter J, Mirsky R, Jessen KR. Control of peripheral glial cell proliferation: enteric neurones exert an inhibitory influence on Schwann cell and enteric glial cell DNA synthesis in culture. Development. 1989;107:107–112. doi: 10.1242/dev.107.1.107. [DOI] [PubMed] [Google Scholar]
- Fay FS, Fujimara K, Rees DD, Fogarty KE. Distribution of Alpha-Actinin in single isolation smooth muscle cells. J Cell Biol. 1983;96:783–795. doi: 10.1083/jcb.96.3.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freshney RI. Culture of Animal Cells. A Manual of Basic Technique. 5. New York: Wiley-Liss; 2005. [Google Scholar]
- Frings M, Haschke G, Heinke B, Schafer KH, Diener M. Spontaneous contractions of intestinal smooth muscle re-aggregates from the new-born rat triggered by thromboxane A2. J Vet Med. 2000;A47:469–475. doi: 10.1046/j.1439-0442.2000.00306.x. [DOI] [PubMed] [Google Scholar]
- Gareth EJ, Murphy SJ, Watt DJ. Segregation of the myogenic cell lineage in mouse muscle development. J Cell Sci. 1990;97:659–667. doi: 10.1242/jcs.97.4.659. [DOI] [PubMed] [Google Scholar]
- Glazier JA, Graner F. Simulation of differential driven adhesion rearrangement of biological cells. Phys Rev E. 1993;47:2128–2154. doi: 10.1103/physreve.47.2128. [DOI] [PubMed] [Google Scholar]
- Goyffon M, Kovoor J. Chatoids Venoms. In: Bettini S, editor. Arthropod Venoms. Vol. 48. Berlin: Springer-Verlag; 1978. pp. 395–418. [Google Scholar]
- Harrington C, Perrino FW. The effects of cytosine arabinoside on RNA-primed DNA synthesis by RNA polymerase-primase. Am Soc Biochem Mol Biol. 1995;270:26664–26669. doi: 10.1074/jbc.270.44.26664. [DOI] [PubMed] [Google Scholar]
- Herovici C. Stain Technol. Vol. 38. 1963. Polychrome stain for differentiating precollagen from collagen; pp. 204–205. [PubMed] [Google Scholar]
- Johnson DS, Heinemann SF. Detection of 5-HT3R-A, a 5HT3 receptor subunit, in submucosal and myenteric ganglia of rat small intestine using in situ hybridization. Neurosci Lett. 1995;184:67–70. doi: 10.1016/0304-3940(94)11170-n. [DOI] [PubMed] [Google Scholar]
- Kwapiszewska G, Meyer M, Bogumil R, et al. Identification of proteins in laser-microdissected small cell numbers by SELDI-TOF and Tandem MS. BMC Biotechnol. 2004;4:30. doi: 10.1186/1472-6750-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarovici P, Yanai P, Pelhate M, Zlotkin E. Insect toxic components from the vemon of a chatoid scorpion, scorpio maurus palmatus (scorpionidae) J Biol Chem. 1982;257:8397–8404. [PubMed] [Google Scholar]
- Lieberman M, Hauschka SD, Hall ZW, Eisenberg BR, Horn R, Walsh JV, et al. Isolated smooth muscle cells as a physiological model. Am J Physiol. 1987;253:C349–C363. doi: 10.1152/ajpcell.1987.253.3.C349. [DOI] [PubMed] [Google Scholar]
- Liu M, Rayport S, Jiang Y, Murphy DL, Gershon MD. Expression and function of 5HT3 receptors in the enteric neurones of mice lacking the serotonin transporter. Am J Physiol Gastrointest Liver Physiol. 2002;283:G1398–G1411. doi: 10.1152/ajpgi.00203.2002. [DOI] [PubMed] [Google Scholar]
- Mawe GM, Branchek TA, Gershon MD. Peripheral neural serotonin receptors: Identification and characterisation with specific antagonists and agonists. Proc Natl Acad Sci USA. 1986;83:9799–9803. doi: 10.1073/pnas.83.24.9799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oishi K, Itoh Y, Isshiki Y, Kai C, Takeda Y, Yamaura K, et al. Agonist-induced isometric contraction of smooth muscle cell-populated collagen fiber. Am J Physiol. 2000;279:C1432–C1442. doi: 10.1152/ajpcell.2000.279.5.C1432. [DOI] [PubMed] [Google Scholar]
- Owens GK. Control of hypertrophic versus hyperplastic growth of vascular smooth muscle cells. Am J Physiol Heart Circ Physiol. 1989;257:H1755–H1765. doi: 10.1152/ajpheart.1989.257.6.H1755. [DOI] [PubMed] [Google Scholar]
- Pelhate M, Zlotkin E. Actions of insect toxin and other toxins derived from the venom of the scorpion Androctonus australis on isolated giant axons of the cockroach (Periplaneta americana) J Exp Biol. 1982;97:67–71. doi: 10.1242/jeb.97.1.67. [DOI] [PubMed] [Google Scholar]
- Possani LD, Bacerril B, Delepierre M, Tytgat J. Scorpion Venom toxins specific for Na+-channels. Eur J Biochem. 1999;264:287–300. doi: 10.1046/j.1432-1327.1999.00625.x. [DOI] [PubMed] [Google Scholar]
- Qu Z, Balkir L, Van Deutekom JCT, Robbins P, Pruchnic R, Huard J. Development of approaches to improve cell survival in myoblast transfer therapy. J Cell Biol. 1998;142:1257–1267. doi: 10.1083/jcb.142.5.1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rando T, Blau HM. Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J Cell Biol. 1994;125:1275–1287. doi: 10.1083/jcb.125.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson BP, Engel G, Donatsch P, Stadler PA. Identification of Serotonin M-receptor subtypes and their specific blockade by a new class of drugs. Nature. 1985;316:216–231. doi: 10.1038/316126a0. [DOI] [PubMed] [Google Scholar]
- Richler C, Yaffe D. The in vitro cultivation and differentiation capacities of myogenic cell lines. Dev Biol. 1970;23:1–22. doi: 10.1016/s0012-1606(70)80004-5. [DOI] [PubMed] [Google Scholar]
- Romey G, Chicheportiche R, Lazdunski M, Rochat H, Miranda F, Lissitzky S. Scorpion neurotoxin – a presynaptic toxin which affects both Na+ and K+ channels in axons. Biochem Biophys Res Commun. 1975;64:115–121. doi: 10.1016/0006-291x(75)90226-0. [DOI] [PubMed] [Google Scholar]
- Schaffer K, Herrmuth H, Mueller J, et al. Bombesin-like peptides stimulate somatostatin release from rat fundic D cells in primary culture. Am J Physiol Gastrointest Liver Physiol. 1997;273:G686–G695. doi: 10.1152/ajpgi.1997.273.3.G686. [DOI] [PubMed] [Google Scholar]
- Schultheiss G, Diener M. Inhibition of spontaneous amooth muscle contractions in rat and rabbit intestine by blockers of the thromboxane A2 pathway. J Vet Med. 1999;46A:123–131. doi: 10.1046/j.1439-0442.1999.00200.x. [DOI] [PubMed] [Google Scholar]
- Skalli O, Ropraz P, Trzeciak A, Benzonana G, Gillessen D, Gabbiani G. A monoclonal antibody against alpha-smooth muscle actin: A new probe for smooth muscle differentiation. J Cell Biol. 1986;103:2787. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulston JE, Schierenberg E, White JG, Thomson JN. The embryonic cell lineage of the nematode Caenorhabditis elegans. Dev Biol. 1983;100:64–119. doi: 10.1016/0012-1606(83)90201-4. (Abstract) [DOI] [PubMed] [Google Scholar]
- Smirnov SV, Zholos AV, Shuba MJF. Potential-dependent inward currents in single isolated smooth muscle cells on the rat ileum. Am J Physiol. 1992;454:549–571. doi: 10.1113/jphysiol.1992.sp019279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thyberg J, Nilson J, Palmerg L, Sjolund M. Adult human arterial smooth muscle cells in primary culture: Modulation from contractile to synthetic phenotype. Cell Tissue Res. 1985;239:69–75. doi: 10.1007/BF00214904. [DOI] [PubMed] [Google Scholar]
- Vachon M. Etudes sur les Scorpions. Institut Pasteur d’Algerie; 1952. p. 483. p. [PubMed] [Google Scholar]
- Yaffe D. Retention of differentiation potentialities during prolonged cultivation of myogenic cells. Proc Natl Acad Sci USA. 1968;61:477–483. doi: 10.1073/pnas.61.2.477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita N, Nishida M, Hoshida S, Kuzuya T, Hori M, Taniguchi N, et al. Induction of manganese peroxide dismutase in rat cardiac myocytes increase tolerante to hypoxia 24 h after preconditioning. J Clin Invest. 1994;94:2193–2199. doi: 10.1172/JCI117580. [DOI] [PMC free article] [PubMed] [Google Scholar]