Abstract
The involvement of the unique saiga nose in vocal production has been neglected so far. Rutting male saigas produce loud nasal roars. Prior to roaring, they tense and extend their noses in a highly stereotypic manner. This change of nose configuration includes dorsal folding and convex curving of the nasal vestibulum and is maintained until the roar ends. Red and fallow deer males that orally roar achieve a temporary increase of vocal tract length (vtl) by larynx retraction. Saiga males attain a similar effect by pulling their flexible nasal vestibulum rostrally, allowing for a temporary elongation of the nasal vocal tract by about 20%. Decrease of formant frequencies and formant dispersion, as acoustic effects of an increase of vtl, are assumed to convey important information on the quality of a dominant male to conspecifics, e.g. on body size and fighting ability. Nasal roaring in saiga may equally serve to deter rival males and to attract females. Anatomical constraints might have set a limit to the rostral pulling of the nasal vestibulum. It seems likely that the sexual dimorphism of the saiga nose was induced by sexual selection. Adult males of many mammalian species, after sniffing or licking female urine or genital secretions, raise their head and strongly retract their upper lip and small nasal vestibulum while inhalating orally. This flehmen behaviour is assumed to promote transport of non-volatile substances via the incisive ducts into the vomeronasal organs for pheromone detection. The flehmen aspect in saiga involves the extensive flexible walls of the greatly enlarged nasal vestibulum and is characterized by a distinctly concave configuration of the nose region, the reverse of that observed in nasal roaring. A step-by-step model for the gradual evolution of the saiga nose is presented here.
Keywords: acoustic communication, comparative anatomy, evolutionary morphology, formant analysis, larynx, mating system, rutting calls, Saiga tatarica, sexual selection, vocal tract
Introduction
The saiga (Saiga t. tatarica, Bovidae, Mammalia) has evolved one of the most extraordinary noses among mammals (Linné, 1766; Pallas, 1777; Murie, 1870; Jacobi, 1921; Lodyshenskaja, 1952; Frey & Hofmann, 1995, 1997; Clifford & Witmer, 2004) (Fig. 1). However, the biological role of this bizarre nose has not yet been fully explained.
Fig. 1.
Photo of an adult rutting male saiga while producing a nasal roar. Inset: Rutting male, rostral view.
The most widely accepted hypothesis for the evolutionary origin of the saiga nose promotes the removal of dust particles from the inhalatory air raised by locomotion of migrating large herds (Bannikov et al. 1961; Jirnov, 1982; Fadeev & Sludsky, 1983; Danilkin, 2005). The preferred locomotory type of the relatively small saiga for energetically advantageous movement in an open flat semi-arid steppe environment is the amble (Heptner et al. 1989). To gain maximal freedom of the shoulder musculature for extension of the forelimb, the neck is kept horizontally during locomotion. This conspicuous locomotory posture has led to a reorientation of the dorsal tubercula of the cervical transverse processes to which the extensor muscles of the neck insert (cf. Fig. 1 of Murie, 1870; cf. Fig. 3b). As a consequence, and by considering the modest shoulder height of 60–70 cm and the ability to cover distances of 80–120 km per day during migratory periods (Bannikov, 1963; Sokolov, 1974), the head is almost permanently immersed in the immense dust cloud produced by the herd. Nasal vestibula of saiga always contain concretions of mucus-cemented dust (Bannikov et al. 1961). It appears that there existed a selection pressure for the evolution of a dust filter in the rostral nose region and for shortening and displacing the sensitive respiratory region caudally to protect it from getting clogged with dust particles (Frey & Hofmann, 1997). This applied to individuals of both sexes and any age.
Fig. 3.
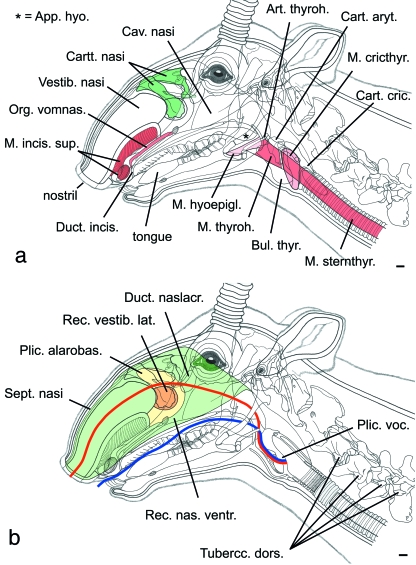
(a) Overlay of the head of an adult male saiga to show topographic positions of skull, cervical vertebrae, nasal vestibulum, hyoid apparatus, pharynx, larynx, tongue, incisive duct and vomeronasal organ. The incisive duct gradually turns into the ventral nasal recess. (b) Lateral vestibular recess, alarobasal fold, nasal septum and dimensions of vocal tract. Nasal (red) and oral (blue) vtls indicated by curved lines. Scale bar = 10 mm.
The evolutionary transformation of the saiga nose entailed several apomorphic structures, e.g. a large flexible nasal vestibulum, oval nostrils and paired lateral nasal recesses, and caudal displacement of other structures, e.g. the alar and basal folds and the ventral nasal concha (cf. Murie, 1870; Lodyshenskaja, 1952; Frey & Hofmann, 1997; Clifford & Witmer, 2004).
Saiga is a ‘young’ species that seems to have originated in the steppe regions of central Asia in the late Tertiary (Pliocene) or early Quaternary (Pleistocene) (Harington & Cinq-Mars, 1995). Morphologically, Pleistocene saigas were almost identical to recent individuals (Sokolov, 1974; Baryshnikov & Tikhonov, 1994). Therefore fossils recorded so far cannot assist us in the reconstruction of the evolution of the saiga nose. Evolution of the unique saiga nose under ecological constraints led to an elongation of the nasal air passages and to a highly mobile nose in both sexes (Bannikov et al. 1961). Superimposed is a pronounced sexual dimorphism of relative nose size. Bannikov (1963, p. 100) reported a particular increase in nose size and mobility in adult males during the rut. He emphasized the elongation of the trunk-like rostral portion, then dangling in front of the mouth.
Saiga is a polygynous species with a harem-like mating system. During the rut from December to January, males establish territories and gather females (sometimes as many as 50) and defend them in severe fights against rival males. Copulation occurs at night (Bannikov, 1963, p. 101, Sokolov, 1974).
The basic theory of vocal production suggests that the source signal, produced by the vocal folds, is subjected to subsequent acoustic filtering by the supralaryngeal vocal tract (vt) (Fant, 1960; Titze, 1994). The length of the vt is the main determinant for vocal resonances (= formant frequencies): longer vts produce more closely spaced formants (Fitch & Hauser, 2002).
In mammals, the potential shifts of formant frequencies are limited by anatomical constraints (Fitch, 2000a; Fitch & Hauser, 2002). Therefore, formant spacing represents a reliable indicator of body size (Fitch, 1997; Riede & Fitch, 1999; Reby & McComb, 2003a, but see Pfefferle & Fischer, 2006).
However, some mammals are capable of vt elongation by retraction of the larynx during vocalization, e.g. rutting male red deer Cervus elaphus (Fitch & Reby, 2001; Reby et al. 2005) and fallow deer Dama dama (Birrer, 2004, 2006; McElligott et al. 2006). Apart from this internal vt elongation, a second mechanism comprises external elongation by extension of the lips in rhesus macaques Macaca mulatta (Hauser et al. 1993) and in Diana monkeys Cercopithecus diana (Riede et al. 2005).
In all mammals, the vt rostral to the pharynx is bifurcated, terminating in the nostrils dorsally and in the oral opening ventrally. As a consequence, in the same way as an extension of the lips, an elongation of the nose might lead to a vt elongation (Fig. 1).
In mammals, flehmen, i.e. checking of female urine or vaginal secretions by the vomeronasal organ of the male, is accompanied by a conspicuous raising of the upper lip, involving the nostrils and the nasal vestibulum (Schneider, 1930, 1931, 1932a,1932b,1934; Estes, 1972; Ladewig & Hart, 1982). Accordingly, this behaviour can be expected to be influenced by the greatly transformed nose of the saiga.
Materials and methods
Functional morphology: computer tomography and dissection
The main source for the anatomical data was a head and neck specimen of an adult 4-year-old male saiga (body mass after death: 33.5 kg) which had died at Zoo Cologne, Germany, in 2002 owing to an accidental injury.
Before dissection, the intact specimen was subjected to computer tomographic (CT) investigation at the clinic for small animals in Düppel, Berlin, using a General Electric Lightspeed 4-slice helical Computer tomograph (GE, Milwaukee, WI). Scans were set at 120.0 kV, 110.0 mA, 1.2-mm slice thickness and at 120.0 kV, 160 mA, 1.2-mm slice thickness, standard and bone algorithms, respectively. A volume rendering software (GE VolumeViewer) was applied for processing of the slice data. Both sectional anatomy and three-dimensional reconstructions were analysed.
Macroscopic dissection was carried out on the specimen submersed in water and without using any preservative. Consecutive dissection steps were recorded by digital photography (Nikon D70s, Nikon Corp., Tokyo, Japan).
Results of a former dissection of the head and neck of a 2-year-old male saiga (body mass after death: 30.0 kg) were used for comparison (cf. Frey & Hofmann, 1995, 1997). This dissection had been recorded on photographic slides (Nikon F3, Nikon Corp.). Representative slides were scanned and digitalized for analysis.
Saiga skulls kept in the morphological collection of the IZW were used for comparison.
Standard veterinary nomenclature (NAV, 2005) was applied to anatomical structures.
Behaviour: video and acoustic analyses
Loud nasal roars and the concomitant behaviour of six mature, wild-born male saigas of similar body size, kept at the Volokolamsk breeding centre of Moscow Zoo, Russia, were recorded at distances of 1–10 m in four successive years (2002–2005). Five males provided winter rutting calls, and one male non-rutting calls only, in summer and autumn. Records were made with a cassette tape recorder Sony WM-D6C (Sony Corp., Tokyo, Japan), microphones Sennheiser K6-ME64 or K6-ME66 (Sennheiser electronic, Wedemark, Germany), video cameras Sony CCD-TRV228E or DCR-TRV-40E, and photo cameras Canon A95 (Canon Inc., Tokyo, Japan) or Nikon D70s.
During rut in January, focal males were released singly to a herd of 5–20 females within an enclosure, varying from 1 to 3 ha in different years, for a period of about 4 weeks. In 2004, two males were released for 2 weeks each in succession. Another focal male provided rutting calls when being isolated by a small wire mesh enclosure preventing physical contact with the females and their harem male. Non-rutting calls were recorded in a small separate wooden enclosure preventing visual contact with conspecifics.
Calls were digitized and analysed using avisoft saslab pro v. 4.33 (Avisoft Bioacoustics, Berlin, Germany) with a sampling frequency of 22.05 kHz and 16-bit resolution. Video clips were digitized using adobe premiere v. 6.0 (Adobe Systems Inc., San José, CA, USA) with 16-bit colors at 25 frames s−1, 720 × 576 pixels. Frame-by-frame analysis allowed examination of body postures and nasal configurations at the beginning, during and at the end of loud nasal roars.
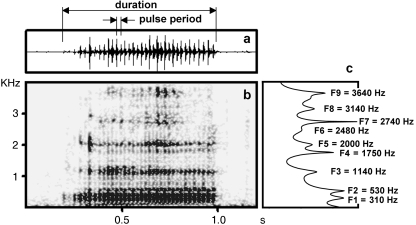
Call duration and mean pulse period were measured in the main window of avisoft with standard marker cursor (time resolution about 1.5 ms) and automatically transferred to excel (Microsoft, Redmond, WA, USA). We supposed that pulse rate represented the vocal source vibration frequency (i.e. fundamental frequency, f0). Accordingly, we calculated f0 for each call as inverse to the mean pulse period value (Fig. 2).
Fig. 2.
(a) Wave-form and (b) spectrogram of the loud nasal roar of a rutting male saiga. Measurements for pulse period and call duration are shown. (c) LPC spectrum of the same nasal roar, showing the first nine formant frequencies (F1, F2, etc.) of the call.
Vtl measurements in the dissected specimen served to establish settings for linear predictive coding (LPC). Formant frequency values were either extracted from an entire call or from part of a call showing clearly visible formants by using ‘To Formants (Burg)’ commands in praat v. 4.3.21 (http://www.praat.org): time step 0.04 s; window analysis 0.08 s; maximum number of formants from 5 to 9, maximum formant frequency 2.2–4.4 kHz. Peak formant values were extracted with ‘To Spectrum (slice)’ commands: minimum frequency resolution 20 Hz, bandwidth reduction 50 Hz (Fig. 2).
Applying the model of a straight uniform tube closed at one end, we calculated formant dispersion (ΔF) for each saiga male by using linear regression according to Reby & McComb (2003a). Based upon formant frequencies of loud nasal roars, vtl was calculated by the equation: vtl = c/2ΔF where c is the speed of sound in air, approximated as 350 m s−1.
Formant values and call duration were measured in six adult saiga males from a total of 305 calls and mean pulse period from 265 calls.
To estimate relative vt elongation resulting from nose extension during calls, we analysed pairs of head profiles from video single frames filmed from the same position. The first showed a saiga male producing a loud nasal roar with extended nose and the second showed the same male just after having produced this roar, with relaxed nose. We tried to select those pairs of profiles where an animal was standing in perfect lateral position to the camera. Head position was checked by considering horn position – superimposed horn contours were taken as indicators of ideal profiles. In cases where such profiles were unavailable, we selected images closest to ideal profiles. Apart from that, vt elongations could not always be measured in frames of maximum tension and relaxation of the nose as a target animal could move to become partially hidden behind obstacles. Accordingly, observation angles deviating from 90° relative to the longitudinal head axis introduced a certain error to the measurements of vt elongation. The video available for the non-rutting male 33 lacked appropriate lateral perspectives, so that we could not measure vt elongation for this male.
Measurements (in pixels) were done with autocad (Autodesk Inc., San Rafael, CA, USA), on a curved line, approximated by seven dots established by hand along the longitudinal centre line of the nose, from the medial eye angle to the tip of the nose. This line was taken as an approximation of the length of the mobile portion of the nose. Each profile was measured three times, and the average was calculated. As the length of the intracranial part of the nasal vt remains unchanged, the ratio of the curved lines with extended versus relaxed noses provides a rough estimate of relative nasal vt elongation.
Statistical analyses
All statistical analyses were made in statistica v. 6.0 (StatSoft Inc., Tulsa, OK, USA). Distribution of values for all acoustical parameters did not differ from normal for each male (Kolmogorov–Smirnov test). Accordingly, we applied one-way anova to compare variability of the measured values within and between individuals and two-tailed t-test to compare between rutting and non-rutting calls. For all measured values, means ± SD are given.
Results
Anatomical results
Topographic relationships of inner nasal structures
The nasal septum divides the nasal vocal tract into two completely separated compartments, the right and the left nasal passage. Each nasal passage basically comprises two parts, a nasal vestibulum rostrally and a nasal cavity proper.
The nasal vestibulum consists of an oval nostril and a greatly enlarged tube-like vestibulum proper, overhanging the mouth opening rostrally and gaining in diameter caudally. Its ventral surface is in contact with a pronounced muscular cushion consisting mainly of the greatly enlarged m. lev. lab. sup. (for definitions of muscles see Appendix 1) (Fig. 3a, Table 1).
Table 1.
Muscles of the saiga nose (after Lodyshenskaja, 1952; Frey & Hofmann, 1997, supplemented)
| Muscle | Portions | Origin | Insertion | Specific features | Function |
|---|---|---|---|---|---|
| m. lev. nasolab. | frontonasal bone, not clearly separable from origin of m. frontalis (not described) | dorsomedial fibres onto m. lev. lab. sup. and connective tissue of nasal vestibulum; ventrolateral fibres spread fan-like on lateral vestibular recess | muscle fibres turning into delicate tendinous fibres rostrally, which, farther rostrally, interweave with connective tissue | longitudinal contraction of dorsal and lateral nasal walls, thereby forming transverse folds, extension or compression of lateral vestibular recess | |
| m. lev. lab. sup. | rostroventral ridge (oblique line) of maxilla between facial tuber and infra-orbital foramen, fibres tendinously reinforced at origin | to fibres of the contralateral muscle and to tough connective tissue at vestibular and nasal dorsum | evenly fan-shaped from its origin on, radiating onto lateral and dorsal vestibular walls | supporting longitudinal nasal vestibular contraction, dorsoventral contraction, lateral nasal vestibular movements, oblique uni- or bilateral longitudinal contractions, extension or compression of lateral vestibular recess | |
| m. canin. | ventral part of oblique line of maxilla, caudally adjacent to infra-orbital foramen | tough connective tissue of vestibular dorsum, lateral surface of nostril | caudal half band-like, rostral half widely fan-shaped, radiating onto lateral and rostrodorsal vestibular walls, in the middle vestibular dorsum its fibres are super- imposed on m. lev. lab. sup. | supporting longitudinal nasal vestibular contraction, dorsoventral contraction, lateral nasal vestibular movements, oblique uni- or bilateral longitudinal contractions, participates in dorsoventral contraction of rostral nasal vestibulum, retraction, dilation and elevation of nostrils | |
| m. depr. lab. sup. | ventral to oblique line of maxilla and dorsal to the upper fourth premolar from surface of maxilla | lateral vestibular wall onto dorsally directed fibres of m. zyg. | vestigial, narrow band-like muscular fascicle, coursing along ventral margin of facial nerve, rostrally covered by m. canin. | negligible influence on nasal shape changes, perhaps maintaining relative position of facial nerve during different stages of nasal vestibular contractions | |
| m. lat. nasi | superficial portion of m. orbic. oris | tough connective tissue rostrally at dorsum of vestibulum and fibres of contralateral muscle | transversely oriented fibres, surrounding rostral portion of nasal vestibulum, i.e. the nostril region | participates in control of nasal aperture (constriction, dilation) | |
| m. zyg. | zygomatic arch, lateral to mandibular joint | fan-like onto superficial maxillary portion of m. orbic. oris | caudodorsal half is tendinous up to rostral edge of m. masseter, near its insertion part of the fibres curve dorsally terminating in the rostrolateral vestibular wall, covered by m. canin. | assists in narrowing and dorsal orientation of the nostril, possibly assists in lateral extension of rostral nasal vestibulum | |
| m. orbic. oris | sphincter muscle of the mouth, only indirectly connected to osseous elements, particularly by m. incis. sup., interwoven with the labial skin | rostrolabial portion intermingled with origin of m. lat. nasi and insertion of m. zyg., thereby involved in protraction and convex bending of rostral vestibulum | assists in retraction and downward movement of nostrils | ||
| m. incis. sup. | ventrally curved spatular tip of incisive bone | radiating dorsocaudally and medially to the entire ventral surface of nasal vestibulum | coursing in the groove between nasal septum and incisive and maxillary bones, greatly enlarged, elongation (from rostral tip of incisive bone to ventral termination of alarobasal fold), concomitant with caudal displacement of insertion site, unusual bend around lateral margin of ventrally curved rostral portion of incisive bone | elevation and depression of nasal vestibular floor, protraction and elongation of nasal vestibulum, lateral movements of nasal vestibulum | |
| m. hyoepigl. | mainly from ceratohyoid, only marginally from basihyoid | rostral surface of epiglottic cartilage | considerable relative length (c. 42 mm) | rostroventral movements of the epiglottis | |
| m. thyroaryt. (2 portions) | m. ventric. | concave inner surface of thyroid bulla | rostral aspect of muscular process up to arycorniculate ligament | caudally overlaps rostral third of m. voc., covers ovoid resilient fat pad laterally | narrowing of glottic cleft, increase of tension and thickness of vocal folds |
| m. voc. | caudal half of thyroid bulla and cricothyroid ligament | ventral tip and caudal aspect of vocal process | narrowing of glottic cleft, increase of tension and thickness of vocal folds | ||
| m. mal. (2 portions) | lacrimal | lacrimal bone near medial angle of the eye | along dorsal edge of molar portion of m. buccinator (not described) | spreads fan-like, crossing tendinous origins of m. lev. lab. sup. and m. canin. | both portions might assist in lateral retraction of nasal vestibulum via connective tissue connections to adjacent muscles, e.g. m. lev. lab. sup., m. cut. fac. |
| caudal | ventromedial section of m. orbicularis oculi (not described) | buccal portion of m. buccinator along dorsal buccal ramus of facial nerve | thin muscular band, 10–20 mm in width, partly covers lacrimal portion | ||
| m. cut. fac. | superficial lamina of cervical fascia along oblique line from laryngeal region to auricular base | obliquely along rostral edge of m. zyg. to mouth angle, buccopharyngeal and masseteric fasciae | crosses parotid gland and m. masseter (not described) | tenses and moves skin in labial, buccal and masseteric regions, pulls mouth angle caudally | |
The main nasal cavity comprises a large crescent-shaped homologue of the combined alar and basal folds, roofing the ventral nasal recess and flanked by the lateral vestibular recess (Fig. 3b).
Lateral vestibular recess
The lateral vestibular recess is a unique paired apomorphic structure of the saiga nose. It consists of a lateral outpocketing of the nasal vestibulum rostral to the pre-orbital gland (Frey & Hofmann, 1997; Clifford & Witmer, 2004). Its rostral opening into the nasal vestibulum proper is situated lateral to the rostral edge of the alarobasal fold (Fig. 3b). The dorsoventral height of the slit-like opening of the lateral vestibular recess measured about 25 mm and its transverse width was around 10 mm. The blindsac extends caudolaterally over the edge of the maxillary bone. The greatest rostrocaudal length of the lateral vestibular recess in the dissected specimen was about 30 mm, and the greatest dorsoventral height about 27 mm.
Parts and flexibility of the nasal septum
The nasal septum consists of three major parts, from rostral to caudal: the membraneous part, the cartilage of the nasal septum, and the osseous part, composed of the perpendicular lamina of the ethmoid bone and of the vomer with its dorsal groove lodging the cartilage of the nasal septum (Clifford & Witmer, 2004).
The rostrocaudal length of the nasal septum, measured in a mediosagittal section along an arched central line from the rostral edge of the nostrils to the caudal edge of the pharyngeal septum, was about 240 mm. The greatest dorsoventral height of the nasal septum (between alarobasal fold and ventral nasal concha) was about 90 mm. The area of the nasal septum was approximately 140 cm2 (Fig. 3b).
Those parts of the nasal septum protruding from the concave rostral skull contours (not covered by skull bones) exhibit a high degree of flexibility.
Nasal cartilages and flexibility of the nasal walls
The premaxillary (= incisive bone), the maxillary and the nasal bones were dramatically reduced in saiga. Concomitantly, those cartilages originally supporting the primitive nostrils underwent considerable caudal displacement. Formerly representing the position of the primitive nostrils, the dorsally fused cartilages are now located far caudally to the actual apomorphic nostrils. In saiga, the rostral cartilage lends support to the crescent-shaped alarobasal fold (Fig. 3a,b).
The resilience of these cartilages provides a high degree of flexibility for those portions of the lateral nasal walls immediately rostrally adjacent to the concave rostral skull contours.
Those parts of the nasal walls positioned rostral to the cartilages consist of interwoven connective tissue and musculature. Accordingly, their flexibility is even greater than that of the cartilage-supported portions.
Incisive duct and vomeronasal organ
The ventrally curved incisive duct, 3–4 mm wide, is about 70 mm long and opens on the lateral aspect of the incisive papilla.
The vomeronasal cartilage, 3–4 mm wide and enveloping the vomeronasal organ, courses parallel to the incisive duct, 3–4 mm dorsal to it. Rostrally it tapers into the vomeronasal duct, which opens into the incisive duct about 10 mm caudal to its opening into the oral cavity (Fig. 3a). The total length of the vomeronasal organ plus vomeronasal duct is about 85 mm. Laterally, the vomeronasal cartilage is protected by the incisive and maxillary bone; medially, it touches the palatinal process of the incisive bone and the vomer.
Muscles capable of extending the flexible parts of the nose
Muscles of the saiga nose have been described already by Murie (1870), Lodyshenskaja (1952) and Frey & Hofmann (1997). All muscles of the flexible proboscis are strongly intermingled with tough connective tissue forming a functional entity so that configuration changes of the proboscis are almost always the result of the co-ordinated contractions of several muscles and not of one single muscle alone. For a summary of muscle functions see Table 1.
Hyoid apparatus
The shape and position of the saiga's hyoid apparatus basically conform to that of other bovids. The rostroventral portion of the thyrohyoid is calcified to some extent, whereas the caudodorsal half is highly flexible. The thyrohyoid connection is made up by a short and highly resilient ligament, about 5 mm long in the relaxed state (Fig. 3a). The flexibility of the thyrohyoid itself and of the thyrohyoid connection in particular may allow for a tilting movement of the larynx that moves the thyroid bulla to a more rostral position.
Larynx
The larynx of saiga has evolved several apomorphic features. The thyroid cartilage at its caudal end forms a conspicuous thyroid bulla (Figs 3a, 4). This thyroid bulla encloses a preglottal ventral laryngeal saccule (Frey et al. 2007), i.e. an extension of the laryngeal cavity proper also lined with laryngeal mucosa.
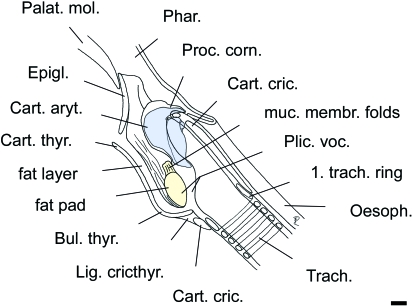
Fig. 4.
Sagittal section of an adult male larynx to show thyroid bulla, orientation, shape and relative length of vocal fold. Rostrally, the vocal fold is supported by a resilient ovoid fat pad. Scale bar = 10 mm.
The laryngeal vestibulum is set obliquely to the longitudinal axis of the trachea at an unusually acute angle of 20–25°. Accordingly, the short epiglottis is raised in the direction of the soft palate. The length of the epiglottis (rostral tip to base in mediosagittal section) is c. 40 mm. The hyoepiglottic muscle is of considerable relative length (Fig. 3a, Table 1). The corniculate process of the arytenoid cartilage is short, almost telescoped dorsoventrally (Figs 3b, 4). The vocal process of the arytenoid cartilage makes up c. 45% of the length of the entire cartilage (18 mm/40 mm). The rostral surface of the vocal process is concavely extended. The m. thyroaryt. consists of two portions, the m. ventric. and the m. voc. (Table 1).
In consequence of the oblique position of the thyroid cartilage, the vocal fold of saiga is set at a comparably acute angle of c. 30° relative to the long axis of the trachea. The vocal fold is protruding into the thyroid bulla. The morphology of the saiga's stout vocal fold is remarkable. Its dorsoventral length is relatively short (c. 25 mm) and its rostrocaudal width is relatively large (c. 20 mm), as is its transverse diameter (c. 13 mm). Rostrally, the vocal fold is supported by a pronounced ovoid, tough and resilient fat pad, the blunt pole of which is directed dorsally (Figs 3b, 4). Its greatest dorsoventral length is c. 24 mm, and its greatest transverse diameter c. 12 mm. This fat pad is laterally covered by the m. ventric. The caudal portion of the vocal fold is covered laterally by the m. voc. A distinct vocal ligament could not be identified.
Vocal tract length
In the dissected specimen, the oral vtl along a central line from the caudal edge of the glottis to the external border of the lower lip was c. 320 mm. Nasal vtl, measured along a central line between dorsal and ventral contour, from the caudal edge of the glottis to the external border of the nostrils was c. 380 mm. As a consequence, the nasal vtl exceeds the oral vtl by 20% (Fig. 3b).
Sexual dimorphism
According to our own unpublished observations, the nose of the female saiga is basically of the same structure as that of the male, and adult female saigas also vocalize through their nose. As distinct from males, however, females call with almost relaxed noses. They may use their nasally emitted calls to keep in contact with their young, particularly during and after the calving season, i.e. in a mother–young context.
The female's rostral skull region is also concavely shaped and accommodates an enlarged nasal vestibulum. The conchae are also shortened, caudally displaced and obliquely inclined. The nostrils are also oval and devoid of any cartilaginous support (Fig. 5).
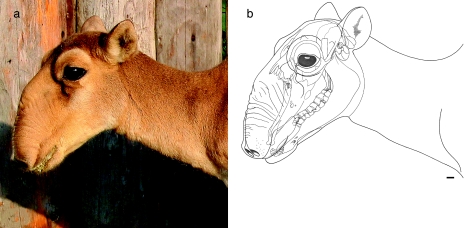
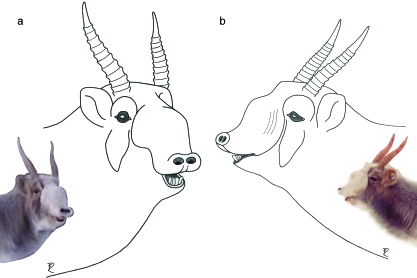
Fig. 5.
Photo (a) and overlay of head contour and skull (b) of an adult female saiga in its summer coat. Despite equally pronounced evolutionary transformations of the rostral skull region as in the male, the female nasal vestibulum is smaller than in the male and does not overhang the mouth opening. The nostrils at rest are more rostrally directed than in the male. Scale bar = 10 mm.
However, despite basic correspondence of nose structure, there is an obvious sexual dimorphism of adult individuals beyond what would be expected from body size proportions. The middle portion of the vestibulum is more voluminous in the male and integumental transverse wrinkles are set wider apart than in the female. The rostral portion of the vestibulum is longer, overhanging the mouth opening in the male but not in the female. Accordingly, the nostrils at rest are directed ventrally in the male but more rostrally in the female. This sexual dimorphism is most pronounced during the rut, presumably involving hormonal effects in the male (cf. Bannikov, 1963; Fig. 6).
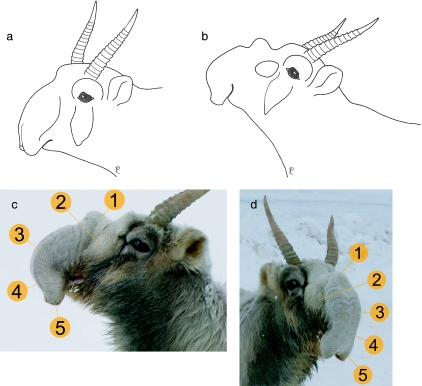
Fig. 6.
Nose extension in an adult male saiga during the rut according to video clip single frames and photos. (a) Resting position, (b) calling posture. (c,d) Main features emerging synchronously with nasal roaring: 1) pronounced folding of nasal dorsum, 2) inflation of lateral vestibular recess, 3) pronounced inflation and distinct curving of nasal vestibulum, 4) elongation of nasal vestibulum, 5) anterior bending, medial tilting and dilation of nostrils. (a,b,c) Left lateral view, (d) rostrolateral view.
Behavioural results
Calling posture and nose extension
When producing their rutting calls, adult males typically adopt a specific calling posture. They hold the neck in an almost horizontal position and raise their head until its longitudinal axis also approximates the horizontal (Fig. 6a–c).
Immediately prior to producing a rutting call, males extend their noses in a highly stereotypic manner. Nose extension is exclusively associated with the production of rutting calls and involves several conspicuous features: (1) folding of the proximal nasal dorsum, (2) lateral constriction in the proximal third of the nose except the lateral recess which is strongly emphasized, (3) convex curving (S-shaping) of the nasal vestibulum and anterior bending of the nostrils, inflation of the middle and distal vestibular portions, (4) elongation of the nasal vestibulum so that the distal nasal tubes overhang the mouth considerably, (5) medial tilting and dilation of the nostrils (Fig. 6c,d). Among five rutting males, interindividual differences in the number of folds of the proximal nasal dorsum and in S-shaping occurred: three males had only one distinct fold, one male had two folds and another had three folds (Fig. 7a–c). The S-shaping in most males was smooth, literally reminiscent of an ‘S’, whereas in one male it was less smooth, more like a zigzag bend.
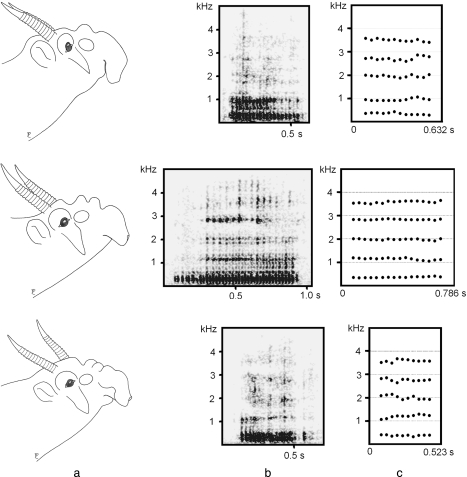
Fig. 7.
Individual differences of nose extension (one fold, two folds, three folds) and concomitant acoustic features between three adult males during the rut. (a) Nose extension, (b) spectrograms of loud nasal roars and (c) tracks of the spectral envelope peaks, approximating the run of formants throughout these roars. The tracks are given from 0.1 to 0.55 s of the roar in the one-fold male (above), from 0.3 to 0.9 s in the 2-fold male (middle) and from 0.1 to 0.5 in the three-fold male (bottom). Head contours according to video clip single frames.
Apparently, the mouth is closed during the production of rutting calls. Condensing water vapour, expelled from the nose synchronously with the calls, revealed that the production of nasal roars occurs during exhalation.
One effect of nose extension is the elongation of vtl compared to the relaxed state. The analysis of pair head profiles from successive video images revealed that nose extension definitely resulted in vt elongation. Averages varied from 17.5 to 41.6% in different rutting males (mean 21.7 ± 11.4) (Table 2). Measurements of vt elongation for the five rutting males varied between 11 and 53%. Apart from interindividual differences, this high variability might result both from an error owing to slightly different observation angles and from forced selection of frames in which the nose was not maximally extended or relaxed.
Table 2.
Call duration, fundamental frequency (f0), central formant frequency for the first formant (F1), calculated values for formant dispersion (ΔF), vocal tract length (vtl) and relative value of vt elongation during calling (vt elongation), are presented. Comparisons of call parameter values between individual rutting males and between all rutting males and the single non-rutting male, are listed at the bottom
| Call parameters | ||||||
|---|---|---|---|---|---|---|
| Animals | Duration (ms) | F0 (Hz) | F1 (Hz) | ΔF (Hz) | vtl (mm) | vt elongation (%) |
| Male 1 | 544 ± 146 | 41.1 ± 2.9 | 368 ± 18 | 398 | 440 | 17.5 ± 5.0 |
| (n = 83) | (n = 80) | (n = 83) | (n = 9) | |||
| Male 2 | 370 ± 146 | 36.9 ± 4.8 | 376 ± 33 | 401 | 436 | 21.7 ± 15.9 |
| (n = 23) | (n = 11) | (n = 23) | (n = 6) | |||
| Male 3 | 304 ± 133 | 40.3 ± 4.0 | 330 ± 37 | 397 | 441 | 25.13 ± 7.2 |
| (n = 34) | (n = 23) | (n = 34) | (n = 2) | |||
| Male 62 | 405 ± 108 | 47.6 ± 3.5 | 402 ± 54 | 401 | 436 | 41.6 |
| (n = 83) | (n = 83) | (n = 83) | (n = 1) | |||
| Male 54 | 291 ± 133 | 52.8 ± 3.7 | 401 ± 45 | 436 | 401 | 23.2 ± 12.8 |
| (n = 35) | (n = 24) | (n = 35) | (n = 8) | |||
| ANOVA F-ratio | F4,252 = 34.7* | F4,215 = 92.6* | F4,252 = 24.0* | |||
| Rutting males | 418 ± 162 | 44.5 ± 5.6 | 379 ± 47 | 407 ± 17 | 431 ± 17 | 21.7 ± 11.4 |
| (n = 258) | (n = 221) | (n = 258) | (n = 5) | (n = 5) | (n = 26) | |
| Non-rutting male 33 | 579 ± 126 | 52.9 ± 5.1 | 459 ± 50 | 493 | 356 | |
| (n = 47) | (n = 44) | (n = 47) | ||||
| t-test | t = 7.62* | t = 9.21* | t = 9.65* | |||
P < 0.001.
Interestingly, the non-rutting male that produced nasal roars only in summer and autumn did not adopt the specific vocal posture accompanying the rutting calls and vocalized with a less extended and only slightly inflated nose.
Flehmen
In the course of flehmen behaviour, rutting saiga males tense their noses in a way opposite to nasal roaring. The nasal vestibulum is retracted, its dorsal contours become concavely depressed and the open nostrils are directed anteriorly (Fig. 8). At the same time, the mouth is opened slightly.
Fig. 8.
Nose tension during flehmen behaviour according to video clip single frames (inset a) and photos (inset b). In contrast to the convex nose configuration while producing rutting roars, the dorsal nose contour is concavely shaped, the nasal vestibulum is retracted and the nostrils are upwardly directed. The mouth is slightly opened. (a) Right frontolateral view, (b) left lateral view.
Acoustic results
Throughout a loud nasal roar, a male keeps its nose extended until the call has ended. Fixed nose extension results in stable, non-descending tracks of the spectral envelope peaks, approximating the run of formants, throughout a call (Fig. 7). For five rutting males and one non-rutting male, we calculated mean formant values of loud nasal roars. Subsequently, we calculated the formant dispersion (ΔF) for each male. The vocal tract was approximated as a straight uniform tube closed at one end (glottis) and open at the other. For each male, a linear regression line fitted closely to the respective sets of observed formant values (0.9954 < r2 < 0.9998, P < 0.001 for all males) suggesting good agreement with the selected vocal tract model.
Table 2 presents mean values for call duration, fundamental frequency and central formant frequency of the first formant for calls of all six saiga males. anova showed highly significant interindividual differences in duration, fundamental and formant frequencies for the five rutting males. Vtls for the five rutting males, calculated from formant dispersion, varied from 401 to 441 mm (mean 431 ± 17 mm), probably as a result of individual differences of nose extension (Fig. 7). Alternatively, individual differences in vtls might result from differences of body size. Unfortunately, we could not estimate this influence because of lacking data on body mass of the subject males. The fundamental frequency of saiga is very low: 44.5 ± 5.6 in the rutting males and 52.9 ± 5.1 in the non-rutting male.
The calls of the non-rutting male were significantly longer, higher in fundamental frequency and higher in central formant frequency for the first formant than the calls of the rutting males (Table 2). The estimated apparent vtl of the non-rutting male was 356 mm, which is in good agreement with the measured nasal vtl of the dissected specimen with relaxed nose. The apparent vtl of the non-rutting male was noticeably shorter than the shortest apparent vtl (401 mm) among rutting males.
The differences in apparent vtls between all rutting males and the non-rutting male demonstrate that males producing rutting calls elongate their vts by approximately 20%. Behavioural results suggest that this vt elongation is effected by the specific change of nose configuration exclusively observed in rutting males.
The nasal roars of rutting males are the most intensive calls in the saiga's vocal repertoire. An amplitude difference between rutting and non-rutting calls was indicated by different respective recording levels while recording at similar distances. Apart from that, nasal roars of rutting saiga males were audible up to distances of 400–500 m, whereas the non-rutting roars were already inaudible at distances around 100 m. Neither females nor subadult saigas could produce calls of comparable intensity (our unpublished data).
Discussion
Nose extension during nasal roaring
The evolutionary reduction of the osseous nasal walls and the caudal displacement of the nasal conchae paved the way for active nose extension that strongly depends on the large and flexible nasal septum and the flexible interwoven musculature and connective tissue so characteristic for the saiga's nasal vestibulum. Nose extension consists of several distinct features adjusted simultaneously in different nose regions (Fig. 6c,d). Considering the anatomical results (Table 1), we may reasonably infer which of the muscles are involved in producing those features.
The pronounced transverse folding of the proximal nasal dorsum most probably is effected by contractions of the m. lev. nasolab. and of caudal fibres of the fan-shaped m. lev. lab. sup. The contracting caudal fibres of the m. lev. lab. sup. and lateroventral fibres of the m. lev. nasolab. should also be involved in the pronounced lateral constriction of the proximal third of the nose. The bulging of the lateral vestibular recess might be caused by inclusion of a small pressurized air volume requiring a tight closure of its rostral opening; thereby, its contours are prominently accentuated. Inflation of the lateral vestibular recess should occur during that inhalation immediately preceding vocal exhalation. Contractions of the rostroventral fibres of the fan-shaped m. canin. are ideally suited to produce the pronounced convex curving of the flexible middle vestibular portion between the lateral vestibular recess and the nostrils. By this action, which synchronously bends the nostrils anteriorly, the rostral two thirds of the nose adopt almost an S-shape. The combined action of three muscles may be responsible for the pronounced pulling of the distal third of the vestibulum ventrally around the closed mouth opening. These muscles are the fan-shaped inserting fibres of m. zyg., the rostrolabial portion of m. orbic. oris and, most important, m. incis. sup. Owing to the conspicuous apomorphic bending of the fibres of m. incis. sup. and their extensive insertion to the ventral wall of the nasal vestibulum (Fig. 3a), their contraction will pull the floor of the nasal vestibulum rostrally. This will result in a pronounced elongation of the nose so that the distal nasal tubes overhang the mouth considerably.
The pronounced inflation of the middle main portion of the nasal vestibulum requires a strong vocal exhalation. Probably, this also provides the pressure by which the air-filled lateral vestibular recess is displaced laterally against the m. lev. lab. sup.
Medial tilting and dilation of the nostrils is supposedly effected by co-operation of three muscles: the fan-shaped insertion of m. zyg., the rostrolabial portion of m. orbic. oris, and the m. lat. nasi. Dilation might result in part from the pronounced exhalatory inflation of the nose. Interindividual differences of nose extension (Fig. 7) might provide a basis for individual acoustic recognition of harem-holding males by male and female conspecifics (Table 2).
Vocal tract elongation by nose extension
Compared with vt elongation by oral roaring in red and fallow deer, rutting saiga males have evolved a strikingly different mechanism of vt elongation. During the oral roaring of red deer and fallow deer, the larynx is retracted to elongate the vt. In the nasal roars of saiga, however, the main part of vt elongation is achieved by temporary rostral protraction of the nasal vestibulum.
The oral roaring of red and fallow deer is emitted with open mouth in a specific roaring posture in which the head is held up and the ventral neck region extended (Fitch & Reby, 2001; McElligott et al. 2006). In red deer, minimum formant frequencies, achieved during maximal retraction of the larynx, are limited by an anatomical constraint and therefore still provide honest information on the body size of a caller (Reby & McComb, 2003a; Reby et al. 2005). The anatomical constraint limiting further retraction of the larynx in fallow deer remains to be investigated. In red deer, a strong relationship between the minimum formant frequencies and reproductive success has been demonstrated (Reby & McComb, 2003b; Charlton et al. 2007). Red deer can achieve an internal temporary vt elongation of about 100%, fallow deer of about 50% (McElligott et al. 2006). Acoustic assessment of a rutting male's body size may prevent unnecessary fighting and at the same time may attract potential mating partners.
Saiga males during their nasal roaring also adopt a specific posture in which the head is held up in an almost horizontal position and the ventral neck region extended (Fig. 6b,c). As in roaring cervids, this produces a certain temporary elongation of the pharyngeal portion of the vt. In rutting saiga males, however, most of the vt elongation is external and achieved by nose extension, in particular by protraction of the vestibular floor and pronounced S-shaping of the nasal vestibulum. Both an acoustic comparison between rutting and non-rutting calls of adult saiga males and an optical comparison between vtls achieved with extended and relaxed noses among rutting males suggest a mean vt elongation of about 20% during the production of rutting calls (Table 2).
A possible anatomical constraint that may limit rostral vt elongation in saiga is the length of the m. incis. sup. (Fig. 3a) effecting the protraction of the vestibular floor. In addition, the size of the nasal vestibulum itself may be restricted by basic requirements for breathing, e.g. to retain tolerable flight performance.
Primary evolution of the saiga nose as a consequence of ecological selection pressures has led to a certain morphological elongation of the rostronasal parts in both sexes. We can reasonably expect that nose size differences between males and females in this phase evolved according to body mass proportions. The continuously improving function of the nasal vestibulum as a dust filter provoked regular forced expirations through the nose to get rid of mucus-enveloped dust particles. The progressive reduction and caudal displacement of the nasal conchae resulted in a literally ‘empty’ nasal vestibulum that certainly absorbs less acoustic energy in comparison with typical noses of other ungulates, e.g. that of sheep, Ovis ammon f. aries, (Fitch, 2000b). Repetitive forced expirations, in combination with an ‘empty’ nasal vestibulum, might have favoured preferential calling through the nose rather than through the mouth in both sexes.
In view of the polygynous mating system of saiga and of male vocal display during the rut, this might have exposed the nose of the males to the influence of sexual selection. Males with longer noses, i.e. with longer nasal vocal tracts, were able to produce lower resonance frequencies and thus to signal larger body size, better fighting abilities (better quality) to male and female conspecifics. By male–male competition and female choice this could have triggered a subsequent evolutionary phase of intra- and inter-sexual selection that ultimately led to the pronounced sexual dimorphism of today's saiga noses.
Apart from active vt elongation, an elongation of vtl per se can be achieved by roaring through the nose rather than via the oral cavity because nasal vtl exceeds oral vtl by 20% (Fig. 3b). Thus, theoretical considerations suggest that rutting saiga males should perform their roaring via the nose to maximize vtl and to minimize formant frequencies.
Judging from behavioural observations, male saigas keep the mouth closed while producing their rutting calls so that they are exclusively emitted via the nose. Therefore, these rutting vocalizations are referred to as ‘nasal roaring’.
Red and fallow deer often begin their oral roaring (groaning) bouts with common roars (groans) while their larynges are not yet fully retracted and vtls have not yet reached their maximum values. As a consequence, formant frequencies are decreasing throughout the initial common roars (groans) of a bout and only the following common and harsh roars (groans) produce minimum formant values representing the intended maximal signal to body size (Fitch & Reby, 2001; Reby & McComb, 2003a; McElligott et al. 2006; Vannoni & McElligott, 2007). However, immediately prior to escalating agonistic bouts of harsh roars into fighting, red deer stags of about equal size maximally retract their larynges right before the onset of roaring, thereby avoiding formant descending that could acoustically betray their actual (smaller) body size (Reby & McComb, 2003b; Reby et al. 2005). In contrast, saiga males always start calling with a perfectly extended nose, i.e. with a maximally elongated vt. This results in a stable, non-descending run of formant frequencies throughout a nasal roar. Compared to oral roaring of red deer (> 1.5 s – cf. Reby & McComb, 2003b), saiga nasal roars are relatively short (418 ms on average in rutting males). Accordingly, if it occurs at all, acoustic perception of formant descending during a saiga nasal roar would tend to be more difficult than in the longer oral roars of red deer. Apart from acoustic aspects, the short duration may reduce the exhalative loss of water vapour and heat, and be energetically advantageous for saiga males during the strenuous rutting period.
Provided our interpretation of the laterally bulging structure, constantly associated with nasal roaring, as the hard-inflated lateral vestibular recess is correct, this feature may point to an additional function of nose-extension. Apart from providing an additional surface of extra seromucous secretions for the collection of inhaled particles (Clifford & Witmer, 2004), the paired air-filled cushion flanking the main vocal exhalatory air stream might act as some kind of resonating chamber. However, we did not follow this line of investigation.
Male elephant seals (Mirounga angustirostris and Mirounga leonina) also inflate their greatly enlarged nasal vestibulum while emitting their loud rutting roars (Sanvito & Galimberti, 2000). In contrast to saiga, however, the mouth is wide open during the roars, suggestive of oral roaring. The recent findings on formant frequencies in the M. leonina suggest that most of the vocal output is produced through the mouth, whereas the proposed nasal formant is very low in amplitude (Sanvito et al. 2007a,b). The hard-inflated proboscis might function as a resonator, increasing the amplitude of the roars, which can be heard in a 500–1000 m range (Bartholomew & Collias, 1962; Southall et al. 2003). Accordingly, the elephant seal nose might primarily serve as a visual signal, and only secondarily meet an acoustic function, as has been discussed for the hood-and-nasal-septum display of rutting male hooded seals (Cystophora cristata) (Terhune & Ronald, 1973; Ballard & Kovacs, 1995).
Larynx position and nasal roaring
Compared to oral roaring in cervids, nasal roaring requires (preferably all of) the exhalatory air to be guided via the choanae through the nostrils. This has some morphofunctional implications with regard to larynx position. Sealing the oral cavity off from the nasal air passage is achieved by keeping the epiglottis dorsally and in close contact with the soft palate (intact velar-epiglottal seal, velar port open). The flexible caudal portion of the thyrohyoid and the resilient thyrohyoid connection suggest a rostrodorsal tilting movement of the larynx around the thyrohyoid articulation in saiga. This would tend to push the epiglottis dorsally and prevent the escape of exhalatory air into the oral cavity. The dorsoventrally telescoped corniculate process certainly facilitates this movement by its less space-demanding shape between laryngeal entrance and dorsal pharyngeal wall. By contrast, a pronounced retraction of the larynx, synchronous to vocalization, would temporarily disrupt the contact between the soft palate and the small epiglottis of saiga.
In humans, with a permanently descended larynx, an alternative to the velar-epiglottal seal can be achieved by synchronous lowering of the soft palate and raising of the body and root of the highly mobile tongue and by keeping both in close apposition, thereby establishing a velar-lingual seal, as, for example, in the human speech sound ‘ng’ in ‘sing’ (cf. Ladefoged, 1996; Stevens, 2000). However, this is less applicable in typical ruminants with their undescended larynx and a much less mobile tongue. Any pronounced retraction of the larynx will automatically depress and likewise retract the tongue, as its root is firmly attached to the basihyoid (Nickel et al. 1979). Therefore, formation of a strong velar-lingual seal, which would be necessary to direct a forced exhalatory air stream exclusively through the nasal vt and to produce a loud nasal roar while the mouth is kept open, is more difficult to achieve, if not impossible. In other words, a strong velar-lingual seal would automatically impede any substantial retraction of the larynx. This dilemma might be overcome by an elongated soft palate and/or an enlarged epiglottis, neither of which are present in saiga.
Thus, a trade-off between nasal roaring and larynx retraction can be assumed. Accordingly, we do not expect a pronounced internal vt elongation by temporary retraction of the larynx in saiga.
Larynx, vocal fold structure and sound source frequency
A thyroid bulla, as in the larynx of the saiga (cf. Fig. 11 of Murie, 1870; Figs 3, 4), has also been reported for other mammals, e.g. several marsupial species and, among bovids, for the eland (Tragelaphus oryx) and the takin (Budorcas taxicolor) (Göppert, 1937, p. 860, Frey & Hofmann, 2000, cf. Frey et al. 2007). However, a functional explanation for its evolution is missing so far. Considering the structure and close topographic relation to the vocal folds, we may speculate that the thyroid bulla functions as a resonating chamber increasing the amplitude of the low frequency vocal fold oscillations.
The acute angle of the saiga's vocal fold relative to the long axis of the trachea is reminiscent of the situation in some pinniped species (Schneider, 1962, 1964). Owing to the oblique position of the vocal fold, more fibres of the thyroarytenoid muscle cross the vocal fold than would be the case in a more parallel arrangement of both structures. In pinnipeds the resulting tighter closure of the intermembranaceous portion of the glottic cleft is advantageous for diving (Schneider, 1962, 1963). As in pinnipeds, the oblique position of the saiga's vocal fold can be expected to effect a more powerful control of the vocal fold and glottic cleft. The considerable relative length of the vocal process of the arytenoid cartilage and the overlapping strong ventricularis and vocalis muscles in the saiga support this view. By contrast to pinnipeds, the necessity for better control might be related to the remarkable structure of the saiga's vocal fold, in particular to its mass increase as a consequence of the ovoid resilient fat pad included in the rostroventral margin of the vocal fold (cf. Fig. 11 of Murie, 1870; Fig. 4). The division of the saiga's vocal fold into two portions, a flexible and a tough resilient portion, is reminiscent of the situation in the roaring felids (Hast, 1986, 1989; Peters & Hast, 1994), in the takin (Frey & Hofmann, 2000) and in the muskox (Ovibos moschatus) (Frey et al. 2006).
The low fundamental frequency of nasal roars of saiga (37–53 Hz in different males) may be caused by the fat pads contained in the vocal folds, as was proposed earlier for roaring felids (Peters & Hast, 1994) and for the takin (Frey & Hofmann, 2000), but not for the muskox (Frey et al. 2006). At the same time, the fundamental frequency of male saiga nasal roars was found to be substantially lower than those reported for the roars of other bovids also possessing fat pads on their vocal folds: about 500 Hz in male Mongolian gazelle (Procapra gutturosa) (Frey & Gebler, 2003), about 210 Hz in the takin (Frey & Hofmann, 2000; Gebler & Frey, 2005), and 90–120 Hz in the muskox (Gebler & Frey, 2005; Frey et al. 2006). However, for the roars of takin and muskox a second, much lower frequency (‘pulse rate’) of about 20 Hz has been reported that is comparable to the fundamental frequency of saiga roars (Gebler & Frey, 2005; Frey et al. 2006).
Cervids show fundamental frequencies of rutting calls that are comparable with saiga: 35 Hz in fallow deer (Reby et al. 1998), 65–142 Hz in red deer (Reby & McComb, 2003a), 34 Hz in the Corsican subspecies of red deer (Reby & McComb, 2003b) and 55 Hz in Scandinavian reindeer (Rangifer tarandus) (Frey et al. 2007). The mean fundamental frequency of female African elephants when producing their contact calls is near 17 Hz (McComb et al. 2003).
As has been proposed for the takin (Frey & Hofmann, 2000), the vocal fold fat pads in male saigas may enhance the amplitude of oscillations and, correspondingly, the intensity of calls. At the moment we do not know whether or not the female's vocal fold is also supported by a fat pad.
Formant frequencies and vtl measurements
As in most studies on non-human mammals applying LPC-analysis to the estimation of formant dispersion, we used a uniform tube model to roughly calculate apparent male saiga vtl. It demonstrated good agreement between real and apparent vtls for rhesus macaques (Fitch, 1997); domestic dogs (Canis lupus f. familiaris) (Riede & Fitch, 1999); African elephants (Loxodonta africana) (McComb et al. 2003); red deer (Fitch & Reby, 2001; Reby & McComb, 2003a) and fallow deer (McElligott et al. 2006). This simple model can also be applied to get estimates of relative vt elongation that occurred during rutting calls with perfectly extended nose vs. non-rutting calls with almost relaxed nose.
Application of an LPC-based analysis to male saigas’ loud nasal roars yielded, however, an unexpected high value for the first formant (about 380 Hz). In addition, odd formants F1, F3, F5, F7 and F9 of all male loud nasal roars were expressed considerably more strongly than the even formants F2, F4, F6 and F8. The poorly distinguishable even formants introduced major difficulties into the analysis of most calls. In some primate species, formants may shift considerably due to the non-uniform vts (Riede et al. 2005, 2006a) or to inflation of a subhyoid air sac (Harris et al. 2006). In addition, data from bird acoustics suggest that the connection of resonators of variable volumes to the vt may explain the paradoxical formant spacing as a masking of intrinsic resonances that causes the appearance of additional strongly expressed power peaks (Riede et al. 2006b). Probably, this also holds for saiga, as cross-sectional areas along the nasal tract vary considerably both in the resting and in the extended nose. Apart from that, nose extension possibly includes the connection of additional resonance volumes to the saiga's vt, e.g. the lateral vestibular recess and the (rostrally closed) oral cavity. Morpho-acoustic investigations of the human nasal and paranasal cavities have convincingly demonstrated that solid-walled additional side chambers function as Helmholtz resonators and profoundly influence the acoustic transmission characteristics by causing antiresonances and changing formant shape (Dang & Honda, 1994, 1996). As the oral cavity and the paranasal sinuses, provided with yielding walls of finite impedance, each had its own antiresonance frequency in humans (Dang & Honda, 1994), we may reasonably expect specific antiresonance frequencies of the oral cavity and of the lateral vestibular recess and corresponding alterations of formants in saiga.
In view of the substantial deviation of the shape of the saiga's vt from that of an ideal uniform tube and of the presence of potential additional resonators, more sophisticated analyses (vt cross-sectional modelling, cineradiographic analysis of vocalizing saiga males, etc.) are required to elucidate relationships of these issues with formant spacing.
Nose corrugation during flehmen behaviour
Flehmen is a behaviour of adult males of many mammalian species aimed to detect the first signs of estrus or to confirm diestrus in females, by leading female urine or vaginal secretions during inhalation through the slightly opened mouth via the incisive ducts into the vomeronasal organs (vno) for analysis (Estes, 1972; Ladewig & Hart, 1980, 1982; Wysocki et al. 1980; Crump et al. 1984). Flehmen is characterized by a distinct facial expression (facial grimace) occurring, for example, after sniffing or licking the genitals or the fresh urine of a female, and thus serves as an external cue that an individual is using its vno (cf. Estes, 1972; Ladewig & Hart, 1980, 1982; Ladewig et al. 1980; Melese-d’Hospital & Hart, 1985).
Flehmen behaviour in rutting saiga males is remarkable as, in consequence of the dramatic evolutionary reductions of the osseous nasal walls, it not only involves the upper lips and nostril region, but also extensive parts of the now flexible wall regions of the nasal vestibulum (Fig. 8a,b). Flehmen does not invoke an inflation of the lateral vestibular recess. Instead, the concerned area is retracted caudally as indicated by rostrally concave skin folds below the eye. In addition to the muscles that effect the convex shape for nasal roaring (Table 1), the m. mal. and the m. cutan. fac. may be involved in this retraction (cf. Frey & Hofmann, 1997).
Basically, the specific concave ‘flehmen-shape’ of the saiga's nose is formed by the same muscles as those identified for flehmen in domestic ruminants and white-tailed deer (Odocoileus virginianus) by Dagg & Taub (1970). In saiga, however, some of these muscles have greatly increased their respective area of action according to the evolutionary reduction of the osseous nasal walls.
The conspicuous concave constriction of the main middle portion of the flexible nasal vestibulum is probably produced by strong combined contractions of the rostral fibres of the m. lev. lab. sup. and the m. canin. This pronounced dorsoventral compression of the middle portion will already cause a certain lifting of the nostrils, which then are directed rostrodorsally. Probably, this action will be enhanced by contractions of the insertional fibres of the m. zyg. and of the m. lat. nasi. Synchronously, the mouth is slightly opened.
In other mammals the nostrils are restricted or closed for the duration of flehmen (Dagg & Taub, 1970; Estes, 1972). A synchronous blocking of the oral passageway to the trachea by the epiglottis may result in a suction of air through the incisive ducts. This would suck out the contents of the vno into the nasal cavity by a Venturi effect; the resulting low pressure within the vno would tend to promote the influx of liquid (or air) into the vno from the oral cavity (Dagg & Taub, 1970; Estes, 1972).
In saiga, however, the nostrils remain open during flehmen. This might be related to its simple circular nostrils, which cannot be closed as easily as the primitive slit-like nostrils supported by specific cartilages entailing a sigmoid air flux. Flehmen with open nostrils, however, would tend to impede inhalation through the incisive ducts. Accordingly, the transport of substances into the vno would have to rely more strongly on the vascular vomeronasal pump (cf. Estes, 1972; Eccles, 1982; Meredith, 1994) than on inspiration through the incisive ducts in saiga. As an alternative, one could assume a blockage of the dorsal nasal airway proximal to the apomorphic nostrils by that conspicuous concave nose tension of saiga. This would be facilitated by the dorsally bulging floor of the nasal vestibulum owing to the powerful m. incis. sup. (Fig. 3a). Despite open nostrils, this would allow for inhalation through the incisive ducts into the separated and narrow ventral nasal recess of saiga, thereby promoting substance transport into the vno.
Modified nasal regions in other species
Apart from saiga, modified nasal regions that are related in some way or other to vocalizations were independently evolved by a number of species among different mammalian taxa, e.g. in some microchiropteran families that emit echo-locating signals via the nose (Megadermatidae, Rhinolophidae, etc.) (Suthers & Fattu, 1973; Pedersen, 1993), proboscis monkeys (Nasalis larvatus) (Kawabe & Mano, 1972; Ravosa, 1991; Owren, 1994; Messeri & Trombi, 2000), elephants (Loxodonta africana, Elephas maximus) (Boas & Paulli, 1908; McComb et al. 2003), tapirs (Tapirus) (Witmer et al. 1999; Gilmore et al. 2006), dikdiks (Madoqua guentheri, Madoqua kirki) (Frey & Hofmann, 1996; Kingswood & Kumamoto, 1996, 1997), elephant seals (Mirounga angustirostris, M. leonina) (Bartholomew & Collias, 1962; Sanvito et al. 2007a,b), hooded seal (Cystophora cristata) (Terhune & Ronald, 1973; Wiig, 1985; Ballard & Kovacs, 1995), and Cetacea, particularly odontocetes (e.g. Physeter macrocephalus, Hyperoodon ampullatus) (Cranford et al. 1996). However, not all of those mammals that vocalize via the nasal tract have dramatically transformed noses. The outward appearance of the noses of Dorcas gazelle (Gazella dorcas) and Speke's gazelle (G. spekei), except for a few rostrodorsal transverse folds, do not deviate much from the typical bovid pattern. G. dorcas and particularly G. spekei can inflate the small rostral vestibular portion of their noses considerably for vocalizing (see Brent Huffmann, http://www.ultimateungulate.com or http://www.csew.com/antelopetag/Professional%20Site/Prof%20Bio% or http://community.webshots.com/photo/42511421/1070864044030272744WDnUkB). Even the American bison (Bison bison), with its typical non-transformed bovid nose, is capable of nasal calling (http://www.moscowzoo.ru/get.asp?id=C135).
Correspondingly, evolutionary transformation of the nasal region affects the correlated osseous features of the skull to varying degrees. In the following list, the respective amounts of osteological transformation appear in the order from the most pronounced to almost unnoticeable: cetacea, elephants, saiga, tapirs, Guenther's dikdik, Kirk's dikdik, elephant seals, hooded seal, proboscis monkey, nasally vocalizing microchiroptera, Dorkas and Speke's gazelle.
The frequencies of vocalizations produced via transformed nasal regions comprise extremely high-pitched ultrasound (Microchiroptera), high-pitched whistling sounds (tapirs, dikdiks) and low-pitched sounds (saiga, elephants). In the Dorcas gazelle the sound produced is reminiscent of the quacking of a duck and in Speke's gazelle, which can inflate its rostral nose region up to half the size of a tennis ball, the sound is similar to that of a muffled pistol shot (Walther, 1979, p. 439).
Calculations of vtl from formant spacing in female elephants suggest that the morphologically greatly elongated nasal tract, including the trunk, and, possibly, a capacious pharyngeal cavity, resulting from a caudally retracted larynx, are involved in contact calls (Shoshani, 1998; McComb et al. 2003). The extraordinary length of the nasal vocal tract, i.e. a very long supralaryngeal filter, promotes the formation of exceptionally low resonance frequencies (but still above the infrasonic level) well suited for long-distance (1.5–3 km) communication (McComb et al. 2003). The elephant trunk, a homologue of the nasal vestibulum (and the upper lip), does not contain any conchae, which, as in saiga, have become evolutionarily shortened, dorsally inclined and caudally displaced.
Hypothetical step-by-step model for the evolution of the saiga nose
Combining sporadic evidence for the use of little specialized bovid noses in vocalization with ecological requirements of a dry-steppe scenario, the following step-by-step model for the evolution of the saiga nose arises.
(1) Increasing use of the amble as an energetically advantageous mode of fast locomotion by a smaller-sized bovid, inhabiting a flat habitat devoid of trees and major obstacles for quick running promoted the initial evolution of the unique saiga nose. Primitive, slit-like nostrils are still supported by specific cartilages. The nasal vestibulum is small and most parts of the nasal cavity are protected by rigid osseous walls.
(2) A low head position is advantageous for ambling (better extension of forelimbs through greater freedom of shoulder musculature). However, large dust clouds are produced by the moving herd. As a consequence, the slit-like nostrils have to be opened more widely and the small nasal vestibulum will become increasingly susceptible to getting clogged with dust concretions potentially resulting in reduced efficiency of breathing.
(3) Evolution of slightly larger nasal vestibula by a slight caudal displacement of the primitive nostrils and formation of short integumental tubes rostral to the primitive nostrils. Thereby, the primitive nostrils become an integral part of the proximal nasal vestibulum. Concomitantly, secondarily derived circular nostrils, lacking any cartilaginous support, evolve at the distal end of the short integumental tubes.
(4) A further increase in the amount of ambling in locomotion will lead to an increasingly larger and flexible nasal vestibulum at the expense of the nasal cavity proper in both sexes. This process comprises profound morpho-anatomical changes that lead to a dramatic shift of relative dimensions of the nasal cavities compared to a typical bovid nose (Frey & Hofmann, 1997; Clifford & Witmer, 2004). Supposedly, these transformations entailed a reduced efficiency of the primitive counter-current exchange of heat and water vapour effected by the respiratory region (cf. Nelson et al. 2007). The advantages of the derived nose structure must overcompensate those disadvantages. In this phase the size of the nasal vestibulum evolved equally in both males and females according to body mass proportions.
(5) The increasing demand for getting rid of filtered and mucus-covered dust particles from the nasal vestibulum provoked regular forced expirations through the nose. This specific behaviour favoured preferential calling through the nose rather than through the mouth in both sexes.
(6) Increasing use of the nasal airway by adult males for producing their rutting calls triggered the evolution of a sexual dimorphism by mechanisms of sexual selection. Nasal instead of oral roaring was favoured by selection because nasal vtl exceeded oral vtl. A longer vt will decrease formants and formant dispersion and signal an exaggerated body size to conspecifics. An anatomical constraint to further reduction of the nasal cavity proper and to further enlargement of the nasal vestibulum will keep the acoustic output an honest indicator of body size and provide information for rivals and for females (cf. Reby & McComb, 2003a; Charlton et al. 2007).
(7) Active elongation and tension of the flexible rostral nasal vestibulum added a further temporary elongation to the anatomical vtl, thereby influencing vocal output.
This study is an example of how limited a morphofunctional investigation must remain without including the investigation of live animals. In the case of the saiga's rutting calls, no one would have assumed such profound active changes of its nasal configuration by anatomical investigations alone.
Acknowledgments
For access to specimens, we thank Dr W. Zimmermann and Dr L. Kolter, Zoo Cologne, Germany. We thank the staff of Volokolamsk breeding centre of Moscow Zoo, particularly E. Kuprikova and V. Kashinin, for assistance in data collection. We thank A. Volodin for technical support. We are grateful to Dr T. Riede and Dr E. Vannoni for valuable discussion and to Dr A. Bannikova for provision of literature. While working with the saigas, we adhered to the ‘Guidelines for the treatment of animals in behavioural research and teaching’ (Anim. Behav. 65: 249–255) and to the laws of the Russian Federation, where the acoustic research was conducted. This work was supported by a grant from the Russian Foundation for Basic Research to IAV and EVV (RFBR 06-04-48400). Finally, we thank two anonymous referees for their constructive and inspiring comments.
Appendix 1: abbreviations
| App. hyo. | hyoid apparatus |
| Art. thyroh. | thyrohyoid articulation |
| Bul. thyr. | thyroid bulla |
| Cart. aryt. | arytenoid cartilage |
| Cart. cric. | cricoid cartilage |
| Cart. thyr. | thyroid cartilage |
| Cartt. nasi | nasal cartilages |
| Cav. nasi | nasal cavity |
| Duct. incis. | incisive duct |
| Duct. naslacr. | nasolacrimal duct |
| Epigl. | epiglottis |
| Lig. cricthyr. | cricothyroid ligament |
| muc. membr. folds | mucous membrane folds |
| m. buccinator | muscle of the cheek |
| m. canin. | caninus muscle |
| m. cricthyr. | cricothyroid muscle |
| m. cut. fac. | facial cutaneous muscle |
| m. depr. lab. sup. | depressor muscle of upper lip |
| m. frontalis | cutaneous muscle of frontal region |
| m. hyoepigl. | hyoepiglottic muscle |
| m. incis. sup. | superior incisive muscle |
| m. lat. nasi | lateral nasal muscle |
| m. lev. lab. sup. | levator muscle of upper lip |
| m. lev. nasolab. | nasolabial levator muscle |
| m. mal. | malaris muscle |
| m. masseter | large lateral muscle of mastication |
| m. orbicularis oculi | orbital muscle of the eye |
| m. orbic. oris | orbicular muscle of the mouth |
| m. sternthyr. | sternothyroid muscle |
| m. thyroaryt. | thyroarytenoid muscle |
| m. thyroh. | thyrohyoid muscle |
| m. ventric. | ventricular muscle |
| m. voc. | vocalis muscle |
| m. zyg. | zygomatic muscle |
| Oesoph. | oesophagus |
| Org. vomnas. | vomeronasal organ |
| Palat. mol. | soft palate |
| Phar. | pharynx |
| Plic. alarobas. | alarobasal fold |
| Plic. voc. | vocal fold |
| Proc. corn. | corniculate process |
| Rec. nas. ventr. | ventral nasal recess |
| Rec. vestib. lat. | lateral vestibular recess |
| Sept. nasi | nasal septum |
| Trach. | trachea |
| 1. trach. ring | 1. tracheal ring |
| Tubercc. dors. | dorsal tubercula of cervical |
| transverse processes | |
| Vestib. nasi | nasal vestibulum |
| Vno | vomeronasal organ |
| Vt | vocal tract |
| Vtl | vocal tract length |
References
- Ballard KA, Kovacs KM. The acoustic repertoire of hooded seals (Cystophora cristata. Can J Zool. 1995;73:1362–1374. [Google Scholar]
- Bannikov AG. Die Neue Brehm Bücherei. Wittenberg-Lutherstadt: A. Ziemsen Verlag; 1963. Die Saiga-Antilope (Saiga tataricaL.) No. 320. [Google Scholar]
- Bannikov AG, Jirnov LV, Lebedeva LS, Fandeev AA. Saiga Biology. Moscow: Agricultural Literature Press; 1961. [in Russian] [Google Scholar]
- Bartholomew GA, Collias NE. The role of vocalization in the social behaviour of the northern elephant seal. Anim Behav. 1962;10:7–14. [Google Scholar]
- Baryshnikov GF, Tikhonov AN. Notes on skulls of Pleistocene saiga of northern Eurasia. Hist Biol. 1994;8:209–234. [Google Scholar]
- Birrer M. Switzerland: Zoological Institute (Animal Behaviour), University of Zürich; The descended larynx in fallow deer (Dama dama) and its role in communication. Diploma thesis. [Google Scholar]
- Birrer M. Wildbiologie. Wildtier Schweiz; 2006. Das Kommunizieren der Damhirsche. Verhalten 8/14. [Google Scholar]
- Boas JEV, Paulli S. The elephant's head (studies in the comparative anatomy of the organs of the head of the Indian elephant and other mammals) First Part – the facial muscles and the proboscis with seventeen plates in colour. Jena: Gustav Fischer Verlag, 79 pp. [Google Scholar]
- Charlton B, Reby D, McComb K. Female red deer prefer the roars of larger males. Biol Lett. 2007 doi: 10.1098/rsbl.2007.0244. doi: 10.1098/rsbl.2007.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford AB, Witmer LM. Case studies in novel narial anatomy: 3. Structure and function of the nasal cavity of saiga (Artiodactyla: Bovidae: Saiga tatarica. J Zool London. 2004;264:217–230. [Google Scholar]
- Cranford TW, Amundin M, Norris KS. Functional morphology and homology in the odontocete nasal complex: implications for sound generation. J Morphol. 1996;228:223–285. doi: 10.1002/(SICI)1097-4687(199606)228:3<223::AID-JMOR1>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Crump D, Swigar AA, West JR, Silverstein RM, Müller-Schwarze D, Altieri R. Urine fractions that release flehmen in black-tailed deer, Odocoileus hemionus columbianus. J Chem Ecol. 1984;10:203–215. doi: 10.1007/BF00987849. [DOI] [PubMed] [Google Scholar]
- Dagg AI, Taub A. Flehmen. Mammalia. 1970;34:686–695. [Google Scholar]
- Dang J, Honda K. Morphological and acoustical analysis of the nasal and the paranasal cavities. J Acoust Soc Am. 1994;96:2088–2100. doi: 10.1121/1.410150. [DOI] [PubMed] [Google Scholar]
- Dang J, Honda K. Acoustic characteristics of the human paranasal sinuses derived from transmission characteristic measurement and morphological observation. J Acoust Soc Am. 1996;100:3374–3383. doi: 10.1121/1.416978. [DOI] [PubMed] [Google Scholar]
- Danilkin AA. Hollow-horned Ruminants (Bovidae) Moscow: KMK Scientific Press; 2005. [in Russian] [Google Scholar]
- Eccles R. Autonomic innervation of the vomeronasal organ of the cat. Physiol Behav. 1982;28:1011–1015. doi: 10.1016/0031-9384(82)90168-8. [DOI] [PubMed] [Google Scholar]
- Estes RD. The role of the vomeronasal organ in mammalian reproduction. Mammal. 1972;36:315–341. [Google Scholar]
- Fadeev VA, Sludsky AA. Saiga. In: Gvozdev EV, Kapitonov VI, editors. Mammals of Kazakhstan. Alma-Ata: Nauka; 1983. pp. 56–92. [in Russian], v.3, pt. 3. [Google Scholar]
- Fant G. Acoustic Theory of Speech Production. The Hague: Mouton; 1960. [Google Scholar]
- Fitch WT. Vocal tract length and formant frequency dispersion correlate with body size in rhesus macaques. J Acoust Soc Am. 1997;102:1213–1222. doi: 10.1121/1.421048. [DOI] [PubMed] [Google Scholar]
- Fitch WT. Skull dimensions in relation to body size in nonhumans mammals: the causal bases for acoustic allometry. Zool Anal Complex Syst. 2000a;103:40–58. [Google Scholar]
- Fitch WT. The phonetic potential of nonhuman vocal tracts: comparative cineradiographic observations of vocalizing animals. Phonetica. 2000b;57:205–218. doi: 10.1159/000028474. [DOI] [PubMed] [Google Scholar]
- Fitch WT, Hauser MD. Unpacking ‘honesty’: vertebrate vocal production and the evolution of acoustic signals. In: Simmons A, Fay RR, Popper AN, editors. Acoustic Communication, Springer Handbook of Auditory Research. New York: Springer; 2002. pp. 65–137. [Google Scholar]
- Fitch WT, Reby D. The descended larynx is not uniquely human. Proc R Soc Lond B. 2001;268:1669–1675. doi: 10.1098/rspb.2001.1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey R, Gebler A. The highly specialized vocal tract of the male Mongolian gazelle (Procapra gutturosaPallas, 1777 – Mammalia, Bovidae) J Anat. 2003;203:451–471. doi: 10.1046/j.1469-7580.2003.00232.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey R, Hofmann RR. Der Kopf der Saiga-Antilope (Saiga tatarica tataricaLinnaeus 1766, Mammalia: Bovidae) – Ausgewählte funktionsmorphologische Aspekte. I. Die Speicheldrüsen, die Mandibula und die Zunge. Zool Beitr (N.F.) 1995;36:169–198. [Google Scholar]
- Frey R, Hofmann RR. Evolutionary morphology of the proboscideal nose of Guenther's dikdik (Rhynchotragus guentheri –Thomas, 1894) (Mammalia, Bovidae) Zool Anz. 1996;235:31–51. [Google Scholar]
- Frey R, Hofmann RR. Skull, proboscis musculature and preorbital gland in the saiga antelope and Guenther's dikdik (Mammalia, Artiodactyla, Bovidae) Zool Anz. 1997;235:183–199. [Google Scholar]
- Frey R, Hofmann RR. Larynx and vocalization of the takin (Budorcas taxicolorHodgson, 1850 – Mammalia, Bovidae) Zool Anz. 2000;239:197–214. [Google Scholar]
- Frey R, Gebler A, Fritsch G. Arctic roars – laryngeal anatomy and vocalization of the muskox (Ovibos moschatusZimmermann, 1780, Bovidae) J Zool. 2006;268:433–448. [Google Scholar]
- Frey R, Gebler A, Fritsch G, Nygrén K, Weissengruber GE. Nordic rattle – the hoarse vocalization and the inflatable laryngeal air sac of reindeer (Rangifer tarandus. J Anat. 2007;210:131–159. doi: 10.1111/j.1469-7580.2006.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebler A, Frey R. Anatomical structures involved in non-human vocalization. In: Fuchs S, Perrier P, Pompino-Marschall B, editors. Speech Production and Perception: Experimental Analyses and Models. Berlin: Zentrum für Allgemeine Sprachwissenschaft, Typologie und Universalienforschung Berlin. ISSN 1435-9588; 2005. pp. 33–43. ZAS papers in Linguistics No. 40/2005, pp. [Google Scholar]
- Gilmore M, Carter T, O’Connell T, Shaw J. 3. Comparative behavioural study of two species of tapirs (Tapirus bairdii and Tapirus indicus; pp. 35–36. [Google Scholar]
- Göppert E. Atmungssystem (Organe der Luftatmung), I. Kehlkopf und Trachea. In: Bolk L, Göppert E, Kallius E, Lubosch W, editors. Handbuch der Vergleichenden Anatomie der Wirbeltiere. Berlin, Vienna: Urban & Schwarzenberg; 1937. pp. 797–866. Bd. 3. [Google Scholar]
- Harington CR, Cinq-Mars J. Radiocarbon dates on saiga antelope (Saiga tatarica) fossils from Yukon and the Northwest Territories. Arctic. 1995;48:1–7. [Google Scholar]
- Harris TR, Fitch WT, Goldstein LM, Fashing PJ. Black and white colobus monkey (Colobus guereza) roars as a source of both honest and exaggerated information about body mass. Ethology. 2006;112:911–920. [Google Scholar]
- Hast MH. The larynx of roaring and non-roaring cats. J Anat. 1986;149:221–222. [PMC free article] [PubMed] [Google Scholar]
- Hast MH. The larynx of roaring and non-roaring cats. J Anat. 1989;163:117–121. [PMC free article] [PubMed] [Google Scholar]
- Hauser MD, Evans CS, Marler P. The role of articulation in the production of Rhesus monkey (Macaca mulatta) vocalizations. Anim Behav. 1993;45:423–433. [Google Scholar]
- Heptner VG, Nasimovich AA, Bannikov AG. Mammals of the Soviet Union. In: Heptner VG, Naumov NP, editors. Ungulates. Vol. 1. Leiden: E.J. Brill; 1989. [Google Scholar]
- Jacobi A. Die Rüsselbildung bei Säugetieren der Gegenwart und Vorzeit. Jen Z Naturwiss. 1921;57:199–218. [Google Scholar]
- Jirnov LV. Moscow: Forest Manufacture Press; Returned to life (ecology, conservation and use of the saiga) [in Russian] [Google Scholar]
- Kawabe M, Mano T. Ecology and behavior of the wild proboscis monkey, Nasalis larvatus(Wurmb.), in Sabah, Malaysia. Primates. 1972;13:213–228. [Google Scholar]
- Kingswood SC, Kumamoto AT. Madoqua guentheri. Mammalian Species. pp. 1–10. No. 539.
- Kingswood SC, Kumamoto AT. Madoqua kirkii. Mammalian Species. pp. 1–10. No. 569.
- Ladefoged P. Elements of Acoustic Phonetics. 2. Chicago and London: University of Chicago Press; 1996. [Google Scholar]
- Ladewig J, Hart BL. Flehmen and vomeronasal organ function in male goats. Physiol Behav. 1980;24:1067–1071. doi: 10.1016/0031-9384(80)90049-9. [DOI] [PubMed] [Google Scholar]
- Ladewig J, Hart BL. Flehmen and vomeronasal function. In: Breipohl W, editor. Olfaction and Endocrine Regulation. London: IRL Press; 1982. pp. 237–247. [Google Scholar]
- Ladewig J, Price EO, Hart BL. Flehmen in male goats: role in sexual behaviour. Behav Neural Biol. 1980;30:312–322. doi: 10.1016/s0163-1047(80)91198-x. [DOI] [PubMed] [Google Scholar]
- Linné C. Systema naturae per regna tria naturae, secundum classes, ordines, genera, species, cum characteribus, differentiis, synonymis, locis. Laurentii Salvii, Holmiae. pp. 1–532. Tomus I. Editio duodecima, reformata.
- Lodyshenskaja WI. Morphology of the upper respiratory pathways of the saiga antelope. Scientific Papers of the Karelo-Finnish-University. 1952;4:17–41. [in Russian] [Google Scholar]
- McComb K, Reby D, Baker L, Moss C, Sayialel S. Long-distance communication of acoustic cues to social identity in African elephants. Anim Behav. 2003;65:317–329. [Google Scholar]
- McElligott AG, Birrer M, Vannoni E. Retraction of the mobile descended larynx during groaning enables fallow bucks (Dama dama) to lower their formant frequencies. J Zool. 2006 doi: 10.1111/j.1469-7998.2006.00144.x. [Google Scholar]
- Melese-d’Hospital PY, Hart BL. Vomeronasal organ cannulation in male goats: evidence for transport of fluid from oral cavity to vomeronasal organ during flehmen. Physiol Behav. 1985;35:941–944. doi: 10.1016/0031-9384(85)90263-x. [DOI] [PubMed] [Google Scholar]
- Meredith M. Chronic recording of vomeronasal pump activation in awake behaving hamsters. Physiol Behav. 1994;56:345–354. doi: 10.1016/0031-9384(94)90205-4. [DOI] [PubMed] [Google Scholar]
- Messeri P, Trombi M. Vocal repertoire of proboscis monkey (Nasalis larvatus, L.) in Sarawak. Folia Primatol. 2000;71:281–282. (abstract) [Google Scholar]
- Murie J. On the Saiga Antelope, Saiga tatarica(Pall.) Proc Zool Soc London. 1870;1870:451–503. [Google Scholar]
- NAV Nomina anatomica veterinaria [online] [2006 February 1];World Assoc Vet Anat. http://www.wava-amav.org/Downloads/nav_2005.pdf. [Google Scholar]
- Nelson JE, Christian KA, Baudinette RV. Anatomy of the nasal passages of three species of Australian bats in relation to water loss. Australian J Zool. 2007;55:57–62. [Google Scholar]
- Nickel R, Schummer A, Seiferle E. The Viscera of the Domestic Mammals. 2. BerlinHamburg: Paul Parey; 1979. [Google Scholar]
- Owren M. Handbook and Abstracts. Jakarta: Indonesian Wildlife Society; 1994. Tonal vocalisations recorded from adult male proboscis monkeys (Nasalis larvatus) show contest-specific formant variation; p. 174. [Google Scholar]
- Pallas PS. Berlin: Christianum Fridericum Voss; Ad Genus Antiloparum complementum, 14. Antilope saiga, 21–36.Descriptio Antilopes saigae, 36–45, Taf. I et III (fig.6 et 9–11). Spicilegia Zoologica quibus novae imprimis et obscurae Animalium Species Iconibus, Descriptionibus atque Commentariis illustrantur 12. [Google Scholar]
- Pedersen SC. Cephalometric correlates of echolocation in the chiroptera. J Morphol. 1993;218:85–98. doi: 10.1002/jmor.1052180107. [DOI] [PubMed] [Google Scholar]
- Peters G, Hast MH. Hyoid structure, laryngeal anatomy, and vocalization in felids (Mammalia: Carnivora: Felidae) Z Säugetierk. 1994;59:87–104. [Google Scholar]
- Pfefferle D, Fischer J. Sounds and size: identification of acoustic variables that reflect body size in hamadryas baboons, Papio hamadryas. Anim Behav. 2006;72:43–51. [Google Scholar]
- Ravosa MJ. The ontogeny of cranial sexual dimorphism in two Old World monkeys: Macaca fascicularis(Cercopithecinae) and Nasalis larvatus(Colobinae) Int J Primatol. 1991;12:403–426. [Google Scholar]
- Reby D, Joachim J, Lauga J, Lek S, Aulagnier S. Individuality in the groans of fallow deer (Dama dama) bucks. J Zool. 1998;245:79–84. [Google Scholar]
- Reby D, McComb K. Anatomical constraints generate honesty: acoustic cues to age and weight in the roars of red deer stags. Anim Behav. 2003a;65:519–530. [Google Scholar]
- Reby D, McComb K. Vocal communication and reproduction in deer. Adv Stud Behav. 2003b;33:231–264. [Google Scholar]
- Reby D, McComb K, Cargnelutti B, Darwin CJ, Fitch WT, Clutton-Brock TH. Red deer stags use formants as assessment cues during intra-sexual agonistic interactions. Proc R Soc Lond B. 2005;272:941–947. doi: 10.1098/rspb.2004.2954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riede T, Fitch T. Vocal tract length and acoustics of vocalization in the domestic dog (Canis familiaris. J Exp Biol. 1999;202:2859–2867. doi: 10.1242/jeb.202.20.2859. [DOI] [PubMed] [Google Scholar]
- Riede T, Bronson E, Hatzikirou H, Zuberbühler K. Vocal production mechanisms in a non-human primate: morphological data and a model. J Human Evol. 2005;48:85–96. doi: 10.1016/j.jhevol.2004.10.002. [DOI] [PubMed] [Google Scholar]
- Riede T, Bronson E, Hatzikirou H, Zuberbühler K. Multiple discontinuities in nonhuman vocal tracts – A response to Lieberman (2006) J Human Evol. 2006a;50:222–225. [Google Scholar]
- Riede T, Suthers RA, Neville HF, Blevins WE. Songbirds tune their vocal tract to the fundamental frequency of their song. PNAS. 2006b;103:5543–5548. doi: 10.1073/pnas.0601262103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanvito S, Galimberti F. Bioacoustics of southern elephant seals. I. Acoustic structure of male aggressive vocalisations. Bioacoustics. 2000;10:259–285. [Google Scholar]
- Sanvito S, Galimberti F, Miller EH. Vocal signalling of male southern elephant seals is honest but imprecise. Anim Behav. 2007a;73:287–299. [Google Scholar]
- Sanvito S, Galimberti F, Miller EH. Having a big nose: Structure, ontogeny, and function of the elephant seal proboscis. Can J Zool. 2007b;85:207–220. [Google Scholar]
- Schneider KM. Das Flehmen. Zool Garten (N.F.) 1930;3:183–198. [Google Scholar]
- Schneider KM. Das Flehmen (II. Teil) Zool Garten (N.F.) 1931;4:349–364. [Google Scholar]
- Schneider KM. Das Flehmen (III. Teil) Zool Garten (N.F.) 1932a;5:200–226. [Google Scholar]
- Schneider KM. Das Flehmen (IV. Teil) Zool Garten (N.F.) 1932b;5:287–297. [Google Scholar]
- Schneider KM. Das Flehmen (V. Teil) Zool Garten (N.F.) 1934;7:182–201. [Google Scholar]
- Schneider R. Vergleichende Untersuchungen am Kehlkopf der Robben (Mammalia, Carnivora, Pinnipedia) (Gegenbaurs) Morph. Jb. 1962;103:177–262. [Google Scholar]
- Schneider R. Der Kehlkopf der Klappmütze (Cystophora cristataErxleben 1777). Ein weiterer Beitrag zur Anatomie des Robbenkehlkopfes. Anat Anz. 1963;112:54–68. [PubMed] [Google Scholar]
- Schneider R. Der Larynx der Säugetiere. In: Helmcke J-G, Lengerken HV, Starck D, Wermuth H, editors. Handbuch der Zoologie. 8. Berlin, New York: Walter de Gruyter; pp. 1–128. [Google Scholar]
- Shoshani J. Understanding proboscidean evolution: a formidable task. TREE. 1998;13:480–487. doi: 10.1016/s0169-5347(98)01491-8. [DOI] [PubMed] [Google Scholar]
- Sokolov VE. Saiga tatarica. Mammal Species. pp. 1–4. No 38.
- Southall BL, Schusterman RJ, Kastak D. Acoustic communication ranges for northern elephant seals (Mirounga angustirostris. Aquat Mamm. 2003;29:202–213. [Google Scholar]
- Stevens K. Acoustic Phonetics. Cambridge, MA: MIT Press; 2000. [Google Scholar]
- Suthers RA, Fattu JM. Mechanisms of sound production by echolocating bats. Am Zool. 1973;13:1215–1226. [Google Scholar]
- Terhune JM, Ronald K. Some hooded seal (Cystophora cristata) sounds in March. Can J Zool. 1973;51:319–321. [Google Scholar]
- Titze IR. Principles of Voice Production. Englewood Cliffs: Prentice Hall; 1994. [Google Scholar]
- Vannoni E, McElligott AG. Individual acoustic variations in fallow deer (Dama dama) common and harsh groans: a source-filter theory perspective. Ethology. 2007;113:223–234. [Google Scholar]
- Walther F. Die Gazellen und ihre Verwandten. In: Grzimek B, editor. Grzimeks Tierleben. Munich: Deutscher Taschenbuch Verlag; 1979. pp. 431–449. Bd. 13. [Google Scholar]
- Wiig Ø. Morphometric variation in the hooded seal (Cystophora cristata. J Zool (Lond) 1985;206:497–508. [Google Scholar]
- Witmer LM, Sampson SD, Solounias N. The proboscis of tapirs (Mammalia: Perissodactyla): a case study in novel narial anatomy. J Zool (Lond) 1999;249:249–267. [Google Scholar]
- Wysocki CJ, Wellington JL, Beauchamp GK. Access of urinary non-volatiles to the mammalian vomeronasal organ. Science. 1980;207:781–784. doi: 10.1126/science.7352288. [DOI] [PubMed] [Google Scholar]