Abstract
Placodontia (Reptilia: Sauropterygia) is a group of enigmatic armored marine reptiles restricted to the Triassic time period. Only a single row of osteoderms dorsal to the spine is present in the basal placodontoid Placodus gigas, whereas derived cyamodontoids superficially resemble turtles in enclosing their body in an armor shell. Despite the extensive occurrence of the dermal armor in the derived cyamodontoid group, little research has focused on its bone histology and development. Here, I present an overview of the bone microstructures that reveals the unique presence of cartilaginous tissue in the postcranial armor plates. Placodont armor plates stand in contrast to osteoderms of other tetrapods that develop intramembraneously or through metaplastic ossification without cartilaginous preformation. The different developmental pathways leading to this ‘postcranial fibro-cartilaginous bone’ tissue found in placodont plates compared to the dermal bone tissues of most other tetrapod osteoderms indicate the non-homology of these structures. A resulting morphogenetic model of histogenesis is given to exemplify how the derived armor morphologies (i.e. spiked, flat polygonal and hexagonal, and rhomboidal shapes) together with the peculiar bone histologies could have developed through differential growth. In accordance with the pachyostotic limb bones of placodonts, the presence of the compact ‘postcranial fibro-cartilaginous bone’ is interpreted as an osteosclerotic trend in the armor plates which aids in buoyancy control and affects maneuverability and swimming speed.
Keywords: cartilage, dermoskeleton, osteoderms, Placodontia, skeletal histology
Introduction
Placodontia first appeared about 245 Ma ago in the lower Anisian, diversified during the Anisian and Ladinian, and died out 200 Ma ago at the end of the Late Triassic (e.g. Pinna, 1990; Pinna & Mazin, 1993). The taxon includes the largely unarmored Placodontoidea (Nopcsa, 1923; Rieppel & Zanon, 1997; Rieppel, 2000) and the heavily armored Cyamodontoidea (Nopcsa, 1923; Peyer & Kuhn-Schnyder, 1955; Westphal, 1975, 1976; Mazin & Pinna, 1993; Rieppel & Zanon, 1997; Rieppel, 2000). According to Rieppel (2000), the Placodontoidea are presumably paraphyletic with relationships being [Paraplacodus (Placodus, Cyamodontoidea)]. Members of Placodontia were thought to be restricted to the western Tethys and adjacent epicontinental seas of Europe and the Middle East (Westphal, 1975; Pinna, 1990; Pinna & Mazin, 1993; Rieppel, 2000), where they inhabited coastal, lagoonal, and shelf regions (Owen, 1858; Huene, 1933; Westphal, 1976; Mazin & Pinna, 1993). However, recent finds of cyamodontoid placodonts from Guizhou Province, China (Li, 2000; Li & Rieppel, 2002a,b), suggest a Tethys-wide distribution.
Dermal armor plates in placodontoids are restricted to the more derived placodontoid Placodus gigas from the Lower and Upper Muschelkalk of the Germanic Triassic. The most basal placodont genus Paraplacodus broilifrom the Anisian/Ladinian boundary (Besano Formation) of Monte San Giorgio, Switzerland, lacks any postcranial armor. P. gigas had a single median row of armor plates directly above its spine. Above the cervicals, the armor plates increase in size and rest directly dorsal to the neural arches. From the seventh cervical vertebra caudal to the beginning of the sacrum, the armor plates sit intermediate between two successive neural spines (Drevermann, 1933; Huene, 1933; Rieppel, 2000). For the caudals, the armor plates again become reduced in size and sit, similar to the cervicals, directly dorsal to the neural spines (Huene, 1933).
An overview of the cyamodontoid armor was recently given by Rieppel (2002). The torso is encased in a turtle-like armor shell (Gregory, 1946; Rieppel, 2002) and the armor is composed of a dorsal carapace that in some taxa can be split in two separate armor shields, and a ventral plastron. The armor usually comprises a pavement of numerous small, flat or spiked armor plates of round, polygonal or strictly hexagonal shape, as in Psephoderma alpinum. Similar to the condition in the turtle shell, the bony armor was covered by keratinous shields in life that generally did not overlap with the bony sutures (Westphal, 1975, 1976; Rieppel, 2002). As an exception, the horn shields in Psephosaurus suevicus may have been overlapping with the bone sutures (Rieppel, 2002: fig. 21 A1). Furthermore, Psephosaurus armor plates are known to diverge in morphology depending on their location. For example, the holotype of Psephosauriscus sinaiticus (HUJ-Pal. T.R.3421; Rieppel, 2002: fig. 28), previously figured and labeled as ‘Psephosaurus’ (Westphal, 1975: fig. 5; 1976: fig. 4), clearly shows differences between the shapes of carapacial (dorsal) and plastral (ventral) armor plates. Whereas the sutured carapacial plates are more hexagonal in shape with a ‘weak central elevation surrounded by a shallow circular zone of depression’, the plastral plates have ‘a cycloid superficial appearance and a rhomboidal basal outline’ (Rieppel, 2002: p. 27). An assignment of isolated armor plates to any given cyamodontoid genus usually remains tentative because most cyamodontoid armor plates show, or are derived from, a basic hexagonal pattern (Westphal, 1975, 1976; Rieppel, 2002). As it is only known from armor fragments, the systematic position of the genus Psephosaurus remains unclear.
The work of Westphal (1976) provided some bone histological data of placodont armor, and Buffrénil & Mazin (1992) and Ricqlès & Buffrénil (2001) discussed bone microstructures of the placodont endoskeleton, i.e. of limb bones. Based on the distinctiveness of the dermoskeleton and the endoskeleton in vertebrates (see Donoghue & Sansom, 2002), Westphal (1976: p. 36) implied that the armor of placodonts is indeed composed of dermal plates, i.e. true osteoderms, stating that ‘... in almost all placodonts the dermal armour had no strict relationship to the endoskeleton’.
During a comparative study (Scheyer, 2007) of armor histology in sauropsid amniotes, cartilage was found to be present in several placodont taxa. This is very surprising in light of the fact that generally among amniotes, postcranial dermal armor, i.e. osteoderms, develop without a preformation in cartilage (e.g. Francillon-Vieillot et al. 1990).
The purpose of this paper is to describe in detail the skeletal histology of placodont armor, to compare it with osteoderms of other tetrapod groups, and to test the hypothesis of the dermal origin of placodont armor. Furthermore, the question arises whether placodont armor plates show similarities or deviations in development compared to postcranial osteoderms of other amniote clades. Bone histological data of several placodont dermal armor elements are therefore briefly compared to the bone histologies of turtle shells and osteoderms of basal and derived archosaurs, lepidosaurs, and xenarthrans (Mammalia). Further comparison to fossil amphibian dermal elements from three temnospondyl taxa is provided to polarize the microstructural characteristics of amniote osteoderms in general. The data are combined into a morphogenetic model of histogenesis of placodont armor, used as a basis for developmental interpretation.
Materials and methods
Sampling strategy
For the current study, bony armor plates of placodont taxa were sampled, including one isolated plate of placodontoid P. gigas and 11 cyamodontoid elements (Fig. 1). The cyamodontoid sample included four elements of Psephoderma sp., four elements of Psephosaurus suevicus, two elements of Psephosaurus sp., and one element of cf. Placochelys sp. The latter specimen was recovered from sediments of the Carnian age (Upper Triassic) of southern Germany and strongly resembles some of the armor plates of the holotype of Placochelys placodonta from Hungary (Rieppel, 2002: p. 12, fig. 12).
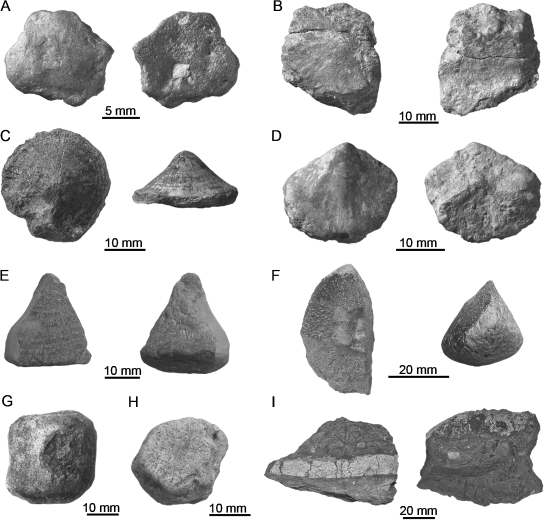
Fig. 1.
Sample of placodont armor plates. (A) External and internal view of roughly hexagonal plate of Psephosaurus suevicus (MHI 1426/3). (B) External and internal view of polygonal plate of P. suevicus (MHI 1426/2). (C) External and lateral view of recumbent spiked plate of P. suevicus (SMNS 91007). (D) External and internal view of procumbent spiked plate of P. suevicus (MHI 1426/1). (E) External and internal view of procumbent spiked plate of cf. Placochelys sp. (SMNS 91010). (F) External and lateral view of keeled plate of Placodus gigas (SMNS 91006). (G) External view of rhomboidal plate (plastron, SMNS 91007) of Psephosaurus sp. (H) External view of roughly hexagonal plate (carapace, SMNS 91008) of Psephosaurus sp. (I) Lateral view and external view of fused plates of Psephoderma sp. (NRM-PZ R.1759a) embedded in limestone matrix.
Four dermal bones of temnospondyl amphibians were sampled, including one element of the capitosaur Mastodonsaurus giganteus, one element of the plagiosaur Gerrothorax pustuloglomeratus, and two elements of the basal temnospondyl Trimerorhachis sp. Due to the fragmentary nature of the material from all three taxa, it could not always be ascertained whether the elements belonged to the skull or shoulder girdle, although, having a sutured margin, the specimen of M. giganteus is interpreted as belonging to the skull. Planes of sectioning of the material were chosen to lie either parallel or transversal to the ornamental sculpturing.
A sagittal thin-section through the proximal epiphysis of a long bone of the small pachypleurosaurid reptile Neusticosaurus pusillus (PIMUZ T4022) was studied for comparison with structures of fossil calcified cartilage.
Additional scanning electron microscope (SEM; Tescan Vega TS5130LM, Brno, Czech Republic) images were taken to analyze the fine structures of placodont armor plates. A polished planar section of specimen SMNS 91007 was etched for 3–5 s with hydrochloric acid (10%). After dilution of the acid with distilled water (to pH neutrality), the section was fixed, gold-coated and analyzed under the SEM (SE detection). All taxa, accession numbers, locality and other relevant morphological data have been compiled in Table 1.
Table 1.
Taxa, accession numbers, localities, and relevant morphological data of specimens used in the current study
| Taxon | Taxonomy | Accession No. | Locality | Morphology |
|---|---|---|---|---|
| Placodontia | ||||
| Placodus gigas Agassiz, 1834 | Placodontoidea | SMNS 91006 | Bühlingen near Rottweil, Germany (?Trochitenkalk Formation, Ladinian, Middle Triassic, ‘Upper Muschelkalk; Lower Hauptmuschelkalk`, ?m7) | Isolated plate from the anterior part of the dorsal vertebral column; round shape, slightly triangular in cross-section, maximum height: ~23 mm |
| Psephoderma sp. | Cyamodontoidea | NRM-PZ R.1759a | Wadi Raman (Makhtesh Ramon), Negev, Israel (Muschelkalk; Middle Triassic) | Row of five fused plates, polygonal (?hexagonal) shapes in dorsoventral view, maximum height: 10 mm |
| Psephosaurus suevicus Fraas, 1896 | Cyamodontoidea | MHI 1426/1-3 | Quarry ‘Hohenloher Steinwerk’, Kirchberg/Jagst (Erfurt Formation, Ladinian, Middle Triassic, k1, ‘Anthrakonit-Bank, Basisbonebed’) | Three isolated plates; one resembling a procumbent spike (MHI 1426/1) with apex and opposite semicircular margin and two with hexagonal/polygonal shapes (MHI 1426/2 & 3) |
| SMNS 91007 | Hoheneck near Ludwigsburg, Germany (Erfurt Formation, Ladinian, Middle Triassic, k1) | Isolated plate; round in externo-internal view with small blunt central spike, maximum height: 11 mm | ||
| Psephosaurus sp. | Cyamodontoidea | SMNS 91008 | Hoheneck near Ludwigsburg, Germany (Erfurt Formation, Ladinian, Middle Triassic, k1) | Isolated flat plate, roughly hexagonal shape, concave internal surface, maximum height: 6 mm |
| Psephosaurus sp. | Cyamodontoidea | SMNS 91009 | Hoheneck near Ludwigsburg, Germany (Erfurt Formation, Ladinian, Middle Triassic, k1) | Isolated plate, rhomboidal shape in externo-internal view; convex external surface with slightly raised off-lefted ridge, maximum. height: 14 mm |
| cf. Placochelys sp. | Cyamodontoidea | SMNS 91010 | Willsbach near Heilbronn, Germany (Grabfeld Formation, Lower Carnian, Upper Triassic, ‘Estherienschichten, Anatinabank’ k2) | Isolated spiked, procumbent plate, triangular shape in externo-internal view, maximum height: 12 mm; maximum length: 27 mm |
| Nothosauria | ||||
| Neusticosaurus pusillus (Fraas, 1881) | Pachypleurosauridae | PIMUZ T4022 | ?Meride Limestone (Middle Triassic), Monte San Giorgio, Southern Switzerland | Sagittal section of proximal epiphysis of long bone |
| Fossil Amphibia | ||||
| Mastodonsaurus giganteus(Jaeger, 1828) | Temnospondyli | SMNS 91011 | Kupferzell, southern Germany (Erfurt-Formation, Ladinian, Upper Triassic, ‘Lettenkeuper’) | Thick bone fragment of cranium (one sutured margin); strong external sculpturing pattern with ridges |
| Gerrothorax pustuloglomeratus(v. Huene, 1922) | Temnospondyli | SMNS 91012 | Kupferzell, southern Germany (Erfurt-Formation, Ladinian, Upper Triassic, ‘Lettenkeuper’) | Bone fragment of cranium or shoulder girdle?; external surface with sculpturing pattern of low ridges and tubercles |
| Trimerorachis sp. | Temnospondyli | TMM 40031-59 | Tit Mountain locality, Archer County, Texas, USA (Petrolia Formation, Lower Permian) | Bone fragment of cranium or shoulder girdle?; external surface with sculpturing pattern of reticular ridges in the left and radially arranged low ridges towards the margins |
| Trimerorachis sp. | Temnospondyli | TMM 40031-60 | Tit Mountain locality, Archer County, Texas, USA (Petrolia Formation, Lower Permian) | Bone fragment of cranium or shoulder girdle?; external surface with sculpturing pattern of reticular ridges in the left and radially arranged low ridges towards the margins |
Methodology and terminology
The study follows standard petrographic thin-sectioning procedures as described in Scheyer & Sander (2004), whereas the terminology of the skeletal (bone) histology is mainly based on Francillon-Vieillot et al. (1990), Scheyer & Sánchez-Villagra (2007) and Scheyer et al. (2007). All thin-sections were studied and documented with a binocular microscope (magnifications: 16× and 63×; normal transmitted light) and with a Leica DMLP® compound polarizing microscope (Wetzlar, Germany; magnifications: 40×, 100×, 400×; normal transmitted and polarized light, additional lambda compensator). The compound polarizing microscope was equipped with a special wide-field lens (1.6×) and a Nikon Coolpix®-LCD camera (E995, Tokyo, Japan).
Because the nature of the placodont armor is to be assessed herein, and based on the fact that osteoderms are not always completely ossified (Moss, 1969), the descriptive term ‘armor plate’ is used throughout the text. Some placodonts have a dorsal and a ventral armor, thus the terms ‘external’ and ‘internal’ instead of ‘dorsal’ and ‘ventral’ are used respectively for providing directions. ‘Internal’ thus means towards the body cavity and ‘external’ towards the epidermis.
Institutional abbreviations
The following abbreviations are used: HUJ-PAL, Paleontological Collections, Department of Evolution, Systematics, and Ecology, The Hebrew University, Jerusalem; IPB, Institute of Palaeontology, University of Bonn, Bonn, Germany; MHI, Muschelkalkmuseum Hagdorn Ingelfingen, Ingelfingen, Germany; NRM, Swedish Museum of Natural History, Stockholm, Sweden; PIMUZ, Paläontologisches Institut & Museum, Universität Zürich, Zürich, Switzerland; SMNS, Staatliches Museum für Naturkunde Stuttgart, Stuttgart, Germany; TMM, Texas Memorial Museum, Austin, Texas, USA.
Results
Skeletal histology of the placodontoid armor
P. gigas
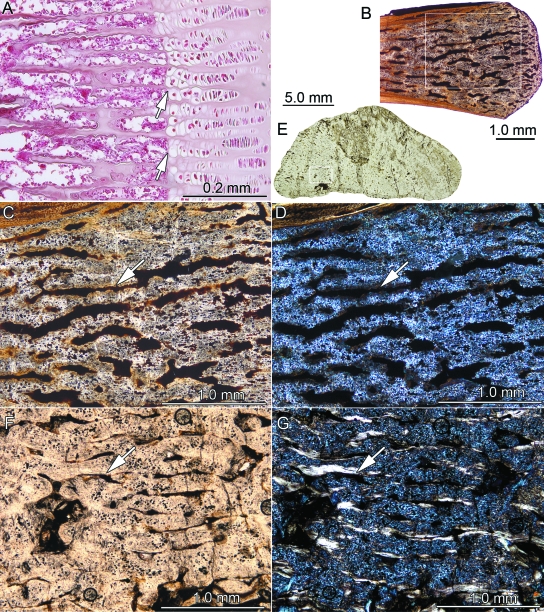
The thin-section shows a differentiation into a compact bone layer surrounding a poorly vascularized interior bone core (Fig. 2A–C). True spongy cancellous bone is not developed, and thus the core still appears compact. The external areas, including the sharp keel of the plate, consist of well developed external cortical bone. The bone tissues of the interior core and the poorly developed internal cortex appear superficially similar. No cartilage was found in the sample.
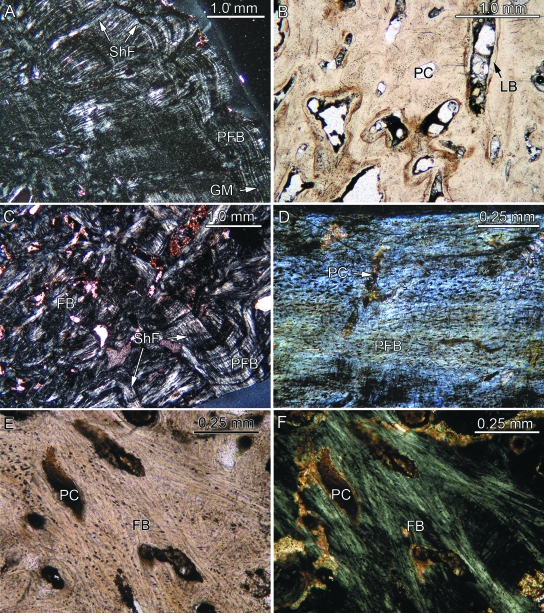
Fig. 2.
Bone histology of placodont armor plates that are completely ossified. With the exception of (B) and (E), which were taken in normal transmitted light, the images (A, C, D, F) were taken using polarized light. (A) Close-up of external cortex of Placodus gigas (SMNS 91006) showing parallel-fibered bone (PFB) with growth marks (GM) and Sharpey's fibers (ShF) extending perpendicularly and obliquely into the PFB. (B) Close-up of interior part of former specimen vascularized by primary vascular canals (PC) and primary osteons lined with lamellar bone (LB). (C) Close-up of internal cortex and part of interior core of former specimen. The interior of the plate consists of interwoven fiber bundles (FB) and prominent ShF. (D) Parallel-fibered bone of the external bone tissue of Psephosaurus suevicus (MHI 1426/2) poorly vascularized with primary canals. (E, F) Interior part of the former specimen. The bone tissue is vascularized by primary canals and consists of interwoven long, coarse FB. Many of the FB extend and focus towards the growth center of the plate (lying in the direction of the upper left corner of the images).
Primary parallel-fibered bone tissue (PFB) that locally grades into lamellar bone (LB) builds up the external cortical bone that covers both sides of the external keel (Fig. 2A). Cyclical growth marks (GM) appear throughout the external cortical bone and extend parallel to the external bone surfaces. The GM are mostly diffuse and are thus not as conspicuous as well defined lines of arrested growth. Numerous thin collagenous fiber bundles extend perpendicular to the PFB and LB of the external cortical bone. The wavy appearance of the external bone surfaces in thin-section results from foramina that insert perpendicularly into the external cortical bone. The foramina continue as vascular canals into the external cortical bone. The primary bone tissue of the external cortical bone is poorly vascularized by a few scattered anastomosing canals.
The interior core of the armor plate is poorly vascularized by scattered primary vascular canals and primary osteons. In general, the primary canals are arranged radially. The core is distinct from the similarly compact external and internal cortical bone in that it lacks PFB and LB. Instead, it contains a meshwork of randomly arranged structural fiber bundles. A few prominent fiber bundles extend from the margins of the interior bone core in the direction of the apex of the external keel.
The thin internal cortical bone at the base and the margins of the armor plate consist of PFB with GM extending sub-parallel to the internal surface of the bone (Fig. 2C). However, compared to the external cortical bone, the layers of the PFB are less distinct here because coarse and thick fiber bundles, i.e. Sharpey's fibers, extend into the PFB. The arrangement of the Sharpey's fibers depends on their exact location within the armor plate. Laterally, on both sides of the external keel, the Sharpey's fibers insert at a steep to moderate angle into the cortical bone and generally point towards the bone core (Fig. 2C). The vascularization of the bone tissue is low, with only a few scattered primary vascular canals that emerge as the foramina seen on the internal bone surface.
Skeletal histology of the cyamodontoid armor
Hexagonal/polygonal armor plates
The plates of Psephoderma sp. (NRM-PZ R.1759a), P. suevicus (MHI 1426/2 & 3), and Psephosaurus sp. (SMNS 91008) all share many bone histological characteristics and are described together. Variation among the taxa is pointed out where appropriate. The procumbent spiked plate of P. suevicus (MHI 1426/1) is included here too because it still retains a roughly hexagonal internal bone surface. The hexagonal plates consist of external cortical bone, distinct marginal areas, and an internal bone tissue including the central core of the plate and the bone towards the internal surface of the plate. A clear distinction between interior bone and a separate internal cortex is not possible. No cancellous bone, extensive secondary remodeling or fibro-cartilaginous tissue was encountered in the hexagonal plates.
The bone tissue of the external cortical bone and the lateral margins consists of intergradations of PFB (Fig. 2D) and LB with some poorly developed GM. Thin fiber bundles that are arranged perpendicular or at a steep angle to the external bone surface cross the bone lamellae of the external cortical bone. Towards the plate margins, the fiber bundles insert more diagonally into the bone tissue and curve slightly towards the interior core of the plate.
The vascularization consists mainly of primary vascular canals (Fig. 2D–F) and, to a limited extent, scattered primary osteons and small erosion cavities. The numerous primary vascular canals radiate outwards from a growth center to the surfaces of the bone. Those radiating canals that extend towards the external and internal cortical bone dominate the vascularization pattern, resulting in an hourglass structure of two vascularized conical areas surrounded by less vascularized areas that extend towards the plate margins. The fiber bundles of the interior core generally mirror the arrangement of the primary vascular canals (Fig. 2E,F). The center of the radiating vascularization pattern is shifted slightly towards the external cortical bone in Psephoderma sp., whereas it is shifted in an internal direction in specimen SMNS 91008 of Psephosaurussp. In specimen MHI 1426/1 of P. suevicus, the center lies near the margin of the plate. These configurations result in disparate distributions of vascular spaces in the samples, in that the conical areas extending towards the external and internal bone surface vary in size.
The interior core and the internal part of the bony plates (i.e. the growth center of the plate) consist of mostly randomly arranged fiber bundles. The vascularization pattern does not change from the interior core towards the internal bone surface, apart from a minor overall reduction in the amount of primary vascular canals.
The sutured plate margins show numerous bone pegs and sockets of various lengths into which the fiber bundles protrude. The sutural relief is generally low, although occasionally bony pegs interdigitate more strongly with adjacent armor plates. There are fiber bundles that extend perpendicularly into the sutural bone tissue.
Psephosaurussp. (SMNS 91009)
The thin-section of the rhomboidal plate exhibits three different tissue types: (1) PFB with radial vascularization, (2) dense regular fibro-cartilaginous tissue with spatially regular bony trabeculae and vascularization, and (3) loose irregular fibro-cartilaginous tissue with irregular short bony trabeculae.
The first tissue type (Fig. 3A) comprises the margins as well as one flank and the apex of the off-centered ridge of the plate, thus forming the majority of the external cortex that rests on the interior core of the plate. This tissue is characterized by PFB and cyclical GM that extend parallel to the external bone surface. Fiber bundles that extend perpendicular to the external bone surface cross the PFB lamellae. Vascularization of the PFB is achieved by anastomosing, radially arranged primary vascular canals and scattered primary osteons. The direction of the radial primary canals is roughly parallel to the perpendicular fiber bundles. The bone lamellae that directly surround the primary vascular canals often consist of fiber bundles that exhibit a fiber orientation deviating slightly from that of the interstitial PFB. Areas of secondary bone resorption and secondary osteons are not observed in the tissue.
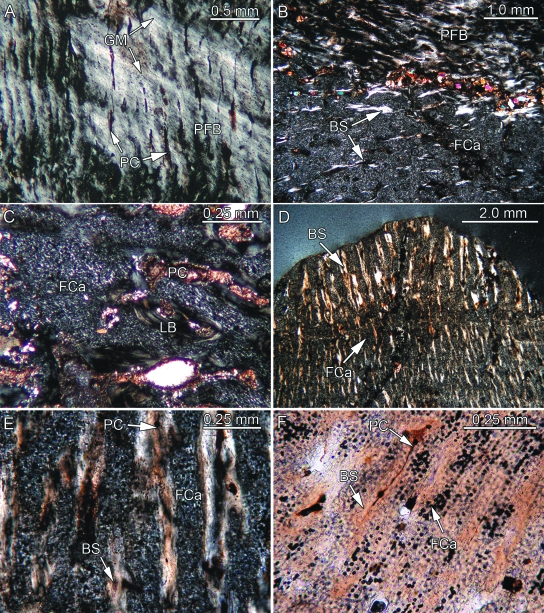
Fig. 3.
Bone histology of placodont armor plates that range from consisting partly to consisting completely of PFCB. Besides (F) all remaining images were taken in polarized light. (A) Close-up of parallel-fibered bone (PFB) and radial primary canals (PC) of external cortex of Psephosaurus sp. (SMNS 91009). (B) Close-up of transition between PFB and PFCB matrix, consisting largely of cartilage cell lacunae (FCa) and bone spiculae (BS), of the external cortex of former specimen. (C) Close-up of externally situated cartilaginous bone of cf. Placochelys sp. (SMNS 91010). Note that only a few BS are present. (D) Transition between coarse apical and fine interior PFCB of P. suevicus (SMNS 91007). Note differences in size and orientation of BS, as well as amount of interstitial FCa. (E) Close-up of the coarse PFCB of former specimen. (F) Close-up of the fine interior PFCB of former specimen. BS and PC alternate with regular layers of FCa.
The second tissue type, ordered fibro-cartilaginous tissue (Fig. 3B), is found as a broad wedge surrounded by the first tissue type described above. This second tissue type exhibits a fibro-cartilaginous matrix and a distinct radial orientation of bony trabeculae and vascularization, respectively. The cartilage cell lacunae show a slightly raised spatial organization, being aligned parallel to the radially arranged primary vascular canals and the associated bony trabeculae. Whereas the trabeculae are shorter and randomly arranged at the transition to the cartilaginous tissue of the core of the plate, their arrangement in the external cortex increasingly resembles the arrangement described for the first tissue type.
The third tissue type, loose fibro-cartilaginous tissue, is found in the interior of the plate, and it extends to the internal surface of the plate. A few isolated large vascular cavities, all surrounded by LB, occur throughout the plate. The core consists of a fibro-cartilaginous matrix similar to the one described above for the specimen SMNS 91007 of P. suevicus. However, the cartilage cell lacunae are arranged to form a loose tissue without a dominant spatial arrangement. Within the cartilaginous matrix, short and thin bony trabeculae are randomly arranged. The trabeculae usually do not connect with each other and do not form a trabecular meshwork. Only in the more lateral areas of the core tissue are a few of the trabeculae aligned more radially. The bony trabeculae are associated with short primary vascular canals and scattered primary osteons. Longer primary canals are not observed in the tissue.
Cf. Placochelys sp. (SMNS 91010)
The spiked armor plate is made up of two areas with different tissue types. The first occupies a triangular area that extends from the apex to about one third of the external surface and about half of the length of the internal surface. It consists of PFB showing three major cyclical GM. The transition to the second tissue type, which encompasses the rest of the armor plate to the oval excavated marginal side opposite the apex, is slightly concave in thin-section. The GM resemble thick, mainly poorly vascularized layers of bone. The vascularization of the PFB, consisting of fine reticular primary vascular canals, is restricted to bone layers intercalating with the avascular layers. The primary canals are arranged sub-parallel to the GM and dominate the reticular pattern. The GM in the PFB are parallel to the internal surface of the spike in the apical part but deviate slightly in an internal direction, thus extending almost parallel to the transition line to the second tissue type.
The second tissue type, which comprises the rest of the spike, has a matrix of large cartilage cell lacunae and isolated thin bony trabeculae (Fig. 3C). The cartilaginous matrix dominates in volume towards the apex, whereas the cartilage to bony trabeculae ratio becomes somewhat more even towards the excavated surface opposite the apex. Apically, the trabeculae gain a little in spatial orientation by increasingly extending sub-parallel to the external and internal bone surface. Towards the excavated surface opposite the apex, the trabeculae appear in a more random arrangement. The bony trabeculae are associated again with primary vascular canals. Some of the canals are completely lined by LB, thus forming primary osteons. In other cases, the cartilage matrix and primary vascular canals are in direct contact. The reticular vascularization pattern of fine anastomosing primary vascular canals observed in the area of bone tissue near the apex is not encountered in the cartilage tissue. However, the canals anastomose more frequently towards the excavated surface opposite the apical region.
P. suevicus(SMNS 91007)
The thin-section reveals a rather homogeneous type of bone tissue throughout the recumbent spike, with only the blunt apex being set off from the rest of the bone. A separation into external and internal cortical bone and interior cancellous bone is not recognized. Furthermore, there is no secondary remodeling of the primary bone tissue. With the exception of the apex of the armor plate, the whole bone down to the internal surface of the bone shows 13 parallel GM. These are best observed at the lateral margins of the armor plate and become less distinct towards the interior center of the bone. A single large vascular canal extends centrally from the internal bone surface towards the apex (not visible in gross morphology because of matrix cover).
The distal-most tissue of the apex does not show any cyclical GM but consists of a loose meshwork of a few coarse sub-parallel bony trabeculae that seldom branch (Fig. 3D). The almost vertically arranged trabeculae extend from the external surface of the bone towards the first GM. The trabeculae consist of PFB with only a few flattened and elongated bone cell lacunae. The apical tissue is poorly vascularized by a few primary vascular canals that are associated with and follow the bony trabeculae. Cartilaginous tissue with a distinct fibrous texture completely fills the spaces between the bony trabeculae (Fig. 3D,E). In this tissue, large cell lacunae are organized in diffuse layers. Based on postdepositional diagenetic alteration (permineralization) of the cartilaginous bone tissue, many of the large cell lacunae are completely dark in normal transmitted and polarized light, whereas others appear more translucent in thin-section. In the majority of the translucent cell lacunae, small round black spherical structures are recognized. The cartilaginous tissue in the apex occupies more space than the bony trabeculae.
Internal to the first GM towards the internal bone surface (Fig. 3D), the bony trabeculae are finer and shorter. Furthermore, a differentiation in the arrangement of the trabeculae between the interior bone tissue and the lateral bone tissue becomes obvious. The interior area of the spiked armor plate is composed of two sets of shorter bony trabeculae being arranged at a steep angle to each other and pointing towards the internal bone surface. In the lateral areas of the plate, however, the tissue is dominated by somewhat longer, fine bone trabeculae that extend sub-parallel to the external bone surface (Fig. 3F). Similarly, the arrangement of the interstitial cartilaginous matrix is less ordered in the medial areas and becomes more ordered towards the margins of the armor plate. Towards the lateral surfaces of the plate, the cartilage cell lacunae are organized in layers parallel to the dominant direction of the bony trabeculae (Fig. 3F) and thus also sub-parallel to the marginal surface of the bone. The cell lacunae in the cartilaginous tissue are of the same type already observed in the apical tissue. Using a lambda compensator in polarized light, fine delineations that are differently colored due to changes in fibrous arrangement become apparent around the individual cartilage cell lacunae. Each of the two opposite delineations of the cell lacunae has similar colorations, thus creating a distinct color grid within the cartilage tissue. The amount of primary vascular canals and scattered primary osteons increases slightly from the apex towards the internal bone surface, resulting in an overall radial vascularization pattern that has its focus in the apex of the spiked armor plate.
Skeletal histology of temnospondyl sculptured bones
Although morphologically diverse, all temnospondyl specimens share a similar bone microstructure. They are thus described together, with variations being pointed out where appropriate.
All samples show a diploe structure (Fig. 4A) with distinct cancellous bone framed by external and internal compact cortical bone. The cortical bone layers are of similar thickness and are well vascularized. The orientation of the primary and secondary osteons is parallel to the bone surface.
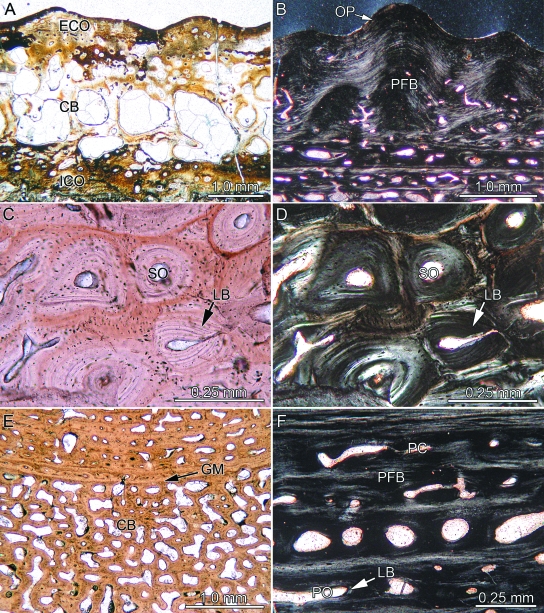
Fig. 4.
Bone histology of temnospondyl amphibians. (A) Complete section of Trimerorhachis sp. (TMM 40031-59) showing interior cancellous bone (CB) framed by external and internal cortical bone (ECO, ICO). (B) Close-up of external ornamentation pattern (OP) and parallel-fibered bone (PFB) of Gerrothorax pustuloglomeratus (SMNS 91012) in polarized light. Close-up of the cancellous bone of external cortex of Mastodonsaurus giganteus (SMNS 91011; transverse section) in (C) normal and (D) polarized light. Note lamellar bone (LB) of older and younger generations of secondary osteons (SO) forming Haversian bone. (E) Close-up of cancellous bone of G. pustuloglomeratus (SMNS 91012; transverse section) showing a compact primary growth mark (GM). (F) Close-up of internal cortex of former specimen (transverse section) strongly vascularized by primary osteons (PO, lined with LB) and primary vascular canals (PC).
In Trimerorhachis sp. the external cortical bone consists of PFB with scattered primary osteons, primary vascular canals, and few secondary osteons. Growth of the PFB follows the wavy surface sculpture consisting of ridges and valleys, but distinctive cyclical GM are not observed (Fig. 4A). Fiber bundles that extend diagonally to the external bone surface in the PFB dominate the bone tissue. The eponymous ornamentation pattern of fine isolated tubercles and short ridges in Gerrothorax pustuloglomeratus consists of wavy PFB (Fig. 4B). Locally, a second compact layer of PFB may be situated internal to the ornamentation pattern. The transition between ornamentation and this second layer of PFB, in which the lamellae extend horizontally, is mostly distinct. The PFB of the internal layer does not reach into the ridges or tubercles. In M. giganteus, the external-most layers of the external cortical bone consist of PFB that changes to primary interwoven structural collagenous fiber bundles (ISF) moving in an internal direction. Poorly developed GM are present in the PFB, though their course is difficult to follow. The bone tissue is vascularized by primary osteons and primary vascular canals. In the center of a sculpture ridge, the fiber bundles of the PFB cross each other at moderate angles. At the areas of overlap, Sharpey's fibers insert at right angles into the external cortical bone. Sharpey's fibers are restricted to the ridges of the ornamentation pattern and are absent from the valleys in between the ridges.
The trabeculae of the cancellous bone appear stout. The vascularization is characterized by a few large cavities. Interstitial primary ISF bone tissue and scattered primary osteons are still found within the centers of larger trabeculae, whereas the trabecular walls are lined with secondary LB. In external and internal directions, newly formed erosion cavities lack a complete LB lining and instead show irregular erosion fronts. In M. giganteus, extensive remodeling affects the whole of the cancellous bone. In some of the compact bone areas, secondary osteons form patches of Haversian bone (Fig. 4C,D). In G. pustuloglomeratus, bone trabeculae are more slender and gracile, and usually lined with secondary LB. GM are visible in the cancellous bone as less vascularized bone layers intercalating with thicker zones of well vascularized tissue (Fig. 4E).
More internally, the ISF of the cancellous bone grades into PFB of the internal cortical bone proper. The nature of the PFB is best studied in sections extending parallel to the ornamental ridges. The majority of the layers of the PFB are well vascularized with scattered primary osteons, secondary osteons and larger erosion cavities (Fig. 4F). Interstratified poorly vascularized to avascular lamellae also appear in the bone tissue. The primary vascular canals resemble tubes in sections parallel to the ornamentation, and have circular shapes in sections transverse to the ornamentation.
The bone element of M. giganteus showed sutured margins with short interdigitating bony pegs and sockets. Towards the center of the cancellous bone, remnants of former growth stages of the bony pegs are observable, although these bone lamellae of the sutural pegs are subject to increased remodeling.
Discussion
Certain histologic traits are common to all sampled plates, including overall compactness of the plates and the resulting lack of interior cancellous bone, distinctive areas within the bone that vary between high and low degrees of spatial organization, cyclical GM, and a radial mode of growth (including a radial vascularization pattern) with a distinct growth center. Secondary bone remodeling (i.e. large erosion cavities, secondary osteons, and Haversian bone) does not occur in the placodont armor samples. Contrary to Westphal's (1975) assessment of growth patterns in placodont armor plates, which was based solely on hexagonal plate forms, quite a few differences in the locations of the centers of growth were found in the studied sample.
The formation and retention of what is here proposed as cartilaginous tissue in placodont armor is not well understood yet, so the tissue in placodont armor plates is preliminarily termed ‘postcranial fibro-cartilaginous bone’, referred to PFCB hereafter.
As is known for the vertebrate skull, the occurrence of cartilage and cartilage-like tissue (e.g. secondary cartilage, chondroid bone, cartilage formation in fracture healing) may be associated with typical dermal or membrane bones that also lack cartilage precursors (Fang & Hall, 1997; Hall, 2005).
Chondroid bone (see Beresford, 1981; Hall, 2005), a tissue that is often connected with rapid bone growth during intramembraneous ossification (Lengelé et al. 1990, 1996) and that disappears during ontogeny (Fang & Hall, 1997; Hall, 2005), may indeed show superficial similarities with the tissue of the placodont armor.
Based on histological comparison with cartilage in epiphyses/growth plates of extant and fossil tetrapod limb bones, however, the tissue in the postcranial armor plates of placodonts is hypothesized to represent a truly cartilaginous tissue (Fig. 5). In the histological section of the extant Sus sp., the columns and layers of hypertrophied hyaline cartilage cells are easily recognized before they are calcified, reabsorbed, and substituted by bone tissue (Fig.5A). Rhodin (1985) did an extensive study of similar chondro-osseous development in turtle long bones. The cartilaginous tissue in placodont plates differs from hyaline cartilage in having a distinctly fibrous nature, similar to fibrocartilage of intervertebral disks, for example (e.g. Hall, 2005).
Fig. 5.
Comparison of cartilaginous tissue in extant and fossil tetrapods. (A) Histological section (PIMUZ AP I-0001) through the growth plate of a phalangeal bone (Sussp.). Note resorption and subsequent substitution (arrows) of the hypertrophied hyaline cartilage cells arranged in columns and layers. (B) Sagittal thin-section through the epiphysis of a long bone of Neusticosaurus pusillus (PIMUZ T4022) in normal transmitted light. Position of the close-up (C, D) is marked by rectangle. Calcified cartilage and bone trabeculae of the epiphysis in (C) normal transmitted light and in (D) polarized light. Note that periosteal bone is visible in the upper left corner of the image. (E) Complete thin-section of the armor plate of Psephosaurussp. (SMNS 91009) in normal transmitted light. Position of the close-up (F, G) is marked by rectangle. Dense regular PFCB tissue in (F) normal transmitted light and in (G) polarized light. Note that in (C, D) and (F, G) bone trabeculae are marked by a white arrow.
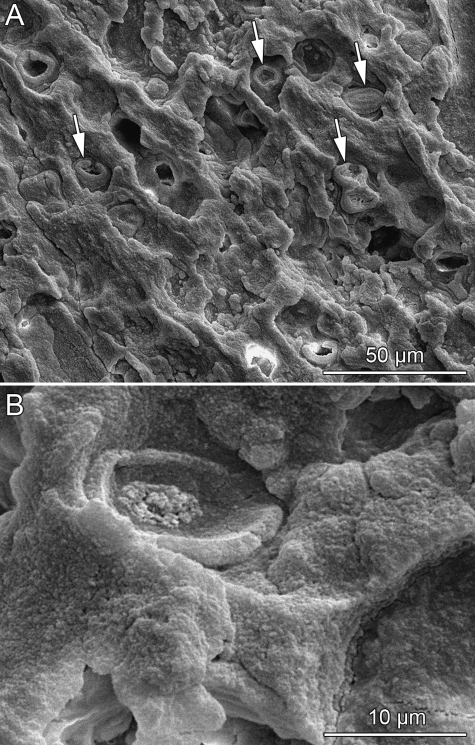
Retention of calcified cartilage in the bones is recognized in many extant and fossil tetrapod groups that are secondarily adapted to an aquatic environment (e.g. Buffrénil et al. 1990;Ricqlès & Buffrénil, 2001). For example, it is found in the epiphyseal regions of the long bones and vertebral centra of fossil aquatic reptiles like pachypleurosaurs and plesiosaurs (Ricqlès & Buffrénil, 2001: figs 2,4). This could be corroborated herein by the study of the epiphyseal region of a long bone of Neusticosaurus pusillus (Fig. 5B–D). The pachypleurosaur sample and the peculiar tissue in the placodont plates (Fig. 5E–G) are very similar in appearance (minor size variation occurs in the bone trabeculae and the vascular canals), with both showing columns and layers of hypertrophied cell lacunae between bone trabeculae. The cell lacunae have similar dimensions and plump circular shapes, and lack structures like canaliculi. Furthermore, the cell lacunae share the same optical properties when viewed in polarized light using a lambda compensator, which relates to the calcification of the tissues. The oval structures in the presumably calcified cartilaginous tissue (Fig. 6) are thus interpreted as cartilage cell lacunae. As observable in the SEM images, the lacunae either have closed circular to ovoid shapes or are half closed and bowl-shaped (Fig. 6B), largely depending on the grinding and etching of the fossil tissue. Because the structures mostly represent lacunae instead of hollow tubes, it is unlikely that they housed elongated collagenous fibers instead of globular cells.
Fig. 6.
SEM images (SE detection) of the armor plate of Psephosaurus suevicus (SMNS 91007). (A) Overview of the PFCB tissue. Supposed cartilage cell lacunae are indicated by white arrows. (B) Close-up of the tissue with focus on an ovoid cartilage cell lacuna. Part of the outer layer of the lacuna is missing, probably due to etching process.
As is generally the case in fossils, tissue staining is not applicable, and differentiating between tissue types is based mainly on comparison with extant taxa. Franc et al. (1995) used immunohistochemical and biochemical staining techniques on ‘recently’ fossilized material from the Pleistocene to verify the cartilaginous nature of a thin pellicle attached to a long bone, after the presence of cartilage was proposed by Lechêne de la Porte et al. (1993) based on light and electron microscopic techniques. However, it seems unlikely that the methods of Franc et al. (1995) are similarly applicable to much older fossils, i.e. from the Mesozoic and Paleozoic time period, which experienced higher diagenetic alteration. The prolonged process of fossilization undoubtedly affects both the mineralized (usually being recrystallized) and especially the non-mineralized part (usually being completely degraded) of the skeletal tissue far more profoundly with time (e.g. Trueman & Martill, 2002).
Because a cartilage precursor is not involved in the classic formation of tetrapod dermal armor, the unique cartilaginous tissue in placodont armor plates requires particular attention. Several amniote and fossil amphibian outgroups are included in the following discussion to understand better the nature of tetrapod dermal armor in general and of the placodont armor in particular.
Outgroup comparison: amniotes
Detailed studies on the skeletal and bone histology of amniote dermal armor is currently available or is in preparation for turtles and pareiasaurs (Scheyer & Sánchez-Villagra, 2007; Scheyer et al. 2007; Scheyer, in press), archosaurs (Scheyer & Sander, 2004; Main et al. 2005; Hill & Lucas, 2006), lepidosaurs (Moss, 1969, 1972; Zylberberg & Castanet, 1985; Levrat-Calviac & Zylberberg, 1986), and xenarthrans (Hill, 2006; Vickaryous & Hall, 2006). Even though quite a wide bone histological diversity is covered by these groups, none of them shows bone microstructures comparable to placodont armor.
Turtles, in which endoskeletal elements that do have a cartilage precursor are incorporated into the otherwise dermoskeletal bony shell (Vallén, 1942; Suzuki, 1963; Zangerl, 1969), do not show any cartilaginous tissue similar to that of placodont armor. As is the case for osteological data (e.g. Gregory, 1946), differences in histogenesis and bone tissue types do not support homology of the placodont armor with the turtle shell.
In extant and fossil reptiles, the osteodermal microstructures are basically interpreted to conform to the structures of the soft integumentary layers in which they develop (e.g. Zylberberg & Castanet, 1985; Levrat-Calviac & Zylberberg, 1986; Hill & Lucas, 2006; Scheyer & Sánchez-Villagra, 2007; Scheyer et al. 2007), because existing soft tissue structures are directly transferred into the hard tissue by metaplastic ossification (Haines & Mohuiddin, 1968). However, the tissue type found in the completely ossified placodont plates (SMNS 91006; SMNS 91008; NRM-PZ R1759a) superficially resembles the ISF encountered in other reptilian osteoderms (Scheyer & Sander, 2004; Scheyer & Sánchez-Villagra, 2007; Scheyer et al. 2007). The tissue type consisting of randomly arranged fiber bundles in the placodonts is hypothesized to develop through complete ossification of the cartilaginous bone instead of direct ossification of dermal structures.
Outgroup comparison: fossil amphibians
Comparison with bone microstructures of basal amphibian armor implies that the retention of cartilage is not expected to be inherited from fossil amphibian ancestors either, because the studied temnospondyl specimens resemble amniote osteoderms in that they develop partly through direct ossification of integumentary structures without a cartilage precursor. All three amphibian taxa share a diploe structure of their bones with distinct external and internal compact bone that frames interior cancellous bone; a feature that was not observed in placodonts. The high overall vascularization of the samples reflects the mainly aquatic lifestyle of the amphibian temnospondyls used in this study (e.g. Schoch, 1999;Holmes, 2000;Hellrung, 2003). All three samples are characterized by strong vascularization, secondary remodeling, and well developed interior cancellous bone instead of the radial vascularization seen in the placodont osteoderms.
Because only elements of the dermatocranium and the shoulder region of temnospondyls could be included in the current study, the direct applicability and significance for placodont postcranial armor plates is debatable. However, I feel confident that based on the presented results (increasing the outgroup data) our understanding of the formation of dermal bone in general is advanced. Future sampling should thus expand also to temnospondyl postcranial osteoderms (e.g. of G. pustuloglomeratus), to complete the data on bone histological structures of this group and to increase the comparability with other anamniote groups (e.g. fish, lissamphibians) and amniotes.
Morphogenetic model of histogenesis in placodont armor plates
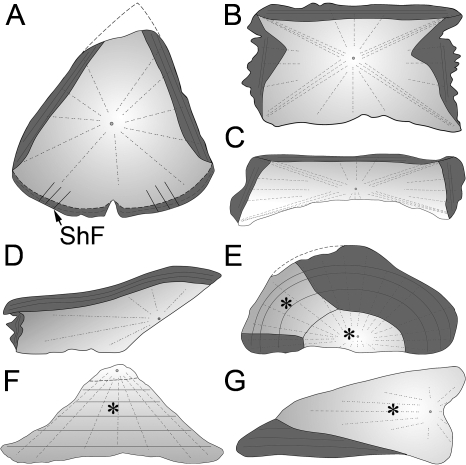
Differential growth may lead to the variety of plate shapes (Fig. 7) found in cyamodontoid placodonts, ranging from polygonal/hexagonal plate forms (NRM-PZ R.1759a; MHI 1426/2 & 3; and SMNS 91008), to procumbent and recumbent spikes (MHI 1426/1; SMNS 91009; SMNS 91010), to rhomboidal forms as in the plastral plate of Psephosaurus sp. (SMNS 91009).
Fig. 7.
Sketches of the thin-sections of the sampled placodontoid (A) and cyamodontoid (B–G) armor plates (all scaled to similar size for comparison). The cyamodontoid plate forms are highly divergent from the plate morphology and histology of the placodontoid specimen. Zones of parallel-fibered bone showing growth marks are marked in dark gray, loosely ordered structural fiber bundles are in light gray, and areas where PFCB is developed are marked with an asterisk. Small circles and dashed lines represent growth centers and orientation of vascular patterns, respectively. (A) Placodus gigas (SMNS 91006). Note prominent Sharpey's fibers (ShF) inserting into the internal part of the plate. (B) Psephodermasp. (NRM PZ R.1759a). Note that this section was chosen to represent also the hexagonal/polygonal plates of Psephosaurus suevicus (MHI 1426/2 & 3). (C) Psephosaurussp. (SMNS 91008). (D) P. suevicus (MHI 1426/1). (E) Psephosaurussp. (SMNS 91009). (F) P. suevicus (SMNS 91007). (G) cf. Placochelyssp. (SMNS 91010).
Furthermore, the placodont armor plate samples range from some that are completely ossified (Fig. 7A–D), to some showing intermediate stages, to one consisting completely of cartilaginous tissue (Fig. 7E–G). The first type is represented by the completely ossified plates of P. gigas (SMNS 91006), Psephosaurus sp. (SMNS 91008), P. suevicus (MHI 1426/1-3), and Psephoderma sp. (NRM-PZ R1759a), where no cartilage is present. The samples of cf. Placochelys sp. (SMNS 91010) and Psephosaurus sp. (SMNS 91009) represent the second type. These samples demonstrate intermediate stages with localized patches of the armor plates free of cartilage and other areas still showing a mixture of bony trabeculae and cartilaginous matrix. The third type is represented by the spiked plate of P. suevicus(SMNS 91007), which has the highest amount of cartilage cell lacunae compared to the overall armor plate volume.
In contrast to the generally accepted osteoderm development, and even though not all specimens explicitly exhibit cartilage cell lacunae, I here propose a cartilaginous preformation for the general development of placodont armor plates. In contrast to the turtle shell, which combines endoskeletal and dermoskeletal bone elements (e.g. Gilbert et al. 2001), the morphological data in placodonts strongly indicate that the armor plates belong to the dermoskeleton (i.e. the relative position of the plates superficial to the ribs and vertebrae as well as the complete independence of the superficial armor plates from the deeper endoskeletal structures; see Westphal, 1975, 1976). This implies that the formation of cartilage in the placodont integument and armor must have involved some novel developmental mechanisms (Fang & Hall, 1997; Webster & Zelditch, 2005).
The different stages of ossification (from highly cartilaginous to intermediate to completely ossified) are interpreted as representing different ontogenetic stages, although sexual dimorphism or heterochronic effects among the species and genera cannot be completely ruled out. Future expanded sampling and study of growth series may provide a test of these alternatives.
Osteosclerosis in limb bones, ribs, and vertebrae of fossil and recent tetrapod groups that are secondarily adapted to a marine lifestyle often involves extensive endosteal bone deposition coupled with the retention of calcified cartilage (e.g. Hua & Buffrénil, 1996; Ricqlès & Buffrénil, 2001). The compact nature of the tissue and the presence of a cartilaginous matrix in the placodont sample could indicate such a form of osteosclerosis in the armor plates. This observation agrees well with the pachyostotic trend (thickening of cortical compact bone) that was previously recognized in the long bone histology of P. gigas (Buffrénil & Mazin, 1992; Ricqlès & Buffrénil, 2001). It is proposed that in addition to the pachyostosis found in limb bones, the osteosclerosis of the armor plates thus contributed to some extent to buoyancy control while swimming and browsing for food, at least in the heavily armored cyamodontoids. Swimming speed, on the other hand, was most likely reduced in the heavily armored taxa (compare to Massare, 1988, 1994).
Developmental implications
The presence of cartilage cell lacunae generally opposes the morphological data that indicate the armor as being part of the dermoskeleton. The question arises as to how the presence of cartilage in the placodont armor plates can be explained. This is linked with the question of whether the placodont armor is neural crest or mesodermally (sclerotomally) derived. Such questions cannot be directly addressed in fossil taxa. Fossils are preserved single stages of development, so the fate of cells cannot be directly followed, and the permineralized and recrystallized tissue cannot be immunostained to elucidate the origin of cells. Developmental studies on living animals have to be consulted to indicate developmental aspects in the fossils.
Only a few years ago it was commonly acknowledged that cranial neural crest cells (cranial ncc) were known for their skeletogenic potential, whereas the trunk neural crest cells (trunk ncc) were thought to lack a respective potential (e.g. Gilbert, 2000). Thus, it was most plausible to assume that skeletal derivatives were either cranial ncc or mesodermally derived. Recently, however, trunk ncc were found to have skeletogenic (i.e. chondrogenic) potential in an amphibian species (Graveson et al. 1997), and McGonnell & Graham (2002: p. 767) showed that trunk ncc of quails exhibit skeletogenic potential when transplanted into developing mandibular and maxillary primordia. At least a partial involvement of trunk ncc has been further proposed in the formation of the turtle shell, especially of the plastron (Clark et al. 2001; Cebra Thomas et al. 2007). To date, this hypothesis is hotly debated but, to my knowledge, no contradicting data have been published discussing this issue in more detail.
A possible hypothesis of placodont armor formation may similarly include the direct involvement of trunk ncc in the development of the proposed dermoskeletal plates. Until further data are available, however, it remains unclear how far evolutionary developmental aspects of turtle shell formation, which itself is quite unique among tetrapods (e.g. Burke, 1989; Gilbert et al. 2001; Kuraku et al. 2005; Nagashima et al. 2007), can be translated to the extinct placodonts.
In the current study, the simplified model of osteogenesis (including little histology) proposed by Westphal (1975) is expanded on a wider base of placodont taxa, morphological shapes, and bone histological characteristics. The location of the center of growth, the radial growth pattern including the vascularization and growth marks, and the occurrence of the different histological tissues within the bones were all taken into account. The presence of a cartilaginous matrix in part of the placodont samples with the complete absence of cartilage in others is interpreted as being the result of different ontogenetic states of the samples. If the developing cartilaginous bone of the placodont dermal armor had a highly reduced potential to fossilize compared to the completely ossified tissue, this could explain why most finds of juvenile placodonts lack postcranial dermal armor plates (see Rieppel, 2002).
Even though placodonts have been extinct for over 200 million years and we have no living relatives to compare the fossils with, the small permineralized pieces of bony armor studied once more underscore the diversity of skeletal structures realized among vertebrates. It also shows the opportunities and restrictions for studying the developmental biology of skeletal structures of extinct groups, as well as the need for further research of extant species.
Acknowledgments
T. Rowe, R. Schoch, H. Hagdorn, H. Furrer, and T. Mörs are thanked for kindly providing specimens for the study. I thank P. M. Sander, H. Müller, and M. Sánchez-Villagra for commenting on the manuscript and O. Dülfer for his help in preparing thin-sections. E. Goldbeck, D. Kranz, G. Heumann, and G. Oleschinski are acknowledged for their support in preparing the manuscript. Three anonymous reviewers are thanked for their constructive criticism, which greatly improved the manuscript. This project was funded by Deutsche Forschungsgemeinschaft Grant #SA-469/15-1/2.
References
- Beresford WA. Chondroid Bone, Secondary Cartilage and Metaplasia. Baltimore and Munich: Urban and Schwarzenberg; 1981. [Google Scholar]
- Buffrénil VD, Mazin J-M. Contribution de l’histologie osseuse à l’interprétation paléobiologique du genre PlacodusAgassiz, 1833 (Reptilia, Placodontia) Revue de Paléobiologie. 1992;11:397–407. [Google Scholar]
- Buffrénil VD, Ricqlès AD, Ray CE, Domning DP. Bone histology of the ribs of the archaeocetes (Mammalia: Cetacea) J Vertebr Paleontol. 1990;10:455–466. [Google Scholar]
- Burke AC. Development of the turtle carapace: implications for the evolution of a novel bauplan. J Morphol. 1989;199:363–378. doi: 10.1002/jmor.1051990310. [DOI] [PubMed] [Google Scholar]
- Cebra-Thomas JA, Betters E, Yin M, Plafkin C, McDow K, Gilbert SF. Evidence that a late-emerging population of trunk neural crest cells forms the plastron bones in the turtle Trachemys scripta. Evol Dev. 2007;9:267–277. doi: 10.1111/j.1525-142X.2007.00159.x. [DOI] [PubMed] [Google Scholar]
- Clark K, Bender G, Murray BP, et al. Evidence for the neural crest origin of turtle plastron bones. Genesis. 2001;31:111–117. doi: 10.1002/gene.10012. [DOI] [PubMed] [Google Scholar]
- Donoghue PCJ, Sansom IJ. Origin and early evolution of vertebrate skeletonization. Microsc Res Tech. 2002;59:352–372. doi: 10.1002/jemt.10217. [DOI] [PubMed] [Google Scholar]
- Drevermann F. Die Placodontier. 3. Das Skelett von Placodus gigasAgassiz im Senckenberg-Museum. Abh Senckenb Naturforsch Ges. 1933;381:319–364. [Google Scholar]
- Fang J, Hall BK. Chondrogenic cell differentiation from membrane bone periostea. Anat Embryol. 1997;196:349–362. doi: 10.1007/s004290050104. [DOI] [PubMed] [Google Scholar]
- Franc S, Marzin E, Boutillon M-M, Lafont R, Lechêne de la Porte P, Herbage D. Immunohistochemical and biochemical analyses of 20 000–25 000-year-old fossil cartilage. Eur J Biochem. 1995;234:125–131. doi: 10.1111/j.1432-1033.1995.125_c.x. [DOI] [PubMed] [Google Scholar]
- Francillon-Vieillot H, Buffrénil VD, Castanet J, Géraudie J, Meunier FJ. Microstructure and mineralization of vertebrate skeletal tissues. In: Carter JG, editor. Skeletal Biomineralization: Patterns, Processes and Evolutionary Trends. New York: Van Nostrand Reinhold; 1990. pp. 471–530. [Google Scholar]
- Gilbert SF. Developmental Biology. 6. Sunderland, Massachusetts: Sinauer Associates, Inc; 2000. [Google Scholar]
- Gilbert SF, Loredo GA, Brukman A, Burke AC. Morphogenesis of the turtle shell: the development of a novel structure in tetrapod evolution. Evol Dev. 2001;3:47–58. doi: 10.1046/j.1525-142x.2001.003002047.x. [DOI] [PubMed] [Google Scholar]
- Graveson AC, Smith MM, Hall BK. Neural crest potential for tooth development in a urodele amphibian: developmental and evolutionary significance. Dev Biol. 1997;188:34–42. doi: 10.1006/dbio.1997.8563. [DOI] [PubMed] [Google Scholar]
- Gregory WK. Pareiasaurs versus placodonts as near ancestors to the turtles. Bull Am Mus Nat Hist. 1946;86:275–326. [Google Scholar]
- Haines RW, Mohuiddin A. Metaplastic bone. J Anat. 1968;103:527–538. [PMC free article] [PubMed] [Google Scholar]
- Hall BK. Bones and Cartilage. Developmental and Evolutionary Skeletal Biology. Amsterdam: Elsevier Academic Press; 2005. [Google Scholar]
- Hellrung H. Gerrothorax pustuloglomeratus, ein Temnospondyle (Amphibia) mit knöcherner Branchialkammer aus dem Unteren Keuper von Kupferzell (Süddeutschland) Stuttg Beitr Naturkd (B Palaeontol) 2003;330:1–130. [Google Scholar]
- Hill RV. Comparative anatomy and histology of xenarthran osteoderms. J Morphol. 2006;267:1441–1460. doi: 10.1002/jmor.10490. [DOI] [PubMed] [Google Scholar]
- Hill RV, Lucas SG. New data on the anatomy and relationships of the Paleocene crocodylian Akanthosuchus langstoni. Acta Palaeontol Polonica. 2006;51:455–464. [Google Scholar]
- Holmes RB. Chapter 7: Palaeozoic temnospondyls. In: Heatwole H, Carroll RL, editors. Amphibian Biology, Palaeontology – The Evolutionary History of Amphibians. Volume 4. Chipping Norton: Surrey Beatty & Sons; 2000. pp. 1081–1120. [Google Scholar]
- Hua S, Buffrénil VD. Bone histology as a clue in the interpretation of functional adaptations in the Thalattosuchia (Reptilia, Crocodylia) J Vertebr Paleontol. 1996;16:703–717. [Google Scholar]
- Huene FV. Die Placodontier. 4. Zur Lebensweise und Verwandtschaft von Placodus. Abh Senckenb Naturforsch Ges. 1933;38:365–382. [Google Scholar]
- Kuraku S, Usuda R, Kuratani S. Comprehensive survey of carapacial ridge-specific genes in turtle implies co-option of some regulatory genes in carapace evolution. Evol Dev. 2005;7:3–17. doi: 10.1111/j.1525-142X.2005.05002.x. [DOI] [PubMed] [Google Scholar]
- Lechêne de la Porte P, Marzin E, Lafont R, et al. Mise en évidence de structures cartilagineuses fossiles. C R Seances Acad Sci, Série 2, Méc Phys Chim Sci Univers Sci Terre. 1993;316:1831–1835. [Google Scholar]
- Lengelé B, Dhem A, Schowing J. Early development of the primitive cranial vault in the chick embryo. J Craniofac Genet Dev Biol. 1990;10:103–112. [PubMed] [Google Scholar]
- Lengelé B, Schowing J, Dhem A. Chondroid tissue in the early facial morphogenesis of the chick embryo. Anat Embryol. 1996;193:505–513. doi: 10.1007/BF00185882. [DOI] [PubMed] [Google Scholar]
- Levrat-Calviac V, Zylberberg L. The structure of the osteoderms in the gekko: Tarentola mauritanica. Am J Anat. 1986;176:437–446. doi: 10.1002/aja.1001760406. [DOI] [PubMed] [Google Scholar]
- Li C. Placodont (Reptilia: Placodontia) from Upper Triassic of Guizhou, Southwest China [in Chinese with English summary] Vertebr PalAsiat. 2000;38:314–317. [Google Scholar]
- Li C, Rieppel O. A new cyamodontoid placodont from Triassic of Guizhou, China [in Chinese] Chin Sci Bull. 2002a;47:156–159. [Google Scholar]
- Li C, Rieppel O. A new cyamodontoid placodont from Triassic of Guizhou, China. Chin Sci Bull. 2002b;47:403–407. [Google Scholar]
- Main RP, Ricqlès AD, Horner JR, Padian K. The evolution and function of thyreophoran dinosaur scutes: implications for plate function in stegosaurs. Paleobiology. 2005;31:291–314. [Google Scholar]
- Massare JA. Swimming capabilities of Mesozoic marine reptiles: implications for methods of predation. Paleobiology. 1988;14:187–205. [Google Scholar]
- Massare JA. Swimming capabilities of Mesozoic marine reptiles: a review. In: Maddock L, Bone Q, Rayner JMV, editors. Mechanics and Physiology of Animal Swimming. Cambridge: Cambridge University Press; 1994. pp. 133–149. [Google Scholar]
- Mazin J-M, Pinna G. Palaeoecology of the armoured placodonts. Paleontol Lombarda Nuova Serie. 1993;2:83–91. [Google Scholar]
- McGonnell IM, Graham A. Trunk neural crest has skeletogenic potential. Curr Biol. 2002;12:767–771. doi: 10.1016/s0960-9822(02)00818-7. [DOI] [PubMed] [Google Scholar]
- Moss ML. Comparative histology of dermal sclerifications in reptiles. Acta Anat. 1969;73:510–533. doi: 10.1159/000143315. [DOI] [PubMed] [Google Scholar]
- Moss ML. The vertebrate dermis and the integumental skeleton. Am Zool. 1972;12:27–34. [Google Scholar]
- Nagashima H, Kuraku S, Uchida K, Ohya YK, Narita Y, Kuratani S. On the carapacial ridge in turtle embryos: its developmental origin, function and the chelonian body plan. Development. 2007;134:2219–2226. doi: 10.1242/dev.002618. [DOI] [PubMed] [Google Scholar]
- Nopcsa F. Die Familien der Reptilien. Fortschr Geol Palaeontol. 1923;2:1–210. [Google Scholar]
- Owen R. Description of the skull and teeth of the Placodus laticeps, Owen, with indications of other new species of Placodus, and evidence of the saurian nature of that genus. Phil Trans R Soc Lond. 1858;148:169–184. [Google Scholar]
- Peyer B, Kuhn-Schnyder E. Placodontia. In: Piveteau J, editor. Traité de Paléontologie. Paris: Masson et Cie; 1955. pp. 459–486. [Google Scholar]
- Pinna G. Notes on stratigraphy and geographical distribution of placodonts. Ital Sci Nat Museo Civ Stor Nat Milano. 1990;131:145–156. [Google Scholar]
- Pinna G, Mazin J-M. Stratigraphy and paleobiogeography of the Placodontia. Paleontol Lombarda Nuova Serie. 1993;2:125–130. [Google Scholar]
- Rhodin AGJ. Comparative chondro-osseous development and growth of marine turtles. Copeia. 1985;1985(3):752–771. [Google Scholar]
- Ricqlès AD, Buffrénil VD. Bone histology, heterochronies and the return of tetrapods to life in water: where are we? In. In: Mazin J-M, Buffrénil VD, editors. Secondary Adaptations of Tetrapods to Life in Water. München: Verlag Dr. Friedrich Pfeil; 2001. pp. 289–310. [Google Scholar]
- Rieppel O. Paraplacodus and the phylogeny of the Placodontia (Reptilia: Sauropterygia) Zool J Linn Soc. 2000;130:635–659. [Google Scholar]
- Rieppel O. The dermal armor of the cyamodontoid placodonts (Reptilia, Sauropterygia): morphology and systematic value. Fieldiana: Geol, New Series. 2002;46:1–41. [Google Scholar]
- Rieppel O, Zanon RT. The interrelationships of Placodontia. Hist Biol. 1997;12:211–227. [Google Scholar]
- Scheyer TM, Sánchez-Villagra MR. Carapace bone histology in the giant pleurodiran turtle Stupendemys geographicus: phylogeny and function. Acta Palaeontol Pol. 2007;52:137–154. [Google Scholar]
- Scheyer TM. Institute of Palaeontology, University of Bonn; Comparative bone histology of the turtle shell (carapace and plastron): implications for turtle systematics, functional morphology and turtle origins. (in press), Ph.D. Thesis. [Google Scholar]
- Scheyer TM, Sander PM. Histology of ankylosaur osteoderms: implications for systematics and function. J Vertebr Paleontol. 2004;24:874–893. [Google Scholar]
- Scheyer TM, Sander PM, Joyce WG, Böhme W, Witzel U. A plywood structure in the shell of fossil and living soft-shelled turtles (Trionychidae) and its evolutionary implications. Org Divers Evol. 2007;7:136–144. [Google Scholar]
- Schoch RR. Comparative osteology of Mastodonsaurus giganteus(Jaeger, 1828) from the Lettenkeuper (Longobardian) of Germany (Baden-Württemberg, Bayern, Thüringen) Stuttg Beitr Naturkd (B Palaeontol) 1999;278:1–175. [Google Scholar]
- Suzuki HK. Studies on the osseous system of the slider turtle. Ann N Y Acad Sci. 1963;109:351–410. doi: 10.1111/j.1749-6632.1963.tb13476.x. [DOI] [PubMed] [Google Scholar]
- Trueman CN, Martill DM. The long-term survival of bone: the role of bioerosion. Archaeometry. 2002;44:371–382. [Google Scholar]
- Vallén E. Beiträge zur Kenntnis der Ontogenie und der vergleichenden Anatomie des Schildkrötenpanzers. Acta Zool. 1942;23:1–127. [Google Scholar]
- Vickaryous MK, Hall BK. Osteoderm morphology and development in the nine-banded armadillo, Dasypus novemcinctus(Mammalia, Xenarthra, Cingulata) J Morphol. 2006;267:1273–1283. doi: 10.1002/jmor.10475. [DOI] [PubMed] [Google Scholar]
- Webster M, Zelditch ML. Evolutionary modifications of ontogeny: heterochrony and beyond. Paleobiology. 2005;31:354–372. [Google Scholar]
- Westphal F. Bauprinzipien im Panzer der Placodonten (Reptilia triadica)-Principles of structure and growth in the dermal armor of placodonts (Reptilia triadica) Paläont Z. 1975;49:97–125. [Google Scholar]
- Westphal F. The dermal armour of some Triassic placodont reptiles. In: Bellairs Ad’A, Cox CB., editors. Morphology and Biology of Reptiles. London: Academic Press; 1976. pp. 31–41. [Google Scholar]
- Zangerl R. The turtle shell. In: Gans C, Bellairs Ad’A, Parsons TS, editors. Biology of the Reptilia, Morphology A. Vol. 1. London: Academic Press; 1969. pp. 311–339. [Google Scholar]
- Zylberberg L, Castanet J. New data on the structure and the growth of the osteoderms in the reptile Anguis fragilisL. (Anguidae, Squamata) J Morphol. 1985;186:327–342. doi: 10.1002/jmor.1051860309. [DOI] [PubMed] [Google Scholar]