Abstract
Effective local ablation of large tumors with radiofrequency has been made possible by recent advancements. Tumor ablation with radiofrequency has been described mainly in the liver, but also recently in the kidney, adrenal gland, lung, and breast. A rapidly growing splenic metastasis from renal cell carcinoma was effectively treated percutaneously, with US guidance. Focal splenic disease may not be a common indication for ablation; however, further work is necessary to evaluate the safety and efficacy of this procedure in this setting.
Keywords: Radiofrequency (RF) ablation; Spleen, neoplasms
PERCUTANEOUS radiofrequency thermal ablation (RFA) is being added to the arsenal of minimally invasive treatments of localized cancer. Recent trends toward minimally invasive surgical options have emphasized decreased cost and time and reduced morbidity and mortality rates. Successful minimally invasive, image-guided local treatments will have to meet these expectations without sacrificing efficacy.
Image-guided cancer therapies are based on the premise that local disease control may result in enhanced survival. Patients with small, solitary liver tumors or adrenocortical carcinoma recurrences are among the first oncology populations to be studied with new ablative techniques because local disease control should result in improved survival, based on extrapolation from the surgical literature. Surgical resection has been shown to improve survival in patients with small or isolated primary and metastatic liver or adrenal neoplasms (1,2).
Recently developed refinements in RFA technology have enabled larger tumor volumes to be treated. This has, in turn, led to wider clinical applications in oncology (3–6). If a needle can be placed safely into a tumor, it is feasible to ablate the tumor with RFA. However, consensus indications are not yet clearly defined; therefore, the interventional radiologist should be cautious with patient selection. Just because it is possible to treat a tumor does not mean it is worthwhile. Rigorous scientific review and long-term prospective trials are needed to help define the role of RFA in cancer treatment.
Splenic embolization, splenic artery occlusion, and transcatheter splenic ablation are routine procedures. Radiofrequency ablation was chosen for this patient with metastatic renal cell carcinoma as a method for local control of the dominant metastatic lesion. This splenic lesion's growth rate had far outpaced the slower “stable” metastatic disease elsewhere in this patient, who had undergone multiple surgical procedures. Splenectomy was considered; however, RFA was chosen as a less invasive method of treatment in this patient with multiple medical problems, stable metastatic disease elsewhere, and a history of multiple surgeries.
Case Presentation and Hospital Course
A 55-year-old man presented with a rapidly enlarging 5-cm × 4-cm solitary splenic metastasis from renal cell carcinoma (Fig 1), the growth rate of which far outpaced the relatively stable, slow-growing lung and retroperitoneal disease. He presented 20 months earlier and underwent right nephrectomy for renal cell carcinoma. He had small pulmonary metastases at that time, and underwent chemotherapy with interleukin-2 and alpha interferon, to which he had a partial response. Disease progressed a year later, with retroperitoneal lymphadenopathy and a solitary femur metastasis with fracture. He underwent stabilization with an intramedullary rod and radiation therapy for the femur metastasis, and received a P-glycoprotein antagonist with vinblastine. Medical history included hypertension and non–insulin-dependent diabetes.
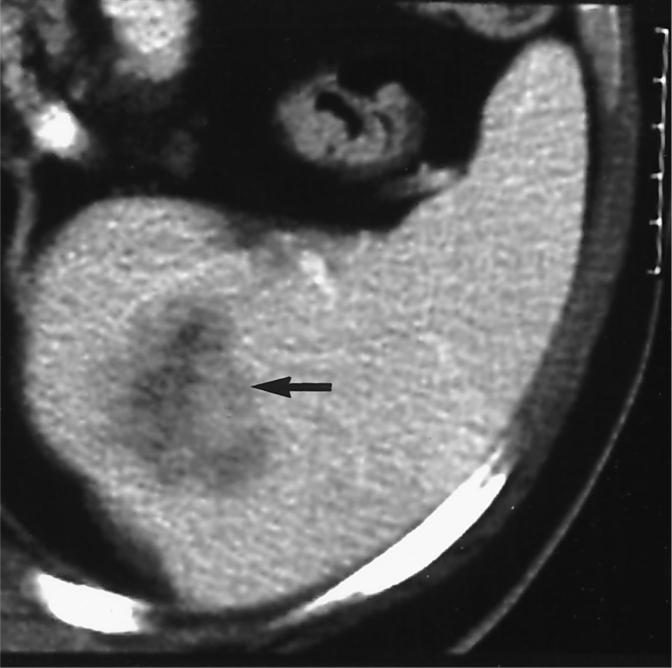
Figure 1.
Pretreatment CT scan shows an enhancing, low-attenuation splenic metastasis from renal cell carcinoma (arrow).
Written informed consent was obtained after an extensive office consultation. Institutional review board approval was not obtained because the ablation system used is FDA 510 K-cleared for soft tissue ablation. He was placed in the supine oblique position in the interventional US suite and anesthetized with 1% lidocaine to the splenic capsule. Conscious sedation was administered with intravenous midazolam and intravenous fentanyl during standard hemodynamic monitoring. Color Doppler and gray-scale US surveys were performed to best plan the safest lateral intercostal approach to the splenic tumor. This path did not traverse major hilar vasculature or pleura, and it crossed as little splenic parenchyma as possible.
The Radionics Cosman CC-1 Coagulator 200-W, 480 kHz generator ablation system (Radionics, Burlington, MA) was set up before the procedure with a cluster Cool-Tip triple-needle probe (Radionics) connected to a water pump and to the generator, which monitored impedance, temperature, power, current, and time. Four grounding pads were attached to the front and back of the thighs. Chilled saline was perfused through the closed-system channels in the three needles to limit charring, which can insulate and limit energy deposition (and treatment volume). The three parallel 17.5-gauge needles with one shared handle were placed through the acrylic stabilizing button, after a 23-gauge fine needle was placed first, so it could be used as a tandem guide for subsequent larger probe placement, so as to minimize probe manipulation.
The output was adjusted to 1.0 amp for 1 minute to let the tissue start to cook slowly, to avoid overcooking early. The output was increased to 1.7 amps for as long as the tissue would tolerate the energy deposition, before tissue impedance rises. Impedance rose exponentially after 8 minutes, at which point we began manual pulsing. Pulsing consisted of decreased current to 0.5 amps for 10−15 seconds to allow for tissue cooling after each impedance rise. This was followed by a return to 1.5 amps, until the impedance rose again (approximately every 60 sec). In pulsing mode, the generator can automatically adjust the current and power based on tissue impedance rise. A single treatment sphere required 12 minutes of heating, and the treated volume was monitored with US. Echogenic organic microbubbles provided a rough estimate of treated tissue. The needle track was cauterized on needle removal.
After the procedure, the patient was kept overnight for monitoring. The patient reported no pain after the procedure, and no opiates or analgesics were necessary after the procedure. In the hours after the procedure, the patient said the entry site was “noticeable,” but not tender.
Contrast enhanced MR and CT were performed immediately after the procedure to evaluate short-term effects and to document lack of complications. MR immediately after RFA showed low-signal coagulative necrosis within the lesion, and the tear-drop shape, whose apex corresponded to the needle track cauterization (Fig 2). CT has been repeated every 3 months after the procedure. The 6-month follow-up CT showed significant shrinkage over time, although the overall shape of the scar was similar (Fig 3). Repeat CT scans will be obtained every 3 months for the first year, to ensure early detection of regrowth.
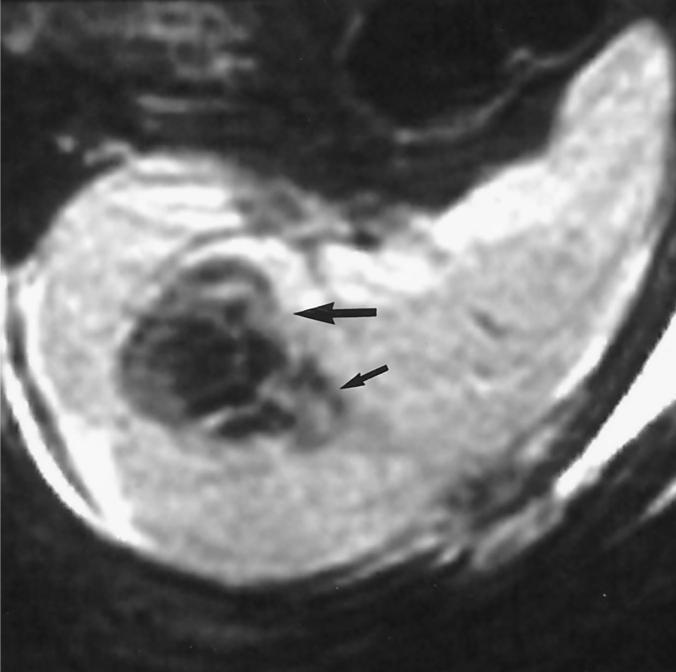
Figure 2.
T2-weighted (repetition time = 3,000; echo time = 112) MR of the splenic metastasis/thermal lesion (large arrow) immediately after ablation shows no enhancement and diffuse low signal consistent with coagulative necrosis. The needle track was cauterized as the probe was removed, resulting in the tear-drop shaped thermal lesion, whose apex corresponds to the needle track (small arrow).
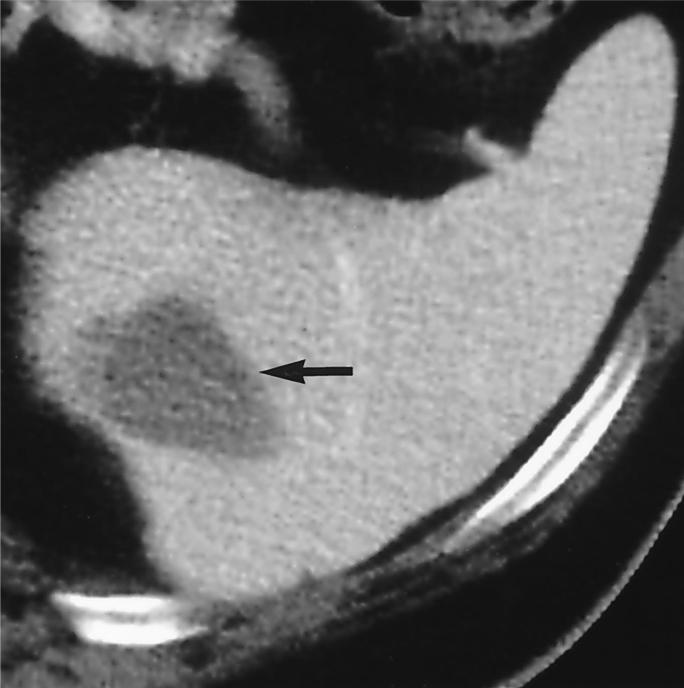
Figure 3.
Contrast-enhanced CT scan of thermal lesion and scar (arrow) at 6 months after ablation. The thermal lesion is much smaller but retains similar shape.
DISCUSSION
Minimally invasive cancer therapy is based on the premise that local treatment of local neoplastic disease may improve survival in certain clinical scenarios. RFA has proved effective in the short-term local control of small (4−5 cm) liver tumors (5,6), kidney tumors, and adrenal tumors (3,4). Radiofrequency deposition at the needle tip leads to ionic agitation and frictional heat. At temperatures higher than 50°C, tissue dessicates and proteins denature, causing coagulative necrosis.
Local neoplasm treatment options have traditionally included surgery and radiation therapy. Tissue thermal ablation with radiofrequency is a minimally invasive treatment which could prove safer and less costly than conventional local therapies in some clinical scenarios. Surgical resection remains the definitive treatment for neoplastic splenic disease; however, an effective minimally invasive option may prove useful in the patient with multiple medical problems, comorbid disease, concomitant tumor, or high surgical risk.
Splenectomy may result in morbidity, requires hospitalization and lengthy recovery, and has high resource cost. Radiofrequency ablation preserves normal splenic parenchyma and, hence, splenic function, and has few complications (6). The complication rate for RFA in the liver is lower than 3% (5,6).
Image-guided fine needle biopsy of the spleen is a safe procedure (7–9). No complications were reported by Soderstrom (8) in more than 1,000 splenic punctures with a 22-gauge needle without imaging guidance. During splenoportography, 19−22-gauge needles have been commonly used, with bleeding rates lower than 2% (9–11). Similar to biopsy, needle placement during ablation should traverse as little spleen as possible before reaching the lesion (12).
After RFA, complete loss of T2-weighted signal and lack of contrast enhancement on CT and MR is consistent with coagulation necrosis (Fig 2). Lack of thick, nodular, irregular enhancement at the periphery of the coagulation necrosis suggests short-term local control of this focal splenic lesion. Presence of this finding on CT and MR in the liver has been shown to correspond with incomplete treatment or tumor regrowth after RFA (13). Although shrinking size would suggest complete treatment, further imaging follow-up is necessary to distinguish whether the subtle areas of low attenuation at the treatment margin (Figure 3, arrow) on CT represent posttreatment granulation tissue, or recurrent or residual tumor. The limited follow-up, and the fact that only one patient was treated, are major limitations to this technique, and no broad conclusions pertaining to efficacy can be made at this time, except that it is possible to perform RFA in the spleen.
Radiofrequency ablation is a safe and predictable method of local tissue destruction and could prove to be an option for failed splenic embolization in the setting of trauma or hypersplenism. Tissue debulking, coagulation, and fulguration are direct results of RFA, similar to electrocautery on a larger scale. Patients with hypersplenism may benefit from embolization before or instead of surgical splenectomy, by improving platelet function and retarding hemolysis and thrombocytolysis (14). Surgical risk may be lessened by preoperative embolization of massive splenomegaly. Focal ablation with use of focused US has been shown to control splenic bleeding from incisions in an animal model (15). Whether radiofrequency ablation could prove useful in these settings remains speculative. However, cauterizing the probe track on the way out may limit back-bleeding and needle track seeding.
Minimally invasive methods of treating isolated splenic tumors may have lower associated morbidity and mortality rates than surgery and entail less toxicity than radiation therapy or systemic chemotherapy. Radiofrequency ablation can be performed in the spleen. Certainly, further study is warranted to examine whether radiofrequency ablation of splenic lesions is safe and whether it provides any lasting, meaningful benefit for specific patients who might be poor candidates for surgical splenectomy.
Abbreviation
- RFA
radiofrequency ablation
References
- 1.Bellantone R, Ferrante A, Boscherini M, et al. Role of reoperation in recurrence of adrenal cortical carcinoma: Results from 188 cases collected in the Italian National Registry for Adrenal Cortical Carcinoma. Surgery. 1997;122:1212–1218. doi: 10.1016/s0039-6060(97)90229-4. [DOI] [PubMed] [Google Scholar]
- 2.Nordlinger B, Gulguet M, Valliant JC, et al. Surgical resection of colorectal carcinoma metastases to the liver. Cancer. 1996;77:1254–1262. [PubMed] [Google Scholar]
- 3.Wood BJ, Fojo A, Levy EB, Gomez-Jorge J, Chang R, Spies J. Radiofrequency ablation of adrenocortical carcinoma: early experience; Presented at: Annual Meeting of the Society of Cardiovascular and Interventional Radiology; San Diego, CA. March 25−30, 2000. [Google Scholar]
- 4.Wood BJ, Levy EB, Gomez-Jorge J, Chang R, Spies J. Radiofrequency ablation of renal tumors; Presented at: Annual Meeting of the Society of Cardiovascular and Interventional Radiology; San Diego, CA. March 25−30, 2000. [Google Scholar]
- 5.Solbiati L. New applications of ultrasonography: interventional ultrasound. Eur J Radiol. 1998;27(suppl 2):S200–S206. doi: 10.1016/s0720-048x(98)00063-1. [DOI] [PubMed] [Google Scholar]
- 6.Curley SA, Izzo F, Delrio P, et al. Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg. 1999;230:1–8. doi: 10.1097/00000658-199907000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O'Malley ME, Wood BJ, Boland GW, Mueller PR. Percutaneous imaging-guided biopsy of the spleen. AJR Am J Roentgenol. 1999;172:661–665. doi: 10.2214/ajr.172.3.10063856. [DOI] [PubMed] [Google Scholar]
- 8.Soderstrom N. How to use cytodiagnostic spleen puncture. Acta Med Scand. 1976;199:1–5. doi: 10.1111/j.0954-6820.1976.tb06683.x. [DOI] [PubMed] [Google Scholar]
- 9.Keogan MT, Freed KS, Paulson EK, et al. Image-guided percutaneous biopsy of focal splenic lesions: update on safety and effectiveness. AJR Am J Roentgenol. 1999;172:933–937. doi: 10.2214/ajr.172.4.10587123. [DOI] [PubMed] [Google Scholar]
- 10.Kadir S. Diagnostic Angiography. WB Saunders; Philadelphia: 1986. Angiography of the liver, spleen, and pancreas. pp. 389–390. [Google Scholar]
- 11.Bergstrand I, Ekman CA. Percutaneous lienoportal venography: technique and complications. Acta Radiol Diagn (Stockh) 1957;47:269–279. doi: 10.3109/00016925709170895. [DOI] [PubMed] [Google Scholar]
- 12.Quinn SF, vanSonnenberg E, Casola G, Wittich GR, Neff CC. Interventional radiology in the spleen. Radiology. 1986;161:289–291. doi: 10.1148/radiology.161.2.3763890. [DOI] [PubMed] [Google Scholar]
- 13.DuPuy D, Mayo-Smith W, Murphy B, et al. The imaging appearance of radiofrequency ablated hepatic tumors: A paradigm for differentiating post ablation changes from recurrent tumor. [abstract] AJR Am J Roentgenol. 2000;174(suppl):35. [Google Scholar]
- 14.Wood BJ, Hahn PF. The spleen. In: Taveras JM, Ferrucci JT, editors. Radiology: Diagnosis, Imaging, Intervention. Vol. 4. Lippincott-Raven; 1998. pp. 1–12. [Google Scholar]
- 15.Vaezy S, Martin R, Keilman G, et al. Control of splenic bleeding by using high intensity ultrasound. J Trauma. 1999;47:521–525. doi: 10.1097/00005373-199909000-00015. [DOI] [PubMed] [Google Scholar]