Abstract
Intrasexual competition favours the evolution of conspicuous fighting ability badges. However, in spite of the fact that chemoreception is important in sexual selection of many animals, such as lizards, the role of chemical signals in males' contests is relatively unknown. Here, we show that proportions of cholesterol in femoral gland secretions of male Iberian rock lizards were related to their body size (which confers a competitive advantage in fights). Males discriminated chemically and responded aggressively to cholesterol stimuli presented on swabs. Moreover, we experimentally increased cholesterol in the scent of males, and staged encounters in neutral cages between two unfamiliar and size-matched males. Focal males lost more agonisitic interactions against males manipulated with cholesterol than in control tests. We suggest that differences in scent composition may reliably signal fighting ability in many lizard species, which would help to avoid the costs of fighting.
Keywords: chemoreception, chemical signals, male–male competition, fighting badges
1. Introduction
Male competition over females or resources important to females often favours the evolution of male traits conferring fighting ability and correlated status signalling badges (Andersson 1994). During social encounters, males may use status badges to judge relative fighting ability, thus avoiding costly aggressive interactions (Maynard Smith 1982). Most studies examining status badges focused on conspicuous visual or acoustic traits (Searcy & Nowicki 2005), generally ignoring chemical signals. Chemoreception is important in intraspecific communication of many animals, including invertebrates, reptiles and mammals (Wyatt 2003), where chemical traits alone may reveal dominance status (e.g. Apps et al. 1988; Moore et al. 1997; Zulandt-Schneider et al. 2001). In lizards, femoral gland secretions might also signal dominance (Alberts et al. 1992; Moreira et al. 2006). Chemical recognition seems to reduce costs of fighting (López & Martín 2002), but no study has examined the chemical basis of this recognition, and whether lizards actually use ‘chemical badges’ during agonistic encounters.
Iberian rock lizards, Lacerta monticola, are small lacertids from the rocky mountains of the Iberian Peninsula. Fights between males are frequent and dominance hierarchies often emerge (Aragón et al. 2004). Males discriminate between self-produced scents and scents of familiar and unfamiliar males (Aragón et al. 2001). The proportion of certain chemicals (i.e. cholesterol) in males' femoral secretions changes with body length (López et al. 2006). Because males with larger body size and larger heads have advantages in contests (López et al. 2002), we predicted that cholesterol might be a fighting ability badge in agonistic interactions.
Here, we examined whether relative proportions of cholesterol in femoral secretions of male L. monticola were related to body size. Then, we tested whether males (i) discriminated chemically cholesterol from other compounds in secretions and (ii) responded aggressively to cholesterol, while responded neutrally to other chemicals. Finally, (iii) we manipulated chemicals of size-matched males to test whether scent may signal fighting ability during agonistic contests.
2. Material and methods
(a) Male body size and chemicals in femoral secretions
We captured by noosing 50 adult male lizards during the May 2005 mating season, in different places over a large area (Guadarrama Mountains, Central Spain). Lizards were individually housed at ‘El Ventorrillo’ Field Station, 5 km from capture sites, in outdoor 80×50 cm PVC terraria containing rocks for cover, and food (mealworms) and water ad libitum. We measured lizards' snout-to-vent length (SVL) with a ruler (±SE=71±1 mm; range=63–78 mm), and head depth (8.3±0.1 mm), length (15.6±0.1 mm) and width (10.7±0.1 mm) with a digital calliper. We used principal component analysis (PCA) to reduce these four variables (log-transformed). The first PC (PC-1) explained 78.8% variance and was positively correlated with all morphological measurements. We used PC-1 scores as a variable (‘body size’) in subsequent analyses.
We collected femoral secretion directly in glass vials with Teflon-lined stoppers that were stored at −20°C. Samples were analysed by gas chromatography–mass spectrometry (ThermoQuest Trace 2000) with a Supelco-Equity-5 column temperature programmed (50—280°C at 5°C min−1 and 280°C for 30 min). The compounds were identified by comparison of mass spectra in the National Institute of Standards and Technology library, and later confirmed with authentic standards. The relative amount of each component was determined as the percentage of the total ion current (TIC) area transformed following Aitchison (1986; for similar analyses and details of chemicals see López et al. 2006).
(b) Chemosensory responses of males to chemical compounds
Lizards react to different chemical stimuli with increased and differential rates of tongue extrusions (Cooper & Burghardt 1990). Tongue-flick (TF) rate can, therefore, be used as a quantitative bioassay of detection of chemicals. We compared TF rate by males (n=16) in response to stimuli arising from cotton applicators bearing (i) dichloromethane (DCM; control) or DCM in which we had dissolved (ii) hexadecanoic acid, (iii) ergosterol or (iv) cholesterol. DCM was used to gauge baseline TF rates in the experimental situation. The other three chemicals were selected because they were the most abundant compounds in femoral secretions (López et al. 2006). We prepared chemical stimuli the same day of the tests by dissolving and mixing 24 mg of each compound (standards from Sigma-Aldrich Chemicals) in 1 ml DCM. In a second test, we measured TF rates to examine whether males can discriminate three different concentrations of cholesterol dissolved in DCM (8, 16 or 24 mg ml−1).
Immediately before the trials, we dipped for 3 s the cotton tip (1 cm) of a wooden applicator attached to a long stick (50 cm) in vials containing chemical stimuli. Swabs with all stimuli were visually similar for humans. A new swab was used in each trial. Every lizard was exposed to all scents (one trial per day) and order of presentation was counterbalanced. Trials were conducted outdoor in the middle of May (mating season), and between 11.00 and 13.00 h (GMT) when lizards were fully active. We slowly approached each lizard's cage and moved the swab to a position 1 cm anterior to the lizard's snout. We recorded numbers of TFs directed to the swab for 60 s beginning with the first TF and numbers of bites to the swabs as a measure of aggressive or defensive responses to the chemicals.
(c) Staged agonistic interactions
During May 2006, we captured different individual males (n=60) in the study area and maintained them as above. We staged encounters between pairs of males randomly chosen, but to avoid the effects of body size differences on the outcome of the contests, we never paired males that differed by more than 1 mm SVL and/or 0.5 g. To avoid the effects of previous experience (López & Martín 2002), the two males never encountered each other before the trials. We planned a repeated measures design in which each focal male (n=20) encountered in a random order two different manipulated males in a neutral arena (to avoid residency effects). We manipulated scent of males by impregnating them with sunflower oil (‘control’ treatment), or with sunflower oil in which we had dissolved cholesterol (24 mg ml−1) (‘cholesterol’ treatment). Preceding each trial, we rubbed the dorsal trunk, cloaca and hindlimbs of males (i.e. those areas most intensely investigated by tongue-flicking during encounters) with cotton swabs moistened in oil. Focal males were also impregnated with sunflower oil alone to control for potential effects of this manipulation in all males.
All tests were made in sunny conditions outdoors during May, between 09.00 and 12.00 h GMT. The test arena was a terrarium (80×50×25 cm) divided into two equally sized compartments with a removable opaque partition. We manipulated males and gently transferred them from their home terraria to the test arena, and gave them 10 min for acclimatization before removing the partition. From a hidden point, we recorded an ‘agonistic interaction’ if a male approached the other male without or with aggressive displays and made the other male retreat or run away, either without contact, or by touching him or, occasionally, by giving a quick bite on the head. The criterion for establishment of dominance was observed avoidance behaviour in one of the contestants (e.g. rapid retreat or running away) (see López et al. 2001). Multiple interactions could occur during each trial, and we noted the number of interactions ‘won’ or ‘lost’ by the focal male during 10 min. Fights did not cause observable injury or physical stress.
3. Results
Relative proportions of cholesterol in femoral secretions ranged between 54 and 84% (mean±s.e.=69±2%). Larger males (those with greater body size PC scores) had relatively greater proportions of cholesterol in their femoral secretions (forward stepwise regression, R2=0.20, F1,48=12.04, p=0.001).
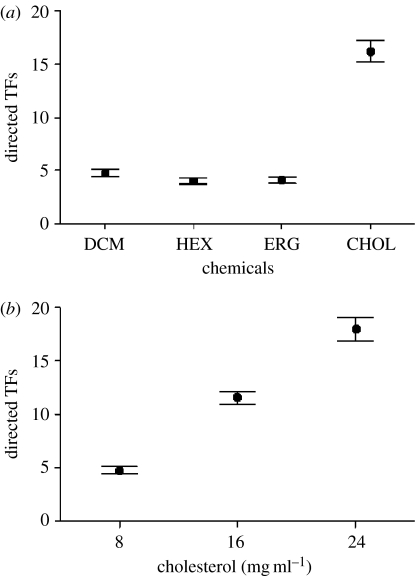
In chemosensory tests, the rate of TFs (log-transformed) directed to swabs differed between chemical stimuli (repeated measures ANOVA: F3,45=85.26, p<0.0001; figure 1a). TF rates to cholesterol were higher than to other stimuli (Tukey's tests: p<0.0002 in all cases) and there were no differences among other chemicals (p>0.70 in all cases). Six individuals bit the swabs with cholesterol vigorously and briefly, whereas no bites were recorded to other scents (binomial test, p=0.0015).
Figure 1.
Mean (±s.e.) number of tongue-flicks (TF) directed by male lizards towards swabs bearing (a) dichloromethane (DCM), cholesterol (CHOL), hexadecanoic acid (HEX) or ergosterol (ERG), all dissolved in DCM, or (b) three different concentrations of cholesterol dissolved in DCM.
In the second test, TF rates differed between concentrations of cholesterol (repeated measures ANOVA: F2,30=167.81, p<0.0001; figure 1b), with increased TF rates to stimuli having increasing concentrations (Tukey's tests: p<0.0001 in all cases). Five lizards bit the applicators bearing ‘high’ concentrations of cholesterol, and one lizard bit the applicators bearing ‘low’ concentrations (binomial test, p=0.022).
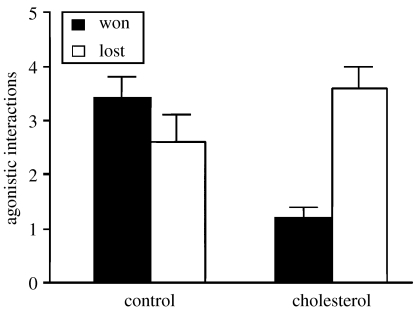
In staged encounters, there were no significant differences in the number of agonistic interactions between treatments (repeated measures two-way ANOVA, F1,19=1.58, p=0.22) and focal males lost more interactions than manipulated males (F1,19=6.47, p=0.019), but the interaction was significant (F1,19=11.61, p=0.003; figure 2). Thus, in ‘cholesterol’ trials, focal males lost more interactions than they won (Tukey's test: p<0.01), but there were no differences in the ‘control’ treatment (p=0.62).
Figure 2.
Mean (+s.e.) number of agonistic interactions won or lost by focal males in staged encounters with males impregnated with sunflower oil (‘control’), or sunflower oil with cholesterol (‘cholesterol’).
4. Discussion
Cholesterol proportions in femoral secretions of male L. monticola were related to their body size. In this lizard, cholesterol is the major lipid in secretions and its production may be size/age dependent. In lizards, social dominance, lipid metabolism, femoral gland production and percentage of lipid in secretions depend on androgen levels (Alberts et al. 1992; Sheridan 1994). Testosterone upregulates cholesterol in humans and birds (McGraw et al. 2006). Thus, it is likely that proportions of cholesterol in secretions depended on sex steroid levels, which would make it a potentially reliable signal of dominance.
Chemosensory tests clearly showed that cholesterol was readily discriminated and elicited aggressive responses. Lizards often exhibit social dominance systems and use pheromones as social signals (Mason 1992), and intrasexual aggression in many lizards is mediated by chemical recognition (Cooper & Vitt 1987; López & Martín 2002). Because a large body size is determinant in agonistic contests, it would be advantageous to assess the size of rivals in the absence of the signallers before being involved in fights. Previous experiments showed that behavioural responses of intruder male L. monticola to scent marks of unfamiliar males depended on the relative differences in body size (Aragón et al. 2001): the current experiment suggests that body size may be assessed from cholesterol proportions in scent.
Moreover, cholesterol may be a signal of fighting ability during agonistic contests too. Visual estimation of size should occur first, but between similarly sized males, chemoreception could allow an individual to quickly assess a rival's fighting ability, such that potentially inferior males may retreat before a costly escalated fight occurs. In fact, most interactions lost in our experimental treatment were retreats of males before manipulated males had started any aggressive approach. We suggest that differences in scent composition, based on cholesterol or other lipids, may reliably signal fighting ability in many lizard species where chemoreception is important, which would help to avoid the costs of unnecessary fighting.
Acknowledgments
We thank two anonymous reviewers for helpful comments, and ‘El Ventorrillo’ MNCN Field Station for the use of their facilities. Financial support was provided by the MEC project CGL2005-00391/BOS. Experiments were approved by the ‘Comunidad de Madrid’ Environmental Agency. All lizards were returned healthy to their capture sites.
References
- Aitchison J. Chapman and Hall; London, UK: 1986. The statistical analysis of compositional data: monographs in statistics and applied probability. [Google Scholar]
- Alberts A.C, Pratt N.C, Phillipis J.A. Seasonal productivity of lizard femoral glands: relationship to social dominance and androgen levels. Physiol. Behav. 1992;51:729–733. doi: 10.1016/0031-9384(92)90109-f. doi:10.1016/0031-9384(92)90109-F [DOI] [PubMed] [Google Scholar]
- Andersson M. Princeton University; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Apps P.J, Rasa A, Viljoen H.W. Quantitative chromatographic profiling of odours associated with dominance in male laboratory mice. Aggr. Behav. 1988;14:451–461. [Google Scholar]
- Aragón P, López P, Martín J. Chemosensory discrimination of familiar and unfamiliar conspecifics by lizards: implications of field spatial relationships between males. Behav. Ecol. Sociobiol. 2001;50:128–133. doi:10.1007/s002650100344 [Google Scholar]
- Aragón P, López P, Martín J. The ontogeny of spatio-temporal tactics and social relationships of adult male Iberian rock lizards, Lacerta monticola. Ethology. 2004;110:1001–1019. doi:10.1111/j.1439-0310.2004.01046.x [Google Scholar]
- Cooper W.E, Burghardt G.M. A comparative analysis of scoring methods for chemical discrimination of prey by squamate reptiles. J. Chem. Ecol. 1990;16:45–65. doi: 10.1007/BF01021267. doi:10.1007/BF01021267 [DOI] [PubMed] [Google Scholar]
- Cooper W.E, Vitt L.J. Intraspecific and interspecific aggression in lizards of the scincid genus Eumeces: chemical detection of conspecific sexual competitors. Herpetologica. 1987;43:7–14. [Google Scholar]
- López P, Martín J. Fighting rules and rival recognition reduce costs of aggression in male lizards, Podarcis hispanica. Behav. Ecol. Sociobiol. 2001;49:111–116. doi:10.1007/s002650000288 [Google Scholar]
- López P, Martín J. Chemical rival recognition decreases aggression levels in male Iberian wall lizards, Podarcis hispanica. Behav. Ecol. Sociobiol. 2002;51:461–465. doi:10.1007/s00265-001-0447-x [Google Scholar]
- López P, Muñoz A, Martín J. Symmetry, male dominance and female mate preferences in the Iberian rock lizard, Lacerta monticola. Behav. Ecol. Sociobiol. 2002;52:342–347. doi:10.1007/s00265-002-0514-y [Google Scholar]
- López P, Amo L, Martín J. Reliable signaling by chemical cues of male traits and health state in male lizards, Lacerta monticola. J. Chem. Ecol. 2006;32:473–488. doi: 10.1007/s10886-005-9012-9. doi:10.1007/s10886-005-9012-9 [DOI] [PubMed] [Google Scholar]
- Mason R.T. Reptilian pheromones. In: Gans C, Crews D, editors. Biology of the reptilia. vol. 18. University of Chicago Press; Chicago, IL: 1992. pp. 114–228. [Google Scholar]
- Maynard Smith J. Cambridge University Press; Cambridge, UK: 1982. Evolution and the theory of games. [Google Scholar]
- McGraw K.J, Correa S.M, Adkins-Regan E. Testosterone upregulates lipoprotein status to control sexual attractiveness in a colorful songbird. Behav. Ecol. Sociobiol. 2006;60:117–122. doi:10.1007/s00265-005-0135-3 [Google Scholar]
- Moore P.J, Reagan-Wallin N.L, Haynes K.F, Moore A.J. Odour conveys status on cockroaches. Nature. 1997;389:25. doi:10.1038/37888 [Google Scholar]
- Moreira P.L, López P, Martín J. Femoral secretions and copulatory plugs convey chemical information about male identity and dominance status in Iberian rock lizards (Lacerta monticola) Behav. Ecol. Sociobiol. 2006;60:166–174. doi:10.1007/s00265-005-0153-1 [Google Scholar]
- Searcy W.A, Nowicki S. Princeton University Press; Princeton, NJ: 2005. The evolution of animal communication. [Google Scholar]
- Sheridan M.A. Regulation of lipid-metabolism in poikilothermic vertebrates. Comp. Biochem. Physiol. B. 1994;107:495–508. doi:10.1016/0305-0491(94)90176-7 [Google Scholar]
- Wyatt T.D. Cambridge University Press; Cambridge, UK: 2003. Pheromones and animal behaviour. [Google Scholar]
- Zulandt-Schneider R.A, Huber R, Moore P.A. Individual and status recognition in the crayfish, Orconectes rusticus: the effects of urine release on fight dynamics. Behaviour. 2001;138:137–153. doi:10.1163/15685390151074348 [Google Scholar]