Abstract
Carotenoids determine the yellow–red colours of many ornaments, which often function as signals of quality. Carotenoid-based signalling may reliably advertise health and should be particularly sensitive to parasite infections. Nematodes are among the commonest parasites of vertebrates, with well-documented negative effects on their hosts. However, to date, little is known about the effects that these parasites may have on carotenoid-based signalling. Tetraonid birds (grouse) exhibit supra-orbital combs, which are bright integumentary ornaments pigmented by carotenoids. We tested the effect of the nematode parasite Trichostrongylus tenuis on signalling in free-living male red grouse Lagopus lagopus scoticus. We show that experimentally reduced nematode infection increases plasma carotenoid concentration and comb redness, demonstrating for the first time that nematodes can influence carotenoid-based signals.
Keywords: carotenoids, comb, nematodes, red grouse Lagopus lagopus scoticus, Trichostrongylus tenuis
1. Introduction
The brightly coloured ornaments of animals often function as reliable signals of quality, indicating better body condition or ability to resist parasites (Hamilton & Zuk 1982). Carotenoid-based signals are among the most familiar criteria for mate choice (Hill & McGraw 2006). Identifying limiting factors along the pathway from nutritional access to coloration is essential for the understanding of how carotenoid-based ornaments have evolved and are maintained as honest signals (Hill & McGraw 2006). Vertebrates cannot produce carotenoids, so carotenoid intake can limit ornament expression (Olson & Owens 1998). Carotenoids also have beneficial physiological functions, being immunostimulants and antioxidants (Møller et al. 2000). The trade-offs resulting from carotenoid use in self-maintenance versus ornamentation may further confer honesty (Faivre et al. 2003). Carotenoid-based signals should be particularly sensitive to parasites (Lozano 1994), though experimental evidence remains limited (e.g. Hill & McGraw 2006). For instance, coccidia can directly reduce carotenoid uptake (Hõrak et al. 2004), and cestodes might negatively influence carotenoid signalling (Figuerola et al. 2005). Nematodes are common intestinal parasites of vertebrates, and often have profound effects on hosts (Wakelin 1978); however, their effects on circulating carotenoids and carotenoid-dependent ornamentation have never been tested experimentally.
We manipulated nematode parasites in male red grouse and investigated the effects on carotenoid-based signalling. Red grouse display red supra-orbital combs pigmented by carotenoids (Mougeot et al. 2007) that function in intra- and inter-sexual selection (Mougeot et al. 2004, 2007). Using an anthelmintic drug, we reduced infection by Trichostrongylus tenuis worms. This main parasite of red grouse negatively impacts condition, productivity and survival (Hudson 1986). We predicted a reduction in T. tenuis would increase plasma carotenoids and the pigmentation of grouse combs.
2. Material and methods
(a) Experiment
In autumn 2005 (16 October–1 November), we caught 37 males on Edinglassie Estate, northeast Scotland (57°12′ N–3°07′ W). Each was ringed, fitted with a radio collar (TW3-necklace tag, Biotrack) and aged (young, i.e. hatched that summer or old). Males were randomly assigned to one of two treatments: dosed (parasite reduction) or control. After collecting faecal samples for parasite counts, control males were given 1 ml oral dose of water, and dosed males were given 1 ml of anthelmintic (levamisole hydrochloride, Nilverm Gold), a drug effective at reducing T. tenuis (Hudson 1986). We recaptured 30 males 18±4 days after treatment. Time between capture and recapture did not differ between groups (general linear model, F1,26=0.22, p=0.643). At each capture, we took a blood sample from the wing vein and a digital photograph of the comb. Blood was centrifuged and plasma kept frozen at −20°C. Males were kept overnight in individual boxes to collect faecal samples.
(b) Comb redness
High-resolution (2272×1704 pixels) pictures of the flattened comb were taken at a standard distance (50 cm) using the flash of the digital camera (Nikon Coolpix 4500). The same grey reference chip was placed beside the comb for each picture. We analysed digital images using Adobe Photoshop v. 7.0, measuring the average component of red (R) from the largest continuous area within the combs and the grey reference using the RGB system (see electronic supplementary material). Comb redness measures were highly repeatable (see electronic supplementary material).
(c) Plasma carotenoid concentration
Carotenoids were quantified by diluting 60 μl of plasma in acetone (1 : 10). The mixture was vortexed and centrifuged at 10 000 r.p.m. for 10 min. The supernatant was examined in a ShimadzuUV-1603 spectrophotometer and we determined the optical density at 446 nm, the wavelength of maximal absorbance for lutein (Mínguez-Mosquera 1993), the most common circulating carotenoid in birds (Hill & McGraw 2006). This wavelength has been considered as a reliable index of total carotenoids (Blount et al. 2003; McGraw et al. 2003). Plasma carotenoid concentration (μg ml−1) was calculated using a standard curve of lutein (Sigma Chemicals).
(d) Parasite abundance
We used faecal egg concentrations to estimate coccidia and T. tenuis abundance. Samples were stored at 4°C to inhibit egg development and analysed within 5 days of collection to ensure reliable estimates (Seivwright et al. 2004, see electronic supplementary material).
(e) Statistical analyses
We used SAS v. 9.1. Counts of coccidia eggs and T. tenuis worms were fitted to generalized linear mixed models (GLMMs) using a Poisson error distribution. Plasma carotenoid concentration and comb redness were fitted to GLMMs using a normal distribution (Shapiro–Wilk tests, NS). When testing for treatment effects, we included ‘individual’ as a random effect, to account for repeated measures. We tested for differences between treatment groups in changes over time of variables by including ‘recapture’ (before versus after treatment), treatment (dosed versus control), age (old and young) and their interactions as fixed effects. Trichostrongylus tenuis and coccidia abundances were log transformed when included as explanatory variables. For analyses of comb redness, R-values were standardized using R-values from the grey reference, included as a covariate in all models. All tests were two-tailed.
3. Results
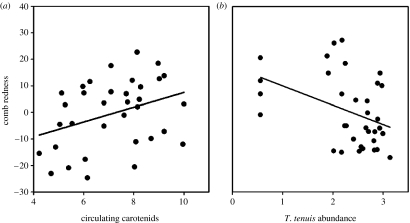
Before treatment, comb redness positively correlated with circulating carotenoids (F2,33=3.17, p=0.084; figure 1a), significantly so when parasites were included as covariates (F1,29=6.67, p=0.015; T. tenuis, p=0.011 and coccidia, F1,29=2.29, p=0.141). Circulating carotenoids tended to decrease with increasing coccidia and T. tenuis intensities (F1,31=3.41, p=0.074 and F1,31=2.53, p=0.122, respectively), significantly so when comb redness was a covariate (coccidia, F1,29=6.13, p=0.019 and T. tenuis, F1,29=4.99, p=0.033; redness, F1,29=6.67, p=0.015; figure 1). Comb redness correlated negatively with T. tenuis abundance (F1,29=7.49, p=0.010; figure 1b). These relationships did not differ between age groups (all p>0.38).
Figure 1.
Relationships between comb redness and (a) plasma carotenoid concentration (μg ml−1). (b) T. tenuis abundance (worms per grouse) before treatment.
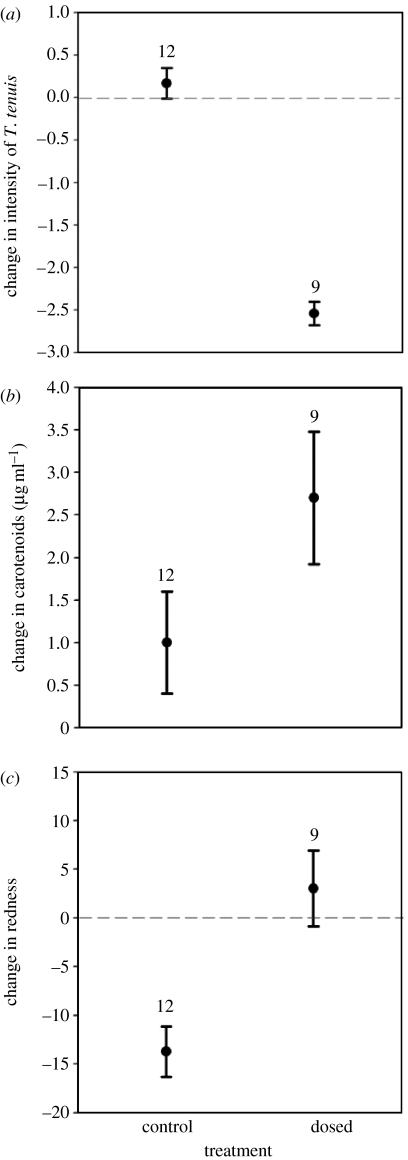
Before treatment, T. tenuis and coccidia prevalences were 89.2 and 100%, respectively. Old males had more T. tenuis than young grouse (table 1). Trichostrongylus tenuis abundance decreased significantly more in dosed than in control males (table 1; figure 2a; see also table S1 in electronic supplementary material) independently of bird age. Coccidia abundance was not affected by treatment (table 1). Young males had more coccidia than old males before treatment, but changes over time in abundance did not differ between treatment groups (table 1), in both old and young birds.
Table 1.
Effects of age, treatment and recapture on coccidia and T. tenuis abundance, plasma carotenoid concentration and comb redness.
| dependent variables | coccidia abundancea | T. tenuis worm abundancea | carotenoids | comb rednessb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| explanatory variables | d.f. | F | p | d.f. | F | p | d.f. | F | p | d.f. | F | p |
| age (A) | 1,34.1 | 4.67 | 0.038 | 1,31.8 | 54.79 | <0.001 | 1,24 | 0.47 | 0.498 | 1,24 | 0.02 | 0.879 |
| treatment (T) | 1,33.7 | 0.50 | 0.486 | 1,26.8 | 5.28 | 0.030 | 1,24 | 0.18 | 0.675 | 1,24 | 0.04 | 0.842 |
| recapture (R) | 1,32.5 | 5.75 | 0.022 | 1,29.1 | 7.45 | 0.011 | 1,24 | 33.23 | <0.001 | 1,24 | 2.51 | 0.126 |
| T×R | 1,33.8 | 0.17 | 0.686 | 1,15.6 | 8.59 | 0.010 | 1,24 | 4.37 | 0.046 | 1,24 | 14.50 | 0.019 |
| A×T×R | 4,17.3 | 0.24 | 0.835 | 4,15.4 | 1.94 | 0.311 | 4,15 | 1.71 | 0.200 | 4,21 | 1.12 | 0.372 |
GLMM models were performed with Poisson error and log link function.
For analyses of comb redness, all models including the R-value of the grey reference as a covariate (p<0.001).
Figure 2.
Mean (±s.d.) changes in (a) T. tenuis abundance (log transformed), (b) plasma carotenoid concentration and (c) comb redness in control and dosed males. Sample sizes above bars are shown.
Prior to treatment, plasma carotenoid concentration did not differ between treatment and age groups (both p>0.46). Circulating carotenoids increased significantly more in dosed than in control birds (table 1, figure 2b), in both young and old males. Comb redness also increased significantly more in dosed than in control birds (table 1; figure 2c and electronic supplementary material) in both young and old males. Coccidia abundance did not influence redness after controlling for treatment effects (F1,17=0.29, p=0.669).
4. Discussion
In untreated males, comb redness increased with circulating carotenoids, significantly so when parasites were taken into account. Thus, the relationship between ornament coloration and circulating carotenoids, which has been found in several other species (Hill & McGraw 2006), was only revealed when parasites provided the appropriate context. Similarly, the negative relationships between carotenoid levels and parasites were better understood when comb colour was taken into account. The negative relationships between both coccidia and T. tenuis parasites and carotenoid levels suggested that both parasites limited carotenoid-based signalling.
Our treatment was effective at reducing T. tenuis worms, increasing circulating carotenoids and ultimately enhancing ornamental coloration. It is known that other intestinal parasites, particularly coccidia (McGraw & Hill 2000; Hõrak et al. 2004) influence carotenoid-based signals in captive birds. Our anthelmintic treatment reduced nematode infection without significantly affecting coccidia parasites. Our experimental results were also consistent with the correlative results, and both indicated that T. tenuis nematodes reduce circulating carotenoids and redness of the comb. We are thus confident that our results indicate a negative effect of nematodes on plasma carotenoids and on carotenoid-based ornamentation. Despite a high prevalence, T. tenuis abundance was low in our study, compared with the range observed in red grouse (up to 30 000 worms, Hudson 1986). Thus, even subtle variations in nematode infection can affect ornamentation.
Nematodes can affect carotenoid signals in several, non-exclusive ways. The thickening of the gut epithelium caused by coccidiosis has been shown to constrain carotenoid absorption (Allen 1987). Adult nematodes inhabit the caeca of red grouse (Seivwright et al. 2004) and cause significant damage to epithelial tissues. Grouse have particularly long caeca to maximize digestion and absorption of plant nutrients. Although we do not know if carotenoid absorption takes place in the caeca, the caecal damage caused by T. tenuis worms could constrain absorption and explain the negative effect of nematodes on circulating carotenoids. Trichostrongylus tenuis worms might also reduce the production of high-density lipoproteins and their incorporation into ornaments (McGraw et al. 2006) or directly compete with the bird for carotenoids (Mawson & Wakabongo 2002). Finally, nematodes can also have other systemic effects on carotenoid availability (Hill et al. 2004) as carotenoids may be diverted to boost the immune system against nematodes instead of being displayed in ornaments (Møller et al. 2000; Blount et al. 2003).
Nematodes are among the commonest parasites of vertebrates (Wakelin 1978), and have the potential to reduce plasma carotenoid availability and carotenoid use for ornamentation, as demonstrated by our experiment. This should stimulate more experiments on wild and captive animals, and more detailed investigation of the mechanisms by which nematode parasites influence carotenoid signals.
Acknowledgments
We thank the landowner and D. Calder from Edinglassie Estate for allowing us to conduct the experiment. J.M.P., F.M. and L.P.R. were supported by post- and pre-doctoral grants (Ministerio de Educación y Ciencia, Spain). J. Vicente, J. Blount and R. Moss improved greatly an early version of the manuscript. GRB was supported by NSERC.
Supplementary Material
In this appendix, we describe how colour and parasites were analysed and determined together with their repeatability assessments. We also show the mean and standard desvition before and after T. tenuis treatment on redness of the comb, carotenoid levels and coccidia and T. tenuis abundances
References
- Allen P.C. Physiological response of chicken gut tissue to coccidial infection: comparative effects of Eimeria acervulina and Eimeria mitis on mucosal mass, carotenoid content, and brush border enzyme activity. Poult. Sci. 1987;66:1306–1315. doi: 10.3382/ps.0661306. [DOI] [PubMed] [Google Scholar]
- Blount J.D, Metcalfe N.B, Birkhead T.R, Surai P.F. Carotenoid modulation of immune function and sexual attractiveness in zebra finches. Science. 2003;300:125–127. doi: 10.1126/science.1082142. doi:10.1126/science.1082142 [DOI] [PubMed] [Google Scholar]
- Faivre B, Gregoire A, Preault M, Cezilly F, Sorci G. Immune activation rapidly mirrored in a secondary sexual trait. Science. 2003;300:103. doi: 10.1126/science.1081802. doi:10.1126/science.1081802 [DOI] [PubMed] [Google Scholar]
- Figuerola J, Torres J, Garrido J, Green A.J, Negro J.J. Do carotenoids and spleen size vary with helminth load in greylag geese? Can. J. Zool. 2005;83:389–395. doi:10.1139/z05-022 [Google Scholar]
- Hamilton W.D, Zuk M. Heritable true fitness and bright birds: a role for parasites? Science. 1982;218:384–387. doi: 10.1126/science.7123238. doi:10.1126/science.7123238 [DOI] [PubMed] [Google Scholar]
- Hill G.E, McGraw K.J. Harvard University Press; Cambridge, MA: 2006. Bird coloration. Mechanisms and measurements. [Google Scholar]
- Hill G.E, Farmer K.L, Beck M.L. The effect of mycoplasmosis on carotenoid plumage coloration in male house finches. J. Exp. Biol. 2004;207:2095–2099. doi: 10.1242/jeb.00998. doi:10.1242/jeb.00998 [DOI] [PubMed] [Google Scholar]
- Hõrak P, Saks L, Karu U, Ots I, Surai P.F, McGraw K.J. How coccidian parasites affect health and appearance of greenfinches. J. Anim. Ecol. 2004;73:935–947. doi:10.1111/j.0021-8790.2004.00870.x [Google Scholar]
- Hudson P.J. The Game Conservancy Trust; Fordingbridge, UK: 1986. The red grouse: the biology and management of a wild gamebird. [Google Scholar]
- Lozano G.A. Caroteniods, parasites, and sexual selection. Oikos. 1994;70:309–311. [Google Scholar]
- Mawson A.R, Wakabongo M. Onchocerciasis-associated morbidity: hypothesis. Trans. R. Soc. Trop. Med. Hyg. 2002;96:541–542. doi: 10.1016/s0035-9203(02)90434-7. doi:10.1016/S0035-9203(02)90434-7 [DOI] [PubMed] [Google Scholar]
- McGraw K.J, Hill G.E. Differential effects of endoparasitism on the expression of the carotenoid- and melanin-based ornamental coloration. Proc. R. Soc. B. 2000;267:1525–1531. doi: 10.1098/rspb.2000.1174. doi:10.1098/rspb.2000.1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw K.J, Gregory A.J, Parker R.S, Adkins-Regan E. Diet, plasma carotenoids, and sexual coloration in the zebra finch (Taeniopygia guttata) Auk. 2003;120:400–410. doi:10.1642/0004-8038(2003)120[0400:DPCASC]2.0.CO;2 [Google Scholar]
- McGraw K.J, Correa S.M, Adkins-Regan E. Testosterone upregulates lipoprotein status to control sexual attractiveness in a colorful songbird. Behav. Ecol. Soc. 2006;60:117–122. doi:10.1007/s00265-005-0135-3 [Google Scholar]
- Mínguez-Mosquera I. Universidad de Sevilla; Sevilla, Spain: 1993. Clorofilas y carotenoides en tecnologia de alimentos. [Google Scholar]
- Møller A.P, Biard C, Blount J.D, Houston D.C, Ninni P, Saino N, Surai P.F. Carotenoid-dependent signals: indicators of foraging efficiency, immunocompetence or detoxification ability? Avian Poult. Biol. Rev. 2000;11:137–159. [Google Scholar]
- Mougeot F, Irvine J.R, Seivwright L, Redpath S.M, Piertney S. Testosterone, immunocompetence, and honest sexual signaling in male red grouse. Behav. Ecol. 2004;15:930–937. doi:10.1093/beheco/arh087 [Google Scholar]
- Mougeot, F., Martínez-Padilla, J., Pérez-Rodríguez, L. & Bortolotti, G. R. 2007 Carotenoid-based coloration and ultraviolet reflectance of the sexual ornaments of grouse. Behav. Ecol. Soc. (doi:10.1007/s00265-006-0304-z)
- Olson V.A, Owens I.P.F. Costly sexual signals: are carotenoids rare, risky or required? Trends Ecol. Evol. 1998;13:510–514. doi: 10.1016/s0169-5347(98)01484-0. doi:10.1016/S0169-5347(98)01484-0 [DOI] [PubMed] [Google Scholar]
- Seivwright L, Redpath S.M, Mougeot F, Watt L, Hudson P.J. Faecal egg counts provide a reliable measure of Trichostrongylus tenuis intensities in free-living red grouse Lagopus lagopus scoticus. J. Helminthol. 2004;78:69–76. doi: 10.1079/joh2003220. doi:10.1079/JOH2003220 [DOI] [PubMed] [Google Scholar]
- Wakelin D. Immunity to intestinal parasites. Nature. 1978;273:617–620. doi: 10.1038/273617a0. doi:10.1038/273617a0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In this appendix, we describe how colour and parasites were analysed and determined together with their repeatability assessments. We also show the mean and standard desvition before and after T. tenuis treatment on redness of the comb, carotenoid levels and coccidia and T. tenuis abundances