Abstract
The Lake Victoria ‘species flock’ of cichlids is puzzling because reproductive isolation often occurs in the absence of substantial ecological differences among species. Theory predicts that this cannot evolve with most genetic mechanisms for mate choice. We provide the first evidence that learning, in the form of sexual imprinting, helps maintain reproductive isolation among closely related cichlid species. Using a cross-fostering experiment, we show that young females develop a sexual preference for males of their foster mothers' species, even reversing species assortative mating preferences. We suggest that learning creates favourable conditions for reproductive isolation to evolve.
Keywords: imprinting, mate choice, speciation.
1. Introduction
One of the least understood processes in speciation is the evolution of reproductive isolation without a geographical barrier. Though long thought implausible, speciation without geographical isolation recently has been suggested for a few cases, among which are the haplochromine cichlid fish (Seehausen & van Alphen 1999). Both Lake Malawi and Lake Victoria harbour large species flocks, endemic to each lake (Salzburger et al. 2005). Adaptations in jaw morphology may explain the radiation into various feeding niches (Kocher 2004). However, often several closely related species are found in sympatry with little morphological and ecological difference while a secondary sexual character, nuptial coloration, has strongly diverged (Seehausen & van Alphen 1999). This may be explained by disruptive sexual selection for conspicuous coloration (Maan et al. 2004) and strong assortative mating (Seehausen & van Alphen 1998). Postzygotic isolation cannot explain the maintenance of these species (Seehausen et al. 1997). In spite of great interest in these species and their evolution, we still do not have a clear understanding of how they have evolved or maintain reproductive isolation.
Learning by young individuals about the maternal phenotype as a model for their later sexual preference could promote reproductive isolation in sympatry. Recent models show that this allows evolution of assortative mating under a wider range of conditions than most scenarios involving a genetic background for mate preferences (Felsenstein 1981; Irwin & Price 1999; Verzijden et al. 2005). Such early learning of a sexual preference is known as sexual imprinting. It is prevalent in birds (ten Cate & Vos 1999) and is also documented in mammals (Kendrick et al. 1998). Females of haplochromine cichlid species show mouth brooding and continued protection of the brood after hatching. This might provide the offspring the opportunity to learn specific characters of their mother. A few studies have addressed imprinting in cichlids (Siepen & Crapon de Caprona 1986; Barlow et al. 1990) but so far no clear examples of parental sexual imprinting in fish are known. Sexual imprinting could promote the evolution of reproductive isolation, as it would strongly link phenotype to mate preference. Here, we test whether mate preferences in females of two Lake Victoria haplochromine cichlid species are affected by their mothers' phenotype.
The closely related species pair Pundamilia pundamilia and Pundamilia nyererei is a model for the study of mate preferences and reproductive isolation in Lake Victoria haplochromine cichlids. They show only slight ecological and morphological differentiation and are sympatric throughout the range of P. nyererei (Seehausen & van Alphen 1999). Male nuptial coloration in P. pundamilia is mostly blue and P. nyererei red (figure 1). To test for early learning, we designed an interspecific cross-fostering experiment between the two Pundamilia species. The virgin daughters from these broods were tested for their preference for males of the two species.
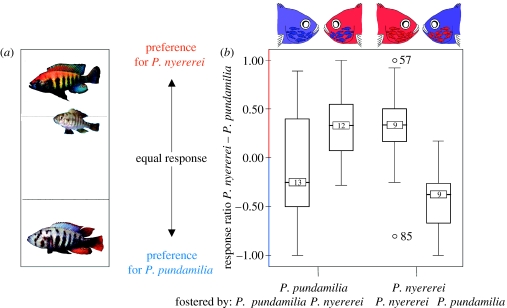
Figure 1.
The cross-fostering experiment. (a) The two-way female mate choice experimental setup. Females can enter male territories through grids, while the larger males cannot leave theirs (photos by Ole Seehausen). (b) The results of the cross-fostering experiment. For this figure, we subtracted the approach ratio to PP from PN. Scores above zero reflect a higher approach ratio to P. nyererei males and scores below zero reflect a higher approach ratio to P. pundamilia males. Graphs represent median, interquartile ranges and full ranges. Numbers on median bars show number of individuals in each treatment group. (Coloration in the drawings are for cartoon purposes only, they do not reflect natural coloration.)
2. Material and methods
(a) Housing and breeding
Wild caught P. pundamilia and P. nyererei from Makobe island, Tanzania, were housed in single species stock tanks (size 1×0.4×0.6 m). For breeding, up to 12 females were housed with one male that was replaced regularly. All tanks were connected to a central recirculation water filter system. Water temperature was 24.5°C, ±1°C, light regime was 12 L : 12 D. Fish were fed daily with fresh shrimp and peas or commercial pellets and flakes.
(b) Cross-fostering and raising of the broods
Brooding females that had spawned approximately at the same time (maximum 4 days apart) were gently forced to spit out their eggs, within 2–5 days after spawning. Eggs were then taken up in a plastic pipette, which was then emptied in another female's mouth, such that each female received the eggs of the other female. All four types of crossings were done, both within and between species. Each treated female was placed in a visually isolated small tank. Mouth brooding takes 3–4 weeks, then the female releases the fry. The fry then start foraging independently, while females guard them for three weeks. When this stopped prematurely, the female was placed behind a perforated transparent sheet within the tank. Females were removed after four weeks. Each brood was later placed in a stock tank (1.5–5 months after spawning) exclusive for that brood. With first signs of nuptial coloration (average 170 days), we visually separated brothers and sisters with a perforated opaque sheet, to prevent them from gaining breeding experience. At sexual maturity, PIT tags (12 mm glass tags, UKID122GL Biomark, Inc.) were implanted in the left belly cavity. Females were then placed in a communal tank with conspecific females of other treatment broods.
(c) Testing
Two grids (mesh size 160×160 mm) divided the experimental tank (2×0.5×0.5 m) into three equal compartments. The outer compartments contained a P. pundamilia and P. nyererei male. Bricks served as territorial ‘rocks’. The males were matched in size (mean difference standard length 0.7 mm s.e.m. ±0.07, range 79–126 mm). Males were placed into the experimental tank 24 h before testing. In total, 34 P. nyererei and 36 P. pundamilia males were used, (re)combined into 53 pairs. Males performed on average in 2.48 tests (±0.19 s.e.m.). A female was placed in the middle compartment 30–60 min prior to testing while opaque screens hid the males from her. A trial started with removal of the screens and lasted 30 min. We tested the females blindly with regard to treatment. The focal female could interact freely with the males which were restricted to the outer compartments of the tank (figure 1). Males courted females when they entered their compartment. Males initiate courtship by a series of behavioural displays, to which females can respond by approaching. Courtship may then proceed and eventually lead to spawning. This was scored as described in Seehausen & van Alphen (1998). In a successful trial, both males displayed at least two quivers and the female approached a quiver twice. Unsuccessful trials were repeated later on. We tested until each female had two successful trials, with different male pairs. Male species position (left or right) was reversed in the second trial. We calculated the relative approach ratio as follows: (number of approaches to conspecific male/number of quiver displays conspecific male)—(number of approaches to heterospecific male/number of quiver displays heterospecific male).
In total, 43 females from 21 broods were tested. Five broods of each species were interspecifically cross-fostered, and five P. nyererei and six P. pundamilia were control-fostered broods. The data is presented in table 1 of the electronic supplementary material.
(d) Colour analysis of males and females
Males and females were photographed with a Sony DSC-F707 camera at the same place under the same lighting conditions, with the same aperture each time. Pictures were analysed with SigmaScan Pro v. 4.0 (SPSS Inc.; Maan et al. 2004). Only the dorsal, most intensely coloured part of the body was used for coloration analysis. Ranges for hue and saturation were maximized for differences between the males of the two species. Those same settings were then used in females. We analysed the red/blue ratio.
All statistical analysis was done in R (R Development Core Team 2005) with nested Generalized Linear Models (GLMM), stepwise deleting factors from a fully saturated model until the minimal adequate GLMM was found, whose factors and significance levels are reported. Models were nested, correcting for any pseudoreplication; broods within treatment and individuals (two trials) within broods.
3. Results
The behaviour of females between two tests was significantly correlated: r=0.33, t41=2.2, p=0.03.
Species of foster mother had a significant effect on the preference of the females. Interspecifically cross-fostered females of both species preferred heterospecific males more than did females of the intraspecific cross-foster treatment (P. nyererei: GLMM, F(1,10)=24.77, p=0.0011; P. pundamilia: GLMM, F(1,9)=5.54, p=0.045; Both species: GLMM, F(1,19)=21.39, p=0.0002, figure 1). Females in three treatment groups showed significant preferences for males of their foster mothers' species (interspecific treatment P. pundamilia and P. nyererei: p=0.0003, t12=−5.02; p=0.0012, t9=−4.63, respectively, and intraspecific treatment P. nyererei: p=0.038, t9=2.41, nested one-sample t-tests). In trials with P. pundamilia females, conspecific males displayed significantly more than heterospecific males: F(1,84)=29.26, p<0.001. Since the number of female approaches best fitted an inverse exponential relation to male display effort (see electronic supplementary material), this lowered their preference scores.
The ratio red/blue was significantly different between the females of the two species (p<0.001, F(1,60)=23.79). More results are given in table 2 of the electronic supplementary material.
4. Discussion
This experiment provides the first strong evidence for cichlids, and for fish in general, that females prefer males of the maternal phenotype as a result of imprinting. In contrast, studies testing for sexual imprinting in substrate spawning Central American cichlids (Siepen & Crapon de Caprona 1986; Barlow et al. 1990) have shown small and inconsistent effects. This may indicate that the ability to imprint has evolved in consort with the mouth brooding in haplochromine cichlids, or that a latent ability for sexual imprinting is expressed with the opportunity provided by the mouth-brooding behaviour.
Nuptial coloration is an important cue in both interspecific (Seehausen & van Alphen 1998) and intraspecific mate choice (Maan et al. 2004), suggesting its importance in reproductive isolation. While sexually mature males are brightly coloured, females are mostly yellow or light brown. However, the females do show some differentiation in colour in the same direction as males (table 2 of electronic supplementary material), which therefore may provide a basis for the learnt preferences. Interestingly, visual early learning was also shown to mediate assortative shoaling preferences in zebra fish (Danio rerio; Engeszer et al. 2004).
Olfactory cues are used in broodcare in parent–offspring communication in cichlids (Kühme 1964). These may be correlated with male coloration (Plenderleith et al. 2005), or reflect species differences, possibly through MHC and related mechanisms (Zavazava & Eggert 1997). This could provide species specific cues to the hatching brood. Imprinting on olfactory cues is therefore also plausible. As both colour and chemical cues could be used at all stages of the experiment, we cannot conclude which cue served as imprinting stimulus.
In a sympatric speciation scenario, disruptive sexual selection on coloration may have initiated divergence of mating cues (Maan et al. 2004) but this alone may not provide full reproductive isolation. However, imprinting can be a very effective mechanism in linking mate preferences to other diverged characters (Verzijden et al. 2005).
In summary, we show that cichlid young imprint on their mothers' phenotype and that this can reverse species assortative mating preferences. All haplochromine cichlids in the East African great lakes are maternal mouth brooders. Imprinting may be widespread among them, given their high degree of relatedness. We suggest that imprinting greatly enhanced their tendency for assortative mating. The presence of imprinting may therefore be critical to explaining the many sympatric species of haplochromine cichlids.
Acknowledgments
The university committee for animal experiments approved this experiment: licence number: DEC03079. The authors thank Peter Dijkstra, Enja Feuth de Bruin, Antti Poikonen, Mohammed Haluna, Kees Hofker, Mhoja Kayeba, Rob Lachlan, Martine Maan, Ole Seehausen, Maria Servedio and Inke van der Sluijs, and two anonymous referees for help and/or for discussions and comments on the manuscript. Financial support: NWO (ALW-810.64.012) and Lucie Burgers Foundation for Comparative Behaviour Research, Arnhem, The Netherlands.
Supplementary Material
Data of male and female behaviour in each preference test and a regression analysis of the interaction between male courtship effort and female tendency to approach. Scores of red and blue colour ranges of males and females of both species
References
- Barlow G.W, Francis R.C, Baumgartner J.V. Do the colors of parents, companions and self influence assortative mating in the polychromatic midas cichlid. Anim. Behav. 1990;40:713–722. doi:10.1016/S0003-3472(05)80700-6 [Google Scholar]
- Engeszer R.E, Ryan M.J, Parichy D.M. Learned social preference in zebrafish. Curr. Biol. 2004;14:881–884. doi: 10.1016/j.cub.2004.04.042. doi:10.1016/j.cub.2004.04.042 [DOI] [PubMed] [Google Scholar]
- Felsenstein J. Skepticism towards Santa Rosalia, or why are there so few kinds of animals? Evolution. 1981;35:124–138. doi: 10.1111/j.1558-5646.1981.tb04864.x. doi:10.2307/2407946 [DOI] [PubMed] [Google Scholar]
- Irwin D.E, Price T. Sexual imprinting, learning and speciation. Heredity. 1999;82:347–354. doi: 10.1038/sj.hdy.6885270. doi:10.1038/sj.hdy.6885270 [DOI] [PubMed] [Google Scholar]
- Kendrick K.M, Hinton M.R, Atkins K, Haupt M.A, Skinner J.D. Mothers determine sexual preferences. Nature. 1998;395:229–230. doi: 10.1038/26129. doi:10.1038/26129 [DOI] [PubMed] [Google Scholar]
- Kocher T.D. Adaptive evolution and explosive speciation: the cichlid fish model. Nat. Rev. Genet. 2004;5:288–298. doi: 10.1038/nrg1316. doi:10.1038/nrg1316 [DOI] [PubMed] [Google Scholar]
- Kühme W. Eine chemisch aufgelöste Brutpflegereaktion bei Cichliden (Pisces) Naturwissenschaften. 1964;51:20–21. [Google Scholar]
- Maan M.E, Seehausen O, Soderberg L, Johnson L, Ripmeester E.A.P, Mrosso H.D.J, Taylor M.I, van Dooren T.J.M, van Alphen J.J.M. Intraspecific sexual selection on a speciation trait, male coloration, in the Lake Victoria cichlid Pundamilia nyererei. Proc. R. Soc. B. 2004;271:2445–2452. doi: 10.1098/rspb.2004.2911. doi:10.1098/rspb.2004.2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plenderleith M, van Oosterhout C, Robinson R.L, Turner G.F. Female preference for conspecific males based on olfactory cues in a Lake Malawi cichlid fish. Biol. Lett. 2005;1:411–414. doi: 10.1098/rsbl.2005.0355. doi:10.1098/rsbl.2005.0355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- R Development Core Team. R foundation for statistical computing; Vienna, Austria: 2005. R: a language and environment for statistical computing.http://www.R-project.org [Google Scholar]
- Salzburger W, Mack T, Verheyen E, Meyer A. Out of Tanganyika: genesis, explosive speciation, key-innovations and phylogeography of the haplochromine cichlid fishes. BMC Evol. Biol. 2005;5:17. doi: 10.1186/1471-2148-5-17. doi:10.1186/1471-2148-5-17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seehausen O, van Alphen J.J.M. The effect of male coloration on female mate choice in closely related Lake Victoria cichlids (Haplochromis nyererei complex) Behav. Ecol. Sociobiol. 1998;42:1–8. doi:10.1007/s002650050405 [Google Scholar]
- Seehausen O, van Alphen J.M. Can sympatric speciation by disruptive sexual selection explain rapid evolution of cichlid diversity in Lake Victoria? Ecol. Lett. 1999;2:262–271. doi:10.1046/j.1461-0248.1999.00082.x [Google Scholar]
- Seehausen O, van Alphen J.J.M, Witte F. Cichlid fish diversity threatened by eutrophication that curbs sexual selection. Science. 1997;277:1808–1811. doi:10.1126/science.277.5333.1808 [Google Scholar]
- Siepen G, Crapon de Caprona M.D. The influence of parental color morph on mate choice in the cichlid fish Cichlasoma nigrofasciatum. Ethology. 1986;71:187–200. [Google Scholar]
- ten Cate C, Vos D.R. Sexual imprinting and evolutionary processes in birds: a reassessment. Adv. Study Behav. 1999;28:1–31. [Google Scholar]
- Verzijden M.N, Lachlan R.F, Servedio M.R. Female mate-choice behavior and sympatric speciation. Evolution. 2005;59:2097–2108. doi:10.1554/04-567.1 [PubMed] [Google Scholar]
- Zavazava N, Eggert F. MHC and behavior. Immunol. Today. 1997;18:8–10. doi: 10.1016/s0167-5699(97)80006-0. doi:10.1016/S0167-5699(97)80006-0 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data of male and female behaviour in each preference test and a regression analysis of the interaction between male courtship effort and female tendency to approach. Scores of red and blue colour ranges of males and females of both species