Abstract
Comparative study of character evolution in the shorebirds is presently limited because the phylogenetic placement of some enigmatic genera remains unclear. We therefore used Bayesian methods to obtain a well-supported phylogeny of 90 recognized genera using 5 kb of mitochondrial and nuclear sequences. The tree comprised three major clades: Lari (gulls, auks and allies plus buttonquails) as sister to Scolopaci (sandpipers, jacanas and allies), and in turn sister to Charadrii (plovers, oystercatchers and allies), as in previous molecular studies. Plovers and noddies were not recovered as monophyletic assemblages, and the Egyptian plover Pluvianus is apparently not a plover. Molecular dating using multiple fossil constraints suggests that the three suborders originated in the late Cretaceous between 79 and 102 Mya, and at least 14 lineages of modern shorebirds survived the mass extinction at the K/T boundary. Previous difficulties in determining the phylogenetic relationships of enigmatic taxa reflect the fact that they are well-differentiated relicts of old, genus-poor lineages. We refrain from suggesting systematic revisions for shorebirds at this time because gene trees may fail to recover the species tree when long branches are connected to deep, shorter branches, as is the case for some of the enigmatic taxa.
Keywords: Charadriiformes, divergence times, phylogeny, molecular clock
1. Introduction
The great diversity observed in morphology, behaviour, breeding systems and other ecological characters in shorebirds (Charadriiformes) provides tests of evolutionary hypotheses of life-history traits (e.g. Myers 1981; Whitfield & Tomkovich 1996; Barbosa & Moreno 1999). A well-supported phylogeny is a prerequisite for comparative studies because it can be used to understand how and when adaptive changes took place. For instance, by mapping morphological characters onto a well-supported phylogeny of the shanks derived from nuclear and mitochondrial DNA gene sequences, Pereira & Baker (2005) identified many characters that have retained ancestral states or have evolved in parallel. Although the phylogenetic relationships within shorebirds are well established at the family level based on nuclear and/or mitochondrial sequences (Ericson et al. 2003; Paton et al. 2003; Paton & Baker 2006), they are uncertain or unknown for many groups of species and genera, including enigmatic taxa such as the ibisbill (Ibidorhyncha struthersii), thick-knees (Burhinidae) and sheathbills (Chionidae). Hence, most of the evolution of life-history traits among shorebirds has yet to be mapped onto a well-structured phylogenetic framework.
To overcome this problem, Thomas et al. (2004) used the matrix representation with parsimony method (also known as supertree method) to propose the most inclusive phylogenetic hypothesis for extant shorebirds to date. However, their framework may seriously compromise interpretations of evolutionary histories of shorebirds because the supertree method lacks measures of nodal support and is known to be highly prone to biases depending on which source trees are included in the analysis, and by treating well-supported and poorly supported source trees as equally likely. Additionally, the supertree presented in Thomas et al. (2004) falls short in providing good resolution for many congeneric species because not enough source trees or none at all exist for these groups.
DNA sequencing is the logical approach to gather enough data and establish well-resolved phylogenetic hypotheses that better represent the evolutionary history of the Tree of Life. Here, we provide a comprehensive phylogeny and divergence times for shorebird genera based on DNA sequences of four genes. Our study provides a scaffold for future studies aimed at resolving phylogenetic relationships among species of shorebirds as well as a temporal framework for the evolution of life-history traits among shorebirds.
2. Material and methods
We sampled 90 out of 96 putative genera of Charadriiformes including two species of the enigmatic Turnix for DNA amplification and sequencing of the small ribosomal subunit (12S rDNA), NADH dehydrogenase subunit 2 (ND2), cytochrome b (cyt b) and recombination-activating protein (RAG-1) gene (species, primers and PCR conditions given in the electronic supplementary material). Gene fragments were concatenated in an alignment of 5198 bp, including gaps. All sequences obtained in this study were deposited in GenBank (table 1 in electronic supplementary material).
Tree inference was performed by a Metropolis-coupled Markov chain Monte Carlo Bayesian approach by running two simultaneous independent runs, each with one cold and five heated chains as implemented in MrBayes v. 3.1.2 (Ronquist & Huelsenbeck 2003). Divergence times were estimated using a Bayesian approach that accounts for uncertainties in branch lengths for individual gene partitions, rates of evolution and time constraints (Thorne & Kishino 2002). For details, see the electronic supplementary material (figures 1 and 2).
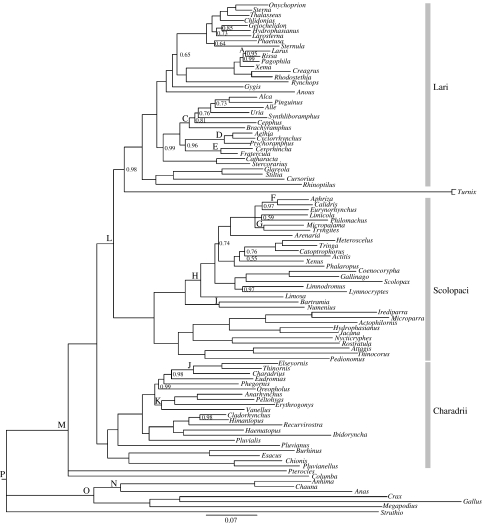
Figure 1.
Bayesian tree for Charadriiformes genera. Letters A to P indicate nodes for which fossil or molecular time constraints were used to estimate divergence times (see table 2 in electronic supplementary material). Numbers at nodes are posterior probabilities (PP), which are not indicated if PP=1.0. Nodes with PP<0.5 are collapsed.
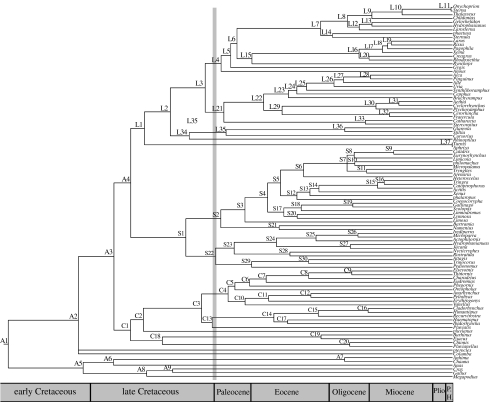
Figure 2.
Bayesian chronogram for Charadriiformes genera. The Cretaceous/Paleocene boundary is marked by a grey bar. Nodes are labelled A, C, L and S for Avian outgroups, Charadrii, Lari and Scolopaci divergences, respectively.
3. Results and discussion
(a) Phylogenetic relationships
The Bayesian tree recovered the three major clades as in recent analyses of both nuclear and mtDNA sequences (Ericson et al. 2003; Paton et al. 2003; Paton & Baker 2006) with the Lari sister to the Scolopaci, which are in turn sister to the Charadrii. The gulls (Larus and allies) plus skimmers (Rhynchops) are sister to the terns (Sterna and allies), but noddies (Gygis and Anous) are successive sister groups to this clade instead as basal members of the tern clade (contra Bridge et al. 2005). The alcids (Alca and Fratercula) plus skuas (Catharacta and Stercorarius) comprise the sister group to the terns, gulls and noddies, and the coursers (Cursorius and Rhinoptilus) and pratincoles (Glareola and Stiltia) are sister to all these clades. Buttonquails (Turnix) are the sister to the rest of the Lari. Phylogenetic relationships among alcid genera are congruent with those in the cyt b plus allozyme tree of Friesen et al. (1996), with the Dovekie (Alle) and auks (Alca, Penguinus, Uria) sister to the synthliboramphine murrelets (Synthliboramphus), and a pectinate sequence of sister groups being formed successively by the guillemots (Cepphus), brachyramphine auklets (Brachyramphus) and a clade containing the true auklets (Ptychoramphus, Cyclorrhynchus, Aethia) plus the puffins (Fratercula, Cerorhinca).
The Scolopaci comprises two major clades of genera, one containing the Scolopacidae (sandpipers, snipes and allies) and the other containing the Jacanidae and Rostratulidae (Jacana and allies and painted snipes Nyctocryphes and Rostratula, respectively) as a sister group to the Pedionomidae plus the Thinocoridae (Plains Wanderer Pedionomus and seedsnipes Thinocorus, respectively). Within the Scolopacidae, the calidridine sandpipers (Calidris and allies) plus the turnstones (Arenaria) are sister to the shanks (Tringa and allies) and phalaropes (Phalaropus). Clades formed by the snipes and woodcock (Gallinago, Coencorypha, Scolopax) and dowitchers (Limnodromus) plus jacksnipe (Lymnocryptes), then the godwits (Limosa), and finally the curlews (Numenius) plus Upland sandpiper (Bartramia) branch off in a pectinate sequence identical to that in the nuclear RAG-1 tree of Paton et al. (2003). Within the jacanas, we found the same phylogenetic relationships among geographically disjunct sister pairs of genera reported by Whittingham et al. (2000). The predominantly Australian Irediparra is sister to the African Microparra and the Asian Hydrophasianus is sister to New World Jacana, consistent with their hypothesis of extinction of intervening forms to explain this biogeographic pattern.
In the Charadrii, the typical plovers (Charadrius and allies) are in one clade with the notable exception that Pluvialis groups with the oystercatchers (Haematopus) and ibisbill (Ibidorhyncha), stilts (Himantopus, Cladorhynchus) and avocets (Recurvirostra), thereby rendering typical plovers paraphyletic. The lapwings (Vanellus) are sister to three Australasian genera of plovers (Anarhynchus, Peltohyas, Erythrogonys). Additionally, the enigmatic Egyptian plover (Pluvianus) is sister to these clades. Thick-knees (Burhinus, Esacus) form a clade with sheathbills (Chionis) and the Magellanic Plover (Pluvianellus) which are the basal sister group to the rest of the Charadrii, as in previous DNA trees (Ericson et al. 2003; Paton et al. 2003; Paton & Baker 2006). Finally, the phylogenetic placement of the sandgrouse (Pterocles) remains unresolved, but they do not appear to be shorebirds.
While it is tempting to suggest systematic revisions of the Charadriiformes based on our results, caution is warranted because anomalous gene trees that differ from the species tree can be common when sequence data are concatenated (Degnan & Rosenberg 2006). This is especially likely to be a problem when recent branches are long and deeper branches in the species tree are short, as occurs in parts of our tree including the branch to Pluvialis. We therefore advocate prudence until clade markers such as CR1 retroposons can be found to check controversial phylogenetic placements of genera.
(b) Divergence times
Based on relaxed clock molecular dating, the most recent common ancestor (MRCA) of the shorebirds was estimated to have occurred 93 Mya (95% CI 84–102 Mya; table 3 in electronic supplementary material), which is considerably older than the 80 Mya estimate obtained by Paton et al. (2003). This is because the latter authors fixed the root age of the Charadriiformes at 78 Mya based on a previous molecular age estimated from whole mitochondrial DNA genomes (Paton et al. 2002). However, this calibration point is much younger than that estimated with a much more comprehensive sampling of vertebrates which had multiple fossil constraints and accounted properly for phylogenetic and fossil age uncertainties (Pereira & Baker 2006).
Moreover, we added 14 other fossil constraints within the Charadriiformes owing to our much larger taxon sampling than in previous studies. With these constraints, the three suborders were estimated to have originated in the late Cretaceous between 79 and 102 Mya (table 3 in electronic supplementary material). Fourteen ancestors of extant lineages apparently pre-date the K/T boundary 65 Mya, as follows: terns plus gulls plus noddies; alcids plus skuas; coursers; pratincoles; buttonquails; sandpipers and allies plus curlews; jacanas plus painted snipes; seedsnipes plus plains wanderer; traditional plovers; oystercatchers and allies including ibisbill; Pluvialis; Egyptian plover; sheathbills and Magellanic plover; and thick-knees.
The survival of so many lineages across the K/T boundary suggests that many more shorebird lineages diversified well before the K/T boundary than assumed by the long fuse model for the evolution of shorebirds (Feduccia 2003). However, diversification of genera within the three suborders predominantly post-dates the K/T boundary. As in many ordinal clades of birds (Pereira et al. 2002; Baker et al. 2006; Tavares et al. 2006), these radiations coincide with the warming in the Eocene when ecosystems were highly productive (Jaramillo et al. 2006). We conclude that shorebirds originated earlier than was estimated previously, and they apparently were not affected by the bolide impact 65 Mya. Finally, the survival of ancient lineages of shorebirds since the late Cretaceous (Cooper & Penny 1997) suggests a possible reason why a number of oddball taxa were so difficult to place phylogenetically, as they are the ancient relicts of a much earlier radiation.
Acknowledgments
A.J.B. was supported by an operating grant from the Natural Sciences and Engineering Research Council of Canada, the Royal Ontario Museum Foundation and the National Science Foundation (Assembling the Tree of Life (AToL) Program—EF-0228693). We are grateful to R. T. Brumfield and D. Dittmann at the LSU Museum of Natural Science Collection of Genetic Resources, USA and Jon Fjeldså at the Zoological Museum of the University of Copenhagen, Denmark for granting tissue loans for this research.
Supplementary Material
References
- Baker A.J, Pereira S.L, Haddrath O.P, Edge K.-A. Multiple gene evidence for expansion of extant penguins out of Antarctica due to global cooling. Proc. R. Soc. B. 2006;273:11–17. doi: 10.1098/rspb.2005.3260. doi:10.1098/rspb.2005.3260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbosa A, Moreno E. Hindlimb morphology and locomotor performance in waders: an evolutionary approach. Biol. J. Linn. Soc. 1999;67:313–330. doi:10.1006/bijl.1998.0282 [Google Scholar]
- Bridge E.S, Jones A.W, Baker A.J. A phylogenetic framework for the terns (Sternini) inferred from mtDNA sequences: implications for taxonomy and plumage evolution. Mol. Phylogenet. Evol. 2005;35:459–469. doi: 10.1016/j.ympev.2004.12.010. doi:10.1016/j.ympev.2004.12.010 [DOI] [PubMed] [Google Scholar]
- Cooper A, Penny D. Mass survival of birds across the Cretaceous–Tertiary boundary: molecular evidence. Science. 1997;275:1109–1113. doi: 10.1126/science.275.5303.1109. doi:10.1126/science.275.5303.1109 [DOI] [PubMed] [Google Scholar]
- Degnan J.H, Rosenberg N.A. Discordance of species trees with their most likely gene trees. PLoS Genet. 2006;2:e68. doi: 10.1371/journal.pgen.0020068. doi:10.1371/journal.pgen.0020068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericson P.G, Envall I, Irestedt M, Norman J.A. Inter-familial relationships of the shorebirds (Aves: Charadriiformes) based on nuclear DNA sequence data. BMC Evol. Biol. 2003;3:16. doi: 10.1186/1471-2148-3-16. doi:10.1186/1471-2148-3-16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feduccia A. ‘Big Bang’ for tertiary birds? Trends Ecol. Evol. 2003;18:172–176. doi:10.1016/S0169-5347(03)00017-X [Google Scholar]
- Friesen V.L, Baker A.J, Piatt J.F. Phylogenetic relationships within the Alcidae (Charadriiformes: Aves) inferred from total molecular evidence. Mol. Biol. Evol. 1996;13:359–367. doi: 10.1093/oxfordjournals.molbev.a025595. [DOI] [PubMed] [Google Scholar]
- Jaramillo C, Rueda M.J, Mora G. Cenozoic plant diversity in the neotropics. Science. 2006;311:1893–1896. doi: 10.1126/science.1121380. doi:10.1126/science.1121380 [DOI] [PubMed] [Google Scholar]
- Myers J.P. Cross-seasonal interactions in the evolution of sandpiper social systems. Behav. Ecol. Sociobiol. 1981;8:195–202. doi:10.1007/BF00299830 [Google Scholar]
- Paton T, Haddrath O, Baker A.J. Complete mitochondrial DNA genome sequences show that modern birds are not descended from transitional shorebirds. Proc. R. Soc. B. 2002;269:839–846. doi: 10.1098/rspb.2002.1961. doi:10.1098/rspb.2002.1961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paton T.A, Baker A.J. Sequences from 14 mitochondrial genes provide a well-supported phylogeny of the Charadriiform birds congruent with the nuclear RAG-1 tree. Mol. Phylogenet. Evol. 2006;39:657–667. doi: 10.1016/j.ympev.2006.01.011. doi:10.1016/j.ympev.2006.01.011 [DOI] [PubMed] [Google Scholar]
- Paton T.A, Baker A.J, Groth J.G, Barrowclough G.F. RAG-1 sequences resolve phylogenetic relationships within Charadriiform birds. Mol. Phylogenet. Evol. 2003;29:268–278. doi: 10.1016/s1055-7903(03)00098-8. doi:10.1016/S1055-7903(03)00098-8 [DOI] [PubMed] [Google Scholar]
- Pereira S.L, Baker A.J. Multiple gene evidence for parallel evolution and retention of ancestral morphological states in the shanks (Charadriiformes: Scolopacidae) Condor. 2005;107:514–526. doi:10.1650/0010-5422(2005)107[0514:MGEFPE]2.0.CO;2 [Google Scholar]
- Pereira S.L, Baker A.J. A mitogenomics timescale for birds detects variable phylogenetic rates of molecular evolution and refutes the standard molecular clock. Mol. Biol. Evol. 2006;23:1731–1740. doi: 10.1093/molbev/msl038. doi:10.1093/molbev/msl038 [DOI] [PubMed] [Google Scholar]
- Pereira S.L, Baker A.J, Wajntal A. Combined nuclear and mitochondrial DNA sequences resolve generic relationships within the Cracidae (Galliformes Aves) Syst. Biol. 2002;51:946–958. doi: 10.1080/10635150290102519. doi:10.1080/10635150290102519 [DOI] [PubMed] [Google Scholar]
- Ronquist F, Huelsenbeck J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics. 2003;19:1572–1574. doi: 10.1093/bioinformatics/btg180. doi:10.1093/bioinformatics/btg180 [DOI] [PubMed] [Google Scholar]
- Tavares E.S, Baker A.J, Pereira S.L, Miyaki C.Y. Phylogenetic relationships and historical biogeography of neotropical parrots (Psittaciformes: Psittacidae: Arini) inferred from mitochondrial and nuclear DNA sequences. Syst. Biol. 2006;55:454–470. doi: 10.1080/10635150600697390. doi:10.1080/10635150600697390 [DOI] [PubMed] [Google Scholar]
- Thomas G.H, Wills M.A, Székely T. A supertree approach to shorebird phylogeny. BMC Evol. Biol. 2004;4:28. doi: 10.1186/1471-2148-4-28. doi:10.1186/1471-2148-4-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne J.L, Kishino H. Divergence time and evolutionary rate estimation with multilocus data. Syst. Biol. 2002;51:689–702. doi: 10.1080/10635150290102456. doi:10.1080/10635150290102456 [DOI] [PubMed] [Google Scholar]
- Whitfield D.P, Tomkovich P.S. Mating system and timing of breeding in Holarctic waders. Biol. J. Linn. Soc. 1996;57:277–290. doi:10.1006/bijl.1996.0015 [Google Scholar]
- Whittingham L.A, Sheldon F.H, Emlen S.T. Molecular phylogeny of jacanas and its implications for morphologic and biogeographic evolution. The Auk. 2000;117:22–32. doi:10.1642/0004-8038(2000)117[0022:MPOJAI]2.0.CO;2 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.