Abstract
Females in many species engage in matings with males that are not their social mates. These matings are predicted to increase offspring heterozygosity and fitness, and thereby prevent the deleterious effects of inbreeding. We tested this hypothesis in a cooperative breeding mammal, the common mole-rat Cryptomys hottentotus hottentotus. Laboratory-based studies suggested a system of strict social monogamy, while recent molecular studies indicate extensive extra-pair paternity despite colonies being founded by an outbred pair. Our data show that extra-pair and within-colony breeding males differed significantly in relatedness to breeding females, suggesting that females may gain genetic benefits from breeding with non-resident males. Extra-colony male mating success was not based on heterozygosity criteria at microsatellite loci; however, litters sired by extra-colony males exhibited increased heterozygosity. While we do not have the data that refute a relationship between individual levels of inbreeding (Hs) and fitness, we propose that a combination of both male and female factors most likely explain the adaptive significance of extra-pair mating whereby common mole-rats maximize offspring fitness by detecting genetic compatibility with extra-pair mates at other key loci, but it is not known which sex controls these matings.
Keywords: relatedness, genetic compatibility, microsatellite, extra-pair paternity, mate choice, Cryptomys
1. Introduction
While evidence abounds demonstrating that females of many species produce young via extra-pair males, the adaptive significance for this behaviour still remains unclear. A number of studies suggest that further to the acquisition of food resources, parental care and ‘good genes’, selection for extra-pair mates may primarily be driven by genetic compatibility and the costs of inbreeding (Tregenza & Weddell 2000; Griffith et al. 2002; Wolff & Macdonald 2004). Negative fitness consequences associated with inbreeding are well documented (Thornhill 1993; Coltman et al. 1998; Keller & Waller 2002; Bean et al. 2004) and evidence for individual levels of inbreeding as fitness predictors is accumulating (Hansson et al. 2001; Hansson & Westerberg 2002; Foerster et al. 2003). By mating with males that have dissimilar alleles, females increase levels of heterozygosity in their offspring; this may preclude the effects of deleterious alleles and probably increases heterozygosity at loci that enhance fitness, e.g. the major histocompatibility (MHC) gene complex (Petrie & Kempenaers 1998; Tregenza & Weddell 2000).
The common mole-rat (Cryptomys hottentotus hottentotus) is widely distributed in South Africa, where colonies comprising two to fourteen individuals permanently occupy a discrete network of burrows, locating food as they blindly extend foraging tunnels (Spinks et al. 2000). Laboratory-based studies suggested that a system of strict social monogamy characterized the mating system of this species, and mark–recapture field studies confirmed that colonies were founded by unrelated pairs (Bennett 1989, 1992). Nevertheless, microsatellite data collected from our field study populations revealed unexpectedly high levels of paternity assigned to males that were not colony residents (extra-colony males, ECMs; Bishop et al. 2004). The common mole-rat is a cooperative breeder that lives in colonies characterized by a generally linear social hierarchy, where the breeding male is the most dominant individual (Bennett 1989). Therefore, why should females mate with males that do not contribute to the maintenance and provisioning of her resident colony? In many social species, constraints on mate choice may result in females paired with genetically similar males (Cohas et al. 2006). Subordinate common mole-rat females do not breed within their home colony. Instead, they appear to delay reproduction until such time that ecological conditions favour dispersal and independent reproduction (Spinks et al. 2000); this may result in social pairings between genetically suboptimal mates, which in turn may favour extra-pair matings.
In this study, we were interested in whether relatedness is an important factor influencing extra-pair mating patterns in the common mole-rat. If female mole-rats engage in extra-pair matings to enhance the fitness of their offspring, we can predict that (i) females would choose ECM that were less genetically similar than within-pair males, (ii) ECM would have higher levels of heterozygosity than within-pair males, and (iii) offspring sired by extra-pair males would have higher levels of heterozygosity than those sired by within-pair males. Because size is an important determinant of male social status, we also investigated whether any difference exists in the size of colony breeding males and those ECM that succeeded in gaining paternity. Resident breeding males within a colony are most probably the largest male or one of the largest males (Bishop et al. 2004); subordinate males, however, are fully reproductively functional and merely behaviourally quiescent (Spinks et al. 1997).
2. Material and methods
We studied 13 colonies of the common mole-rat at two sites representing the broad ecological range of the species. Individuals were live-trapped using modified Hickman traps from September 1992 to November 1996; all individuals were sexed, weighed and marked as part of long-term mark–recapture studies (detailed in Spinks et al. 2000). Seven microsatellite loci were used to investigate paternity for a total of 73 offspring collected from the two study sites (detailed in Bishop et al. 2004); the loci had a combined exclusion probability of more than 0.95 and the mean probability of identity (PID) for potential sires in the two populations was 2.1×10−4 (see electronic supplementary material). In contrast to the suggested mating system of strict monogamy, ECM were assigned paternity with high levels of confidence to approximately 30% of offspring at both sites (Bishop et al. 2004). In this study, we investigate measures of genetic similarity and heterozygosity between females and within-colony breeding males (WCMs), as well as ECMs to which paternity was assigned. WCMs were those males known to have sired young within their resident colony. As a measure of individual inbreeding, we calculated standardized heterozygosity (Hs; proportion of heterozygous loci/mean heterozygosity of typed loci; Coltman et al. 1999). Measures of relatedness (R) within and among colonies and between females and males were calculated using genotypic data from seven microsatellite loci in Relatedness v. 5.0.8 (Queller & Goodnight 1989). Average relatedness among adult males and females in our study populations (expected R=0) was 0.03±0.0009 s.e.m. calculated by jackknifing; in a randomized sample of 100 litters, the average relatedness among full siblings (expected R=0.5) was 0.45±0.01. All test statistics were performed in Statistica v. 7.0 (StatSoft Inc.).
3. Results and discussion
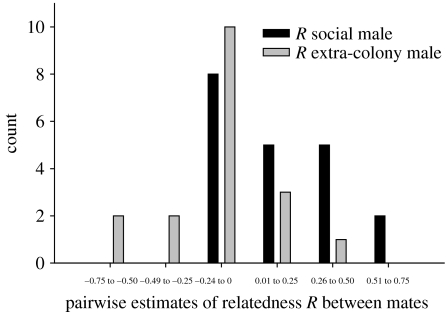
Our results suggest that common mole-rats enhance outbreeding by engaging in extra-colony matings. Females were generally less related to ECMs than to resident WCMs with whom they sired young (figure 1; WCMs, mean R to females ±s.e.m.=0.17±0.06; ECMs, mean R=−0.05±0.06; one-tailed paired t-test, t=2.19, d.f.=17, p<0.025). Furthermore, the mean difference in R between females and ECMs was found to be significantly greater than the observed differences in R within and among colonies of mole-rats (see electronic supplementary material; two-sample randomization test, p=0.04), suggesting that extra-pair mating occurs non-randomly with respect to levels of non-kin relatedness. Overall however, females who mated with non-resident males were not more related to their colony breeding male compared with females whose litters were only sired by the colony male (mean R of females to colony males with extra-colony paternity±s.e.m.=0.22±0.08; mean R of females to colony males without extra-colony paternity±s.e.m.=0.15±0.07; t-test, t=0.51, d.f.=19, p=0.61). This might be because a sample of individuals at any point in time will represent both established and recently founded colonies in which variation in turnover of reproductive females will influence opportunities for mating with ECMs. Reproductive tenure within colonies will provide some females greater opportunity to mate with ECMs, while females that had recently founded a colony would not necessarily engage in extra-pair matings immediately; unfortunately, it is not clear from our data whether older females with long-term reproductive tenure were more likely to engage in extra-colony paternity than young, recently dispersed and mated females.
Figure 1.
Distribution of pairwise estimates of relatedness between females and their colony mates versus extra-colony males. R was calculated according to Queller & Goodnight (1989).
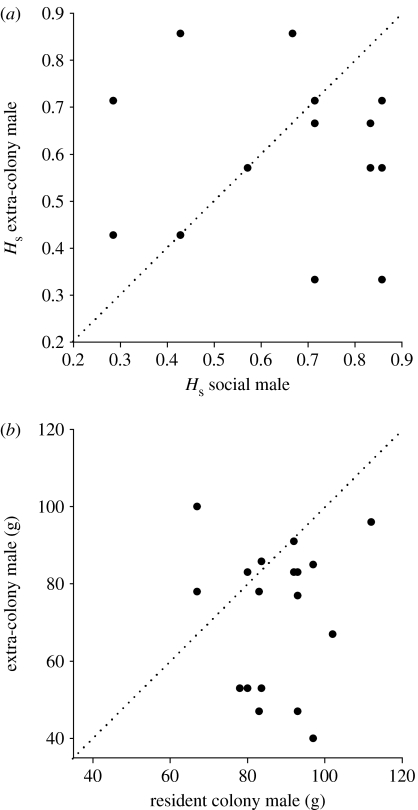
Do females mate with ECM that are more heterozygous than their colony mates? Our results suggest that ECM mating success is not based on heterozygosity criteria at neutral loci, such as microsatellites. Overall, males that lost paternity to ECMs did not differ in heterozygosity from those that sired all their colony offspring (mean Hs±s.e.m. of cuckolded males=0.61±0.05, mean Hs of non-cuckolded males=0.56±0.05; Mann–Whitney U-test, n=12,19; U=103, Z=0.446, p=0.65), and colony males did not differ in microsatellite heterozygosity compared with their cuckolders (mean Hs±s.e.m. of WCMs=0.62±0.04, mean Hs of ECMs=0.61±0.03; Wilcoxon's matched pairs test, n=17, Z=0.21, p=0.83; figure 2a). Nevertheless, litters from extra-pair matings displayed increased levels of heterozygosity; mean Hs for litters sired by ECMs was significantly greater than for those sired by WCMs (mean Hs±s.e.m. of ECM litters=0.65±0.03, mean Hs of WCM litters=0.55±0.02; one-tailed t-test, t=−2.03, d.f.=35, p=0.025). As a result, female mole-rats appear to maximize offspring heterozygosity by mating with genetically unrelated males rather than heterozygous males. At present, we do not have data that associate microsatellite heterozygosity in mole-rats with measures of individual fitness, but we cannot exclude the possibility that females seek increased variation and/or compatibility at additional paternal loci known to influence fitness, and measures of R may reflect differences between males at various important loci under moderate or strong selection (Reed & Frankham 2001).
Figure 2.
Pairwise comparisons of colony male and extra-colony male mate choice (n=18 dyads). (a) Standardized heterozygosity (Hs) and (b) size of male (g). Dotted line represents values that are identical.
Finally, dominant males are generally the largest individuals within colonies and are thought more likely to gain access to oestrous females than smaller subordinate males (Bennett 1992). However, our data suggest that WCMs appear to be cuckolded time and again by generally smaller, foreign males (mean WCM weight±s.e.m.=87.7 g±2.7, mean ECM weight=72.2 g±3.3; Wilcoxon's matched pairs test, n=18, Z=2.59, p=0.009; figure 2b). We believe that this is unlikely to be associated with female choice for size; mole-rats have highly reduced eyes and spend much of their lives within their subterranean burrow systems. They do not use visual cues to determine whether individuals are foreign or conspecifics; instead, they rely on colony odour to identify colony members (Spinks et al. 1998). Thus, a more probable explanation is that breeding females simply have a greater chance of encountering subordinate males from foreign colonies; subordinate males work harder, foraging and extending the tunnel system, and are more likely to disperse than established breeding males or indeed breeding females, and these factors may in turn lead to increased encounters with foreign oestrous females. Undoubtedly though, both dominant and subordinate ECMs increase their reproductive fitness by successfully siring offspring in colonies to whose maintenance they do not contribute.
In summary, our results suggest that female common mole-rats enhance outbreeding by mating with non-resident males that are less related to themselves than their social mate. While this behaviour leads to increased offspring heterozygosity, male reproductive success does not appear to be dependent on individual heterozygosity at microsatellite loci. We propose that both males and females maximize offspring fitness by detecting genetic compatibility with extra-pair mates at additional key loci. Given the life history, ecology and reproductive physiology of the species, we can reject a number of alternative hypotheses explaining the adaptive value of extra-pair mating behaviour (reviewed in Wolff & Macdonald 2004). Detecting genetic compatibility requires a mechanism to determine the degree to which extra-pair mates differ from their colony mates. In the absence of visual cues, mole-rats appear to be an excellent model to test whether odour signatures indicate compatibility between males and females at loci, such as the MHC.
Acknowledgments
The authors thank J. Emmerson who kindly allowed us to study and trap animals on his property. We are grateful to N. C. Bennett, M. J. O'Riain and A. C. Spinks for their insightful discussions and data collection; F. P. D. Cotterill and three anonymous reviewers provided constructive comments on the manuscript. This work was supported by research grants from the South African National Research Foundation and the Cape Town University Research Council.
Supplementary Material
Details and references for calculating the probability of identity and the randomization tests used in the analyses
References
- Bean K, Amos W, Pomeroy P.P, Twiss S.D, Coulson T.N, Boyd I.L. Patterns of parental relatedness and pup survival in the grey seal (Halichoerus grypus) Mol. Ecol. 2004;13:2365–2370. doi: 10.1111/j.1365-294X.2004.02199.x. doi:10.1111/j.1365-294X.2004.02199.x [DOI] [PubMed] [Google Scholar]
- Bennett N.C. The social structure and reproductive biology of the common mole-rat, Cryptomys hottentotus hottentotus and remarks on the trends in reproduction and sociality in the family Bathyergidae. J. Zool. Lond. 1989;219:45–59. [Google Scholar]
- Bennett N.C. Aspects of social behaviour in a captive colony of the common mole-rat, Cryptomys hottentotus hottentotus. Z. Säugertier. 1992;57:294–309. [Google Scholar]
- Bishop J.M, Jarvis J.U.M, Spinks A.C, Bennett N.C, O'Ryan C. Molecular insight into patterns of colony composition and paternity in the common mole-rat Cryptomys hottentotus hottentotus. Mol. Ecol. 2004;13:1217–1229. doi: 10.1111/j.1365-294X.2004.02131.x. doi:10.1111/j.1365-294X.2004.02131.x [DOI] [PubMed] [Google Scholar]
- Cohas A, Yoccoz N.G, Da Silva A, Goosens B, Allainé D. Extra-pair paternity in the monogamous alpine marmot (Marmota marmota): the roles of social setting and female mate choice. Behav. Ecol. Sociobiol. 2006;59:597–605. doi:10.1007/s00265-005-0086-8 [Google Scholar]
- Coltman D.W, Bowen W.D, Wright J.M. Birth weight and neonatal survival of harbour seal pups are positively correlated with genetic variation measured by microsatellites. Proc. R. Soc. B. 1998;265:803–809. doi: 10.1098/rspb.1998.0363. doi:10.1098/rspb.1998.0363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coltman D.W, Pilkington J.G, Smith J.A, Pemberton J.M. Parasite-mediated selection against inbred soay sheep in a free-living island population. Evolution. 1999;53:1259–1267. doi: 10.1111/j.1558-5646.1999.tb04538.x. doi:10.2307/2640828 [DOI] [PubMed] [Google Scholar]
- Foerster K, Delhey K, Johnson A, Lijfield J.T, Kempenaers B. Females increase offspring heterozygosity and fitness through extra-pair matings. Nature. 2003;425:714–717. doi: 10.1038/nature01969. doi:10.1038/nature01969 [DOI] [PubMed] [Google Scholar]
- Griffith S.C, Owens I.P.F, Thuman K.A. Extra pair paternity in birds: a review of interspecific variation and adaptive function. Mol. Ecol. 2002;11:2195–2212. doi: 10.1046/j.1365-294x.2002.01613.x. doi:10.1046/j.1365-294x.2002.01613.x [DOI] [PubMed] [Google Scholar]
- Hansson B, Bensch S, Hasselquist D, Åkesson M. Microsatellite diversity predicts recruitment of sibling great reed warblers. Proc. R. Soc. B. 2001;268:1287–1291. doi: 10.1098/rspb.2001.1640. doi:10.1098/rspb.2001.1640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansson B, Westerberg L. On the correlation between heterozygosity and fitness in natural populations. Mol. Ecol. 2002;11:2467–2474. doi: 10.1046/j.1365-294x.2002.01644.x. doi:10.1046/j.1365-294X.2002.01644.x [DOI] [PubMed] [Google Scholar]
- Keller L.F, Waller D.M. Inbreeding effects in wild populations. Trends Ecol. Evol. 2002;17:230–241. doi:10.1016/S0169-5347(02)02489-8 [Google Scholar]
- Petrie M, Kempenaers B. Extra-pair paternity in birds: explaining variation between species and populations. Trends Ecol. Evol. 1998;13:52–58. doi: 10.1016/s0169-5347(97)01232-9. doi:10.1016/s0169-5347(97)01232-9 [DOI] [PubMed] [Google Scholar]
- Queller D.C, Goodnight K. Estimating relatedness using genetic markers. Evolution. 1989;43:258–275. doi: 10.1111/j.1558-5646.1989.tb04226.x. doi:10.2307/2409206 [DOI] [PubMed] [Google Scholar]
- Reed D.H, Frankham R. How closely correlated are molecular and quantitative measures of genetic variation? A meta-analysis. Evolution. 2001;43:258–275. doi: 10.1111/j.0014-3820.2001.tb00629.x. [DOI] [PubMed] [Google Scholar]
- Spinks A.C, van der Horst G, Bennett N.C. Influence of breeding season and reproductive status on male reproductive characteristics in the common mole-rat, Cryptomys hottentotus hottentotus. J. Reprod. Fert. 1997;109:79–86. doi: 10.1530/jrf.0.1090079. [DOI] [PubMed] [Google Scholar]
- Spinks A.C, O'Riain M.J, Polokow D.A. Intercolonial encounters and xenophobia in the common mole-rat, Cryptomys hottentotus hottentotus (Bathyergidae): the effects of aridity, sex and reproductive status. Behav. Ecol. 1998;69:224–234. [Google Scholar]
- Spinks A.C, Jarvis J.U.M, Bennett N.C. Comparative patterns of philopatry and dispersal in two common mole-rat populations: implications for the evolution of mole-rat sociality. J. Anim. Ecol. 2000;69:224–234. doi:10.1046/j.1365-2656.2000.00388.x [Google Scholar]
- Thornhill N.W. University of Chicago Press; Chicago, IL: 1993. The natural history of inbreeding and outbreeding: theoretical and empirical perspectives. [Google Scholar]
- Tregenza T, Weddell N. Genetic compatibility, mate choice and patterns of parentage: invited review. Mol. Ecol. 2000;9:1013–1027. doi: 10.1046/j.1365-294x.2000.00964.x. doi:10.1046/j.1365-294X.2000.00964.x [DOI] [PubMed] [Google Scholar]
- Wolff J.O, Macdonald D.W. Promiscuous females protect their offspring. Trends Ecol. Evol. 2004;19:127–134. doi: 10.1016/j.tree.2003.12.009. doi:10.1016/j.tree.2003.12.009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Details and references for calculating the probability of identity and the randomization tests used in the analyses