Abstract
Effects of the social environment on age at sexual maturation are assumed to require direct interactions, such as suppression of subordinates through aggression from dominants. Using green swordtails (Xiphophorus helleri), we demonstrate for the first time that females and males adjust their age at maturation in response to visual cues of male sexual ornamentation in the current environment: females matured earlier, whereas males matured later if all the mature males seen had large ornaments. Thus, age at maturation shifted in accordance with the perceived quality of mates (females) or mating competitors (males), demonstrating a capability to use visual cues from the environment to strategically adjust rates of sexual development.
Keywords: life-history strategy, local mate competition, phenotypic plasticity, sexual maturation, social environment
1. Introduction
The timing of the onset of sexual maturation is an important determinant of fitness and involves the resolution of several trade-offs. For example, in species with indeterminate growth patterns, individuals that mature early trade-off the costs of small size (lower fecundity (Andersson 1994) or reduced offspring quality (Clutton-Brock et al. 1988)) against the benefits of maturing early (e.g. reduced risk of dying prior to reproduction, Roff 1992; Stearns 1992). There are also physiological and other costs associated with being sexually mature (Bentley et al. 1998) and thus individuals should delay maturation until the benefits will be maximized. Given that a number of environmental variables, such as resource availability and predation risk, may alter the costs and benefits of maturing at different ages, the optimal age at maturation should vary among environments. One potential environmental variable that may alter the optimal age at maturation is the availability of suitable mates, since this will depend on factors such as the operational sex ratio and the relative quality of available mates or sexual competitors (Rodd et al. 1997). Therefore, various features of the environment in which an individual develops determine the optimal age of maturation, and perception of these features by juveniles might be expected to influence the timing of maturation. Chemical cues have been shown to affect the timing of maturation in a variety of species (e.g. insects, Pereira et al. 2006; fishes, Aday et al. 2003; mammals, Rekwot et al. 2001). Here, we test a different sensory modality, asking whether juvenile animals can use visual cues in the assessment of their future probability of mating, and test the prediction that the timing of sexual maturation should be sensitive to the perceived quality of mature males (as indicated by sexual ornament size).
We used green swordtails (Xiphophorus helleri), live-bearing Poeciliid fish from sub-tropical North and Central America which have well-documented visual sexual signals and flexible timing of sexual maturation. Direct physical interactions with dominant individuals can cause subordinate male swordtails to delay maturation (Borowsky 1973; Campton 1992), and while such responses may be a physiological stress response as a consequence of social suppression, it is also known that they use visual cues when making other reproductive decisions such as choice of mate (Basolo 1990; Johnson & Basolo 2003). Male green swordtails develop a long, brightly coloured and costly (Basolo & Alcaraz 2003; Basolo & Wagner 2004) sexual ornament at maturity (an extension to the caudal fin; the ‘sword’). Females, in mate choice trials based on visual cues, prefer males of large body size, and among males of similar body size, prefer those with longer swords (Basolo 1990; Rosenthal & Evans 1998). Female preference for longer swords appears to represent a bias for larger apparent size, with investment in sword length representing a cheaper way of increasing the apparent size than investment in body length (Basolo 1998; Rosenthal & Evans 1998). While sexual maturation in males triggers the development of the sword, it also causes a reduction in the rate and eventual cessation of body growth (Basolo 1998; Royle et al. in preparation), and so the timing of maturation has a marked effect on a male's competitive ability as an adult, since smaller males are usually subordinate (Beaugrand et al. 1996) and although sword length also has an effect on competitive ability, it is less than that of body size (Benson & Basolo 2006).
2. Material and Methods
(a) Creating groups of juveniles
Thirteen breeding females from different families were used to generate the fry used in this study. Breeding females were first generation offspring of wild-caught females from Belize, Central America.
Each breeding female was mated to a single mature male and consequently all fry produced by a female were full siblings. Each brood of siblings was separated from the mother on the day of birth and kept as a single brood until the juveniles reached two months of age. At this point, each brood was used to form two sibling groups of three to five juveniles, with one group randomly allocated to the long-sworded treatment and another to the short-sworded treatment. Treatment groups were set up when fry were two months of age in order to avoid the period of high juvenile mortality that occurs early in life. Group sizes did not differ between treatments (mean±s.e. for long-sworded treatment=4.69±0.21 fish per group, n=13 groups; mean±s.e. for short-sworded treatment=4.77±1.67, n=13 groups; paired samples t-test t12=1.000, p=0.337).
(b) Experience protocol
Each sibling group (n=26) was maintained in its own experimental glass tank (38 cm×21 cm×20 cm (l×w×h)) on a 12 : 12 h L : D cycle and fed Hikari tropical micropellets (Kyorin, Japan) ad libitum twice daily. Stimulus males were from a stock population of males originating from the same wild population as the juveniles used in this study but unrelated to them. Males were introduced singly into a small compartment at the front of each experimental tank (12 cm×21 cm×20 cm (l×w×h)), which allowed the juveniles in that tank visual, but no tactile or chemical communication with that male and vice versa. Tanks were screened so that the only sexually mature fish that each group of juveniles saw from the day that they were born were the single stimulus males placed in the small compartment in front of their tank. Juveniles in the long-sworded treatment saw stimulus males with swords of 18 mm or greater (mean±s.e. sword length=22.3±0.4 mm; mean±s.e. sword as a percentage of body length=54.6±0.9%), while those in the short-sworded treatment saw males with swords of 12 mm or shorter (mean±s.e. sword length=8.0±0.3 mm; mean±s.e. sword as a percentage of body length=19.5±0.9%). Each stimulus male remained in its compartment for 3 days before being removed. Juveniles saw the first male at approximately 70 days of age and a subsequent male every 30 days thereafter until they had seen a total of four different males. Ages at which juveniles saw males did not differ between the treatment groups (one-way ANOVA, F1,96=1.354, p=0.247) and stimulus males did not differ in standard (i.e. body) length between treatment groups (mean±s.e. for long-sworded treatment=41.1±0.3 mm, mean±s.e. for short-sworded treatment=41.5±0.6 mm; paired samples t-test, t12=−0.601, p=0.559).
(c) Assessing and measuring size at maturation
Juveniles were checked weekly for the first signs of sexual maturation, defined as the development of the gonopodium for males and the gravid spot for females (Marcus & McCune 1999). Upon maturation, a fish was removed from its experimental tank, weighed (to 0.001 g) and measured (standard length, from the tip of snout to the caudal peduncle to the nearest 0.1 mm), and then placed into a separate tank. A total of 95 fish reached sexual maturity, 36 females and 9 males from the long-sworded treatment and 33 females and 17 males from the short-sworded treatment. Mortality always affected the smallest individuals within a tank and was similar between treatments (16/61 for long-sworded and 12/62 for short-sworded; Χ12=0.46, p>0.1). There was no significant difference between the treatments in sex ratio (=number of females/total number of fry) (median (25th, 75th percentile)=1.0 (0.59, 1.0) long-sworded treatment, 1.0 (0.33, 1.0) short-sworded treatment, Mann–Whitney U-test, N=13, Z=−0.799, p=0.479), the order of sexes within the size hierarchy of the group (i.e. whether the largest fish in the group was male or female, etc.) (median (25th, 75th percentile) position of females in the hierarchy=second (1.25, 3.75) long-sworded treatment, third (2.0, 3.0) short-sworded treatment, Mann–Whitney U-test, N=69, Z=−0.210, p=0.834) or the order in which males and females matured (median (25th, 75th percentile)=second (1.0, 2.75) long-sworded treatment females, second (1.0, 2.0) short-sworded treatment females, Mann–Whitney U-test, N=69, Z=−0.122, p=0.903).
(d) Statistical analysis
All data were tested for normality and homogeneity of variance and treated accordingly. Analyses were carried out using SPSS v. 12.0 or S-Plus v. 2000. General linear mixed effects models were used to analyse the age and the size at maturation, with treatment and sex as fixed factors and family as a random factor.
3. Results
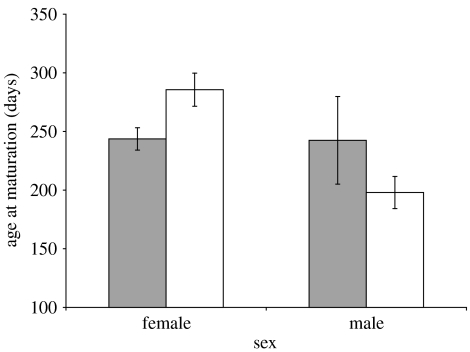
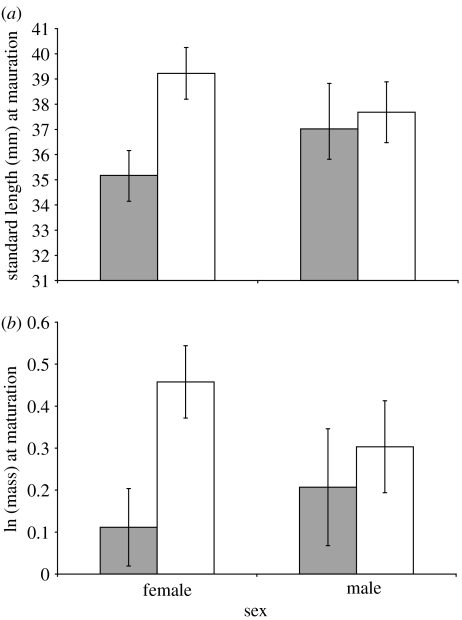
We compared the age and the size at maturation of both males and females maturing from the two treatment groups, while controlling for family identity. Analysis of age at maturation revealed a significant effect of treatment (linear mixed effects model, t79=2.59, p=0.01) and a significant interaction between treatment and sex (linear mixed effects model, t79=−3.01, p=0.004; figure 1). Females reared in the long-sworded treatment matured earlier than those in the short-sworded treatment, but this pattern was reversed in males. Analysis of size at maturation (both standard length and mass) revealed a significant effect of treatment (for standard length, linear mixed effects model, t81=3.07, p=0.003; for mass (ln transformed), linear mixed effects model, t81=2.95, p=0.004), but no interaction between treatment and sex (for standard length, t79=−1.19, p=0.24; for mass (ln transformed), t79=−0.95, p=0.35; thus, these interactions are not included in the previous models; figure 2a,b). Females reared in the long-sworded treatment matured at a smaller size than females reared in the short-sworded treatment, whereas there was no significant difference between the treatments in the size of males.
Figure 1.
Mean age (days) (±s.e.) at maturation for males and females in the two treatments. Dark bars represent the long-sworded treatment, clear bars the short-sworded treatment. See text for the statistical analysis. Females matured earlier in the long-sworded treatment than in the short-sworded treatment, while the converse was true for males.
Figure 2.
(a) Mean standard length (mm) (±s.e.) at maturation for males and females in the two treatments and (b) mean mass (ln transformed) at maturation for males and females in the two treatments (±s.e.). Dark bars represent the long-sworded treatment, clear bars the short-sworded treatment. See text for the statistical analysis. Females from the long-sworded treatment were smaller than females in the short-sworded treatment, whereas males did not differ in size between the treatments.
4. Discussion
The results of this experiment suggest that the age and the size at sexual maturation of green swordtails are plastic in response to visual cues, indicative of the current distribution of male phenotypes in the population. Females matured earlier and at a smaller size (whereas males matured later), when all of the mature males that they had seen had longer as opposed to shorter swords.
Earlier maturation of females exposed to visual cues of longer as opposed to shorter swords should reduce the risk of mortality prior to reproduction by reducing the time spent as a juvenile (Stearns 1992), and allow females to take advantage of the opportunities for mating with males that have large sexual ornaments. In contrast, the delayed maturation of females that saw only shorter-sworded males should allow females to delay the costs associated with being sexually mature and grow for longer. Therefore, by reaching a larger size at maturation, they have the potential for higher fecundity (Andersson 1994) and possibly better offspring quality (Clutton-Brock et al. 1988). An apparent dearth of high-quality males would reduce the perceived costs of this delay in reproduction. In the short-sworded treatment group, males maturing earlier would reduce the risk of mortality before having had an opportunity to reproduce, while escaping costs of early maturation (i.e. a small total size) owing to the perceived lack of high-quality competitors.
To the best of our knowledge, this is the first experimental demonstration in any species that (i) the age and the size at sexual maturation are responsive to solely visual cues, (ii) they are responsive to cues relating to the quality of current mating opportunities, or (iii) these responses can be in opposite directions in males and females. A recent experiment using redback spiders (Latrodectus hasselti) demonstrated that males mature earlier when pheromonal cues of females were present than when they were absent, but this study did not look at the effects of mating opportunities on female maturation (Kasumovic & Andrade 2006). To date, most research into the effects of different stimuli on the timing of sexual maturation in vertebrates has focused primarily on chemical cues. Our study indicates that we must expand that sensory arena, including at least visual and, possibly, acoustic cues in our investigations. It would also be interesting to look at whether or not visual cues of other environmental variables (such as predation risk) can affect these life-history traits.
Acknowledgments
We thank J. Laurie for animal husbandry and K. E. Arnold, D. T. Haydon, D. McLennan, P. Monaghan and one anonymous reviewer for comments on this manuscript. C.A.W was funded on a Natural Environment Research Council (NERC) studentship.
References
- Aday D.D, Wahl D.H, Philipp D.P. A mechanism for social inhibition of sexual maturation in bluegill. J. Fish Biol. 2003;62:486–490. doi:10.1046/j.1095-8649.2003.00033.x [Google Scholar]
- Andersson M. Princeton University Press; Princeton, NJ: 1994. Sexual selection. [Google Scholar]
- Basolo A.L. Female preference predates the evolution of the sword in swordtail fish. Science. 1990;250:808–810. doi: 10.1126/science.250.4982.808. doi:10.1126/science.250.4982.808 [DOI] [PubMed] [Google Scholar]
- Basolo A.L. Shift in investment between sexually-selected traits: tarnishing of the silver spoon. Anim. Behav. 1998;55:665–671. doi: 10.1006/anbe.1997.0634. doi:10.1006/anbe.1997.0634 [DOI] [PubMed] [Google Scholar]
- Basolo A.L, Alcaraz G. The turn of the sword: length increases male swimming costs in swordtails. Proc. R. Soc. B. 2003;270:1631–1636. doi: 10.1098/rspb.2003.2388. doi:10.1098/rspb.2003.2388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basolo A.L, Wagner W.E. Covariation between predation risk, body size and fin elaboration in the green swordtail, Xiphophorus helleri. Biol. J. Linn. Soc. 2004;83:87–100. doi:10.1111/j.1095-8312.2004.00369.x [Google Scholar]
- Beaugrand J.P, Payette D, Goulet C. Conflict outcome in male green swordtail fish dyads (Xiphophorus helleri): interaction of body size, prior dominance/subordination experience, and prior residency. Behaviour. 1996;133:303–319. [Google Scholar]
- Benson K.E, Basolo A.L. Male–male competition and the sword in male swordtails, Xiphophorus helleri. Anim. Behav. 2006;71:129–134. doi:10.1016/j.anbehav.2005.05.004 [Google Scholar]
- Bentley G.E, Demas G.E, Nelson R.J, Ball G.F. Melatonin, immunity and cost of reproductive state in male European starlings. Proc. R. Soc. B. 1998;265:1191–1195. doi: 10.1098/rspb.1998.0418. doi:10.1098/rspb.1998.0418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borowsky R.L. Social control of adult size in males of Xiphophorus variatus. Nature. 1973;245:333–335. doi:10.1038/245332a0 [Google Scholar]
- Campton D.E. Heritability of body size of green swordtails, Xiphophorus helleri 1. Sib analyses of males reared individually and in groups. J. Hered. 1992;83:43–48. [Google Scholar]
- Clutton-Brock T.H, Albon S.D, Guinness F.E. Reproductive success in male and female red deer. In: Clutton-Brock T.H, editor. Reproductive success. University of Chicago Press; Chicago, IL: 1988. pp. 325–343. [Google Scholar]
- Johnson J.B, Basolo A.L. Predator exposure alters female mate choice in the green swordtail. Behav. Ecol. 2003;14:619–625. doi:10.1093/beheco/arg046 [Google Scholar]
- Kasumovic M.M, Andrade M.C.B. Male development tracks rapidly shifting sexual versus natural selection pressures. Curr. Biol. 2006;16:R242–R243. doi: 10.1016/j.cub.2006.03.017. doi:10.1016/j.cub.2006.03.017 [DOI] [PubMed] [Google Scholar]
- Marcus J.M, McCune A.R. Ontogeny and phylogeny in the Northern swordtail clade of Xiphophorus. Syst. Biol. 1999;48:491–522. doi:10.1080/106351599260111 [Google Scholar]
- Pereira R, Teal P.E.A, Sivinski J, Dueben B.D. Influence of male presence on sexual maturation in female Caribbean fruit fly, Anastrepha suspensa (Diptera: Tephritidae) J. Insect Behav. 2006;19:31–43. doi:10.1007/s10905-005-9011-2 [Google Scholar]
- Rekwot P.I, Ogwu D, Oyedipe E.O, Sekoni V.O. The role of pheromones and biostimulation in animal reproduction. Anim. Reprod. Sci. 2001;65:157–170. doi: 10.1016/s0378-4320(00)00223-2. doi:10.1016/S0378-4320(00)00223-2 [DOI] [PubMed] [Google Scholar]
- Rodd F.H, Reznick D.N, Sokolowski M.B. Phenotypic plasticity in the life history traits of guppies: responses to social environment. Ecology. 1997;78:419–433. doi:10.2307/2266018 [Google Scholar]
- Roff D.A. Chapman and Hall; New York, NY: 1992. The evolution of life histories: theory and analysis. [Google Scholar]
- Rosenthal G.G, Evans C.S. Female preference for swords in Xiphophorus helleri reflects a bias for large apparent size. Proc. Natl Acad. Sci. USA. 1998;95:4431–4436. doi: 10.1073/pnas.95.8.4431. doi:10.1073/pnas.95.8.4431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royle, N. J., Metcalfe, N. B. & Lindström, B. In preparation. Compensatory resource allocation and sexual selection in green swordtail fish.
- Stearns S.C. Oxford University Press; Oxford, UK: 1992. The evolution of life histories. [Google Scholar]